Abstract
AKT-mTORC1(mammalian target of rapamycin complex 1) signaling pathway plays a critical role in tumorigenesis and can be targeted by rapamycin. However, the underlying mechanism of how lncRNAs (long noncoding RNA) regulate the AKT-mTORC1 pathway remains unclear. EPIC1 is a Myc-binding lncRNA, which has been previously demonstrated to be overexpressed in multiple cancer types. In a pathway analysis including 4962 cancer patients, we observed that lncRNA EPIC1 expression was positively correlated with the AKT-mTORC1 signaling pathway in more than 10 cancer types, including breast and ovarian cancers. RNA-seq analysis of breast and ovarian cancer cells demonstrated that EPIC1-knockdown led to the downregulation of genes in the AKT-mTORC1 signaling pathway. In MCF-7, OVCAR4 and A2780cis cell lines, EPIC1 knockdown and overexpression respectively inhibited and activated phosphorylated AKT and the downstream phosphorylation levels of 4EBP1 and S6K. Further knockdown of Myc abolished the EPIC1’s regulation of AKT-mTORC1 signaling; suggested that the regulation of phosphorylation level of AKT, ERK, 4EBP1 and S6K by EPIC1 depended on the expression of Myc. Moreover, EPIC1 overexpressed MCF-7, A2780cis, and OVCAR4 cells treated with rapamycin showed significant decreasing in rapamycin mediated inhibition of p-S6K and p-S6 comparing with the control group. In addition, Colony Formation assay and MTT assay indicated that EPIC1 overexpression led to rapamycin resistance in breast and ovarian cancer cell lines. Our results demonstrated the lncRNA EPIC1 expression activated the AKT-mTORC1 signaling pathway through Myc and led to rapamycin resistance in breast and ovarian cancer.
Keywords: lncRNA EPIC1, Myc, AKT-mTORC1, rapamycin resistance
Introduction:
AKT-mTORC1 (mammalian target of rapamycin) has been identified as a critical signaling pathway contributing to cancer initiation and progression. Two major downstream targets of mTORC1, 4EBP1 (eukaryotic initiation factor 4E binding protein 1) and S6K (ribosomal protein S6 kinase1) play major roles in protein synthesis, ribosome biogenesis and lipid synthesis which are all related to cancer cell proliferation.1-3 Besides AKT, ERK (extracellular-regulated kinase) has been demonstrated as another upstream regulator of mTORC1 and regulated its expressions and functions.4-7 Recent studies showed that Myc, as a transcription factor, is a potential regulator of mTORC1 in multiple cancer types8-11. Moreover, the upregulation of Myc leads to drug resistance of mTORC1 inhibitors.12 There are different types of AKT-mTORC1 inhibitors have been developed, and among these inhibitors, rapamycin has been known as a specific inhibitor of mTORC1.13 Currently, rapamycin is a known drug targeting mTORC1 and showing anticancer effect in different cancer types;14-17 including breast cancer.18
Long noncoding RNAs (lncRNAs) are RNA transcripts that are more than 200 nucleotides and do not translate into proteins.19 Recent studies have shown that lncRNAs are involved in tumorigenesis of different cancer progression include cell proliferation, anti-apoptosis, motility by interacting with chromatins, RNAs, and proteins.20,21 Moreover, several lncRNAs have been reported as potential regulators of mTORC1, which correlated with cancer cell metabolism and energy stress.22,23 The nuclear lncRNA EPIC1 (epigenetically-induced lncRNA1) has been shown to regulate cell proliferation through the cell cycle by interacting with transcription factor Myc in breast cancer.24 However, the role of EPIC1 in regulating the AKT-mTORC1 pathway and rapamycin response is not clear.
In this study, we performed a comprehensive investigation into EPIC1’s regulation of the AKT-mTORC1 pathway using the genomics data from 4,962 tumor patients. The EPIC1’s regulation of the AKT-mTORC1 pathway in tumor samples was further confirmed by RNA-seq analysis in EPIC1 knockdown cancer cell lines. We concluded that EPIC1 activate AKT-mTORC1 signaling pathway in breast and ovarian cancer cells through increasing the phosphorylation level of phospho-S6K, phospho-S6, and phospho-4EBP1. Further silencing Myc rescued EPIC1’s activation of S6K, S6, and 4EBP1, suggested EPIC1’s regulation of the AKT-mTROC1 pathway was dependent on Myc. Moreover, we have demonstrated that EPIC1 overexpression led to rapamycin resistance in both breast and ovarian cancer. In conclusion, our study revealed that EPIC1 played an essential role in the rapamycin resistance and AKT-mTROC1 signaling pathway by interacting with Myc in breast and ovarian cancers.
Materials and Method
2.1. Cell culture, stable cell line generation
Human breast cancer cell line MCF-7 was purchased from ATCC (American Type Culture Collection) and cultured with Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and 1% penicillin-streptomycin. Human ovarian cancer cell line OVCAR4 was purchased from NIH/NCI and cultured with RPMI-1640 medium supplemented with 2 mM glutamine, 10% FBS, and 1% penicillin-streptomycin. Human ovarian cancer cell line A2780cis was purchased from ECACC (European Collection of Cell Cultures), supplied by Sigma-Aldrich and cultured with RPMI-1640 medium supplemented with 2 mM glutamine, 10% FBS and 1% penicillin-streptomycin. Stable cell lines were generated according to our previous publication.24 Basically, 293T cells were seeded in 6-cm dish with DMEM medium with only 10% FBS, without any penicillin or streptomycin. After overnight, transfect 293T cells with 3μg of control or EPIC1 plasmid, 2.25μg of psPAX2 (Addgene, #12260), and 0.75 ug of pVSV-G (Addgene, #8454) using Lipofectamine 3000 Reagent (ThermoFisher, 10881775001) as guidelines suggested to produce lentivirus medium. Then, cells were incubated for 6 hr and changed to DMEM medium with only 10% FBS. Cells were then incubated for another 24 hr. The culture medium with lentivirus was collected and went through 0.45 μM filter to remove any floating cells or debris. At the same time, prepare target cells (MCF-7, A2780cis, and OVCAR4) in 6 cm dish. Used the collection lentivirus medium to infect target cells for 24hr with 8 μg/ml polybrene. Finally, select the target cells with puromycin (Fisher BioReagents, BP2956-100) to generate the stable cell line.
2.2. siRNA treatment and cloning
Cells in 6-well culture plates were transfected with 40 nM siRNA targeted EPIC1, Myc, and a control siRNA using Lipofectamine RNAiMAX reagent (ThermoFisher, 13778150) per the instructions by manufactures. After 48 hr, cells were collected, and western blot analysis and quantitative real-time PCR (qRT-PCR) were performed. siRNA sequences are listed as following: siControl (Santa Cruz, sc-37007), siEPIC1#1: CCUUCAGACUGUCUUUGAA; siEPIC1#2: GCUUUCUCUCGGAAACGUG. Full length of EPIC1 expression plasmid was followed the previous description.24 Briefly, to construct EPIC1 expression plasmids, full-length of EPIC1 was cloned into pCDH-CMV-MCS-EF1-Puro (System Biosciences, #CD510B-1) with XbaI and EcoRI enzymes. Full-length of Flag-tagged MYC expression vectors were generated using a human MYC cDNA Clone (OriGene, #SC112715) as a DNA template.
2.3. SDS-PAGE and Western Blot
Cells were lysed in RIPA lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM PMSF, and 1x protease inhibitor cocktail (Sigma-Aldrich, P8340)). Total protein concentrations were measured with BCA protein assay kit (ThermoFisher, 23225). 20 μg of protein with 5x SDS sample buffer were loaded into SDS-PAGE gel and then transferred to PVDF membranes (Bio-Rad, 162-0177). Membranes were blocked in 5% non-fat milk (Lab Scientific, M0841) for 1 hr and then incubated in primary antibodies diluted 1:1000 at 4 °C. ß-Actin antibody was purchased from Sigma-Aldrich (A5441). The following antibodies were purchased from Santa Cruz: p-ERK (sc-7383), total-ERK (sc-514302), PTEN (sc-7974). The rest of antibodies are purchased from Cell Signaling Technology: p-AKT (4060) phosphorylation site, total-AKT (4691), p-S6K (9234), total-S6K (2788), p-S6 (4858), total-S6 (2217), p-4EBP1 (2855), total 4EBP1 (9644), Myc (13987), Flag (14793). On the second day, membranes were incubated in horseradish peroxidase-conjugated secondary antibodies for 1hr (Anit-mouse IgG: ThermoFisher 31430; anti-rabbit IgG: ThermoFisher, 31460). Bands were detected by ECL (electrogenerated chemiluminescence) solution (ThermoFisher, 32106) on films.
2.4. Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using Trizol reagent. cDNAs were generated from 1 μg of RNA using cDNA Reverse Transcription Kit (Applied Biosystems, 4368813). qRT-PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems, 4367659) on 6 Flex Real-Time PCR System (Applied Biosystems). Relative mRNA expression levels were normalized to GAPDH. GAPDH primer sequences were used: forward: 5’-GGTGAAGGTCGGAGTCAACG-3’; and reverse: 5’-TGGGTGGAATCATATTGGAACA-3’. EPIC1 primer sequences were used: forward: 5’-TATCCCTCAGAGCTCCTGCT-3’; and reverse: 5’-AGGCTGGCAAGTGTGAATCT-3’. PTEN primer sequences were used: forward: 5’-TGGATTCGACTTAGACTTGACCT-3’; and reverse: 5’-GGTGGGTTATGGTCTTCAAAAGG-3’.
2.5. RNA immunoprecipitation (RIP)
RIP followed the description of our previous publication.24 Briefly, cells were collected and washed once with pre-chilled 1x PBS solution, then cells were treated for 10 minutes with 0.3% formaldehyde at room temperature on a rotor and add glycine solution to a final concentration of 125 mM to treat for another 5 minutes. Cells were pelleted and lysed with RIP buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 0.5 mM DTT, 1 mM PMSF, and 1 x protease inhibitor cocktail (Sigma, P8340)) and incubate on rotor for 20 minutes at 4 °C. Cell lysates were incubated with Myc, MAX, and normal mouse or rabbit IgG used as a control overnight at 4 °C. On the second day, prewashed Dynabeads Protein G (Invitrogen, 10004D) were added and incubated for 2 hr at 4 °C. Finally, beads were washed six times with RIPA buffer. RNA was isolated with Trizol reagent, and qRT-PCR was performed.
2.6. Colony Formation Assay
5 x 103 cells stably overexpressing EPIC1 or a control vector were seeded into 12-well culture plate and cultured overnight, then cells were treated with a serial of dilution of rapamycin (Selleckchem, S1039) ranging from 1.25, 10, 30, 100, to 200 nM, and DMSO was used as a vehicle control. Medium and drug were changed every two days. MCF-7, A2780cis cells were cultured for 6 days, and OVCAR4 cells were cultured for 7 days. Then, cells were washed once with PBS solution and fixed with 2% formaldehyde for 10 minutes at room temperature, and stained for 20 minutes in 0.01% crystal violet-25% methanol solution. Representative images were scanned and analyzed. The colony quantification is done by using ImageJ software and relative cell survival for each concentration is normalized with the quantification at 0nM.
2.7. MTT Assay
5 x 103 cells were seed into 96-well culture plates and incubated overnight. Cells were treated as indicated with a serial of dilution of rapamycin (Selleckchem, S1039) ranging from 0.05, 0.25, 1.25, 10, 30, 100, 200 nM, and DMSO as a vehicle control. MCF-7 and OVCAR4 cells were treated for 72 hrs, and A2780cis cells were treated for 96 hr. Finally, cells were incubated for 4 hr with MTT solution (Biosynth, 1329524) and solubilizing solution (40% DMF, 16% SDS, 2% Acetic acid, PH4.7) were added to dissolve for overnight. The optical density of 570 nm and 630 nm were measured on the next day.
2.8. Chromatin Immunoprecipitation (ChIP)- sequencing
Use 1x107 cells per sample cross-link with 1.42 % formaldehyde on shaker for 10 minutes at RT. Then quenched by adding 1.25M glycine and shake for another 5 minutes at RT. Cells were rinsed by pre-chilled PBS for two times. Harvested cells into 50 ml tubes and add lysis buffer (50 mM pH 7.5 Tris-HCl, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, and 1% Triton X-100) with proteasome inhibitor and sonicated to shear the chromatin to yield DNA fragment sizes 100-500bp. A small portion of DNA fragment lysis was used as input samples. Then add Dynabeads Protein G (Invitrogen, 10004D) that was pre-incubated with 30μg Myc antibodies for overnight at 4°C into DNA fragment lysis. Incubate beads and lysis for overnight at 4°C. On the next day, wash beads for six times using the lysis buffer and elute the DNA from beads. Reversed cross-link protein and DNA at 65°C for overnight. On the second day, digest protein and RNA used RNase A (Qiagen, 1007885) and Proteinase K (NEB, P8107S), respectively. Extracted DNA and run agarose gel to check the size of DNA fragments. Then performed library construction (BioLabs, E7645S) to enrich the DNA products. At last, send samples for sequencing.
2.9. Computational Analysis
RNA-seq data of MCF-7 and A2780cis cells after EPIC1 knockdown were profiled using the STAR-RSEM pipeline as in our previous study.24,25 To interpret the function of regulated genes after EPIC1 siRNA treatment, GSEA (version 2.2.0) was performed using the 50 cancer hallmark gene sets.26,27 The expression data for 4,962 patients for 20 cancer types were downloaded TCGA Pan-Cancer project (Data Freeze 1.3) processed as our previous publication.24 To identify the pathways that are enriched with genes correlated with EPIC1 expression in tumor samples, we performed a similar GSEA for each cancer type in the TCGA dataset. The False Discovery Rate (FDR) was used to show the significance of the enriched pathways. The Pearson and Spearman correlation was calculated between gene expressions, and significance was defined as p < 0.05.
3. Results
3.1. The high expression level of EPIC1 is correlated with activated mTORC1 signaling pathway
To determine the function of lncRNA EPIC1, we first used Gene Set Enrichment Analysis (GSEA) to identify EPIC1 related signaling pathways in 20 different cancer types. This analysis revealed that the mTORC1 signaling pathway was positively correlated with EPIC1 expression in 12 out of 20 cancer types (Figure 1A), including breast (Figure 1B left) and ovarian cancer (Figure 1B right). EPIC1 has been shown to significantly associate with breast cancer poor prognosis in our previous publication.24 Moreover, we have further performed survival analysis between EPIC1 expression and ovarian cancer patient prognosis. The analysis indicates that EPIC1 overexpressed ovarian cancer tumors show a nonsignificant trend toward poor survival (Supplemental Figure 1C). In addition, RNA-sequencing analysis indicated that genes involved in the mTORC1 signaling pathway were significantly downregulated in the EPIC1-knockdown MCF-7 and A2780cis cell lines (Figure 1A). In the breast (Figure 1C) and ovarian (Supplemental Figure 1A) cancer patient samples, mTROC1 signaling core genes were positively correlated with EPIC1 expression. For example, the expression of AURKA (serine/threonine-protein kinase 6), GSK3B (glycogen synthase kinase 3 beta), and PLK1 (polo-like kinase 1), which were all reported as major regulators of mTORC1 signaling pathway,28-30 were positively associated with EPIC1 expression in breast (Figure 1D) and ovarian (Supplemental Figure 1B) cancer tumors. These analyses suggested EPIC1 might play an important role in promoting the mTORC1 signaling pathway in cancer.
Figure 1: High expression level of EPIC1 is correlated with activated mTORC1 signaling pathway.
A. Correlation between EPIC1 expression and mTORC1 signaling pathway by Gene set enrichment analysis (GSEA) in 20 cancer types and EPIC1 knockdown MCF-7 (m1, m2, m12), A2780cis cells (ac1, ac2, ac12). The heatmap indicates the GSEA scores. B. Association between the enrichment of mTORC1 signaling and EPIC1 expression in breast cancer patients (left) and ovarian cancer patients (right) by GSEA analysis (A). FDR, False Discovery Rate. C. Expression analysis showing that EPIC1 expression is associated with hallmark mTORC1 genes (B) in 927 breast cancer patients. Each column represents one patient. D. Correlation analysis between specific genes that involved in the mTORC1 signaling pathway and EPIC1 expression in breast cancer patients.
3.2. EPIC1 promotes the AKT-mTORC1 pathway through upregulating the phosphorylation level of 4EBP1 and S6K
In order to determine if EPIC1 regulates the AKT-mTORC1 signaling pathway and its downstream targets, we examined the relationship between EPIC1 and p-AKT. Meanwhile, we also studied two downstream mTORC1 targets, p-S6K and p-4EBP1. Out of ten breast and ovarian cancer cell lines, we chose three that had relatively high expression levels of EPIC1 identified by qRT-PCR (Figure 2A) for further studies. We further generated EPIC1 overexpression stable cell lines in MCF-7, OVCAR-4, and A2780cis cells (Figure 2E). Western Blot demonstrated that three EPIC1 overexpressed cells all showed an increased signal of p-AKT, p-S6K, and p-4EBP1 compared with control cells without affecting the total expression level of these proteins (Figure 2B, 2C, and 2D). In addition, we investigated the protein expression level of p-ERK, which was known to be another upstream regulator of mTORC1 besides AKT.4-7 We observed that p-ERK was also highly upregulated in EPIC1 overexpressed cells (Figure 2B, 2C, and 2D). To verify EPIC1’s regulation of mTORC1 signaling, we knockdown EPIC1 by treating these three cell lines with EPIC1 siRNAs (Figure 2F, 2G, and 2H right). Knockdown EPIC1 downregulated the expression of p-ERK and two mTORC1 downstream targets p-S6K and p-4EBP1 without changing the total S6K or 4EBP1 protein level. These data suggested that EPIC1 promoted the AKT-mTORC1 pathway by upregulating the phosphorylation levels of AKT and mTROC1 downstream targets.
Figure 2: EPIC1 promotes AKT-mTORC1 pathway through upregulating the phosphorylation level of 4EBP1 and S6K.
A. qRT-PCR analysis of EPIC1 RNA level in breast and ovarian cancer cell lines. EPIC1 RNA level was normalized to GAPDH. B-D. Western blot of AKT-mTORC1 signaling pathway proteins in MCF-7 (B), A2780cis (C) and OVCAR4 (D) cells stably overexpressing EPIC1 or a control vector. E. PCR validation for GAPDH and EPIC1 overexpression in MCF-7, A2780cis and OVCAR4 cell lines. F-H. Western blot of AKT-mTORC1 signaling pathway proteins in MCF-7 (F), A2780cis (G) and OVCAR4 (H) cells treated with EPIC1 siRNA or a control siRNA. EPIC1 knockdown efficiency was also shown by qRT-PCR (right). Error bars indicate mean ± SD, n = 3. *p < 0.05, **p < 0.01, and ***p< 0.001. The quantification of indicated protein phosphorylation level of western blots were shown above each signal band. All western blots were repeated three times independently with similar results, and representative images are shown.
3.3. Activation of AKT-mTORC1 signaling pathway by EPIC1 is mediated by its interaction with Myc
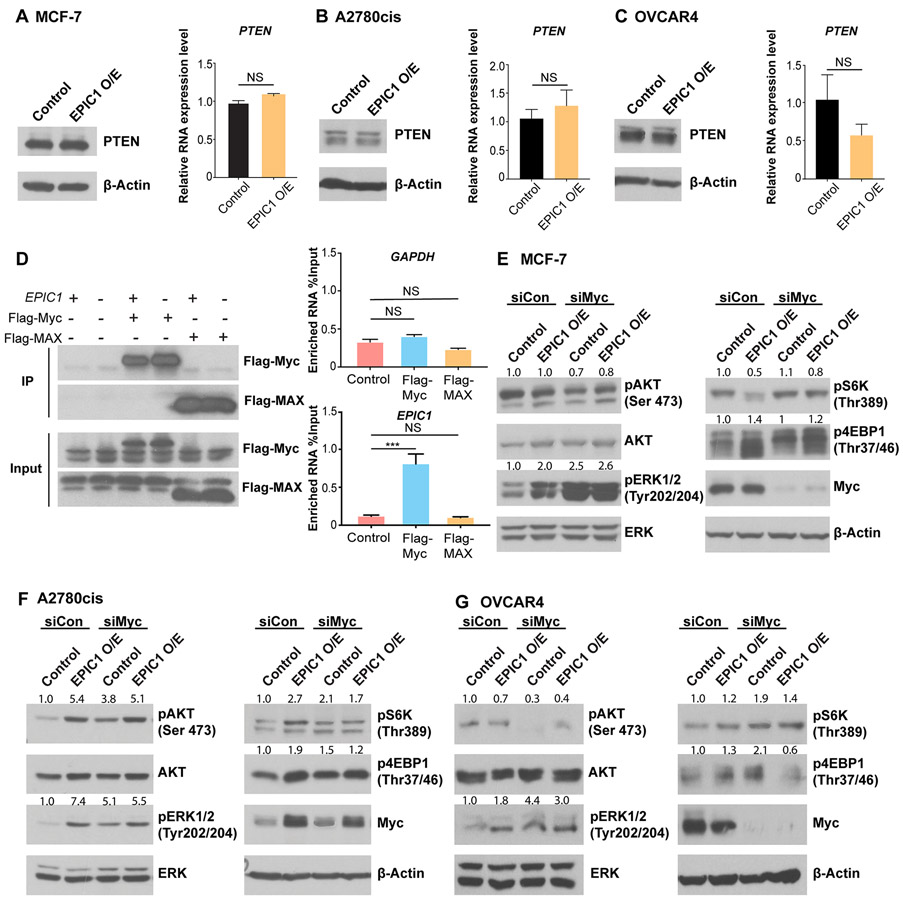
There are several critical proteins that have been established as direct regulators of the AKT-mTORC1 pathway. PTEN is a well-known tumor suppressor gene, which can suppress the AKT-mTORC1 pathway through inhibiting AKT phosphorylation and activation.31 We first examined if EPIC1 regulated PTEN expression level. Western blot data demonstrated that there were no significant differences of PTEN at the protein level between EPIC1 overexpressed cells and control MCF-7, A2780cis, and OVCAR4 cells (Figure 3A, 3B, and 3C left). Furthermore, qRT-PCR indicated comparable PTEN mRNA expression level between control and EPIC1 overexpression cells (Figure 3A, 3B, and 3C right). These observations suggested that EPIC1’s regulation of the AKT-mTORC1 pathway is not mediated by PTEN. Our previous study found that EPIC1 is located in the nucleus and binds with transcription factor Myc24, which has demonstrated as a regulator of AKT-mTORC1 pathway by previous studies. We thus sought to determine if EPIC1 activates the AKT-mTORC1 signaling pathway through interacting with Myc.8,9,11,12 We first verified the binding between EPIC1 and Myc by performing RNA immunoprecipitation assay (RIP) in 293T cells. We also included Flag-tagged MAX (Myc-associated protein X) as a negative control.32 The RIP assay indicated that compared with control and Flag-MAX, only Flag-Myc could successfully pull down EPIC1 after normalized with input (Figure 3D right). We further used siRNA to knockdown Myc in MCF-7 control and EPIC1 overexpressed cells. Western Blot demonstrated that compared with siControl treatment, knockdown of Myc abolished the EPIC1’s activation effect on p-AKT, p-ERK, and p-4EBP1 in EPIC1 overexpression cells (Figure 3E). We have also performed the siMyc treatment in two ovarian cancer cell lines A2780cis (Figure 3F) and OVCAR4 (Figure 3G) and observed similar results. These data suggested that EPIC1 promoted the AKT-mTORC1 signaling pathway through its interaction with Myc.
Figure 3: Activation of AKT-mTORC1 signaling pathway by EPIC1 is through the interaction with Myc.
A-C. Western blot and qRT-PCR analysis of PTEN protein and RNA level in MCF-7 (A), A2780cis (B), and OVCAR4 (C) cells stably overexpressing EPIC1 or a control vector. D. RNA immunoprecipitation (RIP) experiment showing by qRT-PCR analysis of EPIC1 enrichment in Flag-Myc, Flag-MAX, and control samples of 293T cells (right). Western blot of IP efficiency and input was also shown (left). E-G. Western-Blot of AKT-mTROC1 signaling pathway proteins in MCF-7 (E), A2780cis (F), and OVCAR4 (G) stably overexpressing EPIC1 or a control vector after silencing by Myc siRNA or a control siRNA for 48 hours. Error bars indicate mean ± SD, n = 3. *p < 0.05, **p < 0.01, and ***p< 0.001. NS, not significant. The quantification of the indicated protein phosphorylation level of western blots were shown above each signal band. All western blots were repeated three times independently with similar results, and representative images are shown.
3.4. Activation of AKT-mTORC1 by EPIC1 leads to rapamycin resistance
mTORC1 signaling pathway has been established to be an important drug target for cancer treatment.12 Rapamycin, also named Sirolimus is a class I mTOR inhibitor approved to treat breast cancer.18 However, drug resistance of rapamycin and other types of mTOR inhibitors remain a current challenge in treating cancer patients.33,34 Based on our previous studies, we hypothesized that overexpression of EPIC1 could lead to the resistance of mTOR inhibitors. After treated with rapamycin in MCF-7 control and EPIC1 overexpression cells, Indeed, EPIC1 overexpression dampened rapamycin’s inhibition of S6K and S6 phosphorylation (Figure 4A). This result suggested that EPIC1 may play an important role in rapamycin resistance. There was no significant difference in the protein level of another mTORC1 downstream target p4EBP1. This observation was concordant with previous studies, which showed that rapamycin has a strong effect on p-S6K, but not p-4EBP1.35 Consistently, siRNA knockdown of EPIC1 (Figure 4B right) enhanced rapamycin’s inhibition of S6K and S6 phosphorylation (Figure 4B left). MTT experiment was performed by seeding 5,000 MCF-7 control and EPIC1 overexpressed cells in 96-well plate and treated with different concentrations of rapamycin for 72 hr. The cell viability curve indicated that EPIC1 overexpressed cells had a significantly higher IC50 for treating rapamycin (Figure 4C). Further colony formation assay confirmed that EPIC1 overexpressed cells exhibited an increased resistance to rapamycin and higher rate of relative cell survival (Figure 4D). In ovarian cancer cell lines A2780cis and OVCAR4, we also observed that EPIC1 overexpression lead to rapamycin resistance; as indicated by p-S6K and p-S6 (Figure 5A and 5B) and MTT assay (Figure 5C, 5D). These observations suggested that the promotion of the AKT-mTORC1 pathway by EPIC1 could lead to mTORC1 inhibitor-rapamycin resistance in breast and ovarian cancer.
Figure 4: Activation of AKT-mTORC1 by EPIC1 leads to rapamycin resistance in MCF-7 cells.
A. Western-Blot of AKT-mTROC1 signaling pathway proteins in MCF-7 cells stably overexpressing EPIC1 or a control vector after treatment with a serial dosage of rapamycin (Rap: 0, 0.05, 0.1, 0.2, 0.4, 1, 10 nM) for 2 hrs. B. Western-Blot of AKT-mTROC1 signaling pathway proteins in MCF-7 cells transfected for 48 hr with EPIC1 siRNA or a control siRNA then treated with a serial dosage of rapamycin (Rap: 0, 0.05, 0.1, 0.2, 0.4, 1, 10 nM) for 2 hrs. EPIC1 knockdown efficiency was analyzed by qRT-PCR (Right). C. Cell viability was detected by MTT assay after treatment with a serial dosage of rapamycin as indicated for 72 hr in MCF-7 cells stably overexpressing EPIC1 or a control vector. D. Colony formation assay of MCF-7 cells stably overexpressing EPIC1 or a control vector after treatment for 6 days with a serial dosage of rapamycin as indicated. The quantification of relative cell survival is on the right. Error bars indicate mean ± SD, n = 3. *p < 0.05, **p < 0.01, and ***p< 0.001. The quantification of indicated protein phosphorylation level of western blots were shown above each signal band at significant concentrations as indicated. All western blots were repeated three times independently with similar results, and representative images are shown.
Figure 5: Activation of AKT-mTORC1 by EPIC1 leads to rapamycin resistance in A2780cis and OVCAR4 cells.
A-B. Western-Blot of AKT-mTROC1 signaling pathway proteins in A2780cis (A) and OVCAR4 (B) cells stably overexpressing EPIC1 or a control vector after treatment with a serial dosage of rapamycin (Rap: 0, 0.05, 0.1, 0.2, 0.4, 1, 10 nM) 2 hrs. C-D. Cell viability was detected by MTT assay after treat with rapamycin for 72 hr and 96 hr in OVCAR4 and A2780cis cells stably overexpressing EPIC1 or a control vector, respectively. Error bars indicate mean ± SD, n = 3. *p < 0.05, **p < 0.01, and ***p< 0.001. The quantification of indicated protein phosphorylation level of western blots were shown above each signal band at significant concentrations as indicated. All western blots were repeated three times independently with similar results, and representative images are shown.
3.5. Knockdown of EPIC1 results in cells sensitive to rapamycin treatment
In order to further determine the role of EPIC1 in rapamycin resistance of breast and ovarian cancers, we performed cell viability assays in MCF-7, A2780cis and OVCAR4 cell lines treated with rapamycin after knockdown EPIC1 by using siRNA. The cell viability curves of MTT experiments indicated that MCF-7 (Figure 6A), A2780cis (Figure 6B), and OVCAR4 (Figure 6C) parental cells with EPIC1 knockdown showed a significantly lower IC50 while treating rapamycin compared with control cells. Further colony formation assays demonstrated that MCF-7 (Figure 6D), A2780cis (Figure 6E), and OVCAR4 (Figure 6F) cells with EPIC1 knockdown showed a significantly lower number of colonies and lower relative cell survival rate after treated with rapamycin. These results suggested that inhibition of EPIC1 could lead to rapamycin sensitivity in breast and ovarian cancer cells.
Figure 6: Knockdown of EPIC1 results in cells sensitive to rapamycin treatment.
A. Cell viability was detected by MTT assay after treated with a serial dosage of rapamycin as indicated for 72 hr in MCF-7 cells silencing by control siRNA or EPIC1 siRNA. B. Cell viability was detected by MTT assay after treated with a serial dosage of rapamycin as indicated for 96 hr in A2780cis cells silencing by control siRNA or EPIC1 siRNA. C. Cell viability was detected by MTT assay after treated with a serial dosage of rapamycin as indicated for 72 hr in OVCAR4 cells silencing by control siRNA or EPIC1 siRNA. D. Colony formation assay of MCF-7 cells treated with control siRNA or EPIC1 siRNA then treated with a serial dosage of rapamycin as indicated for 6 days. E. Colony formation assay of A2780cis cells treated with control siRNA or EPIC1 siRNA then treated with a serial dosage of rapamycin as indicated for 6 days. F. Colony formation assay of OVCAR4 cells treated with control siRNA or EPIC1 siRNA then treated with a serial dosage of rapamycin as indicated for 7 days. The quantification of relative cell survival is on the right (D-F). Error bars indicate mean ± SD, n = 3. *p < 0.05, **p < 0.01, and ***p< 0.001.
Discussion:
13 Rapamycin, along with its derivatives and other mTOR inhibitors, has been demonstrated to have a therapeutic effect for cancer.36 However, emerging evidence indicated that drug resistance has become a serious problem in patients who have been treated with rapamycin derivatives and other classes of mTOR inhibitors37,38. In the current study, we have demonstrated that Myc binding lncRNA EPIC1 is a potential regulator of the AKT-mTORC1 signaling pathway. In 927 breast and 264 ovarian cancer patients, EPIC1 expression level was significantly correlated with genes involved in ribosome biogenesis and protein synthesis that were affected by the AKT-mTORC1 signaling pathway. These observations suggested that lncRNA EPIC1 played an essential role in mTORC1 mediated tumorigenesis. Indeed, EPIC1 overexpression increased the protein phosphorylation level of crucial kinases involved in the AKT-mTORC1 signaling pathway. We have further shown that knockdown of Myc abolished EPIC1’s regulation of the AKT-mTORC1 signaling pathway. Finally, our study demonstrated that EPIC1 overexpression could lead to rapamycin resistance in breast and ovarian cancer cells.
Recent studies have shown that lncRNAs are promising in serve as potential biomarkers for cancer diagnosis, prognosis, and treatment. For example, one clinical study demonstrated that colorectal cancer patients who had higher expression of lncRNA MALAT1 appeared worse disease-free survival and overall survival compared with patients who had lower MALAT1 expression. This result suggested that the expression level of MALAT1 might serve as a prognostic biomarker in colorectal cancer patients.39 There was another preclinical study revealed that the expression of ten lncRNAs were associated with stage progression in ovarian cancer patients, which suggested that these lncRNAs were able to serve as prognostic biomarkers treating ovarian cancer patients.40 In a recent study that integrated cancer cell line pharmacogenomics data and cancer patient drug response data, our group have also shown that lncRNAs can be used to predict cancer drug response.25 In this study, our computational and experimental result suggested that overexpression of lncRNA EPIC1 might serve as a clinically actionable biomarker in breast and ovarian cancer patients.
From our study, we concluded that EPIC1 regulated the AKT-mTORC1 signaling pathway through its interaction with Myc. However, the specific mechanism of how EPIC1-Myc axis regulates mTORC1 signaling pathway still needs to be answered. In the future, we aim to pinpoint out the targets in the EPIC1-Myc axis that mediates their regulation of mTORC1 signaling pathway. This goal can be achieved by performing Myc ChIP-seq analysis in EPIC1 control and knockdown MCF-7 cells. Our preliminary ChIP-seq results showed that after EPIC1 knockdown, there was a significant downregulation of the binding between Myc transcription factor and IGF1R (Supplemental Figure 2A), PI3KR2 (Supplemental Figure 2B), and AKTIP (Supplemental Figure 2C) at the promoter region. IGF1R encodes insulin growth factor receptor protein, which is a receptor tyrosine kinase binding with growth factor and activating AKT-mTORC1 signaling pathway.41 PI3KR2 encodes p85 protein, which is a regulatory subunit of PI3K. p85 facilitates the function of PI3K enzyme to activate and phosphorylate AKT.42 Decreasing the expression of p85 partially inhibits the function of PI3K enzyme.42 AKTIP gene encodes AKT interacting protein, which directly interacts with AKT and modulates AKT activity by enhancing the phosphorylation of AKT’s regulatory sites.43 Whether EPIC1-MYC axis regulates mTORC1 signaling pathway through transcriptional regulation of IGF1R, PI3KR2, and AKTIP warrant future study.
Supplementary Material
Supplemental Figure 1: High expression level of EPIC1 is correlated with activated mTORC1 signaling pathway in ovarian cancer patients
A. Expression analysis showing that EPIC1 expression is associated with hallmark mTORC1 genes (Figure 1 B right) in 264 ovarian cancer patients. Each column represents one patient.
B. Correlation analysis between specific genes that regulate mTORC1 signaling pathway and EPIC1 expression in ovarian cancer patients.
Supplemental Figure 2: Myc targeted genes that regulates AKT-mTORC1 signaling pathway showed by ChIP-Seq data in MCF-7 control and EPIC1 knockdown cells
A. Peak of IGF1R showed by Myc ChIP-Seq in MCF-7 control (sicontrol) and EPIC1 knockdown (siEPIC1) cells.
B. Peak of PIK3R2 showed by Myc ChIP-Seq in MCF-7 control (sicontrol) and EPIC1 knockdown (siEPIC1) cells.
C. Peak of AKTIP showed by Myc ChIP-Seq in MCF-7 control (sicontrol) and EPIC1 knockdown (siEPIC1) cells.
Acknowledgments:
This study was supported by the Shear Family Foundation (to D.Y.), the American Cancer Society Research Scholar Award (132632-RSG-18-179-01-RMC to D.Y.), and National Cancer Institute (1R01CA222274-01 to D.Y.). We thank the Center for Simulation and Modeling (SaM) at the University of Pittsburgh for computing assistance.
Abbreviations:
- LncRNA
Long noncoding RNA
- AKT
Protein kinase B
- mTORC1
Mammalian-target of rapamycin complex 1
- EPIC1
Epigenetically-induced long noncoding RNA 1
- AKT
Protein kinase B
- ERK
Extracellular-signal-regulated kinase
- S6K
p70S6 kinase
- S6
Ribosomal protein S6
- 4EBP1
Eukaryotic translation initiation factor 4E-binding protein 1
- p-AKT
Phosphorylated protein kinase B
- p-ERK
Phosphorylated extracellular-signal-regulated kinase
- p-S6K
Phosphorylated p70S6 kinase
- p-S6
Phosphorylated ribosomal protein S6
- p-4EBP1
Phosphorylated eukaryotic translation initiation factor 4E-binding protein 1
- PTEN
Phosphatase and tensin homolog
- Rap
Rapamycin
- EPIC1 O/E
Epigenetically-induced long noncoding RNA 1 overexpression
- FDR
False discovery rate
- BRCA
Breast cancer
- OV
Ovarian cancer cell
- IP
Immunoprecipitation
- RIP
RNA immunoprecipitation assay
- qRT-PCR
Quantitative real-time Polymerase chain reaction
- PCR
Polymerase chain reaction
- MAX
Myc-associated protein X
- GS
Glutamine synthetase
- GSEA
Gene set enrichment analysis
- AURKA
Serine/threonine-protein kinase 6
- GSK3B
Glycogen synthase kinase 3 beta
- PLK1
polo-like kinase 1
- IGF1R
Insulin-like growth factor 1 receptor
- PI3KR2
Phosphatidylinositol 3-kinase regulatory subunit beta
- AKTIP
AKT-interacting protein
- ChIP-Seq
Chromatin immunoprecipitation sequencing
- MALAT1
Metastasis associated lung adenocarcinoma transcript 1
- siRNA
Small interfering RNA
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interests.
Data Availability Statement
The data that support the findings of this study are openly available in GEO with reference number [GSE98538].
Reference:
- 1.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. [DOI] [PubMed] [Google Scholar]
- 2.Sabatini DM. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc Natl Acad Sci U S A. 2017;114(45):11818–11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen XG, Liu F, Song XF, et al. Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol Carcinog. 2010;49(6):603–610. [DOI] [PubMed] [Google Scholar]
- 5.Winter JN, Fox TE, Kester M, Jefferson LS, Kimball SR. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am J Physiol Cell Physiol. 2010;299(2):C335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter JN, Jefferson LS, Kimball SR. ERK and Akt signaling pathways function through parallel mechanisms to promote mTORC1 signaling. Am J Physiol Cell Physiol. 2011;300(5):C1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santini E, Feyder M, Gangarossa G, Bateup HS, Greengard P, Fisone G. Dopamine- and cAMP-regulated phosphoprotein of 32-kDa (DARPP-32)-dependent activation of extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin complex 1 (mTORC1) signaling in experimental parkinsonism. J Biol Chem. 2012;287(33):27806–27812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt EV, Ravitz MJ, Chen L, Lynch M. Growth controls connect: interactions between c-myc and the tuberous sclerosis complex-mTOR pathway. Cell Cycle. 2009;8(9):1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CJ, Malina A, Pelletier J. c-Myc and eIF4F constitute a feedforward loop that regulates cell growth: implications for anticancer therapy. Cancer Res. 2009;69(19):7491–7494. [DOI] [PubMed] [Google Scholar]
- 11.Vartanian R, Masri J, Martin J, et al. AP-1 regulates cyclin D1 and c-MYC transcription in an AKT-dependent manner in response to mTOR inhibition: role of AIP4/Itch-mediated JUNB degradation. Mol Cancer Res. 2011;9(1):115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gera JF, Mellinghoff IK, Shi Y, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279(4):2737–2746. [DOI] [PubMed] [Google Scholar]
- 13.Sun SY. mTOR kinase inhibitors as potential cancer therapeutic drugs. Cancer Lett. 2013;340(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noh WC, Mondesire WH, Peng J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10(3):1013–1023. [DOI] [PubMed] [Google Scholar]
- 15.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16(4):525–537. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19(56):6680–6686. [DOI] [PubMed] [Google Scholar]
- 17.Chan S Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91(8):1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23(23):5314–5322. [DOI] [PubMed] [Google Scholar]
- 19.Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest. 2016;126(8):2775–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morlando M, Ballarino M, Fatica A. Long Non-Coding RNAs: New Players in Hematopoiesis and Leukemia. Front Med (Lausanne). 2015;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt AM, Chang HY. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016;29(4):452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Xiao ZD, Han L, et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat Cell Biol. 2016;18(4):431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yacqub-Usman K, Pickard MR, Williams GT. Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. Prostate. 2015;75(7):693–705. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Yang B, Zhang M, et al. lncRNA Epigenetic Landscape Analysis Identifies EPIC1 as an Oncogenic lncRNA that Interacts with MYC and Promotes Cell-Cycle Progression in Cancer. Cancer Cell. 2018;33(4):706–720 e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang Z, Xu J, et al. Systematic identification of non-coding pharmacogenomic landscape in cancer. Nat Commun. 2018;9(1):3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. [DOI] [PubMed] [Google Scholar]
- 28.Pal SK, He M, Tong T, et al. RNA-seq reveals aurora kinase-driven mTOR pathway activation in patients with sarcomatoid metastatic renal cell carcinoma. Mol Cancer Res. 2015;13(1):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo X, Snider WD, Chen B. GSK3beta regulates AKT-induced central nervous system axon regeneration via an eIF2Bepsilon-dependent, mTORC1-independent pathway. Elife. 2016;5:e11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Z, Wen D. The Emerging Role of Polo-Like Kinase 1 in Epithelial-Mesenchymal Transition and Tumor Metastasis. Cancers (Basel). 2017;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis NM, Sokolosky M, Stadelman K, et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention. Oncotarget. 2014;5(13):4603–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251(4998):1211–1217. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene. 2003;22(25):3937–3942. [DOI] [PubMed] [Google Scholar]
- 34.Gruppuso PA, Boylan JM, Sanders JA. The physiology and pathophysiology of rapamycin resistance: implications for cancer. Cell Cycle. 2011;10(7):1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105(45):17414–17419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delbaldo C, Albert S, Dreyer C, et al. Predictive biomarkers for the activity of mammalian target of rapamycin (mTOR) inhibitors. Target Oncol. 2011;6(2):119–124. [DOI] [PubMed] [Google Scholar]
- 37.Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Res. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Houghton PJ. Mechanisms of resistance to rapamycins. Drug Resist Updat. 2001;4(6):378–391. [DOI] [PubMed] [Google Scholar]
- 39.Zheng HT, Shi DB, Wang YW, et al. High expression of lncRNA MALAT1 suggests a biomarker of poor prognosis in colorectal cancer. Int J Clin Exp Pathol. 2014;7(6):3174–3181. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou M, Wang X, Shi H, et al. Characterization of long non-coding RNA-associated ceRNA network to reveal potential prognostic lncRNA biomarkers in human ovarian cancer. Oncotarget. 2016;7(11):12598–12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leroy C, Ramos P, Cornille K, et al. Activation of IGF1R/p110beta/AKT/mTOR confers resistance to alpha-specific PI3K inhibition. Breast Cancer Res. 2016;18(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Y, Jiang Y, Zou F, et al. Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc Natl Acad Sci U S A. 2013;110(17):6829–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao P, Lu C, Ma Y, Xu L, Zhu J, Yu X. Roles of NlAKTIP in the Growth and Eclosion of the Rice Brown Planthopper, Nilaparvata lugens Stal, as Revealed by RNA Interference. Int J Mol Sci. 2015;16(9):22888–22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: High expression level of EPIC1 is correlated with activated mTORC1 signaling pathway in ovarian cancer patients
A. Expression analysis showing that EPIC1 expression is associated with hallmark mTORC1 genes (Figure 1 B right) in 264 ovarian cancer patients. Each column represents one patient.
B. Correlation analysis between specific genes that regulate mTORC1 signaling pathway and EPIC1 expression in ovarian cancer patients.
Supplemental Figure 2: Myc targeted genes that regulates AKT-mTORC1 signaling pathway showed by ChIP-Seq data in MCF-7 control and EPIC1 knockdown cells
A. Peak of IGF1R showed by Myc ChIP-Seq in MCF-7 control (sicontrol) and EPIC1 knockdown (siEPIC1) cells.
B. Peak of PIK3R2 showed by Myc ChIP-Seq in MCF-7 control (sicontrol) and EPIC1 knockdown (siEPIC1) cells.
C. Peak of AKTIP showed by Myc ChIP-Seq in MCF-7 control (sicontrol) and EPIC1 knockdown (siEPIC1) cells.
Data Availability Statement
The data that support the findings of this study are openly available in GEO with reference number [GSE98538].