Abstract
Many cellular stresses induce cellular senescence and the irreversible arrest of cell proliferation in different cell types. Although blocked in their capacity to divide, senescent cells are metabolically active and are characterized by a different metabolic phenotype as compared to non-senescent cells. Changes observed in senescent cells depend from the cell type and lead to an adaptative flexibility in the type of metabolism. This metabolic reprogramming is needed to cope with survival and with the energetic demands of the senescent program that include the increased secretion of senescence-associated secretory phenotype factors.
Keywords: Cellular senescence, metabolism, non-immune cells, immune cells
1. Introduction
Cellular senescence indicates the irreversible arrest of cell proliferation that is induced by different stressors, such as DNA damage, telomere shortening, radiation, production of reactive metabolites, mitogenic and metabolic stressors. Proliferative arrest is mediated by the inhibition of cell cycle progression through p16INK4 and/or the activation of cell cycle arrest through p53/p21. Once a cell becomes senescent, it shows changes in chromatin organization and gene expression (Childs et al., 2015; van Deursen, 2014). Cellular senescence has been shown to be implicated in aging and in a number of age-associated diseases (Campisi and d'Adda di Fagagna, 2007). Senescent cells accumulate in the body during aging and promote tissue degeneration and malignant transformation and their elimination in genetically engineered mice models or by using senolytic drugs results in increased longevity and decreased occurrence of age-related diseases (Baker et al., 2011; Zhu et al., 2015). Senescent cells secrete multiple factors that constitute the senescence-associated secretory phenotype (SASP), that consists of pro-inflammatory factors (cytokines, chemokines, micro-RNAs), soluble receptors (TNF receptors), non-protein soluble factors (nitric oxide), growth factors (EGF, VGEF, NGF) and extracellular matrix macromolecules (Fibronectin, Collagens, Laminin) (Campisi, 2011).
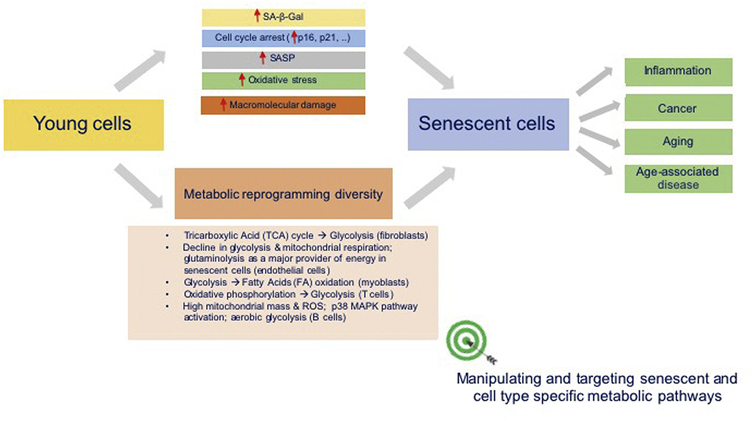
Despite the decline in their proliferative potential, cells with a senescent phenotype show an altered metabolism associated with increased oxidative stress, impaired proteostasis, impairment of specific metabolic pathways and accumulation of oxidized proteins (Hamon et al., 2020). Metabolic reprogramming is therefore required for these cells to cope with the energetic demands of the senescent program that include the increased secretion of SASP factors, increased oxidative stress and increased endoplasmic reticulum stress. The characteristics of senescent cells are shown in Fig. 1. Interestingly, this resulting energy metabolic shift has been found to depend on cell types.
Fig. 1. Characteristics of senescent cells.
Accumulation of senescent cells in the body promote inflammation, cancer, aging and age-associated diseases. Senescent cells are characterized by the expression of senescence-associated β-galactosidase (SA β-gal), the enzyme that catalyzes the hydrolysis of β-galactosides into monosaccharides; by the expression of the cell cycle inhibitors (p16/p21); by increased secretion of pro-inflammatory mediators that constitute the SASP; by increased oxidative stress and macromolecular damage. Senescent cells are also flexible in the utilization of different metabolic pathways. Their metabolic reprogramming allows them to survive in the hostile inflammatory environment and to support their function.
Data on metabolic changes contributing to cell senescence have also been reported. Results have shown that mitochondrial dysfunction, characterized by high production of reactive oxygen species (ROS) and depolarized mitochondria accumulating around the nucleus, drives oncogene-induced senescence via a mechanism mediated by p53 (Moiseeva et al., 2009), whose sustained activation is strictly dependent on malic enzymes (Jiang et al., 2013). ROS is involved in the establishment and stabilization of senescence and elevated levels of ROS are associated with both replicative and stress- or oncogene-induced senescence. ROS can damage DNA directly or can sustain an ongoing DNA-damage response, long-term activation of p21 and cell cycle arrest (Passos et al., 2010). Results from the group of Judith Campisi have shown that cells undergoing mitochondrial dysfunction-associated senescence have a secretory phenotype distinct from the one induced by genotoxic stress, and lack IL-1-dependent SASP factors. This phenotype has been observed in cultured human cells and in the murine model of premature aging through mitochondrial dysfunction (Wiley et al., 2016).
A metabolomics-proteomics combined approach has been employed to identify metabolism-associated molecular events involved in senescence-induced pathways following in vitro treatment of human cancer cells with chemotherapeutic drugs (Wu et al., 2017). Results have shown that tricarboxylic acid (TCA) cycle, the pentose phosphate pathway and nucleotide pathways were all up-regulated in senescent cells, whereas fatty acids (FAs) pathways were down-regulated, thus identifying the metabolic events facilitating ROS elimination and DNA damage repair in senescent cells. These same processes were down-regulated in apoptotic cells in which DNA damage was irreparable.
Although the majority of studies have focused on senescent cells in tissues, the presence of circulating immune cells with a senescent phenotype has also been shown. In the first part of the review, we will summarize published findings on the effects of in vitro senescence on metabolic pathways in non-immune cells (fibroblasts, endothelial cells and myoblasts). In the second part of the review, we will go over the senescence-associated changes on metabolic pathways associated with the regulation of cellular function in immune cells (T and B lymphocytes, and macrophages).
2. Senescence-associated metabolic changes in non-immune cells
2.1. Extracellular and intracellular metabolites analysis during cellular senescence of fibroblasts
Early findings have shown that human fibroblasts undergoing replicative senescence become more glycolytic (Bittles and Harper, 1984; Goldstein et al., 1982), as also demonstrated in by metabolic profiling studies (Zwerschke et al., 2003), and this status is associated with the acquisition of the senescence-associated secretome also referred as to SASP (Wiley and Campisi, 2016). More recently, using unbiased metabolomic studies for the characterization of senescence-associated extracellular metabolome, extracellular metabolites profiling analysis of human senescent fibroblasts in vitro have identified key pathways that may provide useful non invasive biomarkers of aging or cellular senescence in vivo (James et al., 2015). Indeed, many redox homeostasis metabolites such as the gamma-glutamyl amino acids that play an important role in regulating the intra- and extra-cellular exchange of glutathione, were found to accumulate in replicative senescent oral human fibroblasts as compared with growing, quiescent or confluent cells. Moreover, both cysteine-glutathione disulfide and cysteine sulfinic acid which are formed under oxidative stress conditions were increased in replicative senescent fibroblasts while pyridoxate, a vitamin B6 metabolite indicative of oxidative stress, was also found elevated. Other metabolic pathways that were found to be altered in senescent fibroblasts included nucleotide metabolites, as illustrated by the accumulation of nucleotide catabolites with the concomitant depletion of thymidine and lipid metabolism, and by the accumulation of phospholipid catabolites and monohydroxy FAs such as glycerophosphorylcholine, 2-hydroxypalmitate and 2-hydroxystearate. Concerning energy metabolism, metabolites belonging to the TCA cycle, which is the major common pathway for the oxidation of carbohydrates, lipids and certain aminoacids, such as citrate and fumarate were found to accumulate while pyruvate was depleted in the medium of human senescent oral fibroblasts. Interestingly, intracellular citrate and fumarate levels were found to decline while pyruvate accumulated only slightly in human senescent oral fibroblasts. Further analyses of intracellular metabolites of the TCA cycle, pentose phosphate pathway, glycolysis and pyruvate metabolism were indicative of reduced TCA activity with an energy production shift towards glycolysis and gluconeogenesis together with an increased glucose-6-phosphate shuttling to support glutathione detoxification and nicotinamide adenine dinucleotide phosphate (NADPH) regeneration for fighting against oxidative stress and restoring redox homeostasis. Although increased glycolysis in senescent cells was somewhat counterintuitive since p53 generally suppresses glycolysis and stimulates the TCA cycle, senescence appears as dynamic process with transient high levels of p53 followed by p21 and subsequent accumulation of p16.
That the induction of the fibroblast extracellular senescence metabolome is a dynamic process was further demonstrated by the same group in a follow-up study aimed at identifying several new extracellular senescence metabolites that are associated with chronological aging (James et al., 2018) in order to better understand the metabolic signatures of aging and their relationship with cellular senescence. Moreover, intracellular metabolic profiling has also pointed out to alterations in oxidized NAD (NAD+) and nicotinamide metabolism (James et al., 2016). While senescent human oral fibroblasts maintained NAD+ and nicotinamide levels, there were indications of increased nicotinamide turnover and alterations of NAD+ salvage pathway as evidenced by the accumulation of intracellular nicotinamide ribonucleotide and nicotinamide riboside as well as extracellular 1-methyl nicotinamide. Interestingly, the nicotinamide phosphoribosyltransferase (NAMPT) regulated NAD+ salvage pathway has recently been implicated in modulating the strength of the pro-inflammatory senescence SASP as a consequence of the upregulation of NAMPT by high mobility group A proteins in RAS-induced senescent IMR90 fibroblasts through 5’-AMP activated kinase (AMPK) signaling and suppression of p53-mediated inhibition of p38 MAP kinase to enhance NF-KB activity (Nacarelli et al., 2019). Hence, an increase in the oxidized/reduced NAD (NAD+/NADH) ratio would favor the conversion of a low proinflammatory SASP as observed during replicative senescence into a high proinflammatory SASP as observed in oncogene induced senescence. Indeed, suppression of the pro-inflammatory SASP of Ras-induced senescent human IMR-90 fibroblasts was successfully achieved by such metabolic-targeting drugs as etomoxir that inhibits of carnitine palmitoyltransferase 1, the rate-limiting step in mitochondrial oxidation of FAs (Quijano et al., 2012). In addition, mTOR inhibition by rapamycin was also found to suppress the pro-inflammatory SASP of radiation-induced senescent human foreskin fibroblasts (Laberge et al., 2015). Moreover, metabolic differences between senescent and non-senescent cells have already been proven useful for the targeting of the upregulated pathways in senescent cells to promote their elimination as previously reported for the removal of chemotherapy induced senescent tumor cells by using synthetic lethal metabolic targeting of the glycolytic and autophagic pathways (Dorr et al., 2013).
2.2. Intracellular metabolites analysis during replicative senescence of endothelial cells
Beside fibroblasts, endothelial cell fate has been widely addressed during replicative and stress-induced senescence but the metabolic features of these senescent cells are not yet completely understood (Sabbatinelli et al., 2019). Indeed, the occurrence and further accumulation of senescent endothelial cells has been linked to age-associated vascular dysfunction and has been suggested to play an important role in the related pathologies such as atherosclerosis and other cardiovascular diseases (Childs et al., 2014; Jia et al., 2019; Tian and Li, 2014). Interestingly, the investigation of senescence-associated metabolic changes of endothelial cells has shown that they do not behave as previously reported for fibroblasts. Indeed, although quiescent endothelial cells mainly rely on glycolysis for their energy demand (Eelen et al., 2018), the upregulation of glycolysis observed with senescent fibroblasts was not found in human umbilical vein endothelial cells (HUVEC) upon replicative senescence, while glutaminolysis was recognized as the main provider of energy in senescent cells (Unterluggauer et al., 2008). Also, FA oxidation has recently been pointed out to play an important role for maintaining redox homeostasis in quiescent endothelial cells (Kalucka et al., 2018). Only recently, unbiased metabolomics analyses have been performed to explore cellular metabolic profiles as endothelial cells progress towards replicative senescence (Yi et al., 2020). In these experiments, HUVEC were cultured until they reached 61 cumulative population doublings and data points were sequentially taken at 4, 15, 31, 46 and 61 population doublings. The most changing metabolites were those involved in metabolic pathways related to energy metabolism and redox homeostasis, and decreased levels of several antioxidant molecules such as glutathione and creatine were continuously observed. Moreover, several metabolisms including glutathione, taurine/hypotaurine and glycerophospholipid metabolism were significantly altered in population doublings higher than 4 according to metabolic pathway analyses using the MetaboAnalyst 4.0 observer. Hence, replicative senescence of HUVEC was found to be closely associated with such metabolic disorders as increased oxidative stress, impaired energy metabolism and decreased protein synthesis. Concerning energy metabolism, declining levels of energy metabolism related metabolites such as AMP, ADP, ATP, NAD+ and creatine were observed. During the progression of cellular senescence, other metabolites related to the TCA cycle showed significantly changed levels while the lactate level was gradually reduced with no significant change of the glucose level. These results suggested that both declined glycolysis and mitochondrial respiration were contributing to the senescence associated decrease in energy production in HUVEC (Yi et al., 2020). Hence, neutral amino acids such as valine, isoleucine and glycine were metabolized for providing alternative energy sources during HUVEC senescence.
2.3. Intracellular metabolites analysis during cellular senescence of myoblasts as compared with fibroblasts
In our previously published studies, intracellular metabolites profiling analysis was performed by Metabolon (Baraibar and Friguet, 2013; Hamon et al., 2020; Le Boulch et al., 2018), as previously described (Reitman et al., 2011). Cellular pellets (106 cells) from six different batches from young and replicative senescent fibroblasts and myoblasts and from four batches of stress-induced senescent fibroblasts were accessioned into the Metabolon LIMS system. Raw data was extracted, peak-identified and QC processed using Metabolon’s hardware and software (Dehaven et al., 2010). Welch’s two-sample t-test was used to identify biochemicals that differed significantly between experimental groups.
Cellular replicative senescence has been associated with increased oxidative stress as evidenced by the accumulation of protein and lipid oxidation products (Baraibar et al., 2012; Levine and Stadtman, 2001; Stadtman, 2006). However, in our studies replicative senescent fibroblasts and myoblasts appear to combat oxidative stress as there are decreases in 2-hydroxypalmitate, 3-HODE/9-HODE, and 7-α-hydroxycholesterol markers of lipid peroxidation, and markers of oxidative stress such as methionine sulfoxide and 7-β-hydroxycholesterol (Baraibar et al., 2016; Hamon et al., 2020). In contrast, these biochemicals were all elevated in stress-induced premature senescent fibroblasts that had been treated with H2O2 (Le Boulch et al., 2018). In addition, levels of glutathione were increased in both cell types during replicative senescence. This appeared to be due to increased glutathione synthesis in replicative senescent myoblasts as gamma-glutamyl amino acids, cysteine, and ophthalmate were increased. Replicative senescent myoblasts also had higher levels of 6-phosphogluconate and reduced glutathione, indicating conversion of the oxidized form of glutathione to the reduced form. Thus, senescence appeared associated with a more oxidative environment in myoblasts, which they are equipped to neutralize. This hypothesis is also supported by the increased levels of the antioxidant carnosine.
Differential effects on energy metabolism were observed in myoblasts and fibroblasts undergoing replicative senescence. In senescent myoblasts, glycolytic intermediates such as glucose-6-phosphate, fructose-6-phosphate, and the isobar containing fructose 1,6-diphosphate, glucose 1,6-diphosphate, and myoinositol 1,4 or 1,3-diphosphate were decreased, which may indicate decreased metabolism of glucose via glycolysis. Since the functionality of the mitochondrial respiratory chain was not affected in human myoblasts during replicative senescence, the decreased glucose oxidation observed is most likely due to an impairment in glycolysis and/or TCA cycle (Baraibar et al., 2016). In contrast, these glycolytic intermediates were relatively unchanged in senescent fibroblasts while the downstream intermediates 2-phosphoglycerate, 3-phosphoglycerate, and phosphoenolpyruvate were dramatically depleted as compared to young fibroblasts. Together with the observed increase in lactate in senescent fibroblasts, this result suggested increased glycolytic activity in these cells. Consistent with increased glycolysis, acetyl-CoA levels were elevated in fibroblasts during senescence, which likely accounts for the increased TCA intermediates citrate, fumarate, and malate and increased levels of NADH. Depletion of the pentose phosphate pathway intermediate 6-phosphogluconate also suggested an increased activity of this pathway. Furthermore, the increased levels of NADH suggested an impairment of the mitochondrial respiratory chain in senescent fibroblasts since NADH produced by the TCA cycle in the mitochondria is utilized by the electron transport chain for ATP generation by oxidative phosphorylation. In senescent myoblasts, elevated pyrophosphate levels may indicate a decrease in ATP synthesis associated with the observed decreases in glycolysis and/or TCA cycles. No effect on pyrophosphate was observed in senescent fibroblasts.
Several changes in lipid metabolism were indicative of increased mitochondrial β-oxidation and membrane remodeling. Indeed, long chain free FAs (mostly n>18) were decreased while medium chain free FAs were unaffected. This may reflect decreased synthesis of this class of lipids or increased incorporation into cellular membranes. However, multiple acyl-carnitines were also increased in both cell types during replicative senescence. This signature typically indicates increased β-oxidation of FAs for energy production due to increased transport of FAs into the mitochondria. In addition, increased glycerolipid metabolites (eg. ethanolamine, choline, glycerol, glycerol 3-phosphate) as well as increased monoacylglycerols in senescent myoblasts may reflect the usage of fat stores for energy production. The difference between glycerolipid metabolism in senescent fibroblasts versus myoblasts is consistent with the altered energy contribution from glycolysis in myoblasts. Such changes in lipid metabolism may also reflect changes in membrane composition during replicative senescence.
Taken together, these studies illustrate that the metabolic profiles of cells displaying a senescent phenotype can be quite different. Notably, the same metabolic pathways were differentially affected by replicative senescence in myoblasts and fibroblasts. This may be reflective of different cellular responses to increased oxidative stress and/or impaired protein homeostasis that are associated with cellular senescence. This may be also due to differences in basal energy metabolism between the two cell types depending on whether they are quiescent or proliferating. Interestingly, the metabolic profile of senescent fibroblasts was not completely shared by stress-induced premature senescent fibroblasts (Le Boulch et al., 2018).
2.4. Oxi-proteome analysis and identification of proteins involved in energy metabolism that are accumulating as oxidatively modified during cellular senescence of fibroblasts and myoblasts
Since cellular senescence has been associated with increased oxidative stress, impaired protein homeostasis and accumulation of oxidized proteins, the occurrence and identification of such modified proteins have been performed for human fibroblasts and myoblasts upon replicative senescence as well as fibroblasts during oxidative stress-induced premature senescence (Ahmed et al., 2010; Baraibar et al., 2016; Le Boulch et al., 2018). Indeed, accumulation of oxidized proteins is a hallmark of cellular senescence in vitro and organisma aging in vivo (Baraibar and Friguet, 2013; Baraibar et al., 2012). Moreover, damaged proteins that are building up as oxidized during aging, cellular senescence and age-related diseases represent a restricted set of proteins, also referred as to “Oxi-proteome”, indicating that certain proteins are more prone to oxidative modification and subsequent intracellular accumulation. Hence, identification of this restricted set of proteins could give insights into the mechanisms by which the accumulation of these oxidatively modified proteins is affecting cellular function.
Among other functional categories such as protein quality control/ stress response and cellular morphology, several oxidized proteins were also found to fall in energy metabolism. In senescent fibroblasts, oxidatively modified proteins related to energy metabolism were mainly associated with the TCA, FA oxidation, oxidative phosphorylation (OXPHOS) and mitochondrial function since an important proportion of modified proteins were of mitochondrial origin. Conversely in senescent myoblasts for which most of the oxidized proteins were of cytosolic origin, proteins belonging to the glycolytic pathway were mainly affected by oxidation when it comes to energy metabolism related proteins. Interestingly, these findings are pointing out to energy metabolism dysregulation that was also evidenced through the metabolites analysis reported above. Indeed, the metabolome analyses were indicative of a metabolic switch which is different depending on cell type: from TCA cycle and oxidative phosphorylation to glycolysis in senescent fibroblasts and from glycolysis to FA oxidation in senescent myoblasts. In fact, these differential effects on energy metabolism that were observed in myoblasts and fibroblasts undergoing replicative senescence are in line with the non-overlapping sets of oxidized proteins belonging to different energy metabolic pathways in the two cell types. Taken together, these results suggest that the senescence associated impairment of energy metabolism specific pathways might be explained, at least in part, by the accumulation of oxidatively damaged and non functional enzymes in these pathways, hence arguing for a functional link between protein oxidation, protein maintenance and alteration of energy metabolism during cellular senescence. Whether such energy metabolic differences between senescent fibroblasts and myoblast that have been shown in vitro would also be observed in vivo remains to be elucidated, as well as their physiological relevance.
3. Senescence-associated metabolic changes in immune cells
Despite the decline in their proliferative potential, immune cells with a senescent phenotype show high metabolic activity and, in general, acquire a more glycolytic phenotype even in the presence of high levels of oxygen. The choice of glycolysis, away from OXPHOS, leads to a dysbalanced bioenergetic condition that occurs in response to the increased oxidative stress caused by the accumulation of dysfunctional mitochondria. Metabolic reprogramming is needed to cope with the high energetic demands associated with increased secretion of SASP factors and increased oxidative stress.
In this section of the review we will focus on human senescent CD4+ and CD8+ T cells, as these are the immune cells that have been thoroughly investigated and characterized for senescence-associated metabolic changes. Published results on senescent B cells and macrophages will also be summarized below.
3.1. Senescence-associated metabolic changes in T cells
Resting T cells are quiescent and require adenosine triphosphate (ATP) for their basal functions (Maciver et al., 2008). After stimulation with antigens or mitogens, T cells rapidly divide and in addition to ATP also require biosynthetic precursors to support their proliferation (Jones and Thompson, 2007; Maciver et al., 2008). Memory T cells do not need to proliferate anymore ano decrease their glycolytic metabolism (Pearce et al., 2009). The transition of T cells from oxidative to glycolytic pathways and vice versa is crucial for the regulation of cell survival and for the expansion and selection of antigen-specific high-affinity T cell clones (Coloff et al., 2011; Wensveen et al., 2010). Proliferating T cells are characterized by progressive reduction in telomere length, a feature of cell senescence, that ultimately leads to in vitro exhaustion (Effros, 2011). However, exhausted T cells in contrast to senescent T cells have low expression of immunological markers of senescence and reduced secretion of pro-inflammatory cytokines (Crespo et al., 2013; Wherry, 2011).
In humans, T cell senescence has been shown to induce changes in cellular metabolism, although the opposite has also been shown, i.e. that metabolic changes are contributing to T cell senescence. In particular, it has been shown that the age-associated decline in mitochondrial function (Bratic and Larsson, 2013) is linked to T cell dysfunction observed with increasing age (Ron-Harel et al., 2015). An example is the reduced ATP production, due to reduced coupling efficiency, and the defective induction of several metabolites as well as of mitochondrial enzymes of one-carbon metabolism that lead to reduced naïve T cell activation (Ron-Harel et al., 2018). Failure to provide the sufficient amounts of ATP during the initial phases of T cell activation leads to defects in signaling. Reduced Ca++-mediated signaling, due at least in part to Ca++ buffering deficits found in aged mitochondria, has also been shown (Mather and Rottenberg, 2002). In T regulatory (TREG) cells, that exhibit a metabolic profile different from that of effector T cells, with selectivity for glucose metabolism, glucose consumption is accelerated to support their function and this is associated with the acquisition of a senescent phenotype (Li et al., 2019). TLR8 signaling in inhibits glucose uptake and TREG function, and improves anti-tumor immunity in vitro and in vivo, as shown in a melanoma mouse model.
One of the characteristics of T cells becoming late-differentiated and progressing to cell senescence is the loss of surface expression of CD28 (Effros et al., 1994; Fagnoni et al., 1996; Weng et al., 2009), a crucial co-stimulatory molecule involved in T cell activation and in the regulation of important cell functions such as lipid raft formation, IL-2 gene transcription, apoptosis, stabilization of cytokine mRNA and cell adhesion. Early studies have shown that CD28 is also associated with metabolic fitness (Thompson et al., 1989), as indicated by increased respiratory capacity and increased expression of enzymes that facilitate mitochondrial FA oxidation, such as carnitine palmitoyltransferase, needed for ATP and function (secretion of cytokines, and cytotoxic activity) (Klein Geltink et al., 2017). Senescent CD4+ and CD8+ T cells have been identified in different studies as CD28-CD27-, CD28-CD57+, TEMRA (T effector memory re-expressing CD45RA).
Senescent CD4+ T cells characterized by the CD28-CD27- phenotype, show reduced proliferation and short telomeres (Fletcher et al., 2005). These cells engage the intracellular metabolic sensor AMPK to trigger p38 mitogen-activated protein kinase (MAPK) auto-phosphorylation (Lanna et al., 2014), a pathway activated in response to glucose deprivation and/or genotoxic stress. These results have shown for the first time that T cells have an ‘intra-sensory’ pathway for p38 activation that senses intracellular changes. Once triggered, this pathway inhibits T cell proliferation and telomerase activation via p38 MAPK signaling and this can be reversed by inhibiting AMPK or p38 MAPK. In CD28-CD27- CD4+ T cells transduced with a lentiviral vector co-expressing a green fluorescent protein reporter gene and inhibitory shRNAs to Sestrins, the senescent phenotype of these cells is reversed, and enhanced cell proliferation and telomerase activity, diminished DNA damage foci, and re-expression of the T cell receptor (TCR) signalosome components Lck and Zap70 and of the co-stimulatory receptors CD28 and CD27 were observed (Lanna et al., 2017). Sestrins, upstream of AMPK, promote cell survival under stress conditions and regulate AMPK activity, and therefore are crucial regulators of metabolic homeostasis (Budanov and Karin, 2008; Eid et al., 2013; Lee et al., 2010).
Human senescent T cells can also be characterized by the expression of CD45RA, with highly differentiated T cells that re-express CD45RA identified as senescent T cells and called TEMRA. These cells accumulate with age and are characterized by low proliferative activity, high levels of DNA damage and reduced telomerase activity (Henson et al., 2014; Parish et al., 2010). Human CD4+ and CD8+ TEMRA have been shown to senesce at different rates, due to a significant difference in mitochondrial content between the two subsets (Callender et al., 2020). Briefly, CD4+ TEMRA, that acquire an immunosenescent phenotype later than CD8+ TEMRA, have not only higher mitochondrial content but their mitochondria are healthier and more oxidative as compared to those of CD8+ TEMRA, suggesting that the intrinsic metabolic phenotype of the subset drives the susceptibility to become senescent. Moreover, CD4+ TEMRA have better nutrient uptake and higher basal and induced respiration, as evaluated by oxygen consumption rates (OCR), similar to the other CD4+ T cell subsets, whereas extracellular acidification rates (ECAR), which represent a measure of glycolysis, are only increased in part as compared to the CD8+ TEMRA subset. The amount of ATP made by CD4+ TEMRA is also greater. These results altogether suggest that the higher mitochondrial content and better fitness of the CD4+ TEMRA subset allows for a greater flexibility in the type of metabolism.
CD8+ TEMRA exhibit the typical senescent phenotype characterized by low proliferative activity, short telomeres, low telomerase activity and expression of the senescence-associated marker CD57 (Henson et al., 2014). It has recently been shown (Pereira et al., 2020) that CD8+ TEMRA down-regulate canonical TCR signaling pathways and genes associated with TCR signaling such as Trac, Cd3e, Cd3g, Lck, Lat and Plcg1, but upregulate the expression of receptors associated with natural killer (NK) cells, such as the inhibitory receptors KLRG1 and NKG2A and the activatory receptors NKG2C and NKG2D. The NK-like CD8+ TEMRA are characterized by higher cytotoxic activity as compared to the CD8+ TEMRA, because they express the cytotoxic molecules perforin and granzyme B, as well as the NKG2D/DAP12 complex, regulated by Sestrin 2. The acquisition of these features gives the CD8+ TEMRA the advantage to be cytotoxic against cells that express NKG2D ligands. These results altogether indicate that CD8+ TEMRA, although senescent, undergo a Sestrin 2-mediated reprogramming that allows them to efficiently kill target cells also through this acquired NKR-dependent mechanism. This function could be particularly relevant for the elimination of senescent cells from different tissues especially in conditions in which the immune function of CD8+ T cells is down-regulated due to chronic stimulation with persistent viruses (e.g. cytomegalovirus) that accumulate with age. It has also been shown that these cells may engage in a broad-spectrum, innate-like killing activity, as the inhibition of Sestrin 2 decreases the expression of the NKG2D/DAP12 complex and restores canonical TCR signaling in CD8+ TEMRA cells (Pereira et al., 2020). The efficient reprogramming of CD8+ T cells was found to be associated with reduced pyruvate dehydrogenase kinase activity, a marker of OXPHOS, suggesting that these cells preferentially utilize glycolysis, as also shown by other studies (Jeng et al., 2018). Moreover, senescent CD8+ T cells down-regulate Sirtuin 1 (Jeng et al., 2018), a nuclear NAD-dependent protein deacetylase (Chang and Guarente, 2014), with anti-aging activities (Grabowska et al., 2017; Guarente, 2007). The down-regulation of Sirtuin 1, together with the enhanced proteasomal degradation of its downstream transcription factor forkhead box protein O1, promotes the metabolic reprogramming of senescent CD8+ T cells and their enhanced glycolytic activity and granzyme B expression (Jeng et al., 2018).
Senescent T cells have been shown to be more abundant in the blood of individuals with immune-associated disorders. T cell senescence is associated with inflammaging and dysfunctional hepatic glucose homeostasis (Yi et al., 2019). Senescent T cells increase in the blood of pre-diabetic patients, secrete large amounts of pro-inflammatory cytokines and chemokines, and ROS, due to their high glycolytic profile, as shown by elevated ECAR, also consistent with abnormal glucose homeostasis. These dysfunctional properties of senescent CD8+ T cells have been suggested to contribute to increased hepatic inflammation and insulin resistance (IR) in pre-diabetic patients, confirming the existence of an immunometabolic link between T cell senescence and the pathophysiology of diabetes.
3.2. Senescence-associated metabolic changes in B cells
Unstimulated B cells use glucose and FAs to produce ATP. B cell receptor (BCR)-stimulated B cells in both mice and humans upregulate the expression of the glucose transporter Glut1 and activate glycolysis and to a much lesser extent OXPHOS to support their demands of energy for antibody production (Akkaya et al., 2018; Caro-Maldonado et al., 2014).
Only a few studies have evaluated senescence-associated changes in B cell metabolism. In one of these (Kurupati et al., 2019), the authors have shown that switched memory B cells isolated from the peripheral blood of elderly individuals were characterized by higher mitochondrial mass and mitochondrial ROS and lower FOXO1, a transcription factor involved in the regulation of metabolic homeostasis in response to oxidative stress (Link, 2019). Switched memory B cells are among the B cell subsets showing a senescent phenotype, as we (Frasca et al., 2017a) and others (Buffa et al., 2013; Bulati et al., 2014; Martorana et al., 2014) have previously shown. High mitochondrial ROS in switched memory B cells may be responsible for the reduced accumulation of this B cell subset in the elderly, including those specific for the influenza A virus.
B cells exhibit traits typical of senescence in elderly individuals. In humans, B cell percentages and numbers are significantly and progressively decreased with age and there is also a shift in the proportions of the different B cell subsets with a significant increase in the frequencies of the subset called Double Negative (DN) B cells (Frasca et al., 2017a; Frasca et al., 2017b). DN B cells have previously been called late/exhausted memory or tissue-like memory B cells, and they are the most pro-inflammatory B cell subset, reported to be increased also in the blood of patients with autoimmune (Adlowitz et al., 2015; Claes et al., 2016; Wehr et al., 2004) and infectious diseases (Chang et al., 2017; Illingworth et al., 2013; Moir et al., 2008), suggesting that DN B cells may accumulate in vivo in inflammatory conditions and in the presence of chronic stimulation with self antigens or viral/parasitic antigens, and may secrete autoimmune or protective antibodies, respectively.
We initially found (Frasca et al., 2017a) that only memory B cells express SASP markers, such as pro-inflammatory cytokines (TNF-α/IL-6/IL-8), cell cycle regulators (p16INK4), inflammatory micro-RNAs (miRs, miR-155/16/93) and especially the DN B cell subset. This subset also shows spontaneous activation by phosphorylation of AMPK the energy sensing enzyme which is ubiquitously expressed in mammalian cells. DN B cells activate a p38 MAPK signaling pathway, downstream of AMPK, leading to the expression of SASP mediators, while class switch recombination is downregulated. These data altogether showed for the first time that signaling through metabolic pathways is associated with a senescence phenotype, demonstrating for the first time in human B lymphocytes the link between aging, cellular senescence, SASP and metabolism.
More recently (Frasca et al., 2019) we compared frequencies and metabolic requirements of DN B cells in the blood of healthy individuals of different ages and in the blood and the subcutaneous adipose tissue (SAT) of individuals with obesity. We showed that DN B cells from young individuals have minimal metabolic requirements, DN B cells from elderly and obese individuals utilize higher amounts of glucose to perform autoimmune antibody production and enroll in aerobic glycolysis to support their function. DN B cells from the SAT have the highest metabolic requirements as they activate OXPHOS, aerobic glycolysis and FA oxidation. DN B cells from the SAT also show the highest levels of ROS and the highest levels of phosphorylated AMPK and Sestrin 1, both able to mitigate stress and cell death. This metabolic advantage drives DN B cell survival and function (secretion of autoimmune antibodies).
3.3. Senescence-associated metabolic changes in macrophages / monocytes
Macrophages from elderly individuals, as compared to those from younger individuals, are characterized by higher intrinsic inflammation, measured by pro-inflammatory cytokines (IL-6, MCP-1, TNF-α), leading to a chronic activation status which is generally associated with decreased effector functions, such as phagocytosis, antigen presentation and wound healing (Fulop et al., 2016). The age-related decline in macrophage function has been associated not only with cell senescence (Ritzel et al., 2019), but also with defective autophagy (Stranks et al., 2015), reduced availability of NAD (Minhas et al., 2019) and reduced respiration and mitochondrial function, including reduced ATP production and membrane potential and increased ROS (Chougnet et al., 2015; Stranks et al., 2015). NAD decline has been attributed to the activation of CD38 (Covarrubias et al., 2019), a transmembrane protein involved in Ca++-mediated signaling and mobilization, robustly induced by SASP-mediated activation of NF-kB (Amici et al., 2018).
Monocytes from elderly individuals also show reduced respiratory capacity, likely due to reduced NAD levels as a results of CD38 activation by the SASP (Pence and Yarbro, 2018). Interestingly, the results of the TRIMM (Thymus Regeneration, Immunorestoration, and Insulin Mitigation) clinical trial, using using the recombinant human growth hormone, alone or in combination with both dehydroepiandrosterone and metformin (in an attempt to limit the “diabetogenic” effect of the growth hormone) have shown that the reversal of immunosenescence in humans was significantly associated with lower levels of CD38 in monocytes and increased NAD tissue availability (Fahy et al., 2019).
4. Conclusions
Senescent cells secrete an enormous amount of pro-inflammatory products contributing to local and systemic inflammation. Because of the direct effects of inflammation on immunity, senescent cells and their products represent a therapeutic target for the treatment of conditions associated with inflammation, and several interventions have already shown that it is possible to delay or rescue age-associated defects in mitochondrial function, induce mitochondrial rejuvenation and improve immune and non-immune cell function. Studies in mice and non-human primates have clearly indicated that the ability of both exercise (Safdar et al., 2011) and caloric restriction (Messaoudi et al., 2006) to improve T cell immunity during aging could be at least in part linked to their effect in improving mitochondrial function. The finding that senescent cells rely on metabolic reprogramming for survival and function has suggested the exciting possibility that their function could be regulated by manipulating the cell’s metabolism. Current and future studies need to focus on the identification of effective strategies of intervention to selectively target metabolic pathways found switched on or upregulated in a large number of senescent cells and tissues for anti-aging interventions.
Highlights.
Senescent cells are metabolically active
Cellular senescence induces metabolic changes in non-immune and immune cells
Senescent cells are flexible in the use of different metabolic pathways
Metabolic reprogramming in senescent cells leads to increased survival and supports function
Acknowledgments
Study supported by NIH awards AG32576, AG059719, AG023717, and by INSERM cross-cutting program on ageing, Fondation pour la Recherche Médicale grant SPF20170938839.
Abbreviations
- AMP
adenosine monophosphate
- AMPK
AMP-activated protein kinase
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- BCR
B cell receptor
- DN
Double negative
- ECAR
extracellular acidification rate
- EGF
epithelium growth factor
- FA
fatty acid
- FOXO1
Forkhead box protein O1
- HODE
hydroxyoctadecadienoic acid
- HUVEC
human umbilical vein endothelial cell
- IR
insulin resistance
- LIMS
Laboratory Information Management System
- MAPK
mitogen-activated protein kinase
- NAD
nicotinamide adenine dinucleotide phosphate
- NADP
nicotinamide adenine dinucleotide phosphate
- NAMPT
nicotinamide phosphoribosyltransferase
- NF-KB
nuclear factor-kappa B
- NGF
nerve growth factor
- NK
Natural killer
- NKR
Natural killer receptor
- OCR
oxygen consumption rate
- OXPHOS
oxidative phosphorylation
- QC
quality control
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- SAT
subcutaneous adipose tissue
- TCA
tricarboxylic acid
- TCR
T cell receptor
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Footnotes
Ethics declarations
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adlowitz DG, Barnard J, Biear JN, Cistrone C, Owen T, Wang W, Palanichamy A, Ezealah E, Campbell D, Wei C, Looney RJ, Sanz I, Anolik JH, 2015. Expansion of Activated Peripheral Blood Memory B Cells in Rheumatoid Arthritis, Impact of B Cell Depletion Therapy, and Biomarkers of Response. PLoS One 10, e0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed EK, Rogowska-Wrzesinska A, Roepstorff P, Bulteau AL, Friguet B, 2010. Protein modification and replicative senescence of WI-38 human embryonic fibroblasts. Aging Cell 9, 252–272. [DOI] [PubMed] [Google Scholar]
- Akkaya M, Traba J, Roesler AS, Miozzo P, Akkaya B, Theall BP, Sohn H, Pena M, Smelkinson M, Kabat J, Dahlstrom E, Dorward DW, Skinner J, Sack MN, Pierce SK, 2018. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol 19, 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici SA, Young NA, Narvaez-Miranda J, Jablonski KA, Arcos J, Rosas L, Papenfuss TL, Torrelles JB, Jarjour WN, Guerau-de-Arellano M, 2018. CD38 Is Robustly Induced in Human Macrophages and Monocytes in Inflammatory Conditions. Front Immunol 9, 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM, 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraibar MA, Friguet B, 2013. Oxidative proteome modifications target specific cellular pathways during oxidative stress, cellular senescence and aging. Exp Gerontol 48, 620–625. [DOI] [PubMed] [Google Scholar]
- Baraibar MA, Hyzewicz J, Rogowska-Wrzesinska A, Bulteau AL, Prip-Buus C, Butler-Browne G, Friguet B, 2016. Impaired energy metabolism of senescent muscle satellite cells is associated with oxidative modifications of glycolytic enzymes. Aging (Albany NY) 8, 3375–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraibar MA, Liu L, Ahmed EK, Friguet B, 2012. Protein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseases. Oxid Med Cell Longev 2012, 919832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittles AH, Harper N, 1984. Increased glycolysis in ageing cultured human diploid fibroblasts. Biosci Rep 4, 751–756. [DOI] [PubMed] [Google Scholar]
- Bratic A, Larsson NG, 2013. The role of mitochondria in aging. J Clin Invest 123, 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Karin M, 2008. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa S, Pellicano M, Bulati M, Martorana A, Goldeck D, Caruso C, Pawelec G, Colonna-Romano G, 2013. A novel B cell population revealed by a CD38/CD24 gating strategy: CD38(-)CD24 (-) B cells in centenarian offspring and elderly people. Age (Dordr) 35, 2009–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulati M, Buffa S, Martorana A, Candore G, Lio D, Caruso C, Colonna-Romano G, 2014. Trafficking phenotype and production of granzyme B by double negative B cells (IgG(+)IgD(-)CD27(-)) in the elderly. Exp Gerontol 54, 123–129. [DOI] [PubMed] [Google Scholar]
- Callender LA, Carroll EC, Bober EA, Akbar AN, Solito E, Henson SM, 2020. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell 19, e13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, 2011. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev 21, 107–112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F, 2007. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8, 729–740. [DOI] [PubMed] [Google Scholar]
- Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR, Rathmell JC, 2014. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol 192, 3626–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Guarente L, 2014. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LY, Li Y, Kaplan DE, 2017. Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells. J Viral Hepat 24, 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM, 2014. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15, 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Durik M, Baker DJ, van Deursen JM, 2015. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, Janssen EM, 2015. Loss of Phagocytic and Antigen Cross-Presenting Capacity in Aging Dendritic Cells Is Associated with Mitochondrial Dysfunction. J Immunol 195, 2624–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, Hupperts R, Somers V, 2016. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. J Immunol 197, 4576–4583. [DOI] [PubMed] [Google Scholar]
- Coloff JL, Mason EF, Altman BJ, Gerriets VA, Liu T, Nichols AN, Zhao Y, Wofford JA, Jacobs SR, Ilkayeva O, Garrison SP, Zambetti GP, Rathmell JC, 2011. Akt requires glucose metabolism to suppress puma expression and prevent apoptosis of leukemic T cells. J Biol Chem 286, 5921–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias AJ, Lopez-Dominguez JA, Perrone R, Kale A, Newman J, Iyer SS, 2019. Aging-related inflammation driven by cellular senescence enhances NAD consumption via activation of CD38+ macrophages. bioRxiv 609438. [Google Scholar]
- Crespo J, Sun H, Welling TH, Tian Z, Zou W, 2013. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 25, 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaven CD, Evans AM, Dai H, Lawton KA, 2010. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Dabritz JH, Lisec J, Lenze D, Gerhardt A, Schleicher K, Kratzat S, Purfurst B, Walenta S, Mueller-Klieser W, Graler M, Hummel M, Keller U, Buck AK, Dorken B, Willmitzer L, Reimann M, Kempa S, Lee S, Schmitt CA, 2013. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425. [DOI] [PubMed] [Google Scholar]
- Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P, 2018. Endothelial Cell Metabolism. Physiol Rev 98, 3–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, 2011. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol 46, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schachter F, 1994. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol 29, 601–609. [DOI] [PubMed] [Google Scholar]
- Eid AA, Lee DY, Roman LJ, Khazim K, Gorin Y, 2013. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol Cell Biol 33, 3439–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P, 1996. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians. Immunology 88, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy GM, Brooke RT, Watson JP, Good Z, Vasanawala SS, Maecker H, Leipold MD, Lin DTS, Kobor MS, Horvath S, 2019. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 18, e13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN, 2005. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol 175, 8218–8225. [DOI] [PubMed] [Google Scholar]
- Frasca D, Diaz A, Romero M, Blomberg BB, 2017a. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol 87, 113–120. [DOI] [PubMed] [Google Scholar]
- Frasca D, Diaz A, Romero M, D'Eramo F, Blomberg BB, 2017b. Aging effects on T-bet expression in human B cell subsets. Cell Immunol 321, 68–73. [DOI] [PubMed] [Google Scholar]
- Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB, 2019. Metabolic requirements of human pro-inflammatory B cells in aging and obesity. PLoS One 14, e0219545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Dupuis G, Baehl S, Le Page A, Bourgade K, Frost E, Witkowski JM, Pawelec G, Larbi A, Cunnane S, 2016. From inflamm-aging to immune-paralysis: a slippery slope during aging for immuneadaptation. Biogerontology 17, 147–157. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Ballantyne SR, Robson AL, Moerman EJ, 1982. Energy metabolism in cultured human fibroblasts during aging in vitro. J Cell Physiol 112, 419–424. [DOI] [PubMed] [Google Scholar]
- Grabowska W, Sikora E, Bielak-Zmijewska A, 2017. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 18, 447–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, 2007. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol 72, 483–488. [DOI] [PubMed] [Google Scholar]
- Hamon MP, Ahmed EK, Baraibar MA, Friguet B, 2020. Proteome Oxidative Modifications and Impairment of Specific Metabolic Pathways During Cellular Senescence and Aging. Proteomics 20, e1800421. [DOI] [PubMed] [Google Scholar]
- Henson SM, Lanna A, Riddell NE, Franzese O, Macaulay R, Griffiths SJ, Puleston DJ, Watson AS, Simon AK, Tooze SA, Akbar AN, 2014. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J Clin Invest 124, 4004–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, Crompton PD, Marsh K, Ndungu FM, 2013. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol 190, 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James EL, Lane JA, Michalek RD, Karoly ED, Parkinson EK, 2016. Replicatively senescent human fibroblasts reveal a distinct intracellular metabolic profile with alterations in NAD+ and nicotinamide metabolism. Sci Rep 6, 38489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James EL, Michalek RD, Pitiyage GN, de Castro AM, Vignola KS, Jones J, Mohney RP, Karoly ED, Prime SS, Parkinson EK, 2015. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J Proteome Res 14, 1854–1871. [DOI] [PubMed] [Google Scholar]
- James ENL, Bennett MH, Parkinson EK, 2018. The induction of the fibroblast extracellular senescence metabolome is a dynamic process. Sci Rep 8, 12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng MY, Hull PA, Fei M, Kwon HS, Tsou CL, Kasler H, Ng CP, Gordon DE, Johnson J, Krogan N, Verdin E, Ott M, 2018. Metabolic reprogramming of human CD8(+) memory T cells through loss of SIRT1. J Exp Med 215, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Aroor AR, Jia C, Sowers JR, 2019. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta Mol Basis Dis 1865, 1802–1809. [DOI] [PubMed] [Google Scholar]
- Jiang P, Du W, Mancuso A, Wellen KE, Yang X, 2013. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493, 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Thompson CB, 2007. Revving the engine: signal transduction fuels T cell activation. Immunity 27, 173–178. [DOI] [PubMed] [Google Scholar]
- Kalucka J, Bierhansl L, Conchinha NV, Missiaen R, Elia I, Bruning U, Scheinok S, Treps L, Cantelmo AR, Dubois C, de Zeeuw P, Goveia J, Zecchin A, Taverna F, Morales-Rodriguez F, Brajic A, Conradi LC, Schoors S, Harjes U, Vriens K, Pilz GA, Chen R, Cubbon R, Thienpont B, Cruys B, Wong BW, Ghesquiere B, Dewerchin M, De Bock K, Sagaert X, Jessberger S, Jones EAV, Gallez B, Lambrechts D, Mazzone M, Eelen G, Li X, Fendt SM, Carmeliet P, 2018. Quiescent Endothelial Cells Upregulate Fatty Acid beta-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab 28, 881–894 e813. [DOI] [PubMed] [Google Scholar]
- Klein Geltink RI, O'Sullivan D, Corrado M, Bremser A, Buck MD, Buescher JM, Firat E, Zhu X, Niedermann G, Caputa G, Kelly B, Warthorst U, Rensing-Ehl A, Kyle RL, Vandersarren L, Curtis JD, Patterson AE, Lawless S, Grzes K, Qiu J, Sanin DE, Kretz O, Huber TB, Janssens S, Lambrecht BN, Rambold AS, Pearce EJ, Pearce EL, 2017. Mitochondrial Priming by CD28. Cell 171, 385–397 e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurupati RK, Haut LH, Schmader KE, Ertl HC, 2019. Age-related changes in B cell metabolism. Aging (Albany NY) 11, 4367–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J, 2015. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17, 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanna A, Gomes DC, Muller-Durovic B, McDonnell T, Escors D, Gilroy DW, Lee JH, Karin M, Akbar AN, 2017. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat Immunol 18, 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanna A, Henson SM, Escors D, Akbar AN, 2014. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol 15, 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boulch M, Ahmed EK, Rogowska-Wrzesinska A, Baraibar MA, Friguet B, 2018. Proteome oxidative carbonylation during oxidative stress-induced premature senescence of WI-38 human fibroblasts. Mech Ageing Dev 170, 59–71. [DOI] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M, 2010. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327, 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Stadtman ER, 2001. Oxidative modification of proteins during aging. Exp Gerontol 36, 1495–1502. [DOI] [PubMed] [Google Scholar]
- Li L, Liu X, Sanders KL, Edwards JL, Ye J, Si F, Gao A, Huang L, Hsueh EC, Ford DA, Hoft DF, Peng G, 2019. TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy. Cell Metab 29, 103–123 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, 2019. Introduction to FOXO Biology. Methods Mol Biol 1890, 1–9. [DOI] [PubMed] [Google Scholar]
- Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC, 2008. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol 84, 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorana A, Balistreri CR, Bulati M, Buffa S, Azzarello DM, Camarda C, Monastero R, Caruso C, Colonna-Romano G, 2014. Double negative (CD19+IgG+IgD-CD27-) B lymphocytes: a new insight from telomerase in healthy elderly, in centenarian offspring and in Alzheimer's disease patients. Immunol Lett 162, 303–309. [DOI] [PubMed] [Google Scholar]
- Mather MW, Rottenberg H, 2002. The inhibition of calcium signaling in T lymphocytes from old mice results from enhanced activation of the mitochondrial permeability transition pore. Mech Ageing Dev 123, 707–724. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, Douek DC, Mori M, Nikolich-Zugich J, 2006. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A 103, 19448–19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, Contrepois K, Wang Q, Lee BA, Coronado M, Bernstein D, Snyder MP, Migaud M, Majeti R, Mochly-Rosen D, Rabinowitz JD, Andreasson KI, 2019. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol 20, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS, 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 205, 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O, Bourdeau V, Roux A, Deschenes-Simard X, Ferbeyre G, 2009. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol Cell Biol 29, 4495–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, Noma KI, Baur JA, Schug Z, Tang HY, Speicher DW, David G, Zhang R, 2019. NAD(+) metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol 21, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish ST, Wu JE, Effros RB, 2010. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J Clin Immunol 30, 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T, 2010. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y, 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BD, Yarbro JR, 2018. Aging impairs mitochondrial respiratory capacity in classical monocytes. Exp Gerontol 108, 112–117. [DOI] [PubMed] [Google Scholar]
- Pereira BI, De Maeyer RPH, Covre LP, Nehar-Belaid D, Lanna A, Ward S, Marches R, Chambers ES, Gomes DCO, Riddell NE, Maini MK, Teixeira VH, Janes SM, Gilroy DW, Larbi A, Mabbott NA, Ucar D, Kuchel GA, Henson SM, Strid J, Lee JH, Banchereau J, Akbar AN, 2020. Sestrins induce natural killer function in senescent-like CD8(+) T cells. Nat Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano C, Cao L, Fergusson MM, Romero H, Liu J, Gutkind S, Rovira II, Mohney RP, Karoly ED, Finkel T, 2012. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle 11, 1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, He Y, Bigner DD, Vogelstein B, Yan H, 2011. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci U S A 108, 3270–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Doran SJ, Glaser EP, Meadows VE, Faden AI, Stoica BA, Loane DJ, 2019. Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol Aging 77, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron-Harel N, Notarangelo G, Ghergurovich JM, Paulo JA, Sage PT, Santos D, Satterstrom FK, Gygi SP, Rabinowitz JD, Sharpe AH, Haigis MC, 2018. Defective respiration and one-carbon metabolism contribute to impaired naive T cell activation in aged mice. Proc Natl Acad Sci U S A 115, 13347–13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron-Harel N, Sharpe AH, Haigis MC, 2015. Mitochondrial metabolism in T cell activation and senescence: a mini-review. Gerontology 61, 131–138. [DOI] [PubMed] [Google Scholar]
- Sabbatinelli J, Prattichizzo F, Olivieri F, Procopio AD, Rippo MR, Giuliani A, 2019. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front Physiol 10, 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA, 2011. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A 108, 4135–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER, 2006. Protein oxidation and aging. Free Radic Res 40, 1250–1258. [DOI] [PubMed] [Google Scholar]
- Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJ, Puleston DJ, Shenderov K, Watson AS, Veldhoen M, Phadwal K, Cerundolo V, Simon AK, 2015. Autophagy Controls Acquisition of Aging Features in Macrophages. J Innate Immun 7, 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, Leiden JM, June CH, 1989. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A 86, 1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XL, Li Y, 2014. Endothelial cell senescence and age-related vascular diseases. J Genet Genomics 41, 485–495. [DOI] [PubMed] [Google Scholar]
- Unterluggauer H, Mazurek S, Lener B, Hutter E, Eigenbrodt E, Zwerschke W, Jansen-Durr P, 2008. Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerontology 9, 247–259.. [DOI] [PubMed] [Google Scholar]
- van Deursen JM, 2014. The role of senescent cells in ageing. Nature 509, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter HH, Warnatz K, 2004. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol 113, 161–171. [DOI] [PubMed] [Google Scholar]
- Weng NP, Akbar AN, Goronzy J, 2009. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol 30, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensveen FM, van Gisbergen KP, Derks IA, Gerlach C, Schumacher TN, van Lier RA, Eldering E, 2010. Apoptosis threshold set by Noxa and Mcl-1 after T cell activation regulates competitive selection of high-affinity clones. Immunity 32, 754–765. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, 2011. T cell exhaustion. Nat Immunol 12, 492–499. [DOI] [PubMed] [Google Scholar]
- Wiley CD, Campisi J, 2016. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab 23, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J, 2016. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab 23, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Ye H, Shao C, Zheng X, Li Q, Wang L, Zhao M, Lu G, Chen B, Zhang J, Wang Y, Wang G, Hao H, 2017. Metabolomics-Proteomics Combined Approach Identifies Differential Metabolism-Associated Molecular Events between Senescence and Apoptosis. J Proteome Res 16, 2250–2261. [DOI] [PubMed] [Google Scholar]
- Yi HS, Kim SY, Kim JT, Lee YS, Moon JS, Kim M, Kang YE, Joung KH, Lee JH, Kim HJ, Chun K, Shong M, Ku BJ, 2019. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis 10, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Lin K, Jiang T, Shao W, Huang C, Jiang B, Li Q, Lin D, 2020. NMR-based metabonomic analysis of HUVEC cells during replicative senescence. Aging (Albany NY) 12, 3626–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL, 2015. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerschke W, Mazurek S, Stockl P, Hutter E, Eigenbrodt E, Jansen-Durr P, 2003. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem J 376, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]