Abstract
Purpose
The purpose of this paper is to present and validate an originally developed application SkinCare used for skin dose mapping in interventional procedures, which are associated with relatively high radiation doses to the patient’s skin and possible skin reactions.
Methods
SkinCare is an application tool for generating skin dose maps following interventional radiology and cardiology procedures using the realistic 3D patient models. Skin dose is calculated using data from Digital Imaging and Communications in Medicine (DICOM) Radiation Dose Structured Reports (RDSRs). SkinCare validation was performed by using the data from the Siemens Artis Zee Biplane fluoroscopy system and conducting “Acceptance and quality control protocols for skin dose calculating software solutions in interventional cardiology” developed and tested in the frame of the VERIDIC project. XR‐RV3 Gafchromic films were used as dosimeters to compare peak skin doses (PSDs) and dose maps obtained through measurements and calculations. DICOM RDSRs from four fluoroscopy systems of different vendors (Canon, GE, Philips, and Siemens) were used for the development of the SkinCare and for the comparison of skin dose maps generated using SkinCare to skin dose maps generated by different commercial software tools (Dose Tracking System (DTS) from Canon, RadimetricsTM from Bayer and RDM from MEDSQUARE). The same RDSRs generated during a cardiology clinical procedure (percutaneous coronary intervention—PCI) were used for comparison.
Results
Validation performed using VERIDIC's protocols for skin dose calculation software showed that PSD calculated by SkinCare is within 17% and 16% accuracy compared to measurements using XR‐RV3 Gafchromic films for fundamental irradiation setups and simplified clinical procedures, respectively. Good visual agreement between dose maps generated by SkinCare and DTS, RadimetricsTM and RDM was obtained.
Conclusions
SkinCare is proved to be very convenient solution that can be used for monitoring delivered dose following interventional procedures.
Keywords: DICOM RDSR, interventional procedures, skin dose assessment, SkinCare application, software, XR‐RV3 GafChromic films
1. INTRODUCTION
Interventional procedures in radiology and cardiology are associated with relatively high radiation doses to the patient’s skin which may lead to skin reactions. 1 Even though peak skin dose (PSD) assessment can be accomplished with a wide selection of detectors, 2 using software tools is more convenient and economical. At the present time, most equipment vendors have developed online solutions for skin dose calculations. CareMonitor by Siemens and DoseWise by Philips are solutions which basically provide the accumulated peak air kerma in the current projection. 3 On the other hand, Dose Map from GE is an advanced two‐dimensional (2D) solution, 4 , 5 , 6 while Dose Tracking System (DTS) from Canon (Toshiba) represents a state of the art three‐dimensional (3D) solution for skin dose mapping. 7 , 8 , 9 The most important drawback of previously mentioned solutions is that they are vendor specific and cannot be used in fluoroscopy systems from other manufacturers.
Utilizing Digital Imaging and Communications in Medicine (DICOM) Radiation Dose Structured Report (RDSR) generated at the end of the intervention is the only way to make vendor‐independent solutions. 10 RDSR was added to the DICOM standard 11 with intention to standardized format of recording all the information related to the exposure parameters used for each irradiation event undergone by the patient. RDSR contains all the necessary technical, geometric, and dosimetric data necessary to assess the patient skin dose. In addition to online solutions mentioned above, there are commercial offline software tools which utilize DICOM headers and/or RDSRs for skin dose calculations such as em.dose from Esprimed, 12 , 13 RDM by MEDSQUARE, 3 DOSE from Qaelum, 14 NEXO[DOSE]® by Bracco, 15 , 16 RadimetricsTM from Bayer, 17 and Skin Dose Map® tool integrated in DoseWatch® by GE Healthcare. 18 Other software solutions can be found in literature. 19 , 20 , 21 , 22 , 23
The objective of this paper is to present an originally developed skin dose mapping application SkinCare that can be readily used with interventional units from different manufacturers. The application was validated by using the data from the Siemens Artis Zee fluoroscopy system and conducting the “Acceptance and quality control protocols for skin dose calculating software solutions in interventional cardiology” developed and tested in the frame of the Validation and Estimation of Radiation Skin Dose in Interventional Cardiology (VERIDIC) project. 24 VERIDIC project, funded under European Joint Programme for the Integration of Radiation Protection Research, H2020 (Grant agreement No 662287), was focused on the skin dose calculation (SDC) software products in interventional cardiology, with an aim to contribute to the harmonization and the validation of SDC software products in interventional cardiology. 24 Additionally, SkinCare’s dose maps generated using different RDSRs were compared visually with different validated commercial software tools for Canon (Toshiba), GE, Philips, and Siemens fluoroscopy systems in order to verify the correctness of applied geometric algorithm.
2. MATERIALS AND METHODS
2.A. SkinCare
SkinCare is an application tool for generating skin dose maps following interventional radiology and interventional cardiology procedures using the realistic 3D patient models. RDSRs from Canon (Toshiba), GE, Philips, and Siemens were used for development of the application, making SkinCare compatible with major fluoroscopy unit vendors. SkinCare is a standalone desktop application that runs in any of the available web browsers. Easy configuration of the system correction factors and patient models provide fast way for generating skin dose maps and visualization of RDSR content.
2.A.1. Patient modeling
Patient models were created using MB‐Lab, 25 an open‐source plug‐in for free and open‐source 3D computer graphics software Blender. 26 SkinCare has a library of 42 3D patient models of height ranging from 150 cm to 210 cm, with an increment of 10 cm. Male and female models consider three different body types: thin, standard size, and obese. All models have arms‐down pose corresponding to patient supine position. Two additional models of sizes 30 × 30 × 15 cm3 and 35.56 cm × 43.52 cm (14" × 17") represent water phantom and XR‐RV3 Gafchromic film, respectively, for the purpose of quality control (QC) tests.
2.A.2. Patient positioning
Since different manufacturers of the fluoroscopy systems define 3D position of isocenter in relation to proprietary point in space, it is necessary to determine the offsets for RDSR’s attributes Table Lateral Position, Table Longitudinal Position, and Table Height Position. This can be done by positioning the surface of table head end at the isocenter. Values that are then recorded in RDSR need to be inserted in SkinCare’s fields Lateral offset, Longitudinal offset, and Height offset. Additionally, SkinCare also has field Head‐Table distance in order to improve the patient position estimation on the table, by taking into account distance from patient head to the table end (this value should be measured prior to the beginning of every procedure). The orientation of the model is assumed to be supine and to lie in the middle of the table.
2.A.3. Skin dose calculation
Skin dose calculation is based on determining the affected points of the 3D patient model by the x‐ray beam. Once the 3D positions of x‐ray tube focal spot, detector, and patient are found for every irradiation event using data from RDSR, dose is calculated as entrance surface dose, that is, only for 3D points in which x‐ray beam enters the patient model (x‐ray beam exit points are not relevant).
Dose is calculated by using [Eq. (1)]:
| (1) |
where is the air kerma reported at the Interventional Reference Point (IRP), 27 is calibration factor for the KAP meter, is table attenuation factor, is oblique factor defined as relative fraction of transmission between zero and nonzero angles of incidence, is distance from source to IRP, is distance from source to affected 3D point, is backscatter factor interpolated from the coefficients of Benmakhlouf H et al., 28 and is the dose conversion factor from air to soft tissue interpolated from the coefficients of Benmakhlouf H et al. 28
2.B. SkinCare validation
2.B.1. Gafchromic film calibration
XR‐RV3 Gafchromic films calibration was performed in the Secondary Standard Dosimetry Laboratory of Vinča Institute of Nuclear Sciences. Films were cut in small square pieces of 2 cm × 2 cm and the irradiation was carried out free‐in‐air, by positioning films at 1 m distance from the radiation source. During calibration, film pieces were oriented in such a way that the yellow side of the film was facing the x‐ray tube. The film pieces were irradiated to 16 air kerma values between 0 and 10 Gy using RQR8 standard beam quality. 29 The conversion of air kerma to absorbed dose to skin is considered to be equal to one. 30
Scanning of the irradiated films was performed after 24 hr of exposure with the Hewlett‐Packard (HP) Scanjet 7650 flatbed scanner. VueScan scanning software was used for linear (raw) scanning which enables data acquisition straight from the scanner’s sensor without any manipulation from the scanning software. Most software packages that come with consumer scanners do not offer this ability and perform processing on raw data. Film pieces were scanned with a resolution of 300 PPI as 48 Bit RGB TIF files. Scanned images were analyzed in the Python programming language using only the red channel in order to ensure maximum sensitivity. To obtain an image value corresponding to a particular air kerma, a square region of interest (ROI) was formed with dimensions of 120 px (ROI was approximately 1 cm2). The formed ROI was shifted throughout the whole image, and pixel values inside the ROI were averaged. ROI with the lowest average pixel value corresponds to the highest dose. Response of the XR‐RV3 Gafchromic films as a function of film dose was modeled using a rational function proposed by Lewis et al. 31 :
| (2) |
where represents the lowest average pixel value of ROI at dose D, and a, b, and c are coefficients of the rational function. Calibration curve was obtained using Levenberg–Marquardt nonlinear curve fitting algorithm.
Uncertainties related to the use of XR‐RV3 Gafchromic films for patient skin dose assessment in the interventional environment have been estimated in our previous work and published in Medical Physics. 30 It is shown that overall uncertainty of skin dose measurements using XR‐RV3 Gafchromic films ranges from 9% (k = 2) for tightly controlled measurement conditions, adequate laboratory calibrations and well‐defined readout protocol to 78% (k = 2) in the worst case scenario where the conditions of exposure, film handling, and readout are weakly controlled and where corrections for the relevant influence quantities are not made. In this work the overall measurement uncertainty was assumed to be 41% (k = 2) since a well‐defined laboratory calibration is performed, while other influencing parameters related to clinical application of dosimetry films are less controlled. 30
2.B.2. VERIDIC’s acceptance and QC testing protocols
One of the objectives of the VERIDIC project was to develop a protocol for acceptance and QC tests of SDC software to be used by medical physicists in clinical practice. 24 A slightly modified acceptance protocol which is originally composed of 13 fundamental irradiation setups and three simplified clinical procedures intended to represent more realistic conditions, has been used. Fundamental setups in addition to simplified clinical procedures are used to verify that key parameters (collimated field area, table height, C‐arm angulation) which can significantly affect the PSD, were appropriately taken into account by SkinCare. All irradiations were performed using fluorographic (acquisition) mode.
First four fundamental irradiations intended to evaluate and for two beam qualities were not performed since thorough evaluation of and was performed before conducting this protocol in the Siemens service mode over wide range of beam qualities as discussed in the next section. XR‐RV3 Gafchromic films were used as dosimeters to compare PSDs and dose maps obtained through measurements and calculations. For such comparison, a tissue equivalent slab phantom, combined of water and polymethyl methacrylate (PMMA) plates (plates of 1 cm thickness, 30 × 30 cm 2 surface), was used to simulate a simplified patient of different sizes (10, 15, 20, 25, and 30 cm). Acceptability criteria require that the calculated PSD values should not differ more than 40% from the measured values for any of the fundamental irradiation events and the simplified procedures. 24
2.B.3. Calibration factor and Table attenuation factor
Two multiplicative factors that significantly influence skin dose calculations are the calibration factor () and the table attenuation factor (). RDSR provides for entry of a single CF which corrects air kerma reported at the IRP, , in such a way by calibration of the against quality control measurements. The allowable tolerance for the displayed and kerma‐area product () values should not deviate from the actual values by more than ± 35% above 100 mGy and 2.5 Gy‐cm2, respectively. 32 The effect of the patient table and the mattress on the x‐ray beam transmission is taken into account by using which depends on beam quality, field size, and C‐arm angulation. DeLorenzo et al. 33 found that values at 0° for table plus pad ranged from 0.59 to 0.89 for three different fluoroscopy systems. Such a large tolerance for and the fact that is not defined in RDSR could lead to very inaccurate skin dose calculations.
Evaluation of calibration factor
Calibration of the KAP meters in the Siemens Artis Zee Biplane system was performed with a solid‐state semiconductor detector R100B (RTI Electronics AB, Molndal, Sweden), calibrated in the Secondary Standard Dosimetry Laboratory at the Vinča Institute of Nuclear Sciences in standard RQR beam qualities. 29 Setups used for determination of the calibration factors are shown in Fig 1. The frontal tube (Plane A) was set at the under‐table position (primary angle = 0ο, secondary angle = 0ο), and the lateral tube (Plane B) was set at the lateral position (primary angle = ±90ο, secondary angle = 0ο). R100B detector was taped at the beam entrance side of the table for Plane A measurements, while it was taped at the beam entrance side of the PMMA phantom for Plane B measurements. A radiopaque ruler was used to measure dimensions of the x‐ray field which was set to approximately 10 × 10 cm in the plane of the detector.
Fig. 1.
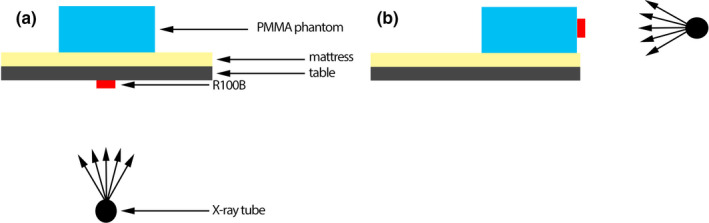
Calibration factor evaluation setup for (a) plane a and (b) plane B. Plane A was set in under‐table position and Plane B was set at the lateral position.
The Siemens Artis Zee Biplane was operated in service mode in order to determine the for different beam qualities. Exposures were made using X‐ray tube voltages ranging from 60 to 120 kVp with 10 kVp increments at six different copper filter thicknesses: 0, 0.1, 0.2, 0.3, 0.6, and 0.9 mm. The measured by the system’s KAP meter and the incident air kerma () measured by R100B were recorded for each exposure. Calibration factor for each exposure was calculated as:
| (3) |
where is the calibration factor, is the incident air kerma measured by R100B, is the field area in the plane of the R100B, and is air kerma‐area product measured by the KAP meter.
Evaluation of table attenuation factor
was measured using the frontal tube (Plane A) set at the under‐table x‐ray tube position (primary angle = 0ο, secondary angle = 0ο) using detector R100B. Setups used for determination of values are shown in Fig 2. Setup 1 was used to measure air kerma rates without the patient table influence, while setup 2 was used to measure air kerma rates when table and mattress were in the beam path. A radiopaque ruler was used to measure dimensions of x‐ray field for setup 2.
Fig. 2.
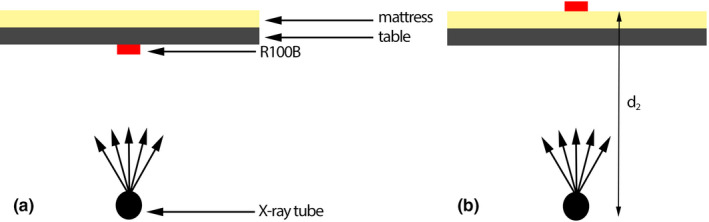
Table attenuation factor measurements for (a) setup 1 and (b) setup 2. Setup 1 was used to measure air kerma rates when there was no influence of table, whereas setup 2 was used to measure air kerma rates when table and mattress were in the beam path.
Service‐mode projections were acquired for each value of the x‐ray tube voltage ranging from 60 to 120 kVp with 10 kVp increments and varying copper thicknesses of 0, 0.1, 0.2, 0.3, 0.6, and 0.9 mm. Also, three field sizes of 10 × 10 cm2, 15 × 15 cm2, and 20 × 20 cm2 in the plane of R100B have been employed. Air kerma rates measured by R100B were recorded for each exposure. Table attenuation factor for each exposure was calculated as:
| (4) |
where is the table attenuation factor, is the air kerma rate from setup 1, is the air kerma rate from setup 2, and the ratio stands for the inverse‐square‐law correction for distance.
For a beam incident on the table surface at a nonperpendicular angle, , should be multiplied with oblique factor, , which is defined as a relative fraction of transmission between zero and nonzero angles of incidence. Using methodology proposed by Rana et al., 9 oblique factor was calculated as:
| (5) |
2.B.4. Comparison of patient skin dose maps generated using different commercial software tools
Apart from acceptance protocol, the accuracy of SkinCare was evaluated by visual comparison of skin dose maps generated by SkinCare and different commercial software tools using DICOM RDSRs generated after cardiology clinical procedures (percutaneous coronary intervention‐PCI) performed on patients. These procedures were performed on fluoroscopy units from different vendors (Canon, GE, Philips and Siemens) and their corresponding RDSRs were used to generate skin dose maps by SkinCare and to compare them to skin dose maps generated by different commercial software tools (DTS from Canon, RadimetricsTM from Bayer and RDM from MEDSQUARE). These skin dose mapping software tools were chosen because they have been validated against the fluoroscopy units from above‐mentioned manufacturers. The main objective of this comparison was to verify the correctness of applied geometric algorithm for different manufacturers, while PSD comparison was not performed because the lack of the knowledge of fluoroscopy system specific values of CF and TAF would result in incorrect comparison.
3. RESULTS
3.A. SkinCare
The graphical user interface (GUI) of SkinCare is shown in Figure 3. It is a single‐page long‐scrolling standalone application that runs in any web browser. Modern design and ease of use enables very fast acquisition of skin dose map and PSD.
Fig. 3.
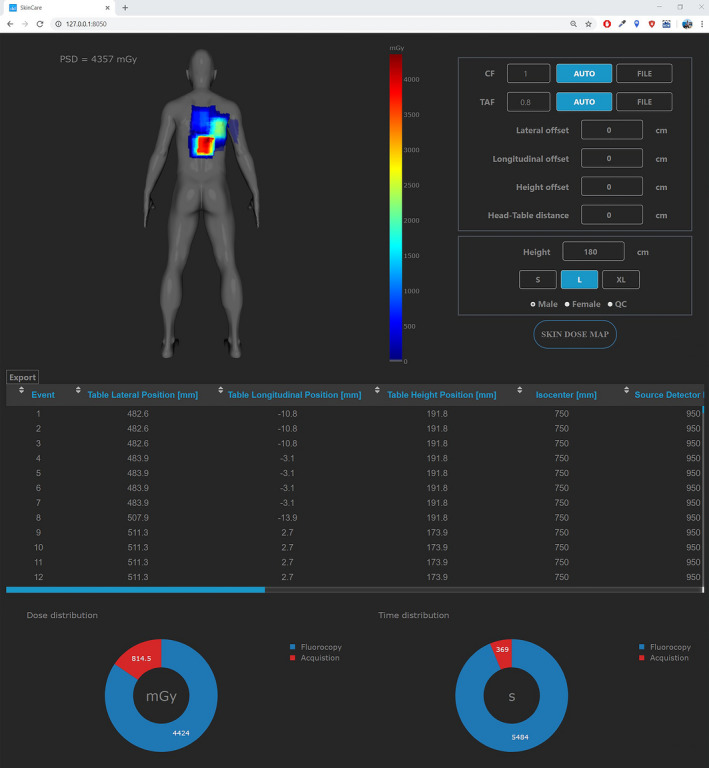
SkinCare user interface.
SkinCare enables usage of single and values through the AUTO mode (by pressing AUTO button) or multiple and values via the FILE mode (by pressing FILE button). In the FILE mode user needs to choose a .csv file filled with values for given template. Table 1 and Table 2 present templates filled with arbitrary values for and determination, respectively. In Table 2 “FOV” corresponds to table top square field size. There is no limit in number of rows for templates, so it is up to medical physicists to decide on number of measurements. Last two columns of templates correspond to and values for fluoroscopy and image acquisition, thus this mode enables very accurate skin dose assessment. In this work we only acquired and for image acquisition but it is possible to acquire separate factors for both fluoroscopy and image acquisition. Interpolated and values are then calculated for every irradiation event based on values inserted in templates.
Table 1.
Template for determination.
| kV [kV] | Cu [mm] | CF fluoroscopy | CF image acquisition |
|---|---|---|---|
| 60 | 0 | 0.80 | 0.80 |
| 80 | 0.1 | 0.81 | 0.81 |
| 100 | 0.2 | 0.82 | 0.82 |
|
120 ⋮ |
0.3 ⋮ |
0.83 ⋮ |
0.83 ⋮ |
Table 2.
Template for determination.
| FOV [cm] | kV [kV] | Cu [mm] | TAF fluoroscopy | TAF image acquisition |
|---|---|---|---|---|
| 7 | 60 | 0 | 0.80 | 0.80 |
| 7 | 80 | 0.1 | 0.81 | 0.81 |
| 7 | 100 | 0.2 | 0.82 | 0.82 |
|
7 ⋮ |
120 ⋮ |
0.3 ⋮ |
0.83 ⋮ |
0.83 ⋮ |
Patient model configuration is performed by setting the height, size, and gender of a patient. Apart from patient models, there are two additional models (slab phantom and XR‐RV3 Gafchromic film) which enable medical physicists to perform QC test of the system. Rotation of the models enables easier visualization of the skin dose map, especially when larger angulations and oblique projections are present.
Below the skin dose map is scrollable table in horizontal and vertical directions which contains data from RDSR (event number, table lateral position, table longitudinal position, table height position, source to isocenter distance, source to detector distance, event type, primary angle, secondary angle, air kerma at IRP, tube voltage, and copper filtration) and data calculated by SkinCare (field‐of‐view, BSF, CF, TAF, field‐of‐view at entrance side of patient, and source to patient distance) for each irradiation event. Two doughnut charts that show image acquisition vs fluoroscopy dose and time distributions are positioned below the table.
Skin dose maps for different RDSRs are shown in Fig 4. Color coded dose map is relative to the PSD for each individual patient. Figure 4 highlights the effectiveness of SkinCare for producing very detailed dose maps from different fluoroscopy system manufacturers on a diverse patient population.
Fig. 4.
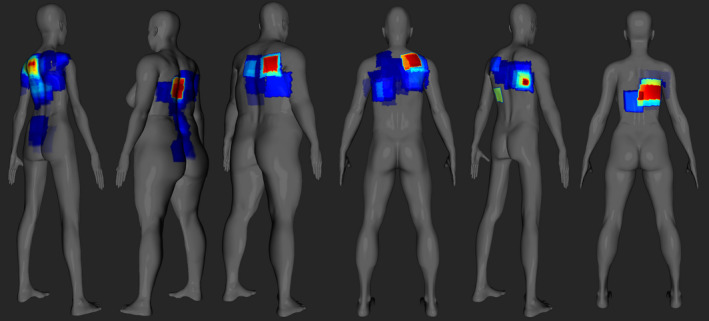
Skin dose maps for different RDSRs. Color map is relative to the PSD for each individual patient.
Figure 5 shows models of the XR‐RV3 Gafchromic film (left) and slab phantom (right). Since the models have flat surfaces and good spatial resolution, medical physicists can perform fast measurements for the purpose of QC tests.
Fig. 5.
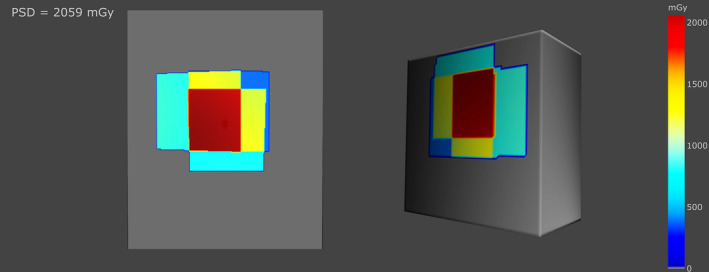
Models of XR‐RV3 Gafchromic film (left) and slab phantom (right) with dose maps.
3.B. SkinCare validation
3.B.1. Gafchromic film calibration
XR‐RV3 Gafchromic calibration curve for determining skin dose is shown in Figure 6. Fitting coefficients have the following values: . Table 3 shows measured values, predicted values, and percent errors for all data used during calibration. Fitting errors in the high dose range (>2 Gy) where tissue reactions may occur to the skin are within 3.2%. Additionally, root mean square error (RMSE) goodness‐of‐fit indicator was calculated in order to evaluate the performance of the fitting equation. Fitting equation produces an RMSE of 92 mGy which demonstrates small error of fitting.
Fig. 6.
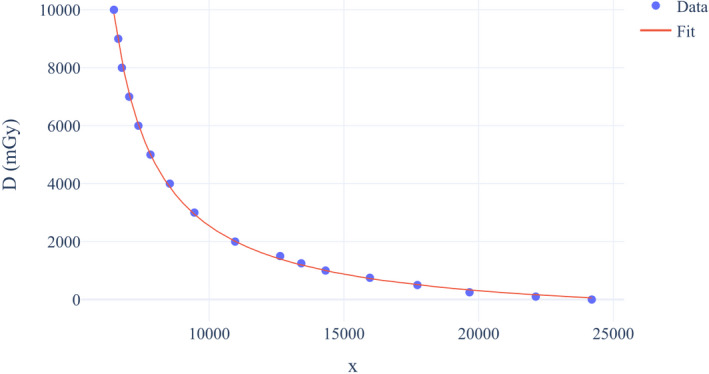
XR‐RV3 Gafchromic calibration curve.
Table 3.
Assessment of fitting function from Equation 2.
| Point | Measured (mGy) | Predicted (mGy) | Error (%) |
|---|---|---|---|
| 1 | 100 | 162.6 | 62.6 |
| 2 | 250 | 330.7 | 32.28 |
| 3 | 500 | 508.1 | 1.62 |
| 4 | 750 | 723.4 | −3.55 |
| 5 | 1000 | 996.4 | −0.36 |
| 6 | 1250 | 1190.4 | −4.77 |
| 7 | 1500 | 1395.5 | −6.97 |
| 8 | 2000 | 2007.8 | 0.39 |
| 9 | 3000 | 2942.5 | −1.92 |
| 10 | 4000 | 3875.1 | −3.12 |
| 11 | 5000 | 5007.5 | 0.15 |
| 12 | 6000 | 6040.0 | 0.66 |
| 13 | 7000 | 7110.3 | 1.57 |
| 14 | 8000 | 8228.8 | 2.86 |
| 15 | 9000 | 8910.1 | −1.00 |
| 16 | 10000 | 9867.0 | −1.33 |
3.B.2. Calibration factor and Table attenuation factor
Calibration factors for each beam quality for both tubes of biplane system are presented in Figures 7 and 8. For each particular filter thickness for all tube voltages, differences between the lowest and the highest calibration factors values were calculated. For Plane A differences ranged from 1.3% to 4.5%, whereas for Plane B differences ranged from 3.2% to 7.0%.
Fig. 7.
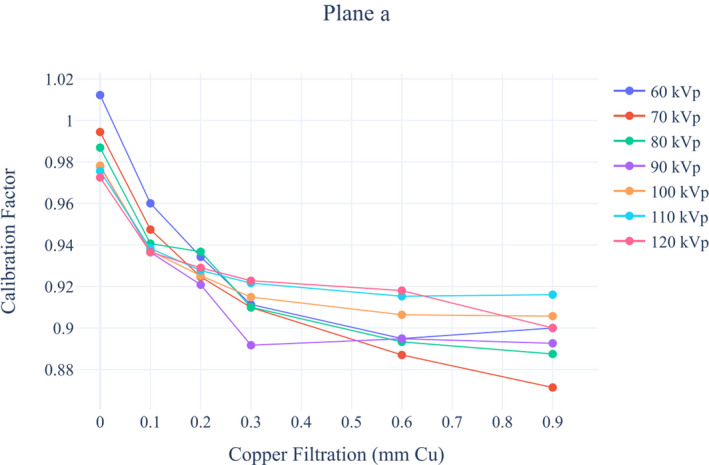
Siemens Artis Zee Biplane fluoroscopy system calibration factors for Plane a.
Fig. 8.
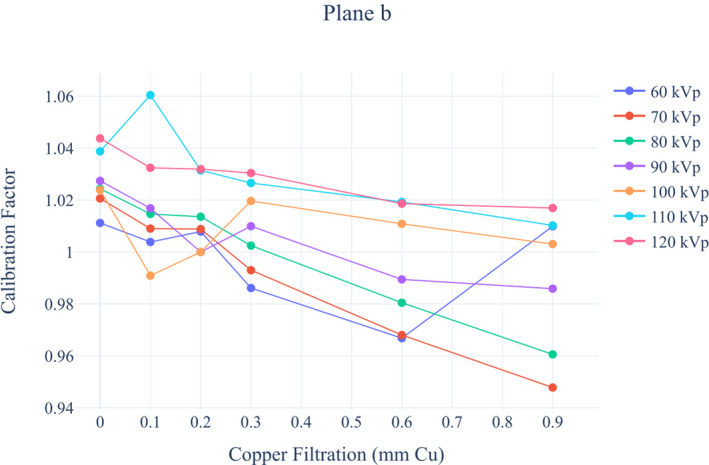
Siemens Artis Zee Biplane fluoroscopy system calibration factors for Plane b.
Table attenuation factors for Siemens Artis Zee Biplane fluoroscopy system can be found in Table 4. With the increase in the x‐ray tube voltage and by increasing the field size and added Cu filtration of the primary x‐ray beam, value has increased. The recorded values ranged from 0.69 to 0.88. Table 5 shows the oblique factor as a function of incident angle, kVp and added Cu filtration for the 10 x 10 cm2 field size. decreased with the increase in the incident angle and at 10ο, 20ο, 30ο, and 40ο ranged from 0.994 to 0.997, 0.977 to 0.989, 0.945 to 0.973, and 0.894 to 0.948, respectively. Field size had a small influence on .
Table 4.
Siemens Artis Zee Biplane fluoroscopy system table attenuation factors.
| Field Size (cm2) | Tube voltage (kV) | Additional filtration (mm Cu) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | 0.6 | 0.9 | ||
| 10 × 10 | 60 | 0.694 | 0.745 | 0.769 | 0.783 | 0.808 | 0.825 |
| 70 | 0.713 | 0.762 | 0.785 | 0.798 | 0.822 | 0.833 | |
| 80 | 0.724 | 0.772 | 0.793 | 0.806 | 0.827 | 0.836 | |
| 90 | 0.734 | 0.779 | 0.799 | 0.810 | 0.829 | 0.836 | |
|
100 110 |
0.743 | 0.785 | 0.805 | 0.814 | 0.831 | 0.836 | |
| 0.750 | 0.790 | 0.808 | 0.817 | 0.832 | 0.838 | ||
| 120 | 0.756 | 0.794 | 0.811 | 0.823 | 0.834 | 0.839 | |
| 15 × 15 | 60 | 0.703 | 0.755 | 0.782 | 0.796 | 0.823 | 0.834 |
| 70 | 0.721 | 0.774 | 0.798 | 0.810 | 0.838 | 0.848 | |
| 80 | 0.732 | 0.783 | 0.807 | 0.819 | 0.842 | 0.851 | |
| 90 | 0.743 | 0.790 | 0.812 | 0.823 | 0.845 | 0.848 | |
| 100 | 0.751 | 0.796 | 0.817 | 0.827 | 0.845 | 0.851 | |
| 110 | 0.759 | 0.801 | 0.821 | 0.830 | 0.845 | 0.852 | |
| 120 | 0.765 | 0.805 | 0.824 | 0.833 | 0.848 | 0.853 | |
| 20 × 20 | 60 | 0.720 | 0.772 | 0.795 | 0.814 | 0.830 | 0.843 |
| 70 | 0.741 | 0.791 | 0.812 | 0.830 | 0.849 | 0.860 | |
| 80 | 0.753 | 0.802 | 0.823 | 0.840 | 0.856 | 0.866 | |
| 90 | 0.765 | 0.811 | 0.830 | 0.847 | 0.862 | 0.870 | |
| 100 | 0.775 | 0.817 | 0.839 | 0.851 | 0.866 | 0.874 | |
| 110 | 0.784 | 0.823 | 0.844 | 0.855 | 0.867 | 0.876 | |
| 120 | 0.790 | 0.828 | 0.849 | 0.858 | 0.871 | 0.878 | |
Table 5.
Siemens Artis Zee Biplane table oblique factors.
| Field Size (cm2) | Tube voltage (kV) | Additional filtration (mm Cu) | 10ο | 20ο | 30ο | 40ο |
|---|---|---|---|---|---|---|
| 10 × 10 | 60‐120 | 0 | 0.994‐0.996 | 0.977‐0.982 | 0.945‐0.958 | 0.894‐0.918 |
| 60‐120 | 0.1 | 0.995‐0.996 | 0.981‐0.985 | 0.955‐0.965 | 0.914‐0.932 | |
| 60‐120 | 0.2 | 0.996‐0.997 | 0.983‐0.987 | 0.960‐0.968 | 0.923‐0.938 | |
| 60‐120 | 0.3 | 0.996‐0.997 | 0.984‐0.988 | 0.963‐0.970 | 0.928‐0.942 | |
| 60‐120 | 0.6 | 0.997‐0.997 | 0.986‐0.988 | 0.968‐0.972 | 0.937‐0.946 | |
| 60‐120 | 0.9 | 0.997‐0.997 | 0.988‐0.989 | 0.971‐0.973 | 0.943‐0.948 |
3.B.3. VERIDIC’s acceptance and QC testing protocols
In Table 6 eight fundamental irradiation setups used to validate SkinCare and PSD comparison between XR‐RV3 Gafchromic films and SkinCare are displayed. During all irradiations the tube voltage and the added filtration were automatically set by the system, while RDSR attributes Table Height position, Distance source to detector, and Collimated field area were set at 15 cm, 120 cm, and 900 cm2, respectively. First five setups were used for the purpose of testing the effect of the phantom scatter, sixth and seventh were used for testing of the effect of field overlap, whereas eighth was used for testing the effect of lateral irradiations. The tests have shown that PSD calculated by SkinCare is within 17% accuracy compared with measurements using XR‐RV3 Gafchromic films.
Table 6.
Fundamental irradiation setups.
| Setup | Configuration | Tube projection | Dose (IRP) total (mGy) | PSD (mGy) | Accuracy | |
|---|---|---|---|---|---|---|
| XR‐RV3 | SkinCare | |||||
| 1* | Phantom 15 cm + table + mattress | PA | 968 | 1362 | 1184 | −13.1% |
| 2* | Phantom 20 cm + table + mattress | PA | 673 | 784 | 765 | −2.4% |
| 3* | Phantom 25 cm + table + mattress | PA | 556 | 605 | 630 | 4.1% |
| 4* | Phantom 30 cm + table + mattress | PA | 1180 | 1463 | 1414 | −3.3% |
| 5* | Phantom 10 cm + table + mattress | PA | 96 | 130 | 108 | −16.9% |
| 6* | Phantom 25 cm + mattress + table | PA + LAO 20ο | 1528 | 2187 | 1947 | −11.0% |
| 7* | Phantom 25 cm + mattress + table | PA + LAO 20ο + PA CRAN 15ο | 1638 | 2240 | 2086 | −6.9% |
| 8* | Phantom 25 cm + mattress + table | LAO 90ο | 1317 | 1777 | 1869 | 5.2% |
Table Height position = 15 cm, Distance source to detector = 120 cm, FOV = 29 cm, tube voltage and additional filtration (mm Cu) ‐ Automated selection.
Table 7 shows setups of the simplified clinical procedures. During all irradiations the tube voltage and the added filtration were automatically set by the system, while the distance source to detector was kept constant at 120 cm. Slab phantom with an addition of 10 cm PMMA layer were used as the phantom. Procedures varied considerably as it can be seen from Table 7 and Fig. 9. Figure 9 shows comparison between scanned XR‐RV3 Gafchromic films and dose maps obtained using SkinCare’s XR‐RV3 Gafchromic phantom for all three simplified clinical procedures. It should be noted that the scanner used for geometrical comparison in Figure 9 was not used for calibration and PSD assessment due to the presence of horizontal lines on scanned images. On both of the images for the same procedures there are letters A–D used for comparison of the spatial distribution of dose and numbers 1–4 used for dose comparison. In Table 8 the comparison results are displayed. The doses calculated by SkinCare were within 16% accuracy compared with measurements using XR‐RV3 Gafchromic films. Spatial distribution of dose as calculated on the SkinCare’s XR‐RV3 Gafchromic phantom were within 9% accuracy compared to spatial distributions recorded on the GafChromic film for all points except for one point where accuracy was −20.7% (2.8 cm in this case).
Table 7.
Simplified clinical procedures.
| Procedure | Primary Angle (degrees) | Secondary Angle (degrees) | Collimated Field Area (cm2) | Table Height Position (cm) | Cumulative Ka,r (mGy) |
|---|---|---|---|---|---|
| 1* | −30 | −10 | 745 | 15 | 498 |
| −30 | −5 | 439 | 15 | 533 | |
| −25 | −5 | 439 | 15 | 431 | |
| 30 | −15 | 751 | 15 | 469 | |
| 30 | −5 | 751 | 15 | 346 | |
| 35 | −15 | 285 | 15 | 372 | |
| 2* | 30 | 5 | 506 | 10 | 191 |
| 25 | −5 | 815 | 10 | 176 | |
| 30 | 0 | 815 | 10 | 183 | |
| −10 | 5 | 234 | 10 | 141 | |
| 20 | 0 | 234 | 10 | 165 | |
| −30 | 30 | 838 | 10 | 221 | |
| 3* | 20 | 10 | 552 | 5 | 248 |
| 50 | 10 | 552 | 5 | 461 | |
| −30 | 15 | 552 | 5 | 277 | |
| 25 | 35 | 552 | 5 | 327 | |
| 30 | 20 | 552 | 5 | 320 | |
| 50 | 10 | 552 | 5 | 703 |
Distance source to detector = 120 cm, tube voltage and additional filtration (mm Cu) ‐ Automated selection.
Fig. 9.
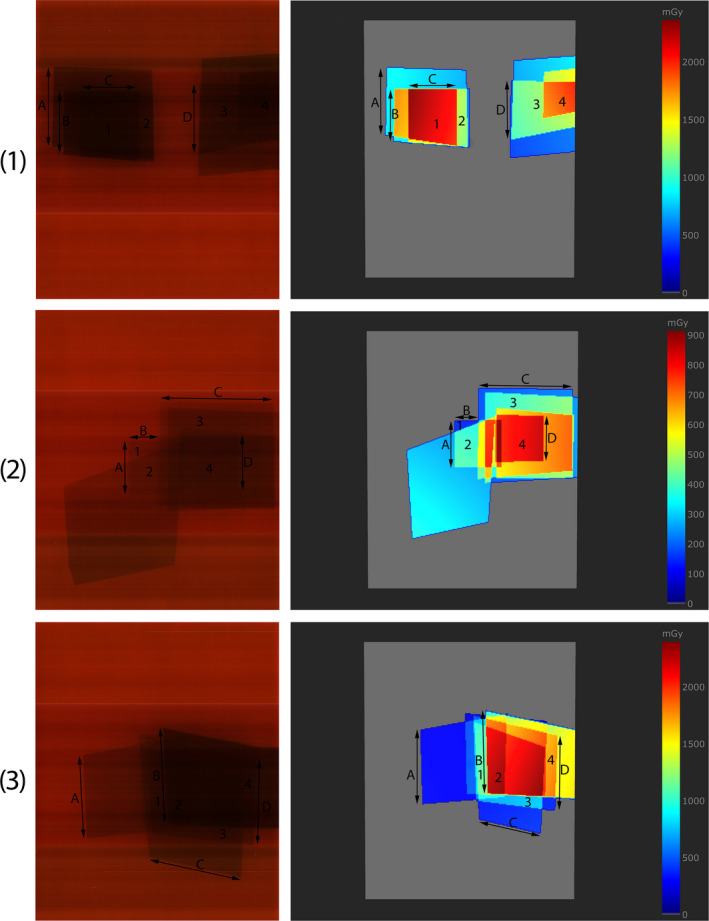
Comparison of the dose maps obtained using XR‐RV3 Gafchromic films (left) and SkinCare’s XR‐RV3 Gafchromic phantom (right) for three simplified clinical procedures. On both images for the same procedures there are letters a‐d used for comparison of the spatial distribution of dose and numbers 1–4 used for dose comparison. First row represents first procedure, second row second procedure, and third row third procedure.
Table 8.
Comparison of the doses and spatial distribution of dose measured by XR‐RV3 Gafchromic films and SkinCare.
| Procedure | Point | Dose (mGy) | Accuracy | Point | Distance (cm) | Accuracy | ||
|---|---|---|---|---|---|---|---|---|
| XR‐RV3 | SkinCare | XR‐RV3 | SkinCare | |||||
| 1 | 1 | 1794 | 1809 | 0.8% | A | 11.4 | 11.4 | 0.0% |
| 2 | 1042 | 1078 | 3.5% | B | 9.0 | 8.9 | −1.1% | |
| 3 | 1068 | 1025 | −4.0% | C | 8.2 | 8.2 | 0.0% | |
| 4 | 1505 | 1552 | 3.1% | D | 10.1 | 9.9 | −2.0% | |
| 2 | 1 | 127 | 133 | 4.7% | A | 7.5 | 8.0 | 6.7% |
| 2 | 310 | 358 | 15.5% | B | 4.4 | 4.0 | −8.6% | |
| 3 | 422 | 370 | −12.3% | C | 16.0 | 16.0 | 0.0% | |
| 4 | 781 | 721 | −7.7% | D | 7.4 | 7.5 | 1.1% | |
| 3 | 1 | 1079 | 1015 | −5.9% | A | 11.9 | 12.5 | 5.0% |
| 2 | 1898 | 1971 | 3.8% | B | 13.8 | 14.1 | 2.2% | |
| 3 | 565 | 647 | 14.5% | C | 13.5 | 10.7 | −20.7% | |
| 4 | 1556 | 1464 | −5.9% | D | 12.4 | 12.6 | 1.6% | |
3.B.4. Comparison of patient skin dose maps generated using different commercial software tools
Figure 10 presents comparison of a skin dose map generated by SkinCare to skin dose maps from different commercial software tools, all based on the same RDSRs generated during routine clinical PCI procedure. In particular, RDRSs from Canon, Philips, and GE/Siemens fluoroscopy units were used to compare skin dose maps from SkinCare to skin dose map from DTS, RadimetricsTM, and RDM, respectively. Good visual agreement between dose maps proved that SkinCare’s geometry methodology is correct for all major vendors.
Fig. 10.
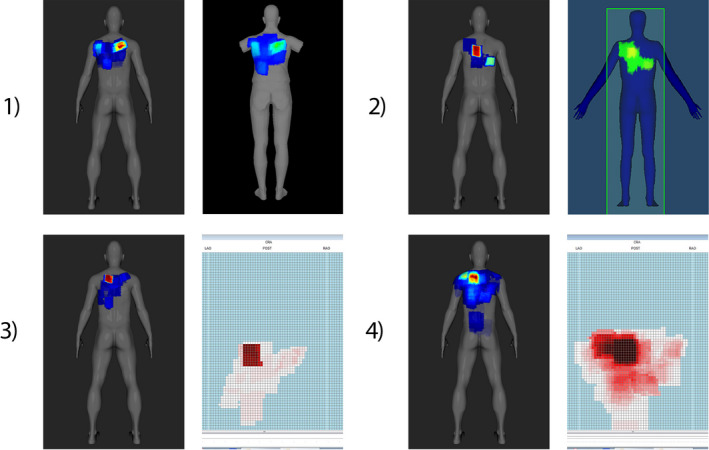
Dose map comparison between SkinCare and 1) DTS for Canon RDSR, 2) RadimetricsTM for Philips RDSR, 3) RDM for GE RDSR, 4) RDM for Siemens RDSR.
From the presented results, it is evident that 3D patient representation clearly shows advantages compared to 2D patient representation, especially in the case of larger angulations and oblique projections. Unlike other software which display color maps relative to the PSD, RadimetricsTM displays color map on an absolute dose scale which makes harder to locate highest irradiated region on patient’s skin. Furthermore, RadimetricsTM has slightly worse spatial resolution of dose maps, which makes edges of dose maps sawtooth‐shaped. In addition, DTS models without arms are good way to address cases when patient’s arms are in overhead position, unlike RadimetricsTM and SkinCare which calculate skin dose for patient’s arms if they are in x‐ray beam path.
4. DISCUSSION
Standalone vendor‐independent application SkinCare presented in this paper is elegant and efficient way for skin dose mapping in interventional radiology and interventional cardiology. Validation performed by using the data from the Siemens Artis Zee Biplane fluoroscopy system and conducting VERIDIC’s “Acceptance and quality control protocols for skin dose calculating software solutions in interventional cardiology” showed that PSD calculated by SkinCare was within 17% accuracy compared with measurements using XR‐RV3 Gafchromic films for fundamental irradiation setups, whereas doses calculated by SkinCare were within 16% accuracy compared with measurements using XR‐RV3 Gafchromic films for simplified clinical procedures. With respect to the overall uncertainty of skin dose measurements using Gafchromic films, that ranges from 9% to 78%, depending on the level of control of various film dosimetry steps, 30 the difference of < 20% is considered to be acceptable.
The obtained results are in the same range as those obtained with similar studies that were carried out by other research groups by evaluating software with predefined protocol. Bordier et al. 5 compared a calculation method that produces dose maps using Gafchromic XR‐RV3 films on an anthropomorphic phantom and found that doses agreed within better than 15% compared with the Gafchromic films. Using the Radiation Dose Monitor tool, Habib Geryes et al. 3 accomplished average difference of 10 ± 7% and 9 ± 7% between the calculated and the measured PSD values for 34 test conditions performed on PMMA phantom using Siemens Artis Zee and GE Innova IGS interventional systems, respectively. Methodology to evaluate software described by Habib Geryes et al. was used in study by Greffier et al. 18 and it was found that average differences between the measured PSD by XR‐RV3 Gafchromic films and the calculated PSD using interactive Skin Dose Map® tool (SDMTool) integrated to the radiation dose management system (RDMS) DoseWatch® were 6% ± 6% (range from − 3% to 22%) for flat phantom and 5% ± 7% (range from − 3% to 25%) for ICRP phantom. Rana et al. 9 found that biplane dose tracking system (Biplane‐DTS) was able to determine the entrance dose within 6% and the spatial distribution of the dose within 4% compared to the measurements with the ionization chamber and film for the SK150 head phantom.
Quantitative analysis by comparing geometry of dose maps between Skincare and other commercial software was not possible to conduct due to lack of information about input parameters for the calibration and table offsets used by other solutions. Nevertheless, good visual agreement between dose maps obtained by SkinCare and commercial software tools (DTS from Canon, RadimetricsTM from Bayer and RDM from MEDSQUARE) proved that SkinCare can be used by medical physicists in practice for most of the modern fluoroscopy units.
Two multiplicative factors that significantly influence skin dose calculations are and . RDSR have only one , whereas is not defined in RDSR. Additionally, a large range of possible values and allowable tolerance of ± 35% above 100 mGy and 2.5 Gy‐cm2 for the displayed and , respectively, could lead to very inaccurate skin dose calculations. Wunderle et al. 34 showed that for typical adult beam qualities, applying a single determined at tube voltage of 100 kV with cooper filtration in the beam results in a deviation of less than 5% due to beam quality variation. Therefore, attribute in RDSR containing multiple values for these factors is mandatory since single and unavailable cannot account for typical beam qualities used during interventional procedures.
SkinCare calculates skin dose map and PSD completely based on RDSR, thus any difference between RDSRs from different vendors greatly affect calculation. For instance, only Siemens and Philips have defined Table Lateral Position and Table Longitudinal Position fields according to the DICOM standard, however, some RDSRs from Philips record these fields in RDSR only for cine acquisitions. Similarly, Siemens and GE report square fields by automatically converting rectangular fields into square equivalent fields which can affect spatial distribution of dose as shown in Table 8, whereas Toshiba’s (Canon) RDSR fields Collimated field height and Collimated field width when multiplied give a result which is larger than the value in Collimated field area. Philips has defined fields Bottom shutter, Left shutter, Right shutter, and Top shutter at 1 m from the focal spot in some DICOM conformance statements while in others definition is not provided so it is up to medical physicist to check this in practice. GE sometimes does not report additional filtration while Philips in some RDSRs does not report Distance Source to Detector for every event.
From above it is obvious that taking into account all possible vendor specific exceptions can be a problem. Thus, harmonizing RDSRs is mandatory for easier and more accurate assessment of PSD. Additionally, if vendors come up with accurate way of assessing patient position on table and implement that in RDSR, then cumulative skin dose could be accurately calculated for repeated procedures.
5. CONCLUSION
This paper presents originally developed standalone desktop application SkinCare for generating skin dose maps and PSD calculation after completion of interventional radiology and cardiology procedures on realistic 3D patient models using DICOM RDSRs from Canon (Toshiba), GE, Philips, and Siemens fluoroscopy systems. SkinCare is proved to be very convenient solution that can be used for monitoring delivered dose following the interventional procedures. The goal of future research is the validation considering the clinical use of SkinCare and expanding SkinCare’s capabilities to support all interventional radiology and cardiology procedures and setups.
CONFLICT OF INTEREST
The authors have no relevant conflict of interest to disclose.
Author Contribution
Marko Krajinović and Olivera Ciraj‐Bjelac conceived of the presented idea. Marko Krajinović developed the software and performed the computations. Nikola Kržanović performed Gafchromic film calibration, R100B calibration, and performed calibration curve fitting. Olivera Ciraj‐Bjelac encouraged Marko Krajinović to evaluate Calibration factors and Table attenuation factors in the Siemens service mode over wide range of beam qualities. Olivera Ciraj‐Bjelac supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Acknowledgments
The authors thank Biljana Toskić and Jasenka Vasić‐Vilić for their assistance with fluoroscopic service mode. The authors thank to the partners of the VERIDIC project (part of the CONCERT project) who provided insight and expertise that greatly assisted the research. The research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia.
REFERENCES
- 1. Cousins C, Miller DL, Bernardi G, et al, ICRP publication 120: radiological protection in cardiology. Annals of the ICRP. 2013;42:1–125. [DOI] [PubMed] [Google Scholar]
- 2. Farah J, Trianni A, Carinou E, et al, Measurement of maximum skin dose in interventional radiology and cardiology and challenges in the set‐up of European alert thresholds. Radiat Prot Dosimetry. 2015;164:138–142. [DOI] [PubMed] [Google Scholar]
- 3. Habib Geryes B, Hadid‐Beurrier L, Waryn MJ, Jeanpierre A, Farah J. Benchmarking the DACS‐integrated radiation dose monitor® skin dose mapping software using XR‐RV3 Gafchromic films. Med Phys. 2018;45:4683–4692. [DOI] [PubMed] [Google Scholar]
- 4. Bordier C, Klausz R, Desponds L. Accuracy of a dose map method assessed in clinical and anthropomorphic phantom situations using Gafchromic films. Radiat Prot Dosimetry. 2015;165:244–249. [DOI] [PubMed] [Google Scholar]
- 5. Bordier C, Klausz R, Desponds L. Patient dose map indications on interventional X‐ray systems and validation with Gafchromic® XR‐RV3 film. Radiat Prot Dosimetry. 2015;163:306–318. [DOI] [PubMed] [Google Scholar]
- 6. Nilsson Althén J, Sandborg M. Verification of indicated skin entrance air kerma for cardiac X‐ray‐guided intervention using Gafchromic film. Radiat Prot Dosimetry. 2016;169:245–248. [DOI] [PubMed] [Google Scholar]
- 7. Bednarek DR, Barbaritis J, Rana VK, Nagaraja SP, Josan MS, Rudin S. Verification of the performance accuracy of a real‐time skin‐dose tracking system for interventional fluoroscopic procedures. Proc SPIE Int Soc Opt Eng. 2011;7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rana VK, Rudin S, Bednarek DR. Updates in the real‐time dose tracking system (DTS) to improve the accuracy in calculating the radiation dose to the patients skin during fluoroscopic procedures. Proc SPIE Int Soc Opt Eng. 2013;8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rana VK, Rudin S, Bednarek DR. A tracking system to calculate patient skin dose in real‐time during neurointerventional procedures using a biplane X‐ray imaging system. Med Phys. 2016;43:5131–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Digital Imaging and Communications in Medicine (DICOM) . Supplement 94: diagnostic X‐ray radiation dose reporting (Dose SR). 2005.
- 11. National Electrical Manufacturers Association . The DICOM Standard 2018. Available on: https://www.dicomstandard.org/. Accessed September 1, 2020.
- 12. Greffier J, Van Ngoc Ty C, Bonniaud G, et al, Assessment of peak skin dose in interventional cardiology: a comparison between GafChromic film and dosimetric software em.dose. Phys Medica. 2017;38:16–22. [DOI] [PubMed] [Google Scholar]
- 13. Magnier F, Poulin M, Van Ngoc Ty C, et al, Comparison of patient skin dose evaluated using radiochromic film and dose calculation software. Cardiovasc Intervent Radiol. 2018;41:762–771. [DOI] [PubMed] [Google Scholar]
- 14. Fitousi N. Patient dose monitoring systems: a new way of managing patient dose and quality in the radiology department. Phys Med. 2017;44:212–221. [DOI] [PubMed] [Google Scholar]
- 15. Calderoni F, Campanaro F, Colombo PE, et al, Analysis of a multicentre cloud‐based CT dosimetric database: preliminary results. Eur Radiol Exp. 2019;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colombo PE, Rottoli F, Felisi M, et al, Validation of a dose tracking software for skin dose map calculation in interventional radiology. Physica Med. 2020;72:122–132. [DOI] [PubMed] [Google Scholar]
- 17. Malchair F, Dabin J, Deleu M, Maccia C, Merce MS. VERIDIC ‐ Standards for digital dose reporting 2018. Available on: https://www.concert‐h2020.eu/en/Publications. Accessed September 1, 2020.
- 18. Greffier J, Grussenmeyer‐Mary N, Larbi A, et al, Experimental evaluation of a radiation dose management system‐integrated 3D skin dose map by comparison with XR‐RV3 Gafchromic® films. Phys Med. 2019;66:77–87. [DOI] [PubMed] [Google Scholar]
- 19. Borrego D, Marshall T, Tran D, Siragusa WB. Physical validation of UF‐RIPSA: a rapid in‐clinic peak skin dose mapping algorithm for fluoroscopically guided interventions. J Appl Clin Med Phys. 2018;19:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson PB, Borrego D, Balter S, Johnson K, Siragusa D, Bolch WE. Skin dose mapping for fluoroscopically guided interventions. Med Phys. 2011;38:5490–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khodadadegan Y, Zhang M, Pavlicek W, et al, Automatic monitoring of localized skin dose with fluoroscopic and interventional procedures. J Digit Imaging. 2011;24:626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takata T, Kotoku J, Maejima H, et al, Fast skin dose estimation system for interventional radiology. J Radiat Res. 2017;59:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hellström M. Estimating patient peak skin dose with fluoroscopic procedures. Umea: Department of Radiation Science, Umea University; 2018. [Google Scholar]
- 24. Dabin J, Deleu M, Feghali JA, Gallagher A, Maccia C, Malchair F, Merce MS. VERIDIC ‐ Acceptance and quality control protocols for skin dose calculating software solutions in interventional cardiology 2020. Available on: https://www.concert‐h2020.eu/en/Publications. Accessed September 1, 2020.
- 25. MB‐Lab, Available on: https://mb‐lab‐community.github.io/MB‐Lab.github.io/. Accessed September 1 2020.
- 26. Blender™, Available on: http://www.blender.org. Accessed September 1, 2020.
- 27. IEC . Medical electrical equipment ‐ Part 2–43: particular requirements for the basic safety and essential performance of X‐ray equipment for interventional procedures, Report No. 60601–2–43, 2010. [DOI] [PubMed]
- 28. Benmakhlouf H, Bouchard H, Fransson A, Andreo P. Backscatter factors and mass energy‐absorption coefficient ratios for diagnostic radiology dosimetry. Phys Med Biol. 2011;56:7179–7204. [DOI] [PubMed] [Google Scholar]
- 29. IEC . Medical diagnostic X‐ray equipment ‐ Radiation conditions for use in the determination of characteristics, Report No. 2005;61267:2005.
- 30. Farah J, Trianni A, Ciraj‐Bjelac O, et al, Characterization of XR‐RV3 GafChromic films in standard laboratory and in clinical conditions and means to evaluate uncertainties and reduce errors. Med Phys. 2015;42:4211–4226. [DOI] [PubMed] [Google Scholar]
- 31. Lewis D, Micke A, Yu X, Chan MF. An efficient protocol for radiochromic film dosimetry and measurement in a single scan. Med Phys. 2012;39:6339–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin P‐JP, Schueler BA, Balter S, et al, Accuracy and calibration of integrated radiation output indicators in diagnostic radiology: a report of the AAPM Imaging Physics Committee Task Group 190. Med Phys. 2015;42:6815–6829. [DOI] [PubMed] [Google Scholar]
- 33. DeLorenzo MC, Yang K, Li X, Liu B. Comprehensive evaluation of broad‐beam transmission of patient supports from three fluoroscopy‐guided interventional systems. Med Phys. 2018;45:1425–1432. [DOI] [PubMed] [Google Scholar]
- 34. Wunderle KA, Rakowski JT, Dong FF. Effect of fluoroscopic X‐ray beam spectrum on air‐kerma measurement accuracy: implications for establishing correction coefficients on interventional fluoroscopes with KAP meters. J Appl Clin Med Phys. 2016;17:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]


