Abstract
Osteomyelitisis a devastating complication of orthopaedic surgeryand commonly caused byStaphylococcus aureus(S. aureus)andGroup B Streptococcus (GBS, S. agalactiae). Clinically, S. aureus osteomyelitis is associated with local inflammation, abscesses, aggressive osteolysis, and septic implant loosening. In contrast, S. agalactiae orthopaedic infections generally involve soft tissue, with acute life-threatening vascular spread. While pre-clinical models that recapitulate the clinical features ofS. aureus bone infectionhave proven useful for research, no animal models of S. agalactiae osteomyelitisexist.Here, we compared the pathology caused by these bacteria in an established murine model of implant-associated osteomyelitis. In vitroscanning electron microscopy and CFU quantification confirmed similar implant inocula for both pathogens (~105 CFU/pin). Assessment of mice at 14 dayspost-infection demonstrated increasedS. aureus virulence, sinceS. agalactiae infected mice had significantly greater body weight, and fewer CFU on the implant, bone and adjacent soft tissue (p<0.05). X-ray, μCT and histologic analyses showed that S. agalactiae induced significantly less osteolysis and implant loosening, and fewer large TRAP+ osteoclaststhanS. aureus without inducingintraosseousabscess formation. Most notably, transmission electron microscopy revealed that although both bacteria are capable of digesting cortical bone,S. agalactiae have a predilection for colonizing blood vessels embedded within cortical bonewhileS. aureus primarily colonizes the osteocyte lacuno-canalicular network. This work establishes the first quantitative animal model of S. agalactiae osteomyelitis, and demonstrates a vasculotropic mode of S. agalactiae infection,in contrast to the osteotropic behavior of S. aureus osteomyelitis.
Keywords: Osteomyelitis, S. agalactiae, S. aureus, abscess, animal model
Introduction
Orthopaedic infections remain a devastating problem that threatens life, wellness, and our healthcare systems1.Specifically, orthopaedic implants introduce a heightened risk for infection, as the implant provides an abiotic surface for pathogens to attachto andsubsequently colonizeadjacent bone and soft tissue, resulting in severe cases of osteomyelitis. Implant-associated infections are considered very difficult to treat due to the need for complex surgical procedures and prolonged antibiotic therapy2. Rigorous intervention studies (i.e. the Surgical Care Improvement Project (SCIP)3) have demonstrated that prosthetic joint infections (PJIs) complicate 0.1–30% of total joint replacements4; 5,and rates of recurrent or persistent infection following a two-stage revision surgery are as high as 33%6. Most recently, an international registry of 192 bone infection patients (157 fracture-related infections7, 86 PJI, and 49 osteomyelitis) demonstrated a cure rate of only 62.1% at one year post-operation. As these poor outcomes have not improved since the development of antibiotic-loaded bone cement (ALBC)8, further research is needed to better understand the pathogenesis of microbial bone infection.
75% of osteomyelitis cases are caused by pathogens of the Staphylococcus genus9, where Staphylococcus aureus is the most common species10. S. aureus is an extremely versatile pathogen with an arsenal of virulence factors enabling infection in nearly every organ system of the human body11. In addition to its presence as an infecting pathogen,S. aureus persistently colonizes 20% of individuals11, making exposure to S. aureus very difficult to avoid. S. aureus is uniquely adapted to survival in implant-associated infections by a variety of mechanisms, including aggregates in synovial fluid12, biofilm formation on the implant and necrotic tissue13; 14, abscess formation in the adjacent soft tissue and bone marrow cavity15; 16, invasion of the osteocyte lacuno-canalicular network (OLCN)17–19, and the formation of small colony variants (SCVs) potentially capable of intracellular persistence20; 21.S. aureus has been the focus of most orthopaedic infection research to date because of its prevalence in the clinic. While this research has elucidated important mechanisms of S. aureus persistence in bone and allowed for more informed treatment strategies, our understanding of the pathophysiology of other pathogens in orthopaedic infections is very limited.
The second most common microbialgenus isolated from PJI’s is Streptococcus22;23,with S. agalactiae, also known as Group B Streptococcus (GBS), being the most common species24; 25. S. agalactiae is a commensal organism that colonizes the intestinal and vaginal flora. It is also anopportunistic pathogen implicated in various infectious diseases, including neonatal septicemia, pneumonia, meningitis, necrotizing fasciitis, and orthopaedic infections26; 27. Despite being known primarily for its prevalence in neonatal infections, studies have shown an increasing burden of invasive S. agalactiae diseases in non-pregnant adults28.
In the context of orthopaedic infection, the pathophysiology of S. agalactiae-induced osteomyelitishas not been investigated. Within cases of streptococcal PJI, S. agalactiae has been reported as an independent risk factor for poorer outcomes23; 25, as well as comorbidities29 and duration of symptoms30.Clinically, there have been conflicting reports related to the specific treatmentofS. agalactiae orthopaedic infection. As a result, the reported cure rates of StreptococcusPJI vary greatly from 35% to 100%23; 29–32.Some studies report strong evidence of better outcomes with debridement, antibiotics and implant retention (DAIR) treatment when the infecting pathogen is S. agalactiae24; 29; 33, while others report that the success of DAIR was not different betweenS. agalactiae andS. aureus infections30; 31.
Taken together, the pathogenesis and optimal treatment for S. agalactiae-induced PJI remains unclear. In part, this is because the mechanisms of S. agalactiae virulence and survival in implant-associated osteomyelitis is not well understood.While this organism is highly susceptible to a variety of antibiotics, its ability to infect and spread rapidly within the blood, fascia and soft tissue makes it difficult to treat33; 34.89% of streptococcalPJI are associated with damaged soft tissue29, which is in agreement with the most common clinical manifestations of S. agalactiae in non-pregnant adults being bacteremia and skin and soft tissue infections34. Therefore, the purpose of this study was to characterize the infection phenotype of S. agalactiae in implant-associated osteomyelitis and compare it to that of the prominent osteomyelitis pathogen,S. aureus. We hypothesized that S. agalactiae preferentially colonizes host vasculature and soft tissue over implant hardware and bone. To test this hypothesis we compared infection with a capsulatedstrainof S. agalactiae, COH1, to a highly virulent and osteolytic strain of methicillin‐resistant S. aureus, USA300, in a well-established murine implant‐associated osteomyelitis model35; 36.
Methods
Strains and Growth Conditions
S. aureus strain, USA300 was grown on tryptic soy agar (TSA) plates or in tryptic soy broth (TSB) at 37˚C with shaking. S. agalactiae strain COH137 was grown on TSA with defibrinated sheep blood (blood agar) or in brain heart infusion broth (BHI) at 37˚C with shaking.
Animal Surgeries
All animal studies were performed in accordance with protocols approved by the University Committee on Animal Resources at the University of Rochester Medical Center and in accordance with the Animal Welfare Act. Tibial pin infection was performed as described previously35; 36; 38; 39. Briefly, 6-week-old, female Balb/C mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed five per cage within their experimental group in two-way housing on a 12-h light/dark cycle. 6-week-old mice are used because of the softness of their bones, which are not prone to fracture during implant insertion, and because they mimic infection of children, which is common in humans40. Following 1-week acclimatization, mice were anesthetized prior to surgery with xylazine (12 mg/kg) and ketamine (130 mg/kg) administered intraperitoneally and given preoperative slow-release buprenorphine (0.5–1.0 mg/kg) administered subcutaneously by URMC veterinary staff. Pins were cut at 4 mm in length from stainless-steel wire with a cross-section of 0.2 mm x 0.5 mm (MicroDyne Technologies, Plainville, CT) and bent into an L-shaped implant as previously described39. The stainless-steel pins were first sterilized, then inoculated by being submerged within an overnight culture of either USA300 or COH1 for 20 minutes at room temperature and then left to air dry for 5 minutes, resulting in an inoculation of approximately 5.0 × 105 CFU/mL. Dry, sterile pins were used for the control, uninfected experimental group. While some components of the bacterial culture media may dry onto infected pins, this is relatively innocuous compared to the bacteria present on pins. Mice were randomly assigned to an infected or uninfected experimental group. The mouse’s left leg was shaved and sterilized with two washes of iodine and one of 70% ethanol. A 5 mm incision was created on the medial surface of thetibiae, and 30- and 26-gauge needles were used to drill holes through the tibiaebefore carefully inserting the infected pin through the holes. Animals were weighed on day 0 and every other day until sacrifice. Incision sites were healed at Day 3 post-infection and were not damaged by housing with other mice. X-ray imaging was performed at day 0 and 14 post-infection using a Faxitron (Tucson, AZ). Radiographs were used to characterize septic implant loosening where the number of pins completely dislodged from the bone was quantified relative to the number of pins stably fixed in the bone within each experimental group, as previously described39. Following sacrifice on day 14, the infected tibiae, trans-tibial implants and soft tissue were harvested and processed for CFU quantification or μCT imaging, followed by histology and transmission electron microscopy (TEM).15 animals were infected with S. aureus and S. agalactiae, where 10 animals from each group were assigned to CFU processing and 5 were assigned to μCT, histology and TEM. 5 animals were implanted with sterile pins and processed for histology. Mice were removed from any of the experimental groups if they died of anesthesia following surgery, therefore the resultant numbers of each group are the following, S. aureus infection (n=15), S. agalactiae infection (n=13) and control sterile pin (n=5). Sample size was determined based on previous studies36.
Colony Forming Unit Quantification
CFU quantification was performed as previously described39. Infected tibiae, transtibial implants and adjacent soft tissue were harvested, placed in sterile PBS and tissues were homogenized using an IKA T-10 handheld homogenizer (Wilmington, NC). Homogenizer maximum speed is 30,000 rpm and maximum power is 50 Hz. Bone and soft tissue were homogenized for approximately 1 minute and transtibial implants were sonicated in PBS for 2 min at 35 kHz (VWR Intl.; Radnor PA) to dislodge adhered bacteria and then vortexed. Tissue homogenate fluid and implant sonicate fluid were serially diluted in PBS and plated on Trypic Soy Agar (TSA) or Blood Agar. Plates were incubated overnight, and resulting colonies were counted. Bone and soft tissue CFUs were normalized to tissue weight.Bacterial colonies are verified to be S. aureus or S. agalactiae by visually inspecting colony morphology. Mouse flora is not cultured by these methods. Internal organs, including spleen, liver, and kidneys, and blood were harvested and placed in sterile microfuge tubes. Organs were homogenized in 1 mL PBS by forcing the tissue through 70 μm filters into 6-well plates. 100 μl of organ homogenate were spread on Blood Agar. 10 μl of whole blood was diluted in 90 μl of PBS and spread on Blood Agar. Plates were incubated overnight, and resulting colonies were counted. Infected tibiae, soft tissue and internal organs were weighed prior to homogenizing and CFUs were ultimately normalized to tissue mass. CFU quantification was not performed on animals with sterile implants, as previous studies have verified that these animals are not infected35.
Micro-computed tomography and Analysis
Micro-computed tomography (μCT) imaging and analysis was performed as previously described39. Infected tibiae were imaged ex vivo by μCT in a VivaCT 40 (Scanco Medical; Bassersdorf, Switzerland) with a 10.5 μm isotropic voxel size, using an integration time of 300 ms, energy of 55 kV, and intensity of 145 μA (n = 5). Resulting DICOM files were analyzed using Amira software (FEI Visualization Sciences Group; Burlington, MA), where bone is isolated by thresholding and hole volumes quantified by manual segmentation of the void area and interpolation through the depth of the tibial cortex. μCT operators were blinded to the experimental group of each animal.
Histology
Bone samples(n = 5) with pins intact were fixed in 10% phosphate-buffered formalin for 72 hrs before removing the pins and 39decalcifying bonein 10% EDTA, and processed for histological sectioning, as previously described. Slides were deparaffinized and stained with H&E, Brown-Brenn or for TRAP activity. H&E staining was used to visualize gross morphology of infected tibiae and inflammatory reactions (n = 5). Brown-Brenn modified Gram stain was used to visualize Gram-positive S. aureus or S. agalactiae, which stained dark purple in a yellow tissue background (n = 5).TRAP staining was used to visualize TRAP+ osteoclasts, which stained red/purple with a blue/green tissue background (n = 5). Slides were digitized using a VS120 Virtual Slide Microscope (Olympus, Waltham, MA).
TRAP Quantification
We developed an Analysis Protocol Package (APP) in Visiopharm (v.2019.07; Hoersholm, Denmark) to detect TRAP+ areas in standard square regions of the tibiae (implant site, diaphysis and metaphysis). The APP utilizes colorimetric histomorphometry to detect TRAP staining (red/purple), fast-green counterstain (blue/green), and background (white) in order to accurately segment TRAP+ area for quantification.Visiopharm operators were blinded to the experimental group of each animal.
TEM “Pop-off” Technique and Imaging
Transmission electron microscopy (TEM) “Pop-off” was performed as previously described39; 41. Briefly, Brown-Brenn stained histologic sections were used to identify regions of interest for TEM imaging, where regions of bone tissue adjacent to the pin hole and colonized by Gram positive bacteria were selected. Next, adjacent non-stained sections were processed for TEM analysis. Slides were deparaffinized and rehydrated, then fixed in 2.5% glutaraldehyde and post-fixed in buffered 1% OsO4. Slides were washed, dehydrated in a graded series ethanol from 50% to 100%, infiltrated with a 1:1 mixture of 100% ethanol and Spurr’s resin and overnight in 100% resin. Regions of interest were covered with Spurr’s resin-filled BEEM capsules and polymerized overnight. Capsules were “popped off” slides by dipping 3–4 times in liquid nitrogen. Thin sections were cut at ~70 nm and placed onto grids for imaging using a Hitachi 7650 TEM (Tokyo, Japan) with a Gatan Erlangshen 11MP CCD camera (Warrendale, PA). The TEM operator was blinded to the experimental group of each animal.
Statistical Analyses
Two-way analysis of variance (ANOVA) with Sidak’s post-hoc test for multiple comparisons was used to compare multiple parameters such as differences in animal body weight over time andCFU quantification. For statistical analysis, CFU data were log-transformed to achieve normal distributions.Fisher’s exact test was used for comparison of nominal data,such as evaluation of implant stability. Unpaired t-test was used when two groups were compared such as hole volume quantification and in vitro pin CFUs. One-way ANOVA, with Tukey’s post-hoc for multiple comparisons was used for grouped data such as TRAP area quantification. All statistics were analyzed using GraphPad Prism (San Diego, CA) and all quantitative data is presented as mean ± standard deviation.
Results
We first determined if the S. agalactiae strain, COH1, andS. aureus strain, USA300, adhered in vitro to pins used to infect murine tibiae. Pins were inoculated withS. agalactiae or S. aureus as described in the methods and sonicated to recover adherent bacteria.Sonicate fluid was serially diluted for CFU quantification. Both S. agalactiae and S. aureus showed ~105 CFU/pin, confirming equivalent inocula (Fig. 1A). SEM of pins inoculated with S. agalactiae demonstrateddirect attachment of ovoid bacteria to the implant and indirect attachment via debrispossibly from the culture medium (Fig. 1B-D), which was similar to previous SEM studies of S. aureus attachment42; 43.
Figure 1.
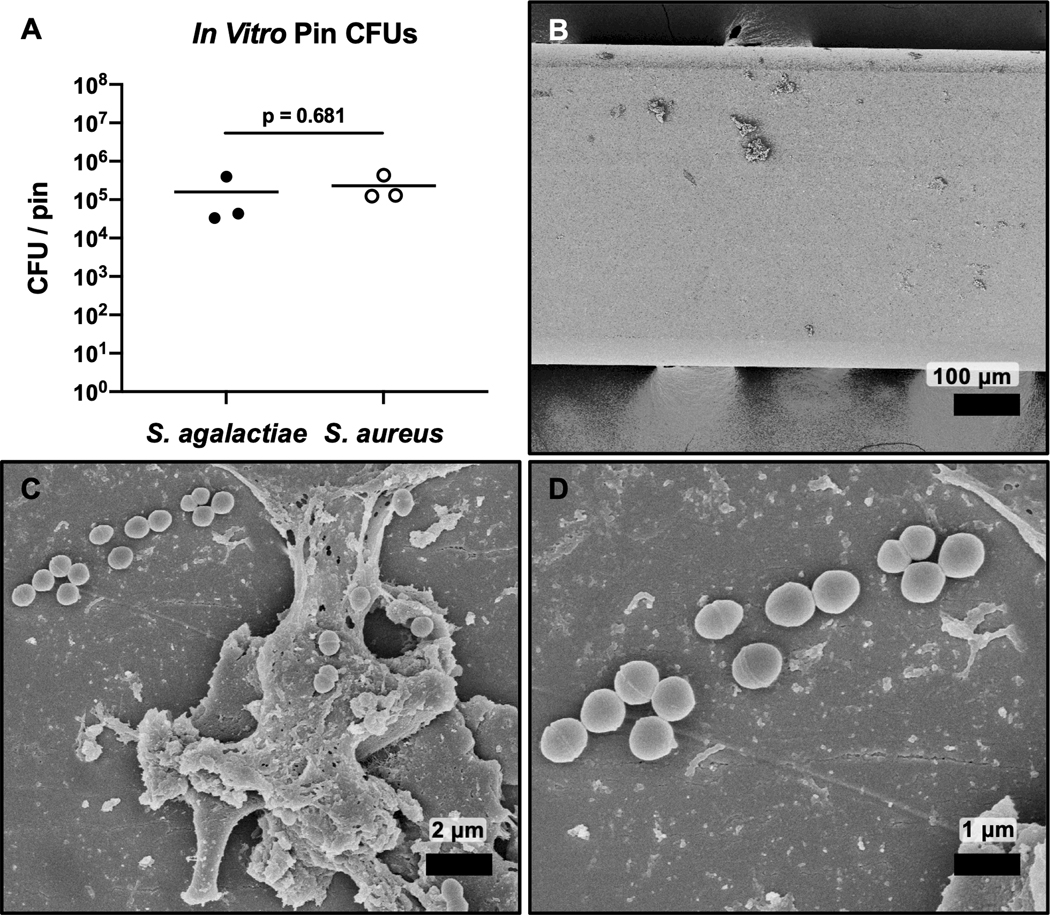
CFU quantification and SEM validation of the S. agalactiae pin inoculum. Stainless steel pins were immersed in overnight cultures of S. agalactiae or S. aureus for 20 min, air dried for 5 min. Pin sonication and serial dilution of the sonicate for CFU quantification confirmed equivocal bacterial attachment for S. agalactiae and S. aureus (~105 CFU) (A, n = 3). SEM of S. agalactiae contaminated shown at x100 (B), x5,000 (C), and x10,000 (D) confirms S. agalactiae attachment. Of note is that the bacteria adhere both directly onto the implant surface (D), and indirectly via adherence to culture debris bound to the implant (C).
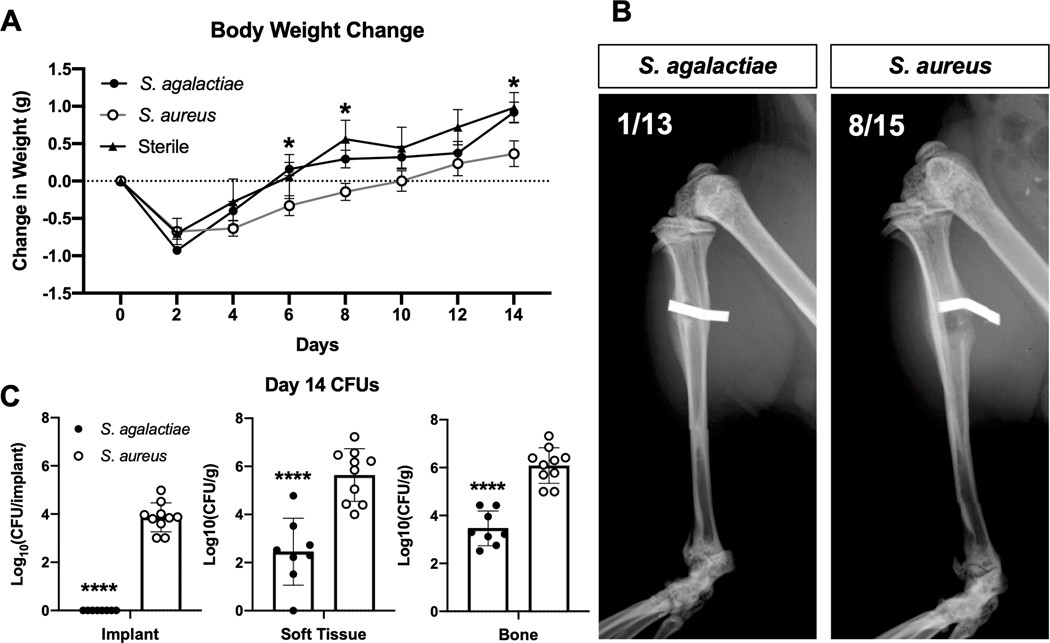
After implantation of pinscontaminated with S. agalactiae or S. aureus as well as control sterile pins into mice in vivo, change in animal weight was tracked longitudinally for 14 days (Fig. 2A).Post-op weight increase ofS. agalactiae-infected mice was similar to the sterile pin control group and showed a significantly faster weight increaseat day 6, 8 and 14-post infectionthan theS. aureus infected animals.
Figure 2.
Distinct clinical features of S. agalactiae vs. S. aureus implant-associated bone infection. Mice were implanted with a transtibial pin contaminated with 105 CFU of S. agalactiae or S. aureus, or a sterile implant, and their change in total body weight over the 14-day infection period is presented as the mean +/− SD (A, n = 13, 15, 5 for S. agalactiae, S. aureus and sterile groups, respectively). Significance evaluated by two-way ANOVA with Sidak’s post-hoc test for multiple comparisons, S. agalactiae vs S. aureus significance (p < 0.05) indicated by * at the indicated time point. Radiographs of infected tibiae were obtained after sacrifice on day 14, and representative radiographs are shown, with the fraction of septic implants that were dislodged from the insertion site (B). Representative images show radiopaque implants stably fixed through the tibia in the S. agalactiae group and dislodged from the tibia in the S. aureus group. Incidence of septic implant loosening (defined as implant dislodgment) was significantly different between S. agalactiae and S. aureus infected tibiae (p = 0.0157 by Fisher’s exact test). The infected implants, soft tissue and tibiae were harvested to determine bacterial load by CFU quantification, and the data for each mouse is presented with mean +/− SD for the group (C) (n = 8, 10 for S. agalactiae and S. aureus groups respectively, ****p < 0.0001 by two-way ANOVA with Sidak’s post-hoc for multiple comparisons). Of note is that zero CFU’s were recovered from any S. agalactiae contaminated pins.
We obtained radiographsof the infected and control tibiae at day 14 post-infection to assess gross osteolysis and septic loosening of the pin implants. All sterile pins remained stably implanted at this time point (data not shown), similar to all previous studies using this animal model35; 36.However, significantly more S. aureus-infected pins were dislodged from the bone (53%) compared to S. agalactiae-infected pins (8%) at day 14 post-infection (Fig. 2B).
CFUs were quantified from the infected implants, surrounding soft tissues, and tibiaeat day 14 post-infection to determine the bacterial load at the infection site. S. agalactiae successfully infected animals at day 14 post-infection, but with significantly lower bacterial burdens in soft tissue and in bone compared to S. aureus(Fig. 2C). Remarkably, all implants initially infected with S. agalactiae were culture negative at day 14 post-infection, indicating that the implant hardware is not a bacterial reservoir for S. agalactiae infection. In addition, bacterial load within the liver, kidney, spleen and blood were quantifiedto evaluate bacterial dissemination from the initial infection site. NeitherS. agalactiae norS. aureus infection groups showed appreciable dissemination to internal organs (Supplemental Fig. 1).
Following harvest on day 14, infected tibiae were processed for μCT scanning and subsequent analysis of peri-implant osteolysis. Tibiae were scanned ex vivo,and DICOMs were 3D reconstructed in Amira software for quantification of medial and lateral implant hole volumes. Qualitatively, both infection groups showed appreciable peri-implant osteolysis. Bone loss on the medial side exceeded that of the lateral side for both groups, likely due to the directionality of pin implantation and resulting differences in inoculation(Fig. 3A). Additionally, reactive new woven bone was observedaround the implant site in both S. agalactiae-and S. aureus-infected groups and not in sterile implant controls(35; 36, and data not shown).Medial and lateral hole volume quantification revealed that S. agalactiae infection results in significantly less pathologic bone destruction than S. aureus infection (Fig. 3B, C).
Figure 3.
S. agalactiae is less osteolytic than S. aureus during implant-associated bone infection. (A) The infected tibiae described in Figure 2 were imaged by μCT, and representative 3D renderings are shown to illustrate the relative levels of peri-implant osteolysis between S. agalactiae and S. aureus in the medial (red) and lateral (green) cortical bone. The medial (B) and lateral (C) pin hole volumes were quantified using Amira software, and the data for each mouse with mean +/− SD for the group are presented (n = 5, *p < 0.05, **p < 0.01 by unpaired t-test).
Histological analysis of infected and sterile tibiae was performed to characterize the pathologic effects ofboth pathogens.H&E staining revealed that sterile implant controlsstimulate a thin collar of fibro-inflammatory tissue, and a thicker layer of peri-implant reactive new woven bone with occasional neutrophils (Fig. 4A-C).S. agalactiae-infected pins caused similar, but thicker, layers of fibro-inflammatory tissue and new woven bone,with more neutrophils (Fig. 4D-F).In contrast, S. aureus induced large abscesses and abundant neutrophils in the fibro-inflammatory tissue, with minimal reactive woven bone formation (Fig. 4G-I). Consistent with the observed increased bacterial burden and acute inflammation in S. aureus-infected tibiae, we observed an apparent increase in peri-implant osteonecrosis in S. aureus-infected tibiae compared to S. agalactiae and sterile controls (Supplemental Fig. 2).
Figure 4.
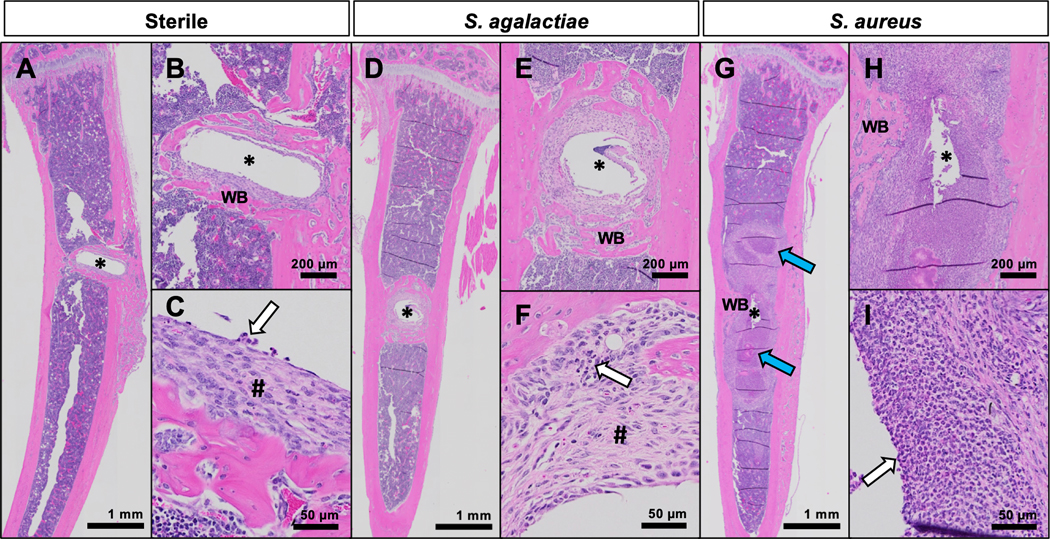
Histologic evidence of S. agalactiae and S. aureus pathology within infected tibiae in mice. Tibiae from mice implanted with sterile (A-C), S. agalactiae-infected (D-F), or S. aureus-infected (G-I) pins were harvested on day 14 for histologic analysis with H&E staining. Representative images of 5 biological replicates shown at x1 (A, D, G), x4 (B, E, H) and x20 (C, F, I) illustrate peri-implant reactions. Around the sterile pin site (*), there is a thin collar of fibro-inflammatory tissue (#), and a thicker layer of reactive new woven bone (WB) with occasional neutrophils (white arrow) (A-C). S. agalactiae-infection results in thicker layers of fibro-inflammatory tissue and new woven bone around the pin site, with more neutrophils present (white arrow) (D-F). In contrast, the S. aureus-infected tibiae show abscess formation (blue arrows) and abundant neutrophils (white arrow) in the fibro-inflammatory tissue, with minimal reactive woven bone (GI).
To investigate differences in bone resorption among sterile,S. agalactiae-and S. aureus-infected tibiae, we stainedsections for TRAP+ osteoclasts in histology sections (Fig. 5). Quantification of the total tibial TRAP+area revealed no differences amongany of the groups (Supplemental Fig. 3). TRAP+ area was quantified within three standardized regions of interest (ROI, Fig. 5A, B): 1) the implant site, 2) the diaphysis and 3) the metaphysis. TRAP stainingshowedstatistically similar osteoclast areas at the implant site in sterile and S. agalactiae groups, whereasS. aureus-infected tibiae had significantly lower osteoclast area at the implant site, likely due to the absence of remodeling woven bone(Fig. 5C). Conversely, increased TRAP area was noted in the diaphysis of S. aureus-infected tibiaecompared to sterile and S. agalactiae-infected tibiae(Fig. 5D). Moreover, visual inspection showed that multinucleated TRAP+osteoclasts were much larger in S. aureus-infected tibiaethan in the other groups, consistent with more active osteolysis. Finally, equivalent osteoclast presence within the periosteum of the metaphysis was observed in all groups, likely as a result of normal bone growth in young mice (Fig. 5E).
Figure 5.
S. agalactiae infection does not induce large osteoclasts in the tibia diaphysis like S. aureus infection. Parallel histologic sections from sterile and infected tibiae were stained for TRAP (red/purple) with fast green counterstain, and three regions of interest (ROI) are schematically illustrated, where 1 = Implant Site, 2 = Diaphysis, 3 = Metaphysis (A). Representative images of the three ROIs in the three experimental groups are shown at x4 (implant site) and x10 (diaphysis and metaphysis) (B). TRAP-stained area was quantified as described in Supplemental Figure 3 and presented for each tibia with the mean +/− SD (C-E) (n = 5, *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA with Tukey’s post-hoc for multiple comparisons). Note TRAP+ area at the implant site is proportional to the amount of new woven bone, and that only the diaphysis of S. aureus infected tibiae contains very large TRAP+ osteoclasts.
Histologic sections were also stained for Gram-positive organisms to elucidate reservoirs of bacteria and biofilm within the infected tibiae.Brown-Brenn staining of sterile tibiae showed no evidence of bacterial colonization (35; 36, and data not shown). S. agalactiae-infected tibiae showed Gram-positivebiofilms colonizing necrotic bone fragments, but no Gram-positive bacteria found in the bone marrow cavity (Fig. 6A-D). In contrast, S. aureus-infected bone contained Gram-positive bacteria in bothstaphylococcal abscess communities (SACs) and colonizing necrotic bone fragments (Fig. 6E-H).
Figure 6.

Histologic evidence of S. agalactiae colonized necrotic bone fragments without marrow abscesses in infected tibiae. Histologic sections were Brown-Brenn stained to identify Gram-positive bacteria (white arrows), and representative images of 5 biological replicates are shown at: x1 to illustrate the whole tibia (A,E), x10 to show the bone marrow cavity (B, F), x4 to show necrotic bone fragments (C, G), and magnified bone fragments at x20 (D, H). Bone fragments are determined to be necrotic by the lack of nuclei within osteocyte lacunae and lack of blood supply. Note that Gram-positive bacteria are readily identified on necrotic bone fragments in S. agalactiae (D, boxed region in C), and S. aureus (H, boxed region in G) infected tibiae. No lesions similar to the staphylococcal abscess communities in S. aureus infected tibiae (F, boxed region in E) were observed in bone marrow of S. agalactiae infected tibiae (B, boxed region in A).
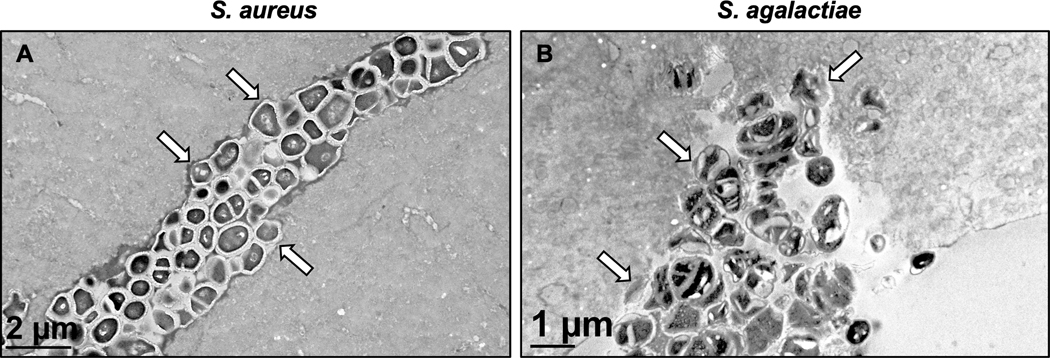
Since S. aureus has been shown to invade and colonize the OLCN of cortical bone17–19, we performed TEM “pop-off” analyses on histologic slides adjacent to Brown-Brenn-stained sections that contained Gram-positive bacteria. Consistent with prior reports, we readily found cocci encased in glycocalyx at the bone soft-tissue interface (Fig. 7A-C),and deformed-submicron bacteria within the OLCN (Fig. 7D-F).S. aureus infection of blood vessels within cortical bone was not detected. In contrast, TEM assessment of S. agalactiae-infected bone revealed that the infection persists as free planktonic bacteria within blood vessels of the cortical bone (Fig. 7G-I). Examination of additional TEM sections cut longitudinally confirmed the vasculotropic behavior ofS. agalactiae in infected tibiae (Fig. 7 J-L).
Figure 7.
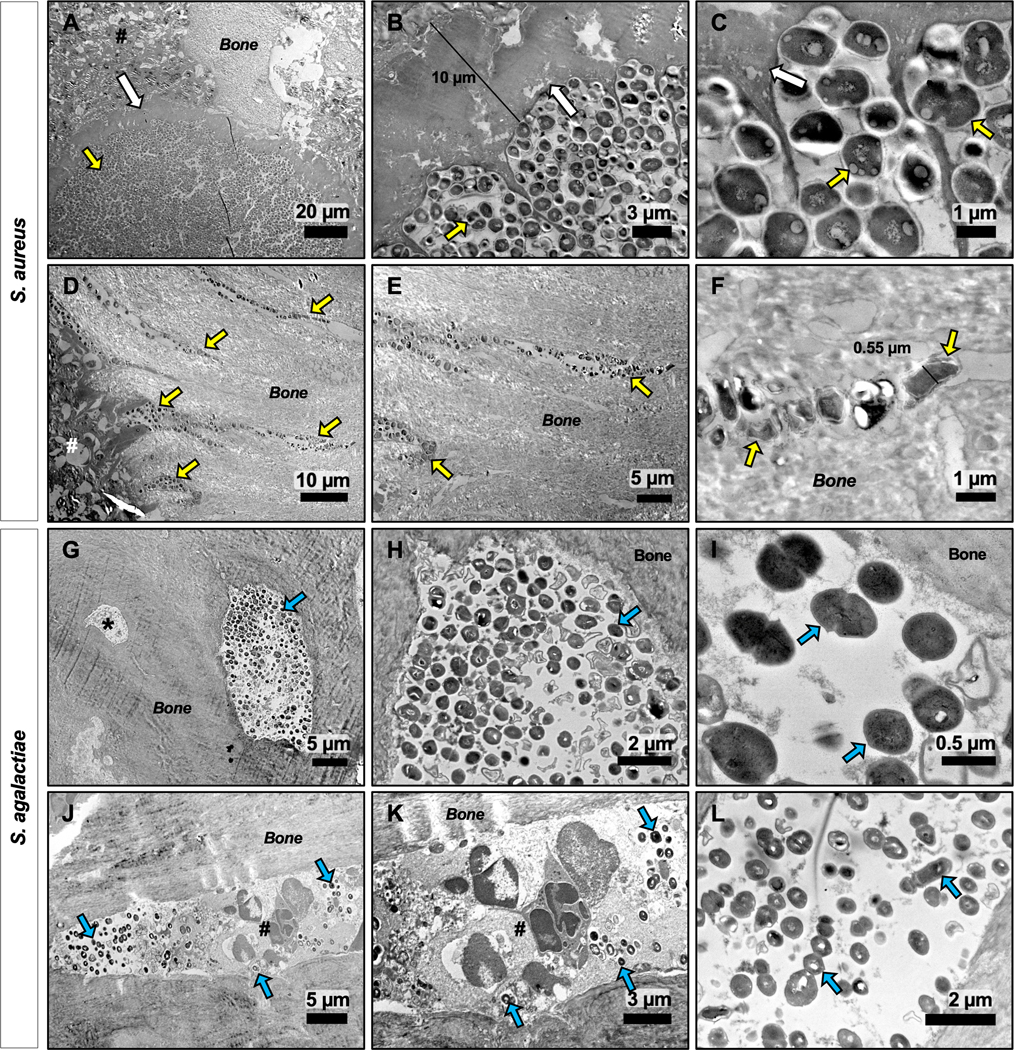
TEM reveals distinct vasculotropic vs. osteotropic features of S. agalactiae vs. S. aureus during implant-associated bone infection. Parallel histologic sections from the S. aureus (A-F) and S. agalactiae (GL) infected tibiae described in Figure 6 were processed for TEM, and representative micrographs of 2–3 biological replicates are shown to illustrate the distinct tropisms of the two organisms. S. aureus and S. agalactiae bacterial cells are observed as dark colored cocci within the light grey bone mineral matrix and are identified with yellow and blue arrows, respectively. S. aureus primarily presented as biofilm bacteria within glycocalyx on the bone surface (arrows in A-C), and submicron sized bacteria dividing within the OLCN embedded within bone tissue (D-F), thereby evading host immune cells (#). In contrast, S. agalactiae bacteria primarily presented as planktonic bacteria within blood vessels embedded within the cortical bone, shown from both cross-sectional (G-I) and longitudinal (J-L) planes of sectioning. This infected region is defined as a blood vessel due to its large size as compared to the adjacent osteocyte (* in G), as well as by the presence of multi-nucleated immune cells (#) present in this cavity (J, K). Biofilm glycocalyx was not observed in S. agalactiae infected tibiae.
Finally, we performed ultrastructural analysis of infected bone by TEMto assess direct bacterial catabolism of the bone mineral and matrix, as evidenced by erosion of the bone surface in contact with bacteria (Fig. 8). This form of bone erosion is oftentimes referred to as “scalloping” due to the pattern of bone loss in direct contact with a lesion44. Consistent with prior reports demonstrating that S. aureus degrades cortical bone directly through the release of lactic acid and collagen-degrading enzymes17–19, TEM identified widened canaliculi with a characteristic scalloped pattern of bone loss surrounding the bacterial cells (Fig. 8A). Although rare, we also observed a similar phenotype in S. agalactiae infected tibiae(Fig. 8B). To our knowledge, this is the first evidence that GBS can directly digest cortical bone during chronic osteomyelitis.
Figure 8.
Evidence of S. agalactiae and S. aureus mediated bone mineral matrix degradation and scalloping. TEM “pop-off” specimens from S. aureus (A) and S. agalactiae (B) infected tibiae were examined at x15,000 magnification to evaluate pathogen mediated bone destruction. Note the regions of bone erosion surrounding individual bacterial cells, resulting in a “scalloped” appearance (white arrows) in both infection groups.
Discussion
Numerous studies of S. aureus infection in models of osteomyelitis have elucidated important characteristics of its virulentpersistence within bone. These key findings have improved our understanding of the host’s response to infection45, as well as the pathogen’s mechanisms of survival within the host46, ultimately informing treatment of implant-associated osteomyelitis in the clinic for better outcomes. In this work, we aimed to expand our understanding of implant-associated osteomyelitis in murine models by investigating infection by the second most prevalent genus, Streptococcus. In so doing, we have established the first quantitative small animal model of chronicS. agalactiae implant-associated infection, which can be utilized to guide novel clinical approaches to Streptococcus implant-associated bone infection.
Previous work established that this model is representative of a chronic-phase infection, in part because of S. aureus biofilm formation and bacterial cell emigration as early as day 7 post-infection36. Here, we have shown for the first time that animals can be infected with S. agalactiae andremain infected for 14 days. In this model of infection, longitudinal weight assessment showed that animals infected with S. agalactiae appear to recover from surgery more rapidly than animals infected with S. aureus. Additionally,S. agalactiae-infected animals showeda significantincrease in weight gain at day 14 post-infection compared to S. aureus-infection. These results suggest that animals infected with S. agalactiaehad less severe diseasethan those infected with S. aureus from a systemic perspective.
Numerous studies have investigated and determined themajor reservoirs of S. aureuspersistence in chronic osteomyelitis to bebiofilms formed on implant hardware and necrotic bone tissue13; 14,abscesses formed in soft tissue and within the bone marrow cavity15; 16, andinvasion of theOLCN of cortical bone18; 47; 48. Unlike S. aureus,PJIs caused by S. agalactiae have been known to infect soft tissue and spread rapidly within the fascia and blood33. Therefore, we aimed to characterize the mode of S. agalactiae infection and persistencein our experimental model of implant-associated osteomyelitis.
For S. aureus, it isunderstood that biofilm formation is a key survival mechanism in PJIs, as the biofilm provides protection from immune cell attack as well as resistance to antibiotic therapies14; 36; 49; 50. In this model of osteomyelitis, bothS. aureus and the less virulent Staphylococcus epidermidis51colonize the implant with approximately 104 CFUs at day 14 post-infection. Importantly, this study revealed that S. agalactiae does not persistently colonize the implant hardware, despite their known ability to form biofilms52. Although implants were initially contaminated with S. agalactiae at day 0 as the site of inoculation, all implant samples were culture-negative by day 14. This result suggests that GBSdoes not adhere toor form robust biofilms upon the implant surface in this setting. Clinically, this is a very important finding as the optimal treatment for GBSimplant-associated infection remains unclear.Whilecurrent guidelines recommend antibiotic treatment with penicillin G and amoxicillin53, many patients are treated with rifampicincombinations to improveeradication of bacterial biofilms on implant surfaces25; 54 despite contradicting evidence that it does not improveoutcomes55. Further, it is unclear whether patients have equivalent outcomes with a less invasive debridement and implant retention procedure versus a more invasive removal of the existing implant for a staged procedure54. As stated previously, some groups argue that S. agalactiaePJI cases have better outcomes with DAIR treatment, where the implant hardware is retained, compared to S. aureus PJI cases24; 29; 33.These improved outcomes are suggested to be due to specific differences in the infecting bacteria’s ability to adhere to and colonize the implant surface33. To date, no studies have investigated the mechanisms associated with this clinical observation. This is the first study to demonstrate that S. agalactiae does not colonize implant hardware in amodel of implant-associated osteomyelitis, suggesting that it may not form biofilms on the implant surface as extensively as other organisms in this setting. Consistent with the lack of implant colonization, we also observed improved implant stability associated with S. agalactiae infection as evidenced by decreased septic loosening, decreased peri-implant osteolysis and increased reactive bone formation compared to S. aureus infection. Together, these key findings suggest an explanation for the apparent clinical success of DAIR therapy when S. agalactiae is the infecting organism.
Interestingly, histologic analyses of infected tibiae revealed that no abscesses were observed in animals infected withS. agalactiae, while abscesses were readily observed inS. aureusinfected animals. Further, S. agalactiae does not establish a durable biofilm on the implant in this infection model, which is known to be the primary location for bacterial biofilm formation36.Together, these results suggest that S. agalactiae does notpersist in a sessile state, asS. aureusdoes, and may preferentially colonize the host in its planktonic state. However, histologic analysis did reveal both pathogenssuccessfully colonizenecrotic fragments of bone, likely in the form of a biofilm, located near the implant site or within the bone marrow cavity.
Previous studies investigatingthe lesser pathogenic organism,S. epidermidis,infection in osteomyelitis showed that its infection phenotype differs from both S. agalactiae and S. aureus. In the same murine model of implant associated osteomyelitis, S. epidermidis infection does not form bone marrow abscesses and does not colonize necrotic bone fragments, but successfully forms robust biofilms on the implant surface51. Taken together, it is clear that infection severity and disease pathology can be dependent on the infecting organism.
Perhaps the most unique finding of this study is that S. agalactiae preferentially colonizes the vasculature embedded within bone, as evidenced by TEM imaging. Of note, infection of blood vessels is an exceedingly rare observation in TEM images of S. aureus-infected bone. However, S. agalactiae-infected animals did not show significantly different weight loss relative to the sterile implant and S. agalactiae infection did not disseminate into blood and internal organs, suggesting that vascular infection by S. agalactiae may be contained to infected regions of bone by the host immune response. Taken together, these results demonstrate that S. agalactiae is a vasculotropic pathogen,relative tothe osteotropic behavior ofS. aureus, during the establishment of implant-associated osteomyelitis.
Finally, this is the first work to describe directS. agalactiae-mediated bone degradation, as evidenced by scalloping of bone. Previously described in S. aureus,but not inS. epidermidisinfection51, the degradation of bone may release a nutrient source to the pathogen as well as expose extracellular matrix proteins for bacterial cell attachment and colonization56.
In summary, this study describes important characteristics of S. agalactiae infection in implant-associated osteomyelitis. We have found that S. agalactiae persists in bone without sessile colonization of the implant or formation of abscesses. Rather, this pathogendisplays preferential vasculotropic colonization of bone. Future work should continue to elucidate features of S. agalactiae orthopaedic infections, first by confirming these findings with additional clinically relevant bacterial strains. Additionally, future studies should investigate the infection dynamics of mono-infection and polymicrobial bone infections with these two pathogens at additional timepoints. Ultimately, the continued development and characterization of infection types in this model should elucidate novel strategies to treat orthopaedic infections.
Supplementary Material
Acknowledgement
This work was supported by a grant from the National Institute of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant number R21AR074571 awarded to Irvin Oh, M.D. This work was also supported by grants from AOTrauma, Clinical Priority Program (Davos, Switzerland), NIAMS P50 AR072000 and NIAMS P30 AR069655 awarded to Edward M Schwarz, PhD and AR43510–23 and 1S10RR027340 to Brendan F. Boyce, MB. Chb.We would like to thank the members of the Histology, Biochemistry & Molecular Imaging Core and the Biomechanics, Biomaterials, and Multimodal Tissue Imaging Core in the Center for Musculoskeletal Research.Electron microscopy was conducted at the URMC Electron Microscope Shared Resource.The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication
Conflict of Interest: Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article. Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research. This work was performed at University of Rochester Medical Center.
References:
- 1.Moriarty TF, Kuehl R, Coenye T, et al. 2016. Orthopaedic device-related infection: current and future interventions for improved prevention and treatment. EFORT Open Reviews 1:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. New England Journal of Medicine 351:1645–1654. [DOI] [PubMed] [Google Scholar]
- 3.Stulberg JJ, Delaney CP, Neuhauser DV, et al. 2010. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA 303:2479–2485. [DOI] [PubMed] [Google Scholar]
- 4.Cram P, Lu X, Kates SL, et al. 2012. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. Jama 308:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz EM, Parvizi J, Gehrke T, et al. 2019. 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. Journal of Orthopaedic Research 37:997–1006. [DOI] [PubMed] [Google Scholar]
- 6.Rosas S, Ong AC, Buller LT, et al. 2017. Season of the year influences infection rates following total hip arthroplasty. World journal of orthopedics 8:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgenstern M, Erichsen C, Militz M, et al. 2020. The AO trauma CPP bone infection registry: Epidemiology and outcomes of Staphylococcus aureus bone infection. J Orthop Res In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz EM, McLaren AC, Sculco TP, et al. 2020. Adjuvant Antibiotic-Loaded Bone Cement: Concerns with Current Use and Research to Make it Work. J Orthop Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arciola CR, An Y, Campoccia D, et al. 2005. Etiology of implant orthopedic infections: a survey on 1027 clinical isolates. The International journal of artificial organs 28:1091–1100. [DOI] [PubMed] [Google Scholar]
- 10.Pulido L, Ghanem E, Joshi A, et al. 2008. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clinical orthopaedics and related research 466:1710–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. [DOI] [PubMed] [Google Scholar]
- 12.Dastgheyb SS, Hammoud S, Ketonis C, et al. 2015. Staphylococcal persistence due to biofilm formation in synovial fluid containing prophylactic cefazolin. Antimicrob Agents Chemother 59:2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nature reviews microbiology 2:95–108. [DOI] [PubMed] [Google Scholar]
- 14.Ricciardi BF, Muthukrishnan G, Masters E, et al. 2018. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Current reviews in musculoskeletal medicine 11:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng AG, DeDent AC, Schneewind O, et al. 2011. A play in four acts: Staphylococcus aureus abscess formation. Trends in microbiology 19:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi SD, Malachowa N, DeLeo FR. 2015. Pathogenesis of Staphylococcus aureus abscesses. The American journal of pathology 185:1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Mesy Bentley KL, Trombetta R, Nishitani K, et al. 2017. Evidence of Staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. Journal of Bone and Mineral Research 32:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Mesy Bentley KL, MacDonald A, Schwarz EM, et al. 2018. Chronic Osteomyelitis with Staphylococcus aureus Deformation in Submicron Canaliculi of Osteocytes: A Case Report. Jbjs Case Connector 8:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masters EA, Salminen AT, Begolo S, et al. 2019. An in vitro platform for elucidating the molecular genetics of S. aureus invasion of the osteocyte lacuno-canalicular network during chronic osteomyelitis. Nanomedicine : nanotechnology, biology, and medicine:102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuchscherr L, Heitmann V, Hussain M, et al. 2010. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. The Journal of infectious diseases 202:1031–1040. [DOI] [PubMed] [Google Scholar]
- 21.Sendi P, Rohrbach M, Graber P, et al. 2006. Staphylococcus aureus small colony variants in prosthetic joint infection. Clinical infectious diseases 43:961–967. [DOI] [PubMed] [Google Scholar]
- 22.Bémer P, Plouzeau C, Tande D, et al. 2014. Evaluation of 16S rRNA gene PCR sensitivity and specificity for diagnosis of prosthetic joint infection: a prospective multicenter cross-sectional study. Journal of clinical microbiology 52:3583–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeller V, Lavigne M, Biau D, et al. 2009. Outcome of group B streptococcal prosthetic hip infections compared to that of other bacterial infections. Joint Bone Spine 76:491–496. [DOI] [PubMed] [Google Scholar]
- 24.Grammatopoulos G, Kendrick B, McNally M, et al. 2017. Outcome following debridement, antibiotics, and implant retention in hip periprosthetic joint infection—an 18-year experience. The Journal of arthroplasty 32:2248–2255. [DOI] [PubMed] [Google Scholar]
- 25.Mahieu R, Dubée V, Seegers V, et al. 2019. The prognosis of streptococcal prosthetic bone and joint infections depends on surgical management—A multicenter retrospective study. International Journal of Infectious Diseases 85:175–181. [DOI] [PubMed] [Google Scholar]
- 26.Pietrocola G, Arciola CR, Rindi S, et al. 2018. Streptococcus agalactiae non-pilus, cell wall-anchored proteins: involvement in colonization and pathogenesis and potential as vaccine candidates. Frontiers in immunology 9:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altrichter CL, Legout L, Assal M, et al. 2005. Severe Streptococcus agalactiae infection of the diabetic foot. Presse medicale (Paris, France: 1983) 34:491–494. [PubMed] [Google Scholar]
- 28.Skoff TH, Farley MM, Petit S, et al. 2009. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clinical Infectious Diseases 49:85–92. [DOI] [PubMed] [Google Scholar]
- 29.Sendi P, Christensson B, Uçkay I, et al. 2011. Group B streptococcus in prosthetic hip and knee joint-associated infections. Journal of Hospital Infection 79:64–69. [DOI] [PubMed] [Google Scholar]
- 30.Marculescu CE, Berbari EF, Hanssen AD, et al. 2006. Outcome of Prosthetic Joint Infections Treated with Debridement and Retention of Components. Clinical Infectious Diseases 42:471–478. [DOI] [PubMed] [Google Scholar]
- 31.Odum SM, Fehring TK, Lombardi AV, et al. 2011. Irrigation and debridement for periprosthetic infections: does the organism matter? The Journal of arthroplasty 26:114–118. [DOI] [PubMed] [Google Scholar]
- 32.Meehan AM, Osmon DR, Duffy MCT, et al. 2003. Outcome of Penicillin-Susceptible Streptococcal Prosthetic Joint Infection Treated with Debridement and Retention of the Prosthesis. Clinical Infectious Diseases 36:845–849. [DOI] [PubMed] [Google Scholar]
- 33.Betz M, Abrassart S, Vaudaux P, et al. 2015. Increased risk of joint failure in hip prostheses infected with Staphylococcus aureus treated with debridement, antibiotics and implant retention compared to Streptococcus. International orthopaedics 39:397–401. [DOI] [PubMed] [Google Scholar]
- 34.Watkins LKF, McGee L, Schrag SJ, et al. 2019. Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008–2016. JAMA internal medicine 179:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D, Gromov K, Søballe K, et al. 2008. Quantitative mouse model of implant‐associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. Journal of Orthopaedic Research 26:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, et al. 2015. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant‐associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. Journal of Orthopaedic Research 33:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin TR, Rubens CE, Wilson CB. 1988. Lung antibacterial defense mechanisms in infant and adult rats: implications for the pathogenesis of group B streptococcal infections in the neonatal lung. Journal of Infectious Diseases 157:91–100. [DOI] [PubMed] [Google Scholar]
- 38.Varrone JJ, de Mesy Bentley KL, Bello‐Irizarry SN, et al. 2014. Passive immunization with anti‐glucosaminidase monoclonal antibodies protects mice from implant‐associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. Journal of Orthopaedic Research 32:1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masters EA, de Mesy Bentley KL, Gill AL, et al. 2020. Identification of Penicillin Binding Protein 4 (PBP4) as a critical factor for Staphylococcus aureus bone invasion during osteomyelitis in mice. PLoS Pathogens 16:e1008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peltola H, Pääkkönen M. 2014. Acute osteomyelitis in children. New England Journal of Medicine 370:352–360. [DOI] [PubMed] [Google Scholar]
- 41.de Mesy Jensen KL, di Sant’Agnese PA. 1992. Large block embedding and “pop-off” technique for immunoelectron microscopy. Ultrastructural pathology 16:51–59. [DOI] [PubMed] [Google Scholar]
- 42.Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, et al. 2015. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J Orthop Res 33:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishikawa M, de Mesy Bentley KL, McEntire BJ, et al. 2017. Surface topography of silicon nitride affects antimicrobial and osseointegrative properties of tibial implants in a murine model. J Biomed Mater Res A 105:3413–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridley WE, Xiang H, Han J, et al. 2018. Endosteal scalloping: Pattern of bone erosion. Journal of medical imaging and radiation oncology 62:131–132. [DOI] [PubMed] [Google Scholar]
- 45.Putnam NE, Fulbright LE, Curry JM, et al. 2019. MyD88 and IL-1R signaling drive antibacterial immunity and osteoclast-driven bone loss during Staphylococcus aureus osteomyelitis. PLoS pathogens 15:e1007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang D, Wijenayaka AR, Solomon LB, et al. 2018. Novel Insights into Staphylococcus aureus Deep Bone Infections: the Involvement of Osteocytes. mBio 9:e00415–00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masters EA, Trombetta RP, de Mesy Bentley KL, et al. 2019. Evolving concepts in bone infection: redefining “biofilm”,“acute vs. chronic osteomyelitis”,“the immune proteome” and “local antibiotic therapy”. Bone research 7:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muthukrishnan G, Masters EA, Daiss JL, et al. 2019. Mechanisms of Immune Evasion and Bone Tissue Colonization That Make Staphylococcus aureus the Primary Pathogen in Osteomyelitis. Current osteoporosis reports 17:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saeed K, McLaren AC, Schwarz EM, et al. 2019. 2018 international consensus meeting on musculoskeletal infection: Summary from the biofilm workgroup and consensus on biofilm related musculoskeletal infections. Journal of Orthopaedic Research 37:1007–1017. [DOI] [PubMed] [Google Scholar]
- 50.Ma D, Shanks RMQ, Davis CM, et al. 2017. Viable bacteria persist on antibiotic spacers following two-stage revision for periprosthetic joint infection. Journal of Orthopaedic Research 36:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomizawa T, Ishikawa M, Bello-Irizarry SN, et al. 2020. Biofilm Producing Staphylococcus epidermidis (RP62A Strain) Inhibits Osseous Integration Without Osteolysis and Histopathology in a Murine Septic Implant Model. J Orthop Res 38:852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosini R, Margarit I. 2015. Biofilm formation by Streptococcus agalactiae: influence of environmental conditions and implicated virulence factors. Frontiers in cellular and infection microbiology 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osmon DR, Berbari EF, Berendt AR, et al. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clinical infectious diseases 56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 54.Fiaux E, Titecat M, Robineau O, et al. 2016. Outcome of patients with streptococcal prosthetic joint infections with special reference to rifampicin combinations. BMC Infect Dis 16:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akgün D, Trampuz A, Perka C, et al. 2017. High failure rates in treatment of streptococcal periprosthetic joint infection: results from a seven-year retrospective cohort study. The bone & joint journal 99:653–659. [DOI] [PubMed] [Google Scholar]
- 56.Junka A, Szymczyk P, Ziółkowski G, et al. 2017. Bad to the Bone: On in vitro and ex vivo microbial biofilm ability to directly destroy colonized bone surfaces without participation of host immunity or osteoclastogenesis. PloS one 12:e0169565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.