Abstract
Purpose:
Descriptions of seizure manifestations (SM), or semiology, can help localize the symptomatogenic zone and subsequently included brain regions involved in epileptic seizures, as well as identify patients with dissociative seizures (DS). Patients and witnesses are not trained observers, so these descriptions may vary from expert review of seizure video recordings of seizures. To better understand how reported factors can help identify patients with DS or epileptic seizures (ES), we evaluated the associations between more than 30 SMs and diagnosis using standardized interviews.
Methods:
Based on patient- and observer-reported data from 490 patients with diagnoses documented by video-electoencephalography, we compared the rate of each SM in five mutually exclusive groups: epileptic seizures (ES), DS, physiologic seizure-like events (PSLE), mixed DS and ES, and inconclusive testing.
Results:
In addition to SMs that we described in a prior manuscript, the following were associated with DS: light triggers, emotional stress trigger, pre-ictal and post-ictal headache, post-ictal muscle soreness, and ictal sensory symptoms.
The following were associated with ES: triggered by missing medication, aura of déjà vu, and leftward eye deviation. There were numerous manifestations separately associated with mixed ES and DS.
Conclusions:
Reported SM can help identify patients with DS, but no manifestation is pathognomonic for either ES or DS. Patients with mixed ES and DS reported factors divergent from both ES-alone and DS-alone.
Keywords: psychogenic nonepileptic seizures, functional seizures, semiology, symptomatogenic zone
1. Introduction:
A core element of the clinical history for patients with seizures is eliciting a description of seizure manifestations (SM) from the patient and any available witnesses. In patients with epileptic seizures (ES), this assists in hypothesized seizure classification and neuroanatomic localization of the symptomatogenic zone, defined as the area of cortex that, when activated by epileptiform activity, produces ictal symptoms [1]. In addition, this description can help identify patients with dissociative seizures (DS), which also are known as functional seizures or psychogenic nonepileptic seizures (PNES) [2-4]. While the exact mechanism of DS is unknown, these seizures most commonly reflect abnormally prominent, involuntary physical manifestations of chronic and, in some cases, acute psychological stressors [5-7]. Identifying patients with DS early can lead to triage towards cognitive behavioral therapy designed for DS, or potentially psychodynamic therapy, and avoidance of adverse effects of antiseizure medication and intubation for prolonged dissociative seizures [8-10].
While reported SM are not as reliable as video recordings, especially with concomitant electroencephalography (EEG), evidence-based decision support tools have been developed that have 70-85% accuracy in differentiating patients with DS from ES [11-16]. In our prior work, we evaluated more than 30 reported manifestations based on retrospective chart review and were unable to evaluate the diagnostic value of additional manifestations due to lack of reporting [17]. In this prospective evaluation, patients underwent a standardized interview during admission for video-EEG monitoring (VEM) that specifically inquired about additional SMs (Table 1).
Table 1:
List of all the seizure manifestations that we evaluated. Tabs indicate specifications within a category. Abbreviations: medications (meds), movements (mvmts), number (#), seizure (sz).
| Seizure Manifestations | ||
|---|---|---|
| Seizure Duration | Sz From Sleep | Head mvmts |
| Sz Freq | Falling | Left Version |
| # Sz Types | Lapse of Awareness | Right Version |
| # Limbs Moving | Dialeptic | Side-to-side |
| Injury from Sz | Maximum Intensity at Onset | Staring |
| Trigger: Sleep Dep | Eye mvmts | |
| Catamenial | Amnesia | Left Version |
| Trigger: Light | Aphasia | Right Version |
| Trigger: Food | Oral injury | Upwards |
| Trigger: Missing Meds | Muscle jerks | Rolling |
| Trigger: Loud Noises | Tonic-clonic mvmts | Fluttering |
| Trigger: Stress | Whole Body mvmts | Eye closure |
| Trigger: Emotional Stress | Part Body mvmts | Eye opening |
| Trigger: Alcohol | Stiffening | Freezing |
| Aura | Shaking | Ictal Cry |
| Headache | Flailing | Ictal Anxiety |
| Urinary Urge | Numbness | Post-ictal Confusion Or Fatigue |
| Epigastric Rising | Tingling | |
| Anxiety | Dizziness | Post-ictal Muscle |
| Déjà vu | Hallucinations | Soreness |
| Scent | Incontinence | Post-ictal Headache |
| Pleasant | Limb Automatisms | |
| Unpleasant | Oral Automatisms | |
| Metallic | Hip thrusting | |
Specifically, we characterized seizure triggers; types of aura, eye movements and head movements; the specific words used to describe the movements; and description of the post-ictal symptoms. By evaluating the extent to which each reported factor was associated with DS, ES, or mixed ES plus DS, we aimed to improve clinician’s understanding and interpretation of patients’ and caregivers’ reports. A clinical purpose of interpreting reported SMs is to determine if the patient has probable DS and warrants further confirmation of diagnosis with ictal VEM prior to prescribing another antiseizure medications [18-20], therefore we focus on characterizing the patient as a whole, as compared to each individual seizure type that a patient may experience.
2. Methods:
Our patient population includes all patients admitted to an adult vEEG monitoring unit from May 2015 to December 2019. Diagnosis was based on expert clinical opinion and met the International League Against Epilepsy definition of “documented” based on the available clinical history, physical exam, vEEG, structural and diffusion MRI, FDG-PET, MEG and SPECT [18]. We placed patients in five mutually exclusive categories: DS, ES, mixed DS plus ES, physiologic seizure-like events (PSLE), and inconclusive monitoring. Our statistical modeling recognizes that these are heterogeneous populations, but the prediction of subtypes is outside the scope of this investigation. In contrast to non-epileptic seizures caused by dissociation, we define PSLE as non-epileptic seizures caused by non-psychological factors including syncope, complex migraines, dementia, and movement disorders [21]. We use mixed seizures only when referring to patients with both DS and ES. As suggested by our results, we keep mixed seizures and DS separate because their SMs were described differently and treatment protocols are different [22]. There were no patients with PSLE and ES.
Inconclusive monitoring occurred when a patient did not have occurrences of their characteristic events to determine a diagnosis for all types of seizures. Inclusion of these patients reduces selection bias while not otherwise affecting the conclusions regarding the other diagnostic categories. Publication of data from these patients with uncertain diagnoses promotes data transparency and hypothesis generation. However, because our data pertains to patients for whom video-EEG was conclusive, our results may not generalize to patients for whom video-EEG would not be conclusive.
Patients underwent a standardized interview with a trained non-neurologist interviewer (EAJ, SRD, MA, JB, CHA, AK) or WTK within 48 hours of the vEEG admission. No information from the health record, beyond age and sex, was used to supplement the history. Age was recorded at the time of the standardized interview. The standardized interview was conducted in the patient’s private hospital room and available caregivers, family, or friends were not dismissed, mirroring a typical neurological interview. The structure of this interview mirrored the Center for Medicare Services billing requirements of an initial neurological history without the physical exam and included sections on medical comorbidities, medications, epilepsy risk factors, head trauma, psychological trauma, family history, allergies, quality of life, and peri-ictal manifestations [17, 23-25]. Caregivers and loved ones were dismissed for questions regarding psychological trauma and suicidal ideation. Peri-ictal manifestations were elicited by asking the patient to describe their typical seizure from start to finish. After this open question, the interviewer would clarify details that were not otherwise specified. As many patients were amnestic of their seizures, no distinction was made between details or descriptions provided by the patient and caregivers. When patients and caregivers disagreed, although this was rare, we used the patients’ report. In total, this detailed standardized interview takes between 15 and 90 minutes.
All patients consented for the use of their records in research, and the UCLA Institutional Review Board approved this study. This work is consistent with Declaration of Helsinki. De-identified raw data and code are at SeizureDisorderCenterResearchGroup.org.
2.1. Description of Seizure Manifestations
We included each SM that was described for at least 1 type of seizure that the patient had. For example, an individual patient could report eye closure in one type of seizures and, simultaneously, eye opening during another type of seizure. In this case, patients would be recorded to have both. Therefore, SM were analyzed per patient and were not analyzed per patient and seizure type. This reflects the perspective that clinicians look to diagnose the patient, as compared to diagnosing each seizure type. While there are clear and clinically important exceptions to this (e.g. tuberous sclerosis complex or Lennox-Gastaut Syndrome), it is uncommon for patients to have multifocal epilepsy, generalized-onset and focal-onset epilepsy, or mixed ES and DS. For patients with mixed ES and DS, we report our results to better understand this complex population.
Seizure manifestations indicators were selected based on previous literature and factors not evaluated in our previous retrospective dataset (Table 1, [17]). Briefly, we describe why each SM was included.
Versive head and eye movements are a reliable lateralizing features for focal ES [26]. In discussion of other eye and head movements, we also evaluated other common reports including upward eye movements, rolling eye movements. Upward eye movements were described similarly to versive eye movements but were directed upward. Eye rolling included motion of the eyes that was not forced or versive. Other descriptions of eye movements were coded as eye movements without additional specifications. Eye closure has been associated with DS [17, 27-34], so we evaluated the converse, eye opening, as well as the rapid alternation between opening and closing, which was termed fluttering [11]. Staring was commonly described during seizures but was not included previously. Similarly, we evaluated ictal lapse of awareness according to the current definitions for epileptic seizures [35]. To reference the literature describing preserved awareness with bilateral movements, we added factors describing bilateral movements as compared to unilateral movements. We found that the physical description of seizures was difficult to analyze, so we included multiple words that were commonly used by patients including stiffening, shaking, and flailing. Although we did not hear these descriptions often, we also added characterization of sensory symptoms of tingling, numbness, and dizziness. To add to the prior evaluation of injury from seizure [36], we also evaluated if the patient falls during seizure.
In addition to ictal features, we evaluated peri-ictal features describing the events immediately before or after ictus, as well as the triggers or factors associated with ictus. An electrographic feature of DS is absence of post-ictal EEG changes [37, 38], so we included clinical post-ictal features of confusion or fatigue, muscle soreness, and headache. We also further evaluated the aura to include typical limbic auras of epigastric rising, déjà vu, and scents other than metallic tastes. Our prior work evaluated metallic tastes [17]. Due to the observation that epileptic scents likely are unpleasant, we specified if the scent of an aura was pleasant or unpleasant. To further characterize incontinence that has been discussed previously [17, 39-41], we added aura of urinary urgency that may localize to insula or temporal lobe epilepsy [42].
2.2. Statistical Modeling
We evaluated the population-level differences in each reported seizure manifestation using pairwise comparison of each diagnostic group using either linear or log-linear heteroskedastic t-tests or Fisher exact tests. When a manifestation was reported in no patients in a group, the binomial exact confidence intervals were calculated assuming that “one half” of a patient in that group reported the factor. This provides the most conservative comparison between groups by assuming the highest potential prevalence that would be rounded down to the actual observed prevalence. The purpose of this analysis was to highlight specific clinical features that clinicians may consider in diagnostic and decision-making situations, rather than quantitatively determining the predictive ability of any feature. Thus, we did not apply a correction for multiple statistical tests [43-45]. Some patients and caregivers were unable to quantify seizure duration, seizure frequency, or age of onset of seizures. For population-level analysis, we excluded patients with missing entries that pertained to the factor modeled in that regression.
3. Results:
In our sample of 490 patients, 77 had DS alone (16% of total), 16 had mixed DS and ES (3% of total, 17% of all patients with DS or DS and ES), 19 had PSLE (4% of total, 20% of patients with DS or PSLE), and 241 had ES alone (49% of total). Of the patients with ES, 105 (44%) had temporal lobe ES, 22 (9%) had frontal lobe ES, 89 (37%) had other or poorly localized focal onsets, 15 (6%) had generalized-onset ES, 3 (1.2%) had both focal and generalized-onset ES, 2 (0.8%) had other epileptic syndromes, and 47 (20%) had ES with unclear localization. Table 2 summarizes the number of patients in each diagnostic group.
Table 2:
The number and percent of patients based on seizure etiology. Horizontal bars separate classes of diagnoses and tabs indicate specification within a category. For patients with ES, we include a breakdown of the localization of epilepsy and the percent (%) contribution of each subgroup to the total sample of patients with ES. Similarly, we calculate the relative proportion of patients with DS who have ES (Mixed DS and ES) and who do not have ES (DS-alone).
| Diagnosis | Number | % Total | % of Class |
|---|---|---|---|
| Dissociative Seizures (DS) | 77 | 16. | 83. |
| Mixed DS and ES | 16 | 3.3 | 17. |
| Inconclusive Monitoring | 137 | 28. | |
| PSLE | 19 | 3.9 | |
| Epileptic Seizures (ES) | 241 | 49. | |
| Generalized | 15 | 3.1 | 6. |
| Frontal lobe | 22 | 4.5 | 9. |
| Temporal lobe | 105 | 21. | 44. |
| Parietal lobe | 2 | 0.4 | 0.8 |
| Occipital lobe | 3 | 0.6 | 1.2 |
| Other Focal or Multifocal | 61 | 12.4 | 25. |
| Focal unclear localization | 28 | 5.7 | 12. |
| Focal and Generalized | 3 | 0.6 | 1.2 |
| Other Epilepsy Syndrome | 2 | 0.4 | 0.8 |
| Not otherwise specified | 47 | 9.6 | 20. |
Patients with PSLE included paroxysmal kinesigenic dyskinesia, other dyskinesia, complex migraines, syncope with and without dysautonomia, orthostatic tremor, sleep myoclonus, obstructive sleep apnea, Tourette’s syndrome, behavioral events in nonverbal or otherwise intellectually disabled patients, and non-epileptic vertigo or dizziness.
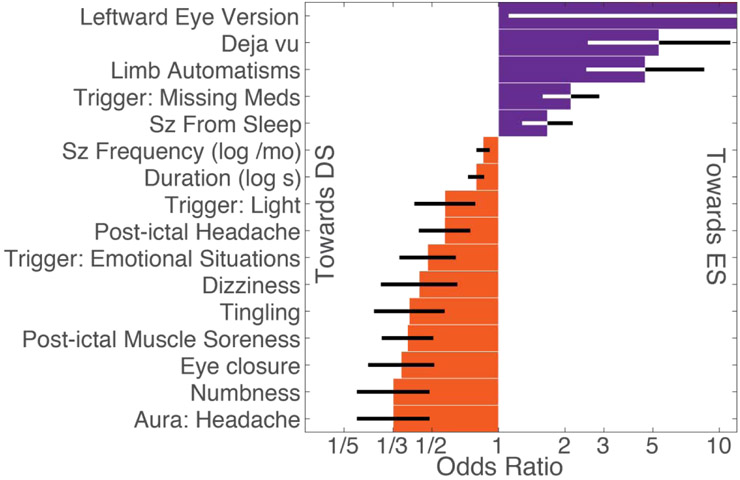
The univariate odds ratios of factors that were significantly different between patients with DS and ES are illustrated in Figure 1. The population level prevalence of each patient-reported factor is summarized in Supplementary Table 1. Seizure duration and seizure frequency were missing in 9% and 5% of patients, respectively.
Figure 1:
Patient reported seizure manifestations (SM) with different prevalence between patients with DS and ES (α<0.10, uncorrected). SMs are ordered based on magnitude. Black and white error bars reflect standard error plotted on a scale of log-odds ratios with tick labels reflecting odds ratios. Leftward eye version was only reported in ES, therefore significance was as determined by a Fisher exact test. Abbreviations: seizure (sz), month (mo), second (s).
For patients with ES, the average time from seizure onset to VEM was 18 years (95% linear confidence interval (CI): 16-21 years; median 15, interquartile range (IQR) 6-26 years). For patients with DS, the average time to VEM was 8.6 years (95% CI: 6-11 years; median 4, IQR 1.25-11.5 years). For patients with mixed seizures, the average time to VEM was 23 years (95% CI: 13-33 years; median 21.5, IQR 5-38 years).
The seizure manifestations that were more common in DS compared to ES included seizure trigger involving light (19% DS, 16% ES, p=0.09); seizures triggered by emotional stress (27% DS, 19% ES, p=0.02), aura of headache (16% DS, 7% ES, p=0.005), ictal numbness (19% DS, 7% ES, p=0.004), ictal tingling (19% DS, 8% ES, p=0.01), ictal dizziness (16% DS, 7% ES, p=0.04), post-ictal muscle soreness (61% DS, 38% ES, p=0.0005), and post-ictal headache (62% DS, 49% ES, p=0.04).
The seizure manifestations that were more common in ES compared to DS included seizures triggered by missing medications (19% DS, 41% ES, p=0.01), aura of déjà vu (1.2% DS, 12% ES, p=0.01), and leftward eye deviation (0% DS, 19% ES, p=0.009).
The seizure manifestations that in the limited number of patients with mixed seizures that differed from those with DS or ES included more seizure types (average 3 mixed, 1.7 DS, 1.7 ES, pmixedvsDS=0.0008, pmixedvsES=0.0006), injury from seizure (81% mixed, 39% DS, 45% ES, pmixedvsDS=0.002, pmixedvsES=0.008), ictal muscle jerks (50% mixed, 15% DS, 11% ES, pmixedvsDS=0.005, pmixedvsES=0.0003), ictal incontinence (63% mixed, 26% DS, 33% ES, pmixedvsDS=0.07, pmixedvsES=0.03), ictal numbness (25% mixed, 7% ES, pmixedvsES=0.04), ictal head movements (62% mixed, 36% ES, pmixedvsES=0.06), ictal leftward head movement (25% mixed, 5% DS, 11% ES, pmixedvsDS=0.03), ictal side to side head movements (25% mixed, 9% DS, 7% ES, pmixedvsES=0.03), ictal staring (69% mixed, 38% DS, 39% ES, pmixedvsDS=0.03, pmixedvsES=0.03), ictal leftward eye movements (13% mixed, 0% DS, 7% ES, pmixedvsDS=0.03), ictal cry or scream (38% mixed, 13% DS, 21% ES, pmixedvsDS=0.03).
In comparison to our prior retrospective work, this prospective dataset independently confirmed differences in the prevalence or value of the following patient-reported SM: seizure duration (average 2:48 min:sec DS, 1:28 ES, p=0.0092), seizures directly from sleep (44% DS, 57% ES, p=0.07), limb automatisms (4% DS, 16% ES, p=0.006), and eye closure (23% DS, 9% ES, p=0.006).
The following factors did not significantly differ between patients with DS, mixed, or ES: catamenial seizures; trigger of loud noises; trigger of stress, trigger of alcohol, the presence of any aura, an epigastric rising aura, aura of fear or anxiety, an aura involving a scent or taste, falling during seizures, ictal amnesia, ictal aphasia, oral injury during seizure, tonic-clonic movements, stiffening, shaking, flailing, hallucinations, hip thrusting, rightward head movements, any eye movements, rightward eye movements, eyes rolling upwards, eye fluttering, eye opening, ictal freezing, and post-ictal confusion or fatigue.
4. Discussion:
These results provide meaningful data to assist clinicians in interpreting many reported seizure manifestations, even though we did not address all of the hundreds of seizure manifestations that were potentially suggestive of DS. While some SMs can raise the likelihood of DS, none fully excluded ES, therefore the diagnosis of DS or ES should not rely on the reporting of a single SM suggestive of DS. Instead, the experienced clinician should consider the totality of SMs as well as other data to formulate a diagnosis and provide suggestions for evaluation and management. We also address the difficult population of patients with mixed DS and ES.
A significant portion of functional neurological disorders (FNDs) includes an element of physical pain. The increased reports of pre- and post-ictal headache as well as post-ictal muscle soreness could reflect this phenomenon. In other series, pre-ictal headache was present in 16% (n=258, [46]) and 87% (n=63, [28]) of patients with DS, and is considered rare in epilepsy [47]. When it occurs in temporal lobe epilepsy, pre-ictal migraine is ipsilateral [48]. In contrast, post-ictal headache was common in both populations including 49% of patients with ES. Therefore, while both pre- and post-ictal headache were more common in DS than ES, our results suggest that timing matters: the positive predictive value of pre-ictal headache (92%, 95% CI 89-96%) was higher than that of post-ictal headache (51%, 95% CI 45-58%).
This differential rate of pain also translates to post-ictal muscle soreness. Even though dissociative seizures tend to be longer [17], the quantitative pattern of muscle involvement can be different from either focal, focal-to-bilateral and generalized or bilateral epileptic seizures [49-52]. Serum studies of anion gap, leukocytosis, creatine kinase and other objective markers of muscle fatigue suggest that ES produce more metabolic demand than DS [53-56], but these results are inconsistent. However, even though a similar percent of patients with DS and ES noted bilateral motor involvement, patients with DS perceived increased muscle soreness after seizures. This parallels a consistent finding in FND that patients’ perception and description of the severity of their symptoms may be higher than suggested from objective physiological measures [57-61].
Similarly, sensory symptoms are subjective. While the ictal sensory symptoms of tingling, numbness and dizziness were present in less than 20% of patients with DS, they were present in less than 10% of patients with ES. Dizziness was the second most common aura in DS [46] and, when seen in 9% of epilepsy in other meta-analyses, ictal dizziness localized to the temporal lobe or temporal-parietal-occipital junction [62]. The temporo-parietal junction has been implicated in functional movement disorders and dissociative seizures [63-66]. Similarly, ictal paresthesias and numbness are reported in a minority of DS and, when rarely present in epilepsy, localize to the parietal lobe or insula [67-70]. This association of sensory symptoms with DS mirrors our prior finding that sensory hallucinations were more common in DS than ES, although that difference was not statistically different in this dataset (13% DS versus 8% ES, p=0.18). Therefore, while ictal sensory symptoms are rare, they may raise suspicion for DS.
The ability to recognize and successfully cope with stressors is a key part of the mechanism of DS and other functional neurological disorders [71, 72]. However, psychological and physiologic stress also reduce seizure threshold on patients with epilepsy [73-76]. This was reflected in our results by the dichotomy between seizures triggered by general stress as compared to emotional stressors, in particular. General stress triggering seizures was common in both groups (57% DS versus 49% ES, p=0.24), but stressful emotional triggers were more prevalent in DS (32% versus 19%, p=0.02), as has been described elsewhere [77]. In our experience, patients needed to be prompted to recognize this difference, therefore providers can follow up a reported trigger of stress by asking about emotional stress in particular.
This type of dichotomy also was seen regarding eye movements. While it is not diagnostic in isolation, ictal eye closure is established to be suggestive of DS [17, 27-34]. Some of this literature refers to “forced eye closure” but we found that without a video, patients and caregivers had difficulty understanding the difference between forced and unforced eye closure and tended to state that all ictal eye closure was forced. Logically, one would presume that the converse—ictal eye opening—would suggest ES, as had been shown in a single 120-patient series [11]. However, ictal eye opening, fluttering, rolling and upward deviation were all nondiagnostic in our dataset (all p-values>0.14).
Another typical finding in focal to bilateral epileptic seizures is versive movements that typically include forced eye and head deviation to one side [26]. While patient and caregiver reports had difficulty differentiating forced versus unforced version, we observed that leftward eye or head movements were quite rare in DS (0% and 5%, respectively) and were present in a minority of patients with ES (7% and 7%, respectively). Nonintuitively, this difference was not present for rightward eye or head movements and was not correlated with handedness (data not shown). While eye deviation towards the ground, termed geotropic, has been reported in DS [78], lateral head and eye version similar to that seen in focal ES have not been described in DS previously.
Other findings suggestive of limbic onset focal to bilateral epileptic seizures were more reliable indicators of ES. Specifically, an aura of déjà vu and simple limb automatisms were not uncommon in patients with ES (12% and 16%, respectively), and were rare in patients with DS (3% and 4%, respectively).
This finding that typical limbic behaviors suggested epilepsy reflects the increased prevalence of limbic epilepsy in our VEM dataset and also reflects the intuitive concept that if a patient’s seizure manifestation closely resembles that of a particular epilepsy syndrome or localization, then the seizures might be more likely to be epilepsy. However, the prevalence of these specific epilepsy syndromes is low so that even in our large, unselected, consecutive series of patients with VEM, there was insufficient data to evaluate specific collections of seizure manifestations in a data-driven fashion.
While we had limited data from the complex population of patients with mixed DS and ES, the data we have suggested that this population may be different from patients with DS alone or ES alone. To a certain extent, some of the manifestations that were suggestive of DS in our dataset were even more prevalent in mixed seizures. In addition to these manifestations, side to side head movements and muscle jerks, as compared to tonic-clonic movements, have previously been described as suggestive of DS and were more common in mixed seizures [14, 71, 79-82], but were not observed to be different between DS alone and ES alone. Conversely, the leftward head and eye version that was suggestive of ES were even more common in mixed seizures. Further, manifestations that were typically associated with ES in prior literature also were exceptionally common in mixed seizures, even if they were not different between DS and ES in our dataset, including injury from seizure, ictal incontinence, and ictal scream. This mixed ictal manifestation in mixed seizures reflects that the average patient had 3 seizure types, whereas patients with DS alone or ES alone reported 1 to 2 seizure types. The rate of ictal staring was very prevalent in mixed seizures (69%), suggesting that this last seizure type may in part include more bland seizures.
This divergence of the interpretation of these seizure manifestations suggests that patients with these difficult to interpret descriptions and multiple seizure types should be evaluated further, likely with VEM. This would suggest that these complex patients should be referred earlier, but the median time from seizure onset to VEM in mixed seizures was 21.5 years, which was longer than patients with either DS or ES (4 and 15 years, respectively). This may reflect a reluctance to refer patients with medication-resistant epilepsy for an epilepsy surgery evaluation of epilepsy, when they also have DS, even though they can be good surgical candidates [83].
While this dataset includes a wide range of ages, many patients and localizations of epilepsy, our data pertains just to adults with DS and ES. The descriptions of seizure manifestation were provided by a combination of the patient and any available witnesses. While this may differ from separate descriptions of the patient and witnesses [16], but our approach mirrors a typical clinical encounter. Our approach of an initial open question followed by specific questions balances an open, patient-centered clinical approach with the need for reliable data for research. As suggested by conversation analysis across cultures, patients with epilepsy more readily provide specific details regarding their seizures, as compared to patients with dissociative seizures who tend to focus on the environment and impact of their seizures [84-87]. Therefore, this prospective design can overcome some limitations inherent to deriving specific factors from chart review of patients with dissociative seizures, while also addressing the difference in communication patterns between patients with epilepsy and dissociative seizures.
The seizure manifestations of children and adolescents with seizures is different from adults [39]. Therefore, we caution the generalization of our results to younger patients. Further, the differential diagnosis of seizures with atypical behaviors focuses primarily on frontal lobe epilepsy (FLE). However, the prevalence of FLE is low even compared to the prevalence of DS, even in our larger dataset (Of all 490 unique admissions, 4.4% (22) were FLE and 16% (77) were DS). Therefore, it remains difficult to distinguish between patients with DS and FLE in the absence of direct observation of the seizure by a seizure specialist with or without concomitant EEG.
The single-variable associations that we discussed used data to provide helpful information to interpret clinically reported peri-ictal manifestations of DS, ES, and mixed seizures, but we caution that these insights are qualitative and only indirectly comment on the underlying pathophysiology of these conditions. While associative studies provide a short list of candidate findings, interventional or other mechanistic studies are needed to demonstrate causal links between findings and DS, ES, or mixed seizures.
Additionally, unlike some of our prior work [17, 23-25, 88], we did not evaluate how these factors can be applied to diagnose individual patients with seizures. Future work is needed to evaluate how these population-level differences can be integrated with other information from patients to reliably diagnose individual patients. Population-level differences do not always translate to reliable individual-level predictions [24]. The diagnosis of seizure-like events is complex because clinicians must consider ES, DS, mixed ES plus DS, as well as PSLE. In comparison to ES and DS, patients with mixed seizures or PSLE are relatively rare in video-EEG datasets, and substantially more data is needed to accurately differentiate these clinically important populations [22]. This is particularly challenging for patients with PSLE who needed video-EEG for diagnosis because of the wide heterogeneity of observed etiologies. However, due to this heterogeneity, it is particularly difficult to interpret SMs in this population. We report data on these populations to facilitate future meta-analyses and multicenter studies that could build a large enough sample to characterize patients with mixed seizures or PSLE.
Just as patients with PSLE are diagnostically heterogeneous, individual patients may experience multiple seizure types. This analysis focused on diagnosing patients instead of individual seizure types. Differentiation of the epileptic from dissociative seizures in a patient with mixed seizures is particularly important, but this analytical level of complexity was outside the scope of this work.
4.4. Conclusions
No reported seizure manifestation is pathognomonic for DS. Ictal sensory symptoms and both pre-ictal and post-ictal headache were more common in patients with DS than ES. Conversely, an aura of déjà vu and, intriguingly, leftward but not rightward eye deviation was very uncommon in patients with DS and was present in some patients with ES. The seizure manifestation of patients with mixed seizures appears quite different from both DS and ES in isolation. Based on the limited predictive value, reported seizure manifestation can suggest “probable” DS; further diagnostic evaluation including observation of the seizures by a seizure specialist is needed to guide treatment.
Supplementary Material
Highlights:
Pre-ictal headache was more specific for dissociative seizures (DS) than post-ictal headache.
Ictal sensory symptoms and seizures triggered by emotional stress were associated with DS.
Epileptic seizures (ES) were associated with déjà vu, left eye deviation and missed medications.
Patients with both ES and DS reported seizure manifestations different from ES-alone and DS-alone
5. Acknowledgements:
The authors thank Kirk Shattuck, Marc Nuwer, and Edward P. Lau for organization support, access to the data, and technical support. This work was supported by the NIH R25 NS065723, UCLA-California Institute of Technology Medical Scientist Training Program (NIH T32 GM08042), the William M. Keck Foundation, research grants to JE (NS03310 & NS080181), the UCLA Departments of Psychiatry & Biobehavioral Sciences and Biomathematics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts & Ethical Publication:
Drs. Engel, Stern, Kerr and Al Banna have clinical practices that include the diagnosis and treatment of patients with epilepsy and non-epileptic seizures. The remaining authors have no declared conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References:
- 1.Rosenow F and Luders H, Presurgical evaluation of epilepsy. Brain, 2001. 124(Pt 9): p. 1683–700. [DOI] [PubMed] [Google Scholar]
- 2.Asadi-Pooya AA, et al. , Terminology for psychogenic nonepileptic seizures: Making the case for “functional seizures”. Epilepsy Behav, 2020. 104(Pt A): p. 106895. [DOI] [PubMed] [Google Scholar]
- 3.Kerr WT and Stern JM, We need a functioning name for PNES: Consider dissociative seizures. Epilepsy Behav, 2020. 105: p. 107002. [DOI] [PubMed] [Google Scholar]
- 4.Beghi M, Peroni F, and Cornaggia CM, Reply to: We need a functioning name for PNES: Considering dissociative seizures. Epilepsy Behav, 2020. 109: p. 107084. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson P and Looper KJ, Psychogenic nonepileptic seizures: a current overview. Epilepsia, 2012. 53(10): p. 1679–89. [DOI] [PubMed] [Google Scholar]
- 6.Baslet G, Roiko A, and Prensky E, Heterogeneity in psychogenic nonepileptic seizures: understanding the role of psychiatric and neurological factors. Epilepsy Behav, 2010. 17(2): p. 236–41. [DOI] [PubMed] [Google Scholar]
- 7.Reuber M, et al. , Diagnostic delay in psychogenic nonepileptic seizures. Neurology, 2002. 58(3): p. 493–5. [DOI] [PubMed] [Google Scholar]
- 8.Kapur J, et al. , Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N Engl J Med, 2019. 381(22): p. 2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein LH, et al. , Cognitive behavioural therapy for adults with dissociative seizures (CODES): a pragmatic, multicentre, randomised controlled trial. Lancet Psychiatry, 2020. 7(6): p. 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libbon R, et al. , The feasibility of a multidisciplinary group therapy clinic for the treatment of nonepileptic seizures. Epilepsy Behav, 2019. 98(Pt A): p. 117–123. [DOI] [PubMed] [Google Scholar]
- 11.Syed TU, et al. , Can semiology predict psychogenic nonepileptic seizures? A prospective study. Ann Neurol, 2011. 69(6): p. 997–1004. [DOI] [PubMed] [Google Scholar]
- 12.Syed TU, et al. , A self-administered screening instrument for psychogenic nonepileptic seizures. Neurology, 2009. 72(19): p. 1646–52. [DOI] [PubMed] [Google Scholar]
- 13.Benge JF, et al. , Diagnostic utility of the Structured Inventory of Malingered Symptomatology for identifying psychogenic non-epileptic events. Epilepsy Behav, 2012. 24(4): p. 439–44. [DOI] [PubMed] [Google Scholar]
- 14.Avbersek A and Sisodiya S, Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry, 2010. 81(7): p. 719–25. [DOI] [PubMed] [Google Scholar]
- 15.Reuber M, et al. , Value of patient-reported symptoms in the diagnosis of transient loss of consciousness. Neurology, 2016. 87(6): p. 625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, et al. , Value of witness observations in the differential diagnosis of transient loss of consciousness. Neurology, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Kerr WT, et al. , Reliability of reported peri-ictal behavior to identify psychogenic nonepileptic seizures. Seizure, 2019. 67: p. 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaFrance WC Jr., et al. , Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia, 2013. 54(11): p. 2005–18. [DOI] [PubMed] [Google Scholar]
- 19.Alessi R and Valente KD, Psychogenic nonepileptic seizures: should we use response to AEDS as a red flag for the diagnosis? Seizure, 2014. 23(10): p. 906–8. [DOI] [PubMed] [Google Scholar]
- 20.Kerr WT, et al. , Diagnostic delay in psychogenic seizures and the association with anti-seizure medication trials. Seizure, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St Louis EK and Cascino GD, Diagnosis of Epilepsy and Related Episodic Disorders. Continuum (Minneap Minn), 2016. 22(1 Epilepsy): p. 15–37. [DOI] [PubMed] [Google Scholar]
- 22.Baroni G, et al. , Variables associated with co-existing epileptic and psychogenic nonepileptic seizures: a systematic review. Seizure, 2016. 37: p. 35–40. [DOI] [PubMed] [Google Scholar]
- 23.Kerr WT, et al. , An objective score to identify psychogenic seizures based on age of onset and history. Epilepsy Behav, 2018. 80: p. 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr WT, et al. , Diagnostic implications of review-of-systems questionnaires to differentiate epileptic seizures from psychogenic seizures. Epilepsy Behav, 2017. 69: p. 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr WT, et al. , Identifying psychogenic seizures through comorbidities and medication history. Epilepsia, 2017. 58(11): p. 1852–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groppel G, et al. , Clinical symptoms in psychogenic seizures. Wien Klin Wochenschr, 1999. 111(12): p. 469–75. [PubMed] [Google Scholar]
- 27.Alessi R, et al. , Semiology of psychogenic nonepileptic seizures: age-related differences. Epilepsy Behav, 2013. 27(2): p. 292–5. [DOI] [PubMed] [Google Scholar]
- 28.Patidar Y, et al. , Clinical profile of psychogenic non-epileptic seizures in adults: A study of 63 cases. Ann Indian Acad Neurol, 2013. 16(2): p. 157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoerth MT, et al. , Clinical predictors of psychogenic nonepileptic seizures: a critically appraised topic. Neurologist, 2008. 14(4): p. 266–70. [DOI] [PubMed] [Google Scholar]
- 30.Syed TU, et al. , Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia, 2008. 49(5): p. 898–904. [DOI] [PubMed] [Google Scholar]
- 31.Chen DK, et al. , Sensitivity and specificity of video alone versus electroencephalography alone for the diagnosis of partial seizures. Epilepsy Behav, 2008. 13(1): p. 115–8. [DOI] [PubMed] [Google Scholar]
- 32.Chung SS, Gerber P, and Kirlin KA, Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology, 2006. 66(11): p. 1730–1. [DOI] [PubMed] [Google Scholar]
- 33.Korucuk M, et al. , Semiological characteristics of patients with psychogenic nonepileptic seizures: Gender-related differences. Epilepsy Behav, 2018. 89: p. 130–134. [DOI] [PubMed] [Google Scholar]
- 34.Brigo F, et al. , Clinical utility of ictal eyes closure in the differential diagnosis between epileptic seizures and psychogenic events. Epilepsy Res, 2013. 104(1-2): p. 1–10. [DOI] [PubMed] [Google Scholar]
- 35.Fisher RS, et al. , Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia, 2017. 58(4): p. 522–530. [DOI] [PubMed] [Google Scholar]
- 36.Asadi-Pooya AA, Emami M, and Emami Y, Ictal injury in psychogenic non-epileptic seizures. Seizure, 2014. 23(5): p. 363–6. [DOI] [PubMed] [Google Scholar]
- 37.Adamolekun B and Foreman A, Post-ictal alpha activity in supplementary motor seizures mimics nonepileptic seizures. Epilepsy Behav, 2010. 18(3): p. 317–21. [DOI] [PubMed] [Google Scholar]
- 38.Guberman A, Psychogenic pseudoseizures in non-epileptic patients. Can J Psychiatry, 1982. 27(5): p. 401–4. [DOI] [PubMed] [Google Scholar]
- 39.Sawchuk T, et al. , Clinical characteristics of psychogenic nonepileptic seizures across the lifespan: An international retrospective study. Epilepsy Behav, 2020. 102: p. 106705. [DOI] [PubMed] [Google Scholar]
- 40.Asadi-Pooya AA, et al. , Clinical characteristics of functional (psychogenic nonepileptic) seizures: An international retrospective study. Epilepsy Behav, 2020. 111: p. 107197. [DOI] [PubMed] [Google Scholar]
- 41.Asadi-Pooya AA, Semiological classification of psychogenic nonepileptic seizures: A systematic review and a new proposal. Epilepsy Behav, 2019. 100(Pt A): p. 106412. [DOI] [PubMed] [Google Scholar]
- 42.Loddenkemper T, et al. , Ictal urinary urge: further evidence for lateralization to the nondominant hemisphere. Epilepsia, 2003. 44(1): p. 124–6. [DOI] [PubMed] [Google Scholar]
- 43.Bertolaccini L, Viti A, and Terzi A, Are the fallacies of the P value finally ended? J Thorac Dis, 2016. 8(6): p. 1067–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasserstein RL and Lazar NA, The ASA Statement on p-Values: Context, Process, and Purpose. American Statistician, 2016. 70(2): p. 129–133. [Google Scholar]
- 45.McDonald JH, Handbook of Biological Statistics. 3rd ed. 2014, Baltimore, Maryland: Sparky House Publishing. [Google Scholar]
- 46.Asadi-Pooya AA and Bahrami Z, Auras in psychogenic nonepileptic seizures. Seizure, 2019. 69: p. 215–217. [DOI] [PubMed] [Google Scholar]
- 47.Kingston WS and Schwedt TJ, The Relationship Between Headaches with Epileptic and Non-epileptic Seizures: a Narrative Review. Curr Pain Headache Rep, 2017. 21(3): p. 17. [DOI] [PubMed] [Google Scholar]
- 48.Bernasconi A, et al. , Lateralizing value of peri-ictal headache: A study of 100 patients with partial epilepsy. Neurology, 2001. 56(1): p. 130–2. [DOI] [PubMed] [Google Scholar]
- 49.Naganur VD, et al. , The utility of an automated and ambulatory device for detecting and differentiating epileptic and psychogenic non-epileptic seizures. Epilepsia Open, 2019. 4(2): p. 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusmakar S, et al. , Automated Detection of Convulsive Seizures Using a Wearable Accelerometer Device. IEEE Trans Biomed Eng, 2019. 66(2): p. 421–432. [DOI] [PubMed] [Google Scholar]
- 51.Kusmakar S, et al. , Novel features for capturing temporal variations of rhythmic limb movement to distinguish convulsive epileptic and psychogenic nonepileptic seizures. Epilepsia, 2019. 60(1): p. 165–174. [DOI] [PubMed] [Google Scholar]
- 52.Kusmakar S, et al. , Gaussian mixture model for the identification of psychogenic non-epileptic seizures using a wearable accelerometer sensor. Conf Proc IEEE Eng Med Biol Soc, 2016. 2016: p. 1006–1009. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, et al. , Potential use of leukocytosis and anion gap elevation in differentiating psychogenic nonepileptic seizures from epileptic seizures. Epilepsia Open, 2019. 4(1): p. 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, et al. , Anion gap can differentiate between psychogenic and epileptic seizures in the emergency setting. Epilepsia, 2017. [DOI] [PubMed] [Google Scholar]
- 55.Cuthill FM and Espie CA, Sensitivity and specificity of procedures for the differential diagnosis of epileptic and non-epileptic seizures: a systematic review. Seizure, 2005. 14(5): p. 293–303. [DOI] [PubMed] [Google Scholar]
- 56.Cragar DE, et al. , A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev, 2002. 12(1): p. 31–64. [DOI] [PubMed] [Google Scholar]
- 57.Boesten N, Myers L, and Wijnen B, Quality of life and psychological dysfunction in traumatized and nontraumatized patients with psychogenic nonepileptic seizures (PNES). Epilepsy Behav, 2019. 92: p. 341–344. [DOI] [PubMed] [Google Scholar]
- 58.Salinsky M, et al. , Health-related quality of life in Veterans with epileptic and psychogenic nonepileptic seizures. Epilepsy Behav, 2019. 94: p. 72–77. [DOI] [PubMed] [Google Scholar]
- 59.Gendre T, et al. , Quality of life in functional movement disorders is as altered as in organic movement disorders. J Psychosom Res, 2019. 116: p. 10–16. [DOI] [PubMed] [Google Scholar]
- 60.Anderson KE, et al. , Impact of psychogenic movement disorders versus Parkinson's on disability, quality of life, and psychopathology. Mov Disord, 2007. 22(15): p. 2204–9. [DOI] [PubMed] [Google Scholar]
- 61.Robson C, et al. , Catastrophising and normalising in patient's accounts of their seizure experiences. Seizure, 2012. 21(10): p. 795–801. [DOI] [PubMed] [Google Scholar]
- 62.Tarnutzer AA, et al. , Clinical and electrographic findings in epileptic vertigo and dizziness: a systematic review. Neurology, 2015. 84(15): p. 1595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demartini B, et al. , The pathophysiology of functional movement disroders. Neurosci Biobehav Rev, 2020. S0149-7634(20): p. 30623. [DOI] [PubMed] [Google Scholar]
- 64.Gallucci-Neto J, et al. , Ictal SPECT in Psychogenic Nonepileptic and Epileptic Seizures. Psychosomatics, 2020. [DOI] [PubMed] [Google Scholar]
- 65.Peterson KT, et al. , Right Temporoparietal Junction Transcranial Magnetic Stimulation in the Treatment of Psychogenic Nonepileptic Seizures: A Case Series. Psychosomatics, 2018. 59(6): p. 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez DL, et al. , An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci, 2015. 46(1): p. 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jobst BC, et al. , The Insula and Its Epilepsies. Epilepsy Curr, 2019. 19(1): p. 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hendrickson R, et al. , Panic attack symptoms differentiate patients with epilepsy from those with psychogenic nonepileptic spells (PNES). Epilepsy Behav, 2014. 37: p. 210–4. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto J, et al. , Seizures arising from the inferior parietal lobule can show ictal semiology of the second sensory seizure (SII seizure). J Neurol Neurosurg Psychiatry, 2003. 74(3): p. 367–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blume WT, et al. , Seizures involving secondary sensory and related areas. Brain, 1992. 115 ( Pt 5): p. 1509–20. [DOI] [PubMed] [Google Scholar]
- 71.Popkirov S, et al. , The aetiology of psychogenic non-epileptic seizures: risk factors and comorbidities. Epileptic Disord, 2019. 21(6): p. 529–547. [DOI] [PubMed] [Google Scholar]
- 72.Espay AJ, et al. , Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurol, 2018. 75(9): p. 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joels M, Stress, the hippocampus, and epilepsy. Epilepsia, 2009. 50(4): p. 586–97. [DOI] [PubMed] [Google Scholar]
- 74.Parihar J, Tripathi M, and Dhamija RK, Seizures and Epilepsy in Times of Corona Virus Disease 2019 Pandemic. J Epilepsy Res, 2020. 10(1): p. 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wanleenuwat P, Suntharampillai N, and Iwanowski P, Antibiotic-induced epileptic seizures: mechanisms of action and clinical considerations. Seizure, 2020. 81: p. 167–174. [DOI] [PubMed] [Google Scholar]
- 76.Biagini G, Panuccio G, and Avoli M, Neurosteroids and epilepsy. Curr Opin Neurol, 2010. 23(2): p. 170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brodtkorb E, Common imitators of epilepsy. Acta Neurol Scand Suppl, 2013(196): p. 5–10. [DOI] [PubMed] [Google Scholar]
- 78.Henry JA and Woodruff GH, A diagnostic sign in states of apparent unconsciousness. Lancet, 1978. 2(8096): p. 920–1. [DOI] [PubMed] [Google Scholar]
- 79.Nezadal T, et al. , Psychogenic non-epileptic seizures: our video-EEG experience. Neurol Res, 2011. 33(7): p. 694–700. [DOI] [PubMed] [Google Scholar]
- 80.An DM, et al. , Clinical features of psychogenic nonepileptic seizures: a study of 64 cases in southwest China. Epilepsy Behav, 2010. 17(3): p. 408–11. [DOI] [PubMed] [Google Scholar]
- 81.Leis AA, Ross MA, and Summers AK, Psychogenic seizures: ictal characteristics and diagnostic pitfalls. Neurology, 1992. 42(1): p. 95–9. [DOI] [PubMed] [Google Scholar]
- 82.Saygi S, et al. , Frontal lobe partial seizures and psychogenic seizures: comparison of clinical and ictal characteristics. Neurology, 1992. 42(7): p. 1274–7. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez Otarula KA, et al. , Psychogenic nonepileptic seizures in patients with surgically treated temporal lobe epilepsy: Presurgical and de novo postsurgical occurrence. Epilepsy Behav, 2017. 75: p. 252–255. [DOI] [PubMed] [Google Scholar]
- 84.Papagno C, et al. , Differentiating PNES from epileptic seizures using conversational analysis. Epilepsy Behav, 2017. 76: p. 46–50. [DOI] [PubMed] [Google Scholar]
- 85.Jenkins L, et al. , Neurologists can identify diagnostic linguistic features during routine seizure clinic interactions: results of a one-day teaching intervention. Epilepsy Behav, 2016. 64(Pt A): p. 257–261. [DOI] [PubMed] [Google Scholar]
- 86.Cornaggia CM, et al. , Conversation analysis in the differentiation of psychogenic nonepileptic and epileptic seizures in pediatric and adolescent settings. Epilepsy Behav, 2016. 62: p. 231–8. [DOI] [PubMed] [Google Scholar]
- 87.Cornaggia CM, et al. , Conversation analysis in the differential diagnosis of Italian patients with epileptic or psychogenic non-epileptic seizures: a blind prospective study. Epilepsy Behav, 2012. 25(4): p. 598–604. [DOI] [PubMed] [Google Scholar]
- 88.Kerr WT, et al. , Objective score from initial interview identifies patients with probable dissociative seizures. Epilepsy Behav, 2020. 2020(113): p. 107525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.