Abstract
Trypanosoma cruzi, and the T. brucei group of parasites cause neglected diseases that affect millions of people around the world. These unicellular microorganisms have complex life cycles involving an insect vector and a mammalian host. Both groups of pathogens possess an inositol 1,4,5-trisphosphate (IP3)/diacylglycerol (DAG) signaling pathway, and an IP3 receptor, but with lineage-specific adaptations that make them different from their mammalian counterparts. The phospholipase C (PLC), which hydrolyzes phosphatidyl inositol 4,5-bisphosphate (PIP2) to IP3 is N-terminally myristoylated and palmitoylated. Acidocalcisomes, which are lysosome-related organelles rich in polyphosphate, are the main intracellular Ca2+ stores. The inositol 1,4,5-trisphosphate receptor (IP3R) localizes to acidocalcisomes instead of the endoplasmic reticulum. The trypanosome IP3R is stimulated by luminal phosphate and pyrophosphate, which are hydrolysis products of polyphosphate (polyP), and inhibited by tripolyphosphate (polyP3), which is the most abundant polyP in acidocalcisomes. Ca2+ signaling is important for host cell invasion and differentiation and to maintain cellular bioenergetics.
Keywords: Acidocalcisome, calcium, inositol phosphates, IP3 receptor, mitochondria, polyphosphate
1. Trypanosomes and acidocalcisomes.
T. cruzi is the etiologic agent of Chagas disease or American trypanosomiasis, which is vector-borne disease endemic from the South of the United States to the South or Argentina and Chile. However, cases of this disease have been detected all over the world because of migratory activity [1]. Its life cycle involves vector stages (epimastigote, and metacyclic trypomastigote) and mammalian stages (bloodstream trypomastigote, and intracellular amastigote). When the infected vector (triatomine) takes a blood meal it releases metacyclic trypomastigotes in its feces near the site of the bite wound. These trypomastigotes enter the host through the wound or through intact mucosal membranes, such as the conjunctiva, and invade nucleated cells where they transform into intracellular amastigotes. After replicating for 4–5 days, amastigotes are converted to cell-derived trypomastigotes, which are released into the circulation to infect other cells or to be ingested by another vector. In the vector, cell-derived trypomastigotes differentiate into epimastigotes, duplicate by binary fission in the midgut, and transform into metacyclic trypomastigotes in the hind gut to start a new cycle. The T. brucei group of parasites includes subspecies that cause human African trypanosomiasis (sleeping sickness), and nagana in cattle, and their occurrence is limited to the African continent. Two main stages of these parasites are usually studied: procyclic trypomastigotes (vector stage) and bloodstream trypomastigotes (mammalian stage). When the infected vector (tsetse fly) takes a blood meal it releases metacyclic trypomastigotes into the skin. The parasites enter the lymphatic system and pass into the bloodstream where they transform into bloodstream trypomastigotes, which reach different tissues and replicate while in circulation. The tsetse fly gets infected with bloodstream trypomastigotes when taking a blood meal on the mammalian host. The parasites transform into procyclic trypomastigotes in the midgut of the vector, multiply by binary fission, leave the midgut, and transform into epimastigotes. The epimastigotes reach the salivary glands and continue multiplication by binary fission to finally differentiate into metacyclic trypomastigotes to close the cycle.
Trypanosomes are characterized by possessing acidocalcisomes as their main Ca2+ storage organelle. Acidocalcisomes are acidic calcium stores first described in trypanosomes [2, 3]. Acidocalcisomes of trypanosomes possess a Ca2+-ATPase for Ca2+ uptake [4, 5] and an IP3 receptor for Ca2+ release [6, 7], and several other membrane pumps, channels, and exchangers (Fig. 1) [7, 8]. Acidification of the organelles is through two electrogenic pumps: a vacuolar H+-ATPase [2, 3, 8, 9] and a vacuolar H+-pyrophosphatase [10, 11]. Other transporters include orthologs to the yeast vacuolar iron transporter (VIT), a polyamine transporter [8], and a zinc transporter [8, 12], as well as channels: an aquaporin in T. cruzi [13, 14] and a K+ channel in T. brucei [15]. There is also physiological evidence of the presence of Na+/H+ and Ca2+/H+ exchangers [16]. Acidocalcisomes are rich in Pi, PPi, and polyphosphate (polyP) [17–19] and they possess an enzymatic complex named vacuolar transporter chaperone complex (VTC) for the synthesis and translocation of polyP, formed by at least two subunits, VTC1 and VTC4 [20–22]. There is also evidence for the presence of enzymes possibly linked to polyP hydrolysis within acidocalcisomes: an exopolyphosphatase [18], a vacuolar soluble pyrophosphatase (VSP) [23–26], and an acid phosphatase [8]. VSP has pyrophosphatase activity in the presence of Mg2+ and exopolyphosphatase activity in the presence of Zn2+ [24]. There is also a Na+/phosphate symporter orthologue to PHO91 of yeast, involved in Na+ and Pi release [8, 27–29]. PolyP, a polymer of three to hundreds of orthophosphate units is abundant within acidocalcisomes, could reach molar concentrations, and is complexed with cations, such as calcium, magnesium, potassium, sodium, zinc, and iron [27]. This chemical composition is probably the reason for the high electron-density of acidocalcisomes when examined by electron microscopy. They are spherical, have an average diameter of 0.2–0.3 μm and can also be identified by staining with DAPI, that labels polyP, or dyes that accumulate in acidic compartment, such as acridine orange or LysoTracker [30]. Functions of the acidocalcisomes include cation and Pi storage [27], osmoregulation [14, 31, 32], parasite persistence [24], Ca2+ signaling [33], and regulation of cell bioenergetics [34].
Figure 1.
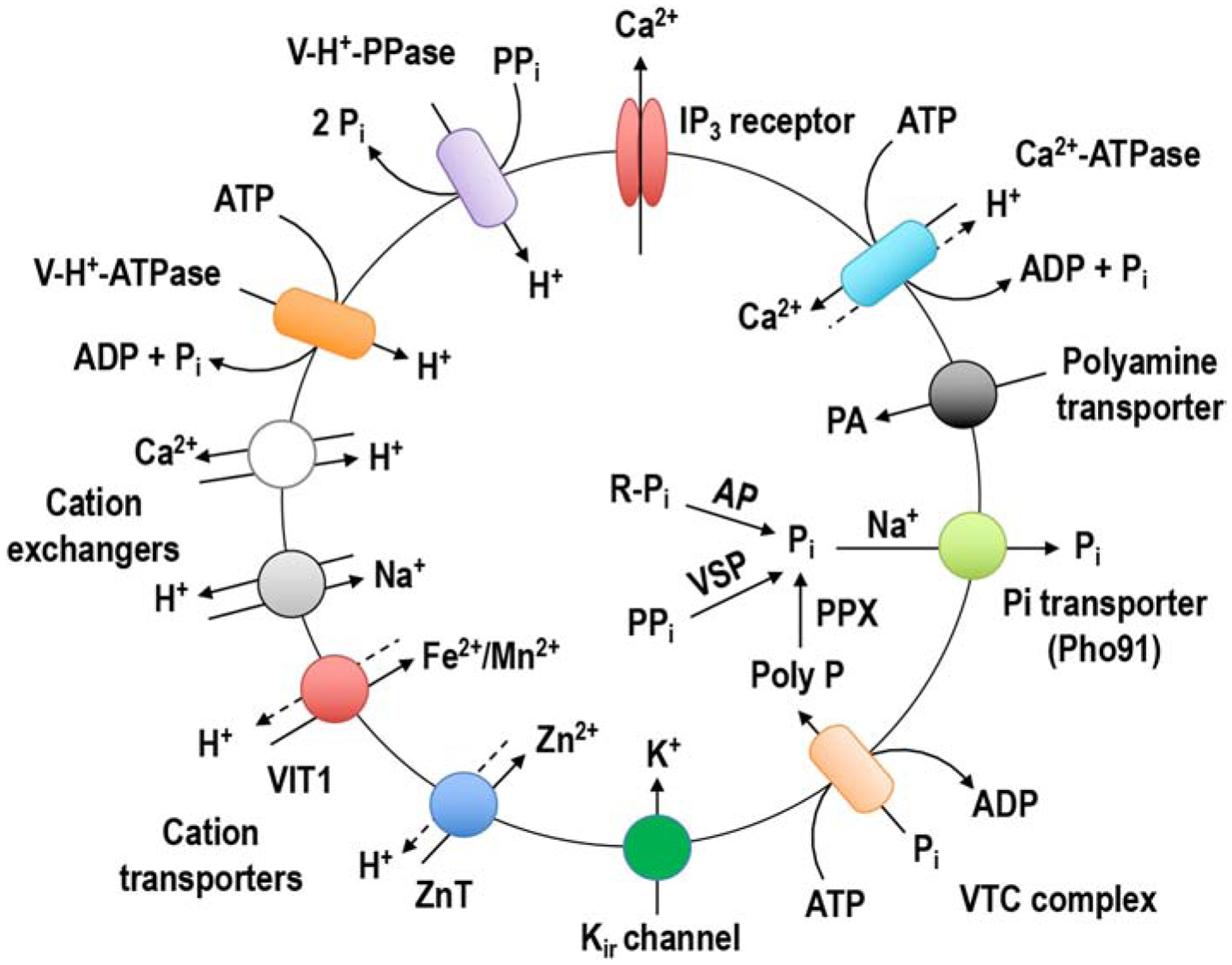
Schematic representation of the acidocalcisome of T. brucei. In the T. brucei acidocalcisomes, Ca2+ is taken up by a H+-counter-transporting Ca2+-ATPase and released by the inositol 1,4,5,trisphosphate (IP3) receptor. H+ is pumped in electrogenically by either the V-H+-PPase or the multisubunit V-H+-ATPase. Ca2+/H+ and Na+/H+ exchangers could be used for Ca2+ release in exchange for Na+ uptake. A vacuolar iron transporter (VIT1) can be used for either Mn2+ or Fe2+ uptake and a Zn2+ transporter (ZnT) for Zn2+ uptake. There is also a polyamine (PA) transporter. A VTC with at least two subunits (Vtc1 and Vtc4) synthesizes polyP using ATP and translocates it into the organelle. A Na+/Pi symporter (Pho91) releases Na+ and Pi from acidocalcisomes. Within acidocalcisomes there is a vacuolar soluble pyrophosphatase (VSP), an exopolyphosphatase (PPX) and an acid phosphatase (AP). An inward rectifier potassium channel (Kir) also localizes to the acidocalcisome.
2. The IP3/diacylglycerol signaling pathway
This pathway was first described in T. cruzi [35] and later found in T. brucei [36]. Using [32P]Pi and [3H]inositol as precursors it was possible to detect phosphatidylinositol (PI), phosphatidylinositol 4-phosphate (PIP) and phosphatidylinositol 4,5-bisphosphate (PIP2), and their derivatives inositol phosphate (IP), inositol 1,4-bisphosphate (IP2) and IP3, respectively, in epimastigotes of T. cruzi. Calcium stimulated the formation of IP2, IP3, and diacylglycerol in permeabilized cells [35]. The use of [3H]inositol labeling also identified the presence of IP, IP2 and IP3 in T. cruzi amastigotes [37] and trypomastigotes [38], and in procyclic and bloodstream forms of T. brucei [36, 39].
A novel phosphoinositide-specific phospholipase C (PI-PLC), which is responsible for the hydrolysis of PIP2 to IP3 was also identified in T. cruzi [31, 34, 40–43] and T. brucei [44, 45]. The T. cruzi enzyme is different from mammalian PI-PLCs: a) it is stimulated by [Ca2+] at above 10 nM; b) lacks a pleckstrin homology (PH) domain; c) has a highly charged linker region between the catalytic X and Y domains; d) has an N-myristoylation consensus sequence at its amino-terminal end, and is dually acylated in vivo by myristate, and palmitate or stearate; and e) is targeted to the plasma membrane [40] (Fig. 2A). Myristoylation is in a glycine residue in the 2nd position while palmitoylation or stearylation is in the cysteine in the 4th position of the protein [31]. The amino-terminal 20 amino acids of TcPI-PLC are necessary and sufficient to target a fused GFP to the surface of epimastigotes and amastigotes [31]. Interestingly, TcPI-PLC localizes to the outer surface of amastigotes, and dual acylation is required for this localization [31].
Figure 2.
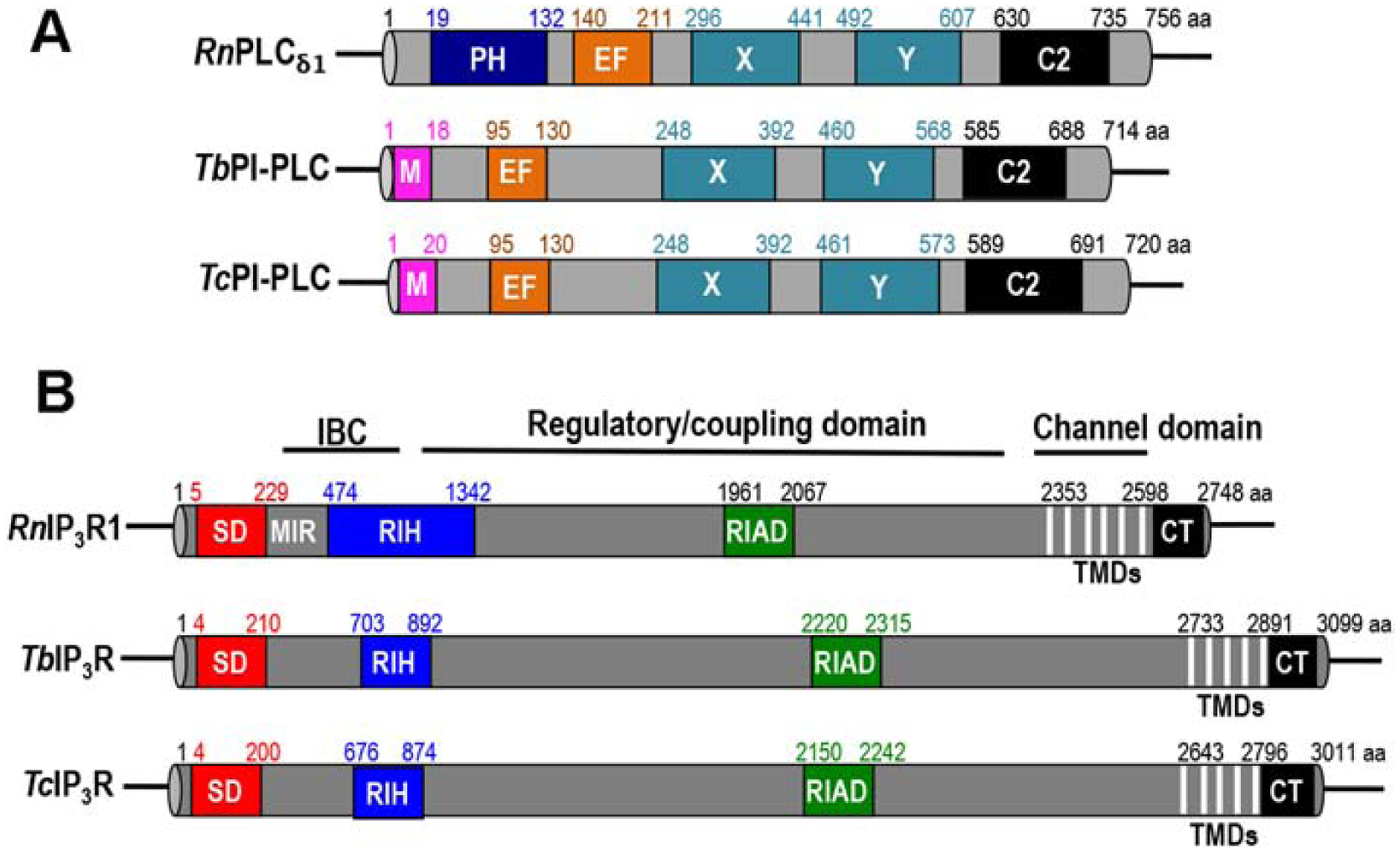
(A) Schematic domain organization of PLC proteins, from Trypanosoma brucei (TbPI-PLC, Tb927.11.5970), Trypanosoma cruzi (TcPI-PLC, TcCLB.504149.160), and Rattus norvegicus (RnPLCδ1, NP_058731.1). M, myristoylation domain; PH, Pleckstrin homology domain; EF, EF-hand domain; X and Y, catalytic domains; C2, C2 domain. (B) Schematic domain organization of IP3R proteins, from T. brucei (TbIP3R, Tb927.8.2770), T. cruzi (TcIP3R, TcCLB.509461.90), and R. norvegicus (RnIP3R1, NM_001007235.2). SD, suppressor domain; MIR, (mannosyltransferase, IP3R and RyR) domain; RIH, (RyR and IP3R homology) domain; RIAD, RIH-associated domain; TMDs, transmembrane domains (also called as channel or pore-forming domain); CT, cytosolic-terminus domain (also called as gate keeper domain); IBC, IP3 binding core domain. The conserved functional domains of the PLC or IP3R proteins were identified with InterPro (ebi.ac.uk/interpro).
TcPI-PLC shows two peaks of surface expression in amastigotes, the first immediately after differentiation of trypomastigotes into amastigotes and the second before differentiation of amastigotes to trypomastigotes [41].
Two potential roles of the surface expression of TcPI-PLC are: 1) a role in shedding GPI-anchor proteins; and 2) a role in cell signaling in the host. Concerning the first role, a number of GPI-anchored proteins from T. cruzi (trans-sialidases, mucins, Ssp4) are shed to the medium by an endogenous PLC [46–48], and it has been shown that the presence of ceramide in the lipid portion of the GPI anchor of these proteins is related to the shedding by endogenous PLC [48]. In this regard, TcPI-PLC is equally capable of cleaving phosphatidylinositol and inositol phosphoceramide, which is the lipid portion of these GPI-anchored proteins [49]. Concerning the second role, surface expression and secretion of TcPI-PLC coincide with PIP2 depletion in the host cell membrane and increase in IP3, suggesting a role of TcPI-PLC in cell signaling in the host [41].
TcPI-PLC expression and production of IP3 are induced during in vitro differentiation of trypomastigotes to amastigotes at low pH [40, 42]. This differentiation is inhibited by treatment of the cells with antisense oligonucleotides against TcPI-PLC, and stimulated by TcPI-PLC overexpression, suggesting that this pathway is involved in this differentiation step [42].
T. brucei PI-PLC (TbPI-PLC) is structurally similar to the T. cruzi enzyme. It lacks a PH domain, has a negatively charged region between the catalytic regions X and Y, and an amino-terminal N-myristoylation consensus sequence that is important for targeting it to intracellular vesicles close to the plasma membrane in procyclic forms (Fig. 2A). The enzyme is active at Ca2+ concentrations below the cytosolic levels, suggesting that it is constitutively active. Its downregulation by RNAi did not result in growth inhibition in procyclic forms and its overexpression increased the activity of lysates, demonstrating its function. Overexpression of TbPI-PLC in bloodstream forms, which is localized to the inner face of the plasma membrane, has no effect on growth but derepresses silent telomeric expression sites (ES) leading to the expression of multiple variant surface glycoproteins (VSG) and this effect is ablated by mutation of its catalytic site [45]. Endogenously tagged TbPI-PLC in procyclic forms [44] and overexpressed TcPI-PLC in bloodstream forms [45] co-localize with their substrate PIP2.
In conclusion, the IP3/diacyglycerol signaling pathway is present in both T. cruzi and T. brucei. The PI-PLCs of both trypanosomatids have similar structural characteristics that distinguishes them from the mammalian enzymes. While TcPI-PLC appears to have roles in differentiation of trypomastigotes to amastigotes, and potential roles in shedding of GPI-anchor proteins and host cell signaling, TbPI-PLC is somehow involved in repression of telomeric ES.
3. The IP3 receptor
The IP3 receptor was first characterized in T. brucei [6]. Sequence analysis using the InterPro and TMHMM servers predicted 5 transmembrane domains (TMDs) in the C-terminal of the IP3R of T. brucei (6) and T. cruzi (34), as also described in TriTrypDB (http://tritrypdb.org/tritrypdb/). Structural studies will be needed to reveal whether this change affects the topology of the receptor as compared with the mammalian orthologs that possess 6 TMDs. The receptor has a series of conserved domains including a suppressor domain-like (SD), ryanodine receptor IP3R homology (RIH), and RIH-associated (RIAD) domains [50] (Fig. 2B). A Ca2+-selectivity filter (GGGVGD), similar to those present in the mammalian IP3Rs, is in the intraluminal loop between TMDs at the C-terminal region. Only 5 of the 10 residues that form the basic pocket that binds IP3 [51, 52] are present in TbIP3R [6]. IP3 was able to stimulate Ca2+ release from DT40–3KO cells transfected with TbIP3R in a dose-dependent manner [6]. DT40–3KO cells are chicken lymphocytes in which the three endogenous vertebrate IP3Rs have been knocked out. IP3 was also able to stimulate Ca2+ release from digitonin-permeabilized procyclic forms or from isolated acidocalcisomes, demonstrating its activity in vitro [6]. UV light photolysis of caged IP3 increased intracellular Ca2+ in live procyclic trypanosomes. Knock down of TbIP3R in bloodstream forms produced growth defects in vitro and reduced mouse infectivity in vivo [6].
Experiments with DT40–3KO cells expressing TbIP3R revealed that IP3-mediated Ca2+ release depends on Ca2+ but not on ATP concentration, is inhibited by heparin, caffeine, and 2-aminomethoxydiphenyl borate (2-APB), and stimulated by adenophostin A [53]. In contrast to the results with DT40–3KO cells expressing rat type I IP3R, DT40–3KO, excised patch clamp recordings from nuclear membranes of DT40–3KO cells expressing TbIP3R showed that luminal orthophosphate (Pi) or pyrophosphate (PPi) and neutral or alkaline pH stimulated IP3-generated currents. However, long chain polyphosphate or acidic pH did not generate currents, while tripolyphosphate (polyP3), the most abundant polyP of acidocalcisomes, inhibited these currents [53]. When extrapolated to what could occur in acidocalcisomes, these results would explain that under the normal acidic conditions of the organelle, the IP3R channel would be closed but when intra-organelle pH increases, for example by increased amino acid catabolism and production of NH3 and its sequestration in acidic compartments as ammonium (NH4+), the channel could open and favor Ca2+ release. Alkalization would also activate polyP hydrolysis by the vacuolar soluble pyrophosphatase, which hydrolyze polyP to Pi and PPi (Fig. 1), favoring the opening of the channel. In agreement with this suggestion it was found that alkalization of acidocalcisomes in permeabilized cells by exposure to ionophores or NH4Cl led to Ca2+ release [53].
T. cruzi IP3R (TcIP3R) is structurally very similar to TbIP3R sharing 45% of amino acid identity. It was also functionally expressed in the ER of DT40–3KO cells to demonstrate IP3-mediated Ca2+ release [34, 54]. Knockdown of TcIP3R led to defects in growth, metacyclogenesis and infectivity in vitro and reduced parasitemia in mice [54]. Recent work using the CRISPR/Cas9 technique to knockout the expression of TcIP3R showed that the channel is required for Ca2+ uptake by mitochondria, regulation of pyruvate dehydrogenase dephosphorylation, and mitochondrial O2 consumption [34]. Knockout mutants increased ammonia production and the AMP/ATP ratio, and increased autophagy, revealing a modulatory activity of TcIP3R-mediated acidocalcisome Ca2+ release on cell bioenergetics [34] (Fig. 3A), which is favored by the presence of acidocalcisome-mitochondrion membrane contact sites [55] (Fig. 3B).
Figure 3.
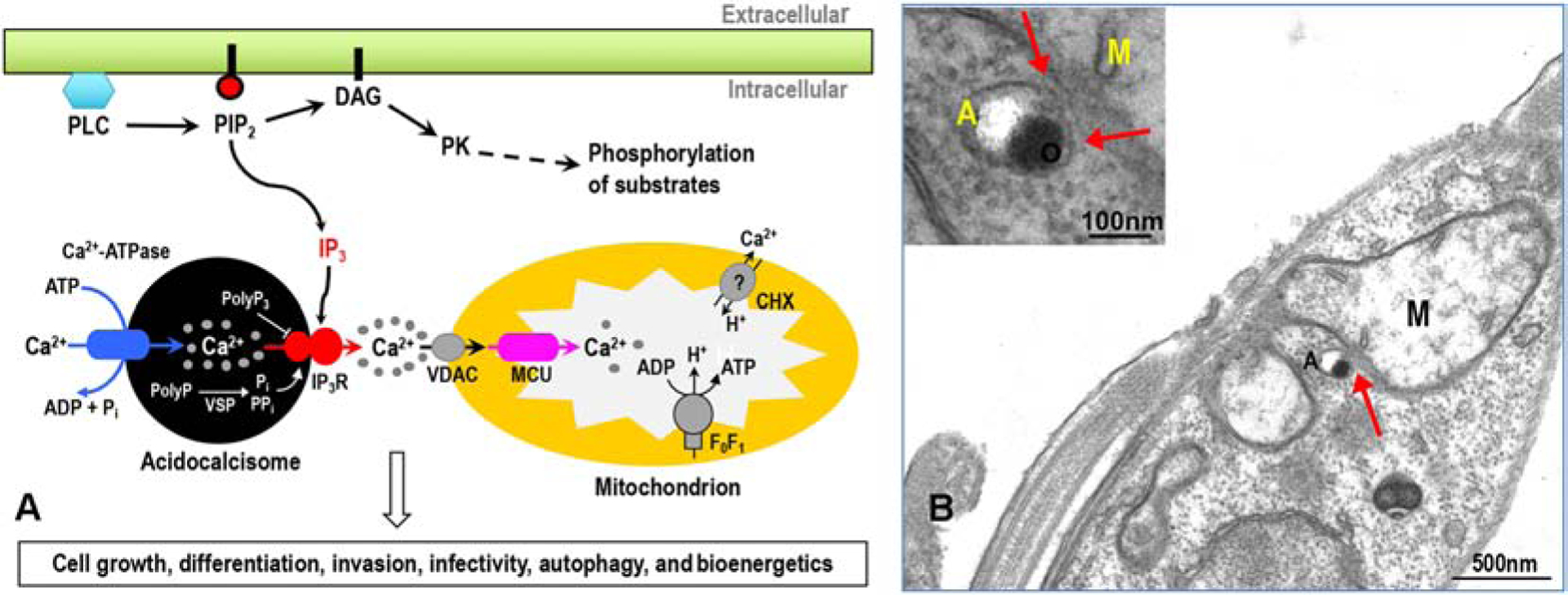
(A). Schematic representation of IP3-mediated Ca2+ signaling pathway in trypanosomes. Inositol 1,4,5-trisphosphate (IP3) is generated by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), catalyzed by phosphoinositide-specific PLC (PI-PLCs), which also generates diacylglycerol (DAG), possibly activating protein kinase (PK) that catalyzes phosphorylation of a variety of intracellular proteins. IP3 binds to IP3 receptor (IP3R) and stimulates Ca2+ release from acidocalcisomes through the IP3R into the cytosol, thereby activating various Ca2+ regulated signal molecules, which regulate the parasite’s cell growth, differentiation, invasion, and infectivity. The IP3-mediated Ca2+ release from acidocalcisome can also be modulated by the activation of the luminal orthophosphate (Pi) or pyrophosphate (PPi) and by the inhibition of tripolyphosphate (polyP3). Importantly, when Ca2+ release from acidocalcisomes is stimulated, Ca2+ permeates a mitochondrial voltage-dependent anion-selective channel (VDAC) through microdomains of high Ca2+ concentration (gray balls) present in their vicinity, and subsequently Ca2+ can be efficiently taken up by the mitochondrial calcium uniporter (MCU), thereby regulating mitochondrial metabolism, autophagy, and cell bioenergetics. CHX, Ca2+/H+ exchanger. (B) Transmission electron microscopy of procyclic forms (PCF) of T. brucei shows membrane contacts between acidocalcisome (A) and mitochondrion (M) of this parasite, which may function for efficient Ca2+ transfer as described in (A). An acidocalcisome appears as a rounded organelle containing electron-dense material that adheres to one side of the membrane, and is seen adjacent to the mitochondrion double membrane. Reproduced with permission from reference 55.
4. Ca2+ signaling
The best evidence for the involvement of Ca2+ signaling in T. cruzi has been the detection of intracellular Ca2+ concentration ([Ca2+]i) increase in Fura 2-labeled trypomastigotes upon contact with host cells [56], and the prevention of host cell invasion by preincubation of the parasites with intracellular Ca2+ chelators [56, 57]. In agreement with these results, increasing intracellular Ca2+ by treatment with ionomycin led to a higher host cell invasion [57]. The trypomastigotes receptor that triggers this Ca2+ increase has not been identified although there is some evidence that Ca2+ release from acidocalcisomes could be involved in this process [34]. Ablation of the gene that encodes the TcIP3R led to decreased host cell invasion [34]. Similarly, treatment of parasites with a combination of ionomycin plus NH4Cl or nigericin, which are known to deplete acidocalcisome Ca2+ [2], reduces the infectivity of metacyclic forms [33].
Attachment and invasion of host cells is a process that requires energy [58] and it has been shown that IP3-mediated Ca2+ release from acidocalcisomes is important for mitochondrial Ca2+ uptake and generation of ATP [34]. Knockout of the genes involved in mitochondrial Ca2+ uptake [59, 60] or in mitochondrial activation of bioenergetic metabolism [61], inhibit host cell invasion.
A role for Ca2+ signaling in metacyclogenesis was also inferred after detection of a rise in [Ca2+]i in epimastigotes by addition of Triatoma infestants (one of the vectors) homogenates. Both Ca2+ rise and metacyclogenesis were blocked by treatment with an intracellular Ca2+ chelator (BAPTA-AM) but not by extracellular EGTA [62], suggesting an intracellular origin of Ca2+.
Evidence for Ca2+ signaling during differentiation of T. brucei is more indirect and based on changes in [Ca2+]i during the differentiation from long slender to short stumpy bloodstream forms [63].
Finally, IP3-dependent Ca2+ release from acidocalcisomes is important to maintain cellular bioenergetics through mitochondrial Ca2+ uptake and stimulation of energy generation in both T. cruzi [34] and T. brucei [6, 64].
Outlook
Trypanosomes have distinct acidic Ca2+ stores named acidocalcisomes that have a Ca2+-ATPase for Ca2+ uptake and an IP3 receptor for Ca2+ release. IP3 is formed by hydrolysis of PIP2 catalyzed by a peculiar phospholipase C that lacks a PH domain for plasma membrane localization, and instead is N-terminal myristoylated and palmitoylated. Surface expression of PI-PLC could have potential roles in shedding of GPI-anchored proteins from the parasite and cell signaling in the host. The IP3 receptor is also peculiar with only 5 TMD, and only 5 of the 10 amino acids described in the mammalian enzymes needed for binding IP3. The inositol phosphate/diacylglycerol pathway is important for Ca2+ signaling, which is required for T. cruzi host cell invasion and metacyclogenesis, potentially for T. brucei differentiation, and for maintaining cellular bioenergetics in both parasites. In conclusion, differences between trypanosomatid and mammalian IP3 signaling represent good targets for the development of new strategies to combat these organisms.
Highlights.
Acidocalcisomes are acidic Ca2+ stores rich in polyphosphate
The IP3 receptor of trypanosomes is in the acidocalcisomes
The IP3 receptor is luminally stimulated by polyphosphate hydrolysis products
The trypanosome PI-PLC is lipid modified and surface-localized
Ca2+ in trypanosomes is important for differentiation, autophagy, and infectivity
Acknowledgements
R.D. was supported by grants of the U.S. National Institutes of Health (grants AI140421 and AI108222), and the Barbara and Sanford Orkin Eminent Scholar Endowment Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Schmunis G, Status of and cost of Chagas disease worldwide, Lancet Infect Dis, 13 (2013) 283–284. [DOI] [PubMed] [Google Scholar]
- [2].Docampo R, Scott DA, Vercesi AE, Moreno SN, Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi, Biochem J, 310 (1995) 1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vercesi AE, Moreno SN, Docampo R, Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei, Biochem J, 304 (1994) 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Luo S, Rohloff P, Cox J, Uyemura SA, Docampo R, Trypanosoma brucei plasma membrane-type Ca2+-ATPase 1 (TbPMC1) and 2 (TbPMC2) genes encode functional Ca2+-ATPases localized to the acidocalcisomes and plasma membrane, and essential for Ca2+ homeostasis and growth, J Biol Chem, 279 (2004) 14427–14439. [DOI] [PubMed] [Google Scholar]
- [5].Lu HG, Zhong L, de Souza W, Benchimol M, Moreno S, Docampo R, Ca2+ content and expression of an acidocalcisomal calcium pump are elevated in intracellular forms of Trypanosoma cruzi, Mol Cell Biol, 18 (1998) 2309–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang G, Bartlett PJ, Thomas AP, Moreno SN, Docampo R, Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity, Proc Natl Acad Sci U S A, 110 (2013) 1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lander N, Chiurillo MA, Storey M, Vercesi AE, Docampo R, CRISPR/Cas9-mediated endogenous C-terminal tagging of Trypanosoma cruzi genes reveals the acidocalcisome localization of the I=inositol 1,4,5-trisphosphate receptor, J Biol Chem, 291 (2016) 25505–25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang G, Ulrich PN, Storey M, Johnson D, Tischer J, Tovar JA, Moreno SN, Orlando R, Docampo R, Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells, PLoS Pathog, 10 (2014) e1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lemercier G, Dutoya S, Luo S, Ruiz FA, Rodrigues CO, Baltz T, Docampo R, Bakalara N, A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei, J Biol Chem, 277 (2002) 37369–37376. [DOI] [PubMed] [Google Scholar]
- [10].Scott DA, de Souza W, Benchimol M, Zhong L, Lu HG, Moreno SN, Docampo R, Presence of a plant-like proton-pumping pyrophosphatase in acidocalcisomes of Trypanosoma cruzi, J Biol Chem, 273 (1998) 22151–22158. [DOI] [PubMed] [Google Scholar]
- [11].Rodrigues CO, Scott DA, Docampo R, Characterization of a vacuolar pyrophosphatase in Trypanosoma brucei and its localization to acidocalcisomes, Mol Cell Biol, 19 (1999) 7712–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferella M, Nilsson D, Darban H, Rodrigues C, Bontempi EJ, Docampo R, Andersson B, Proteomics in Trypanosoma cruzi--localization of novel proteins to various organelles, Proteomics, 8 (2008) 2735–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Montalvetti A, Rohloff P, Docampo R, A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi, J Biol Chem, 279 (2004) 38673–38682. [DOI] [PubMed] [Google Scholar]
- [14].Rohloff P, Montalvetti A, Docampo R, Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi, J Biol Chem, 279 (2004) 52270–52281. [DOI] [PubMed] [Google Scholar]
- [15].Steinmann ME, Schmidt RS, Butikofer P, Maser P, Sigel E, TbIRK is a signature sequence free potassium channel from Trypanosoma brucei locating to acidocalcisomes, Sci Rep, 7 (2017) 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vercesi AE, Docampo R, Sodium-proton exchange stimulates Ca2+ release from acidocalcisomes of Trypanosoma brucei, Biochem J, 315 (1996) 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moreno B, Urbina JA, Oldfield E, Bailey BN, Rodrigues CO, Docampo R, 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Evidence for high levels of condensed inorganic phosphates, J Biol Chem, 275 (2000) 28356–28362. [DOI] [PubMed] [Google Scholar]
- [18].Ruiz FA, Rodrigues CO, Docampo R, Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi, J Biol Chem, 276 (2001) 26114–26121. [DOI] [PubMed] [Google Scholar]
- [19].Urbina JA, Moreno B, Vierkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SN, Docampo R, Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs, J Biol Chem, 274 (1999) 33609–33615. [DOI] [PubMed] [Google Scholar]
- [20].Fang J, Rohloff P, Miranda K, Docampo R, Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect, Biochem J, 407 (2007) 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ulrich PN, Lander N, Kurup SP, Reiss L, Brewer J, Soares Medeiros LC, Miranda K, Docampo R, The acidocalcisome vacuolar transporter chaperone 4 catalyzes the synthesis of polyphosphate in insect-stages of Trypanosoma brucei and T. cruzi, J Eukaryot Microbiol, 61 (2014) 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lander N, Ulrich PN, Docampo R, Trypanosoma brucei vacuolar transporter chaperone 4 (TbVtc4) is an acidocalcisome polyphosphate kinase required for in vivo infection, J Biol Chem, 288 (2013) 34205–34216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lemercier G, Espiau B, Ruiz FA, Vieira M, Luo S, Baltz T, Docampo R, Bakalara N, A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice, J Biol Chem, 279 (2004) 3420–3425. [DOI] [PubMed] [Google Scholar]
- [24].Galizzi M, Bustamante JM, Fang J, Miranda K, Soares Medeiros LC, Tarleton RL, Docampo R, Evidence for the role of vacuolar soluble pyrophosphatase and inorganic polyphosphate in Trypanosoma cruzi persistence, Mol Microbiol, 90 (2013) 699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kotsikorou E, Song Y, Chan JM, Faelens S, Tovian Z, Broderick E, Bakalara N, Docampo R, Oldfield E, Bisphosphonate inhibition of the exopolyphosphatase activity of the Trypanosoma brucei soluble vacuolar pyrophosphatase, J Med Chem, 48 (2005) 6128–6139. [DOI] [PubMed] [Google Scholar]
- [26].Yang Y, Ko TP, Chen CC, Huang G, Zheng Y, Liu W, Wang I, Ho MR, Hsu ST, O’Dowd B, Huff HC, Huang CH, Docampo R, Oldfield E, Guo RT, Structures of Trypanosome Vacuolar Soluble Pyrophosphatases: Antiparasitic Drug Targets, ACS Chem Biol, 11 (2016) 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN, Acidocalcisomes - conserved from bacteria to man, Nat Rev Microbiol, 3 (2005) 251–261. [DOI] [PubMed] [Google Scholar]
- [28].Potapenko E, Cordeiro CD, Huang G, Docampo R, Pyrophosphate stimulates the phosphate-sodium symporter of Trypanosoma brucei acidocalcisomes and Saccharomyces cerevisiae vacuoles, mSphere, 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Potapenko E, Cordeiro CD, Huang G, Storey M, Wittwer C, Dutta AK, Jessen HJ, Starai VJ, Docampo R, 5-Diphosphoinositol pentakisphosphate (5-IP7) regulates phosphate release from acidocalcisomes and yeast vacuoles, J Biol Chem, 293 (2018) 19101–19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Docampo R, Moreno SN, Acidocalcisomes, Cell calcium, 50 (2011) 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].de Paulo Martins V, Okura M, Maric D, Engman DM, Vieira M, Docampo R, Moreno SN, Acylation-dependent export of Trypanosoma cruzi phosphoinositide-specific phospholipase C to the outer surface of amastigotes, J Biol Chem, 285 (2010) 30906–30917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Niyogi S, Jimenez V, Girard-Dias W, de Souza W, Miranda K, Docampo R, Rab32 is essential for maintaining functional acidocalcisomes, and for growth and infectivity of Trypanosoma cruzi, J Cell Sci, 128 (2015) 2363–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maeda FY, Cortez C, Alves RM, Yoshida N, Mammalian cell invasion by closely related Trypanosoma species T. dionisii and T. cruzi, Acta Trop, 121 (2012) 141–147. [DOI] [PubMed] [Google Scholar]
- [34].Chiurillo MA, Lander ES, Vercesi AE, Docampo R, IP3 receptor-mediated Ca2+ release from acidocalcisomes regulates mitochondrial bioenergetics and prevents autophagy in Trypanosoma cruzi, Cell calcium, 92 (2020). [DOI] [PubMed] [Google Scholar]
- [35].Docampo R, Pignataro OP, The inositol phosphate/diacylglycerol signalling pathway in Trypanosoma cruzi, Biochem J, 275 (1991) 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moreno SN, Docampo R, Vercesi AE, Calcium homeostasis in procyclic and bloodstream forms of Trypanosoma brucei. Lack of inositol 1,4,5-trisphosphate-sensitive Ca2+ release, J Biol Chem, 267 (1992) 6020–6026. [PubMed] [Google Scholar]
- [37].Moreno SN, Vercesi AE, Pignataro OP, Docampo R, Calcium homeostasis in Trypanosoma cruzi amastigotes: presence of inositol phosphates and lack of an inositol 1,4,5-trisphosphate-sensitive calcium pool, Mol Biochem Parasitol, 52 (1992) 251–261. [DOI] [PubMed] [Google Scholar]
- [38].Docampo R, Moreno SN, Vercesi AE, Effect of thapsigargin on calcium homeostasis in Trypanosoma cruzi trypomastigotes and epimastigotes, Mol Biochem Parasitol, 59 (1993) 305–313. [DOI] [PubMed] [Google Scholar]
- [39].Cordeiro CD, Saiardi A, Docampo R, The inositol pyrophosphate synthesis pathway in Trypanosoma brucei is linked to polyphosphate synthesis in acidocalcisomes, Mol Microbiol, 106 (2017) 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Furuya T, Kashuba C, Docampo R, Moreno SN, A novel phosphatidylinositol-phospholipase C of Trypanosoma cruzi that is lipid modified and activated during trypomastigote to amastigote differentiation, J Biol Chem, 275 (2000) 6428–6438. [DOI] [PubMed] [Google Scholar]
- [41].Martins Vde P, Galizzi M, Salto ML, Docampo R, Moreno SN, Developmental expression of a Trypanosoma cruzi phosphoinositide-specific phospholipase C in amastigotes and stimulation of host phosphoinositide hydrolysis, Infect Immun, 78 (2010) 4206–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Okura M, Fang J, Salto ML, Singer RS, Docampo R, Moreno SN, A lipid-modified phosphoinositide-specific phospholipase C (TcPI-PLC) is involved in differentiation of trypomastigotes to amastigotes of Trypanosoma cruzi, J Biol Chem, 280 (2005) 16235–16243. [DOI] [PubMed] [Google Scholar]
- [43].Nozaki T, Toh-e A, Fujii M, Yagisawa H, Nakazawa M, Takeuchi T, Cloning and characterization of a gene encoding phosphatidyl inositol-specific phospholipase C from Trypanosoma cruzi, Mol Biochem Parasitol, 102 (1999) 283–295. [DOI] [PubMed] [Google Scholar]
- [44].King-Keller S, Moore CA, Docampo R, Moreno SN, Ca2+ regulation of Trypanosoma brucei phosphoinositide phospholipase C, Eukaryot Cell, 14 (2015) 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cestari I, Stuart K, Inositol phosphate pathway controls transcription of telomeric expression sites in trypanosomes, Proc Natl Acad Sci U S A, 112 (2015) E2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Andrews NW, Robbins ES, Ley V, Hong KS, Nussenzweig V, Developmentally regulated, phospholipase C-mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi, J Exp Med, 167 (1988) 300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pollevick GD, Di Noia JM, Salto ML, Lima C, Leguizamon MS, de Lederkremer RM, Frasch AC, Trypanosoma cruzi surface mucins with exposed variant epitopes, J Biol Chem, 275 (2000) 27671–27680. [DOI] [PubMed] [Google Scholar]
- [48].Agusti R, Couto AS, Campetella OE, Frasch AC, de Lederkremer RM, The trans-sialidase of Trypanosoma cruzi is anchored by two different lipids, Glycobiology, 7 (1997) 731–735. [DOI] [PubMed] [Google Scholar]
- [49].Salto ML, Furuya T, Moreno SN, Docampo R, de Lederkremer RM, The phosphatidylinositol-phospholipase C from Trypanosoma cruzi is active on inositolphosphoceramide, Mol Biochem Parasitol, 119 (2002) 131–133. [DOI] [PubMed] [Google Scholar]
- [50].Prole DL, Taylor CW, Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites, PLoS One, 6 (2011) e26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K, Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor, J Biol Chem, 271 (1996) 18277–18284. [DOI] [PubMed] [Google Scholar]
- [52].Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M, Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand, Nature, 420 (2002) 696–700. [DOI] [PubMed] [Google Scholar]
- [53].Potapenko E, Negrao NW, Huang G, Docampo R, The acidocalcisome inositol-1,4,5-trisphosphate receptor of Trypanosoma brucei is stimulated by luminal polyphosphate hydrolysis products, J Biol Chem, 294 (2019) 10628–10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hashimoto M, Enomoto M, Morales J, Kurebayashi N, Sakurai T, Hashimoto T, Nara T, Mikoshiba K, Inositol 1,4,5-trisphosphate receptor regulates replication, differentiation, infectivity and virulence of the parasitic protist Trypanosoma cruzi, Mol Microbiol, 87 (2013) 1133–1150. [DOI] [PubMed] [Google Scholar]
- [55].Ramakrishnan S, Asady B, Docampo R, Acidocalcisome-mitochondrion membrane contact sites in Trypanosoma brucei, Pathogens, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moreno SN, Silva J, Vercesi AE, Docampo R, Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion, J Exp Med, 180 (1994) 1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yakubu MA, Majumder S, Kierszenbaum F, Changes in Trypanosoma cruzi infectivity by treatments that affect calcium ion levels, Mol Biochem Parasitol, 66 (1994) 119–125. [DOI] [PubMed] [Google Scholar]
- [58].Schenkman S, Robbins ES, Nussenzweig V, Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton, Infect Immun, 59 (1991) 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bertolini MS, Chiurillo MA, Lander N, Vercesi AE, Docampo R, MICU1 and MICU2 play an essential role in mitochondrial Ca2+ uptake, growth, and infectivity of the human pathogen Trypanosoma cruzi, mBio, 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chiurillo MA, Lander N, Bertolini MS, Vercesi AE, Docampo R, Functional analysis and importance for host cell infection of the Ca2+-conducting subunits of the mitochondrial calcium uniporter of Trypanosoma cruzi, Mol Biol Cell, 30 (2019) 1676–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lander N, Chiurillo MA, Bertolini MS, Storey M, Vercesi AE, Docampo R, Calcium-sensitive pyruvate dehydrogenase phosphatase is required for energy metabolism, growth, differentiation, and infectivity of Trypanosoma cruzi, J Biol Chem, 293 (2018) 17402–17417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lammel EM, Barbieri MA, Wilkowsky SE, Bertini F, Isola EL, Trypanosoma cruzi: involvement of intracellular calcium in multiplication and differentiation, Exp Parasitol, 83 (1996) 240–249. [DOI] [PubMed] [Google Scholar]
- [63].Stojdl DF, Clarke MW, Trypanosoma brucei: analysis of cytoplasmic Ca2+ during differentiation of bloodstream stages in vitro, Exp Parasitol, 83 (1996) 134–146. [DOI] [PubMed] [Google Scholar]
- [64].Huang G, Vercesi AE, Docampo R, Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter, Nature Commun, 4 (2013) 2865. [DOI] [PMC free article] [PubMed] [Google Scholar]


