Abstract
We investigated the effects of low pH on the photosynthesis, chlorophyll fluorescence, and mineral contents of the leaves of ginger plants under salt stress. This experiment involved four treatments: T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity). This study showed that photosynthesis (Pn, Gs, WUE and Tr) and chlorophyll fluorescence (qP, Φ PSII, and Fv/Fm) significantly decreased under salt stress; however, all the parameters of the ginger plants under the low-pH treatment and salt stress recovered. Moreover, low pH reduced the content of Na and enhanced the contents of K, Mg, Fe and Zn in the leaves of ginger plants under salt stress. Taken together, these results suggest that low pH improves photosynthesis efficiency and nutrient acquisition and reduces the absorption of Na, which could enhance the salt tolerance of ginger.
Keywords: Ginger, Salt stress, Low pH, Photosynthesis, Chlorophyll fluorescence, Mineral contents
Introduction
Soil salinity has severe effects on plant growth and development and plant productivity in arid and semiarid regions worldwide (Kochian et al., 2015; Krasensky & Jonak, 2012; Ryu & Cho, 2015). The increase in soil salinity and acidification is due to poor irrigation practices, improper fertilizer application, industrial pollution, and seawater intrusion caused by global warming (Gaudio et al., 2015; Ding et al., 2020). In particular, growers are advised to apply large amounts of chemical fertilizers for high yields during long-term vegetable production processes. This leads to a nutrient imbalance and excess salt accumulation in the soil. Salt causes several adverse effects on plant growth and development, including decreased leaf size, yellowing of the leaves, short internodes, short plant height, early flowering and decreased yields (Acosta et al., 2011; Thouvenot, Haury & Thiébaut, 2012; Jan et al., 2017; Ahmad et al., 2018)
Photosynthesis is an important biological process for maintaining plant life and plays a very important role in the evolution of ecosystems on Earth. Photosynthesis provides the energy and carbon required for the biosynthesis of organic compounds necessary for the growth and biomass production of plants. Increasing photosynthesis efficiency is critical to increasing crop yields to meet human demand for food (Long, Marshall-Colon & Zhu, 2015; Zhu, Long & Ort, 2010). Many researchers have studied the effects of salt stress on plant photosynthesis. There is considerable evidence for significant changes in the chlorophyll content (Chl) (Kalaji et al., 2016), net photosynthesis rate (Pn) (Jiang et al., 2017), stomatal conductance (Gs) (Janda et al., 2016), transpiration rate (Tr), maximum photochemical efficiency (Fv/Fm) (Singh, Singh & Prasad, 2016), amount of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisco) and photochemical quenching (qP) (Moles et al., 2016). Chloroplast development and chlorophyll metabolism are important biological activities of photosynthesis in green plants. Studies have revealed chlorophyll synthesis-related enzymes and key regulators involved in chloroplast development (Bollivar, 2006; Cortleven & Schmulling, 2015). Tang et al. (2018) showed that NaCl stress significantly decreased the contents of Chl a, Chl b, and total chlorophyll in cucumber leaves. Ribose-1,5-bisphosphate ribulose carboxylation/oxygenase is a key enzyme that is involved in plant photosynthesis and controls both CO2 fixation and carbon. Rubisco is the key enzyme in the Calvin cycle, converting free CO2 in the atmosphere into energy-storage molecules, such as sucrose, and it plays a direct role in the photosynthesis rate.
Ginger (Zingiber officinale Rose), a perennial plant species of the family Zingiberaceae, is native to tropical rainforest regions. Ginger has been cultivated and widely used for more than 2000 years in China as a spice and as an important ingredient in traditional Chinese medicine. Because the bioactive constituents in ginger are valuable and have been accepted gradually by people (Ali et al., 2008; Li et al., 2015a), ginger’s demand has increased annually worldwide. According to statistics from the FAO (Food and Agriculture Organization of the United Nations), global ginger production was 813 340 tons in 2010 and 1 218 710 tons in 2016 but was 426 032 tons and 938 000 tons in mainland China, respectively.
Many studies on the photosynthesis and chlorophyll content of ginger have focused mainly on antibiotics and drought (Li et al., 2013; Li et al., 2015b; Liu et al., 2018). Salt stress was shown to decrease the growth and biomass yield (leaf fresh weight and root fresh weight) of ginger seedlings in a preliminary study. Moreover, low pH significantly alleviated this inhibition under salt stress, perhaps by lowering the Na content, alleviating osmotic stress, and enhancing plant nutrient uptake (Yin, Cao & Xu, 2019; Yin et al., 2020). However, several studies have focused on the effects of low pH on the photosynthesis and chlorophyll content of ginger under salt stress. As such, the objectives of this study were to investigate the changes in photosynthesis and chlorophyll content in response to acidic salt stress, which is important for understanding the mechanism underlying plant tolerance to acidic salt stress.
Materials & Methods
Plant materials and experimental treatments
The plant materials and experiment treatment reference Yin et al. (2020). In Tai’an, Shandong Province, China pot culture experiment was performed from April to October 2017. On May 14, the Zingiber officinale cultivar Shannong No. 1 were sown in pots (diameter, 25 cm; height, 30 cm) filled with cleaned quartz sand (Yin et al., 2020). Neutral salt stress was simulated with NaCl and Na2SO4(NaCl/Na2SO4=1/1), and pure water with different pH values (HCl/H2SO4=1/1) was used to simulate hydrochloric acid stress treatments. This experiment involved four treatments: T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity). Each treatment was replicated three times, with six individual plants in each replicate. Each pot received 400 mL of treatment solution.
Analytical methods
Photosynthesis parameters
Functional leaves of ginger were selected, and the Pn, Gs, Tr and Ci were measured by a portable photosynthesis system (Ciras-3, PP Systems, USA) using the method of Li et al. (2013), with slight modifications. When the Pn reached a steady state at each light intensity level, data were recorded 5 times per treatment, and the average value was calculated to determine the final photosynthesis parameters. Natural light was used, and the CO2 gas source was part of an open system.
Pigment concentrations
The chlorophyll content was measured according to the methods of Holm (1954).
Chlorophyll fluorescence
The qP, NPQ, ΦPSII, and Fv/Fm were measured according to the methods of Hendrickson et al. (2005) and Liu et al. (2018). At the time of measurements, 5 plants were averaged for each treatment.
Photosynthesis enzyme (RuBPCase, FBPase, and FBA) activity assays
Fructose 1,6-diphosphatase (FBPase) activity was measured according to the methods of Lazro et al. (1974).
RuBPCase activity was determined using an ELISA kit (Suzhou Keming), and fructose 1,6-bisphosphate aldolase (FBA) activity was determined using an ELISA kit (GenMed).
Sugar metabolism
Sucrose synthase (SS) and sucrose phosphate synthase (SPS) activity were estimated following the methods of Batta & Singh (1986).
Reducing sugars and sucrose contents were measured according to the methods of Handel (1968), using a standard graph of glucose.
The starch content was calculated according to the methods of Hannachi & Van Labeke (2018).
Mineral analysis
Ginger leaves were dried for 48 h at 75 °C and ground separately in a Wiley mill to pass through a 20-mesh screen. Afterward, 0.5 g of dried plant tissue was analyzed to determine the following major and minor elements: N, P, K, Ca and Mg. The nitrogen concentration in the plant tissues was determined by the Kjeldahl method after mineralization with sulfuric acid (Bremner, 1965).
Phosphorus concentrations were determined by titration with molybdenum antimony reagent in the presence of dinitrophenol (Shankar, Kumar & Agrawal, 2016).
K, Ca, Fe, Zn and Mg concentrations were determined by dry ashing at 400 °C for 24 h, dissolving the ash in 1/20 HNO3, and assaying the solution obtained using an inductively coupled plasma emission spectrometer (iCAP 7000 Series, Thermo Scientific).
Observations of ginger leaf chloroplast ultrastructure
Functional leaves were sampled (1 mm ×1 mm), quickly placed in a 2.5% glutaraldehyde fixative solution, and then transferred to a 4 °C refrigerator. The material was rinsed with 0.1 M PB (pH 7.4). Tissues avoid light post fixed with 1% OsO4 in 0.1 M PB (pH 7.4) for 7 h at room temperature., after which it was rinsed 3 times with 0.1 M PB (pH 7.4) again for 15 min each time. After that, the leaf tissue was then subjected to dehydration and infiltration, after which it was embedded. Afterward, the material was sectioned (Leica UC7), stained, the cuprum grids are observed under transmission electron microscope (HT7700, Hitachi) and take images.
Statistical analysis
The data are presented as means ± one standard deviation (SD) for three independent replicates. The data were processed with DPS software. All graphs were created using the program SigmaPlot 10.0.
Results
Photosynthesis parameters
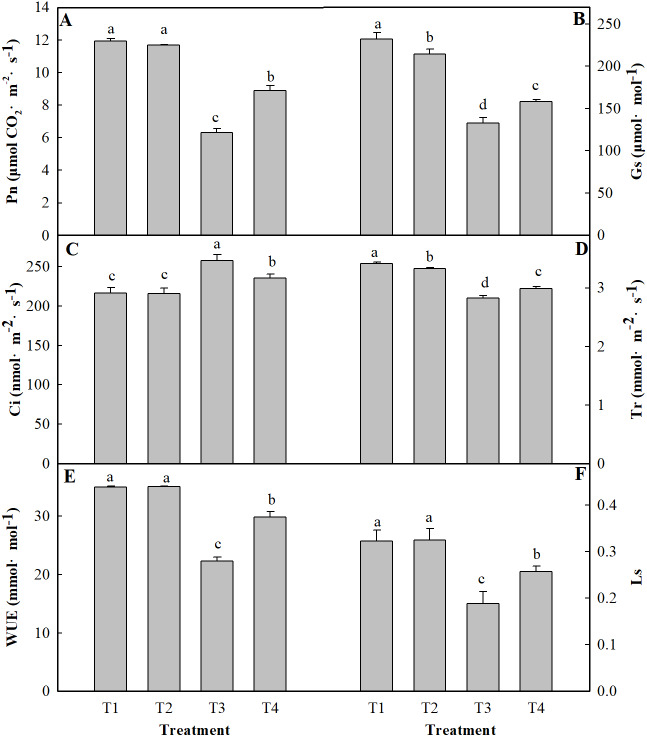
Figure 1 shows the Pn, Gs, Ci, Tr, WUE and Ls of the leaves of ginger plants under salt stress with or without H+ application. Compared with those under the control treatment (T1), the Pn, Gs, Tr, and WUE decreased markedly (by 47.21%, 42.67%, 17.19%, 36.26%, respectively) under the T3 treatment, and the Ci increased by 18.92%. Moreover, compared with those under the control treatment (T1), the Pn, Gs, Tr, and WUE under the T4 treatment decreased by 25.42%, 31.90%, 12.50%, and 14.76%, respectively, and the Ci increased by 8.62%. Compared with that under the control treatment (T1), the Ls under the T3 and T4 treatments decreased by 41.75% and 20.26%, respectively. However, the pH treatment had only a certain effect on the Pn and WUE of ginger seedling leaves, but they did not significantly differ at the level of P < 0.05.
Figure 1. Effects of low pH on photosynthesis parameters of the leaves of ginger plants under salt stress.
T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity). The different small letters in a column of the same treatment days indicate significance at the 5% level.
Pigment contents
As shown in Table 1, compared with the control treatment (T1), the T3 treatment reduced the contents of Chl a, Chl b, Car, and Chl a+b by 9.33%, 28.28%, 7.59% and 13.01%, respectively. Moreover, the contents of Chl a, Chl b, Car, and Chl a+b slightly increased under the T4 treatment compared to the T3 treatment. There was no considerable difference caused by low pH in the Chl a, Chl b, Car, and Chl a+b contents in the leaves. Salt stress alone decreased the root activity by 26.94%. However, at low pH, salt stress decreased the root activity by only 19.57% (Table 2).
Table 1. Effects of low pH on Chlorophyll contents in ginger leaves under salt stress.
T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity). Different small letters in a column of the same treatment days indicate significance at the 5% level.
| Treatment | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Car (mg g−1 FW) | Chl a+b (mg g−1 FW) | Root activity (µg h−1 g FW) |
|---|---|---|---|---|---|
| T1 | 1.93 ± 0.01a | 0.99 ± 0.0211a | 0.79 ± 0.0122a | 2.92 ± 0.013a | 64.84 ± 1.36a |
| T2 | 1.8 ± 0.01b | 0.82 ± 0.0049b | 0.76 ± 0.0166b | 2.62 ± 0.016b | 61.29 ± 1.21b |
| T3 | 1.75 ± 0.01c | 0.71 ± 0.0142c | 0.71 ± 0.0068c | 2.45 ± 0.017c | 47.37 ± 0.62d |
| T4 | 1.78 ± 0.01c | 0.74 ± 0.0102c | 0.73 ± 0.0115c | 2.51 ± 0.019c | 52.15 ± 1.46c |
Table 2. Effects of low pH on the activities of Rubisco, FBA and FBPase in ginger leaves under salt stress.
T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity) Different small letters in a column of the same treatment days indicate significance at the 5% level.
| Treatment | Rubisco | FBA | FBPase |
|---|---|---|---|
| nmol min−1 g−1 | nmol min−1 g−1 | nmol min−1 g−1 | |
| T1 | 105.65 ± 3.43a | 155.81 ± 13.38a | 81.23 ± 3.46b |
| T2 | 94.5 ± 2.28b | 162.17 ± 6.07a | 91.22 ± 4.51a |
| T3 | 65.35 ± 4.66d | 116.65 ± 4.33c | 65.55 ± 1.35c |
| T4 | 74.65 ± 0.83c | 134.55 ± 8.67b | 76.4 ± 3.26b |
Chlorophyll fluorescence
To analyze the changes in different ginger light systems in response to salt stress, chlorophyll fluorescence parameters were measured. These chlorophyll fluorescence parameters display significant negative effects under salt stress (Fig. 2). These effects were manifested by decreased Fv/Fm, qP, and ΦPSII values and an increased NPQ compared to the those of controls (the T3 and T4 treatments). Salt stress alone (T3) reduced the Fv/Fm, qP, and ΦPSII by 9.62%, 12.90%, and 28.96%, respectively, under the T3 treatment and by 6.85%, 7.87%, and 14.35%, respectively, under the T4 treatment compared to those under the normal conditions (T1). In contrast, the value of NPQ increased by 23.27% under the T3 treatment and by 14.35% under the T4 treatment compared to those under the control (T1).
Figure 2. Effects of low pH on chlorophyll parameters of the leaves of ginger plants under salt stress.
T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity). The different lowercase letters in a column of the same treatment days indicate significance at the 5% level.
Photosynthesis enzyme activities
The results related to the activities of photosynthesis enzymes are depicted in Table 2. Low pH increased FBA and FBP activities by 4.08 and 12.30% and decreased Rubisco activity by 10.55% in the absence of salt stress, respectively, compared to those of the control seedlings (Table 2). Salt stress significantly decreased Rubisco, FBA, and FBP activities. Compared with the T3 treatment, the T4 treatment increased the Rubisco, FBA, and FBP activities by 14.23%, 15.34%, and 16.55%, respectively.
Reducing sugar, sucrose, and starch contents
In the salt-affected ginger leaves, the reducing sugar, sucrose and starch contents decreased significantly (Table 3). Salt stress alone (T3) reduced the reducing sugar, sucrose, and starch contents by 51.75, 63.42, and 54.33%, respectively, compared to those of the control. However, at low pH, salt stress decreased reducing sugar, sucrose, and starch contents by only 35.06, 48.62, and 31.48%, respectively, compared to those under the T1 treatment. Table 3 shows that low pH had a weak effect on reducing sugar, sucrose, and starch contents, but they did not significantly differ at the level of P < 0.05.
Table 3. Effects of low pH on Reducing sugar, Sucrose and Starch content in ginger leaves under salt stress.
DW stands for dry weight. T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity) Different small letters in a column of the same treatment days indicate significance at the 5% level.
| Treatment | Reducing sugar | Sucrose | Starch |
|---|---|---|---|
| mg g−1 DW | mg g−1 DW | mg g−1 DW | |
| T1 | 46.32 ± 0.73a | 14.46 ± 0.59a | 323.89 ± 2.68a |
| T2 | 40.51 ± 1.15b | 12.31 ± 0.35b | 319.44 ± 2.71a |
| T3 | 22.35 ± 1.05d | 5.29 ± 0.08d | 147.92 ± 5.12c |
| T4 | 30.08 ± 0.64c | 7.43 ± 0.29c | 221.94 ± 7.87b |
Activity of SS and SPS
SS and SPS are key enzymes involved in carbon metabolism. The different treatments had significant effects on the activity of SS and SPS in the ginger leaves (Fig. 3). Salt stress alone reduced SS and SPS activity by 41.57 and 30.34%, respectively, compared to that of the control (T1). The activity of SS and SPS was reduced by 30.3 and 6.15%, respectively, under the salt +low pH treatment (T4) compared to the control treatment (T1).
Figure 3. Effects of low pH on the activity of SS (A) and SPS (B) in the leaves of ginger plants under salt stress.
T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity). The different small letters in a column of the same treatment days indicate significance at the 5% level.
Content of phosphorus and nitrogen
Figure 4 shows the results of the content of P and N in the leaves of ginger plants under salt stress or under low pH. Compared with the control (T1), salt stress alone (T3) reduced the content of P and N by 57.90 and 16.35%, respectively. Low pH with salt stress (T4) decreased the contents of P and N by 47.48 and 11.08%, respectively, compared to those of the control. Under low pH, the contents of P and N were slightly affected, but they did not significantly differ at the level of P < 0.05.
Figure 4. Effects of low pH on the contents of P (A) and N (B) in the leaves of ginger plants under salt stress.
T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity).
Mineral composition content
As shown in Fig. 5, salt stress significantly increased the Na content in leaves of the plants and significantly decreased the contents of K, Mg, Ca, Fe and Zn. The content of Na in ginger seedling leaves increased by 101.55% under salt stress alone and increased by 40.62% under low pH and salt stress. Salt stress alone significantly reduced the contents of K, Mg, Ca, Fe and Zn by 27.27%, 32.52%, 28.08%, 47.01% and 41.73%, respectively, compared to those of the control. The contents of K, Mg, Ca, Fe and Zn decreased by 17.36, 29.38, 13.78, 40.64 and 31.75%, respectively, under low pH with salt stress (T4), respectively, compared to those under the control treatment (T1).
Figure 5. Effects of low pH on the contents of Na (A), K (B), Mg (C), Ca (D), Fe (E) and Zn (F) in the leaves of ginger plants under salt stress.
T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity).
Ultrastructure morphological changes
Changes in whole mesophyll cells and chloroplasts are shown in Fig. 6. Seedlings grown under normal conditions exhibited regular cell shape and typical chloroplasts; moreover, there were several well-packed starch grains (Fig. 6). However, cell morphological disturbance and plasmolysis occurred when the seedlings were treated with salt stress alone. The shapes of chloroplasts were severely swollen. Furthermore, there was an abundance of osmiophilic granules and fewer starch grains in the chloroplasts compared with those of control (Fig. 6). For low-pH-treated seedlings under salt stress conditions, there was a small improvement in cell morphology. The chloroplasts contained more starch grains than did those of seedlings under salt stress alone (Fig. 6).
Figure 6. Effects of low pH on the leaf ultrastructure in the leaves of ginger plants under salt stress: T1 (pH 6, 0 salinity), T2 (pH 4, 0 salinity), T3 (pH 6, 100 mmol L−1 salinity) and T4 (pH 4, 100 mmol L−1 salinity).
GL: Granum lamellae; S: Starch grains; ch: Chloroplast; N: Cell nucleus.
Discussion
Photosynthesis
The damage caused by salt to plants is primarily attributed to the inhibition and disruption of photosynthesis, and the decrease of photosynthetic efficiency is one of the important reasons for the decrease of plant biomass under salt stress. The Pn decreases because of stomatal limitation or nonstomatal limitation. If Gs and Ci are positively correlated, then the reason for the Pn decrease is related to stomatal limitation; if the two showed no correlation or contrast, then it is related to nonstomatal limitation. In the present experiment, the Pn and Gs decreased under the T3 treatment, and the Ci increased. This suggests that the main factor of photosynthesis limitation is nonstomatal limitation. This is consistent with the research results of Tang et al. (2018). However, damaged photosynthetic structures may be another factor affecting the photosynthesis rate. The photosynthesis enzyme (Rubisco, FBA, and FBP) activities were related to the degree of damage to the photosynthetic structure. 1,5-Ribulose diphosphate oxygenase/shuttle enzyme is an important enzyme involved in CO2 fixation in plant leaves and plays an important role in maintaining photosynthesis (Parry et al., 1997). In this study, the activity of Rubisco, FBA and FBP decreased under the T3 treatment. These results suggest that the reason for photosynthesis under salt stress alone is nonstomatal limitation. Similar results were reported previously (Feng et al., 2014; Ning et al., 2018) However, low pH with salt stress reduced the Ci and increased the Pn; Gs; and the activities of Rubisco, FBA and FBP. Taken together, these results indicated that low pH could protect photosynthetic structures and increase the photosynthesis enzyme activities, thereby increasing the photosynthesis rate.
The water-use efficiency (WUE) of leaves is an important factor affecting whether plants can adapt to extreme environmental conditions (Martin, Tauer & Lin, 1999). In our experiment, the WUE value decreased under salt stress alone. This decrease was related to the reduced leaf transpiration rate, which was caused by the decreased Gs (Bacha et al., 2017). These conditions were unfavorable for substance transport in ginger. However, low pH increased the WUE of leaves under salt stress and improved substance transport in ginger.
Chlorophyll fluorescence
The absorption and transformation of light energy by plants are mainly divided into three closely related parts: chlorophyll fluorescence, qP-related photosynthetic electron transport, and qN-related heat consumption (Schreiber, Schliwa & Bilger, 1986). As an important physiological index for evaluating plant growth and development characteristics, the chlorophyll content can reflect plant health and adaptability of plants (Guo et al., 2015; Xiao, Sang & Wang, 2008). Salt stress causes the decrease of chlorophyll and carotenoid contents in mung bean leaves, which may be caused by the expansion of chloroplast membrane and/or excess Na+ ions in the leaves (Alharby et al., 2019). In this study, the levels of Chl a, Chl b, and carotenoids decreased under salt stress alone. This is consistent with the research results of Do et al. (2018) and Patil et al. (2016). Chlorophyll content has an obvious correlation with the photosynthesis ability of leaves (Dhanapal et al., 2016), and a decrease in chlorophyll content can lead to an irreversibly decreased photosynthesis rate.
The maximal photochemical efficiency of PSII (Fv/Fm) was used to evaluate the primary conversion efficiency of light energy in the PSII reaction center (Jagerbrand & Kudo, 2016). ΦPSII is the actual light-harvesting efficiency of PSII when the reaction center is partially closed, and ΦPSII reflects the ratio of energy consumed by photosynthetic transmission of electrons when the leaves absorb energy. The qP reflects the relative proportion of light energy captured by light-harvesting pigments for photochemical electron transfer (Awlia et al., 2017). The values of Fv/Fm, ΦPSII, and qP were significantly reduced under salt stress, suggesting that salinity induced the inhibition of PSII electron transport and dissipated the excess excitation in the form of heat. This resulted in the reduction in the fluorescence quantum yield (Mehta et al., 2010). The decrease in qP also indicated an increase in the fraction of reduced QA in PSII (Bacha et al., 2017; Hu, Yan & Yu, 2016). The NPQ value, which represents heat dissipation, increased by 23.27%, which indicated that a greater share of excess energy was released as heat, whereas the ability to utilize light energy decreased. The Fv/Fm, ΦFPSII, and qP values increased significantly under low pH with salt stress, indicating that the photochemical activity and electron transfer in ginger leaves were positively affected and thereby enhanced the light energy conversion efficiency of PSII.
Metal elements
Salt stress leads to specific ion toxicity and plant growth inhibition (Park, Kim & Yun, 2016). Excessive accumulation of Na+ is harmful to plant cells, which can lead to significant changes in metabolism and malnutrition (Liang et al., 2018). Nitrogen is an important major element in plant growth and development. It is a component of many plant cell components, including amino acids, proteins, and nucleic acids. The results show that salt treatment induced a decrease in N concentration in ginger plants in our study. However, the plants that were grown under low pH had consistently higher N concentration than the normal plants under salt stress. The decrease in N concentration due to salt stress may be caused by interference by salinity in N acquisition and utilization. Our study corroborates the findings of Zhang et al. (2016), who reported that phosphorus concentration decreased under salt stress. The effect is compounded by the deficiency of other elements (K, Mg, etc.) due to the excessive Na content, which also severely reduces photosynthesis (Lu et al., 2017). In this study, as expected, in the salt-stressed plants, Na accumulated excessively in the leaves. On the other hand, the K content under salt stress alone was markedly lower than that without salt stress treatment, which implied that there is a competitive relationship between K+ and Na+ in ginger leaves. A similar result was reported by Wakeel et al. (2011). However, low pH with salt stress decreased the Na content and increased the K content. Moreover, the results showed that low pH with salt stress resulted in a stronger ability for the absorption and transport of K+ to ensure an adequate concentration of the ions that participate in key metabolic activities (e.g., photosynthetic metabolites) in leaves. As is well known, Mg also plays an important role in photosynthetic metabolism. Mg accumulations were shown to be significantly positively correlated with the relative photosynthesis rate under salt stress (Ning et al., 2018). In the present study, the Mg content decreased under salt stress alone. These results suggest that salt stress reduced chlorophyll concentrations and photosynthesis by imparting a negative impact on Mg2+ uptake. Under salt stress, the concentrations of micronutrients (Fe and Zn in) ginger leaves decreased. Similarly, the concentration of Fe and Zn in chickpea plants decreased with NaCl stress, as reported by Shankar, Kumar & Agrawal (2016). However, compared with salt stress alone, low pH with salt stress resulted in a significantly higher concentration of micronutrients in plants. This may be attributed to the low pH reducing the Na content and thus enhancing the absorption of trace micronutrients.
Conclusions
Salt stress is one of the major abiotic stresses that inhibit plant growth. As shown in Fig. 7, salt stress significantly inhibited the growth and decreased the photosynthesis, pigment contents and mineral contents of ginger leaves. Low pH with salt stress enhanced the activities of RuBPCase, FBPase, and FBA and increased the pigment contents, increasing the photosynthesis rate. Moreover, it is worth noting that low pH simultaneously increased the accumulation of K, Mg, Ca, Fe, and Zn. In summary, the improvement of photosynthesis, pigment contents, and accumulation of minerals due to low pH ultimately increased the biomass accumulation of ginger seedlings under salt stress.
Figure 7. Impacts of low pH on photosynthesis processes of ginger under salt stress.
“ ↓ ” indicates a decrease, “ ↑ ” indicates an increase.
Supplemental Information
Funding Statement
This work was supported by the TaiShan Industrial Experts Program, China (Grant No. tscy20190105), the Project of agricultural excellent germplasm in Shandong province (Grant No. 2020LZGC006), the Industrial technology system of national specialty vegetables (Grant No. CARS-24-A-09), and the National Natural Science Foundation of China (Grant No. 31972399). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Fengman Yin conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Shanying Zhang performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Bili Cao performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Kun Xu conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw measurements are available as a Supplemental File.
References
- Acosta et al. (2011).Acosta JA, Faz A, Jansen B, Kalbitz K, Martínez-Martínez S. Assessment of salinity status in intensively cultivated soils under semiarid climate, Murcia, SE Spain. Journal of Arid Environments. 2011;75:1056–1066. doi: 10.1016/j.jaridenv.2011.05.006. [DOI] [Google Scholar]
- Ahmad et al. (2018).Ahmad P, Abd Allah EF, Alyemeni MN, Wijaya L, Alam P, Bhardwaj R, Siddique KHM. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate-glutathione cycle and secondary metabolites. Scientific Reports. 2018;8:13515. doi: 10.1038/s41598-018-31917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharby et al. (2019).Alharby HF, Al-Zahrani HS, Hakeem KR, Iqbal M. Identification of physiological and biochemical markers for salt (NaCl) stress in the seedlings of mungbean [Vigna radiata (L.) Wilczek] genotypes. Saudi Journal of Biological Sciences. 2019;26:1053–1060. doi: 10.1016/j.sjbs.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali et al. (2008).Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food and Chemical Toxicology: an International Journal Published for the British Industrial Biological Research Association. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Awlia et al. (2017).Awlia M, Fajkus J, Oakey H, Panzarov aK, Trílek M, Negráo S, Roy SJ, Tester M, M. Julkowska M. Mapping genetic components underlying early salt stress responses in Arabidopis thaliana HapMap population. Journal of Horticultural Science and Biotechnology. 2017;92:31e38. [Google Scholar]
- Bacha et al. (2017).Bacha H, Tekaya M, Drine S, Guasmi F, Touil L, Enneb H, Triki T, Cheour F, Ferchichi A. Impact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv. Microtom leaves. South African Journal of Botany. 2017;108:364–369. doi: 10.1016/j.sajb.2016.08.018. [DOI] [Google Scholar]
- Bollivar (2006).Bollivar DW. Recent advances in chlorophyll biosynthesis. Photosynthesis Research. 2006;90:173–194. doi: 10.1007/s11120-006-9076-6. [DOI] [PubMed] [Google Scholar]
- Bremner (1965).Bremner JM. Total nitrogen. In: Black CA, Evans DD, White IL, Ensminger LE, Clark FE, editors. Methods of soil analysis. Agronomy monograph (Part 2) vol. 9. Madison: American Society of Agronomy, Inc.; 1965. pp. 1149–1178. [Google Scholar]
- Cortleven & Schmulling (2015).Cortleven A, Schmulling T. Regulation of chloroplast development and function by cytokinin. Journal of Experimental Botany. 2015;66:4999–5013. doi: 10.1093/jxb/erv132. [DOI] [PubMed] [Google Scholar]
- Dhanapal et al. (2016).Dhanapal AP, Ray JD, Singh SK, Hoyos-Villegas V, Smith JR, Purcell LC, Fritschi FB. Genome-wide association mapping of soybean chlorophyll traits based on canopy spectral reflectance and leaf extracts. BMC Plant Biology. 2016;16:174. doi: 10.1186/s12870-016-0861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding et al. (2020).Ding Z, Koriem MA, Ibrahim SM, Antar AS, Ewis MA, He Z, Kheir AMS. Seawater intrusion impacts on groundwater and soil quality in the northern part of the Nile Delta, Egypt. Environmental Earth Sciences. 2020;79:313. doi: 10.1007/s12665-020-09069-1. [DOI] [Google Scholar]
- Do et al. (2018).Do TD, Vuong TD, Dunn D, Smothers S, Patil G, Yungbluth DC, Chen P, Scaboo A, Xu D, Carter TE. Mapping and confirmation of loci for salt tolerance in a novel soybean germplasm, Fiskeby III. TAG Theoretical and Applied Genetics Theoretische Und Angewandte Genetik. 2018;131:513–524. doi: 10.1007/s00122-017-3015-0. [DOI] [PubMed] [Google Scholar]
- Feng et al. (2014).Feng ZT, Deng YQ, Fan H, Sun QJ, Sui N, Wang BS. Effects of NaCl stress on the growth and photosynthetic characteristics of Ulmus pumila L. seedlings in sand culture. Photosynthetica. 2014;52:313–320. doi: 10.1007/s11099-014-0032-y. [DOI] [Google Scholar]
- Gaudio et al. (2015).Gaudio N, Belyazid S, Gendre X, Mansat A, Nicolas M, Rizzetto S, Sverdrup H, Probst A. Combined effect of atmospheric nitrogen deposition and climate change on temperate forest soil biogeochemistry: a modeling approach. Ecological Modelling. 2015;306:24–34. doi: 10.1016/j.ecolmodel.2014.10.002. [DOI] [Google Scholar]
- Guo et al. (2015).Guo R, Yang Z, Li F, Yan C, Zhong X, Liu Q, Xia X, Li H, Zhao L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biology. 2015;15:170. doi: 10.1186/s12870-015-0546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel (1968).Handel E. Direct microdetermination of sucrose. Analytical Biochemistry. 1968;22:280–283. doi: 10.1016/0003-2697(68)90317-5. [DOI] [PubMed] [Google Scholar]
- Hannachi & Van Labeke (2018).Hannachi S, Van Labeke M-C. Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongena L.) Scientia Horticulturae. 2018;228:56–65. doi: 10.1016/j.scienta.2017.10.002. [DOI] [Google Scholar]
- Hendrickson et al. (2005).Hendrickson L, Forster B, Pogson BJ, Chow WS. A simple chlorophyll fluorescence parameter that correlates with the rate coefficient of photoinactivation of Photosystem II. Photosynthesis Research. 2005;84:43–49. doi: 10.1007/s11120-004-6430-4. [DOI] [PubMed] [Google Scholar]
- Holm (1954).Holm G. Chlorophyll mutations in barley. Acta Agriculturae Scandinavica. 1954;4(1):457–471. doi: 10.1080/00015125409439955. [DOI] [Google Scholar]
- Hu, Yan & Yu (2016).Hu WH, Yan XH, Yu JQ. Importance of the mitochondrial alternative oxidase (AOX) pathway in alleviating photoinhibition in cucumber leaves under chilling injury and subsequent recovery when leaves are subjected to high light intensity. The Journal of Horticultural Science and Biotechnology. 2016;92:31–38. doi: 10.1080/14620316.2016.1219239. [DOI] [Google Scholar]
- Jagerbrand & Kudo (2016).Jagerbrand AK, Kudo G. Short-term responses in maximum quantum yield of PSII (Fv/Fm) to ex situ temperature treatment of populations of bryophytes originating from different sites in Hokkaido, Northern Japan. Plants. 2016;5:22. doi: 10.3390/plants5020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan et al. (2017).Jan AU, Hadi F, Midrarullah, Nawaz MA, Rahman K. Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.) Plant Physiology and Biochemistry. 2017;116:139–149. doi: 10.1016/j.plaphy.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Janda et al. (2016).Janda T, Darko E, Shehata S, Kovacs V, Pal M, Szalai G. Salt acclimation processes in wheat. Plant Physiology and Biochemistry. 2016;01:68–75. doi: 10.1016/j.plaphy.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Jiang et al. (2017).Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Scientific Reports. 2017;7:42039. doi: 10.1038/srep42039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaji et al. (2016).Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiologiae Plantarum. 2016;38:102. doi: 10.1007/s11738-016-2113-y. [DOI] [Google Scholar]
- Kochian et al. (2015).Kochian LV, Pineros MA, Liu J, Magalhaes JV. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annual Review of Plant Biology. 2015;66:571–598. doi: 10.1146/annurev-arplant-043014-114822. [DOI] [PubMed] [Google Scholar]
- Krasensky & Jonak (2012).Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany. 2012;63:1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazro et al. (1974).Lazro JJ, Chueca A, Gorge JL, Mayor F. Fructose 1, 6-diphosphatase from spinach leaf chloroplasts: purification and heterogeneity. Phytochemistry. 1974;13:2455–2461. doi: 10.1016/S0031-9422(00)86920-4. [DOI] [Google Scholar]
- Li et al. (2015a).Li C, Chen J, Wang J, Ma Z, Han P, Luan Y, Lu A. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. The Science of the Total Environment. 2015a;521–522:101–107. doi: 10.1016/j.scitotenv.2015.03.070. [DOI] [PubMed] [Google Scholar]
- Li et al. (2015b).Li H, Wang Y, Xiao J, Xu K. Reduced photosynthetic dark reaction triggered by ABA application increases intercellular CO2 concentration, generates H2O2 and promotes closure of stomata in ginger leaves. Environmental and Experimental Botany. 2015b;113:11–17. doi: 10.1016/j.envexpbot.2015.01.002. [DOI] [Google Scholar]
- Li et al. (2013).Li H-D, Zhang YZ, Xiao J, Xu K. Photosynthetic dark reaction is more sensitive to ABA signaling caused by osmotic stress than Ca2+ signaling in ginger leaves. Scientia Horticulturae. 2013;164:73–76. doi: 10.1016/j.scienta.2013.09.003. [DOI] [Google Scholar]
- Liang et al. (2018).Liang W, Ma X, Wan P, Liu L. Plant salt-tolerance mechanism: a review. Biochemical and Biophysical Research Communications. 2018;495:286–291. doi: 10.1016/j.bbrc.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu X, Lv Y, Xu K, Xiao X, Xi B, Lu S. Response of ginger growth to a tetracycline-contaminated environment and residues of antibiotic and antibiotic resistance genes. Chemosphere. 2018;201:137–143. doi: 10.1016/j.chemosphere.2018.02.178. [DOI] [PubMed] [Google Scholar]
- Long, Marshall-Colon & Zhu (2015).Long SP, Marshall-Colon A, Zhu XG. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell. 2015;161:56–66. doi: 10.1016/j.cell.2015.03.019. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2017).Lu Y, Lei JQ, Zeng FJ, Zhang B, Liu GJ, Liu B. Effect of NaCl-induced changes in growth, photosynthetic characteristics, water status and enzymatic antioxidant system of Calligonum caput-medusae seedlings. Photosynthetica. 2017;55:96–106. doi: 10.1007/s11099-016-0234-6. [DOI] [Google Scholar]
- Martin, Tauer & Lin (1999).Martin B, Tauer CG, Lin RK. Carbon isotope discrimination as a tool to improve water-use efficiency in tomato. Crop Science. 1999;39:1775–1783. doi: 10.2135/cropsci1999.3961775x. [DOI] [Google Scholar]
- Mehta et al. (2010).Mehta P, Jajoo A, Mathur S, Bharti S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiology and Biochemistry. 2010;48:16–20. doi: 10.1016/j.plaphy.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Moles et al. (2016).Moles TM, Pompeiano A, Reyes THuarancca, Scartazza A, Guglielminetti L. The efficient physiological strategy of a tomato landrace in response to short-term salinity stress. Plant Physiology and Biochemistry. 2016;109:262–272. doi: 10.1016/j.plaphy.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Ning et al. (2018).Ning L, Kan G, Shao H, Yu D. Physiological and transcriptional responses to salt stress in salt-tolerant and salt-sensitive soybean (Glycine max [L.] Merr.) seedlings. Land Degradation & Development. 2018;29:2707–2719. doi: 10.1002/ldr.3005. [DOI] [Google Scholar]
- Park, Kim & Yun (2016).Park HJ, Kim WY, Yun DJ. A new insight of salt stress signaling in plant. Molecules and Cells. 2016;39:447–459. doi: 10.14348/molcells.2016.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry et al. (1997).Parry MAJ, Andralojc PJ, Parmar S, Keys AJ, Habash D, Paul MJ, Alres R, Quick WP, Servaites JC. Regulation of Rubisco by inhibitors in the light. Plant, Cell and Environment. 1997;20(4):528–5340. doi: 10.1046/j.1365-3040.1997.d01-85.x. [DOI] [Google Scholar]
- Patil et al. (2016).Patil G, Do T, Vuong TD, Valliyodan B, Lee JD, Chaudhary J, Shannon JG, Nguyen HT. Genomic-assisted haplotype analysis and the development of high-throughput SNP markers for salinity tolerance in soybean. Scientific Reports. 2016;6:19199. doi: 10.1038/srep19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu & Cho (2015).Ryu H, Cho YG. Plant hormones in salt stress tolerance. Journal of Plant Biology. 2015;58:147–155. doi: 10.1007/s12374-015-0103-z. [DOI] [Google Scholar]
- Schreiber, Schliwa & Bilger (1986).Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Research. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Shankar, Kumar & Agrawal (2016).Shankar V, Kumar D, Agrawal V. Assessment of antioxidant enzyme activity and mineral nutrients in response to NaCl stress and its amelioration through glutathione in Chickpea. Applied Biochemistry and Biotechnology. 2016;178:267–284. doi: 10.1007/s12010-015-1870-1. [DOI] [PubMed] [Google Scholar]
- Singh, Singh & Prasad (2016).Singh M, Singh VP, Prasad SM. Nitrogen modifies NaCl toxicity in eggplant seedlings: Assessment of chlorophyll a fluorescence, antioxidative response and proline metabolism. Biocatalysis and Agricultural Biotechnology. 2016;7:76–86. doi: 10.1016/j.bcab.2016.05.007. [DOI] [Google Scholar]
- Batta & Singh (1986).Batta SK, Singh R. Sucrose metabolism in sugar cane grown under varying climatic conditions synthesis and storage of sucrose in relation to the activities of sucrose synthase, sucrose phosphate synthase and invertase. Phytochemistry. 1986;25(11):2431–2437. doi: 10.1016/S0031-9422(00)84484-2. [DOI] [Google Scholar]
- Tang et al. (2018).Tang YY, Yuan YH, Shu S, Guo SR. Regulatory mechanism of NaCl stress on photosynthesis and antioxidant capacity mediated by transglutaminase in cucumber (Cucumis sativus L.) seedlings. Scientia Horticulturae. 2018;235:294–306. doi: 10.1016/j.scienta.2018.02.045. [DOI] [Google Scholar]
- Thouvenot, Haury & Thiébaut (2012).Thouvenot L, Haury J, Thiébaut G. Responses of two invasive macrophyte species to salt. Hydrobiologia. 2012;686:213–223. doi: 10.1007/s10750-012-1013-4. [DOI] [Google Scholar]
- Wakeel et al. (2011).Wakeel A, Farooq M, Qadir M, Schubert S. Potassium substitution by sodium in plants. Critical Reviews in Plant Sciences. 2011;30:401–413. doi: 10.1080/07352689.2011.587728. [DOI] [Google Scholar]
- Xiao, Sang & Wang (2008).Xiao CW, Sang WG, Wang RZ. Fine root dynamics and turnover rate in an Asia white birch forest of Donglingshan Mountain, China. Forest Ecology and Management. 2008;255:765–773. doi: 10.1016/j.foreco.2007.09.062. [DOI] [Google Scholar]
- Yin, Cao & Xu (2019).Yin FM, Cao BL, Xu K. Effects of simulated soil acidification and salt interaction on mineral elements and osmotic substance in ginger. Plant Physiology Journal. 2019;55(6):814–820. doi: 10.13592/j.cnki.ppj.2019.0116. [DOI] [Google Scholar]
- Yin et al. (2020).Yin FM, Liu XN, Cao BL, Xu K. Low pH altered salt stress in antioxidant metabolism and nitrogen assimilation in ginger (Zingiber officinale) seedlings. Physiologia Plantarum. 2020;168(3):648–659. doi: 10.1111/ppl.13011. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang K, Liu H, Song J, Wu W, Li K, Zhang J. Physiological and comparative proteome analyses reveal low-phosphate tolerance and enhanced photosynthesis in a maize mutant owing to reinforced inorganic phosphate recycling. BMC Plant Biology. 2016;16:129. doi: 10.1186/s12870-016-0825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Long & Ort (2010).Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology. 2010;61:235–261. doi: 10.1146/annurev-arplant-042809-112206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Raw measurements are available as a Supplemental File.