Abstract
Liver cancer is a leading cause of cancer deaths worldwide. Hepatocellular carcinoma (~75–85%) and cholangiocarcinoma (~10–15%) account for the majority of primary liver malignancies. Patients with primary liver cancer are often diagnosed with unresectable diseases and do not respond well to current therapies. The liver is also a common site of metastasis. Liver metastasis is difficult to treat, and the prognosis is poor. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells with immunosuppressive activity. MDSCs are an important component of the tumor microenvironment and promote tumor progression through various mechanisms. MDSCs expand in both liver cancer patients and mouse liver cancer models. Importantly, MDSCs correlate with poor clinical outcomes for liver cancer patients. The tumor-promoting functions of MDSCs have also been shown in mouse liver cancer models. All these studies suggest that targeting MDSCs can potentially benefit liver cancer treatment. This review summarizes the current findings of MDSC regulation in liver cancer and related disease conditions.
Liver Cancer
Liver cancer is a leading and growing cause of cancer-related deaths worldwide [1–3]. Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) make up the majority of primary liver cancer. HCC predominantly affects men, comprises 75–85% of primary liver cancer, and ranks as the fifth most common malignancy[1, 2]. HCC often arises from cirrhosis and is closely related to chronic liver diseases[2, 4]. Viral hepatitis and excessive alcohol consumption are major risk factors for HCC. Recently, nonalcoholic fatty liver disease (NAFLD) has been established as an important risk factor for HCC[5, 6]. CCA is the second most common (~10–15%) primary hepatic malignancy and arises from bile duct epithelium. Established risk factors for CCA include primary sclerosing cholangitis (PSC), choledochal cysts, fibropolycystic liver disease, hepatolithiasis, parasitic infection, and toxic exposure[3, 7]. However, CCA patients often do not have any of these risk factors. Less established risk factors include inflammatory bowel disease, viral hepatitis, cirrhosis, obesity, diabetes, alcohol consumption and tobacco use[8, 9]. Surgical resection and liver transplantation can potentially cure early liver cancer; however, most liver cancer patients are diagnosed with unresectable disease and generally respond poorly to current treatment options[1, 2, 7]. Despite the improvements of modern cancer treatments and increased survival, liver cancer is among the few cancers with a rising mortality rate.
Metastasis, the spreading of cancer cells from the primary site to surrounding tissue and distant organs, is the primary cause of cancer-related death[10]. The liver is a common site for metastatic spread of primary gastrointestinal, breast, and lung cancers. Metastatic liver disease accounts for the majority (95%) of all hepatic cancers[11]. Treating liver metastasis is difficult, and often patients have a poor prognosis.
Hepatic accumulation of tumoral MDSCs
The abnormal expansion of immature myeloid populations is common in cancer. [12–14]. The increased immature myeloid cells are also functionally altered and demonstrate potent immunosuppressive activities. Thus, these cells are named myeloid-derived suppressor cells (MDSCs). MDSCs are highly heterogeneous and are grouped into two primary subsets: polymorphonuclear MDSC (PMN-MDSCs) and monocytic MDSCs (M-MDSCs)[12–14]. Mouse MDSCs are CD11b+Gr1+ cells and contain CD11b+Ly6G+Ly6Clo PMN-MDSCs and CD11b+Ly6G−Ly6Chi M-MDSCs. Although human MDSCs can be divided into PMN-MDSCs and M-MDSCs as well, the surface markers are more complex. The detailed phenotyping of MDSC has been reviewed[15]. MDSCs originate from normal myeloid lineage differentiation. PMN-MDSCs resemble neutrophils, and M-MDSCs share markers with monocytes, macrophages and dendritic cells. MDSCs maintain the capacity to differentiate into mature myeloid cells such as dendritic cells or macrophages[16]. Although several MDSC specific markers such as Lectin-type oxidized LDL receptor-1 (LOX-1) and MDSC associated gene signatures have been suggested, it is often challenging to phenotypically separate MDSCs from their myeloid counterparts, thereby immunosuppressive function remains the standard for defining MDSCs [15, 17, 18].
Tumor-derived factors such as CM-CSF are critical to trigger MDSC proliferation and differentiation[19–21]. After being generated in the bone marrow, MDSCs migrate to peripheral organs and tumor tissue. It appears that MDSCs use the same chemotactic mechanisms as their myeloid counterparts for recruitment[19, 22]. CCL2 (MCP-1) and CCL5 (RANTES) are the main chemokines for monocyte/M-MDSC migration. CXC chemokines, including CXCL1 (KC), CXCL5, CXCL6, CXCL8 (IL-8) and CXCL12 (SDF-1) mediate the recruitment of granulocytes/PMN-MDSCs. Additionally, local hematopoiesis contributes to tissue-specific MDSC expansion.
Hepatic MDSC accumulation occurs in various gastrointestinal (GI) diseases. The liver receives ~75% of blood supply via portal circulation, which carries a large amount of intestinal microbial products. The gut microbiome influences the liver through the gut-liver axis. To defend against potential intestinal microbial invasion, the liver contains high levels of various immune cells and harbors the largest macrophage population, Kupffer cells, in the body[23, 24]. While Kupffer cells are resident and self-renewing at homeostasis, in the event of Kupffer cell deletion, the liver has a high capacity to recover myeloid populations by hepatic recruitment of blood monocytes [25, 26]. Dysbiosis and increased gut permeability are commonly present in many chronic GI diseases such as NAFLD, cirrhosis, PSC and colitis. Emerging evidence shows that the gut microbiome is an important modulator for controlling myeloid cells in the liver. In NAFLD mice fed with a western diet, myeloid cells expanded through LPS/TLR4 signaling[27]. In cirrhosis, bacterial translocation reportedly induced IL-10 producing myeloid cells in the liver, which contribute to impaired antibacterial immunity and infection-associated mortality in liver fibrosis[28]. Our recent studies found that both PSC and colitis cause dramatic MDSC accumulation in the liver. CXCR2+PMN-MDSCs are the predominantly expanded population, and the accumulation is mediated by increased CXCL1 from hepatocytes through an LPS/TLR4/CXCL1/CXCR2 dependent mechanism [29].
The liver is a preferred organ for tumor MDSC accumulation. The homing of tumor MDSCs to the liver was first investigated by using fluorescent dye-labeled MDSCs isolated from the spleen of tumor-bearing mice[30]. The transferred MDSCs were mainly found in the liver and spleen, regardless of the tumor-bearing condition of the recipient mice. Studies from groups, including our own, confirmed that hepatic MDSC accumulation is a general phenomenon in mouse tumor models[31, 32]. An increase of both M-MDSCs and PMN-MDSCs was observed, although the degree varies among cancer types and is influenced by tumor burden. Liver expression of CCL2 and CXCL1 mediate hepatic MDSC recruitment[31], and hepatic hematopoiesis also contributed to liver MDSC expansion[30].
As a component of the tumor microenvironment, MDSCs suppress anti-tumor immunity and promote tumor progression[12, 14]. We discuss the current knowledge of the role of MDSC in liver cancer and related disease conditions.
MDSCs in obesity and NAFLD
Obesity increases the risk of liver cancer[33]. Obesity presents with chronic low-grade inflammation and elevations in pro-inflammatory cytokines, including IL-6, TNFα, and prostaglandin E2 (PGE2), which induce MDSC expansion and differentiation[34]. As expected, obese mice have increased MDSC frequencies. In both diet-induced obesity e.g. high-fat diet fed mice and genetic-induced obese mice e.g. leptin-deficient ob/ob mice, immunosuppressive CD11b+Gr1+ cells systemically accumulate in circulating blood, liver, spleen, and adipose tissue[35, 36]. Consistently, an increase of CD11b+CD33+CD14+HLA-CRlo/− M-MDSCs in the blood of obese patients has also been reported[37]. Leptin may contribute to the obesity-induced MDSC accumulation, and blocking leptin signaling using soluble leptin receptor decreased MDSC in HF diet-fed mice[36]. The increased MDSCs seemed to have a protective effect against obesity-associated metabolic dysfunction, and MDSC depletion with anti-Gr1 antibody exacerbated insulin resistance and enhanced adipose inflammation[35, 36]. Despite the beneficial metabolic effects of MDSCs, obesity-induced MDSC accumulation promoted tumor growth of orthotopic 4T1 breast cancer and mediated the spontaneous 4T1 metastasis to the liver [36]. Since obesity promotes HCC, the influence of MDSCs on obesity progression may potentially affect the obesity-HCC transition. Currently, the role of MDSCs in obesity-promoted HCC is still unknown, and more investigations are needed.
Obesity often coexists with NAFLD, a disease condition with excessive accumulation of lipids inside the liver. NAFLD is a spectrum of liver diseases ranging from simple steatosis to more severe nonalcoholic steatohepatitis (NASH) [5, 6, 38]. The association between obesity and NAFLD is observed in mice. Both diet-induced obesity and genetic-induced obesity mouse models commonly result in NAFLD[39]. NAFLD is an important risk factor for HCC[5, 6, 33, 38] Various mouse models recapitulated the HCC-promoting effect of NAFLD [40–43]. Interestingly, the methionine-choline deficient (MCD) diet NASH model does not produce obese mice but causes body weight loss. However, MCD treatment can still promote HCC, which supports an obesity-independent, HCC-promoting mechanism of NAFLD[43]. The increasing prevalence of obesity coincides with NAFLD becoming the most common cause of liver dysfunction globally, and its contribution to HCC prevalence will likely increase.
Several mouse NAFLD models consistently exhibit the hepatic accumulation of MDSCs, yet their role in NAFLD disease remains unclear. In high-fat diet fed mice, the increased hepatic CD11b+Gr1+ cells are comprised of both CD11b+Gr1high and two monocytic subsets, SSChigh CD11b+Gr1dim and SSClowCD11b+Gr1dim[44]. Only the SSClowCD11b+Gr1dim subset demonstrates immunosuppressive functions against autologous T cells through a nitrous oxide (NO)-dependent mechanism, and the SSClowCD11b+Gr1dim population increased through NAFLD progression. The SSClowCD11b+Gr1dim subset express CCR2 and their hepatic recruitment seem to be mediated by the CCL2/CCR2 axis. Interestingly, the SSClowCD11b+Gr1dim cells also express macrophage marker F4/80[44]. Both liver resident Kupffer cells and recruited macrophages play an essential role in NAFLD progression[45]. The liver of NASH patients have an increased amount of macrophages[46], and NAFLD mouse models show the hepatic accumulation of F4/80+ macrophages [47]. Studies that deplete macrophages using clodronate liposomes or gadolinium chloride demonstrate the important role of macrophages in promoting steatosis, hepatic inflammation and NAFLD progression [48–50]. However, both macrophage depletion and inhibition of CCR2+ cell recruitment also target the SSClowCD11b+Gr1dim M-MDSCs. Further complicating the separation of MDSC from inhibitory macrophages, the heterogeneous macrophage populations contain both M1 pro-inflammatory macrophages and M2 immune-inhibitory macrophages [45]. Currently, it is technically challenging to selectively target MDSCs without affecting other macrophage populations for in vivo functional studies. Thus, the development of MDSC-specific targeting methods is needed to clarify the role of MDSC in NAFLD and the consequent effect on HCC development.
MDSCs and HCC
HCC patients have expanded MDSC populations, and in HCC patients, CD14+HLA-DR−/low MDSCs are increased in circulating blood and present in tumors[51–53]. The increase of circulating MDSCs by a different set of markers, Lin−CD33+HLA-DR− MDSCs was reported in HCC patients[54]. These results are reproducible in mice models. In mice lacking hepatic inflammation, MDSC accumulate seems to be a late event during hepatocarcinogenesis and can be found in HCC models caused by carcinogen diethylnitrosoamine (DEN) or MYC oncogene overexpression, [31]. In contrast, implantation of established HCC tumors quickly induces a systemic increase of MDSC[31]. The difference in MDSC accumulation kinetics is likely due to the higher production of tumor-derived factors from implanted tumors.
Accumulating evidence suggests that MDSCs play an important role in HCC development (Figure 1). In HCC patients, tumor volume linearly correlated with circulating levels of CD33+HLA-DR− MDSCs [54, 55]. Importantly, circulating CD14+HLA-DR−/low MDSC frequency was associated with HCC progression and inversely correlated with recurrence-free survival after curative radiofrequency ablation (RFA) therapy or hepatic arterial infusion chemotherapy (HAIC)[53, 56]. A recent meta-analysis showed that elevated MDSCs are associated with an increased risk for disease progression and worse prognosis of HCC [57]. Targeting MDSCs may promote anti-tumor treatments, and several MDSC-targeting approaches are being tested in cancer patients (Table 1). In a recent mouse study, blocking hepatic stellate cell-induced monocyte-intrinsic p38 MAPK signaling suppressed MDSC formation and their accumulation in fibrotic livers. Subsequently, this treatment inhibited HCC growth and boosted the efficacy of anti-PD-L1 therapy[58]. The importance of hepatic stellate cells in the regulation of liver MDSC migration in the context of HCC was also reported by another research group [59]. The CCL2/CCR2 axis is critical for monocyte recruitment to the liver. Similarly, in a separate report, inhibiting tumor monocyte recruitment by blocking the CCL2/CCR2 axis with CCR2 antagonist RDC018 or CCR2 knockout suppressed HCC tumor growth and postsurgical recurrence in mice[60]. In this report, the HCC promoting myeloid population was named TAMs, yet these cells also demonstrated strong immunosuppressive activity and inhibition of CD8+ T cell proliferation. Several studies have suggested blocking the CCL2-CCR2 axis as an effective strategy for HCC treatment[60, 61]; however, our study indicates that the effect is context dependent[62]. In mouse livers with established tumors, blocking the CCL2-CCR2 axis suppresses the senescence-recruited myeloid cells and reduces HCC growth. By contrast, in the absence of tumors, the CCL2-CCR2 axis is necessary for the elimination of premalignant senescent hepatocytes. Tumor-derived factors in the tumor-bearing liver environment, which polarize anti-tumor macrophages to pro-tumor immature myeloid cells (iMCs), caused an opposite effect on the CCL2-CCR2 axis in HCC [62]. These iMCs show immunosuppressive functions and inhibit NK cell activity and are considered MDSCs. This study represents an example of the high plasticity of myeloid populations and explains the controversial observations that tumor associate myeloid cells can have both pro- and anti-tumor functions. The high degree of myeloid plasticity also implies that MDSCs can potentially be converted from immune suppressive to immune promoting cells. Indeed, in both mice and humans, in vitro activation of CD40 signaling using an agonistic antibody decreases Arg1 expression and blocks the immunosuppressive activity of MDSCs[63].
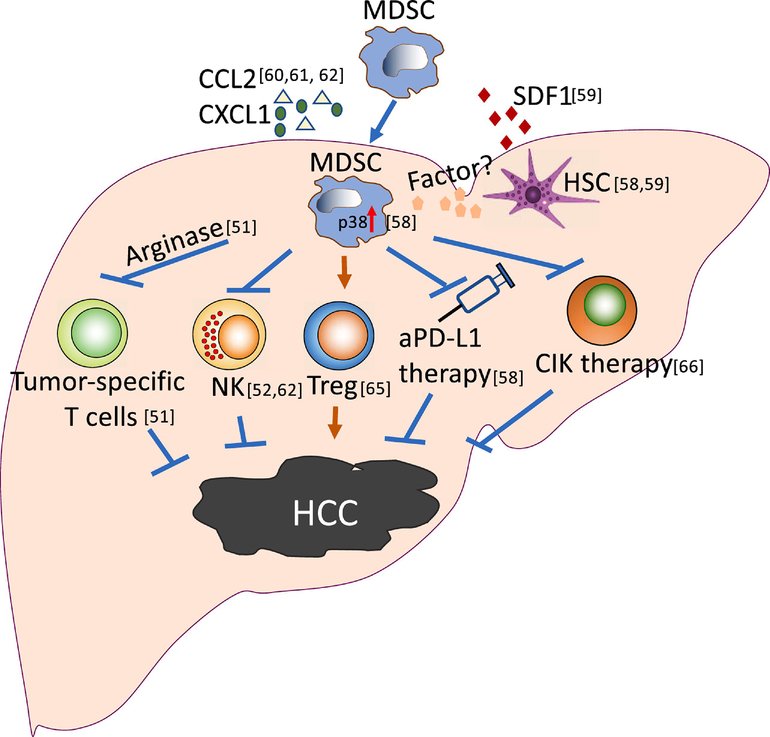
Figure 1. MDSCs promote HCC and inhibit immunotherapy.
MDSCs promote HCC tumor development and influence anti-PD-L1 or cytokine-induced killer cells (CIK) therapies. Hepatic stellate cells (HSC) can recruit MDSC through producing SDF1, and induce a p38-dependent reprogramming of hepatic M-MDSCs to enhance immunosuppressive activity. Many other chemokines, such as CCL2 and CXCL1, contribute to hepatic MDSC recruitment. The increased MDSCs promote HCC by suppressing anti-tumor immunity, including inhibiting T cells and NK cell function and inducing Treg. These mechanisms also negatively impact the efficacy of HCC immunotherapy e.g. anti-PD-L1 and CIK.
Table 1. Strategies for targeting tumor MDSC and potential agents for human cancer treatment.
Currently, three strategies subsist for targeting MDSCs in cancer treatment: (1) blocking MDSC tumor recruitment (2) reducing MDSC population by decreasing MDSC generation and survival (3) inhibiting the immunosuppressive activity of MDSCs. The representative agents and their potential clinical uses are listed.
| Strategies for MDSC inhibition | Potential MDSC-targeting agents for human cancer treatment |
|---|---|
| Blocking MDSC recruitment | CCR2 blockade AZD5069 In a phase 1b/2 trial for advanced solid tumors and metastatic squamous cell carcinoma (NCT02499328) Reparixin In a phase 2 trial for TNBC (NCT02370238) CCR5 antagonist Maraviroc In a phase 1 trial for metastatic colorectal cancer (NCT01736813) CSF-1R inhibitor Plexidartinib In a phase 2 trial for recurrent glioblastoma (NCT01349036) |
| Reducing MDSC generation/survival | Vitamins All trans retinoic acid (ATRA) Reduced MDSC differentiation and improved T cell response in patients with various cancer types.[16] Tyrosine-kinase inhibitors Sunitinib Depleted MDSCs in cancer patients[95]. Chemotherapy agents Gemcitabine Selectively induced MDSC apoptosis in tumor-bearing mice and decreased blood MDSC levels in pancreatic cancer patients[96]. 5-Fluorouracil Decreased MDSC levels in colorectal cancer patients and improved survival[97]. Cisplatin Reduced MDSC population in preclinical tumor models[97]. |
| Inhibiting MDSC Immunosuppressive activity | STAT3 inhibitor AZD9150 (a STAT3 antisense oligonucleotide) Combined with immune checkpoint inhibitors in a phase 1/2 trial for several cancer including HCC (NCT01839604) HDAC inhibitor Entinostat Reduced MDSC function in serval preclinical models and ongoing clinical trial in patients with breast cancer (NCT02115282). PDE5 inhibitor Tadalafil Decreased MDSC function and prolonged survival in patients with head and neck squamous cell carcinoma or melanoma[98] |
The immune-suppressive function of MDSCs may be a factor in HCC progression (Figure 1). Adaptive immunity is critical for hepatocarcinogenesis[64]. Importantly, immune checkpoint blockade therapy provides therapeutic benefit and is FDA approved for HCC treatment. MDSCs from HCC patients and HCC-bearing mice have strong T cell suppressive activity. PMN-MDSCs and M-MDSCs utilize different mechanisms for immune suppression[22]. PMN-MDSCs primarily exert immune suppression by generating a large amount of ROS and often need cell-cell contact with target cells. In contrast, M-MDSCs act through producing a high level of NO, arginase and immune-suppressive cytokines. CD14+HLA-DR−/low MDSCs from HCC patients have monocytic-like features, inhibiting autologous T cell proliferation and cytokine production through arginase activity[51]. In tumor-bearing mice, MDSCs inhibit both antigen-specific and nonspecific T cell responses[14, 22]. Regulatory T cells (Treg) suppress immune activity and are critical in the progression of various types of cancer. CD14+HLA-DR−/low MDSCs from HCC patients can also exert the immunosuppressive function by inducing Treg[51]. In mice, MDSC induced Treg expansion through direct cell-contact and without requiring arginase activity[65]. Along with suppressing the adaptive immunity, human CD14+HLA-DR−/low MDSCs can also directly inhibit the tumor lytic activity of NK cells through a cell-contact dependent, arginase-activity independent manner[52]. In mice, we also found that senescence-promoted HCC progression is mediated through the suppression of NK cell function by MDSCs[62]. Also, MDSCs suppress the tumor lytic function of cytokine-induced killer cells (CIK), and in combination with MDSC inhibition enhances the therapeutic efficacy of CIK against HCC in mice[66].
Although many studies have explored MDSC function in HCC, questions remain. The local environment has a marked influence on MDSC function e.g. tumor MDSCs appear to have a stronger immunosuppressive function than blood MDSCs. Tumor MDSCs express ligands for inhibitory checkpoint receptors such as PD-L1 and CTLA-4, and tumor-associated hypoxia via hypoxia-inducible factor 1-α (HIF1α) helps upregulate PD-L1 expression on the surface of tumor MDSCs[67]. The increased expression of Arg1, iNOS, CAT2 (cationic acid amino transporter 2), and TGF-β by tumor MDSCs contribute to the enhanced suppressive function[68]. Existing evidence suggests that the liver environment also affects MDSC function. In the fibrotic liver, hepatic stellate cells (HSC)-derived factors induce MDSCs to proliferate, obtain immune suppressive function, and crosstalk between HSC and MDSCs[58]. We also observed that hepatic MDSC have stronger T cell suppressive activity than their splenic counterparts[69]. However, the detailed, functional regulation of MDSCs in the various HCC tumor microenvironments and the surrounding liver tissues is generally untested.
Besides immunosuppression, MDSCs can promote carcinogenesis through many other mechanisms such as angiogenesis[70, 71]. Human HCC tumors are commonly hypervascular, and anti-angiogenic therapies, such as anti-VEGF monoclonal antibodies, are approved for HCC treatment[72]. Targeting MDSCs may in part contribute to the efficacy of anti-VEGF therapy. Intratumoral MDSCs of ovarian cancer express VEGF receptors and blocking VEGF signal is associated with decreased MDSC tumor infiltration[73]. Importantly, in various murine tumor models, including B16 melanoma, EL4 lymphoma and LLC lung cancer, tumor MDSC accumulation renders tumors refractory to anti-VEGF therapy [74]. However, the role of MDSCs in HCC angiogenesis and anti-VEFG therapy against HCC is currently unknown. Further investigations are needed to address these questions.
MDSCs and CCA
Currently, the knowledge of MDSCs in CCA is limited. An increased circulating CD14+CD11b+HLA-DR− MDSC has been reported in CCA patients compared to healthy controls [75]. In mice, depleting MDSC abrogated the tumor-promoting effect of cancer-associated fibroblasts using a subcutaneous CCA tumor model, suggesting a contribution of MDSC to CCA progression [76].
Recently, we observed that PSC and colitis promoted CCA using various mouse models[29]. Importantly, the tumor-promoting effect was mediated by PMN-MDSCs (Figure 2). PSC is a well-recognized risk factor for CCA and presents as a progressive inflammatory and fibrotic injury to the bile ducts. However, the role of myeloid cells in PSC is largely unknown. Macrophages likely have a role in PSC [77]. An increase in hepatic recruitment of monocytes was observed in mouse PSC models using both Mdr2−/− mice and an injection of the inhibitor of apoptosis antagonist BV6. Furthermore, blocking liver macrophage accumulation attenuated liver injury and fibrosis in mouse PSC [78]. Mdr2−/− mice also accumulated hepatic Gr1+ myeloid cells [79]. Using both Mdr2−/− and a bile duct ligation mouse model, we recently found that PSC causes liver accumulation of CD11b+Ly6G+Ly6Clo PMN-MDSCs, which present strong immunosuppressive activity [29]. Importantly, gut sterilization attenuated the buildup of PMN-MDSCs in the liver of PSC mice, suggesting a critical role of the gut microbiome in this process. Our findings agree with observations that PSC is associated with gut dysbiosis and increased gut permeability [80, 81]. Colitis also contributes to CCA formation. Although colitis led to MDSC accumulation in the colon, the regulation of hepatic MDSC in the context of colitis remains unclear[82]. As in PSC, we found that the gut microbiome regulates the hepatic accumulation of PMN-MDSC in colitis mice [29]. The hepatic accumulation of MDSC in PSC and colitis indicates their potential contribution to CCA development. Indeed, by targeting the gut microbiome/LPS/TLR4/CXCL1/CXCR2 pathways with various methods, we demonstrated that PMN-MDSCs promoted CCA progression in the context of PSC and colitis[29]. Our study unveiled a novel mechanism: the gut microbiome promotes CCA development through upregulating liver MDSCs.
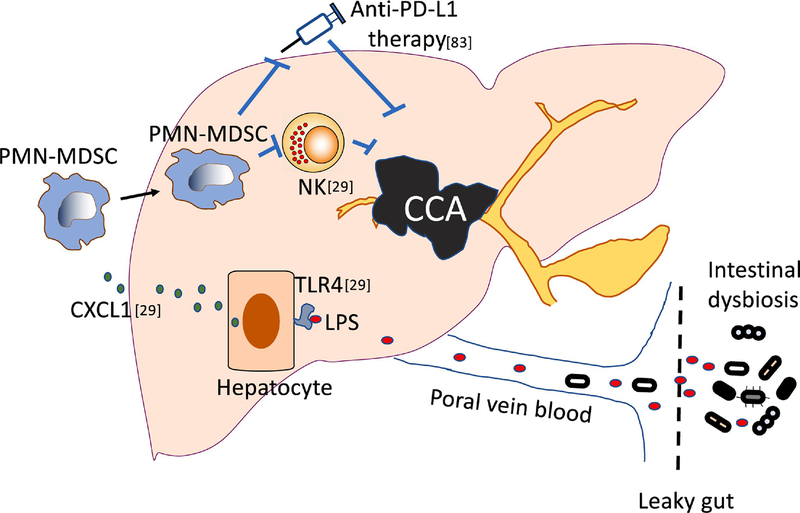
Figure 2. MDSCs enhance CCA and impair anti-PD-L1 immunotherapy.
PMN-MDSCs impact CCA progression in the context of leaky gut and affect anti-PD-L1 therapy against CCA. Our recent study found that in the context of impaired intestinal barrier, liver had an increased exposure to gut microbial products (such as LPS) even intact bacteria through portal circulation. Increased LPS bound to TLR4 on hepatocytes which led to increased CXCL1 production and subsequent MDSC (particularly PMN-MDSC) accumulation. The increased MDSCs impaired NK cell function and promoted CCA development.
Our finding is in line with a recent report which supports that PMN-MDSCs are critical for CCA progression (Figure 2)[83]. The study focused on improving anti-PD-L1 therapy against CCA using an orthotopic mouse CCA tumor model. Similar to clinic observations that CCA patients do not respond well to immune checkpoint blockade, anti-PD-L1 therapy failed to control orthotopic CCA in mice. However, concurrent depletion of PMN-MDSCs and macrophages sensitized CCA to anti-PD-L1 therapy. This is the first study to demonstrate the role of PMN-MDSCs in CCA development, suggesting that PMN-MDSCs are a potential target for immunotherapy against CCA.
MDSCs and liver metastasis
The “seed and soil” theory hypothesizes that both the “seed” (cancer cells) and the “soil” (the local microenvironment) are key determinants for the metastatic formation and outgrowth of metastatic tumor cells [84]. Importantly, animal studies suggest that tumors can shape the microenvironment of distant organs to facilitate tumor spreading. Both resident cells and recruited cells direct the premetastatic niche e.g. recruited myeloid cells are for metastasis[85]. In a study of lung metastasis, the tumor-derived soluble factors were sufficient to induce the pulmonary recruitment of VEGFR1+ bone marrow-derived myeloid progenitor cells to form a pro-metastatic niche [86]. Likewise, hepatic myeloid cell accumulation commonly occurs in tumor-bearing mice even without hepatic lesions, suggesting communication between the liver and distant tumors[87, 88]. In a recent animal study investigating liver metastasis from pancreatic cancer, tumor-derived IL-6 was critical in directing hepatocytes to form a pro-metastatic niche through an IL6-STAT3-serum amyloid A1/2 pathway (Figure 3)[88]. The accumulation of myeloid cells and an increase of fibronectin and type I collagen characterize the pro-metastatic niches in the liver. Although this study was not focused on niche-associated myeloid cells, in considering IL-6 as an important factor for MDSC differentiation, it is likely that these myeloid cells have immunosuppressive functions[89]. Indeed, in mice bearing microscopic pancreatic intraepithelial neoplasia, MDSCs accumulated in the liver [90]. Similarly, the abdominal tumors induced the buildup of hepatic MDSCs leading to B16 tumor liver metastasis[90]. These hepatic MDSCs exhibited strong immunosuppressive functions, inhibited T cell proliferation and CD8+ T cell-mediated cytotoxicity, and induced Treg differentiation.
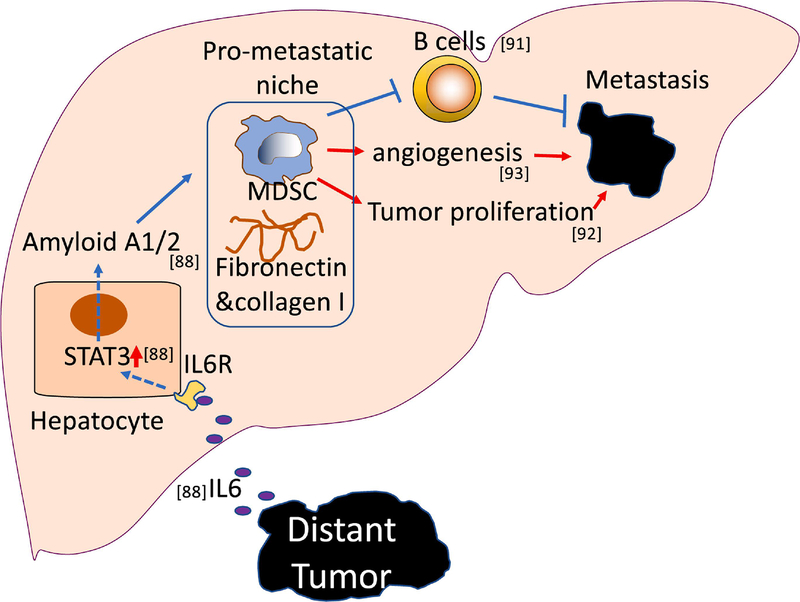
Figure 3. MDSCs promote liver metastasis.
MDSCs contribute to the formation of pro-metastatic niches in the liver. Extrahepatic, distant tumor-derived IL-6 acts on hepatocytes to induce STAT3-dependent production of amyloid A1/2, which subsequently causes accumulation of MDSCs, fibronectin and collagen I. Subsequently, MDSCs promote liver metastasis by suppressing B cells, enhancing angiogenesis and tumor proliferation.
Hepatic MDSCs also have a critical role in the progression of metastatic liver cancer (Figure 3). Hepatic infiltration of CD11b+Gr1+ cells are found in mice bearing liver metastasis from various cancers, such as MC38 colon cancer and LLC[91, 92]. Removing the accumulated CD11b+Gr1+ cells can effectively reduce metastatic tumor burden, indicating their important role in disease progression. Although metastasis-expanded hepatic CD11b+Gr1+ cells are known to have T cell suppressive functions, several studies suggest that these cells can promote liver metastasis through additional mechanisms. In one report, hepatic CD11b+ myeloid cells promoted liver metastasis by inhibiting B cells[91]. Using μMT mice, which lack B cells, the study demonstrated that B cells have an anti-metastatic function and delay MC38 liver metastasis after intrasplenic injection of tumor cells. In tumor-bearing livers, hepatic B cells have reduced expression of costimulatory molecule CD80 and an impaired ability to stimulate T cells. The accumulation of CD11b+ myeloid cells through CD11b-dependent and cell-contact dependent mechanisms caused B cell dysfunction. The CCL2/CCR2 axis reportedly mediated an increase in CD11b+Gr1mid subsets in liver metastasis and depleting the CD11b+Gr1mid cells markedly reduced the proliferation of the metastatic tumor cells[92]. Interestingly, using SCID mice, the study showed that the tumor growth-promoting effect of the CD11b+Gr1mid cells does not require adaptive immunity. Hepatic CD11b+ cells can promote liver metastasis by stimulating angiogenesis[93]. By depleting hepatic CD11b+ cells with diphtheria toxin in CD11b-DTR mice, liver metastasis was inhibited via depletion of tumor vasculature. ANGPTL7 (angiopoietin-like 7) has strong anti-angiogenic function[93]. Using both in vivo and in vitro assays, the metastasis-induced hepatic CD11b+ cells reduced ANGPTL7 expression in tumor cells thus enhanced tumor angiogenesis. This finding translates to the similar downregulation of ANGPTL7 expression in human colon and breast tumor compared to normal tissues.
Summary
Liver cancer is often diagnosed at late stages and effective treatments are lacking. Its rising incidence and mortality urge the development of new therapeutic strategies. Liver cancer often arises in the presence of chronic inflammatory diseases, and immunotherapy has become the standard of care first-line therapy for HCC [94]. However, the current response rate of liver cancer to immunotherapy remains somewhat limited. A better understanding of the immune regulation in liver cancer is necessary to improve immunotherapies. MDSCs are a critical component of the tumor microenvironment and promote tumor progression through various mechanisms. Preclinical studies prove the liver tumor-promoting function of MDSCs, and clinical evidence shows an association between MDSCs and poor outcomes for patients with HCC, thereby targeting MDSCs can improve liver cancer treatments. More investigations are needed to develop selective MDSC targeting methods and combine them with existing immunotherapy to treat liver cancer.
Liver cancer has expanded MDSCs.
MDSCs promote liver cancer progression.
Targeting MDSCs improves immune checkpoint therapy against liver cancer
Acknowledgments
T.F.G., C.M. were supported by the Intramural Research Program of the NIH. The authors thank Justin D. McCallen for editing and proofreading the review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V, Liver cancer: Approaching a personalized care, J Hepatol, 62 (2015) S144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Villanueva A, Hepatocellular Carcinoma, N Engl J Med, 380 (2019) 1450–1462. [DOI] [PubMed] [Google Scholar]
- [3].Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D, Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA), Nat Rev Gastroenterol Hepatol, 13 (2016) 261–280. [DOI] [PubMed] [Google Scholar]
- [4].Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A, Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability, Carcinogenesis, 30 (2009) 1073–1081. [DOI] [PubMed] [Google Scholar]
- [5].Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M, From NASH to HCC: current concepts and future challenges, Nat Rev Gastroenterol Hepatol, 16 (2019) 411–428. [DOI] [PubMed] [Google Scholar]
- [6].Michelotti GA, Machado MV, Diehl AM, NAFLD, NASH and liver cancer, Nat Rev Gastroenterol Hepatol, 10 (2013) 656–665. [DOI] [PubMed] [Google Scholar]
- [7].Razumilava N, Gores GJ, Cholangiocarcinoma, Lancet, 383 (2014) 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA, Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study, Clin Gastroenterol Hepatol, 5 (2007) 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tyson GL, El-Serag HB, Risk factors for cholangiocarcinoma, Hepatology, 54 (2011) 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lambert AW, Pattabiraman DR, Weinberg RA, Emerging Biological Principles of Metastasis, Cell, 168 (2017) 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Disibio G, French SW, Metastatic patterns of cancers: results from a large autopsy study, Arch Pathol Lab Med, 132 (2008) 931–939. [DOI] [PubMed] [Google Scholar]
- [12].Ostrand-Rosenberg S, Fenselau C, Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment, J Immunol, 200 (2018) 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gabrilovich DI, Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system, Nat Rev Immunol, 9 (2009) 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tesi RJ, MDSC; the Most Important Cell You Have Never Heard Of, Trends Pharmacol Sci, 40 (2019) 4–7. [DOI] [PubMed] [Google Scholar]
- [15].Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI, Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards, Nat Commun, 7 (2016) 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI, Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells, Cancer Res, 67 (2007) 11021–11028. [DOI] [PubMed] [Google Scholar]
- [17].Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, Gabrilovich DI, Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients, Sci Immunol, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, Nee K, Hernandez G, Evans K, Torosian L, Silva A, Walsh C, Kessenbrock K, Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics, Sci Immunol, 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lim HX, Kim TS, Poh CL, Understanding the Differentiation, Expansion, Recruitment and Suppressive Activities of Myeloid-Derived Suppressor Cells in Cancers, Int J Mol Sci, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V, Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells, J Clin Invest, 116 (2006) 2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Monu NR, Frey AB, Myeloid-derived suppressor cells and anti-tumor T cells: a complex relationship, Immunol Invest, 41 (2012) 595–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumar V, Patel S, Tcyganov E, Gabrilovich DI, The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment, Trends Immunol, 37 (2016) 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE, Kupffer cells in the liver, Compr Physiol, 3 (2013) 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Krenkel O, Tacke F, Liver macrophages in tissue homeostasis and disease, Nat Rev Immunol, 17 (2017) 306–321. [DOI] [PubMed] [Google Scholar]
- [25].Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, Devoogdt N, Lambrecht BN, Beschin A, Guilliams M, Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells, Nat Commun, 7 (2016) 10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bonnardel J, T’Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, Vanneste B, De Prijck S, Nedospasov SA, Kremer A, Van Hamme E, Borghgraef P, Toussaint W, De Bleser P, Mannaerts I, Beschin A, van Grunsven LA, Lambrecht BN, Taghon T, Lippens S, Elewaut D, Saeys Y, Guilliams M, Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche, Immunity, 51 (2019) 638–654 e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Krenkel O, Hundertmark J, Abdallah AT, Kohlhepp M, Puengel T, Roth T, Branco DPP, Mossanen JC, Luedde T, Trautwein C, Costa IG, Tacke F, Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis, Gut, 69 (2020) 551–563. [DOI] [PubMed] [Google Scholar]
- [28].Hackstein CP, Assmus LM, Welz M, Klein S, Schwandt T, Schultze J, Forster I, Gondorf F, Beyer M, Kroy D, Kurts C, Trebicka J, Kastenmuller W, Knolle PA, Abdullah Z, Gut microbial translocation corrupts myeloid cell function to control bacterial infection during liver cirrhosis, Gut, 66 (2017) 507–518. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, Ma L, Roy S, Fu Q, Brown ZJ, Wabitsch S, Thovarai V, Fu J, Feng D, Ruf B, Cui LL, Subramanyam V, Frank KM, Wang S, Kleiner DE, Ritz T, Rupp C, Gao B, Longerich T, Kroemer A, Wang XW, Ruchirawat M, Korangy F, Schnabl B, Trinchieri G, Greten TF, Gut microbiome directs hepatocytes to recruit MDSC and promote cholangiocarcinoma, Cancer Discov, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ilkovitch D, Lopez DM, The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression, Cancer Res, 69 (2009) 5514–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schrader J, The role of MDSCs in hepatocellular carcinoma--in vivo veritas?, J Hepatol, 59 (2013) 921–923. [DOI] [PubMed] [Google Scholar]
- [32].Hammerich L, Tacke F, Emerging roles of myeloid derived suppressor cells in hepatic inflammation and fibrosis, World J Gastrointest Pathophysiol, 6 (2015) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ, Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults, N Engl J Med, 348 (2003) 1625–1638. [DOI] [PubMed] [Google Scholar]
- [34].Kern L, Mittenbuhler MJ, Vesting AJ, Ostermann AL, Wunderlich CM, Wunderlich FT, Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers, Cancers (Basel), 11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L, Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity, J Biol Chem, 286 (2011) 23591–23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Clements VK, Long T, Long R, Figley C, Smith DMC, Ostrand-Rosenberg S, Frontline Science: High fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells, J Leukoc Biol, 103 (2018) 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bao Y, Mo J, Ruan L, Li G, Increased monocytic CD14(+)HLADRlow/− myeloid-derived suppressor cells in obesity, Mol Med Rep, 11 (2015) 2322–2328. [DOI] [PubMed] [Google Scholar]
- [38].Streba LA, Vere CC, Rogoveanu I, Streba CT, Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question, World J Gastroenterol, 21 (2015) 4103–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Van Herck MA, Vonghia L, Francque SM, Animal Models of Nonalcoholic Fatty Liver Disease-A Starter’s Guide, Nutrients, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, Lorentzen A, Einer C, Schulz S, Clavel T, Protzer U, Thiele C, Zischka H, Moch H, Tschop M, Tumanov AV, Haller D, Unger K, Karin M, Kopf M, Knolle P, Weber A, Heikenwalder M, Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes, Cancer Cell, 26 (2014) 549–564. [DOI] [PubMed] [Google Scholar]
- [41].Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M, Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression, Cell, 140 (2010) 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gomes AL, Teijeiro A, Buren S, Tummala KS, Yilmaz M, Waisman A, Theurillat JP, Perna C, Djouder N, Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma, Cancer Cell, 30 (2016) 161–175. [DOI] [PubMed] [Google Scholar]
- [43].Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF, NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis, Nature, 531 (2016) 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yao L, Abe M, Kawasaki K, Akbar SM, Matsuura B, Onji M, Hiasa Y, Characterization of Liver Monocytic Myeloid-Derived Suppressor Cells and Their Role in a Murine Model of Non-Alcoholic Fatty Liver Disease, PLoS One, 11 (2016) e0149948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kazankov K, Jorgensen SMD, Thomsen KL, Moller HJ, Vilstrup H, George J, Schuppan D, Gronbaek H, The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, Nat Rev Gastroenterol Hepatol, 16 (2019) 145–159. [DOI] [PubMed] [Google Scholar]
- [46].Lotowska JM, Sobaniec-Lotowska ME, Lebensztejn DM, The role of Kupffer cells in the morphogenesis of nonalcoholic steatohepatitis - ultrastructural findings. The first report in pediatric patients, Scand J Gastroenterol, 48 (2013) 352–357. [DOI] [PubMed] [Google Scholar]
- [47].Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS, Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production, J Biol Chem, 287 (2012) 40161–40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O’Doherty RM, Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance, Diabetes, 59 (2010) 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Neyrinck AM, Cani PD, Dewulf EM, De Backer F, Bindels LB, Delzenne NM, Critical role of Kupffer cells in the management of diet-induced diabetes and obesity, Biochem Biophys Res Commun, 385 (2009) 351–356. [DOI] [PubMed] [Google Scholar]
- [50].Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW Jr., C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis, Diabetes, 59 (2010) 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F, A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells, Gastroenterology, 135 (2008) 234–243. [DOI] [PubMed] [Google Scholar]
- [52].Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F, Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor, Hepatology, 50 (2009) 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S, Increase in CD14+HLA-DR −/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis, Cancer Immunol Immunother, 62 (2013) 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shen P, Wang A, He M, Wang Q, Zheng S, Increased circulating Lin(−/low) CD33(+) HLA-DR(−) myeloid-derived suppressor cells in hepatocellular carcinoma patients, Hepatol Res, 44 (2014) 639–650. [DOI] [PubMed] [Google Scholar]
- [55].Lee WC, Wang YC, Cheng CH, Wu TH, Lee CF, Wu TJ, Chou HS, Chan KM, Myeloid-derived suppressor cells in the patients with liver resection for hepatitis B virus-related hepatocellular carcinoma, Sci Rep, 9 (2019) 2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S, Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma, Cancer Immunol Immunother, 65 (2016) 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang X, Fu X, Li T, Yan H, The prognostic value of myeloid derived suppressor cell level in hepatocellular carcinoma: A systematic review and meta-analysis, PLoS One, 14 (2019) e0225327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu M, Zhou J, Liu X, Feng Y, Yang W, Wu F, Cheung OK, Sun H, Zeng X, Tang W, Mok MTS, Wong J, Yeung PC, Lai PBS, Chen Z, Jin H, Chen J, Chan SL, Chan AWH, To KF, Sung JJY, Chen M, Cheng AS, Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma, Gut, 69 (2020) 365–379. [DOI] [PubMed] [Google Scholar]
- [59].Xu Y, Fang F, Jiao H, Zheng X, Huang L, Yi X, Zhao W, Activated hepatic stellate cells regulate MDSC migration through the SDF-1/CXCR4 axis in an orthotopic mouse model of hepatocellular carcinoma, Cancer Immunol Immunother, 68 (2019) 1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H, Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma, Gut, 66 (2017) 157–167. [DOI] [PubMed] [Google Scholar]
- [61].Teng KY, Han J, Zhang X, Hsu SH, He S, Wani NA, Barajas JM, Snyder LA, Frankel WL, Caligiuri MA, Jacob ST, Yu J, Ghoshal K, Blocking the CCL2-CCR2 Axis Using CCL2-Neutralizing Antibody Is an Effective Therapy for Hepatocellular Cancer in a Mouse Model, Mol Cancer Ther, 16 (2017) 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, Medina-Echeverz J, Longerich T, Forgues M, Reisinger F, Heikenwalder M, Wang XW, Zender L, Greten TF, Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression, Cancer Cell, 30 (2016) 533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Medina-Echeverz J, Ma C, Duffy AG, Eggert T, Hawk N, Kleiner DE, Korangy F, Greten TF, Systemic Agonistic Anti-CD40 Treatment of Tumor-Bearing Mice Modulates Hepatic Myeloid-Suppressive Cells and Causes Immune-Mediated Liver Damage, Cancer Immunol Res, 3 (2015) 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schneider C, Teufel A, Yevsa T, Staib F, Hohmeyer A, Walenda G, Zimmermann HW, Vucur M, Huss S, Gassler N, Wasmuth HE, Lira SA, Zender L, Luedde T, Trautwein C, Tacke F, Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer, Gut, 61 (2012) 1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Park MJ, Lee SH, Kim EK, Lee EJ, Baek JA, Park SH, Kwok SK, Cho ML, Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice, Sci Rep, 8 (2018) 3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yu SJ, Ma C, Heinrich B, Brown ZJ, Sandhu M, Zhang Q, Fu Q, Agdashian D, Rosato U, Korangy F, Greten TF, Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma, J Hepatol, 70 (2019) 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S, PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation, J Exp Med, 211 (2014) 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cimen Bozkus C, Elzey BD, Crist SA, Ellies LG, Ratliff TL, Expression of Cationic Amino Acid Transporter 2 Is Required for Myeloid-Derived Suppressor Cell-Mediated Control of T Cell Immunity, J Immunol, 195 (2015) 5237–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ma C, Kapanadze T, Gamrekelashvili J, Manns MP, Korangy F, Greten TF, Anti-Gr-1 antibody depletion fails to eliminate hepatic myeloid-derived suppressor cells in tumor-bearing mice, J Leukoc Biol, 92 (2012) 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Johnson BW, Achyut BR, Fulzele S, Mondal AK, Kolhe R, Arbab AS, Delineating Pro-Angiogenic Myeloid Cells in Cancer Therapy, Int J Mol Sci, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Vetsika EK, Koukos A, Kotsakis A, Myeloid-Derived Suppressor Cells: Major Figures that Shape the Immunosuppressive and Angiogenic Network in Cancer, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS, The Role of Angiogenesis in Hepatocellular Carcinoma, Clin Cancer Res, 25 (2019) 912–920. [DOI] [PubMed] [Google Scholar]
- [73].Horikawa N, Abiko K, Matsumura N, Hamanishi J, Baba T, Yamaguchi K, Yoshioka Y, Koshiyama M, Konishi I, Expression of Vascular Endothelial Growth Factor in Ovarian Cancer Inhibits Tumor Immunity through the Accumulation of Myeloid-Derived Suppressor Cells, Clin Cancer Res, 23 (2017) 587–599. [DOI] [PubMed] [Google Scholar]
- [74].Shojaei F, Ferrara N, Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells, Cancer Res, 68 (2008) 5501–5504. [DOI] [PubMed] [Google Scholar]
- [75].Xu XD, Hu J, Wang M, Peng F, Tian R, Guo XJ, Xie Y, Qin RY, Circulating myeloid-derived suppressor cells in patients with pancreatic cancer, Hepatobiliary Pancreat Dis Int, 15 (2016) 99–105. [DOI] [PubMed] [Google Scholar]
- [76].Lin Y, Li B, Yang X, Cai Q, Liu W, Tian M, Luo H, Yin W, Song Y, Shi Y, He R, Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment, Neoplasia, 21 (2019) 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chen YY, Arndtz K, Webb G, Corrigan M, Akiror S, Liaskou E, Woodward P, Adams DH, Weston CJ, Hirschfield GM, Intrahepatic macrophage populations in the pathophysiology of primary sclerosing cholangitis, JHEP Rep, 1 (2019) 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Guicciardi ME, Trussoni CE, Krishnan A, Bronk SF, Lorenzo Pisarello MJ, O’Hara SP, Splinter PL, Gao Y, Vig P, Revzin A, LaRusso NF, Gores GJ, Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice, J Hepatol, 69 (2018) 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nishio T, Hu R, Koyama Y, Liang S, Rosenthal SB, Yamamoto G, Karin D, Baglieri J, Ma HY, Xu J, Liu X, Dhar D, Iwaisako K, Taura K, Brenner DA, Kisseleva T, Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice, J Hepatol, 71 (2019) 573–585. [DOI] [PubMed] [Google Scholar]
- [80].Liao L, Schneider KM, Galvez EJC, Frissen M, Marschall HU, Su H, Hatting M, Wahlstrom A, Haybaeck J, Puchas P, Mohs A, Peng J, Bergheim I, Nier A, Hennings J, Reissing J, Zimmermann HW, Longerich T, Strowig T, Liedtke C, Cubero FJ, Trautwein C, Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis, Gut, 68 (2019) 1477–1492. [DOI] [PubMed] [Google Scholar]
- [81].Zhou T, Kyritsi K, Wu N, Francis H, Yang Z, Chen L, O’Brien A, Kennedy L, Ceci L, Meadows V, Kusumanchi P, Wu C, Baiocchi L, Skill NJ, Saxena R, Sybenga A, Xie L, Liangpunsakul S, Meng F, Alpini G, Glaser S, Knockdown of vimentin reduces mesenchymal phenotype of cholangiocytes in the Mdr2(−/−) mouse model of primary sclerosing cholangitis (PSC), EBioMedicine, 48 (2019) 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lee CR, Kwak Y, Yang T, Han JH, Park SH, Ye MB, Lee W, Sim KY, Kang JA, Kim YC, Mazmanian SK, Park SG, Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-beta during Murine Colitis, Cell Rep, 17 (2016) 3219–3232. [DOI] [PubMed] [Google Scholar]
- [83].Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, Pavelko KD, Li Y, O’Brien D, Wang C, Graham RP, Smoot RL, Dong H, Ilyas S, Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma, J Clin Invest, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Langley RR, Fidler IJ, The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs, Int J Cancer, 128 (2011) 2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wang Y, Ding Y, Guo N, Wang S, MDSCs: Key Criminals of Tumor Pre-metastatic Niche Formation, Front Immunol, 10 (2019) 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D, VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche, Nature, 438 (2005) 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Eggert T, Medina-Echeverz J, Kapanadze T, Kruhlak MJ, Korangy F, Greten TF, Tumor induced hepatic myeloid derived suppressor cells can cause moderate liver damage, PLoS One, 9 (2014) e112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, Black TA, Chien AL, Majmundar KS, Thompson JC, Yee SS, O’Hara MH, Aggarwal C, Xin D, Shaked A, Gao M, Liu D, Borad MJ, Ramanathan RK, Carpenter EL, Ji A, de Beer MC, de Beer FC, Webb NR, Beatty GL, Hepatocytes direct the formation of a pro-metastatic niche in the liver, Nature, 567 (2019) 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jiang M, Chen J, Zhang W, Zhang R, Ye Y, Liu P, Yu W, Wei F, Ren X, Yu J, Interleukin-6 Trans-Signaling Pathway Promotes Immunosuppressive Myeloid-Derived Suppressor Cells via Suppression of Suppressor of Cytokine Signaling 3 in Breast Cancer, Front Immunol, 8 (2017) 1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Connolly MK, Mallen-St Clair J, Bedrosian AS, Malhotra A, Vera V, Ibrahim J, Henning J, Pachter HL, Bar-Sagi D, Frey AB, Miller G, Distinct populations of metastases-enabling myeloid cells expand in the liver of mice harboring invasive and preinvasive intra-abdominal tumor, J Leukoc Biol, 87 (2010) 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Thorn M, Point GR, Burga RA, Nguyen CT, Joseph Espat N, Katz SC, Liver metastases induce reversible hepatic B cell dysfunction mediated by Gr-1+CD11b+ myeloid cells, J Leukoc Biol, 96 (2014) 883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, Beech J, Allen D, Smart S, Muschel RJ, Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis, Hepatology, 57 (2013) 829–839. [DOI] [PubMed] [Google Scholar]
- [93].Lim SY, Gordon-Weeks A, Allen D, Kersemans V, Beech J, Smart S, Muschel RJ, Cd11b(+) myeloid cells support hepatic metastasis through down-regulation of angiopoietin-like 7 in cancer cells, Hepatology, 62 (2015) 521–533. [DOI] [PubMed] [Google Scholar]
- [94].Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, Investigators IM, Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma, N Engl J Med, 382 (2020) 1894–1905. [DOI] [PubMed] [Google Scholar]
- [95].Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH, Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients, Clin Cancer Res, 15 (2009) 2148–2157. [DOI] [PubMed] [Google Scholar]
- [96].Annels NE, Shaw VE, Gabitass RF, Billingham L, Corrie P, Eatock M, Valle J, Smith D, Wadsley J, Cunningham D, Pandha H, Neoptolemos JP, Middleton G, The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer, Cancer Immunol Immunother, 63 (2014) 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wang Z, Till B, Gao Q, Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells, Oncoimmunology, 6 (2017) e1331807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, Nazarian R, Califano J, Borrello I, Serafini P, Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma, Clin Cancer Res, 21 (2015) 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]