Abstract
Objective:
Data regarding the ability of antidepressants to enter fetal, newborn and infant fluids have become gradually available, but mechanisms of antidepressant transfer remain poorly understood. Here we calculated penetration ratios in an array of matrices from combined samples of pregnant/breastfeeding women taking antidepressants.
Methods:
We performed a systematic literature search of PubMed and EMBASE to identify studies with concentrations of antidepressants from maternal blood, amniotic fluid, umbilical cord blood and/or breast milk. Penetration ratios were calculated by dividing the concentrations in amniotic fluid, umbilical cord plasma or breast milk by the maternal plasma concentration. When data from multiple studies were available, we calculated combined penetration ratios, weighting the study mean by study size.
Results:
Eighty-five eligible studies were identified. For amniotic fluid, the highest penetration ratios were estimated for venlafaxine (mean 2.77, range 0.43–4.70 for the active moiety) and citalopram (mean 2.03, range 0.35–6.97), while the lowest ratios were for fluvoxamine (mean 0.1) and fluoxetine (mean 0.11, range 0.02–0.2 for the active moiety). For umbilical cord plasma, nortriptyline had the highest ratio (mean 2.97, range 0.25–26.43) followed by bupropion (mean 1.14, range 0.3–5.08). For breast milk, the highest ratios were observed for venlafaxine (mean 2.59, range 0.85–4.85), mianserin (mean 2.22, range 0.80–3.64) and escitalopram (mean 2.19, range 1.68–3.00).
Conclusion:
We observed considerable variability across antidepressants regarding their ability to enter fetal, newborn and infant fluids. Measuring antidepressant concentrations in a maternal blood sample can provide a reliable estimate of fetal/infant exposure, although further evidence for concentration-dependent effects is required.
Keywords: antidepressants, breast milk, amniotic fluid, pregnancy, lactation
1. Introduction
Antidepressants represent the most commonly prescribed medications in women during pregnancy (Pariente et al., 2016), with antidepressant use increasing over the past years (Molenaar et al., 2020). Concerns regarding maternal and neonatal safety have been raised as antidepressant use during pregnancy and lactation may be associated with an increased risk of congenital malformations, preterm birth, poor neonatal adaptation syndrome, and persistent pulmonary hypertension of the newborn (Biffi et al., 2020; Ewing et al., 2015; Masarwa et al., 2019), whereas maternal health risks may include preeclampsia and postpartum hemorrhage (Palmsten et al., 2020). Nevertheless, antidepressants are largely considered safe, although it is widely embraced that antidepressant treatment during pregnancy and lactation requires an effective balancing of risks with benefits. Accordingly, advice regarding perinatal antidepressant use requires further evidence, identifying high-risk patient subgroups and accounting for confounding factors (Biffi et al., 2020). Regarding implicated mechanisms underlying the effects on both the fetus and the newborn, some hypotheses based on the pharmacologic profile of each agent have been postulated; for example, poor neonatal adaptation has been considered as related to the serotonergic overstimulation caused by fetal exposure to selective serotonin reuptake inhibitors (Yang et al., 2017), while genetic correlates have also received some interest (Laine et al., 2004).
While underlying pathways for adverse effects of antidepressants in the mother and fetus remain poorly understood, the assessment of antidepressant exposure of the fetus and newborn may offer valuable information when investigating safety issues. In fact, there is some evidence of pharmacokinetic correlates for antidepressant adverse effects on fetal health (Oberlander et al., 2004). Thus, therapeutic drug monitoring (TDM), i.e. quantification of antidepressant drug concentrations in different matrices of pregnant or lactating mothers, such as maternal blood, amniotic fluid, umbilical cord blood and breast milk, can provide an overarching measure of the ability of medications to enter the fetal and newborn circulation (Schoretsanitis et al., 2020b). Across mechanisms of fetal antidepressant transfer, passive diffusion is considered the main pathway (Ewing et al., 2015), but the physicochemical properties of the drugs might nevertheless be of importance (Hutson et al., 2011). For breast milk, the drug’s physicochemical profile as well as milk-related variables, such as pH and lipid content, are important factors (Whitby and Smith, 2005). A large variability in drug concentrations has been reported between fore-milk and hind-milk for lipophilic drugs including many antidepressants, mostly related to the increase in lipid concentration during the course of a feeding (Ohman et al., 1999). Additional benefits of regular TDM in the pharmacotherapy of pregnant women include the monitoring of changes in antidepressant disposition caused by perinatal changes in physiology (Deligiannidis et al., 2014; Westin et al., 2018). Changes in drug disposition may impact treatment outcomes, especially in the third trimester (Schoretsanitis et al., 2020a). TDM can thus be a unique tool to quantify alterations of drug disposition in each trimester and guide dose adjustment when considered alongside clinical assessment. Despite the potential of TDM integration in standard pharmacotherapy of pregnant and breastfeeding women, practical recommendations on when to perform TDM are hardly available.
Aims of the Study
In this article we systematically review TDM data for antidepressants in maternal blood samples (e.g. serum or plasma), amniotic fluid, umbilical cord blood samples (e.g. serum or plasma) and breast milk, aiming to provide excretion patterns into these matrices.
2. Material and Methods
The study was conducted with use of PRISMA guidelines (Hutton et al., 2015) and registered with PROSPERO (registration number CRD42020149698). Studies with antidepressant concentrations in maternal blood (serum or plasma), amniotic fluid, umbilical cord blood (serum or plasma), or breast milk were identified by searching the PubMed and EMBASE databases using the following search strategy: “antidepressant*” AND “pregnancy” AND “blood OR amniotic OR umbilical OR milk”. Data of neonatal concentrations were not the focus of this review, but were included for reasons of completeness. Only studies in English were included. Databases were searched in September 2019 for publications since data inception independently by two authors (GS and MP). References from identified studies were scanned for additional reports and articles from the authors’ own collections were also screened. Finally, searches in PubMed were also performed using the specific substance names of the antidepressants, but no additional studies were identified.
2.1. Inclusion criteria
Studies including pregnant or breastfeeding antidepressant-treated women with concentrations of antidepressants measured in maternal blood, amniotic fluid, umbilical cord blood, breast milk or infant blood were considered. No restrictions with regard to diagnosis, dosage or duration of antidepressant treatment were used.
2.2. Data extraction
Two authors (GS and MP) independently extracted data including sample sizes, daily antidepressant dose, antidepressant concentrations (mean and ranges or standard deviations depending on how data were provided) in maternal blood, amniotic fluid, umbilical cord blood and breast milk (fore-milk and hind-milk when available). Previously published conversion factors were used to convert data provided in substance concentration units (e.g. nmol/L) to mass concentration units (ng/mL) (Hiemke et al., 2018). When the term “blood” is used in the following, is refers to concentrations measured in plasma or serum. Moreover, for simplicity reasons maternal and umbilical cord concentrations will be referred to as plasma concentrations, although in some studies concentrations are measured in serum. For reasons of completeness we also included infant blood (serum or plasma) concentrations when available.
2.3. Outcomes & statistical analysis
Our primary outcomes were the penetration ratios of antidepressants into amniotic fluid, umbilical cord blood and breast milk, i.e. the antidepressant concentrations in amniotic fluid, umbilical cord blood or breast milk, divided by the maternal blood concentration. When antidepressants concentrations in both hind-milk and fore-milk were available, we used the mean milk concentrations as the primary outcome, but with ratios for fore-milk and hind-milk calculated separately. When multiple assessments from one single patient were available and no area under the time-concentration curve (AUC) values were provided, mean concentrations were used for ratio calculation. For amitriptyline, clomipramine, doxepin, imipramine, fluoxetine and venlafaxine, the active moiety (the sum of the parent compound and the active metabolite) was used (Hiemke et al., 2018); in the following text when the names of these antidepressants are used, they refer to the active moiety. Accordingly, data for desvenlafaxine and nortriptyline do not refer to the active metabolites in patients treated with venlafaxine or amitriptyline, but patients receiving the independently marketed compounds. When data from more than one study were available, we calculated combined (pooled) penetration ratios, where the mean of each study was weighted by its size, with larger studies given more weight. We have in a previous review of antipsychotics used this type of analysis (Schoretsanitis et al., 2020b). Combined penetration ratios and ranges are provided in plots produced using Matplotlib, which is a plotting library of Python. When data for an antidepressant exclusively derived from a single case report, only, they were not included in the plots. Additional plots included violin plots for penetration ratios when individual patient data were available for antidepressants with measures for more than five patients (Krzywinski & Altman, 2011).
2.4. Quality assessment
We assessed the quality of the included studies using the ClinPK guidelines (Kanji et al., 2015), an established tool for the evaluation of reporting for pharmacokinetic studies.
3. Results
The search yielded 63 articles from PubMed, 21 additional articles from Embase, 47 additional articles from article references and 45 additional articles from the authors’ own collections, i.e. a total of 176 articles (Figure 1). Abstract screening resulted in rejection of 44 of these (e.g. animal reviews and previous reviews). Full-text screening led to rejection of another 47 articles due to either lack of TDM data in maternal blood and/or amniotic fluid, umbilical cord blood or breast milk, or the lack of TDM data in an analyzable form (no individual patient data or means/ranges). Moreover, analytical method validation articles, articles concerning pharmacokinetic modelling and articles with overlapping data were also excluded. Finally, 85 articles were selected and used for data extraction (Figure 1).
Figure 1.
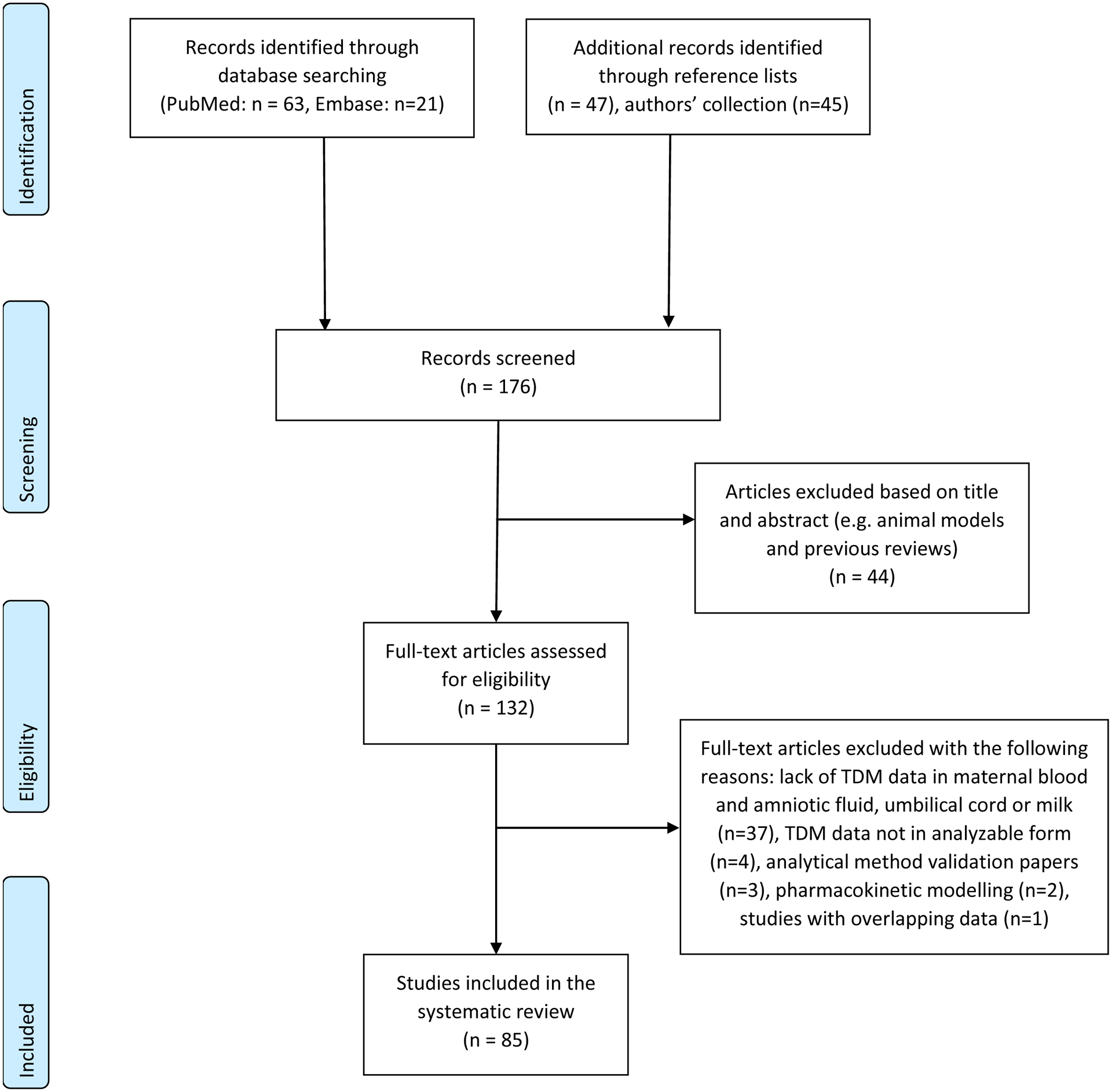
Prisma Checklist Study Flow Diagram
Table 1 summarizes the 82 studies where exact concentrations of antidepressants in maternal blood during pregnancy, at delivery or postpartum and in amniotic fluid, umbilical cord blood, breast milk and/or infant plasma were given, either in running text or in tables In the three remaining papers (Hagg et al., 2000; Pons et al., 1990; Verbeeck et al., 1986), concentration data were provided only in figures, but as the authors of the original studies themselves had calculated exact penetration ratios, these values could be inserted in our analysis.
Table 1.
Concentrations of antidepressants (in alphabetical order) in maternal plasma, amniotic fluid, umbilical cord plasma and infant blood.
| Antidepressant | Reference | N (n) | Daily dose (mg) | Maternal plasma concentration (ng/mL) | Amniotic fluid concentration (ng/mL) | Umbilical cord plasma concentration (ng/mL) | Breast milk concentration (ng/mL)a | Infant plasma concentration (ng/mL) | Quality score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fore-milk | Hind-milk | |||||||||
| Amitriptyline | (Bader & Newman, 1980) | 1 | 100 | 142.0–227.0 | 187.0–210.0 | <10.0 | 9 | |||
| (Brixen-Rasmussen et al., 1982) | 1 (1) | 25–75 | 38.0–121.0 | 60.0–179.0 | <20.0 | 11 | ||||
| (Pittard & O’Neal, 1986) | 1(1) | 100 | 117.0–167.0 | 77.0–143.0 | 11 | |||||
| (Yoshida et al., 1997a) | 2 (2) | 100–175 | 128.0–228.0b | 30.0b | 113.0–197.0b | 11 | ||||
| 1443.0–7156.0c | 185.0c | 707.0–4077.0c | ||||||||
| Amoxapine | (Gelenberg, 1979) | 1 (1) | 250 | 97.0 | <20.0 | 9 | ||||
| Bupropion | (Briggs et al., 1993) | 1 (1) | 100 | 9.0–72.0 | 58.0–189.0 | 10 | ||||
| (Davis et al., 2009) | 3 (3) | 150–300 | 13.5–150.0 | 8.5–11.5 | 11 | |||||
| (Fokina et al., 2016) | 18 (18) | 75–300 | 0.13–53.7 | 0.7–48.0 | 0.22–28.5 | 14 | ||||
| (Haas et al., 2004) | 10 (10) | 300 | 25.0±24.6 | 45.2±49.5 | 14 | |||||
| Citalopram | (Berle et al., 2004) | 9 (10) | 20–50 | 18.5–164.9 | 51.0–235.4 | <2.6 | 12 | |||
| (Franssen et al., 2006) | 1 (1) | 40 | 113.0–160.1 | 230.2–320.1 | 1.2–2.3 | 8 | ||||
| (Heikkinen et al., 2002) | 9 (9) | 20–40 | 40.3–64.6 | 90.3–144.5 | 1.1–9.7 | 13 | ||||
| (Hendrick et al., 2003) | 4 (4) | 20–40 | 12.0–64.0 | 17.0–36.0 | 11 | |||||
| (Jensen et al., 1997) | 1 (1) | 20 | 33.8–60.1 | 87.7–140.3 | 2.3 | 9 | ||||
| (Laine et al., 2003) | 9 (9) | 20–40 | 18.83–69.48 | 11.36–70.45 | 13 | |||||
| (Loughhead et al., 2006a) | 1 (1) | 50 | 262.0 | 94.0 | 13 | |||||
| (Paulzen et al., 2017b) | 12 (12) | 10–40 | 7.0–84.7 | 10.1–123.0d | 5.1–61.7 | 14 | ||||
| (Pogliani et al., 2017) | 2 (2) | 5–10 | 9.3–10.1 | 5.5–14.3 | 15 | |||||
| (Rampono et al., 2000) | 7 (7) | Unclear | 56.0–138.0 | 53.0–168.0 | <2.3 | 13 | ||||
| (Rampono et al., 2009) | 8 (8) | 20–40 | 8.0–84.0 | 6.0–71.0 | 12 | |||||
| (Schmidt et al., 2000) | 1 (1) | 20–40 | 49.0–98.9 | 205.0 | 4.5–12.7 | 8 | ||||
| (Schoretsaniti s et al., 2019) | 6 (6) | 10–30 | 36.4–174.0 | 41.5–260.0 | 13 | |||||
| (Sit et al., 2011) | 1 (1) | 50 | 41.4 | 23.0 | 14 | |||||
| (Spigset et al., 1997) | 2 (2) | 20–40 | 24.7–69.5 | 28.6–117.2 | 12 | |||||
| (Weisskopf et al., 2017) | 1 (1) | 20 | 65.0 | 123.0 | 149.0 | 8 | ||||
| (Weissman et al., 2004) | 2 (2) | 20 | 44.0–63.0 | 41.0–68.0 | <2.1 | 12 | ||||
| Clomipramine | (Loughhead et al., 2006b) | 7 (7) | 50–175 | 27.0–264.0 | 40.0–190.0 | 11 | ||||
| (Schimmell et al., 1991) | 1 (1) | 125–150 | 208.4–509.8 | 215.8–624.2 | 9.8–266.6 | 11 | ||||
| (Yoshida et al., 1997a) | 2 (2) | 75–125 | 89.9–198.0b | 32.0–212.0b | 32.0–432.0b | 11 | ||||
| 69.0–400.0c | 43.0–200.0c | 61.0–283.0c | ||||||||
| Desipramine | (Stancer & Reed, 1986) | 1 (1) | 300 | 257.0–271.0 | 316.0–328.0 | <1.0 | 9 | |||
| Desvenlafaxine | (Rampono et al., 2011) | 10 (10) | 50–150 | 142.0–733.0 | 275.0–1309.0 | 1.0–37.0 | 15 | |||
| Dothiepin | (Buist et al., 1993b) | 28 (28) | 75–225 | 15.0–118.0 | 12.0–140.0 | 11 | ||||
| (Ilett et al., 1992) | 8 (8) | 25–225 | 11.0–170.0 | 5.0–207.0 | 20.0–475.0 | 15 | ||||
| (Rees et al., 1976) | 1 (1) | 75–300 | 10.0–33.0 | 10.0–11.0 | 8 | |||||
| (Yoshida et al., 1997a) | 2 (2) | 50–225 | 27.0–712.0b | 54.0–988.0b | 121.0–1250.0b | 11 | ||||
| 180.0–2623.0c | 124.0–3730.0c | 309.0–4737.0c | ||||||||
| Doxepin | (Frey et al., 1999) | 1 (1) | 35 | 60.0 | 60.0–100.0 | <40.0 | 10 | |||
| (Matheson et al., 1985) | 1 (1) | 75 | 47.0–87.0 | 7.0–38.0 | 7 | |||||
| (Kemp et al., 1985) | 1 (1) | 150 | 136.4±30.0 | 136.6±27.1 | 206.2±51.2 | 10 | ||||
| Duloxetine | (Boyce et al., 2011) | 1 (1) | 60 | 151.0–245.0 | 18.0 | 50.8 | 2.0 | 10 | ||
| (Briggs et al., 2009) | 1 (1) | 60 | 24.0–53.0 | 65.0 | 31.0–64.0 | <1.0 | 10 | |||
| (Collin- Levesque et al., 2018) | 1 (1) | 60 | 39.7–60.6 | 16.1 | 23.6–25.2 | 14.3–29.3 | 11 | |||
| (Lobo et al., 2008) | 6 (6) | 80 | 15.6–63.2 | 3.9–18.3 | 15 | |||||
| Escitalopram | (Castberg & Spigset, 2006) | 1 (1) | 5–10 | 10.0–37.5 | 24.9–76.1 | 11 | ||||
| (Loughhead et al., 2006a) | 1 (1) | 5 | 17.0 | 3.0 | 13 | |||||
| (Rampono et al., 2006) | 8 (8) | 10–20 | 9.0–49.0 | 27.0–99.0 | 15 | |||||
| (Rampono et al., 2009) | 8 (8) | 10–30 | 4.5–64.0 | 4.4–54.0 | 12 | |||||
| (Sit et al., 2011) | 2 (2) | 10 | 5.6–13.5 | ≤6.7 | 14 | |||||
| (Weisskopf et al., 2017) | 1 (1) | 20 | 94.0 | 173.0 | 195.0 | 8 | ||||
| Fluoxetine | (Berle et al., 2004) | 1 (1) | 20 | 222.4e | 66.15 | 13.95 | 12 | |||
| (Burch & Wells, 1992) | 1 (1) | 20 | 265.0–284.0 | 119.0 | 30.0 | 7 | ||||
| (Hale et al., 2001) | 1 (1) | 40 | 875.0 | 238.0 | <182.0 | 5 | ||||
| (Heikkinen et al., 2003) | 11 (11) | 20–40 | 140.6–233.3 | 81.5–116.7 | 6.7–72.4 | 12 | ||||
| (Hendrick et al., 2003) | 15 (15) | 10–60 | 54.0–553.0 | 25.0–383.0 | 11 | |||||
| (Hendrick et al., 2001) | 9 (9) | 10–40 | 64.0–1180 | 49.0–457.0 | <265.0 | 11 | ||||
| (Isenberg, 1990) | 1 (1) | 20 | 295.0 | 70.4 | 6 | |||||
| (Kim et al., 2006) | 30 (30) | 10–30 | 226.3–569.0 | 239.6 | 317.0 | 265.5 | 12 | |||
| (Kristensen et al., 1999) | 14 (14) | 20–80 | 108.0–809.0 | 54.0–705.0 | 15 | |||||
| (Laine et al., 2003) | 6 (6) | 20–40 | 96.06–209.7e | 63.3–110.915 | 13 | |||||
| (Loughhead et al., 2006a) | 11 (11) | 20–80 | 200.0–872.0 | 4.0–80.0 | 13 | |||||
| (Rampono et al., 2004) | 4 (4) | 20–40 | 76.0–451.0 | 42.0–401.0 | 32.0–110.0 | 12 | ||||
| (Rampono et al., 2009) | 2 (2) | 20–40 | 178.0–486.0 | 136.0–367.0 | 12 | |||||
| (Weisskopf et al., 2017) | 1 (1) | 20 | 228.0 | 55.0 | 46.0 | 8 | ||||
| (Weissman et al., 2004) | 1 (1) | 20 | 309.0 | 689.0 | 13.0 | 12 | ||||
| (Yoshida et al., 1998) | 4 (4) | 20–40 | 138.0–427.0 | 39.0–131.0 | 57.0–177.0 | <20.0 | 11 | |||
| Fluvoxamine | (Arnold et al., 2000) | 1 (1) | 75 | 20.0 | 24.0–40.0 | 9.0 | 9 | |||
| (Hostetter et al., 2000) | 1 (1) | 100–150 | 7.0–41.0 | 4.0 | 5.0 | 11 | ||||
| (Kristensen et al., 2002) | 2 (2) | 50–150 | 29.7–190.8 | 35.9–256.3 | 13 | |||||
| (Rampono et al., 2009) | 1 (1) | 150 | 27.0 | 21.0 | 12 | |||||
| (Sit et al., 2011) | 1 (1) | 400 | 62.7 | 4.9 | 14 | |||||
| (Weisskopf et al., 2017) | 1 (1) | 50 | 30 | 23.0 | 27.0 | 8 | ||||
| (Wright et al., 1991) | 1 (1) | 200 | 310.0 | 90.0 | 10 | |||||
| (Yoshida et al., 1997b) | 1 (1) | 100 | 170.0 | 50.0 | 7 | |||||
| Imipramine | (Sovner & Orsulak, 1979) | 1 (1) | 200 | 62.0 | 21.0–59.0 | 10 | ||||
| (Yoshida et al., 1997a) | 4 (4) | 75–150 | 38.0–360.0b | 34.0–408.0b | 48.0–622.0b | 11 | ||||
| 29.0-340-0c | 40.0–509.0c | 110.0–610.0c | ||||||||
| Mianserin | (Buist et al., 1993a) | 2 (2) | 40–60 | 22.0–25.0 | 20.0–80.0 | <10.0 | 9 | |||
| Mirtazapine | (Aichhorn et al., 2004) | 1 (1) | 30 | 7.0–25.0 | 7.0–28.0 | 18.0–34.0 | 13 | |||
| (Kristensen et al., 2007) | 8 (8) | 30–120 | 47.0f | 53.0 | 14 | |||||
| (Tonn et al., 2009) | 1 (1) | 15 | 27.0 | 15.0 | 10.0 | 8 | ||||
| Nortriptyline | (Loughhead et al., 2006b) | 9 (9) | 30–150 | 2.8–71.3 | 4.7–66.4 | 11 | ||||
| (Matheson & Skjaeraasen, 1988) | 1 (1) | 75–125 | 104.0–298.0 | 90.0–404.0 | 10 | |||||
| (Sit et al., 2011) | 1 (1) | 75 | 22.6 | 10.6 | 14 | |||||
| (Sjoqvist et al., 1972) | 1 (1) | 75 | 1200.0 | 430.0 | 180.0 | 12 | ||||
| Paroxetine | (Begg et al., 1999) | 6 | 20–30 | 32.2–117.8 | 19.8–61.7 | <4.0 | 8 | |||
| 4 | 10–20 | 12.0–85.0 | 4.0–37.0 | 5.0–42.0 | <4.0 | |||||
| (Berle et al., 2004) | 6 (6) | 10–30 | 7.2–69.1 | 5.9–50.0 | <1.6 | 12 | ||||
| (Hendrick et al., 2003) | 8 (8) | 10–40 | 10.0–59.0 | 3.0–31.0 | 11 | |||||
| (Hendrick et al., 2000) | 1 (1) | 10 | 10.0 | <2.0 | <1.0 | 7 | ||||
| (Misri et al., 2000) | 23 (23) | 10–40 | 1.1–276.1 | 1.4–101.8 | <0.1 | 15 | ||||
| (Loughhead et al., 2006a) | 2 (2) | 20–40 | 14.0–39.0 | ≤3.0 | 13 | |||||
| (Oberlander et al., 2004) | 33 (33) | 10–40 | 4.4–33.3 | 1.5–10.9 | 10 | |||||
| (Ohman et al., 1999) | 7 (7) | 10–40 | 16.0–164.0 | 8.0–92.0 | 15 | |||||
| (Pogliani et al., 2017) | 2 (2) | 20–25 | 23.4–48.6 | 9.1–10.3 | 15 | |||||
| (Rampono et al., 2004) | 2 (3) | 20 | 10.0–13.0 | 5.0–7.0 | 12 | |||||
| (Rampono et al., 2009) | 1 (1) | 30 | 13.0 | 2.0 | 15 | |||||
| (Spigset et al., 1996) | 1 (1) | 20–40 | 163.0 | 7.6 | 8 | |||||
| (Weisskopf et al., 2017) | 1 (1) | 20 | 27.0 | 16.0 | 24.0 | 8 | ||||
| (Weissman et al., 2004) | 3 (3) | 10–30 | 17.0–77.0 | 10.0–54.0 | <1.5 | 12 | ||||
| Reboxetine | (Hackett et al., 2006) | 4 (4) | 4–10 | 115.0–321.0 | 6.7–16.3 | 2.3–5.0 | 14 | |||
| Sertraline | (Altshuler et al., 1995) | 1 (1) | 100 | 48.0 | 26.6 | <0.5 | 7 | |||
| (Berle et al., 2004) | 6 (6) | 50–100 | 14.1–39.4 | 29.7–70.3 | <1.5 | 12 | ||||
| (Hendrick et al., 2003) | 11 (11) | 100–150 | 3.0–50.0 | 1.0–14.0 | 11 | |||||
| (Hostetter et al., 2000) | 1 (1) | 150–175 | 45.0–53.0 | <2.0 | 21.0 | 11 | ||||
| (Kristensen et al., 1998) | 8 (8) | 50–200 | 15.7–92.0 | 27.9–193.3 | <5.0 | 15 | ||||
| (Loughhead et al., 2006a) | 5 (5) | 50–250 | 52.0–248.0 | ≤25.0 | 13 | |||||
| (Paulzen et al., 2017a) | 6 (6) | 25–100 | 9.7–30.1 | 4.0–14.0 | 3.1–9.3 | 13 | ||||
| (Pogliani et al., 2017) | 10 (10) | 25–100 | 7.4–89.0 | <5.0–35.5 | 15 | |||||
| (Rampono et al., 2004) | 4 (4) | 50 | 9.0–64.0 | 5.0–24.0 | 12 | |||||
| (Rampono et al., 2009) | 5 (5) | 25–100 | 4.0–53.0 | 1.0–22.0 | 15 | |||||
| (Schoretsaniti s et al., 2019) | 6 (6) | 25–100 | 3.3–17.8 | 3.6–35.7 | 13 | |||||
| (Sit et al., 2011) | 9 (9) | 50–200 | 5.8–32.4 | 2.7–11.0 | 14 | |||||
| (Stowe et al., 1997) | 26 (26) | 25–250 | 12.0–206.0 | 8.4–290.0 | ≤87.0 | 11 | ||||
| (Stowe et al., 2003) | 12 (12) | 25–200 | 8.0–92.0 | 17.0–173.0 | ≤3.0 | 11 | ||||
| (Weisskopf et al., 2017) | 1 (1) | 50 | 29.0 | 29.0 | 46.0 | 8 | ||||
| (Weissman et al., 2004) | 20 (20) | 50–100 | 10.0–42.0 | 12.0–90.0 | <1.5 | 12 | ||||
| Venlafaxine | (Paulzen et al., 2020) | 9 (9) | 37.5–225 | 33.9–338.0 | 309.5–1052.5 | 21.1–448.4 | 15 | |||
| (Berle et al., 2004) | 3 (3) | 75–225 | 110.0–387.0e | 273.5–865.7e | 8.4–34.6e | 12 | ||||
| (Boucher et al., 2009) | 7 (7) | 37.5–300 | 7.8–26.1 | 1.4–638.0 | 5.8–163.7 | 12 | ||||
| (Hostetter et al., 2000) | 1 (2) | 200–300 | 432.0 | 329.0 | 879.0–887.0 | 11 | ||||
| (Ilett et al., 1998) | 1 (1) | 150 | 231.0 | 940.0 | 12 | |||||
| (Ilett et al., 2002) | 6 (6) | 225–300 | 264.9–600.7 | 856.0–1464.0 | 15 | |||||
| (Loughhead et al., 2006a) | 3 (3) | 150–225 | 169.0–452.0 | 146.0–1792.0 | 13 | |||||
| (Newport et al., 2009) | 11 (11) | 112.5–300 | 292.0–1210.0 | 739.2–2231.3 | <4.0–248.0 | 12 | ||||
| (Pogliani et al., 2017) | 1 (1) | 150 | 199.8 | 78.9 | 15 | |||||
| (Rampono et al., 2004) | 1 (1) | 150 | 612.0 | 638.0 | 12 | |||||
| (Rampono et al., 2009) | 10 (10) | 75–450 | 64.0–2283.0 | 64.0–1694.0 | 15 | |||||
| (Schoretsaniti s et al., 2019) | 5 (5) | 37.5–150 | 94.0–462.0 | 79.5–1086.0 | 13 | |||||
| (Sit et al., 2011) | 2 (2) | 75 | 156.4–216.3 | 124.6–151.4 | 14 | |||||
N: number of patients; n: number of infants. For amitriptyline, clomipramine, doxepin, fluoxetine, imipramine and venlafaxine provided levels refer to active moiety, i.e. the sum of parent compound and active metabolite. The quality score column represents the quality scores assigned to the study using the ClinPK checklist (for details, see Supplementary table 2).
One value is provided, when authors did not specify fore-milk or hind-milk.
Measured by gas chromatography.
Measured by enzyme immunoassay.
Values were converted from nmol/L using a conversion factor (CF) of 3.3, fairly close to 3.23 (CF for fluoxetine) and 3.39 (CF for N-desmethyl-fluoxetine)
Values were converted from nmol/L using a conversion factor (CF) of 3.7, fairly close to 3.61 (CF for venlafaxine) and 3.80 (CF for O-desmethyl-venlafaxine)
For 7 patients
3.2. Excretion into amniotic fluid
For a total of 52 patients, data were available allowing the calculation of penetration ratios into amniotic fluid for the following antidepressants: bupropion, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. The highest penetration ratios were reported for venlafaxine (i.e. the active moiety venlafaxine plus O-desmethylvenlafaxine) (mean 2.77, range 0.42–3.96), citalopram (mean 2.03, range 0.35–6.97) and bupropion (mean 0.78, range 0.37–1.66), while fluvoxamine (mean 0.1, n=1, no range), fluoxetine (i.e. the active moiety fluoxetine plus norfluoxetine) (mean 0.11, range 0.02–0.2) and paroxetine (mean 0.13, range 0.05–0.21) had the lowest ratios (Table 2 and Figure 2). Amniotic fluid samples were collected at birth in four trials (Fokina et al., 2016; Paulzen et al., 2017a, Paulzen et al., 2017b; Paulzen et al., 2020) and at amniocentesis in the second or third trimester in two trials (Hostetter et al., 2000; Loughhead et al., 2006a).
Table 2.
Penetration ratios (combined means and ranges) of antidepressants into amniotic fluid, umbilical cord and breast milk.
k: number of studies; n: number of patients. Studies for moclobemide and trazodone included single-dose data and do not reflect steady-state conditions. For amitriptyline, clomipramine, doxepin, fluoxetine, imipramine and venlafaxine provided levels refer to the active moiety, i.e. the sum of the parent compound and the active metabolite.
When two numbers are given, the first represents the plasma protein binding for the parent substance, while the second represents the plasma protein binding for the active metabolite
Figure 2.
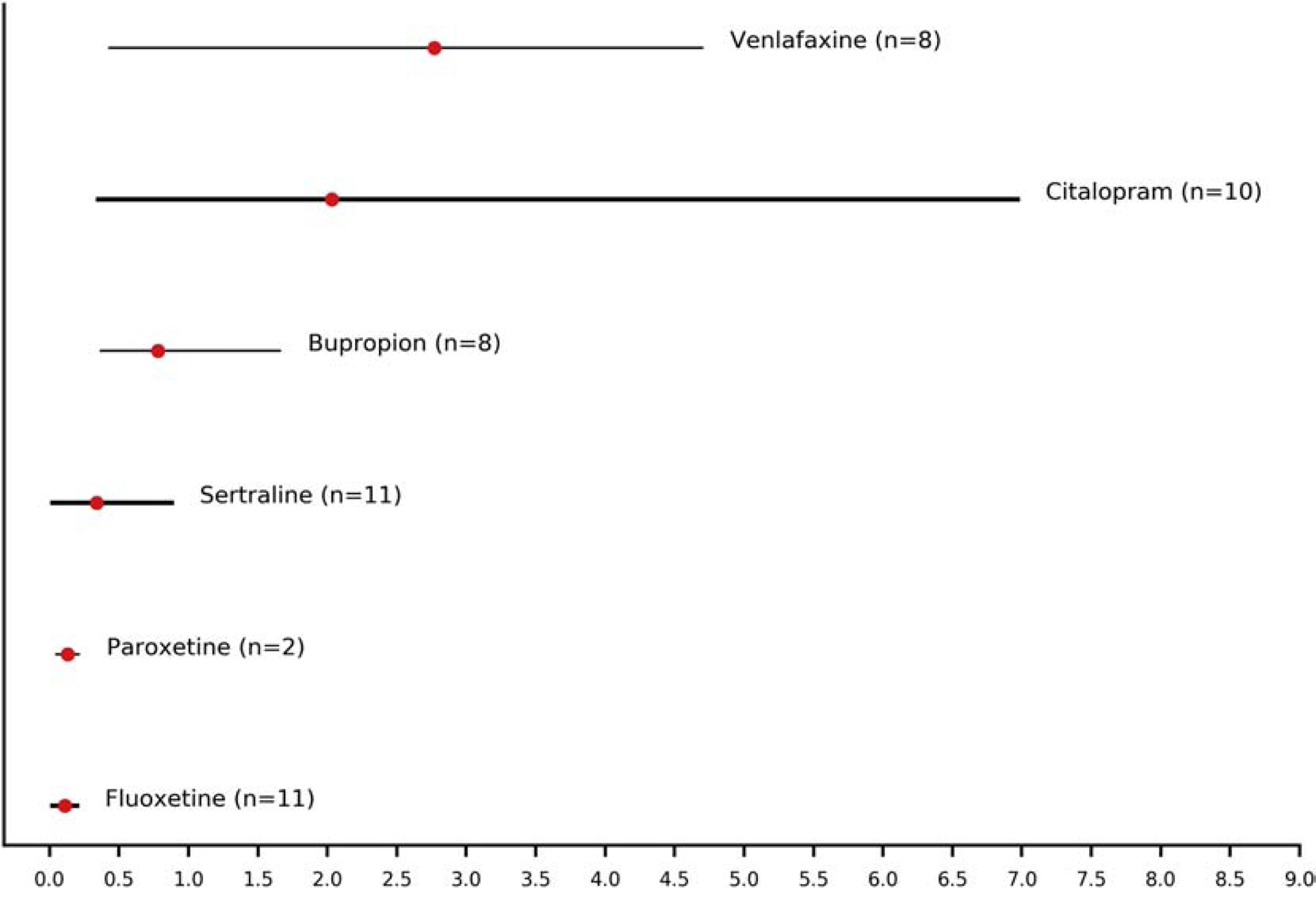
Penetration ratios (combined means and ranges) of antidepressants into amniotic fluid. The width of lines is proportional to the number of patients providing data for the estimation of the penetration ratios (n: number of patients).
3.3. Excretion in umbilical cord plasma
Data from a total of 227 patients allowed the calculation of penetration ratios into umbilical cord plasma for bupropion, citalopram, clomipramine, duloxetine, escitalopram, fluoxetine, fluvoxamine, nortriptyline, paroxetine, sertraline and venlafaxine (Table 2, Figure 3). The highest penetration ratios were reported for nortriptyline (mean 2.97, range 0.25–26.43), followed by bupropion (mean 1.14, range 0.3–5.08) and duloxetine (mean 1.08, range 0.12–2.7). The lowest ratios were observed for paroxetine (mean 0.40, range 0.15–0.91), sertraline (mean 0.44, range 0.14–1.2) and fluvoxamine (mean 0.52, range 0.08–0.78) (Figure 3).
Figure 3.
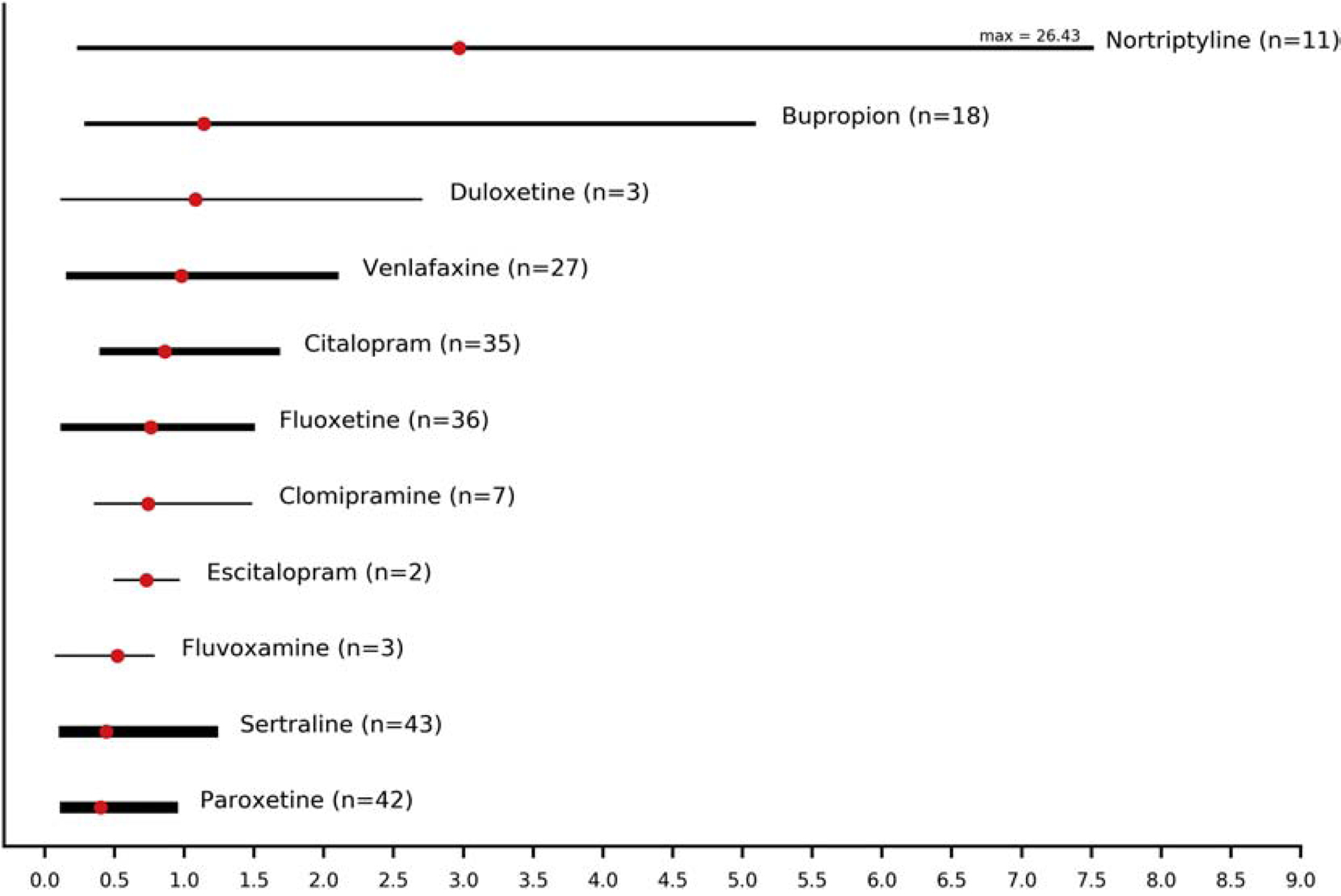
Penetration ratios (combined means and ranges) of antidepressants into umbilical cord. The width of lines is proportional to the number of patients providing data for the estimation of the penetration ratios (max: maximum; n: number of patients).
3.4. Excretion into breast milk
For a total of 373 patients, penetration ratios into breast milk could be calculated for amitriptyline, amoxapine, bupropion, citalopram, clomipramine, desipramine, desvenlafaxine, dothiepin, doxepine, duloxetine, escitalopram, fluoxetine, fluvoxamine, imipramine, moclobemide, mianserin, mirtazapine, nortriptyline, paroxetine, reboxetine, sertraline and venlafaxine. The penetration ratio in this context is identical to the milk/plasma (M/P) ratio, which is often reported in studies on excretion of drugs in breast milk (Spigset and Hagg, 2000). The highest ratios were observed for venlafaxine (mean 2.59, range 0.85–4.85) mianserin (mean 2.22, range 0.8–3.64) and escitalopram (mean 2.19, range 1.68–3.0) (Figure 4). Reboxetine (mean 0.06, range 0.05–0.08), trazodone (mean 0.14±0.04) and amoxapine (<0.21) had the lowest ratios. Data for trazodone derived from a single-dose study and therefore do not reflect steady state conditions (Verbeeck et al., 1986).
Figure 4.
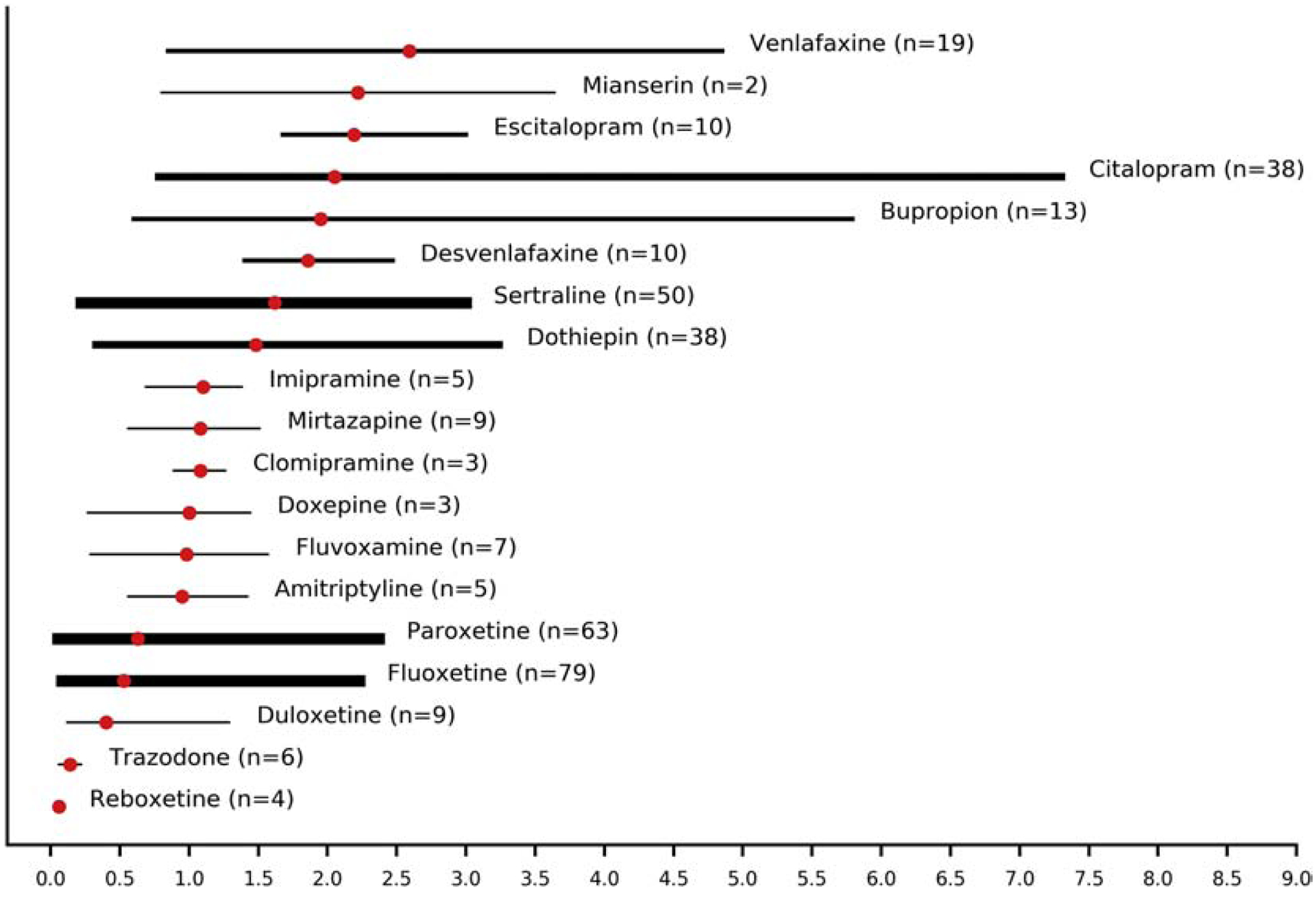
Penetration ratios (combined means and ranges) of antidepressants into breast milk. The width of lines is proportional to the number of patients providing data for the estimation of the penetration ratios (n: number of patients).
Evidence for differences of antidepressant concentrations between fore-milk and hind-milk were very scarce (n=30 from 9 studies, Supplementary table 1). For all antidepressants, data derived from single studies except for dothiepin (n=10 from 2 studies), fluoxetine (n=5 from 3 studies) and paroxetine (n=5 from two studies); the combined penetration ratios of dothiepin and paroxetine were twofold higher in hind-milk than in fore-milk (0.96 vs 1.9 and 0.67 vs 1.16 respectively), while the differences for fluoxetine were marginal (0.31 vs. 0.35).
3.5. Quality assessment
The quality of the studies was acceptable with an average rating score of 11.2 (approximately 70% of the maximum possible score). Details are provided in supplementary table 1. Some variation was due to the lower quality in case reports, where authors provided fewer details than as assessed by the ClinPK checklist.
4. Discussion
This systematic review aimed to capture patterns of antidepressant transfer into fetal and newborn circulation via amniotic fluid, umbilical cord blood and breast milk. Previous systematic reviews and pooled analyses of TDM data have provided valuable insights into the exposure to antidepressants, but mainly via breast milk (Berle and Spigset, 2011; Malone et al., 2004; Pons et al., 1994; Weissman et al., 2004; Wisner et al., 1996; Yoshida et al., 1999). Our updated review additionally included TDM data from amniotic fluid and umbilical cord blood to provide an even more comprehensive overview of the ability of antidepressants to enter fetal and newborn circulation. Evidence was more robustly available for breast milk displaying highest penetration ratios for venlafaxine and mianserin, while umbilical cord blood and, particularly, amniotic fluid data were scarce with highest ratios observed for venlafaxine and citalopram, and nortriptyline and bupropion respectively.
Despite the limited data for amniotic fluid, penetration ratios of about 2–3 were calculated for the venlafaxine active moiety and citalopram. For the venlafaxine active moiety, penetration ratio values into amniotic fluid were driven by the penetration ratios of the active metabolite, which were higher than those of the parent compound. The small number of studies (≤2) and included patients per antidepressant did not allow for analyses to unravel predictors of high (or low) penetration ratios. However, the estimated penetration ratios imply that fetal development takes place in more than twofold higher concentrations than what are found in maternal plasma. The mechanisms underlying those ratio patterns may encompass several factors. We have previously, when reviewing antipsychotic agents, suggested that differences in the affinities e.g. to the efflux pump P-glycoprotein (P-gp) could explain differences in penetration ratios between drugs (Schoretsanitis et al., 2020b). Further, differences in plasma protein binding may also contribute; venlafaxine has a low protein binding compared to all other antidepressants (Cohen and De Vane, 1996). A low plasma protein binding allows a larger part of the measured (total) concentration in plasma to be excreted into amniotic fluid, as only the unbound drug concentration is able to cross cell membranes (Wang et al., 2008). Protein binding differences may also account for the relatively high penetration ratios of citalopram and venlafaxine into umbilical cord blood, followed by nortriptyline, bupropion and duloxetine, all of which having penetration ratios exceeding 1.0. Antidepressants and other psychotropic drugs are primarily bound to alpha-1 acid glycoprotein in plasma, where the concentration in newborns is only about half of that in adults, and even lower in premature infants (Anell-Olofsson et al., 2018). Consequently, when comparing penetrations ratios in umbilical cord plasma, the differences in ratios between drugs do not necessarily represent the ratios found if the free concentrations had been measured. The strikingly high penetration ratio of nortriptyline is most probably driven by one patient, who showed an unusually high penetration ratio (Loughhead et al., 2006b). After removing this patient from the analysis, a combined penetration ratio of 0.62 for the remaining 10 patients was calculated.
An additional mechanism accounting for our findings might include differences in molecular weight, as the molecular weight is important for the diffusion properties through the placenta. Although molecular weight cannot fully explain the ranking suggested by our findings, agents with lower molecular weight, such as venlafaxine, had higher penetration ratios than compounds with higher molecular weight such as paroxetine and fluoxetine (Baumann et al., 2004). However, as molecular weight differences between antidepressants are nevertheless relatively small, its contribution to differences in penetration ratios is likely minor.
Penetration ratios into both amniotic fluid and umbilical cord plasma were invariably higher for antidepressants than other psychotropic agents such as antipsychotics (Schoretsanitis et al., 2020b). However, it is not possible to recommend (or discourage) use of specific antidepressant drugs instead of others during pregnancy based on penetration ratios alone. As an example, paroxetine, which had low penetration ratios for both amniotic fluid and umbilical cord plasma, has been associated with an increased absolute risk of first trimester teratogenicity (Gao et al., 2018).
Evidence is more robust for antidepressant transfer into breast milk, with available data allowing the calculation of penetration ratios for a large group of antidepressants. Differences between antidepressants can be understood in the light of the high oral bioavailability for some of these agents, e.g. citalopram and venlafaxine compared to other antidepressants (Bezchlibnyk-Butler et al., 2000; Troy et al., 1997). The role of protein binding also has to be considered (Weissman et al., 2004), but cannot always account for excretion patterns (Schoretsanitis et al., 2019). Additionally, milk-specific characteristics, such as its lipid content, need to be taken into consideration, given that the majority of antidepressants are lipophilic substances (Kam and Chang, 1997). Unfortunately, most studies including milk samples did not specify for fore-milk or hind-milk and thus evidence on excretion related to the lipid content was available only for a small number of patients (Supplementary table 1). Despite the scarcity of the data, penetration ratios into hind-milk were overall higher than into fore-milk for all antidepressants except for duloxetine, where minimal differences was detected in a single patient (Collin-Levesque et al., 2018). In order to minimize infant exposure to high concentrations some authors have recommended that breastfeeding women receiving antidepressants should discard hind-milk (Academy of Breastfeeding Medicine Protocol, 2008); nevertheless, given the important nutrient composition of hind-milk for the infant, further evidence is required till this strategy can be embraced. On the other hand, although at steady state the elimination half-life may not affect the penetration ratios to a large extent, for antidepressants with shorter half-lives, causing a higher variation in the concentration during a dose interval, it might be easier to find a time point for lactation (i.e. shortly before the next dose) leading to transfer of lower doses to the infant compared to antidepressants with longer half-lives. Nevertheless, we acknowledge that in real-life clinical scenarios it may be not practical to schedule breastfeeding.
During lactation, is also important to consider the half-life of the drug in the infant, as the elimination of drugs is generally slower and the risk of adverse effects consequently higher in premature and newborn infants than in older infants and adults, even though the penetration ratio (or the absolute concentration in milk) will be constant. Almost no adverse reactions have been reported in suckling infants older than three months of age, irrespective of which drug the mother has been taking and what is the penetration ratio or the milk concentration.
Pharmacogenetic variability in the maternal metabolism of antidepressants could also contribute to higher drug concentrations in amniotic fluid, umbilical cord blood and breast milk. Maternal extreme metabolizer types have led to higher exposure of drugs to the infant in the past with the consequences of severe adverse effects (Koren et al., 2006; Laine et al., 2004). The use of TDM can assist clinicians to detect unusually high antidepressant exposure, suspect poor metabolizer phenotypes and characterize high-risk dyads. Consequently, TDM-based strategies could target reduced infant exposure based on the assumption that antidepressant drug concentrations in maternal blood provide a proxy of breast milk excretion.
4.1. Package inserts on antidepressant transfer into amniotic fluid, umbilical cord blood & breast milk
For the vast majority of antidepressants there is no information regarding transfer into human amniotic fluid, umbilical cord or breast milk in their package inserts. Instead, provided information mainly derives from animal models that may be challenging to extrapolate to humans. For two antidepressants, amitriptyline and fluoxetine, package inserts discuss pharmacokinetic data in nursing women (Sandoz Inc, 2014; Eli Lilly, 1987); in a nursing woman receiving 100 mg/day of amitriptyline, amitriptyline levels ranging between 83.0–141.0 ng/mL and 135.0–151.0 ng/mL were reported in maternal serum and milk respectively (Sandoz Inc, 2014). These values are within the range described in available literature (Bader and Newman, 1980; Brixen-Rasmussen et al., 1982; Pittard and O’Neal, 1986; Yoshida et al., 1997a). For fluoxetine, the package insert reports one case of a fluoxetine-treated nursing patient with active moiety (fluoxetine plus norfluoxetine) levels of 70.4 and 295.0 ng/mL in maternal milk and maternal plasma, respectively (Eli Lilly, 1987); these values allow the estimation of a penetration ratio of 0.24, which is within the range of our estimated ratios (0.08–2.23) based on available literature (Berle et al., 2004; Burch and Wells, 1992; Hale et al., 2001; Heikkinen et al., 2003; Hendrick et al., 2001; Isenberg, 1990; Kim et al., 2006; Kristensen et al., 1999; Weisskopf et al., 2017; Weissman et al., 2004; Yoshida et al., 1998).
Furthermore, for several compounds estimates of infant exposure in the package inserts have been given based on milk samples; however, as raw levels of antidepressants in blood and milk are not provided it is not possible to discuss these estimates in light of our review. Specifically, in the package insert for sertraline infant exposure was estimated around 2% of maternal sertraline serum levels in a cohort of 53 mother-infant pairs (Pfizer, 2019). For bupropion an infant daily exposure around 2% of the maternal weight-adjusted dose was estimated based on milk samples (GlaxoSmithKline, 2019). Finally, for duloxetine an infant daily exposure of 0.14% of the maternal dose was estimated in the package insert (Eli Lilly, 2010).
For the rest of antidepressants no specific information on the ability of these medications to enter fetal/newborn circulation is provided in the package inserts.
4.2. Limitations and clinical implications
Our findings should be interpreted with caution, as they mainly reflect the pharmacokinetic profiles of the investigated antidepressants. Despite several years of experience, TDM findings have not informed clinical practice for the most part. In fact, the intuitive notion of concentration-dependent toxicity risk requires large-scale clinical data that is not available yet and penetration ratios cannot be as used safety surrogates. More specific indices, such as relative infant dose for the degree of drug exposure through breast milk may relate more directly to potential pharmacological adverse effects. However, for patients with unusually high levels of antidepressants or high penetration ratios in maternal blood or milk outside of the ranges described here, clinicians may monitor closely including TDM use to early detect potential risks for the mother-infant pair. Another limitation of the current literature derives from the fact that studies did not exclude patients receiving co-medications with enzyme-inhibiting or -inducing properties with direct effects on the pharmacokinetics of the assessed antidepressants (Boucher et al., 2009; Castberg and Spigset, 2006). The effects of drug-drug interactions on antidepressant transfer can be only hypothesized, as they remain poorly understood. Future research will also need to address potential moderators of antidepressant transfer, such as age, body composition measures and race, which were frequently overseen. Lastly, some variation in pharmacokinetic parameters may derive from differences in the analytical methods; high performance liquid chromatography was probably the most common method in former times, being gradually replaced by liquid chromatography with tandem mass spectrometry nowadays. Within this realm, very few studies specified whether the umbilical cord concentrations were from venous, arterial or mixed arteriovenous samples.
The majority of the available data reflected single time point measures; future studies may adopt AUC estimates for blood and milk to increase precision. Except for two single-dose studies (Pons et al., 1990, Verbeeck et al., 1986), studies mainly included patients at steady state with assessed levels of antidepressants being trough. However, several studies did not provide sufficient details, so that it cannot be excluded that some levels of antidepressants were neither trough nor acquired at steadystate.
Future studies investigating antidepressant concentrations across maternal and infant matrices are required to improve the understanding of excretion mechanisms as an underlying factor for the variations in penetration ratios between antidepressants: Considering the limitations of the included studies, the final question regarding the clinical utility of TDM in pregnancy and lactation remains unanswered. We hope that our systematic review will stimulate the collection of larger-scale data to provide better grounds for the understanding of antidepressant transfer into amniotic fluid, umbilical cord blood and breast milk.
Supplementary Material
Highlights.
Knowledge of antidepressant transfer into different matrices by measuring concentrations in pregnant/lactating patients is sparse.
Penetration ratios into amniotic fluid and umbilical cord plasma differ considerably, between antidepressants and matrices.
Penetration ratios into breast milk varied with venlafaxine, mianserin and escitalopram having the highest ratios.
Acknowledgements
Authors are extremely grateful to Konstantinos Ntogkas, Department of Electrical & Computer Engineering, University of Patras, Greece for producing the included plots. Authors are also deeply indebted to Dr. Kenneth Ilett, Pharmacology, Pharmacy and Anaesthesiology Unit, School of Medicine and Pharmacology, University of Western Australia, Crawley, Australia, and the Clinical Pharmacology and Toxicology Laboratory, Path West Laboratory Medicine, Nedlands, Australia and Dr. Dorota A. Doherty, Division of Obstetrics and Gynaecology, The University of Western Australia, Nedlands, Perth, Western Australia, Australia for sharing valuable information on their studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Georgios Schoretsanitis, The Zucker Hillside Hospital, Psychiatry Research, Northwell Health, Glen Oaks, New York, USA.
Andreas A. Westin, Department of Clinical Pharmacology, St Olav University Hospital, Trondheim, Norway.
Julia C. Stingl, Institute of Clinical Pharmacology, University Hospital of RWTH Aachen, Aachen, Germany.
Kristina M. Deligiannidis, The Zucker Hillside Hospital, Psychiatry Research, Northwell Health, Glen Oaks, New York, USA; Zucker School of Medicine, Hempstead, New York and The Feinstein Institutes for Medical Research, Manhasset, New York, USA.
Michael Paulzen, Alexianer Hospital Aachen, Aachen, Germany and Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany; JARA - Translational Brain Medicine, Aachen, Germany.
Olav Spigset, Department of Clinical Pharmacology, St Olav University Hospital, Trondheim, Norway; Department of Clinical and Molecular Medicine, Norwegian University of Science and Technology, Trondheim, Norway.
References
- Academy of Breastfeeding Medicine Protocol, C., 2008. ABM clinical protocol #18: use of antidepressants in nursing mothers. Breastfeed. Med 3(1), 44–52. [DOI] [PubMed] [Google Scholar]
- Aichhorn W, Whitworth AB, Weiss U, Stuppaeck C, 2004. Mirtazapine and breast-feeding. Am. J. Psychiatry 161(12), 2325. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Burt VK, McMullen M, Hendrick V, 1995. Breastfeeding and sertraline: a 24-hour analysis. J. Clin. Psychiatry 56(6), 243–245. [PubMed] [Google Scholar]
- Anell-Olofsson M, Ahmadi S, Lonnqvist PA, Eksborg S, von Horn H, Bartocci M, 2018. Plasma concentrations of alpha-1-acid glycoprotein in preterm and term newborns: influence of mode of delivery and implications for plasma protein binding of local anaesthetics. Br. J. Anaesth 121(2), 427–431. [DOI] [PubMed] [Google Scholar]
- Arnold LM, Suckow RF, Lichtenstein PK, 2000. Fluvoxamine concentrations in breast milk and in maternal and infant sera. J. Clin. Psychopharmacol 20(4), 491–493. [DOI] [PubMed] [Google Scholar]
- Bader TF, Newman K, 1980. Amitriptyline in human breast milk and the nursing infant’s serum. Am. J. Psychiatry 137(7), 855–856. [DOI] [PubMed] [Google Scholar]
- Baumann P, Hiemke C, Ulrich S, Eckermann G, Gaertner I, Gerlach M, Kuss HJ, Laux G, Muller-Oerlinghausen B, Rao ML, Riederer P, Zernig G, Arbeitsgemeinschaft fur neuropsychopharmakologie und, p., 2004. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry 37(6), 243–265. [DOI] [PubMed] [Google Scholar]
- Begg EJ, Duffull SB, Saunders DA, Buttimore RC, Ilett KF, Hackett LP, Yapp P, Wilson DA, 1999. Paroxetine in human milk. Br. J. Clin. Pharmacol 48(2), 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berle JO, Spigset O, 2011. Antidepressant Use During Breastfeeding. Curr Womens Health Rev 7(1), 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berle JO, Steen VM, Aamo TO, Breilid H, Zahlsen K, Spigset O, 2004. Breastfeeding during maternal antidepressant treatment with serotonin reuptake inhibitors: infant exposure, clinical symptoms, and cytochrome p450 genotypes. J. Clin. Psychiatry 65(9), 1228–1234. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk-Butler K, Aleksic I, Kennedy SH, 2000. Citalopram--a review of pharmacological and clinical effects. J. Psychiatry. Neurosci 25(3), 241–254. [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Cantarutti A, Rea F, Locatelli A, Zanini R, Corrao G, 2020. Use of antidepressants during pregnancy and neonatal outcomes: An umbrella review of meta-analyses of observational studies. J Psychiatr. Res 124, 99–108. [DOI] [PubMed] [Google Scholar]
- Boucher N, Koren G, Beaulac-Baillargeon L, 2009. Maternal use of venlafaxine near term: correlation between neonatal effects and plasma concentrations. Ther. Drug. Monit 31(3), 404–409. [DOI] [PubMed] [Google Scholar]
- Boyce PM, Hackett LP, Ilett KF, 2011. Duloxetine transfer across the placenta during pregnancy and into milk during lactation. Arch. Womens. Ment. Health 14(2), 169–172. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Ambrose PJ, Ilett KF, Hackett LP, Nageotte MP, Padilla G, 2009. Use of duloxetine in pregnancy and lactation. Ann. Pharmacother 43(11), 1898–1902. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Samson JH, Ambrose PJ, Schroeder DH, 1993. Excretion of bupropion in breast milk. Ann. Pharmacother 27(4), 431–433. [DOI] [PubMed] [Google Scholar]
- Brixen-Rasmussen L, Halgrener J, Jorgensen A, 1982. Amitriptyline and nortriptyline excretion in human breast milk. Psychopharmacology (Berl) 76(1), 94–95. [DOI] [PubMed] [Google Scholar]
- Buist A, Norman TR, Dennerstein L, 1993a. Mianserin in breast milk. Br. J. Clin. Pharmacol 36(2), 133–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist A, Norman TR, Dennerstein L, 1993b. Plasma and breast milk concentrations of dothiepin and northiaden in lactating women. Hum. Psychopharmacol 8, 29–33. [Google Scholar]
- Burch KJ, Wells BG, 1992. Fluoxetine/norfluoxetine concentrations in human milk. Pediatrics 89(4 Pt 1), 676–677. [PubMed] [Google Scholar]
- Castberg I, Spigset O, 2006. Excretion of escitalopram in breast milk. J. Clin. Psychopharmacol 26(5), 536–538. [DOI] [PubMed] [Google Scholar]
- Cohen LJ, De Vane CL, 1996. Clinical implications of antidepressant pharmacokinetics and pharmacogenetics. Ann. Pharmacother 30(12), 1471–1480. [DOI] [PubMed] [Google Scholar]
- Collin-Levesque L, El-Ghaddaf Y, Genest M, Jutras M, Leclair G, Weisskopf E, Panchaud A, Ferreira E, 2018. Infant Exposure to Methylphenidate and Duloxetine During Lactation. Breastfeed. Med 13(3), 221–225. [DOI] [PubMed] [Google Scholar]
- Davis MF, Miller HS, Nolan PE Jr., 2009. Bupropion levels in breast milk for 4 mother-infant pairs: more answers to lingering questions. J. Clin. Psychiatry 70(2), 297–298. [DOI] [PubMed] [Google Scholar]
- Deligiannidis KM, Byatt N, Freeman MP, 2014. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J. Clin. Psychopharmacol 34(2), 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly Eli. CYMBALTA® (duloxetine hydrochloride) Delayed-Release Capsules for Oral Use, Indianapolis, IN 2010Eli Lilly. PROZAC® fluoxetine hydrochloride Delayed-Release Capsules for Oral Use, Indianapolis, IN: 1987 [Google Scholar]
- Ewing G, Tatarchuk Y, Appleby D, Schwartz N, Kim D, 2015. Placental transfer of antidepressant medications: implications for postnatal adaptation syndrome. Clin. Pharmacokinet 54(4), 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokina VM, West H, Oncken C, Clark SM, Ahmed MS, Hankins GD, Nanovskaya TN, 2016. Bupropion therapy during pregnancy: the drug and its major metabolites in umbilical cord plasma and amniotic fluid. Am. J. Obstet. Gynecol 215(4), 497 e491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen EJ, Meijs V, Ettaher F, Valerio PG, Keessen M, Lameijer W, 2006. Citalopram serum and milk levels in mother and infant during lactation. Ther. Drug. Monit 28(1), 2–4. [DOI] [PubMed] [Google Scholar]
- Frey OR, Scheidt P, von Brenndorff AI, 1999. Adverse effects in a newborn infant breast-fed by a mother treated with doxepin. Ann. Pharmacother 33(6), 690–693. [DOI] [PubMed] [Google Scholar]
- Gao SY, Wu QJ, Sun C, Zhang TN, Shen ZQ, Liu CX, Gong TT, Xu X, Ji C, Huang DH, Chang Q, Zhao YH, 2018. Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: a systematic review and meta-analysis of cohort studies of more than 9 million births. BMC. Med 16(1), 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg AJ, 1979. Single case study. Amoxapine, a new antidepressant, appears in human milk. J. Nerv. Ment. Dis 167(10), 635–636. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline. WELLBUTRIN SR (bupropion hydrochloride) sustained-release tablets, for oral use Research Triangle Park, NC 2019
- Haas JS, Kaplan CP, Barenboim D, Jacob P 3rd, Benowitz NL, 2004. Bupropion in breast milk: an exposure assessment for potential treatment to prevent post-partum tobacco use. Tob. Control 13(1), 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett LP, Ilett KF, Rampono J, Kristensen JH, Kohan R, 2006. Transfer of reboxetine into breastmilk, its plasma concentrations and lack of adverse effects in the breastfed infant. Eur. J. Clin. Pharmacol 62(8), 633–638. [DOI] [PubMed] [Google Scholar]
- Hagg S, Granberg K, Carleborg L, 2000. Excretion of fluvoxamine into breast milk. Br. J. Clin. Pharmacol 49(3), 286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TW, Shum S, Grossberg M, 2001. Fluoxetine toxicity in a breastfed infant. Clin. Pediatr (Phila) 40(12), 681–684. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Ekblad U, Kero P, Ekblad S, Laine K, 2002. Citalopram in pregnancy and lactation. Clin. Pharmacol. Ther 72(2), 184–191. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Ekblad U, Palo P, Laine K, 2003. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin. Pharmacol. Ther 73(4), 330–337. [DOI] [PubMed] [Google Scholar]
- Hendrick V, Stowe ZN, Altshuler LL, Hostetter A, Fukuchi A, 2000. Paroxetine use during breast-feeding. J. Clin. Psychopharmacol 20(5), 587–589. [DOI] [PubMed] [Google Scholar]
- Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D, 2003. Placental passage of antidepressant medications. Am. J. Psychiatry 160(5), 993–996. [DOI] [PubMed] [Google Scholar]
- Hendrick V, Stowe ZN, Altshuler LL, Mintz J, Hwang S, Hostetter A, Suri R, Leight K, Fukuchi A, 2001. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol. Psychiatry 50(10), 775–782. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, Eckermann G, Egberts K, Gerlach M, Greiner C, Grunder G, Haen E, Havemann-Reinecke U, Hefner G, Helmer R, Janssen G, Jaquenoud E, Laux G, Messer T, Mossner R, Muller MJ, Paulzen M, Pfuhlmann B, Riederer P, Saria A, Schoppek B, Schoretsanitis G, Schwarz M, Gracia MS, Stegmann B, Steimer W, Stingl JC, Uhr M, Ulrich S, Unterecker S, Waschgler R, Zernig G, Zurek G, Baumann P, 2018. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51(1–02), 9–62. [DOI] [PubMed] [Google Scholar]
- Hostetter A, Ritchie JC, Stowe ZN, 2000. Amniotic fluid and umbilical cord blood concentrations of antidepressants in three women. Biol. Psychiatry 48(10), 1032–1034. [DOI] [PubMed] [Google Scholar]
- Hutson JR, Garcia-Bournissen F, Davis A, Koren G, 2011. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin. Pharmacol. Ther 90(1), 67–76. [DOI] [PubMed] [Google Scholar]
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala-Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D, 2015. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern Med 162(11), 777–784. [DOI] [PubMed] [Google Scholar]
- Ilett KF, Hackett LP, Dusci LJ, Roberts MJ, Kristensen JH, Paech M, Groves A, Yapp P, 1998. Distribution and excretion of venlafaxine and O-desmethylvenlafaxine in human milk. Br. J. Clin. Pharmacol 45(5), 459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilett KF, Kristensen JH, Hackett LP, Paech M, Kohan R, Rampono J, 2002. Distribution of venlafaxine and its O-desmethyl metabolite in human milk and their effects in breastfed infants. Br. J. Clin. Pharmacol 53(1), 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilett KF, Lebedevs TH, Wojnar-Horton RE, Yapp P, Roberts MJ, Dusci LJ, Hackett LP, 1992. The excretion of dothiepin and its primary metabolites in breast milk. Br. J. Clin. Pharmacol 33(6), 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg KE, 1990. Excretion of fluoxetine in human breast milk. J. Clin. Psychiatry 51(4), 169. [PubMed] [Google Scholar]
- Jensen PN, Olesen OV, Bertelsen A, Linnet K, 1997. Citalopram and desmethylcitalopram concentrations in breast milk and in serum of mother and infant. Ther. Drug. Monit 19(2), 236–239. [DOI] [PubMed] [Google Scholar]
- Kam PC, Chang GW, 1997. Selective serotonin reuptake inhibitors. Pharmacology and clinical implications in anaesthesia and critical care medicine. Anaesthesia 52(10), 982–988. [DOI] [PubMed] [Google Scholar]
- Kanji S, Hayes M, Ling A, Shamseer L, Chant C, Edwards DJ, Edwards S, Ensom MH, Foster DR, Hardy B, Kiser TH, la Porte C, Roberts JA, Shulman R, Walker S, Zelenitsky S, Moher D, 2015. Reporting Guidelines for Clinical Pharmacokinetic Studies: The ClinPK Statement. Clin. Pharmacokinet 54(7), 783–795. [DOI] [PubMed] [Google Scholar]
- Kemp J, Ilett KF, Booth J, Hackett LP, 1985. Excretion of doxepin and N-desmethyldoxepin in human milk. Br. J. Clin. Pharmacol 20(5), 497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Riggs KW, Misri S, Kent N, Oberlander TF, Grunau RE, Fitzgerald C, Rurak DW, 2006. Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br. J. Clin. Pharmacol 61(2), 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ, 2006. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 368(9536), 704. [DOI] [PubMed] [Google Scholar]
- Kristensen JH, Hackett LP, Kohan R, Paech M, Ilett KF, 2002. The amount of fluvoxamine in milk is unlikely to be a cause of adverse effects in breastfed infants. J. Hum. Lact 18(2), 139–143. [DOI] [PubMed] [Google Scholar]
- Kristensen JH, Ilett KF, Dusci LJ, Hackett LP, Yapp P, Wojnar-Horton RE, Roberts MJ, Paech M, 1998. Distribution and excretion of sertraline and N-desmethylsertraline in human milk. Br. J. Clin. Pharmacol 45(5), 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen JH, Ilett KF, Hackett LP, Yapp P, Paech M, Begg EJ, 1999. Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br. J. Clin. Pharmacol 48(4), 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen JH, Ilett KF, Rampono J, Kohan R, Hackett LP, 2007. Transfer of the antidepressant mirtazapine into breast milk. Br. J. Clin. Pharmacol 63(3), 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M, Altman N, 2014. Visualizing samples with box plots. Nat. Methods 11, 119–120. [DOI] [PubMed] [Google Scholar]
- Laine K, Heikkinen T, Ekblad U, Kero P, 2003. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch. Gen. Psychiatry 60(7), 720–726. [DOI] [PubMed] [Google Scholar]
- Laine K, Kytola J, Bertilsson L, 2004. Severe adverse effects in a newborn with two defective CYP2D6 alleles after exposure to paroxetine during late pregnancy. Ther. Drug. Monit 26(6), 685–687. [DOI] [PubMed] [Google Scholar]
- Lobo ED, Loghin C, Knadler MP, Quinlan T, Zhang L, Chappell J, Lucas R, Bergstrom RF, 2008. Pharmacokinetics of duloxetine in breast milk and plasma of healthy postpartum women. Clin. Pharmacokinet 47(2), 103–109. [DOI] [PubMed] [Google Scholar]
- Loughhead AM, Fisher AD, Newport DJ, Ritchie JC, Owens MJ, DeVane CL, Stowe ZN, 2006a. Antidepressants in amniotic fluid: another route of fetal exposure. Am. J. Psychiatry 163(1), 145–147. [DOI] [PubMed] [Google Scholar]
- Loughhead AM, Stowe ZN, Newport DJ, Ritchie JC, DeVane CL, Owens MJ, 2006b. Placental passage of tricyclic antidepressants. Biol. Psychiatry 59(3), 287–290. [DOI] [PubMed] [Google Scholar]
- Malone K, Papagni K, Ramini S, Keltner NL, 2004. Antidepressants, antipsychotics, benzodiazepines, and the breastfeeding dyad. Perspect. Psychiatr. Care 40(2), 73–85. [DOI] [PubMed] [Google Scholar]
- Masarwa R, Bar-Oz B, Gorelik E, Reif S, Perlman A, Matok I, 2019. Prenatal exposure to selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors and risk for persistent pulmonary hypertension of the newborn: a systematic review, meta-analysis, and network meta-analysis. Am. J. Obstet. Gynecol 220(1), 57 e51–57 e13. [DOI] [PubMed] [Google Scholar]
- Matheson I, Pande H, Alertsen AR, 1985. Respiratory depression caused by N-desmethyldoxepin in breast milk. Lancet 2(8464), 1124. [DOI] [PubMed] [Google Scholar]
- Matheson I, Skjaeraasen J, 1988. Milk concentrations of flupenthixol, nortriptyline and zuclopenthixol and between-breast differences in two patients. Eur. J. Clin. Pharmacol 35(2), 217–220. [DOI] [PubMed] [Google Scholar]
- Misri S, Kim J, Riggs KW, Kostaras X, 2000. Paroxetine levels in postpartum depressed women, breast milk, and infant serum. J. Clin. Psychiatry 61(11), 828–832. [DOI] [PubMed] [Google Scholar]
- Molenaar NM, Bais B, Lambregtse-van den Berg MP, Mulder CL, Howell EA, Fox NS, Rommel AS, Bergink V, Kamperman AM, 2020. The international prevalence of antidepressant use before, during, and after pregnancy: A systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J. Affect. Disord 264, 82–89. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Ritchie JC, Knight BT, Glover BA, Zach EB, Stowe ZN, 2009. Venlafaxine in human breast milk and nursing infant plasma: determination of exposure. J. Clin. Psychiatry 70(9), 1304–1310. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W, 2004. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J. Clin. Psychiatry 65(2), 230–237. [DOI] [PubMed] [Google Scholar]
- Ohman R, Hagg S, Carleborg L, Spigset O, 1999. Excretion of paroxetine into breast milk. J. Clin. Psychiatry 60(8), 519–523. [DOI] [PubMed] [Google Scholar]
- Palmsten K, Chambers CD, Wells A, Bandoli G, 2020. Patterns of prenatal antidepressant exposure and risk of preeclampsia and postpartum haemorrhage. Paediatr. Perinat. Epidemiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente G, Leibson T, Carls A, Adams-Webber T, Ito S, Koren G, 2016. Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review. PLoS. Med 13(11), e1002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulzen M, Goecke TW, Stickeler E, Grunder G, Schoretsanitis G, 2017a. Sertraline in pregnancy - Therapeutic drug monitoring in maternal blood, amniotic fluid and cord blood. J. Affect. Disord 212, 1–6. [DOI] [PubMed] [Google Scholar]
- Paulzen M, Goecke TW, Stingl JC, Janssen G, Stickeler E, Grunder G, Schoretsanitis G, 2017b. Pregnancy exposure to citalopram - Therapeutic drug monitoring in maternal blood, amniotic fluid and cord blood. Prog. Neuropsychopharmacol. Biol. Psychiatry 79(Pt B), 213–219. [DOI] [PubMed] [Google Scholar]
- Paulzen M, Schoretsanitis G, Grunder G, Franz C, Stingl JC, Augustin M, 2020. Pregnancy exposure to venlafaxine-Therapeutic drug monitoring in maternal blood, amniotic fluid and umbilical cord blood and obstetrical outcomes. J. Affect. Disord 266, 578–584. [DOI] [PubMed] [Google Scholar]
- Pittard WB 3rd, O’Neal W Jr., 1986. Amitriptyline excretion in human milk. J. Clin. Psychopharmacol 6(6), 383–384. [DOI] [PubMed] [Google Scholar]
- Pfizer. ZOLOFT (sertraline hydrochloride) tablets, for oral use. NY, NY: 2016 [Google Scholar]
- Pogliani L, Falvella FS, Cattaneo D, Pileri P, Moscatiello AF, Cheli S, Baldelli S, Fabiano V, Cetin I, Clementi E, Zuccotti G, 2017. Pharmacokinetics and Pharmacogenetics of Selective Serotonin Reuptake Inhibitors During Pregnancy: An Observational Study. Ther. Drug. Monit 39(2), 197–201. [DOI] [PubMed] [Google Scholar]
- Pons G, Rey E, Matheson I, 1994. Excretion of psychoactive drugs into breast milk. Pharmacokinetic principles and recommendations. Clin. Pharmacokinet 27(4), 270–289. [DOI] [PubMed] [Google Scholar]
- Pons G, Schoerlin MP, Tam YK, Moran C, Pfefen JP, Francoual C, Pedarriosse AM, Chavinie J, Olive G, 1990. Moclobemide excretion in human breast milk. Br. J. Clin. Pharmacol 29(1), 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampono J, Hackett LP, Kristensen JH, Kohan R, Page-Sharp M, Ilett KF, 2006. Transfer of escitalopram and its metabolite demethylescitalopram into breastmilk. Br. J. Clin. Pharmacol 62(3), 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampono J, Kristensen JH, Hackett LP, Paech M, Kohan R, Ilett KF, 2000. Citalopram and demethylcitalopram in human milk; distribution, excretion and effects in breast fed infants. Br. J. Clin. Pharmacol 50(3), 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampono J, Proud S, Hackett LP, Kristensen JH, Ilett KF, 2004. A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate. Int. J. Neuropsychopharmacol 7(3), 329–334. [DOI] [PubMed] [Google Scholar]
- Rampono J, Simmer K, Ilett KF, Hackett LP, Doherty DA, Elliot R, Kok CH, Coenen A, Forman T, 2009. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry 42(3), 95–100. [DOI] [PubMed] [Google Scholar]
- Rampono J, Teoh S, Hackett LP, Kohan R, Ilett KF, 2011. Estimation of desvenlafaxine transfer into milk and infant exposure during its use in lactating women with postnatal depression. Arch. Womens. Ment. Health 14(1), 49–53. [DOI] [PubMed] [Google Scholar]
- Rees JA, Glass RC, Sporne GA, 1976. Serum and Breast Milk Concentrations of Dothiepin. The practitioner 217, 686. [Google Scholar]
- Sandoz Inc. Amitriptyline Hydrochloride Tablets, USP. Princeton, NJ: 2014 [Google Scholar]
- Schimmell MS, Katz EZ, Shaag Y, Pastuszak A, Koren G, 1991. Toxic neonatal effects following maternal clomipramine therapy. J. Toxicol. Clin. Toxicol 29(4), 479–484. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Olesen OV, Jensen PN, 2000. Citalopram and breast-feeding: serum concentration and side effects in the infant. Biol. Psychiatry 47(2), 164–165. [DOI] [PubMed] [Google Scholar]
- Schoretsanitis G, Augustin M, Sassmannshausen H, Franz C, Grunder G, Paulzen M, 2019. Antidepressants in breast milk; comparative analysis of excretion ratios. Arch. Womens. Ment. Health 22(3), 383–390. [DOI] [PubMed] [Google Scholar]
- Schoretsanitis G, Spigset O, Stingl JC, Deligiannidis KM, Paulzen M, Westin AA, 2020a. The impact of pregnancy on the pharmacokinetics of antidepressants: a systematic critical review and meta-analysis. Expert. Opin. Drug. Metab. Toxicol, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoretsanitis G, Westin AA, Deligiannidis KM, Spigset O, Paulzen M, 2020b. Excretion of Antipsychotics Into the Amniotic Fluid, Umbilical Cord Blood, and Breast Milk: A Systematic Critical Review and Combined Analysis. Ther. Drug. Monit 42(2), 245–254. [DOI] [PubMed] [Google Scholar]
- Sit D, Perel JM, Wisniewski SR, Helsel JC, Luther JF, Wisner KL, 2011. Mother-infant antidepressant concentrations, maternal depression, and perinatal events. J. Clin. Psychiatry 72(7), 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoqvist F, Bergfors PG, Borga O, Lind M, Ygge H, 1972. Plasma disappearance of nortriptyline in a newborn infant following placental transfer from an intoxicated mother: evidence for drug metabolism. J. Pediatr 80(3), 496–500. [DOI] [PubMed] [Google Scholar]
- Sovner R, Orsulak PJ, 1979. Excretion of imipramine and desipramine in human breast milk. Am. J. Psychiatry 136(4A), 451–452. [PubMed] [Google Scholar]
- Spigset O, Carieborg L, Ohman R, Norstrom A, 1997. Excretion of citalopram in breast milk. Br. J. Clin. Pharmacol 44(3), 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigset O, Carleborg L, Norstrom A, Sandlund M, 1996. Paroxetine level in breast milk. J. Clin. Psychiatry 57(1), 39. [PubMed] [Google Scholar]
- Spigset O, Hagg S, 2000. Analgesics and breast-feeding: safety considerations. Paediatr. Drugs 2(3), 223–238. [DOI] [PubMed] [Google Scholar]
- Stancer HC, Reed KL, 1986. Desipramine and 2-hydroxydesipramine in human breast milk and the nursing infant’s serum. Am. J. Psychiatry 143(12), 1597–1600. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Cohen LS, Hostetter A, Ritchie JC, Owens MJ, Nemeroff CB, 2000. Paroxetine in human breast milk and nursing infants. Am. J. Psychiatry 157(2), 185–189. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Hostetter AL, Owens MJ, Ritchie JC, Sternberg K, Cohen LS, Nemeroff CB, 2003. The pharmacokinetics of sertraline excretion into human breast milk: determinants of infant serum concentrations. J. Clin. Psychiatry 64(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Owens MJ, Landry JC, Kilts CD, Ely T, Llewellyn A, Nemeroff CB, 1997. Sertraline and desmethylsertraline in human breast milk and nursing infants. Am J Psychiatry 154(9), 1255–1260. [DOI] [PubMed] [Google Scholar]
- Suri R, Stowe ZN, Hendrick V, Hostetter A, Widawski M, Altshuler LL, 2002. Estimates of nursing infant daily dose of fluoxetine through breast milk. Biol. Psychiatry 52(5), 446–451. [DOI] [PubMed] [Google Scholar]
- Tonn P, Reuter SC, Hiemke C, Dahmen N, 2009. High mirtazapine plasma levels in infant after breast feeding: case report and review of the literature. J. Clin. Psychopharmacol 29(2), 191–192. [DOI] [PubMed] [Google Scholar]
- Troy SM, Parker VP, Hicks DR, Pollack GM, Chiang ST, 1997. Pharmacokinetics and effect of food on the bioavailability of orally administered venlafaxine. J. Clin. Pharmacol 37(10), 954–961. [DOI] [PubMed] [Google Scholar]
- Verbeeck RK, Ross SG, McKenna EA, 1986. Excretion of trazodone in breast milk. Br. J. Clin. Pharmacol 22(3), 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Zhu HJ, Gibson BB, Markowitz JS, Donovan JL, DeVane CL, 2008. Sertraline and its metabolite desmethylsertraline, but not bupropion or its three major metabolites, have high affinity for P-glycoprotein. Biol. Pharm. Bull 31(2), 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf E, Panchaud A, Nguyen KA, Grosjean D, Hascoet JM, Csajka C, Eap CB, Ansermot N, collaborators of the S.-B.M.s., 2017. Simultaneous determination of selective serotonin reuptake inhibitors and their main metabolites in human breast milk by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci 1057, 101–109. [DOI] [PubMed] [Google Scholar]
- Weissman AM, Levy BT, Hartz AJ, Bentler S, Donohue M, Ellingrod VL, Wisner KL, 2004. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am. J. Psychiatry 161(6), 1066–1078. [DOI] [PubMed] [Google Scholar]
- Westin AA, Reimers A, Spigset O, 2018. Should pregnant women receive lower or higher medication doses? Tidsskr. Nor. Laegeforen 138(17). [DOI] [PubMed] [Google Scholar]
- Whitby DH, Smith KM, 2005. The use of tricyclic antidepressants and selective serotonin reuptake inhibitors in women who are breastfeeding. Pharmacotherapy 25(3), 411–425. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Findling RL, 1996. Antidepressant treatment during breast-feeding. Am. J. Psychiatry 153(9), 1132–1137. [DOI] [PubMed] [Google Scholar]
- Wright S, Dawling S, Ashford JJ, 1991. Excretion of fluvoxamine in breast milk. Br. J. Clin. Pharmacol 31(2), 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Ciolino JD, Pinheiro E, Rasmussen-Torvik LJ, Sit DKY, Wisner KL, 2017. Neonatal Discontinuation Syndrome in Serotonergic Antidepressant-Exposed Neonates. J. Clin. Psychiatry 78(5), 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Smith B, Craggs M, Kumar RC, 1997a. Investigation of pharmacokinetics and of possible adverse effects in infants exposed to tricyclic antidepressants in breast-milk. J. Affect. Disord 43(3), 225–237. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Smith B, Craggs M, Kumar RC, 1998. Fluoxetine in breast-milk and developmental outcome of breast-fed infants. Br. J. Psychiatry 172, 175–178. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Smith B, Kumar R, 1999. Psychotropic drugs in mothers’ milk: a comprehensive review of assay methods, pharmacokinetics and of safety of breast-feeding. J. Psychopharmacol 13(1), 64–80. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Smith B, Kumar RC, 1997b. Fluvoxamine in breast-milk and infant development. Br. J. Clin. Pharmacol 44(2), 210–211. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


