Abstract
Current risk stratification strategies do not fully explain cardiovascular disease (CVD) risk. We aimed to evaluate the association of low-density lipoprotein (LDL-P) and high-density lipoprotein (HDL-P) particles with progression of coronary artery calcium and carotid wall injury. All participants in the Multi-Ethnic Study Atherosclerosis (MESA) with LDL-P and HDL-P measured by ion mobility, coronary artery calcium score (CAC), carotid intima-media thickness (IMT) and carotid plaque data available at Exam 1 and 5 were included in the study. CAC progression was annualized and treated as a categorical or continuous variable. Carotid IMT and plaque progression were treated as continuous variables. Fully adjusted regression models included established CVD risk factors, as well as traditional lipids. Mean (±SD) follow-up duration was 9.6±0.6 years. All LDL-P subclasses as well as large HDL-P at baseline were positively and significantly associated with annualized CAC progression, however, after adjustment for established risk factors and traditional lipids, only the association with medium and very small LDL-P remained significant (β −0.02, p=0.019 and β 0.01, p=0.003, per 1 nmol/1 increase, respectively). Carotid plaque score progression was positively associated with small and very small LDL-P (p<0.01 for all) and non-HDL-P (p=0.013). Only the association with very small LDL-P remained significant in a fully adjusted model (p=0.035). Mean IMT progression was not associated with any of the lipid particles. In conclusion, in the MESA cohort, LDL-P measured by ion mobility was significantly associated with CAC progression as well as carotid plaque progression beyond the effect of traditional lipids.
Keywords: CAC progression, carotid atherosclerosis, lipoprotein particles, ion mobility
Introduction
Cardiovascular disease (CVD) risk prediction paradigms, based on scoring systems that combine information on traditional risk factors, do not fully explain CVD risk. There is considerable interest in the use of advanced lipid testing to identify individuals who are at elevated CVD risk and who could be targeted for preventive measures1,2. Most studies have reported determination of lipoprotein subfractions without a physical separation of lipoproteins by interpreting the nuclear resonance (NMR) signal of terminal methyl groups of triglycerides and cholesterol esters. The exquisite correlation between NMR measured low density lipoprotein particles (LDL-P) and apoB suggests that apoB measurements were used as part of the calibration algorithm raising doubt whether LDL-P by NMR provided information beyond apoB measurements. In this analysis, we measured lipoprotein particles using ion mobility – a method that separates lipoproteins by size based on the movement of charged particles in a gas-phase under the influence of an electric field3. We aimed to evaluate the performance of ion mobility derived lipoprotein particle measures in determining the association of low-density lipoprotein (LDL-P) and high-density lipoprotein particles (HDL-P) with progression of coronary and carotid atherosclerosis in the MESA cohort.
Methods
The design and objectives of the Multi-Ethnic Study of Atherosclerosis (MESA), sponsored by the National Heart, Lung, and Blood Institute, have been described elsewhere 4. Briefly, MESA study is a prospective cohort study of a multiethnic population-based sample of 6814 men and women aged 45 to 84 years who were free of known CVD at baseline, recruited from 6 U.S. sites. All participants gave informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Protocols were approved by the institutional review boards of the field and reading centers.
This paper used 3 outcome variables: (1) coronary artery calcium (CAC) progression, defined as yearly absolute change in CAC between Exam 1 and 5; (2) change in mean carotid intima and media thickness (cIMT), defined as yearly absolute change in mean cIMT between Exam 1 and 5; (3) progression of carotid plaque (increase in carotid plaque score) between Exam 1 and 5. The increase in carotid plaque score was not annualized due to ordinal rather than continuous nature of the variable. Mean cIMT was calculated as the mean of the mean left and right far wall distal common carotid artery wall thicknesses.
This analysis was restricted to subjects who had baseline and follow-up measures of any of the three outcomes. Cases were treated as missing if values at baseline or follow-up were missing. Progression was calculated as follow-up minus baseline and divided by follow-up time. Subjects who underwent a coronary revascularization procedure before follow-up were excluded from the analysis of CAC progression.
Carotid artery ultrasound performed and scored at the University of Wisconsin Imaging Research Program. At baseline and follow-up, B-mode ultrasound was used to image the near and far walls of the right and left distal common carotid artery (CCA), carotid bulb, and proximal internal carotid (ICA) using a Logiq 700 ultrasound system (13 MHz transducer; General Electric Medical Systems, Wauwatosa, WI). The carotid bifurcations and internal carotid arteries were interrogated thoroughly at 9 MHz from both longitudinal and transverse approaches to identify the thickest regions. Mean and maximal IMT of the far wall of distal CCA (distal 1 cm, proximal to the carotid bifurcation point, where the distal CCA diameter remains uniform) and the proximal 1 cm of the proximal internal carotid were measured in triplicate using a semiautomated border detection program (Syngo Arterial Health package; Siemens Medical Solutions, Malvern, PA) blinded to subject demographic and medical information5.
Carotid plaque presence was defined as a focal abnormal wall thickness (IMT >1.5 mm) or a focal thickening of >50% of the surrounding IMT. A total carotid plaque score (range, 0–12) was calculated to describe carotid plaque burden. Of the 12 segments analyzed for each participant, 1 point per plaque was allocated for the near and far walls of each segment (CCA, bulb, and ICA) of each carotid artery that was interrogated. The excellent reproducibility of the University of Wisconsin Ultrasound Reading Center’s carotid ultrasound measurements using MESA images has been previously described in detail5.
Methods for computed tomography (CT) scanning and interpretation have been reported previously6, 7. CAC was assessed at all six MESA sites at baseline by using either a cardiac-gated electron-beam CT scanner (Chicago, Los Angeles, and New York Field Centers) or a multidetector CT system (Baltimore, Forsyth County, and St. Paul Field Centers). CAC was determined by the Agatston score with excellent reproducibility7.
Traditional lipoproteins (LDL, HDL, triglycerides) were measured as previously described3,8. Ion mobility lipoprotein particles were measured at Quest Diagnostics Nichols Institute (San Juan Capistrano, CA)9. Briefly, following isolation by dextran sulfate precipitation, the lipoproteins were fractionated and quantitated in a single scan using gas-phase electrophoresis. The analysis provided large (I, IIa, range 22.0–23.33 nm), medium (IIb, 21.41–22.0 nm), small (IIIa, 20.82–21.41 nm) and very small (IIIb, IVa to c, 18.0–20.82 nm) LDL particles, large (10.5–14.5 nm) and small HDL-P (7.65–10.5 nm), large (25.0–29.6 nm) and small (23.33–25.0 nm) intermediate density lipoprotein particles (IDL-P), and large (42.4–52.0 nm), medium (33.5–42.4), and small (29.6–33.5) very low density lipoprotein particles (VLDL-P)9
Change in CAC score between baseline and follow-up was analyzed as a continuous or categorical variable (0, 1-99, 100-199, 200-300, >300 Agatston units). Approximately half of the subjects had a CAC score and carotid plaque score of 0 at baseline, hence due to the highly skewed nature of the data, CAC score was log-transformed and carotid plaque were analyzed as ln (carotid plaque score +1) and as transformed and untransformed score ranging from 0 to 12 to allow for more-direct comparison. Annualized CAC change was calculated as (ln CAC + 1 at follow-up – ln CAC + 1 at baseline/ years of follow up). Yearly change in carotid IMT was analyzed as a continuous variable and estimated as IMT at follow-up minus IMT at baseline divided by years of follow up. In cases of CAC, cIMT, carotid plaque regression, the values were treated as continuous variables in the analyses.
Lipoprotein particles were analyzed as continuous variables. Univariate comparisons (ANOVA analysis) and multivariate comparisons using robust linear regression analysis were performed. Robust linear regression, which down-weights observations with large residuals, was used for the analysis of CAC, cIMT and carotid plaque.
Model 1 included baseline (Exam 1) parameters such as age (years), race/ethnicity (white, Chinese, Black, Hispanic), body-mass index, current cigarette smoking (compared to never/former), parent history of myocardial infarction, systolic blood pressure, diastolic blood pressure, diabetes status (insulin use or fasting glucose >140), fasting glucose, high sensitivity C-reactive protein, lipid-lowering medication use, and family income. Model 2 (fully adjusted model) included Model 1 plus LDL-C, HDL-C, and triglycerides.
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA). A p value of <0.05 was considered significant.
Results
Mean follow-up was 9.6 ± 0.6 years. 2,510 and 3,305 subjects were included in the analyses for cIMT and carotid plaque, and CAC, respectively (Figure 1).
Figure 1. Study flowchart.
Overall, 5,104 subjects with CAC (n=3,305) and carotid IMT/plaque (n=2,510) data available at Exams 1 and 5 were included in the analysis.
CAC, coronary artery calcium, IMT, intima media thickness.
Baseline characteristics of these subgroups are provided in Table 1. In addition, 715 (21.6%) subjects had zero CAC both at baseline and follow-up.
Table 1.
Baseline characteristics of study participants
| Variable | CAC (n=3,305) |
cIMT/ Carotid plaque (n=2,510) |
|---|---|---|
| Age (years) | 60.1±9.4 | 59.8±9.3 |
| Men | 1571(47.5%) | 1286(47.4%) |
| White | 1303(39.4%) | 1080(39.8%) |
| Chinese | 384(11.6%) | 325(12.0%) |
| Black | 884(26.7%) | 708(26.1%) |
| Hispanic | 734(22.2%) | 600(22.1%) |
| Height (cm) | 166.9±9.9 | 166.9±9.9 |
| Weight (kg) | 79.4±17.0 | 78.7±16.9 |
| BMI (kg/m2) | 28.4±5.3 | 28.2±5.1 |
| Waist circumference (cm) | 97.8±14.0 | 97.2±13.9 |
| Systolic BP (mmHg) | 125.8±20.3 | 125.0±19.9 |
| Diastolic BP (mmHg) | 72.9±10.6 | 72.7±10.7 |
| Non-HDL particle number (nmol/l) | 1748.4±453.6 | 1742.3±451.2 |
| LDL-C (mg/dL) | 117.8±30.9 | 117.8±30.7 |
| HDL-C (mg/dL) | 50.7±14.7 | 51.0±14.8 |
| Triglycerides (mg/dl) | 130.9±82.0 | 129.2±83.0 |
| Fasting plasma glucose (mg/dL) | 95.3±26.4 | 94.5±25.3 |
| hs-CRP (mg/L) | 3.5±5.2 | 3.4±5.0 |
| Hypertension | 1361(41.2%) | 1054(38.8%) |
| Current cigarette smoker | 392(11.9%) | 327(12.1%) |
| Diabetes mellitus | 312(9.4%) | 235(8.7%) |
| Lipid-lowering medication | 528(16.0%) | 420(15.5%) |
| CAC=0 | 1011(30.6%) | 1868(68.9%) |
| CAC (Agatston score) | 287.5±595.2 | 273.3±587.1 |
| Carotid plaque | 2110(68.3%) | 1841(67.9%) |
| Mean carotid plaque score | 2.2±2.4 | 2.3±2.5 |
| Mean cIMT (mm) | 0.9±0.5 | 0.9±0.2 |
BP – blood pressure, CAC – coronary artery calcium, hs-CRP – high sensitivity C reactive protein, cIMT – carotid intima media thickness, HDL – high density lipoprotein cholesterol, LDL-P – low density lipoprotein cholesterol, HDL-P – high density lipoprotein cholesterol. Data presented as mean±SD.
Higher concentrations of total LDL-P, very small (IIIb to IVc) to small (IIIa) LDL-P, and non-HDL-P, also lower levels of large LDL-P (I to IIa) and large HDL-P subfractions were significantly associated with greater CAC progression, defined as the increase in absolute CAC per year (Figure 2). Although medium LDL-P (IIb) was also associated with annual CAC progression, the trend was non-linear.
Figure 2. Association of lipoprotein subfractions with annual CAC change.
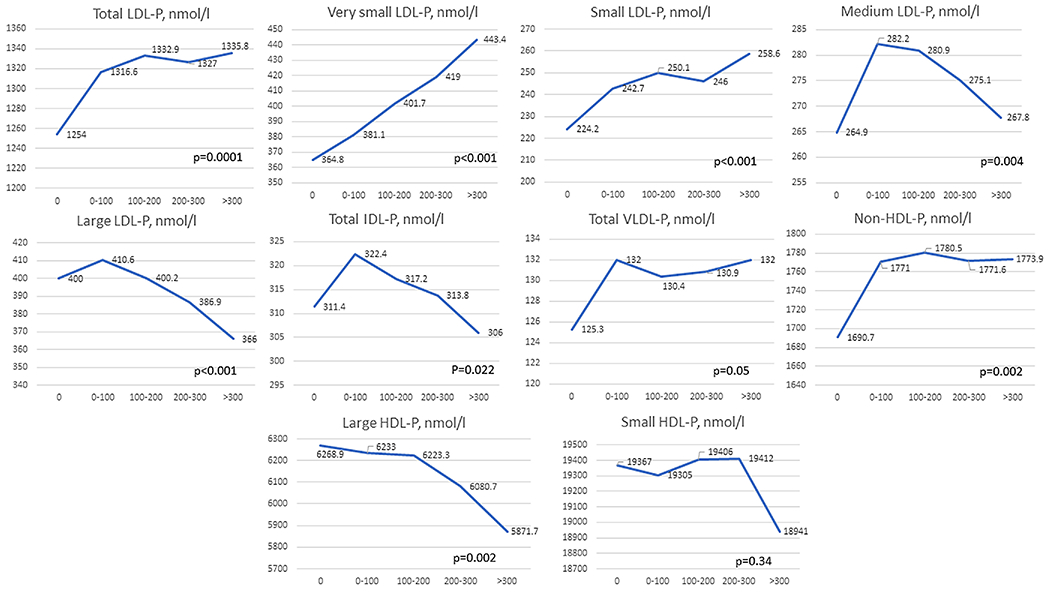
Higher concentrations of total LDL-P, very small (IIIb to IVc) to small (IIIa) LDL-P, and non-HDL-P, also lower levels of large LDL-P (I to IIa) and large HDL-P subfractions were significantly associated with greater CAC progression, defined as the increase in absolute CAC per year (Figure 2). Although medium LDL-P (IIb) was also associated with annual CAC progression, the trend was non-linear.
CAC, coronary artery calcium, LDL-P, low density lipoprotein particle, HDL-P, high density lipoprotein particle. Data presented as mean±SD.
Annual change in CAC was significantly associated with all baseline LDL-P subfractions in unadjusted analyses (Table 2). When adjusted for major CVD risk factors (Model 1) and additionally for conventional lipids (Model 2), annual change in CAC retained significant positive associations with very small LDL-P (IIIb to IVc) and an inverse association with large and medium LDL-P, but not with IDL-P or HDL-P (Table 2).
Table 2.
Association between annual change in coronary artery calcium and lipoprotein subtractions in univariate and multivariate analyses
| Lipoprotein particles | Unadjusted |
Model 1 |
Model 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef | 95% CI | P | Coef | 95% CI | P | Coef | 95% CI | P | |
| LDL-P Total (nmol/l) | 0.004 | −0.001,0.008 | 0.117 | 0.005 | 0.001,0.009 | 0.019 | 0.001 | −0.001,0.01 | 0.747 |
| LDL-P I (nmol/l) | −0.033 | −0.049, −0.017 | <0.001 | −0.019 | −0.034, −0.003 | 0.020 | −0.01 | −0.03,0.01 | 0.446 |
| LDL-P IIa (nmol/l) | −0.047 | −0.070, −0.024 | <0.001 | −0.025 | −0.047, −0.003 | 0.023 | −0.03 | −0.05, −0.01 | 0.045 |
| LDL-P IIb (medium) (nmol/l) | −0.018 | −0.034, −0.002 | 0.028 | −0.006 | −0.022,0.009 | 0.403 | −0.02 | −0.04, −0.00 | 0.019 |
| LDL-P IIIa (small) (nmol/l) | 0.02 | 0.006,0.035 | 0.005 | 0.019 | 0.006,0.033 | 0.007 | 0.01 | −0.02,0.02 | 0.959 |
| LDL-P IIIb (nmol/l) | 0.059 | 0.037,0.082 | <0.001 | 0.050 | 0.029,0.071 | <0.001 | 0.03 | 0.01,0.06 | 0.029 |
| LDL-P Iva (nmol/l) | 0.059 | 0.039,0.079 | <0.001 | 0.050 | 0.030,0.067 | <0.001 | 0.004 | 0.02,0.06 | 0.001 |
| LDL-P IVb (nmol/l) | 0.08 | 0.047,0.113 | <0.001 | 0.065 | 0.034,0.097 | <0.001 | 0.05 | 0.01,0.09 | 0.008 |
| LDL-P IVc (nmol/l) | 0.064 | 0.010,0.119 | 0.020 | 0.054 | 0.002,0.105 | 0.004 | 0.03 | −0.02,0.09 | 0.264 |
| LDL-P large (I and IIa combined) (nmol/l) | −0.021 | −0.030, −0.011 | <0.001 | −0.011 | −0.021, −0.002 | 0.017 | −0.01 | −0.02,0.01 | 0.177 |
| LDL-P very small (IIIb to IVc combined) (nmol/l) | 0.023 | 0.015,0.030 | <0.001 | −0.011 | 0.011,0.026 | <0.001 | 0.01 | 0.001,0.02 | 0.003 |
| VLDL-P total (nmol/l) | 0.004 | −0.029,0.036 | 0.834 | 0.021 | −0.009,0.052 | −0.173 | −0.003 | −0.07,0.01 | 0.204 |
| VLDL-P large (nmol/l) | 0.153 | −0.027,0.333 | 0.096 | 0.206 | 0.037,0.375 | 0.017 | −0.01 | −0.34,0.14 | 0.419 |
| VLDL-P medium (nmol/l) | 0.029 | −0.047,0.105 | 0.453 | 0.069 | −0.002,0.140 | 0.056 | −0.06 | −0.16,0.04 | 0.224 |
| VLDL-P small (nmol/l) | −0.034 | −0.106,0.038 | 0.358 | 0.008 | −0.059,0.076 | 0.806 | −0.06 | −0.14,0.03 | 0.183 |
| IDL-P total (nmol/l) | −0.019 | −0.036, −0.001 | 0.034 | −0.01 | −0.027,0.006 | 0.213 | −0.02 | −0.04,0.01 | 0.097 |
| IDL-P large (nmol/l) | −0.006 | −0.039,0.026 | 0.704 | 0.004 | −0.027,0.034 | 0.813 | −0.03 | −0.07,0.01 | 0.091 |
| IDL-P small (nmol/l) | −0.049 | −0.079, −0.020 | 0.002 | −0.033 | −0.061, −0.006 | 0.019 | −0.02 | −0.06,0.01 | 0.183 |
| Non-HDL-P (nmol/l) | 0.001 | −0.002,0.005 | 0.420 | 0.003 | −0.000,0.006 | 0.089 | −0.001 | −0.001,0.01 | 0.798 |
| HDL-P large (nmol/l) | −0.001 | −0.002, −0.001 | 0.003 | −0.001 | −0.002,−0.001 | 0.007 | 0.001 | −0.001,0.01 | 0.824 |
| HDL-P small (nmol/l) | −0.001 | −0.00,0.001 | 0.274 | −0.001 | −0.001,0.001 | 0.274 | −0.001 | −0.001,0.01 | 0.573 |
CAC – coronary artery calcium, LDL-P – low density lipoprotein particle, VLDL-P – very low density lipoprotein particle, IDL-P – intermediate density lipoprotein particle, HDL-P – high density lipoprotein particle. CAC change shows the yearly change between the baseline coronary artery calcium and a follow-up visit approximately 10 years later. Robust linear regression, which down-weights observations with large residuals, was used due to a few cases with exceptionally large CAC values. Model 1: age (years), race/ethnicity (white, Chinese, Black, Hispanic), BMI, current cigarette smoking (compared to never/former), parent history of myocardial infarction, systolic blood pressure, diastolic blood pressure, diabetes status (insulin use or fasting glucose >140), fasting glucose, high sensitivity C-reactive protein (ln) and lipid-lowering medication use, family income. Model 2: Model 1 + LDL-C, HDL-C, triglycerides (ln)
In unadjusted or adjusted (both Model 1 and Model 2) analyses, annual change in mean cIMT was not associated with any of the lipoprotein particles (Table 3).
Table 3.
Association between annual change in mean cIMT and ion mobility measured lipoprotein subfractions
| Lipoprotein particles | Unadjusted | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef | 95% CI | P | Coef | 95% CI | P | Coef | 95% CI | P | |
| LDL-P total (nmol/l) | 0.001 | −0.001,0.003 | 0.232 | 0.001 | −0.001,0.003 | 0.167 | −0.001 | −0.001,0.001 | 0.729 |
| LDL-P I (nmol/l) | 0.001 | −0.005,0.007 | 0.683 | 0.001 | −0.005,0.007 | 0.713 | −0.001 | −0.01,0.01 | 0.726 |
| LDL-P IIa (nmol/l) | 0.004 | −0.005,0.012 | 0.373 | 0.004 | −0.005,0.012 | 0.419 | −0.001 | −0.01,0.01 | 0.758 |
| LDL-P IIb (medium) (nmol/l) | 0.004 | −0.002,0.010 | 0.147 | 0.005 | −0.001,0.01 | 0.122 | 0.001 | −0.01,0.01 | 0.981 |
| LDL-P IIIa (small) (nmol/l) | 0.003 | −0.002,0.008 | 0.265 | 0.004 | −0.001,0.009 | 0.153 | −0.001 | −0.01,0.01 | 0.963 |
| LDL-P IIIb (nmol/l) | 0.001 | −0.007,0.009 | 0.803 | 0.002 | −0.006,0.011 | 0.583 | −0.001 | −0.02,0.01 | 0.514 |
| LDL-P IVa (nmol/l) | 0.001 | −0.007,0.008 | 0.880 | 0.002 | −0.006,0.009 | 0.762 | −0.001 | −0.01,0.01 | 0.712 |
| LDL-P IVb (nmol/l) | 0.003 | −0.009,0.015 | 0.632 | 0.003 | −0.009,0.016 | 0.616 | 0.001 | −0.01,0.02 | 0.669 |
| LDL-P IVc (nmol/l) | 0.001 | −0.019,0.021 | 0.948 | 0.001 | −0.020,0.021 | 0.981 | −0.001 | −0.01,0.01 | 0.785 |
| LDL-P large (I and IIa combined) (nmol/l) | 0.001 | −0.002,0.005 | 0.530 | 0.001 | −0.003,0.005 | 0.569 | −0.001 | −0.01,0.01 | 0.730 |
| LDL-P very small (IIIb to IVc combined) (nmol/l) | 0.001 | −0.003,0.003 | 0.787 | 0.001 | −0.002,0.004 | 0.661 | −0.001 | −0.01,0.01 | 0.757 |
| VLDL-P total (nmol/l) | 0.006 | −0.006,0.018 | 0.356 | 0.006 | −0.006,0.019 | 0.320 | −0.001 | −0.02,0.02 | 0.964 |
| VLDL-P large (nmol/l) | 0.029 | −0.037,0.096 | 0.385 | 0.027 | −0.041,0.096 | 0.437 | 0.02 | −0.08,0.12 | 0.649 |
| VLDL-P medium (nmol/l) | 0.013 | −0.015,0.041 | 0.379 | 0.013 | −0.016,0.042 | 0.388 | −0.001 | −0.04,0.04 | 0.879 |
| VLDL-P small (nmol/l) | 0.012 | −0.015,0.038 | 0.395 | 0.015 | −0.013,0.042 | 0.287 | −0.001 | −0.04,0.03 | 0.905 |
| IDL-P total (nmol/l) | 0.001 | −0.005,0.008 | 0.667 | 0.002 | −0.004,0.009 | 0.521 | −0.001 | −0.01,0.01 | 0.511 |
| IDL-P large (nmol/l) | 0.005 | −0.007,0.017 | 0.397 | 0.007 | −0.005,0.019 | 0.273 | −0.001 | −0.02,0.01 | 0.684 |
| IDL-P small (nmol/l) | −0.001 | −0.011,0.011 | 0.973 | 0.001 | −0.011,0.012 | 0.923 | −0.001 | −0.02,0.01 | 0.451 |
| Non-HDL-P (nmol/l) | 0.001 | −0.001,0.002 | 0.268 | 0.001 | −0.001,0.002 | 0.193 | −0.001 | −0.02,0.01 | 0.675 |
| HDL-P large (nmol/l) | −0.001 | −0.001,0.001 | 0.130 | −0.001 | −0.001,0.001 | 0.285 | 0.001 | −0.001,0.01 | 0.911 |
| HDL-P small (nmol/l) | −0.001 | −0.001,0.001 | 0.797 | 0.001 | −0.001,0.001 | 0.966 | 0.001 | −0.001,0.01 | 0.817 |
IMT – intima media thickness, LDL-P – low density lipoprotein particle, VLDL-P – very low density lipoprotein particle, IDL-P – intermediate density lipoprotein particle, HDL-P – high density lipoprotein particle.
Model 1: age (years), race/ethnicity (white, Chinese, Black, Hispanic), BMI, current cigarette smoking (compared to never/former), parent history of myocardial infarction, systolic blood pressure, diastolic blood pressure, diabetes status (insulin use or fasting glucose >140), fasting glucose, high sensitivity C-reactive protein (ln) and lipid-lowering medication use, family income.
Model 2: Model 1 + LDL-C, HDL-C, triglycerides (ln)
In unadjusted and Model 1 adjusted linear regression analyses, change in carotid plaque score was significantly associated with total LDL-P, as well as small to very small LDL-P (IIIa to IVc), but not large or medium LDL-P, or HDL-P (Table 4). In the fully adjusted model (Model 2), additionally adjusted for traditional lipoproteins, change in carotid plaque score was significantly associated only with very small LDL-P (LDL-P IIIb, p=0.035).
Table 4.
Association between carotid plaque progression and ion mobility measured lipoprotein subtractions
| Lipoprotein particles | Unadjusted |
Model 1 |
Model 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef | 95% CI | P | Coef | 95% CI | P | Coef | 95% CI | P | |
| LDL-P total (nmol/l) | −0.001 | −0.001,0.001 | 0.002 | −0.001 | −0.001,0.001 | <0.001 | 0.001 | −0.001,0.001 | 0.316 |
| LDL-P I (nmol/l) | −0.001 | −0.001,0.001 | 0.091 | −0.001 | −0.001,0.001 | 0.623 | −0.001 | −0.001,0.001 | 0.287 |
| LDL-P IIa (nmol/l) | −0.001 | −0.001,0.001 | 0.744 | 0.001 | −0.001,0.001 | 0.503 | −0.001 | −0.001,0.001 | 0.397 |
| LDL-P IIb (medium) (nmol/l) | 0.001 | −0.001,0.001 | 0.083 | 0.001 | 0.001,0.002 | 0.018 | −0.001 | −0.001,0.001 | 0.761 |
| LDL-P IIIa (small) (nmol/l) | 0.001 | 0.001,0.002 | <0.001 | 0.001 | 0.001,0.002 | <0.001 | 0.001 | −0.001,0.001 | 0.057 |
| LDL-P IIIb (nmol/l) | 0.001 | 0.001,0.002 | <0.001 | 0.001 | 0.001,0.002 | <0.001 | 0.001 | 0.001,0.002 | 0.035 |
| LDL-P IVa (nmol/l) | 0.001 | 0.001,0.002 | 0.0028 | 0.001 | 0.001,0.002 | 0.011 | 0.001 | −0.001,0.001 | 0.179 |
| LDL-P IVb (nmol/l) | 0.001 | 0.001,0.002 | 0.0029 | 0.001 | −0.001,0.001 | 0.096 | 0.001 | −0.001,0.001 | 0.215 |
| LDL-P IVc (nmol/l) | 0.001 | −0.001,0.001 | 0.284 | 0.001 | −0.001,0.001 | 0.330 | 0.001 | −0.001,0.001 | 0.667 |
| LDL-P large (I and IIa combined) (nmol/l) | −0.001 | −0.001,0.001 | 0.247 | −0.001 | −0.001,0.001 | 0.995 | −0.001 | −0.001,0.001 | 0.314 |
| LDL-P very small (IIIb to IVc combined) (nmol/l) | 0.001 | 0.001,0.002 | <0.001 | 0.001 | 0.001,0.002 | <0.001 | 0.001 | −0.001,0.001 | 0.095 |
| VLDL-P total (nmol/l) | 0.001 | −0.001,0.001 | 0.374 | 0.001 | −0.001,0.001 | 0.172 | −0.001 | −0.001,0.001 | 0.208 |
| VLDL-P large (nmol/l) | 0.001 | −0.001,0.001 | 0.123 | 0.001 | −0.001,0.001 | 0.158 | −0.001 | −0.001,0.001 | 0.574 |
| VLDL-P medium (nmol/l) | 0.001 | −0.001,0.001 | 0.127 | 0.001 | −0.001,0.001 | 0.062 | −0.001 | −0.001,0.001 | 0.486 |
| VLDL-P small (nmol/l) | −0.001 | −0.001,0.001 | 0.917 | 0.001 | −0.001,0.001 | 0.494 | −0.001 | −0.001,0.001 | 0.067 |
| IDL-P total (nmol/l) | −0.001 | −0.001,0.001 | 0.798 | 0.001 | −0.001,0.001 | 0.630 | −0.001 | −0.001,0.001 | 0.116 |
| IDL-P large (nmol/l) | 0.001 | −0.001,0.001 | 0.294 | 0.001 | −0.001,0.001 | 0.135 | −0.001 | −0.001,0.001 | 0.125 |
| IDL-P small (nmol/l) | −0.001 | −0.001,0.001 | 0.168 | −0.001 | −0.001,0.001 | 0.587 | −0.001 | −0.001,0.001 | 0.187 |
| Non-HDL-P (nmol/l) | 0.001 | 0.001,0.002 | 0.013 | 0.001 | 0.001,0.002 | 0.002 | 0.001 | −0.001,0.001 | 0.762 |
| HDL-P large (nmol/l) | −0.001 | −0.001,0.001 | 0.105 | −0.001 | −0.001,0.001 | 0.06 | 0.001 | −0.001,0.01 | 0.937 |
| HDL-P small (nmol/l) | −0.001 | −0.001,0.001 | 0.769 | −0.001 | −0.001,0.001 | 0.556 | −0.001 | −0.001,0.01 | 0.857 |
LDL-P – low density lipoprotein particle, VLDL-P – very low density lipoprotein particle, IDL-P – intermediate density lipoprotein particle, HDL-P – high density lipoprotein particle.
Model 1: age (years), race/ethnicity (white, Chinese, Black, Hispanic), BMI, current cigarette smoking (compared to never/former), parent history of myocardial infarction, systolic blood pressure, diastolic blood pressure, diabetes status (insulin use or fasting glucose >140), fasting glucose, high sensitivity C-reactive protein (ln if needed) and lipid-lowering medication use, family income.
Model 2: Model 1 + LDL-C, HDL-C, triglycerides (ln)
Discussion
As previously in most of the studies lipoprotein particle testing was performed with NMR, this study is one of the few that used ion mobility to determine lipoprotein particle concentration. Rather than imputing particle data based on its presumed composition, ion mobility separates and analyzes the lipoprotein particles themselves. Ion mobility measures lipoprotein concentration for the entire size spectrum of lipoprotein particles ranging from 5 nm to 53 nm at a high size resolution (<0.1 nm diameter on average)3.
In this study, we observed a significant positive association of small and very small LDL-P with two measures of atherosclerosis: CAC progression and carotid plaque progression even after adjustment for traditional lipids. It is known that small LDL particles contain substantially less cholesterol than large LDL-P, such that at the same serum concentration of LDL cholesterol, individuals with predominantly small LDL have greater total concentration of LDL particles than those with predominantly large LDL, and thus may have greater CVD risk10. This may explain why LDL particles are associated with atherosclerosis and CVD outcomes independently from total LDL cholesterol that is a general measure of lipid pool. Small LDL-P may be more atherogenic due to their susceptibility to oxidation and greater affinity for proteoglycans, increasing subendothelial permeability and accumulation11. Moreover, oxidized LDL may promote an inflammatory response that could lead to plaque formation and vulnerability12.
This is in agreement with the Malmo Prevention Project study indicating that very small LDL-P measured by ion mobility were significantly associated with CVD events independently of traditional risk factors and traditional lipids13. Moreover, the simulation model was constructed to further suggest that lipid particles included into a functional risk score were associated with CVD events after adjustment for traditional risk factors14. Furthermore, a recent cross-sectional study reported an association between ion-mobility measured lipid particles with the presence of CAC in subjects with diabetes or metabolic syndrome15.
The HDL Atherosclerosis Treatment Study that measured lipoprotein subfractions using four methods, including NMR and ion mobility, found that small dense LDL were independently related to coronary artery stenosis progression, although the extent of these associations differed depending on the method used16. In a previous cross-sectional MESA baseline analysis (n=5538), an association of NMR-derived LDL size and small LDL-P with carotid intima-media thickness was no longer significant after accounting for lipoprotein subclasses and risk factors17. However, Otvos et al. demonstrated that in case of discordance between LDL-C and NMR measured LDL-P, LDL-P better predicted incident CVD events and cIMT in a MESA cohort, compared to LDL-C18. Moreover, in a post-hoc analysis of the JUPITER trial that investigated the association of ion mobility measured lipoprotein particles with CVD events, LDL-P and smaller subfractions of LDL-P and VLDL-P, but not baseline LDL-C, were related to CVD events9.
In our analysis, large, but not small, HDL-P were significantly negatively associated with CAC progression, but this association became insignificant after adjustment for standard lipid measures. In the Malmö Prevention Project Study, high levels of HDL-C, but not HDL-P subfractions, were associated with incident CVD13. The varying association of HDL particles with progression of subclinical atherosclerosis may be in part explained by the heterogeneity of the HDL particles, genetic factors, and also impacted by cholesterol efflux capacity that was not measured in this study19.
A possible limitation for this study was that ion mobility was performed on samples that were previously frozen and stored for a prolonged period of time. The impact of storage and freezing on lipoprotein analysis is not known. However, lipoprotein profiles from MESA stored samples have been shown to be consistent with those obtained from fresh frozen specimens per Quest Diagnostics laboratory. Moreover, multiple comparisons were performed increasing the probability of a Type I error.
In agreement with previously reported data from outcome studies9, this analysis demonstrated that lipoprotein particles measured with ion mobility were associated with atherosclerosis progression in the coronary and carotid vessels independently from conventional methods. The importance of this finding in overall and residual cardiovascular risk prediction should be further evaluated in prospective studies using ion mobility testing.
In conclusion, LDL particles measured with ion mobility were independently associated with progression of atherosclerosis in a MESA cohort. A significant positive association of small and very small LDL-P with progression of CAC and carotid plaque score was observed. Large HDL particles were significantly inversely associated with CAC and carotid plaque progression; however, this association was attenuated after adjustment for traditional lipids.
Highlights.
Small and very small LDL-P were associated with progression of atherosclerosis.
Advanced lipid testing may identify individuals who are at elevated CVD risk.
Lipid ion mobility testing should be included into prospective outcome studies.
Acknowledgments
Source of Funding:
This research was supported by R01 HL071739 and a grant from Quest Diagnostics, and MESA was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1 TR 001079, and UL1-RR-025005 from National Center for Research Resources. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of conflicting interests
MB received consultant fees/honoraria from Janssen, Pfizer, research grants from General Electric, Speaker’s bureau - Astra Zeneca.
JS receives royalties from the Wisconsin Alumni Research Foundation for a patent related to carotid ultrasound and arterial aging. This product was not used in this study.
Other authors do not have any relevant conflict of interest.
All authors have approved the final manuscript.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D’Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death, N Engl J Med 2006;355:2631–2639. [DOI] [PubMed] [Google Scholar]
- 2.Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, Kuulasmaa K, Yarnell J, Schnabel RB, Wild PS, Munzel TF, Lackner KJ, Tiret L, Evans A, Salomaa V, MORGAM Project. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project, Circulation 2010;121:2388–2397. [DOI] [PubMed] [Google Scholar]
- 3.Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis, Clin Chem 2008;54:1307–1316. [DOI] [PubMed] [Google Scholar]
- 4.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design, Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 5.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis, Circ Cardiovasc Imaging 2015;8: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study, Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 7.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study, Radiology 2005;236:477–484. [DOI] [PubMed] [Google Scholar]
- 8.Mackey RH, Mora S, Bertoni AG, Wassel CL, Carnethon MR, Sibley CT, Goff DC Jr. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis, Diabetes Care 2015;38:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora S, Caulfield MP, Wohlgemuth J, Chen Z, Superko HR, Rowland CM, Glynn RJ, Ridker PM, Krauss RM. Atherogenic Lipoprotein Subfractions Determined by Ion Mobility and First Cardiovascular Events After Random Allocation to High-Intensity Statin or Placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial, Circulation 2015;132:2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease, Curr Atheroscler Rep 2004;6:381–387. [DOI] [PubMed] [Google Scholar]
- 11.Tabas I, Williams KJ, Boren J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis, Circulation 2007;116:1832. [DOI] [PubMed] [Google Scholar]
- 12.Otsuka F, Kramer MC, Woudstra P, Yahagi K, Ladich E, Finn AV, de Winter RJ, Kolodgie FD, Wight TN, Davis HR, Joner M, Virmani R. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study, Atherosclerosis 2015;241:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiffman D, Louie JZ, Caulfield MP, Nilsson PM, Devlin JJ, Melander O. LDL subfractions are associated with incident cardiovascular disease in the Malmö Prevention Project Study, Atherosclerosis 2017;263:287–292. [DOI] [PubMed] [Google Scholar]
- 14.Rowland CM, Shiffman D, Caulfield M, Garcia V, Melander O, Hastie T. Association of cardiovascular events and lipoprotein particle size: Development of a risk score based on functional data analysis, PLoS One 2019;14:e0213172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aneni EC, Osondu CU, De La Cruz J, Martin SS, Blaha MJ, Younus A, Feldman T, Agatston AS, Veledar E, Nasir K. Lipoprotein Sub-Fractions by Ion-Mobility Analysis and Its Association with Subclinical Coronary Atherosclerosis in High-Risk Individuals, J Atheroscler Thromb 2019;26:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams PT, Zhao XQ, Marcovina SM, Otvos JD, Brown BG, Krauss RM. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease, Atherosclerosis 2014;233:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC Jr, O’Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA), Atherosclerosis 2007;192:211–217. [DOI] [PubMed] [Google Scholar]
- 18.Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC Jr. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number, J Clin Lipidol 2011;5:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Superko HR, Pendyala L, Williams PT, Momary KM, King SB 3rd, Garrett BC. High-density lipoprotein subclasses and their relationship to cardiovascular disease, J Clin Lipidol 2012;6:496–523. [DOI] [PubMed] [Google Scholar]


