Abstract
Priapism, a prolonged penile erection in the absence of sexual arousal, is common among sickle cell disease (SCD) patients. Hypogonadism is also common in SCD patients. While administration of exogenous testosterone reverses hypogonadism, it is contraceptive. We hypothesized that stimulation of endogenous testosterone production decreases priapism by normalizing molecular signaling involved in penile erection without decreasing intratesticular testosterone production, which would affect fertility. Treatment of SCD mice with FGIN-1–27, a ligand for TSPO that mobilizes cholesterol to the inner mitochondrial membrane, resulted in eugonadal levels of serum testosterone without decreasing intratesticular testosterone production. Normalized testosterone levels, in turn, decreased priapism. At the molecular level, TSPO restored phosphodiesterase (PDE)5 activity and decreased NADPH oxidase-mediated oxidative stress in the penis, which are major molecular signaling molecules involved in penile erection and are dysregulated in SCD. These results indicate that pharmacologic activation of TSPO could be a novel, targetable pathway for treating hypogonadal men, particularly patients with SCD, without adverse effects on fertility.
Keywords: PDE5, oxidative stress, NADPH oxidase, penis, erection
1. INTRODUCTION
Sickle cell disease (SCD) is the most common inherited blood disorder in the United States, which affects an estimated 100,000 Americans, mostly African-Americans, and millions of people throughout the world (Telen et al., 2019). Priapism, a prolonged and painful penile erection in the absence of sexual arousal or desire, is common in male patients with SCD. Repeated episodes of priapism may cause tissue damage and fibrosis of the corpora cavernosa, leading to irreversible erectile dysfunction (ED). The prevalence of ED associated with recurrent ischemic priapism in SCD patients is as high as 47.5% (Anele et al., 2015).
A principal molecular mechanism of priapism involves downregulated endothelial nitric oxide synthase (eNOS) / phosphodiesterase type 5 (PDE5) expressions in the penis (Musicki & Burnett, 2020), allowing unrestrained penile tissue relaxation. At present, pharmacologic treatments of SCD-associated priapism are often ineffective, and surgical interventions are commonly necessary treatment options (Anele et al., 2015).
Male patients with SCD exhibit decreased serum testosterone levels, with rates of testosterone deficiency as high as 25%. This is associated with impaired physical and sexual maturation, as well as other commonly described hypogonadism symptomatic effects, e.g., reduced libido, ED, decreased physical strength, and easy fatigability (Huang & Muneyyirci-Delale, 2017). The mechanism for reduced testosterone production in SCD has been elucidated in a SCD mouse model. Increased oxidative stress in the testis, related to NADPH oxidase activation, is associated with reduced protein expression of a steroidogenic acute regulatory protein (STAR). Reduced STAR expression limits the supply of cholesterol to the mitochondria in the Leydig cells, contributing to primary hypogonadism (Musicki et al., 2015). A proof-of principle study reported that giving exogenous testosterone to SCD mice prevented priapism through upregulation of PDE5 in the penis, which controls excessive erectile tissue relaxation (Musicki et al., 2018).
Administration of exogenous testosterone is, however, associated with multiple adverse side effects. Administering exogenous testosterone increases serum testosterone, which, through a negative feedback effect on LH, suppresses Leydig cell-stimulated testosterone production, resulting in reduced spermatogenesis (Hamada et al., 2012). Because of this contraceptive effect, exogenous testosterone is inappropriate for young men with hypogonadism who wish to retain reproductive function. Fertility preservation is an increasingly advocated health objective in SCD (Smith-Whitley 2014). Alternative means by which to increase serum testosterone levels, preferably through increasing endogenous testosterone production, would be highly desirable.
Translocator protein (TSPO) is a protein constituent of the transduceosome. The transduceosome is an ensemble of mitochondrial and cytosolic proteins responsible for cholesterol translocation from intracellular stores to the inner mitochondrial membrane, which is the rate-limiting step in steroid formation (Papadopoulos et al., 2018). TSPO takes up free cholesterol from the cytoplasm in response to ligand induction and transfers it to the inner mitochondrial membrane for cleavage to pregnenolone. Animal and cell studies demonstrated that high affinity TSPO drug ligands increase Leydig cell testosterone production (Chen et al., 2019; Chung et al., 2013; Chung et al., 2020). Similarly, clinical studies showed that TSPO-activating ligands increase neurosteroid formation in men (Rupprecht et al., 2010).
We hypothesized that pharmacologic activation of TSPO with a specific drug ligand FGIN-1–27 induces SCD mouse Leydig cells to produce testosterone; normalized testosterone levels, in turn, decrease priapism by normalizing molecular signaling involved in penile erection without decreasing intratesticular testosterone production.
2. MATERIALS AND METHOS
2.1. Mouse model of human SCD
Transgenic SCD and WT male mice, 6–8 months old, were used. Berkeley SCD mice have targeted deletions of murine α and β globins with a transgene containing human α and β globins (Paszty et al., 1997), thus SCD mice represent a suitable animal model for human SCD. Breeding pairs for SCD mice (strain number 3342) were obtained from Jackson Laboratory (Bar Harbor, ME), and were housed and bred in-house. Genotyping was performed by Transnetyx, Inc (Cordova, TN). Mice received routine NIH rodent chow and water. All animal procedures were conducted at the Johns Hopkins University in accordance with the ethical standards of the Johns Hopkins University School of Medicine Guidelines for the Care and Use of Laboratory Animals, and they complied with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol and procedures employed were approved by Johns Hopkins University Animal Care and Use Committee (animal protocol number MO19M442). After completion of the procedure, animals were euthanized by intracardial injection of a saturated solution of potassium chloride to anesthetized animals. All efforts were made to minimize animal suffering.
2.2. FGIN-1–27 treatment and tissue collection
WT and SCD mice were injected with a synthetic ligand for TSPO, N,N-Dihexyl-2-(4-fluorophenyl)indole-3-acetamide (FGIN-1–27), 0.5 mg/kg/day, intraperitoneal injections daily, or vehicle (6% DMSO in saline) for nine days (Chung et al., 2013). Three hours after the last FGIN injection, anesthetized mice (isoflurane 1–1.5% inhalation) underwent erectile function measurements, followed by blood and testis collection for LH and serum and intratesticular testosterone, and testicular TSPO measurements, respectively, and penis collection for molecular or organ bath studies. Seminal vesicles (testosterone-responsive tissue) were removed and weighed, and their weight per body weight was used as a bioassay for testosterone concentrations (van Roijen et al., 1997). To reduce daily fluctuations in testosterone levels, blood and tissue collections were performed in the morning.
2.3. Blood sampling and testosterone measurement
Blood was collected by cardiac puncture from anesthetized mice, kept for 1h at 4 C for testosterone measurements, or at room temperature for LH measurements, then centrifuged at 3,000 x g for 10 minutes. Serum was separated and kept at −80 C. Serum LH was measured at the University of Virginia, Center for Research in Reproduction, by immunoradiometric assay (Fallest et al., 1995). Serum and intratesticular testosterone levels were measured by radioimmunoassay (RIA). The detection limit was 10 pg/ml and intra- and inter-variabilities were 8.7% and 11.2%, respectively (Musicki et al., 2015).
2.4. In vivo erection studies
Intracavernosal pressure (ICP) responses induced by cavernous nerve electrical stimulation were measured as described previously (Musicki et al., 2018). Briefly, under anesthesia, the cavernous nerve was isolated via a midline abdominal incision, and the crura of the penis were identified. A 30-gauge needle connected to polyethylene tubing (PE-10) was inserted into the crura and connected to a pressure transducer (DI-190; Dataq Instruments, Akron, OH). The cavernous nerve was stimulated at 1, 2, and 4 V at 16 Hz with a 5-ms square-wave duration for 1 minute using a Grass Instruments S48 stimulator (Quincy, MA). ICP was recorded using the DI-190 system from 1 min before the start of electrical stimulation until 3 min after stimulation ended. To monitor mean arterial pressure (MAP), the right carotid artery was cannulated with PE-10 tubing filled with heparinized saline (100 U/ml). Response parameters, calculated using MATLAB software, were corrected for baseline, and expressed per MAP. Statistical analysis was performed on detumescence time (a period from the end of cavernous nerve electrical stimulation to a point showing 50% of maximal ICP) averaged for all voltages.
2.5. Functional studies in cavernous tissue
Penile tissue was removed under a dissection microscope, and the penile shaft was separated from the glans penis. The penile shafts were placed in ice-cold Krebs solution of the following composition (in mM): NaCl 130, KCl 4.7, KH2PO4 1.18, MgSO4 1.18, NaHCO3 14.9, dextrose 5.6, CaCl2 1.56, in distilled water. The urethra, dorsal vein, and connective tissues were carefully excised from the penile shaft in chilled Krebs solution. Erectile tissue was mounted in a DMT 820MS muscle strip myograph system (Danish Myo Technology, Aarhus, Denmark) coupled to a PowerLab 8/30 data acquisition system (ADInstruments, Colorado Springs, CO) for isometric tension measurement and recording with LabChart Pro software (ADInstruments). Tissues were bathed in Krebs solution maintained at 37°C and continuously bubbled with a 95% O2 and 5% CO2 mixture. Tissues were allowed to equilibrate for 1 hour, stretched to a resting tension of 4 mN, followed by an additional 1 hour of equilibration. Tissue viability and contractile function were tested with high potassium (120 mM) Krebs solution, with KCl substituted for NaCl. Following successive washes with Krebs solution to achieve a stable resting tension, tissues were constricted with 10 μM phenylephrine (PE), and the sensitivity to NO was tested with a cumulative dose-response (0.001–3.0 μM) of the NO-donor sodium nitroprusside (SNP). Relaxations to SNP were normalized as a percentage restoration to the resting tension from the PE pre-constricted value (La Favor et al., 2013).
2.6. Western blot analysis
Penile and testis samples were snap-frozen in liquid nitrogen, and stored at −80°C until processing for Western blot analyses. Penes and testes were homogenized as previously described (Hurt et al., 2002). Protein concentration was determined using Bicinchoninic Acid method. Homogenates (50–70 μg) were resolved on 4–20%Tris gels and transferred to PVDF membranes. Membranes were blocked for 1 hour at room temperature in PBS (pH 7.4) containing 0.1% Tween-20 and 5% nonfat dry milk, and then probed overnight at 4 C in PBS containing 0.1% Tween-20 and 3% nonfat dry milk with primary antibodies. For Western blots of penile samples, the following antibodies were used: rabbit anti-PDE5 (1:500 dilution, Abcam Inc., catalog number ab64179), rabbit anti phospho (P)-PDE5 (Ser-92) (1:450 dilution, Fabgennix, catalog number PPD5A-140AP), rabbit anti-nitrotyrosine (1:2,000 dilution, Abcam Inc., catalog number ab42789) or rabbit anti-p47phox (1:1,000 dilution, ThermoFisher Scientific, catalog number PA1–9073). For Western blots of testis samples, rabbit anti-TSPO antibody (1:1,000 dilution, Millipore Sigma, catalog number SAB1405525) was used. All signals were standardized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:5,000 dilution, Santa Cruz Biotechnology, catalog number 15734) or β-actin (1:7,000 dilution, Millipore Sigma, catalog number A5316) on stripped membranes (Musicki et al., 2018; Musicki et al., 2012). Bands were detected by horseradish peroxidase conjugated anti-mouse or anti-rabbit secondary antibodies (1:7,000 dilutions, GE Healthcare, catalog numbers NA931V and NA934V). Protein bands were visualized by Amersham ECL Western Blotting Detection Reagent (catalog number RPN2106) and quantified using NIH Image 1.29 software. Results were expressed relative to that of WT mice treated with vehicle. The analysis of nitrotyrosine is a densitometric composite of all proteins in each lane. Due to high cost of antibodies, membranes were cut before performing western blots, except for nitrotyrosine.
2.7. Statistical analysis
The program GraphPad Prism 6 (GraphPad Software) was used for statistical analysis. Statistical analysis was performed by using one-way analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test (between treatment groups) or by modified t-test (between control and each treatment group for Western blot results). Group differences for myograph experiments were determined by two-way repeated measures ANOVA followed by Tukey’s multiple comparisons post-hoc testing. The quantitative values were represented as mean ± standard error of the mean (SEM). A value of P < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. TSPO protein expression is reduced in the testis of SCD mice
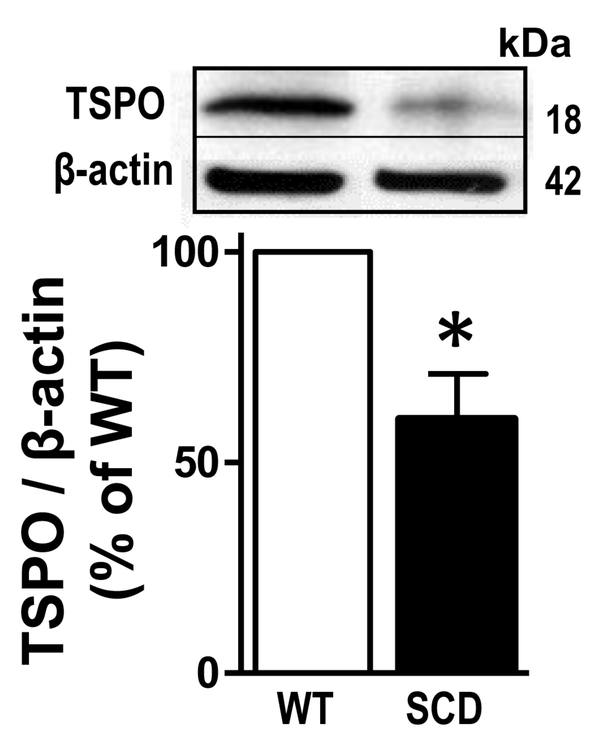
Protein expression of TSPO, a major protein within the transduceosome that is involved in cholesterol translocation into the mitochondria of Leydig cells, was significantly (P<0.05) decreased in SCD mouse testis compared to that of WT (n=4) (Fig. 1). This finding identified a possible mechanism for reduced testosterone biosynthesis in SCD, associated with decreased cholesterol transport in Leydig cells of the testis, supporting our rationale to use a TSPO ligand for treating testosterone deficiency.
Fig. 1.
TSPO protein expression was decreased in the testis of SCD compared to WT mouse testis. Representative Western blot and quantification of TSPO in the testis isolated from WT and SCD mice. Protein level of TSPO was analyzed by immunoblotting and normalized to β-actin expression. Data are expressed as mean ± SEM. Statistical analysis was performed by modified t-test. The value of the control group is regarded as 100%. n = 4. *P < 0.05. SCD, sickle cell disease.
3.2. FGIN-1–27 treatment restores serum and intratesticular testosterone and serum LH levels in SCD mice
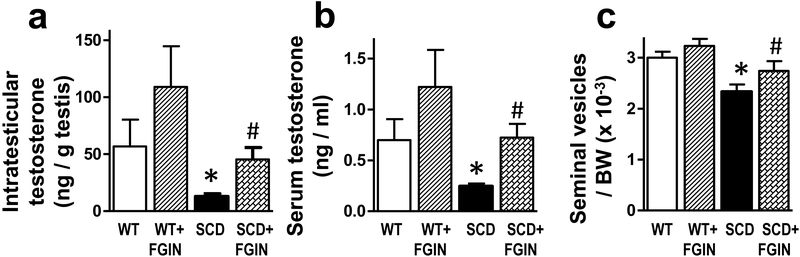
Serum testosterone levels, intratesticular testosterone levels, and seminal vesicles (testosterone-responsive tissue) weight per body weight were significantly (P<0.05) lower in vehicle-treated SCD mice as compared to vehicle-treated WT mice (n=10–17) (Fig. 2a–c). TSPO high affinity agonist FGIN-1–27 treatment increased all three measured parameters in SCD mice to levels comparable to that of WT mice, indicating restored serum and intratesticular testosterone concentrations in SCD mice by the treatment. FGIN-1–27 did not affect testosterone levels or seminal vesicle weights in WT mice.
Fig. 2.
Intratesticular testosterone, serum testosterone, and the weight of seminal vesicles/body weight were decreased in SCD compared to WT mice, and were increased in SCD mice by FGIN-1–27 treatment. WT and SCD mice were injected with a TSPO ligand FGIN-1–27 (0.5 mg/kg/day, intraperitoneal injections daily) or vehicle (6% DMSO in saline) for nine days. (a) Intratesticular and (b) serum testosterone were measured by radioimmunoassay (RIA). (c) Seminal vesicles were removed and weighed, and their weight per body weight was used as a bioassay for testosterone concentrations. Data are expressed as mean ± SEM. Statistical analysis was performed by one-way analysis of variance, followed by Newman-Keuls multiple comparison test. n = 10–17. *, P<0.05 vs WT; #, P<0.05 vs SCD. SCD, sickle cell disease.
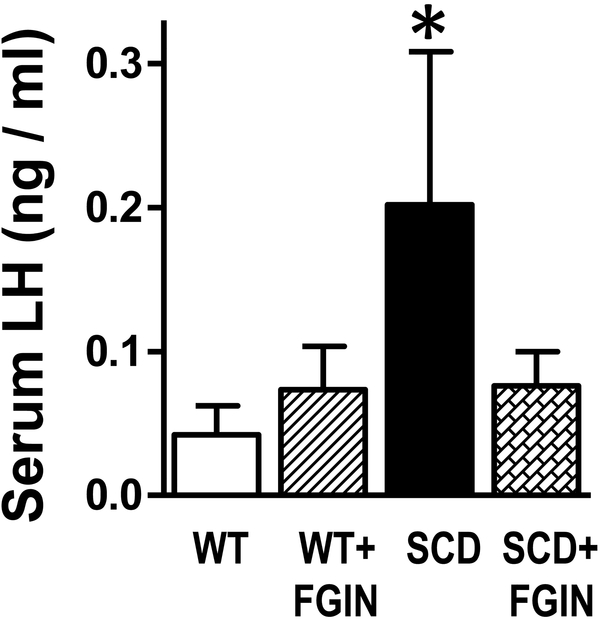
Serum LH levels were significantly (P<0.05) increased in vehicle-treated SCD mice as compared to vehicle-treated WT mice measurements (n=7–10) (Fig. 3), consistent with the observation that serum testosterone was reduced. FGIN-1–27 treatment decreased serum LH levels in SCD mice 2.6-fold, but due to high variability, these values did not differ significantly (P=0.1074) from the values in vehicle-treated SCD mice. FGIN-1–27 did not affect LH levels in WT mice.
Fig. 3.
Serum LH levels were increased in SCD compared to WT mice, and were decreased by FGIN-1–27 treatment. Serum LH was measured by immunoradiometric assay in WT and SCD mice treated with a TSPO ligand or vehicle for nine days. Data are expressed as mean ± SEM. Statistical analysis was performed by one-way analysis of variance, followed by Newman-Keuls multiple comparison test. n = 7–10. *P < 0.05 vs WT. SCD, sickle cell disease.
3.3. FGIN-1–27 treatment corrects prolonged detumescence in SCD mice
Following termination of cavernous nerve electrical stimulation, detumescence time/MAP, indicative of priapism, was significantly (P<0.05) increased in vehicle-treated SCD compared with vehicle-treated WT mice (n=5–7) (Fig. 4a). FGIN-1–27 significantly (P<0.05) decreased detumescence time/MAP in SCD mice, indicating correction of prolonged poststimulation erectile responses by this treatment. FGIN-1–27 did not affect detumescence in WT mice.
Fig. 4.
Priapic activity and reactivity to NO were increased in SCD compared to WT mice and were decreased by FGIN-1–27 treatment. (a) Priapic activity was determined by measuring the time to detumescence following one minute of electrical stimulation of the cavernous nerve in WT and SCD mice treated with a TSPO ligand or vehicle for nine days. Data are expressed as mean ± SEM. Statistical analysis was performed by one-way analysis of variance, followed by Newman-Keuls multiple comparison test. (b) The smooth muscle-dependent relaxation response to the NO donor SNP was assessed with a cumulative dose-response (0.001–3.0 μM) of SNP to mouse corpus cavernosum strips pre-constricted with 10 μM phenylephrine (PE). Group differences were determined by two-way repeated measures ANOVA followed by Tukey’s multiple comparisons post-hoc testing. n=5–7. * P<0.05 SCD vs WT; # P<0.05 SCD vs SCD + FGIN. SCD, sickle cell disease; SNP, donor sodium nitroprusside.
3.4. FGIN-1–27 treatment decreases NO-sensitivity in SCD mouse corpus cavernosum
Relaxation of the corpus cavernosum to the NO-donor sodium nitroprusside (SNP) was significantly (P<0.01) increased at intermediate doses in vehicle-treated SCD compared with vehicle-treated WT mice (n=5–7) (Fig. 4b). FGIN-1–27 treatment of SCD mice reduced the relaxation response to SNP at these doses (P<0.05) compared to that of vehicle-treated SCD mice. FGIN-1–27 treatment did not affect SNP responsiveness in WT mice.
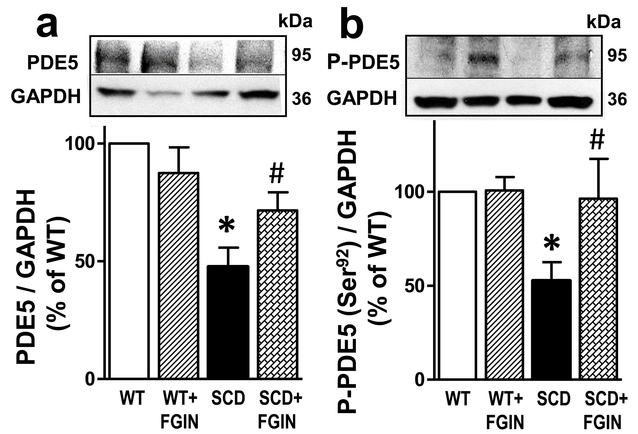
3.5. FGIN-1–27 treatment corrects downregulated PDE5 and P-PDE5 (Ser-92) protein expressions in the SCD mouse penis.
PDE5 and P-PDE5 (Ser-92) protein expressions were significantly (P<0.05) decreased in the penis of vehicle-treated SCD compared to vehicle-treated WT mice (n=5–8) (Fig. 5a,b). FGIN-1–27 treatment of SCD mice significantly (P<0.05) increased PDE5 and P-PDE5 (Ser-92) protein expressions to levels similar to that found in WT mice, indicating that testosterone normalized downregulated PDE5 activity and protein expression in the SCD mouse penis. FGIN-1–27 did not affect P-PDE5 or PDE5 protein expressions in the penis of WT mice.
Fig. 5.
Protein expressions of PDE5 and P-PDE5 (Ser-92) were decreased in the SCD compared to WT mouse penis, and were increased in the SCD mouse penis by FGIN-1–27 treatment. Representative Western blots and quantification of PDE5 (a) and P-PDE5 (Ser-92) (b) in penes isolated from WT and SCD mice treated with a TSPO ligand or vehicle for nine days. Protein levels of PDE5 and P-PDE5 (Ser-92) were analyzed by immunoblotting and normalized to GAPDH protein expression. Data are expressed as mean ± SEM). Statistical analysis was performed by using one-way analysis of variance, followed by Newman-Keuls multiple comparison test (between treatment groups) and by modified t-test (between control and each treatment group). n=5–8. *, P<0.05 vs WT; #, P<0.05 vs SCD. SCD, sickle cell disease; PDE5, phosphodiesterase type 5
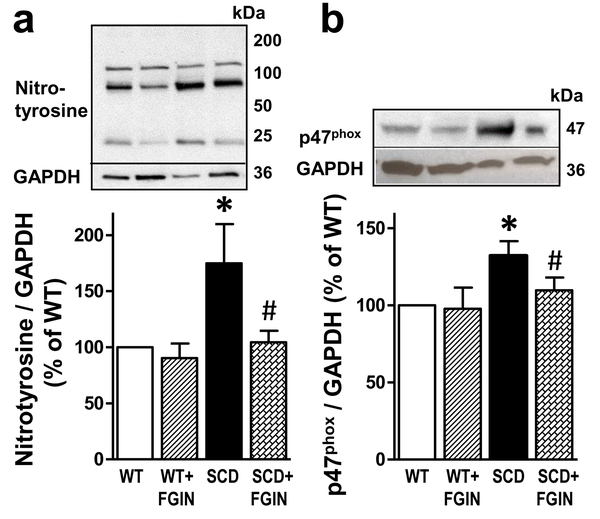
3.6. FGIN-1–27 treatment corrects increased oxidative stress and upregulated protein expression of NADPH oxidase subunit gp47phox in the SCD mouse penis.
Compared with that of the WT mouse penis, the amount of nitrotyrosine, a biomarker for peroxynitrite (the reaction product of NO with superoxide (Bartesaghi & Radi, 2018)), was significantly (P<0.05) increased in the penis of vehicle-treated SCD mice (n=5–6) (Fig. 6a). FGIN-1–27 significantly (P<0.05) decreased nitrotyrosine to levels similar to that found in WT mice. Similarly, protein expression of NADPH oxidase regulatory subunit gp47phox was significantly (P<0.05) increased in the penis of vehicle-treated SCD compared with vehicle-treated WT mice, and was significantly (P<0.05) reduced by FGIN-1–27 treatment (n=5–6) (Fig. 6b). FGIN-1–27 did not affect nitrotyrosine or gp47phox protein expressions in the penis of WT mice.
Fig. 6.
Protein expressions of nitrotyrosine and p47phox subunit of NADPH oxidase were increased in the SCD compared to WT mouse penis, and were decreased in the SCD mouse penis by FGIN-1–27 treatment. Representative Western blots and quantification of nitrotyrosine (a) and p47phox (b) in penes isolated from WT and SCD mice treated with a TSPO ligand FGIN-1–27 or vehicle for nine days. Protein levels of nitrotyrosine and p47phox were analyzed by immunoblotting and normalized to GAPDH expression. Statistical analysis was performed by using one-way analysis of variance, followed by Newman-Keuls multiple comparison test (between treatment groups) and by modified t-test (between control and each treatment group). Data represent means ± SEM (n=5–6). *, P<0.05 vs WT; #, P<0.05 vs SCD. SCD, sickle cell disease.
4. DISCUSSION
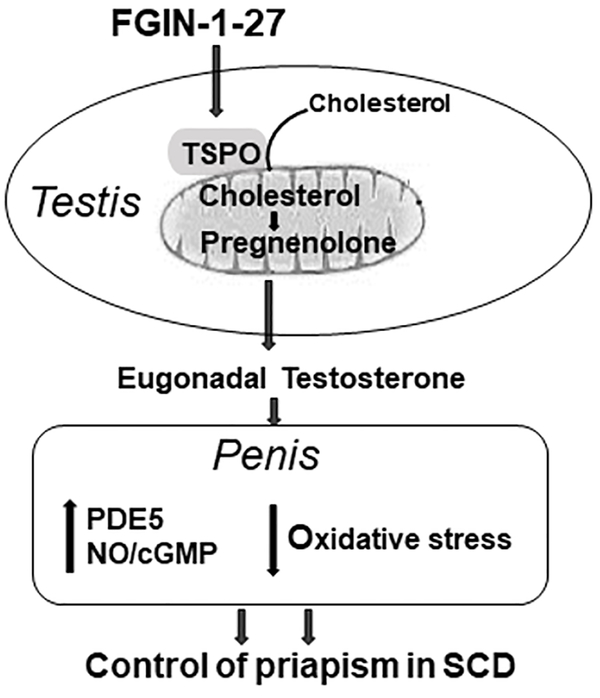
The results of the present study demonstrate that increasing testicular testosterone production using a TSPO ligand reversed primary hypogonadism and decreased priapism in SCD mice in association with restored PDE5 activity and decreased NADPH oxidase-mediated oxidative stress in the penis. The summary of FGIN-1–27 effect on testosterone levels and molecular signaling in the penis in the SCD mouse model is presented in Figure 7. Importantly, administering FGIN-1–27 to SCD mice, by stimulating the Leydig cells themselves to produce testosterone, increased serum testosterone to eugonadal levels and partially normalized serum LH levels, but did not diminish intratesticular testosterone levels. These findings establish that normalized testicular production of testosterone is important for controlling priapism in SCD without affecting intratesticular testosterone levels, which would ultimately preserve fertility.
Fig. 7.
Testosterone deficiency in patients and mice with SCD is associated with priapism. Normalization of testosterone levels in SCD mice, by stimulating endogenous testicular testosterone production, reverses molecular abnormalities involving PDE5/oxidative stress signaling in the penis and decreases priapism susceptibility. The success of reducing priapism by restoring physiologic testosterone levels without reducing intratesticular testosterone suggests a novel, targetable pathway for treating hypogonadal men, including individuals with SCD, without adversely affecting fertility. SCD, sickle cell disease; PDE5, phosphodiesterase type 5.
FGIN-1–27 increased serum testosterone levels in SCD mice to eugonadal levels, comparable to the levels measured in WT mice. The mechanism conceivably involves stimulation of testosterone formation by increasing TSPO-mediated cholesterol translocation to the inner mitochondrial membrane of the testicular Leydig cells required for steroidogenesis, as reported in previous studies (Chen et al., 2019; Chung et al., 2013; Chung et al., 2020). Cholesterol translocation is the rate-determining step in testosterone formation. Furthermore, a recent study reported at least transient increases in serum LH as well as serum testosterone in response to TSPO drug ligand administration, thus raising the possibility that this approach increases Leydig cell testosterone production not only by stimulating cholesterol translocation but also by increasing LH synthesis or release (Chen et al., 2019).
We acknowledge conflicting evidence of the precise role and extent of involvement of TSPO in mitochondrial cholesterol import (Selvaraj et al., 2018). Several studies reported no effect of TSPO knockout on steroidogenesis, questioning the role of TSPO in this process (Morohaku et al., 2014; Tu et al., 2014). However, other recent studies reported the loss of hormone-stimulated steroidogenesis by global or TSPO-specific knockout (Fan et al., 2015; Fan et al., 2018). These and other studies in Leydig cells and other steroidogenic cells (Chung et al., 2013; Chung et al., 2020; Cavallaro et al., 1992; Costa et al., 2018), and our present study, provide evidence that TSPO is involved in mediating steroid hormone formation.
Here, we show that FGIN-1–27 reversed the excessive post-stimulation erectile response in SCD mice, suggesting decreased priapic activity. These findings are in line with a study showing that exogenously administered testosterone in the form of pellets decreased priapism in SCD mice (Musicki et al., 2018), as well as with a recent report of reduced priapism occurrences in testosterone-deficient men with SCD receiving long-acting testosterone undecanoate injections (Morrison et al., 2013). Normalization of erectile function in SCD mice by testosterone presumably resulted from normalization of PDE5 function and decreased oxidative stress in the penis.
Decreased PDE5 activity in the penis of SCD mice (present results and Musicki et al., 2018; Silva et al., 2016a; Champion et al., 2005; Bivalacqua et al., 2013; Lagoda et al., 2014; Ning et al., 2014) and patients with SCD exhibiting priapism (Lagoda et al., 2013) is considered a primary molecular mechanism of priapism. As a result of PDE5 deficiency, cGMP excessively accumulates (because it cannot be degraded) in the erectile tissue upon neurologically mediated sexual stimulation or sleep-related erection and produces unrestrained corporal tissue relaxation resulting in priapism (Musicki & Burnett, 2020). Decreased PDE5 activity in the penis of SCD mice is also evidenced by heightened relaxation of penile strips to an NO donor SNP (because the NO effector, cGMP, cannot be degraded). Increased testosterone production by FGIN-1–27 treatment normalized PDE5 protein expression and activity in the penis of SCD mice. Because of the lack of an androgen response element in the PDE5 promoter (Lin et al., 2013), this effect of testosterone is believed to be indirect, mediated by increased NO-induced increased accumulation of intracellular cGMP. cGMP increases PDE5 protein expression through cGMP response sequences in the PDE5 promoter (Lin et al., 2001). cGMP-mediated activation of PKG also leads to phosphorylation of PDE5 on Ser-92, which increases its catalytic activity by stimulating binding of cGMP to the regulatory domain of the enzyme (Corbin et al., 2000). While in this study we did not measure NO or eNOS activity, we (Musicki et al., 2018) and others (Podlasek et al., 2016) have previously shown a stimulatory effect of testosterone on eNOS protein expression and activity in the penis. Normalized PDE5 activity in the SCD mouse penis by FGIN-1–27 treatment is further evidenced by attenuated relaxation of phenylephrine-contracted penile strips to increasing concentrations of the NO donor SNP (because the NO effector cGMP can now be degraded). Normalized PDE5 function in cavernous smooth muscle prevents excessive accumulation of cGMP upon neurostimulation and decreases priapic activity.
Testosterone deficiency is associated with oxidative stress in the vasculature, including that of the penis (Podlasek et al., 2016). Testosterone has been postulated to improve vascular function by acting as a vasodilator and reducing or inhibiting inflammation and oxidative stress (Kelly & Jones, 2013). Oxidative stress is increased in the erectile tissue of patients (Lagoda et al., 2013) and mice (Musicki et al., 2012; Silva et al., 2016a; Bivalacqua et al., 2013; Lagoda et al., 2014; Musicki et al., 2014; Kanika et al., 2010) with SCD. Increased ROS production in the SCD mouse penis has been attributed to upregulation of NADPH oxidase (Musicki et al., 2012; Bivalacqua et al., 2013; Musicki et al., 2014), activation of xanthine oxidase (Bivalacqua et al., 2013), and eNOS uncoupling (Musicki et al., 2012; Bivalacqua et al., 2013). NADPH oxidases are a family of enzymes that catalyze electron transfer from cytosolic NADPH to molecular oxygen to generate superoxide as its primary product (Zhang et al., 2019). Increased expression of NADPH oxidase subunits gp91phox, p47phox, and p67phox in the penis has been confirmed at the human level in association with SCD-associated priapism, but not for priapism that is unrelated to SCD (Lagoda et al., 2013). We now show that FGIN-1–27 reduced protein expression of NADPH oxidase regulatory subunit and reduced oxidative stress in the penis of SCD mice. Reduced oxidative stress lessens impaired endothelial NO signaling and eNOS function, contributing to normalized PDE function (Elahi et al., 2009). While the mechanism of testosterone’s effect on NADPH oxidase expression is not known, it may involve the improvement of endothelial function. In human endothelial cells, exogenous NO suppresses superoxide production by inhibiting NADPH oxidase activity via direct S-nitrosylation of p47phox (Selemidis et al., 2007) and inhibition of gp91phox and p47phox expressions (Duerrschmidt et al., 2006). In the mouse aorta, endogenous NO suppresses vascular p47phox protein expression and superoxide production (Harrison et al., 2010).
Several limitations of the current study should be acknowledged. First, only one mouse model of SCD, a Berkley mouse, was used. Berkley SCD mice are well characterized and mimic many pathologies seen in patients with SCD (Manci et al., 2006). Another mouse model of SCD, the Townes SCD mouse, was developed by the replacement of the mouse α-globin genes by human α-globin genes and mouse β-globin genes by human Aγ and βS globin genes (Wu et al., 2006). While these mice also exhibit a priapism phenotype, as shown in one study (Silva et al., 2016b), they are not well characterized for this purpose. Second, we acknowledge the limitation of the mouse species for studying spermatogenesis, because the mouse lacks androgen binding protein in the testis and does not require high intratesticular T, as required for humans or other mammalian species (Wang et al., 1989). Thus, we did not test spermatogenic endpoints with this TSPO ligand treatment. Third, we used only one TSPO ligand, FGIN-1–27. Future studies may include alternative TSPO drug ligands, such as benzodiazepine 4’-chlorodiazepam (Ro5–4864), XBD173, olesoxime (TRO19622), or PIGA1138 (Chung et al., 2013; Scholz et al., 2015; Bordet et al., 2007; Costa et al., 2016; Da Pozzo et al., 2016) to further establish the relevance of TSPO for testosterone biosynthesis in the SCD testis. Furthermore, future studies may also be extended to evaluating alternative protein components of the transduceosome (e.g., AAA domain-containing protein 3A [ATAD3A]) (Rone et al., 2012) for their testosterone biosynthetic roles and role in priapism.
In conclusion, normalization of testosterone levels in SCD mice, by stimulating endogenous testicular testosterone production, reverses molecular abnormalities involving PDE5/oxidative stress signaling in the penis and consequently decreases priapism susceptibility. The success of reducing priapism by restoring physiologic testosterone levels without reducing intratesticular testosterone in a SCD mouse model holds promise as a plausible therapy for hypogonadal men, including individuals with SCD, without adversely affecting fertility.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health [grant number NIH/NIDDK RO1DK093917, NIH/NIA R37AG021092, and NIH/NIDDK K01DK115540–01]. The University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) grant U54-HD28934. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
The data to support the findings of the present study are available from the corresponding author upon request.
REFERENCES
- Anele UA, Le BV, Resar LM, & Burnett AL (2015). How I treat priapism. Blood, 125, 3551–3558. 10.1182/blood-2014-09-55188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartesaghi S & Radi R (2018) Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biology, 14, 618–625. 10.1016/j.redox.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivalacqua TJ, Musicki B, Hsu LL, Berkowitz D,E, Champion HC, & Burnett AL. (2013). Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS. One 8, e68028 10.1371/journal.pone.0068028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet T, Buisson B, Michaud M, Drouot C, Galéa P, Delaage P, Akentieva NP, Evers AS, Douglas FC, Mariano AO, Lacapère J-J, Massaad C, Schumacher M, Steidl E-M, Maux D, Delaage M, Henderson CE, Pruss RM. (2007). Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J Pharmacol Exp Ther, 322, 709–20. 10.1124/jpet.107.123000. [DOI] [PubMed] [Google Scholar]
- Cavallaro S, Korneyev A, Guidotti A & Costa E (1992). Diazepam-binding inhibitor (DBI)-processing products, acting at the mitochondrial DBI receptor, mediate adrenocorticotropic hormone-induced steroidogenesis in rat adrenal gland. Proceedings of the National Academy of Sciences of the United States of America, 89, 10598–10602. 10.1073/pnas.89.22.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, & Burnett A,L (2005). Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proceedings of the National Academy of Sciences of the United States of America, 102, 1661–1666. 10.1073/pnas.0407183102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Lu H, Chen P, Zhao X, Guan X, Liang Q, Zirkin BR, Ye L, & Chen H (2019). Acute effects of the translocator protein (TSPO) drug ligand FGIN-1–27 on serum testosterone and LH levels in male sprague dawley rats. Biology of Reproduction, 100, 824–832. 10.1093/biolre/ioy220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Chen H, Midzak A, Burnett AL, Papadopoulos V & Zirkin BR (2013). Drug ligand-induced activation of translocator protein (TSPO) stimulates steroid production by aged brown Norway rat Leydig cells. Endocrinology, 154, 2156–2165. 10.1210/en.2012-2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Brown S, Chen H, Liu J, Papadopoulos V, & Zirkin B (2020). Effects of pharmacologically induced Leydig cell testosterone production on intratesticular testosterone and spermatogenesis. Biology of Reproduction, 102, 489–498. 10.1093/biolre/ioz174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JD, Turko IV, Beasley A & Francis SH (2000). Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. European Journal of Biochemistry 267, 2760–2767. 10.1046/j.1432-1327.2000.01297 [DOI] [PubMed] [Google Scholar]
- Costa B, Da Pozzo E, Giacomelli C, Barresi E, Taliani S, Settimo FD., Martini C. (2016). TSPO ligand residence time: a new parameter to predict compound neurosteroidogenic efficacy. Sci Rep. 11, 6, 18164 10.1038/srep18164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Da Pozzo E, Martini C (2018). Translocator protein and steroidogenesis. Biochem J. 475, 901–904. 10.1042/BCJ20170766. [DOI] [PubMed] [Google Scholar]
- Da Pozzo E, Giacomelli C, Costa B, Cavallini C, Taliani S, Barresi E, Settimo FD., Martini C. (2016). TSPO PIGA Ligands Promote Neurosteroidogenesis and Human Astrocyte Well-Being. Int J Mol Sci. 17, 1028 10.3390/ijms17071028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerrschmidt N, Stielow C, Muller G, Pagano PJ, & Morawietz H (2006). NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. Journal of Physiology 576, 557–567. 10.1113/jphysiol.2006.111070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi MM, Kong YX, & Matata BM (2009). Oxidative stress as a mediator of cardiovascular disease. Oxidative Medicine and Cellular Longevity, 2, 259–269. 10.4161/oxim.2.5.9441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallest PC, Trader GL, Darrow JM, & Shupnik MA (1995). Regulation of rat luteinizing hormone B gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biology of Reproduction, 53, 103–109. 10.1095/biolreprod53.1.103 [DOI] [PubMed] [Google Scholar]
- Fan J, Campioli E, Midzak A, Culty M, & Papadopoulos V (2015). Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proceedings of the National Academy of Sciences of the United States of America, 112, 7261–7266. 10.1073/pnas.1502670112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Wang K, Zirkin B & Papadopoulos V (2018). CRISPR/Cas9–mediated Tspo gene mutations lead to reduced mitochondrial membrane potential and steroid formation in MA-10 mouse tumor Leydig cells. Endocrinology, 159, 1130–1146. 10.1210/en.2017-03065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada AJ, Montgomery B & Agarwal A (2012). Male infertility: a critical review of pharmacologic management. Expert Opinion on Pharmacotherapy, 13, 2511–2531. 10.1517/14656566.2012.740011 [DOI] [PubMed] [Google Scholar]
- Harrison CB, Drummond GR, Sobey CG, & Selemidis S (2010). Evidence that nitric oxide inhibits vascular inflammation and superoxide production via a p47phox-dependent mechanism in mice. Clinical and Experimental Pharmacology and Physiology, 37, 429–434. 10.1111/j.1440-1681.2009.05317.x [DOI] [PubMed] [Google Scholar]
- Huang AW, & Muneyyirci-Delale O (2017). Reproductive endocrine issues in men with sickle cell anemia. Andrology, 5, 679–690. 10.1111/andr.12370 [DOI] [PubMed] [Google Scholar]
- Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, & Burnett AL (2002). Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proceedings of the National Academy of Sciences of the United States of America, 99, 4061–4066. 10.1073/pnas.052712499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanika ND, Melman A & Davies KP (2010). Experimental priapism is associated with increased oxidative stress and activation of protein degradation pathways in corporal tissue. International Journal of Impotence Research 22, 363–373. 10.1038/ijir.2010.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DM & Jones TH (2013). Testosterone: A vascular hormone in health and disease. Journal of Endocrinology 217, R47–R71. 10.1530/JOE-12-0582 [DOI] [PubMed] [Google Scholar]
- La Favor JD, Anderson E,J, Dawkins JT, Hickner RC & Wingard CJ (2013). Exercise prevents Western diet-associated erectile dysfunction and coronary artery endothelial dysfunction: response to acute apocynin and sepiapterin treatment. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 305, R423–R434. 10.1152/ajpregu.00049.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagoda G, Sezen SF, Cabrini MR, Musicki B, & Burnett AL (2013). Molecular analysis of erection regulatory factors in sickle cell disease associated priapism in the human penis. Journal of Urology, 189, 762–768. 10.1016/j.juro.2012.08.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagoda G, Sezen S,F, Hurt K,J, Cabrini MR, Mohanty DK, & Burnett AL (2014). Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. Faseb Journal, 28, 76–84. 10.1096/fj.13-228817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Chow S, Lau A, Tu R & Lue TF (2001). Identification and regulation of human PDE5A gene promoter. Biochemical and Biophysical Research Communications, 280, 684–692. 10.1006/bbrc.2000.4220 [DOI] [PubMed] [Google Scholar]
- Lin CS, Xin Z, Namiki M, Albersen M, Muller. D, & Lue TF. (2013). Direct androgen regulation of PDE5 gene or the lack thereof. International Journal of Impotence Research 25, 81–85. 10.1038/ijir.2013.11 [DOI] [PubMed] [Google Scholar]
- Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, & Coller BS (2006). Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood, 107, 1651–1658. 10.1182/blood-2005-07-2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BF, Reid M, Madden W,& Burnett AL (2013). Testosterone replacement therapy does not promote priapism in hypogonadal men with sickle cell disease: 12-month safety report. Andrology, 1, 576–582. 10.1111/j.2047-2927.2013.00084.x [DOI] [PubMed] [Google Scholar]
- Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, & Selvaraj V (2014). Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology, 155, 89–97. 10.1210/en.2013-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Liu T, Sezen S,F & Burnett AL. (2012). Targeting NADPH oxidase decreases oxidative stress in the transgenic sickle cell mouse penis. Journal of Sexual Medicine, 9, 1980–1987. 10.1111/j.1743-6109.2012.02798.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Bivalacqua TJ, Champion HC, & Burnett AL (2014). Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. Journal of Sexual Medicine 11, 424–430. 10.1111/jsm.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Zhang Y, Chen H, Brown T,R, Zirkin BR,. & Burnett A,L (2015). Mechanism of testosterone deficiency in the transgenic sickle cell mouse. PLoS One, 10, e0128694 10.1371/journal.pone.0128694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Karakus S, Akakpo W, Silva FH, Liu J, Chen H, Zirkin BR, & Burnett AL (2018). Testosterone replacement in transgenic sickle cell mice controls priapic activity and upregulates PDE5 expression and eNOS activity in the penis. Andrology, 6, 184–191. 10.1111/andr.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, & Burnett AL (2020). Mechanisms underlying priapism in sickle cell disease: targeting and key innovations on the preclinical landscape. Expert Opinion on Therapetic Targets, 24, 439–450. 10.1080/14728222.2020.1745188 [DOI] [PubMed] [Google Scholar]
- Ning C, Wen J, Zhang Y, Dai Y, Wang W, Zhang W,Qi L, Grenz A, Eltzsching HK, Blackburn MR, Kellems RE, & Xia Y (2014). Excess adenosine A2B receptor signaling contributes to priapism through HIF-1α mediated reduction of PDE5 gene expression. FASEB Journal 28, 2725–2735. 10.1096/fj.13-247833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Fan J, & Zirkin B (2018). Translocator protein (18 kDa): an update on its function in steroidogenesis. Journal of Neuroendocrinology, 30:10.1111/jne.12500. doi: 10.1111/jne.12500 10.1111/jne.12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, & Rubin EM (1997). Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science, 278, 876–878. 10.1126/science.278.5339.876 [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Mulhall J, Davies K, Wingard CJ, Hannan JL, Bivalacqua TJ, Musicki B, Khera M, González-Cadavid N, & Burnett AL (2016). Translational Perspective on the Role of Testosterone in Sexual Function and Dysfunction. Journal of Sexual Medicine 13, 1183–1198. 10.1016/j.jsxm.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, Ye X, Blonder J, Veenstra T, & Papadopoulos V (2012). Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Molecular Endocrinology 26, 1868–1882. 10.1210/me.2012-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, & Schumacher M (2010). Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nature Reviews Drug Discovery, 9, 971–988. 10.1038/nrd3295 [DOI] [PubMed] [Google Scholar]
- Scholz R, Caramoy A, Bhuckory MB, Rashid K, Chen M, Xu H, Grimm C, & Langmann T (2015). Targeting translocator protein (18 kDa) (TSPO) dampens pro-inflammatory microglia reactivity in the retina and protects from degeneration. Journal of Neuroinflammation, 12, 201 10.1186/s12974-015-0422-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, & Drummond GR (2007). Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovascular Research 75, 349–358. 10.1016/j.cardiores.2007.03.030 [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Stocco DM & Clark BJ (2018). Current knowledge on the acute regulation of steroidogenesis. Biology of Reproduction, 99, 13–26. 10.1093/biolre/ioy102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva F,H, Karakus S, Musicki B, Matsui H, Bivalacqua TJ, Dos Santos JL, Costa FF, & Burnett AL (2016a). Beneficial effect of the nitric oxide donor compound 3-(1,3-Dioxoisoindolin-2-yl)Benzyl nitrate on dysregulated phosphodiesterase 5, NADPH oxidase, and nitrosative stress in the sickle cell mouse penis: implication for priapism treatment. Journal of Pharmacology and Experimental Therapeutics, 359, 230–237. 10.1124/jpet.116.235473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FH, Claudino MA, Calmasini FB, Alexandre EC, Franco-Penteado C, Burnett AL, Antunes E, & Costa FF (2016b) Sympathetic hyperactivity, increased tyrosine hydroxylase and exaggerated corpus cavernosum relaxations associated with oxidative stress plays a major role in the penis dysfunction in Townes sickle cell mouse. PLoS One 11, e0166291 10.1371/journal.pone.0166291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Whitley K (2014). Reproductive issues in sickle cell disease. Blood 124, 3538–3543. 10.1182/blood-2014-07-577619 [DOI] [PubMed] [Google Scholar]
- Telen MJ, Malik P, & Vercellotti GM (2019). Therapeutic strategies for sickle cell disease: towards a multi-agent approach. Nature Reviews Drug Discovorey, 18, 139–158. 10.1038/s41573-018-0003-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, & Selvaray V (2014). Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. Journal of Biological Chemistry, 289, 27444–27454. 10.1074/jbc.M114.578286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roijen JH, Ooms MP, Weber RF, Brinkmann AO, Grootegoed JA, & Vreeburg JT (1997). Comparison of the response of rat testis and accessory sex organs to treatment with testosterone and the synthetic androgen methyltrienolone (R1881). Journal of Andrology, 18, 51–61. [PubMed] [Google Scholar]
- Wang YM, Sullivan PM, Petrusz P, Yarbrough W, Joseph DR. (1989) The androgen-binding protein gene is expressed in CDI mouse testis. Mol Cell Endocrinol. 6, 85–92.. 10.1016/0303-7207(89)90084-1. [DOI] [PubMed] [Google Scholar]
- Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, & Townes TM (2006). Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood, 108, 1183–1188. 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Murugesan P, Huang K, & Cai H (2019). NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nature Reviews Cardiology, 17, 170–194. 10.1038/s41569-019-0260-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.