Abstract
Post-traumatic stress disorder (PTSD) and alcohol use disorder (AUD) are highly comorbid. Additionally, individual differences in response to stress suggest resilient and susceptible populations. The current study exposed male and female Long Evans rats to the synthetically produced predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) to examine individual differences in stress-reactive behaviors (digging and immobility) and whether these differences were related to subsequent alcohol drinking. Male and female Long Evans rats were trained on operant alcohol self-administration. After 9 sessions, rats underwent exposure to TMT or water (Control) in a distinct context. 6 days after TMT exposure, rats underwent re-exposure to the TMT-paired context (without TMT), and a series of behavioral assessments (acoustic startle, zero maze, light/dark box), after which rats resumed alcohol self-administration. TMT subgroups were created using a ratio of digging to immobility behavior during TMT exposure and rats with a ratio score < 1.0 or > 1.0 were grouped into TMT-1 (low digging/high immobility) or TMT-2 (high digging/low immobility), respectively. All male rats exposed to TMT met criteria for TMT-1, while female rats were divided into the two subgroups. In females, high digging/low immobility behavior during TMT exposure (TMT-2) was related to increased alcohol self-administration, but this was not observed in males or females that engaged in low digging/high immobility (TMT-1). These data show that individual differences in stress-reactivity can lead to lasting behavioral changes which may lead to a better understanding of increases in alcohol drinking following stress in females.
Keywords: Predator odor stress, TMT, individual differences, alcohol, sex differences, post-traumatic stress disorder, alcohol use disorder
1. Introduction
Post-traumatic stress disorder (PTSD) is an anxiety, trauma-and stressor-related disorder that manifests after exposure to a traumatic event. Diagnostic criteria include exposure to a traumatic stressor, intrusive symptoms, avoidance or negative alterations in cognition and mood, and alterations in arousal and reactivity [1]. Lifetime prevalence of PTSD in the United States is approximately 8.3% for adults, with females (12.8%) being more likely to have a lifetime prevalence of PTSD compared to males (5.7%) [2, 3]. Furthermore, it is well known that PTSD is highly comorbid with alcohol use disorder (AUD) [4, 5]. According to the National Comorbidity Study [4], 26.2% of women and 10.3% of men in a general population with alcohol dependence have met the criteria for PTSD. Some individuals with PTSD consume alcohol as an attempt to alleviate symptoms, which can increase the risk of developing a drinking problem [6]. In addition, experiences of trauma despite the diagnosis of PTSD, can induce high levels of alcohol craving and lead to an increase in consumption [7].
Not all individuals exposed to trauma develop PTSD. Approximately 5–10% of individuals develop PTSD after experiencing a traumatic stressor [2]. Studies examining stress resilience and susceptibility in humans show one’s ability to adapt to stressful encounters are key factors that can predict resilient outcomes to stress [8]. Well-adapted behavioral responses such as active coping mechanisms [9] and cognitive reappraisal strategies [10] prompt a level of resiliency that can protect against developing PTSD [11, 12]. Therefore, it is necessary to identify novel animal models to target behavioral and neurobiological mechanisms associated with individual variability in response to stress and trauma to better understand differences in vulnerability to developing PTSD.
Animal models have become increasingly important in stress research to examine behaviors that can inform our understanding of clinical PTSD symptoms. These models can be used to investigate the relationship between increased alcohol consumption and stress including examining the relationship between individual differences in symptom profiles of traumatic stress and excessive alcohol drinking [13, 14]. Specifically, predator odor exposure including soiled cat litter [15] and bobcat urine [16] have been shown to produce escalations in alcohol drinking in rats, while dirty rat bedding has been shown to increase alcohol consumption in mice [17]. Additionally, exposure to 2,3,5-trimethyl-3-thiazoline (TMT; an extract of fox feces) has been shown to produce alcohol reinstatement (e.g., relapse-like behavior) in mice [18]. Animal models can also be used to examine relevant individual variability in responses to stress [19–24], including stress resilience. Previous studies have utilized a variety of methods to classify animals into specific phenotype groups to examine individual variability following the stress exposure. For example, classification can be based on avoidance behavior during re-exposure to a bobcat urine paired context [16, 25], behavior during an elevated-plus maze and acoustic startle response following TMT exposure [16, 25], and anxiety-like behavior in an elevated plus maze and context avoidance behavior [23, 24]. These characterization methods focus on grouping rats based on behavioral changes that occur after exposure to stress. The focus of the current study was to determine whether quantifying behavior during the stressor exposure could provide an index of stress-reactivity that could be used to predict long-term consequences of stress including context reactivity, anxiety-like behavior, hyperarousal behavior, and increases in alcohol self-administration in male and female rats.
The current study uses TMT exposure as the stressor because it activates a hardwired “learned-independent system” shown to induce innate fear and defensive behaviors [26]. An important advantage of using predator odor exposure, including TMT, as a stressor, is the ability to measure stress-reactive behaviors during stressor exposure, including defensive digging and immobility [27], which can be used as an index of stress-reactivity. Digging is a species-and strain-dependent behavior that has been implemented in rodent models of PTSD [27, 28]. It is interpreted as a proactive response to stress [29–31], such that it reflects an active coping response or fear-related behavior [32] and predator-stress responsiveness [33]. Freezing behavior is a well-characterized fear-like behavior that animals have been shown to engage in during TMT exposure [27, 34–36]. The present study sought to examine individual differences in stress-reactive behaviors (digging and immobility) during TMT exposure in male and female Long Evans rats to determine whether these individual differences were related to subsequent increases in context reactivity, hyperarousal and anxiety-like behavior and alcohol self-administration. Digging and immobility during TMT were specifically chosen as the measures to calculate ratio score because they represent two distinctly different types of stress-induced behavioral coping strategies [29, 31, 37, 38] (digging = active, immobility = passive) that can be used to reflect individual differences in stress responsivity. TMT-1 represented rats with ratio scores below 1.0 (i.e., low digging/high immobility), indicative of low levels of active coping behavior and higher levels of passive coping behaviors and TMT-2 represented rats with ratio scores greater than 1.0 (i.e., high digging/low immobility), indicative of higher levels of active coping and lower levels of passive coping behavior. We hypothesized that higher digging/immobility ratio would be related to increases in reactivity to the TMT-paired context upon re-exposure, anxiety-like and hyperarousal behaviors, and subsequent increases in alcohol self-administration.
The current study supports several important findings including 1) female but not male rats, show individual variability in the engagement of stress-reactive behaviors during TMT, and 2) stress reactivity during TMT exposure can be an important factor in subsequent alcohol self-administration, specifically in female rats. These lasting consequences of exposure to the synthetically produced predator odor TMT can provide a better understanding of stress-induced increases in alcohol drinking, as well as how individual differences and heterogeneity in stress-reactive behaviors using TMT may be helpful in understanding the lasting consequences of stress.
2. Materials and Methods
2.1. Subjects
Male and female young adult (arrived at 7 weeks old) Long Evans rats (n=64) were used for these experiments. Animals were double housed by sex in ventilated cages (Tecniplast, West Chester, PA) upon arrival to the vivarium with ad libitum food and water. For at least 1 week prior to the start of alcohol self-administration training, all rats were single housed and remained single-housed for the duration of the experiment. Rats were maintained in a temperature and humidity-controlled colony with a 12-hour light/dark cycle (lights on at 07:00). All experiments were conducted during the light cycle. Animals were handled for five days prior to the start of the experiment. Animals were under continuous care and monitoring by veterinary staff from the Division of Comparative Medicine at UNC-Chapel Hill. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and institutional guidelines.
2.2. Self-Administration Apparatus
Self-administration chambers (31 × 32 × 24 cm; Med Associates Inc.; St. Albans, VT) were individually located within standard-attenuating cubicles equipped with an exhaust fan that provided both ventilation and masking of external sounds. Chambers were fitted with a retractable lever on the left and right walls and a white cue light was centered 7-cm above each lever with a liquid receptacle centered on each wall. Lever responses on the left lever (i.e. active lever) activated a syringe pump (Med Associates) that delivered 0.1 ml of solution into the receptacle during a 1.66-s period. The white cue light and tone located above the active lever were activated during pump activation. Responses on the right lever (i.e. inactive lever) had no programmed consequence. The chambers also had infrared photobeams which divided the floor of the chamber into 4 zones to record general locomotor activity throughout each session.
2.3. Alcohol Self-Administration Training
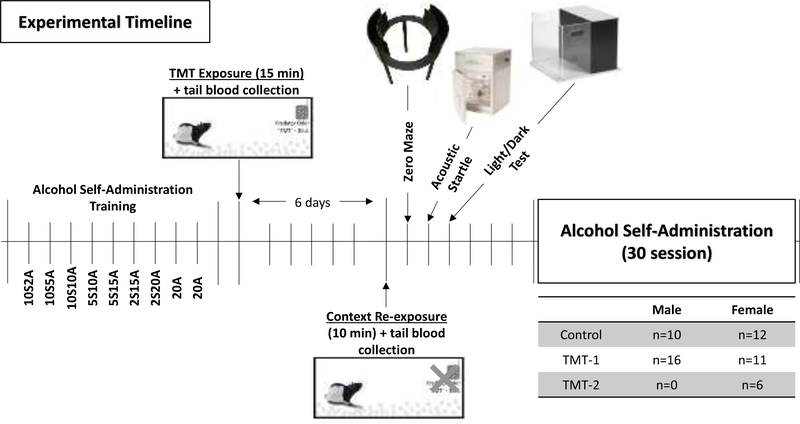
The experimental timeline is illustrated in Figure 1. Rats (approximately 9 weeks old at the start of self-administration training) were trained on operant alcohol self-administration training using a sucrose-fading procedure in which alcohol was gradually added to the 10% (w/v) sucrose solution, similar to [39]. The exact order of daily exposures was as follows: 2% (v/v) alcohol/10% (w/v) sucrose (2A/10S), 5A/10S, 10A/10S, 10A/5S, 15A/5S, 15A/2S, 20A/2S, 20A with one day at each concentration except for 20A which occurred for two days after which the rats remained on 15A as the reinforcer for the remainder of the study. Self-administration sessions (30 minutes) took place 5 days per week (M-F) with the active lever on a fixed ratio 2 schedule (FR2) of reinforcement such that every second response resulted in delivery of alcohol.
Figure 1. Experimental Timeline.
Male and female Long-Evans rats were trained on alcohol self-administration for 9 days. 24 hr post last self-administration training day, male and female rats were exposed to water or TMT. 6 days later, rats were re-exposed to the initial TMT-paired contextual environment in the absence of TMT. 24 hr later rats underwent testing for anxiety-like behavior (zero maze, hyperarousal (acoustic startle response, ASR) and anxiety-like behavior as measured by approach/avoidance behavior (light/dark test and zero maze). Three days after behavioral tests, rats returned to alcohol self-administration for 30 sessions (15A). The sample size of each treatment group for male and female rats is also included.
2.4. Predator Odor Exposure and Context Re-exposure
Approximately 24 hr after nine days of self-administration training, rats underwent predator odor exposure. Rats were transferred from the home cage to Plexiglas exposure chambers (45.72 × 17.78 × 21.59 cm; UNC Instrument Shop, Chapel Hill, NC, USA). The length of the back wall of the chambers was opaque white with two opaque black side walls and a clear front wall to allow for video recordings. A metal basket (17.8 cm above the floor) was hung on the right-side wall. This basket held a piece of filter paper on which was placed 10 μl of TMT or water (for controls) so that the filter paper was inaccessible to the rat. Approximately 600 mL of white bedding (Shepherds ALPHA-dri) was added to the bottom of the exposure chamber prior to the animal being placed in the chamber. After the rat was placed into the chamber, a clear Plexiglas top was slid and secured into place. The exposure session was 15 min in duration and recorded by a video camera for later analysis using ANY-maze ™ Video Tracking System (Stoelting Co. Wood Dale, IL, USA). After completion of the exposure, fecal boli was counted for each rat as a measure of physiological responses to TMT. Following the exposure, rats were immediately transferred to a separate procedure room where tail blood was collected for later analysis of plasma corticosterone levels. Following the completion of blood collection, each rat was returned to the homecage. Blood was collected into heparinized tubes and centrifuged at 4°C for 5 minutes at 2000 rcf. Approximately 20–30 μL of plasma supernatant was collected and stored at −80°C until analysis. Plasma samples (5 μL) were analyzed for corticosterone levels in duplicate using colorimetric EIA kit (ArborAssays, Ann Arbor, MI) per the manufacture instructions.
Six days following predator odor exposure, reactivity to the TMT-paired context was examined. Animals were transferred from their home cage and placed in the context in which they had been previously exposed to water or TMT for 10 min (no TMT present). Sessions were recorded by a video camera and analyzed using ANY-maze ™ Video Tracking System (Stoelting Co. Wood Dale, IL, USA). Following the 10 min context re-exposure, rats were immediately transferred to a separate procedure room where tail blood was collected for later analysis of plasma corticosterone levels. Following the completion of blood collection, each rat was returned to the homecage.
2.5. Elevated Zero Maze
24 hr after the context re-exposure, rats underwent testing in an elevated zero maze to assess anxiety-like behavior. The zero maze was composed of a circular platform with a diameter of 99 cm raised above the floor to a height of approximately 70 cm and divided equally into four quadrants. Two enclosed quadrants contain two walls with one back wall [33.02 cm (H)] and front wall [25.4 in (H)]. The exposed quadrants are located on opposite sides of the circular platform and are bordered with approximately 5 mm rim in order to prevent the rat from falling off the circular platform. Rats were placed in one open quadrant at the start of the test for 5 min in the zero maze before being placed back into their home cage. Zero maze was conducted under red light.
2.6. Acoustic Startle Response
On the following day, rats underwent acoustic startle response testing to assess changes in arousal response using an acoustic startle response system (S-R Lab; San Diego Instruments, San Diego, CA). Rats were placed in a cylinder Plexiglas animal enclosure located within a sound-attenuating test chamber that included an exhaust fan, a sound source, and an internal light that was turned off during the test. At the start of each test, rats underwent a 5-min habituation period during which 60 dB of background white noise was present. The background noise was present during the entire test session. The test session consisted of 30 trials of a 100 ms burst of a 110 dB startle. Each trial was separated by a 30 to 45-s randomized intertrial interval. Startle amplitude was measured with a high-accuracy accelerometer mounted under the animal enclosure and analyzed with SR-Lab software.
2.7. Light/Dark Test
The day after the acoustic startle test (72 hr after the context re-exposure test), animals underwent testing for anxiety-like behavior as measured by approach/avoidance behavior in the light/dark chamber. A dark box insert (44.4 × 22.9 × 30.5 cm) was placed in the left side of an open field chamber (150 lux) (23.31 × 27.31 × 20.32 cm; Med Associates Inc.; St. Albans, VT) to divide the chamber into a dark and light side (150 lux in center of light side). The chamber was located within standard-attenuating cubicles equipped with an exhaust fan that provided both ventilation and masking of external sounds. Time and distance spent on each side of the chamber was measured with 4 parallel beams across the chamber floor. Animals were transported into the testing room in the home cage at least 20 min prior to the start of the 5 min test and each rat was placed in the light side facing the posterior wall.
2.8. Alcohol Self-Administration
Rats resumed alcohol self-administration 4 days after completion of the light/dark test. 30-min self-administration sessions took place 5 days per week (M-F) for 21 sessions (15% v/v, alcohol). After these 21 sessions, self-administration sessions occurred 3 days per week (MWF) for 3 weeks for the remainder of the study.
2.9. Data Analyses
2.9.1. TMT and Context Re-exposure:
TMT Subgroup Classification. Rats were classified into TMT subgroups by a digging/immobility ratio score based on their behavior during the TMT exposure. This score was calculated by dividing the total time spent digging by the total time spent immobile. A cut-off strategy was used such that rats with a criterion ratio score below 1.0 were classified as TMT-1 and rats with a ratio score above 1.0 were classified as TMT-2. Using ANY-maze software, the length of the rectangular TMT exposure chamber was divided into two equal compartments for analysis (TMT side and non-TMT side) to allow for analysis of time spent and time spent digging on the side in which the TMT is located. Digging behavior was quantified manually. Immobility was operationally defined as lack of movement for more than 2 seconds as assessed using ANY-maze software. Therefore, immobility likely captures both inactivity and freezing, which is characteristic of a fear response in rodents and observed during TMT exposure [34]. All ratio scores for male rats exposed to TMT were below 1.0, indicating that no males met the criteria for TMT-2. Therefore, grouping for male rats included controls and TMT-1. T-tests were used to compare differences in stress-reactive behaviors (digging, immobility, time spent on TMT side and corticosterone) in control and TMT-1 in males and one-way analysis of variance (ANOVA) tests were used to compare differences in stress-reactive behaviors between control and TMT subgroups in female rats. A video recording error occurred during the TMT exposure for six female rats, resulting in the last 5 min of the exposure not being recorded. To account for this error, we analyzed the first 10 min of exposure for all animals in the experiment. Unfortunately, two of these rats did not have a full 10 min of video analysis and had to be excluded from the study. One male TMT rat determined to be a statistical outlier based on digging behavior during TMT (greater than 2 standard deviations from the mean) was excluded from the study. Cumulative time spent digging, immobile and time spent on TMT side were analyzed by two-way RM ANOVA with TMT exposure as a between-subjects factor and time as a within-subjects factor. Tukey multiple comparisons tests were used to follow up significant main effects of groups and interactions. All data are reported as mean ± S.E.M. Significance was set at p≤0.05.
2.9.2. Light/Dark Test, Acoustic Startle Response and Zero Maze Test:
The % time in light compartment for the light/dark test, average startle amplitude for acoustic startle response and % open arm time for the zero maze were analyzed by a one-way RM ANOVA. Tukey multiple comparisons tests was used to analyze significant main effects of groups. All data are reported as mean ± SEM. Significance was set at p≤0.05.
2.9.3. Self-Administration:
For alcohol self-administration, alcohol lever responses and alcohol intake (g/kg) are presented as 3-session averages. Alcohol intake was estimated from reinforcers received relative to body weight (kg). These data were analyzed by a two-way RM-ANOVA. Tukey multiple comparisons tests was used to analyze significant main effects of groups and interactions. General locomotor activity during the session was measured and total beam breaks across the session was divided by the session length (30 min) to determine locomotor rate (beam breaks/min). A baseline criterion was set such that rats that had below an average of at least 0.30 g/kg between the two 20A baseline training days prior to TMT exposure were excluded from the study. Two control-male rats, three TMT-male rats and one TMT-female did not meet this criterion. All data are reported as mean ± SEM. Significance was set at p≤0.05.
3. Results
Prior to TMT exposure, there were no differences in alcohol self-administration as measured by lever responses across training days, total alcohol drinking history (Table 1, p > 0.05), locomotor activity or inactive lever responses (p > 0.05) between TMT subgroups and controls in male or female rats.
Table 1.
Alcohol lever responses and alcohol intake (g/kg) from sucrose fading phase during self-administration training
| Alcohol Lever Responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10S2E | 10S5E | 10S10E | 5S10E | 5S15E | 2S15E | 2S20E | 20E | 20E | ||
| Male | Control | 81.5 ± 22.1 | 85.6 ± 18.3 | 106.7 ± 19.8 | 96.5 ± 15.8 | 69.6 ± 14.0 | 33.4 ± 6.0 | 50.2 ± 8.1 | 25.9 ± 4.7 | 30.0 ± 5.2 |
| TMT-1 | 73.4 ± 12.7 | 65.3 ± 13.5 | 82.4 ± 8.1 | 92.7 ± 10.0 | 66.2 ± 6.2 | 41.2 ± 4.7 | 34.9 ± 4.8 | 28.5 ± 4.8 | 32.9 ± 2.8 | |
| Female | Control | 54.9 ± 14.7 | 58.2 ± 13.6 | 63.4 ± 11.0 | 73.9 ± 10.0 | 54.8 ± 9.0 | 28.6 ± 2.2 | 29.9 ± 4.1 | 21.5 ± 3.1 | 26.8 ± 3.6 |
| TMT-1 | 58.6 ± 13.4 | 62.8 ± 11.9 | 57.2 ± 10.1 | 76.9 ± 13.0 | 53.0 ± 5.3 | 30.2 ± 6.1 | 28.2 ± 5.0 | 23.9 ± 2.4 | 29.8 ± 4.8 | |
| TMT-2 | 58.6 ± 13.4 | 62.8 ± 11.9 | 57.2 ± 10.1 | 76.9 ± 13.0 | 53.0 ± 5.3 | 30.2 ± 6.1 | 28.2 ± 5.0 | 23.9 ± 2.4 | 29.8 ± 4.8 | |
| Alcohol Intake (g/kg) | ||||||||||
| Male | Control | 0.2 ± 0.1 | 0.5 ± 0.1 | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.6 ± 0.1 | 1.1 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| TMT-1 | 0.2 ± 0.0 | 0.4 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | |
| Female | Control | 0.2 ± 0.0 | 0.5 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.3 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 |
| TMT-1 | 0.2 ± 0.1 | 0.6 ± 0.1 | 1.0 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 | |
| TMT-2 | 0.3 ± 0.1 | 0.8 ± 0.2 | 1.4 ± 0.3 | 1.6 ± 0.3 | 1.4 ± 0.5 | 0.7 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.8 ± 0.2 | |
Mean ± SEM
3.1. TMT exposure produces distinct subgroups in female but not male rats
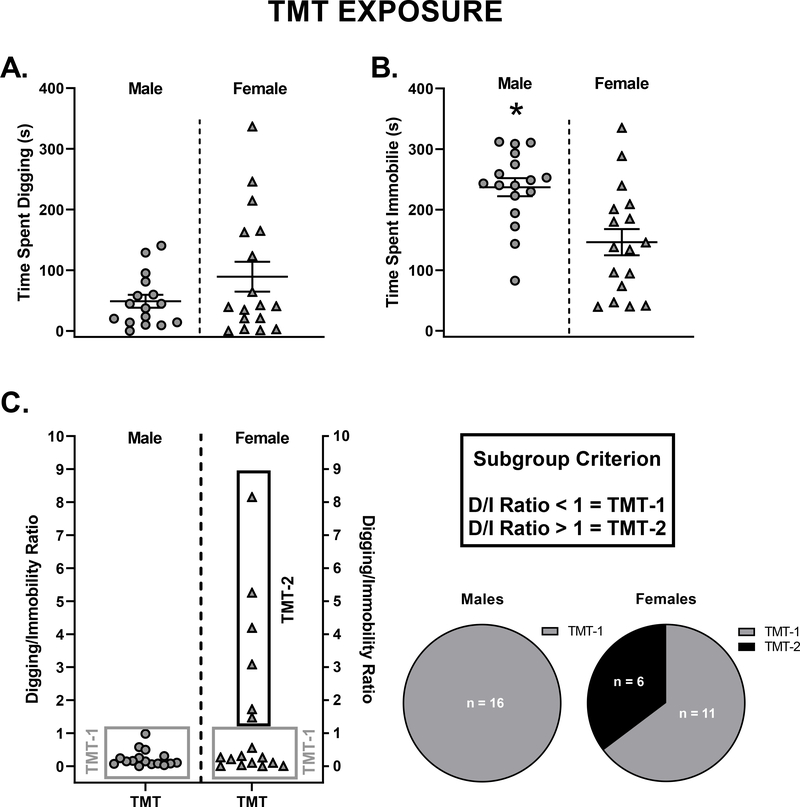
Distribution plots (Figure 2A and B) represent the range of stress-reactive behaviors (digging and immobility) in male and female rats during TMT exposure. Males and females spent similar time engaged in defensive digging behavior during the TMT exposure, although the females had a greater range than the males (Figure 2A; Male: 0.0 to 140.6 sec; Female: 0 to 336.7 sec). Time spent immobile was significantly higher in males compared to females during the TMT exposure (Fig. 2B, t(32) =3.44, p < 0.05), indicating a sex difference in the engagement of immobility during the predator odor stress exposure. Using these stress-reactive behaviors, a digging/immobility ratio was calculated for each rat in the TMT group by dividing the total time spent digging by the total time spent immobile as shown in Figure 2C. Ratio scores below the criterion score of 1.0 were classified as TMT-1 and ratio scores above 1.0 were classified as TMT-2. Venn diagrams illustrate number of rats in each TMT subgroup for males and females. All ratio scores for male rats exposed to TMT were below 1.0, indicating no males met the criteria for TMT-2. Therefore, grouping for male rats included controls and TMT-1. From this point forward, males and females were analyzed separately.
Figure 2. Distribution plots to illustrate range of stress-reactive behaviors between male and female rats exposed to TMT.
(A) Male and female rats exposed to TMT showed no difference in total defensive digging. (B) Male rats exposed to TMT spend significantly more total time immobile compared to female rats exposed to TMT. (C) Representation of digging/immobility scores in male and female TMT-groups 1 and 2. No male rats met the criteria for TMT-2., *p < 0.05. Mean ± SEM.
3.2. Effects of TMT exposure on stress-reactive behaviors, context reactivity, and alcohol self-administration in male rats.
TMT Exposure.
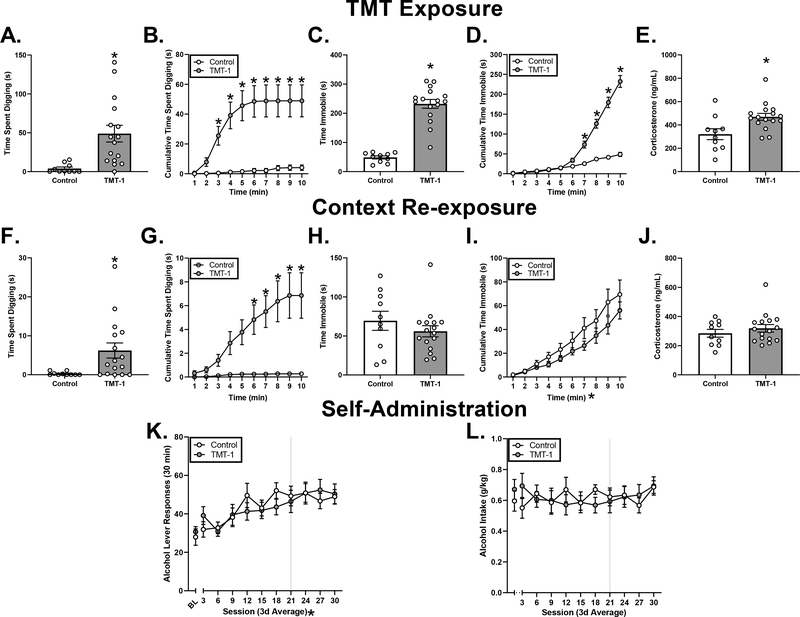
During the TMT exposure, male rats in TMT-1 engaged in significantly more digging (Fig. 3A, t(24) = 3.25, p < 0.05) with 66% of the time digging on the TMT side, and immobility (Fig. 3C, t(24) = 9.35, p < 0.05) compared to controls. Cumulative digging was used to examine the pattern of digging and immobility across time. A two-way RM ANOVA showed a significant main effect of exposure (Fig. 3B, F(1,24) = 11.20, p < 0.05), time (Fig. 3B, F(9,216) = 13.25, p < 0.05) and a significant exposure x time interaction (Fig. 3B, F(9,216) = 10.10, p < 0.05), with significantly greater digging behavior in the TMT group compared to controls from minute 3 throughout the remainder of the exposure (Fig. 3B, p < 0.05). To examine the pattern of immobility over time, a two-way RM ANOVA showed a significant main effect of exposure (Fig. 3D, F(1,24) = 50.45, p < 0.05), time (Fig. 3D, F(9,216) = 138.7, p < 0.05), and a significant time x exposure interaction (Fig. 3D, F(9,216) = 63.97, p < 0.05), with more time immobile in the TMT group compared to controls from minute 7 throughout the remainder of the exposure (Fig. 3D, p < 0.05). Time spent on the TMT side of the chamber was examined as a measure of avoidance behavior, and the TMT group spent less time on the TMT side than controls (Control: 361.5 ± 20.4; TMT-1: 142. ± 17.5; t(24) = 4.37, p < 0.05). Analysis of the distribution of time on the TMT side showed a significant main effect of exposure (Table 2, F (1,24) = 48.39, p < 0.05), time (Table 2, F (9,216) = 227.4, p < 0.05), and a significant group x time interaction (Table 2, F (9,216) = 49.14, p < 0.05), with rats in the TMT group spending significantly less time on the TMT side by minute 4 and throughout the remainder of the session (Table 2, p < 0.05).
Figure 3. Effects of TMT exposure on stress-reactive behaviors, context reactivity, and alcohol self-administration in male rats.
. All male rats exposed to TMT were grouped into TMT-1. Male rats in TMT-1 performed significantly more defensive digging compared to controls (A), as well as across 10 min of the TMT exposure (B). Male rats in TMT-1 exhibited significantly more time immobile (C), as well as across time (D). Male rats in TMT-1 exhibited higher plasma corticosterone compared to controls (E). During context re-exposure, males in TMT-1 performed significant more digging (F), as well as across time (G) compared to controls. Males previously exposed to TMT showed no changes in total time immobile (H) or across time (I), as well as no changes in plasma corticosterone (J) compared to controls. Lastly, males in TMT-1 exhibited no significant increases in alcohol lever responses (K) or alcohol intake (g/kg) (L). For panels K and L, BL (baseline average) is represented to the left of the axis break. *p < 0.05. Mean ± SEM. * by group labels = main effect of group, X-axes * = main effect of time or session.
Table 2.
Cumulative Time Spent on TMT side (s) during TMT exposure and Context Re-exposure
| TMT Exposure (min) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Male | Control | 36.0 ± 2.5 | 76.7 ± 4.9 | 11.2 ± 5.3 | 149.8 ± 6.7 | 186.0 ± 8.9 | 213.9 ± 10.2 | 249.4 ± 12.9 | 327.0 ± 17.6 | 327. ± 17.6 | 361.5 ± 20.4 |
| TMT-1 | 23.4 ± 2.4 | 42.6 ± 4.0 | 67.6 ± 6.4 | 96.7 ± 8.1* | 116.6 ± 9.9* | 127.1 ± 11.6* | 133.2 ± 12.6* | 137.0 ± 14.1* | 139.3 ± 15.*3 | 142. ± 17.5* | |
| Female | Control | 36.9 ± 2.2 | 73.9 ± 2.2 | 106.1 ± 3.1 | 139.5 ± 2.4 | 172.1 ± 6.4 | 209.9 ± 5.6 | 256.0 ± 7.4 | 296.9 ± 8.6 | 339.5 ± 10.4 | 385.2 ± 9.6 |
| TMT-1 | 19.0 ± 1.7 | 19.0 ± 1.7* | 34.3 ± 2.8* | 55.3 ± 4.5* | 76.6 ± 4.7* | 96.2 ± 4.6* | 110.2 ± 3.6* | 113.7 ± 3.9* | 114.3 ± 4.1* | 114.3 ± 4.1* | |
| TMT-2 | 18.5 ± 4.2 | 18.5 ± 4.2* | 43.8 ± 6.3* | 74.5 ± 6.8* | 110.4 ± 8.7*+ | 144.2 ± 13.4*+ | 177.5 ± 17.5*+ | 217.8 ± 17.7*+ | 240.4 ± 20.4*+ | 275.5 ± 31.7*+ | |
| Context Re-exposure (min) | |||||||||||
| Male | Control | 35.1 ± 2.1 | 72.6 ± 2.0 | 110.6 ± 5.0 | 149.6 ± 6.3 | 184.5 ± 8.2 | 220.9 ± 12.5 | 258.0 ± 16.1 | 295.9 ± 17.8 | 346.2 ± 20.7 | 383.0 ± 24.5 |
| TMT-1 | 31.2 ± 2.6 | 60.3 ± 2.7 | 91.5 ± 4.6 | 126.3 ± 6.0 | 162.3 ± 7.4 | 194.3 ± 10.5 | 232.2 ± 13.6 | 271.0 ± 15.7 | 311.0 ± 16.5 | 345.3 ± 17.9 | |
| Female | Control | 33.9 ± 2.3 | 64.3 ± 2.9 | 105.1 ± 4.8 | 141.7 ± 7.1 | 181.5 ± 86 | 213.8 ± 9.5 | 248.9 ± 10.0 | 284.7 ± 13.1 | 319.1 ± 18.1 | 347.0 ± 20.9 |
| TMT-1 | 34.3 ± 2.1 | 65.3 ± 3.6 | 100.9 ± 5.0 | 140.0 ± 5.5 | 178.5 ± 7.6 | 218.3 ± 6.9 | 251.2 ± 8.6 | 290.9 ± 9.2 | 331.0 ± 11.8 | 367.6 ± 12.7 | |
| TMT-2 | 33.4 ± 4.6 | 71.7 ± 3.7 | 110.5 ± 5.4 | 149.4 ± 8.5 | 177.4 ± 9.2 | 216.3 ± 12.3 | 246.5 ± 12.6 | 290.3 ± 13.9 | 333.8 ± 16.5 | 364.7 ± 22.0 | |
Mean ± SEM
p ≤ 0.05 vs. controls
p ≤ 0.05 vs. TMT-1
Fecal boli production during TMT exposure and corticosterone levels following TMT exposure were used as indices of physiological responses to TMT exposure. Fecal boli did not differ (t(24) = 1.36, p > 0.05; Control: 1.70 ± 0.90, TMT-1: 3.19 ± 0.66,). However, plasma corticosterone levels were significantly increased following TMT exposure (Fig. 3E, t(24) = 2.86, p < 0.05).
Context re-exposure and behavioral screens.
During context re-exposure, male rats in TMT-1 engaged in significantly more digging behavior compared to controls (Fig. 3F, t(24) = 2.40, p < 0.05), but not immobility (Fig. 3H) or avoidance (Table 2) behavior (p > 0.05). Analysis of digging behavior across time showed a significant main effect of group (Fig. 3G, F(1,24) = 8.10, p < 0.05), time (Fig. 3G, F(9,216) = 7.16, p < 0.05) and a significant group x time interaction (Fig. 3G, F(9,216) = 6.16, p < 0.05), with the TMT group engaging in significantly greater digging compared to controls from minute 6 throughout the remainder of the session (Fig. 3G, p < 0.05). Analysis of immobility behavior across time showed a significant main effect of time (Fig. 3I, F (9,216) = 60.78, p < 0.05), but no significant main effect of group or significant group x time interaction (Fig. 3I, p < 0.05). Analysis of time spent on TMT side across time showed a significant main effect of time (Table 2, F(9,216) = 389.2, p < 0.05) but no main effect of group or significant group x time interaction (Table 2, p > 0.05). There was also no significant group difference in fecal boli (Control: 0.0 ± 0.0; TMT-1: 0.6 ± 0.4, p > 0.05), or plasma corticosterone (Fig. 3J, t(24) = 0.87, p > 0.05).
Behavior during the light dark test, zero maze, and acoustic startle test did not differ between Controls and TMT-1 (p > 0.05, Table 3).
Table 3.
Behavioral Screen Data
| Light/Dark Test | Acoustic Startle | Zero Maze | |||
|---|---|---|---|---|---|
| Group | Time in Light (%) | Total Distance (cm) | Avg. Startle Amplitude (mV) | Open Arm Time (%) | |
| Male | Control | 18.6 ± 5.7 | 1461.2 ± 113.7 | 110.3 ± 17.2 | 9.4 ± 2.0 |
| TMT-1 | 18.7 ± 2.8 | 1275.0 ± 86.2 | 139.7 ± 31.3 | 8.1 ± 1.7 | |
| Female | Control | 22.6 ± 4.2 | 1462.8 ± 128.2 | 74.1± 8.8 | 10.3 ± 2.0 |
| TMT-1 | 20.5 ± 5.0 | 1787.7 ± 169.3 | 110.6 ± 13.2# | 12.4 ± 1.2 | |
| TMT-2 | 27.1 ± 5.5 | 1717.0 ± 71.4 | 61.3 ± 13.3 | 10.4 ± 1.9 | |
Mean ± SEM
p ≤ 0.05 vs. TMT-2
Alcohol self-administration.
During alcohol self-administration post-TMT exposure, male rats showed a significant main effect of session for alcohol lever responses (Fig. 3K, F (9,216) = 8.61, p < 0.05), but no main effect of group or significant time x group interaction (p > 0.05). For alcohol intake (g/kg), male rats showed no significant main effect of time, group and no significant time x group interaction (Fig. 3L, p > 0.05). These results show that TMT exposure did not affect ongoing alcohol self-administration in male rats following TMT exposure.
3.3. Effects of TMT exposure on stress-reactive behaviors, context reactivity, and alcohol self-administration in female rats.
TMT exposure.
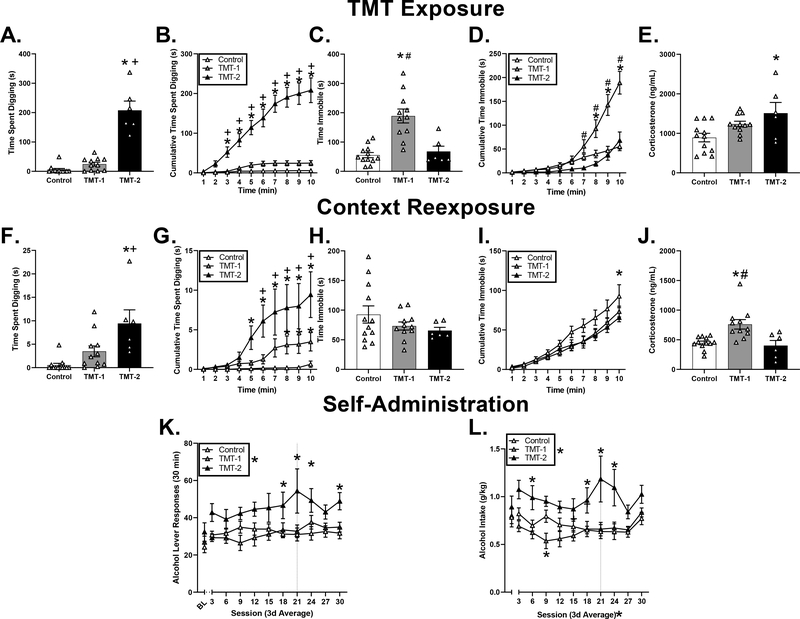
Female rats in TMT-2 engaged in significantly more digging behavior compared to controls and TMT-1 (Fig. 4A, Fig. 4A, F(2,26) = 65.03, p < 0.05), with 40% of the time digging on the TMT side for TMT-1 and 83% for TMT-2. Analysis of cumulative digging showed a significant main effect of group (Fig. 4B, F(2,26) = 65.35, p < 0.05), time (Fig. 4B, F(2,26) = 99.34, p < 0.05) and a significant group x time interaction (Fig. 4B, F(18,234) = 52.42, p < 0.05). Females in TMT-2 spent significantly more time digging from minute 3 and throughout the remainder of the exposure relative to the controls and TMT-1 (Fig. 4B, p < 0.05), indicating the emergence of digging behavior early in the session. Female rats in TMT-1 spent more time immobile than controls or TMT-2 (Fig. 4C, F(2,26) = 18.28, p < 0.05). Analysis of immobility across time showed a significant main effect of group (Fig. 4D, F(2,26) = 9.03, p < 0.05), time (Fig. 4D, F(9,234) = 59.05 and a significant group x time interaction (Fig. 4D, F(2,26) = 14.32, p < 0.05). TMT-1 showed significantly greater time immobile compared to TMT-2 by minute 7 and controls by minute 8 throughout the remainder of the exposure (p < 0.05), indicating that immobility behavior emerged later in the session.
Figure 4. Effects of TMT exposure on stress-reactive behaviors, context reactivity, and alcohol self-administration in female rats.
Female rats in TMT-2 performed significantly more defensive digging compared to controls and TMT-1 (A), as well as across the 10 min TMT exposure (B). Female rats in TMT-1 exhibited significantly more time immobile compared to controls and TMT-2 (C), as well as across 10 min of the TMT exposure (D). Female rats in TMT-2 exhibited higher plasma corticosterone levels compared to controls (E). During context re-exposure, female rats in TMT-2 performed greater total time digging (F), as well as across time (G) compared to controls. There were no changes in total time immobile in TMT-2 (H); however, across time, TMT-2 performed significantly lower immobility behavior compared to controls (I). Female rats in TMT-1 exhibited greater plasma corticosterone levels compared to controls and TMT-2. Female rats in TMT-2 performed significantly greater alcohol lever responses compared to controls (K) and alcohol intake (g/kg) (L) compared to controls. For panels K and L, BL (baseline average) is represented to the left of the axis break. * p < 0.05 vs. controls, # p < 0.05 vs. TMT-2, + p < 0.05 vs. TMT-1. Mean ± SEM. Dotted line denotes moving to MWF sessions. * by group labels = main effect of group, X-axes * = main effect of time or session.
Rats in both TMT subgroups showed less time on the TMT side relative to controls (Control: 385.2 ± 9.6, TMT-1: 114.3 ± 4.1, TMT-2: 275.5 ± 31.7; F(2,26) = 124.4, p < 0.05), and TMT-1 avoided the TMT side more than TMT-2 (p < 0.05). Analysis of the distribution of time on the TMT side across the session showed a significant main effect of group (Table 2, F(2,26) = 178.9, p < 0.05), time (Table 2, F(9,234) = 548.0, p < 0.05) and a significant group x time interaction (Table 2, F(2,26) = 56.96, p < 0.05). TMT-1 and 2 spent significantly less time on the TMT side compared to controls from minute 2 and throughout the remainder of the session (p < 0.05). Interestingly, TMT-2 spent significantly more time on the TMT side compared to TMT-1 from minute 5 throughout the remainder of the session, likely due to the continuation of engagement of digging behavior on the TMT side.
Examination of fecal boli showed a significant main effect of TMT (F(2,26) = 7.71, p < 0.05) with both TMT-1 (2.73 ± 0.66) and 2 (4.2 ± 1.5) showing more fecal boli compared to controls (0.3 ± 0.2; p < 0.05). Plasma corticosterone levels were significantly increased following TMT exposure (Fig. 4E, F(2,26) = 5.05, p < 0.05), with significantly higher plasma corticosterone levels in TMT-2 compared to controls (Fig. 4E, p < 0.05).
Context re-exposure and behavioral screens.
During context re-exposure TMT-2 engaged in significantly more digging compared to controls and TMT-1 (Fig. 4F, F(2,26) = 9.834, p < 0.05). Examination of cumulative digging across time showed a significant main effect of group (Fig. 4G, F(2,26) = 9.62), time (Fig. 4G, F(18,234) = 20.55), and a significant group x time interaction (Fig. 4G, F(2,26) = 6.69, p < 0.05). TMT-2 engaged in significantly more digging compared to controls and TMT-1 from minute 5 through the duration of the exposure (p ≤ 0.05), indicating context-induced behavioral reactivity TMT-1 also engaged in significantly more digging compared to controls from minute 8 through the duration of the experiment (p < 0.05).
Examination of total time immobile during context re-exposure showed no significant main effect of TMT in females (Fig. 4H) (p > 0.05). Analysis of cumulative time immobile across time showed a significant main effect of time (Fig. 4I, F (9,234) = 88.27, p < 0.05), a significant time x group interaction (Fig. 4H, F(18,234) = 1.7, p < 0.05), but no main effect of group (p > 0.05). Female rats in TMT-2 spent significantly less time immobile compared to controls by minute 10 of the context re-exposure.
Analysis of total time spent on the side in which TMT was located showed no significant main effect of TMT (Table 2, p > 0.05). Examination of cumulative time spent on TMT side across time showed a significant main effect of time (Table 2, F(9,234) = 710.9, p < 0.05) but no significant main effect of group or significant group x time interaction (p > 0.05). There was also no main effect of TMT in fecal boli production (Control: 0.00 ± 0.00, TMT-1: 0.64 ± 0.39, TMT-2: 0.00 ±0.00; p > 0.05). Analysis of plasma corticosterone levels showed significant group difference (Fig. 4J, F(2,26) = 9.22, p < 0.05), with higher levels of TMT-1 compared to controls and TMT-2 (Fig. 4J, p < 0.05).
Analysis of anxiety-like behavior during the light dark test and the zero maze showed no significant differences between TMT subgroups and controls (Table 3). However, in the acoustic startle test, there was a significant main effect of TMT group (Table 3; F (2,26) = 4.40, p < 0.05), however, this difference was driven by TMT-1 having higher average startle amplitude relative to TMT-2 (p<0.05), and neither subgroup differed from controls.
Alcohol self-administration.
Analysis of self-administration showed a significant main effect of group on alcohol lever responses (Fig. 4K, F(2,26) = 6.62, p < 0.05), in which TMT-2 had significantly greater alcohol lever responses compared to TMT-1 and controls. There was no main effect of session and no interaction. However, to further explore the group difference in self-administration and based on the a priori hypothesis that TMT exposure would increase alcohol self-administration, planned comparisons tests using Bonferroni correction for multiple comparisons were conducted to compare TMT-1 and 2 to controls. TMT-2 had higher alcohol lever responses compared to controls from session 18 through 24 and on session 30 (p < 0.05). Furthermore, examination of alcohol intake (g/kg), showed a significant main effect of group (Fig. 4L, F (2,26) = 11.21, p < 0.05), in which TMT-2 had significantly greater alcohol intake compared to TMT-1 and controls. There was also a significant main effect of session (F (9,234) = 2.35, p < 0.05), but no significant session by group interaction (p > 0.05). Similarly, to alcohol lever responses, to further explore the group difference Bonferroni correction for multiple comparisons were conducted to compare TMT-1 and 2 to controls. TMT-2 had higher alcohol intake compared to controls during session 6 and from session 18–24 (p ≤ 0.05). Furthermore, TMT-1 showed significantly lower alcohol intake (g/kg) compare to controls during session 9. There no changes in locomotor behavior or inactive lever responses between controls and TMT-in male and female rats (Table 4). These results indicate female rats in TMT-2 that engage in a greater proportion of digging behavior (active coping behavior) to immobility behavior (passive coping behavior) during TMT are more likely to show increases in alcohol self-administration.
Table 4.
Locomotor rate and Inactive Lever Responses during 30 session of self-administration
| Locomotor Rate (Bean breaks/min) (3d Avg.) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | BL | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | |
| Male | Control | 27.4 ± 4.6 | 25.1 ± 6.3 | 25.2 ± 6.1 | 23.5 ± 6.2 | 23.1 ± 6.2 | 22.5 ± 4.4 | 24.0 ± 5.5 | 22.7 ± 6.2 | 23.9 ± 6.1 | 24.1 ± 7.9 | 24.0 ± 8.4 |
| TMT-1 | 27.5 ± 10.6 | 24.7 ± 4.0 | 23.0 ± 3.0 | 23.0 ± 5.0 | 21.1 ± 4.6 | 21.6 ± 3.3 | 20.7 ± 4.3 | 21.6 ± 4.7 | 21.7 ± 4.1 | 21.5 ± 5.1 | 22.3 ± 5.7 | |
| Female | Control | 27.3 ± 1.5 | 30.3 ± 1.7 | 26.7 ± 1.3 | 28.0 ± 1.8 | 28.4 ± 1.9 | 28.1 ± 1.7 | 29.9 ± 2.3 | 26.1 ± 2.2 | 30.0 ± 2.4 | 31.5 ± 2.5 | 27.3 ± 2.6 |
| TMT-1 | 26.2 ± 1.8 | 29.3 ± 1.6 | 27.2 ± 1.6 | 29.7 ± 1.9 | 28.8 ± 2.2 | 28.2 ± 1.8 | 29.5 ± 2.1 | 29.7 ± 1.8 | 30.1 ± 2.3 | 28.9 ± 2.4 | 29.6 ± 2.5 | |
| TMT-2 | 34.5 ± 2.9 | 31.9 ± 1.4 | 26.2 ± 1.9 | 28.9 ± 2.6 | 27.9 ± 1.8 | 27.8 ± 2.4 | 29.0 ± 2.6 | 28.7 ± 2.2 | 29.3 ± 1.9 | 30.0 ± 1.9 | 29.5 ± 2.3 | |
| Inactive Lever Responses (3d Avg.) | ||||||||||||
| Male | Control | 2.7 ± 1.5 | 2.6 ± 2.9 | 3.3 ± 3.5 | 3.3 ± 4.4 | 2.9 ± 3.5 | 2.2 ± 1.8 | 3.5 ± 4.8 | 2.3 ± 3.2 | 3.6 ± 4.6 | 2.6 ± 3.0 | 3.4 ± 3.3 |
| TMT-1 | 2.9 ± 2.1 | 3.2 ± 1.7 | 2.3 ± 0.9 | 1.5 ± 1.1 | 1.7 ± 1.0 | 1.5 ± 0.8 | 1.2 ± .9 | 1.4 ± 1.5 | 2.0 ± 1.3 | 1.6 ± 1.6 | 3.4 ± 2.1 | |
| Female | Control | 2.9 ± 0.7 | 2.8 ± 0.6 | 1.9 ± 0.6 | 2.5 ± 0.5 | 2.0 ± 0.5 | 1.9 ± 0.3 | 2.1 ± 0.7 | 1.3 ± 0.4 | 1.3 ± 0.3 | 2.3 ± 0.6 | 2.0 ± 0.6 |
| TMT-1 | 1.7 ± 0.65 | 2.0 ± 0.5 | 2.7 ± 0.6 | 2.1 ± 0.5 | 2.4 ± 0.7 | 1.8 ± 0.5 | 1.5 ± 0.5 | 1.7 ± 0.6 | 1.5 ± 0.5 | 1.2 ± 0.5 | 1.6 ± 0.3 | |
| TMT-2 | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.5 ± 0.4 | 2.2 ± 0.6 | 2.5 ± 0.7 | 2.4 ± 0.5 | 2.4 ± 0.6 | 2.2 ± 0.6 | 2.8 ± 0.6 | 1.6 ± 0.4 | 1.6 ± 0.4 | |
Mean ± SEM
4. Discussion
In the current study we sought to examine if individual differences in stress-reactive behaviors during TMT exposure (a ratio of defensive digging/immobility) were related to subsequent context reactivity, hyperarousal, anxiety-like behavior and alcohol self-administration in male and female rats. The results demonstrate several important findings. First, during TMT exposure, male rats showed low digging/immobility ratio scores resulting in all male rats to be grouped into TMT-1 (no TMT-2). This data pattern indicates that male rats engaged in a higher proportion of passive coping behavior to active coping behavior; however, in female rats, there were two distinct subgroup of rats that engaged in more active coping behaviors (TMT-2; high digging/immobility ratio scores) or passive coping behaviors (TMT-1; low digging/immobility scores). Third, males and females exposed to TMT showed enhanced behavioral reactivity to the TMT-paired context, suggesting a contextually-cued stress memory. Lastly, female rats that engaged in greater active coping behaviors during TMT exposure (TMT-2) showed increases in alcohol self-administration relative to controls that emerged 30 days (18 sessions) following TMT exposure, indicating a lasting consequence of stressor exposure. In contrast, males and females that engaged in greater passive coping behavior during the TMT exposure showed decreased or no increases in self-administration relative to controls. Together, these data suggest that stress-reactive behaviors during predator odor stressor exposure using TMT may be helpful in understanding individual differences in stress-reactivity and the subsequent impact of stress on alcohol drinking.
TMT elicits innate fear and stress-related behavioral responses including freezing [34, 40], immobility [27, 36] and avoidance behavior [27, 35, 36, 41]. Here, bedding was present in the TMT exposure context so that we could examine an additional stress-reactivity behavior, defensive digging [27]. Defensive digging during TMT exposure involved the rat actively moving bedding material toward the corner of the chamber directly below the basket that held the TMT. Previous studies have defined such behavior as defensive burying [29, 30]; however, as the rat was not actively burying an object but burrowing the head, forepaws or entire body into the bedding and performing shoveling movements, we categorized the behavior as defensive digging. Here, we show male and female rats engaged in similar amount of total time digging during the TMT exposure (Figure 2B), consistent with previous work defensive burying [42] and defensive digging [35] findings. Interestingly, during TMT exposure, both sexes showed a shift in behavioral responses, transitioning from defensive digging to immobility and avoidance behavior. This is reflected in examination of the cumulative digging and immobility graphs that show in male and female rats in the TMT-1 group, digging behavior plateaued (i.e., no further engagement in the behavior), after minute 6 (Figure 3B, 4B, respectively), while immobility behavior started to increase after minute 5 and throughout the remainder of the exposure (Figure 3D, 4D, respectively). In the female TMT-2 group, the rate of digging behavior began to slow near the end of the session (after min 8; Figure 4B), around the same time the rats started to engage in immobility behavior (Figure 4D). This transition has been described as a stress-induced shift in coping behavior, in which rats will shift from active to passive coping strategies [31, 43].
Males engaging in greater immobility behavior than females is in accordance with previous studies showing freezing behavior is a male-typical behavior that has been frequently studied in fear conditioning and extinction studies [44, 45]. Studies using TMT show male rats engage in diverse defensive behaviors including immobility and avoidance when exposed in larger chambers, whereas in a smaller inescapable chamber, rats engage in freezing behavior, suggestive of a fear response [34]. [35]. This high level of immobility behavior in males resulted in all males being classified into TMT-1, indicating that all of the male rats engaged in passive coping responses during TMT exposure. Using a cut-off strategy allowed the formation of subgroups based on the stress reactivity behavior, however, it is important to consider the limitations of this strategy. For example, in the female TMT-2 group, there are 2 rats that have a ratio <2 (but >1). Therefore, it is important to consider if there could be a meaningful biological difference between these rats and the remainder of TMT-2. Future work, using larger sample sizes to dissect these differences will be an important direction.
We show that following TMT exposure increased plasma corticosterone was observed in males of TMT-1 and females of TMT-2, indicating a TMT-induced physiological response. It is also important to acknowledge the high corticosterone levels in the female control rats, which indicates the likelihood that the water exposure induced a stress response, although not elevated to the same degree as TMT-2. As the controls are placed in the same type of exposure chamber as the TMT group, this elevation in corticosterone level is likely a reflection of being placed in the novel inescapable environment, which alone is likely to produce an elevation in corticosterone.
Two out of the four clusters of diagnostic criteria for PTSD within the DSM-5 include 1) intrusive distressing memories of the traumatic event(s) and 2) persistent avoidance of the stimuli associated with the traumatic event(s). In animal models, re-exposing animals to stress-related stimuli or the environment in which the stressor was presented can induce contextual fear or stress responses that can serve as an index of memory of that context. Behaviorally, in the present study, this was evidenced by increased digging in the male rats in TMT-1 and female rats in both TMT subgroups relative to controls during the context re-exposure. It is important to note that the overall degree of digging behavior during context re-exposure was much less than during the TMT exposure, likely given the different conditions of the exposure (i.e., with TMT and without). While it may not be unexpected that female rats in TMT-2 that engaged in digging behavior during TMT exposure also showed digging behavior during context re-exposure, this is an important finding that shows recruitment of the same coping strategy in the absence of the TMT stressor. In fact, the males in TMT-1 showed increased digging later in the session during context re-exposure, and the females in TMT-1 also showed the emergence of increased digging late in the session. Presumably, re-exposure to the TMT context in the absence of TMT is a less stressful experience than the TMT exposure. Therefore, it is possible to consider that there is a threshold for the engagement in digging behavior such that an animal that uses a passive coping strategy under more stressful conditions shows a shift to a more active strategy under less stressful condition. Furthermore, avoidance of the TMT-paired side of the chamber was not observed during context re-exposure. This is not altogether surprising given that it has been shown that although TMT produces significant avoidance behavior during exposure, TMT has not been shown to induce context conditioning through avoidance behavior ([26]). Interestingly, females in TMT-1 showed increased corticosterone levels during context re-exposure. Collectively, these data indicate that TMT can produce contextual conditioning, which is consistent with previous work [22], as evidenced by rodents engaging in similar stress-reactive behaviors as during the initial stressor exposure.
Increased startle response during the acoustic startle paradigm is an indicator of hyperarousal behavior, which is a core symptom profile of PTSD [1]. Animal models of traumatic stress, including TMT [46], can trigger increases in hyperarousal responses. We hypothesized that rats with higher digging/immobility ratio scores, would show anxiety-like and hyperarousal behavior post-TMT exposure. Contrary to our hypothesis, we did not observe changes in startle response (i.e. hyperarousal) in TMT-subgroups compared to controls in the males or females. The lack of a hyperarousal response following TMT remains unclear, but future work could manipulate different startle intensity or examine prepulse inhibition to further dissect this behavioral difference. Additionally, there was no change in approach/avoidance behavior (e.g., anxiety-like behavior) in the light/dark test and zero maze in males or females following TMT exposure. This was consistent with previous work from our lab showing male rats exposed to a single or repeated TMT exposure showed no changes in anxiety-like behavior, measured by the elevated plus maze, or hyperarousal behavior, measured by acoustic startle response [48]. In the current study, these behavioral tests occurred 7–9 days post-TMT exposure, which is consistent with timing from previous studies that show elevated anxiety-like and hyperarousal behavior following predator odor stress (including TMT) that persist over extended periods [22, 24, 49]. However, these previous studies used Sprague-Dawley rats in the elevated plus maze, while the current study assessed anxiety-like behavioral tests using light/dark test and elevate zero maze in Long-Evans male and female rats. Therefore, it is possible the lack of effect in anxiety-like behavior is due to differences in strain and testing paradigms.
Examining how individual differences in response to stress alters alcohol self-administration can lead to a better understanding of behavioral adaptations that persist to modulate long lasting increases in alcohol consumption. Previous studies have examined individual patterns in response to stress and how these differences may potentiate alcohol drinking [13, 15, 16]. However, only a limited number of studies [14] have examined these patterns in both males and females. In the current work, we sought to determine whether individual differences in stress reactivity during TMT exposure as determined by the TMT subgroups would be related to subsequent alcohol drinking. First, we observed increases in self-administration in female rats in TMT-2 (high digging/low immobility) relative to controls following TMT exposure. In contrast, the females in TMT-1 (low digging/high immobility) showed a decrease in alcohol intake (g/kg) on session 9 relative to controls, and males in TMT-1 group did not differ from controls. Together, these findings suggest a relation between the engagement of an active coping strategy and escalated alcohol self-administration. Males and females in TMT-1, engaged in a higher proportion of passive coping behavior, which suggests that this stress coping strategy may act as a protective behavioral coping strategy to prevent increases in alcohol drinking after stress exposure. Second, these increases in alcohol self-administration began to emerge 24 days (12 sessions) after exposure to TMT, suggesting a delay in the increase of alcohol drinking after stress exposure. This is in line with other studies that have shown increases and long-term (1–3 weeks) persistence in alcohol drinking after exposure to different models of predator odor stress [13, 15, 16], and other work from our lab showing changes in gene expression that emerge 4 weeks following TMT exposure [27]. Previous work from our lab has shown TMT-induced increases in alcohol consumption in male rats; however, in this experiment rats were exposed to TMT in a smaller exposure chamber without bedding and animals consumed sweetened alcohol during self-administration [48]. While there is existing literature on the impact of predator stress and predator odor stress on alcohol drinking in male and female rodents, the results vary between species, strain and stress parameters. For example, a single exposure to dirty rat bedding in mice increased female drinking during access to 2-bottle choice immediately following stressor exposure but suppressed consumption in males [50], while repeated exposure only produced an increase in alcohol intake in a subset of female mice regardless of prior alcohol history [17]. Therefore, this study adds to the limited preclinical data on differences in stress-alcohol interactions by examining individual differences in TMT-induced long-term alcohol consumption in an operant conditioning model in both male and female Long-Evans rats.
There are some potential neurobiological mechanisms that could explain why female, but not male rats showed increases in alcohol self-administration after stress. First, the mechanisms by which stress impacts alcohol drinking may be different between male and female rats. For example, female, but not male rats show greater ethanol-induced inhibition on action potential firing in basolateral amygdala (BLA) neurons following exposure to the single prolonged stress (SPS) model, suggesting that ethanol plays a larger role in modulating stress-induced excitability in females [51]. Additionally, female rats that show an increased in ethanol intake after exposed to dirty rat bedding [17], also show an increase in p450scc (PFC and hippocampus), GABAARα2 (PFC-only) and synaptophysin (hippocampus-only), all of which play a potential role in addictive behaviors and behavioral responses to stress [52]. Future studies will focus on understanding the neurobiological changes in males and females after stressor exposure and how these changes could elucidate potential mechanisms associated with resiliency and susceptible populations to stress.
In conclusion, the current study showed that a rodent model of inescapable, uncontrollable predator odor stress (TMT), produced individual differences in stress-reactive behaviors, specifically in females. In contrast, all males exhibited a lower proportion of digging to immobility behavior during TMT, indicating engagement in a great proportion of passive coping responses compared to active coping during stressor exposure. Furthermore, in females, high defensive digging/low immobility behavior during TMT exposure were related to increases in alcohol self-administration, but this was not observed in males or females that engaged in low digging/high immobility. These data patterns suggest that immobility behavior during TMT exposure may act as a protective behavioral coping strategy to prevent increases in alcohol drinking after stress exposure. All together, these data suggest that stress-reactive behaviors during predator odor stressor exposure using TMT can elucidate specific behavioral phenotypes in male and female rats offering insight into individual differences in stress-reactivity. In addition, these results suggest lasting consequences of TMT on alcohol self-administration in females that engage in more active coping, which can help to further understand the impact of stress on subsequent drinking.
Highlights.
Exposure to TMT produces distinct behavioral phenotypes in male and female rats.
Prior exposure to TMT produces enhanced behavioral reactivity to TMT-paired context.
Active coping behavior during TMT related to increased alcohol drinking in females.
Stress-reactivity produces individual differences to stress and alcohol drinking.
Individual differences in stress-reactivity can impact alcohol drinking.
Acknowledgement
This work was supported in part by the National Institute of Health AA026537 and AA011605 (JB) and by the Bowles Center for Alcohol Studies. LCO was supported by Diversity Supplement to AA026537. RET was supported by NS007431. The authors thank Jiaqi Liu, Benjamin Weinberg and Abigail Garcia-Baza for their help with behavioral analysis.
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
Data available on request from the authors.
References
- 1.Association, A.P. Diagnostic and statistical manual of mental disorders 5th ed. 2013, Washington, DC. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, and Walters EE Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 2005. 62(6): p. 593–602. [DOI] [PubMed] [Google Scholar]
- 3.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, and Friedman MJ National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress, 2013. 26(5): p. 537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, and Anthony JC Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry, 1997. 54(4): p. 313–21. [DOI] [PubMed] [Google Scholar]
- 5.Sonne SC, Back SE, Diaz Zuniga C, Randall CL, and Brady KT Gender differences in individuals with comorbid alcohol dependence and post-traumatic stress disorder. Am J Addict, 2003. 12(5): p. 412–23. [PubMed] [Google Scholar]
- 6.Lehavot K, Stappenbeck CA, Luterek JA, Kaysen D, and Simpson TL Gender differences in relationships among PTSD severity, drinking motives, and alcohol use in a comorbid alcohol dependence and PTSD sample. Psychol Addict Behav, 2014. 28(1): p. 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralevski E, Southwick S, and Petrakis I Trauma- and Stress-Induced Craving for Alcohol in Individuals Without PTSD. Alcohol Alcohol, 2020. 55(1): p. 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor KM and Davidson JR Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety, 2003. 18(2): p. 76–82. [DOI] [PubMed] [Google Scholar]
- 9.Russo SJ, Murrough JW, Han MH, Charney DS, and Nestler EJ Neurobiology of resilience. Nat Neurosci, 2012. 15(11): p. 1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers A and Clark DM A cognitive model of posttraumatic stress disorder. Behav Res Ther, 2000. 38(4): p. 319–45. [DOI] [PubMed] [Google Scholar]
- 11.Charney DS Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry, 2004. 161(2): p. 195–216. [DOI] [PubMed] [Google Scholar]
- 12.Yehuda R and LeDoux J Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron, 2007. 56(1): p. 19–32. [DOI] [PubMed] [Google Scholar]
- 13.Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, and Gilpin NW Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry, 2013. 3: p. e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albrechet-Souza L, Schratz CL, and Gilpin NW Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biol Sex Differ, 2020. 11(1): p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manjoch H, Vainer E, Matar M, Ifergane G, Zohar J, Kaplan Z, and Cohen H Predator-scent stress, ethanol consumption and the opioid system in an animal model of PTSD. Behav Brain Res, 2016. 306: p. 91–105. [DOI] [PubMed] [Google Scholar]
- 16.Weera MM, Schreiber AL, Avegno EM, and Gilpin NW The role of central amygdala corticotropin-releasing factor in predator odor stress-induced avoidance behavior and escalated alcohol drinking in rats. Neuropharmacology, 2020. 166: p. 107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn DA, Helms ML, Nipper MA, Cohen A, Jensen JP, and Devaud LL Sex differences in the synergistic effect of prior binge drinking and traumatic stress on subsequent ethanol intake and neurochemical responses in adult C57BL/6 J mice. Alcohol, 2018. 71: p. 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King CE and Becker HC Oxytocin attenuates stress-induced reinstatement of alcohol seeking behavior in male and female mice. Psychopharmacology (Berl), 2019. 236(9): p. 2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dopfel D, Perez PD, Verbitsky A, Bravo-Rivera H, Ma Y, Quirk GJ, and Zhang N Individual variability in behavior and functional networks predicts vulnerability using an animal model of PTSD. Nat Commun, 2019. 10(1): p. 2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen H and Zohar J An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann N Y Acad Sci, 2004. 1032: p. 167–78. [DOI] [PubMed] [Google Scholar]
- 21.Shallcross J, Hamor P, Bechard AR, Romano M, Knackstedt L, and Schwendt M The Divergent Effects of CDPPB and Cannabidiol on Fear Extinction and Anxiety in a Predator Scent Stress Model of PTSD in Rats. Front Behav Neurosci, 2019. 13: p. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwendt M, Shallcross J, Hadad NA, Namba MD, Hiller H, Wu L, Krause EG, and Knackstedt LA A novel rat model of comorbid PTSD and addiction reveals intersections between stress susceptibility and enhanced cocaine seeking with a role for mGlu5 receptors. Transl Psychiatry, 2018. 8(1): p. 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodnik ZD, Black EM, and Espana RA Accelerated development of cocaine-associated dopamine transients and cocaine use vulnerability following traumatic stress. Neuropsychopharmacology, 2020. 45(3): p. 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodnik ZD, Black EM, Clark MJ, Kornsey KN, Snyder NW, and Espana RA Susceptibility to traumatic stress sensitizes the dopaminergic response to cocaine and increases motivation for cocaine. Neuropharmacology, 2017. 125: p. 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albrechet-Souza L and Gilpin NW The predator odor avoidance model of post-traumatic stress disorder in rats. Behav Pharmacol, 2019. 30(2 and 3-Spec Issue): p. 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen JB, Asok A, and Chakraborty T The smell of fear: innate threat of 2,5-dihydro-2,4,5-trimethylthiazoline, a single molecule component of a predator odor. Front Neurosci, 2015. 9: p. 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyler RE, Weinberg B, Lovelock D, Ornelas LC, and Besheer J Exposure to the predator odor TMT induces early and late differential gene expression related to stress and excitatory synaptic function throughout the brain in male rats. Genes, Brain and Behavior, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbitsky A, Dopfel D, and Zhang N Rodent models of post-traumatic stress disorder: behavioral assessment. Transl Psychiatry, 2020. 10(1): p. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Boer SF and Koolhaas JM Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol, 2003. 463(1–3): p. 145–61. [DOI] [PubMed] [Google Scholar]
- 30.Arakawa H Ontogeny of sex differences in defensive burying behavior in rats: effect of social isolation. Aggress Behav, 2007. 33(1): p. 38–47. [DOI] [PubMed] [Google Scholar]
- 31.Fucich EA and Morilak DA Shock-probe Defensive Burying Test to Measure Active versus Passive Coping Style in Response to an Aversive Stimulus in Rats. Bio Protoc, 2018. 8(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riittinen ML, Lindroos F, Kimanen A, Pieninkeroinen E, Pieninkeroinen I, Sippola J, Veilahti J, Bergstrom M, and Johansson G Impoverished rearing conditions increase stress-induced irritability in mice. Dev Psychobiol, 1986. 19(2): p. 105–11. [DOI] [PubMed] [Google Scholar]
- 33.Neal S, Kent M, Bardi M, and Lambert KG Enriched Environment Exposure Enhances Social Interactions and Oxytocin Responsiveness in Male Long-Evans Rats. Front Behav Neurosci, 2018. 12: p. 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace KJ and Rosen JB Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci, 2000. 114(5): p. 912–22. [DOI] [PubMed] [Google Scholar]
- 35.Homiack D, O’Cinneide E, Hajmurad S, Dohanich GP, and Schrader LA Effect of acute alarm odor exposure and biological sex on generalized avoidance and glutamatergic signaling in the hippocampus of Wistar rats. Stress, 2018. 21(4): p. 292–303. [DOI] [PubMed] [Google Scholar]
- 36.Homiack D, O’Cinneide E, Hajmurad S, Barrileaux B, Stanley M, Kreutz MR, and Schrader LA Predator odor evokes sex-independent stress responses in male and female Wistar rats and reduces phosphorylation of cyclic-adenosine monophosphate response element binding protein in the male, but not the female hippocampus. Hippocampus, 2017. 27(9): p. 1016–1029. [DOI] [PubMed] [Google Scholar]
- 37.Metna-Laurent M, Soria-Gomez E, Verrier D, Conforzi M, Jego P, Lafenetre P, and Marsicano G Bimodal control of fear-coping strategies by CB(1) cannabinoid receptors. J Neurosci, 2012. 32(21): p. 7109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, and Blokhuis HJ Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev, 1999. 23(7): p. 925–35. [DOI] [PubMed] [Google Scholar]
- 39.Makhijani VH, Van Voorhies K, and Besheer J The mineralocorticoid receptor antagonist spironolactone reduces alcohol self-administration in female and male rats. Pharmacol Biochem Behav, 2018. 175: p. 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asok A, Ayers LW, Awoyemi B, Schulkin J, and Rosen JB Immediate early gene and neuropeptide expression following exposure to the predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT). Behav Brain Res, 2013. 248: p. 85–93. [DOI] [PubMed] [Google Scholar]
- 41.Hwa LS, Neira S, Pina MM, Pati D, Calloway R, and Kash TL Predator odor increases avoidance and glutamatergic synaptic transmission in the prelimbic cortex via corticotropin-releasing factor receptor 1 signaling. Neuropsychopharmacology, 2019. 44(4): p. 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Falconer EM and Galea LA Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res, 2003. 975(1–2): p. 22–36. [DOI] [PubMed] [Google Scholar]
- 43.Fucich EA, Paredes D, and Morilak DA Therapeutic Effects of Extinction Learning as a Model of Exposure Therapy in Rats. Neuropsychopharmacology, 2016. 41(13): p. 3092–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gruene TM, Flick K, Stefano A, Shea SD, and Shansky RM Sexually divergent expression of active and passive conditioned fear responses in rats. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shansky RM Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress, 2015. 1: p. 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebb AL, Zacharko RM, Gauthier M, and Drolet G Exposure of mice to a predator odor increases acoustic startle but does not disrupt the rewarding properties of VTA intracranial self-stimulation. Brain Res, 2003. 982(2): p. 195–210. [DOI] [PubMed] [Google Scholar]
- 47.Pooley AE, Benjamin RC, Sreedhar S, Eagle AL, Robison AJ, Mazei-Robison MS, Breedlove SM, and Jordan CL Sex differences in the traumatic stress response: PTSD symptoms in women recapitulated in female rats. Biol Sex Differ, 2018. 9(1): p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makhijani VH, Franklin JP, Van Voorhies K, Fortino B, and Besheer J The synthetically produced predator odor 2,5-dihydro-2,4,5-trimethylthiazoline increases alcohol self-administration and alters basolateral amygdala response to alcohol in rats. Psychopharmacology (Berl), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim J, Igarashi M, Jung KM, Butini S, Campiani G, and Piomelli D Endocannabinoid Modulation of Predator Stress-Induced Long-Term Anxiety in Rats. Neuropsychopharmacology, 2016. 41(5): p. 1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cozzoli DK, Tanchuck-Nipper MA, Kaufman MN, Horowitz CB, and Finn DA Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol, 2014. 48(8): p. 741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ornelas LC and Keele NB Sex Differences in the Physiological Response to Ethanol of Rat Basolateral Amygdala Neurons Following Single-Prolonged Stress. Front Cell Neurosci, 2018. 12: p. 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devaud LL, Alavi M, Jensen JP, Helms ML, Nipper MA, and Finn DA Sexually divergent changes in select brain proteins and neurosteroid levels after a history of ethanol drinking and intermittent PTSD-like stress exposure in adult C57BL/6J mice. Alcohol, 2020. 83: p. 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.