Abstract
As the coronavirus disease 2019 (COVID-19) pandemic, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is spreading rapidly worldwide, it has emerged as a leading cause of mortality, resulting in >1 million deaths over the past 10 months. The pathophysiology of COVID-19 remains unclear, posing a great challenge to the medical management of patients. Recent studies have reported an unusually high prevalence of thromboembolic events in COVID-19 patients, although the mechanism remains elusive. Several studies have reported the presence of aPLs in COVID-19 patients. We have noticed similarities between COVID-19 and APS, which is an autoimmune prothrombotic disease that is often associated with an infective aetiology. Molecular mimicry and endothelial dysfunction could plausibly explain the mechanism of thrombogenesis in acquired APS. In this review, we discuss the clinicopathological similarities between COVID-19 and APS, and the potential role of therapeutic targets based on the anti-phospholipid model for COVID-19 disease.
Keywords: COVID-19, thrombosis, anti-phospholipid antibodies, anti-phospholipid syndrome, autoimmunity, molecular mimicry, oxidative stress, anticoagulation
Key messages
There is an unusually high prevalence of thromboembolic events in COVID-19 patients, involving both the arterial and the venous circulation.
Clinical and pathological features of COVID-19 thrombosis resemble APS.
Acquired APS, via molecular mimicry and endothelial dysfunction, could plausibly explain thrombogenesis in COVID-19.
Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected 36 361 054 people worldwide, resulting in 1 056 186 deaths as of 9 October 2020 [1]. ARDS leading to multi-organ failure has been described as the major cause of mortality in COVID-19 [2, 3]. Recently, several studies have reported an unusually high incidence of thrombotic complications associated with COVID-19, implicating a potential role of thrombotic complications in mortality [4, 5]. However, the exact mechanism for thromboembolic manifestations in COVID-19 has yet to be elucidated.
The association of viral infections with the presence of aPLs is well described in the literature. Individuals infected with viruses such as HIV, HBV, HCV, EBV and Parvovirus B19 are commonly associated with this condition [6]. However, there have been limited data linking aPLs with coronavirus infections.
An earlier study reported the presence of aCL in convalescent severe acute respiratory syndrome (SARS), which is caused by SARS-CoV, in patients with osteonecrosis [7]. Regarding SARS-CoV-2, a study reported the presence of aPLs in three patients who presented with strokes [8]. Subsequently, three larger studies found LA positivity rates of 91%, 87.7% and 45% amongst 34, 57 and 56 COVID-19 patients, respectively [9–11]. A more recent study found aPLs in 52% of 172 COVID-19-positive samples and demonstrated the potential thrombogenicity of these antibodies using mouse models [12]. Although these studies have described the presence of aPLs in coronavirus infections, the exact mechanisms of antibody formation and associated pathogenicity remain unclear. Additionally, it is debatable whether the presence of aPLs is the cause or an epiphenomenon of thromboembolic events in COVID-19 patients.
In this review, we discuss the pathogenesis of thromboembolic manifestations in COVID-19 using an APS model, highlighting similarities in the clinical and pathological features of severe COVID-19 and catastrophic APS (CAPS), and its implication in the treatment of COVID-19.
APS and COVID-19
APS is an autoimmune prothrombotic disease characterized by persistently elevated aPLs, resulting in recurrent arterial and venous thromboembolic events [13]. In women, APS can be associated with pregnancy-related complications, such as eclampsia, pre-eclampsia and recurrent miscarriages [14]. APS is diagnosed by the presence of any of the clinical criteria described above along with a positive laboratory test for at least one specific antibody [14], which includes aCL, LA antibodies and anti-β2-glycoprotein I antibodies [15]. LA antibodies are identified through a coagulation-based assay, where they demonstrate prolongation of a phospholipid-dependent clotting time [16]. aCLs and anti-β2-glycoprotein I antibodies are detected by immunoassays that measure reactivity to cardiolipin, a phospholipid, and β2-glycoprotein I, a phospholipid-binding protein, respectively [17].
Although aPLs are associated with APS, the prevalence of the presence of aPLs varies from 1–5% in healthy young individuals [18] to ∼50% in elderly individuals with chronic diseases [19]. The pathogenesis of APS remains elusive owing to the marked heterogeneity in clinical manifestations. Some patients with aPLs remain asymptomatic, others may develop spontaneous thrombosis of large vessels affecting a single site, whereas a small proportion may develop rapidly progressive, life-threatening, multi-organ microvascular thromboses.
CAPS is a rare and severe form of APS. Initial descriptions of CAPS had ≤50% mortality rate; therefore, the prefix catastrophic was used for this form of APS [20]. The diagnostic criteria for CAPS include multiple organ dysfunction developing over a short span of time, histopathological evidence of multiple small vessel occlusions and high titres of aPLs [21]. Histopathological confirmation may not be possible in most CAPS patients owing to the severity of the illness and associated coagulopathy [22]. The international CAPS registry (https://ontocrf.grupocostaisa.com/web/caps/home) has played a pivotal role in the development of diagnostic algorithms, classification criteria and therapeutic guidelines for CAPS [21, 23, 24]. Nearly half of the cases in this registry reported bacterial or viral infections as the main inciting factor, followed by surgical procedures and malignancies [24].
Pathogenesis of aPLs in COVID-19
Coronaviruses have four structural proteins: spike (S), membrane (M), envelope (E) and nucleocapsid (N) [25]. The trimeric, transmembrane S glycoprotein, consisting of two subunits, determines the diversity of the virus and host tropism [26]. The S1 subunit is responsible for the attachment of the virus to the host cell receptor, and the S2 subunit assists in the fusion of the viral capsid with the host cell membrane [27]. Once bound to the receptor, the S protein undergoes host protease-mediated cleavage, resulting in endocytosis of the virus particle. Angiotensin-converting enzyme 2 (ACE2) has been identified as a functional receptor that allows the binding of SARS-CoV-2 to its host cells [28]. What is unique in SARS-CoV-2 compared with SARS-CoV is the presence of a furin-like cleavage site (682RRAR/S686) at the S1/S2 site, which allows complete separation from the viral capsid [29].
We postulate that the generation of aPLs in individuals infected with SARS-CoV-2 could be explained by two possible mechanisms that are not mutually exclusive: molecular mimicry and neoepitope formation. The first possibility is that the S1 and S2 subunits of S protein might form a phospholipid-like epitope that induces the generation of aPLs [30]. Antibodies generated against these phospholipid-like determinants of SARS-CoV-2 and other similar viruses can trigger an immunogenic response if those determinants are shared with native tissues [31]. The other possibility is that the conformation of β2-glycoprotein I in host cells is changed owing to oxidative stress caused by SARS-CoV-2 [32, 33], creating a neoepitope for the antibody generation [34]. β2-Glycoprotein I is a plasma protein that is crucial in maintaining haemostasis and is the most common target of pathogenic aPLs [35]. Various groups have demonstrated that oxidative stress is increased in APS [13, 36, 37], which can lead to a disulfide bond formation in domains I and V of β2-glycoprotein I through the thiol exchange reaction [38]. As a result, the corresponding conformational change in the β2-glycoprotein I exposes the crucial B cell epitope, making it immunogenic [13].
aPLs are not thrombogenic per se, as previously demonstrated in animal models [39], which indicates that if aPLs are a part of the pathogenic pathway, there must be additional factors [40]. A similar two-hit hypothesis could possibly explain the activation of thrombogenesis in the context of SARS-CoV-2 infection [40]. The potential second hit might be triggered by endothelial injuries [41, 42] caused by SARS-CoV-2 infection that result in further disruption of the redox balance owing to increased production of reactive oxygen species by macrophages and endothelial cells [32, 33]. Pathologically, the virus downregulates the antioxidant pathway by inhibiting ACE2, nitric oxide and endothelial nitric oxide synthase pathways [32, 33]. The loss of these protective antioxidant pathways results in thrombus generation and activation of the coagulation cascade [39].
Mechanism of thrombosis in COVID-19 by anti-phospholipid model
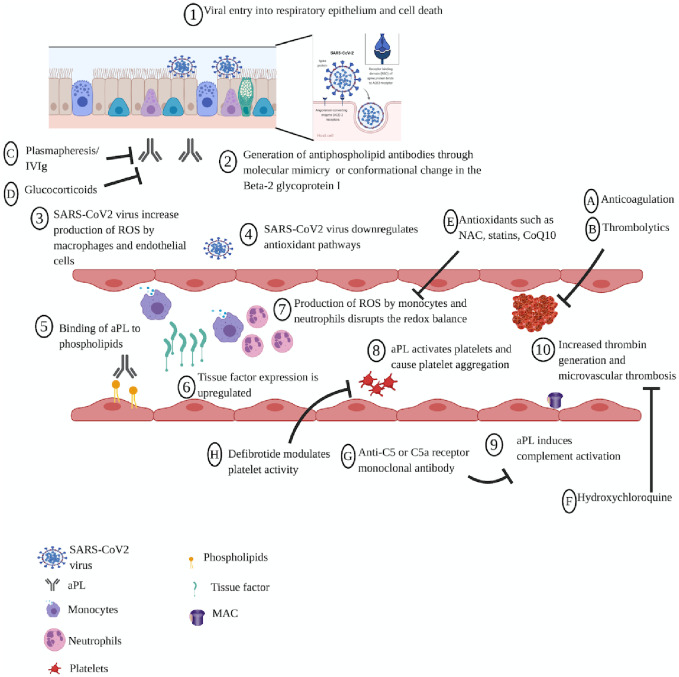
aPLs in COVID-19 patients can cause thrombosis by several possible mechanisms (Fig. 1). First, they can induce the expression of adhesion molecules and tissue factor by binding to endothelial cells and monocytes [43]. Tissue factor is an inducible membrane glycoprotein that plays a major role in initiating the coagulation cascade [44] and fibrin deposition in immunological and inflammatory conditions [45]. These antibodies can also upregulate IL-6, IL-8 and VEGF and induce nitric oxide synthase [46]. Mechanistically, these processes are mediated by p38 mitogen-activated protein kinase (MAPK) phosphorylation and nuclear factor-κB activation [47].
Fig. 1.
Proposed mechanisms of APS pathophysiology and potential therapeutic targets in coronavirus disease 2019
Pathophysiological mechanism of coronavirus disease 2019 (COVID-19). Viral infection of the respiratory epithelium results in endothelial damage, which triggers the production of aPLs, either through molecular mimicry of the SARS-CoV-2 with the innate β2-glycoprotein I or with the generation of a neoepitope resulting from oxidative stress leading to a conformational change in the β2-glycoprotein I. The SARS-CoV-2 induces the production of reactive oxygen species (ROS) by monocytes and endothelial cells and suppresses the antioxidant pathways at the same time. aPL binds to phospholipids and upregulates tissue factor expression, inducing monocytes and neutrophils to produce reactive oxygen species (ROS), which disrupt the redox balance and activate platelets, leading to platelet aggregation and increased thrombin generation. Proposed interventions include anticoagulation, plasmapheresis, IVIG, antioxidants, HCQ, anti-C5 or anti-C5a receptor mAb and defibrotide to reverse APS pathophysiology in COVID-19 patients. MAC, Membrane Attack Complex; NAC, N-acetylcysteine.
Second, oxidative stress [13] and mitochondrial dysfunction [48] might have a major role in thrombogenesis in APS. aPLs can induce nitric oxide and superoxide production, resulting in increased production of a pro-oxidant molecule, peroxynitrite [49]. Concomitantly, these antibodies can result in reduced activity of paraoxonase, an antioxidant enzyme, culminating in oxidative damage to lipid and proteins [37]. The uptake of aPLs into the endosome activates NADPH oxidase and generates superoxide, which upregulates the expression of Toll-like receptors 7 and 8 and sensitizes the cells to these ligands [50]. The disruption of the redox balance through the production of reactive oxygen species by monocytes and neutrophils, accompanied by a reduction in the antioxidant capacity and the observed mitochondrial dysfunction, lead to pro-inflammatory and prothrombotic states in patients with APS [48].
Third, aPLs can also activate platelets, with increased expression of glycoprotein IIb–IIIa [51] and synthesis of thromboxane A2 [52]. This results in platelet aggregation through stimulation with suboptimal doses of adenosine diphosphate, thrombin, collagen or thrombin receptor agonist peptide [51]. Moreover, increased levels of thromboxane A2 can further promote platelet aggregation and vasoconstriction [53].
Fourth, anti-β2-glycoprotein I autoantibodies can disrupt the annexin A5 shield, predisposing to vascular thrombosis and pregnancy loss in APS [54]. Annexin A5 is found in placental and vascular endothelium and functions as an anticoagulant protein [55].
Finally, aPLs can induce complement activation to generate complement split products that attract inflammatory cells to initiate thrombosis and further tissue injury. Activated complement fragments can induce a procoagulant phenotype by direct action through the C5b-9 (membrane attack complex) [56] or the C5a receptor-mediated effects [57].
CAPS and severe COVID-19
Pathological similarities
CAPS can present with features akin to severe sepsis because both these conditions exhibit a pro-inflammatory state represented by elevated levels of cytokines, such as TNF-α, IFN-γ and IL-1 [22]. This process leads to a prothrombotic state and the development of a systemic inflammatory response syndrome.
Occasionally, the term thrombotic storm is used to describe hypercoagulability in patients who progress from a single thrombosis to multiple thromboses at various sites over a short span of time [58]. The putative mechanism involves activation of the coagulation cascade with enhanced thrombin generation mediated by thromboplastic substances and procoagulant proteins released from injured tissues [58]. Concurrently, the downregulation of innate thrombin inhibitors and the fibrinolytic system culminates in further progression of thrombogenesis. Most patients with a thrombotic storm have raised ESR, CRP, fibrinogen and factor VIII levels, consistent with an acute inflammatory state [59]. The pathophysiology of CAPS is similar to that of thrombotic storm, indicating a disease continuum, whereby thrombotic storm predominantly results in macrovascular thrombosis [60]. The pathological features of severe COVID-19 encompass microvascular and macrovascular thromboses, suggesting a spectrum similar to CAPS and thrombotic storm [4, 5]. Additionally, it has been reported recently that the downregulation of fibrinolysis is a main contributor to thrombotic manifestations in severe COVID-19 [61].
Clinical similarities
The myriad of clinical presentations seen in patients with severe COVID-19 bear uncanny resemblances to those of patients with CAPS. COVID-19 patients present with a range of clinical manifestations from asymptomatic to mild upper respiratory symptoms to severe respiratory distress, culminating in multi-organ failure. The significant overlap between clinical manifestations of severe COVID-19 and CAPS is summarized in Table 1.
Table 1.
Clinical similarities between catastrophic APS and severe coronavirus disease 2019
| Clinical features | CAPS registry (%) | Reference | Severe COVID-19 (%) | Reference |
|---|---|---|---|---|
| Renal disease | 73 | [22, 24] | 78–89.1 | [62, 63] |
| ARDS | 36 | [24] | 28.8–85 | [2, 64, 65] |
| Pulmonary emboli | 26 | [24] | 23–87 | [66, 67] |
| Cerebral disease | 56 | [24] | 45.5 | [68] |
| Headache | 8 | [24] | 17 | [68] |
| Cardiac disease | 50 | [22] | 19.7–59.6 | [69, 70] |
| Myocardial infarction | 44 | [22] | 39.3 | [71] |
| Skin manifestations | 47 | [22] | 20.4–34 | [72, 73] |
| Livedo reticularis | 43 | [24] | 6 | [74] |
| Elevated liver enzymes | 63 | [24] | 15–93.4 | [75, 76] |
| Venous thrombosis | 69 | [24] | 46.1–79 | [77, 78] |
| Laboratory findings | ||||
| Thrombocytopenia | 67 | [22] | 57.7–71 | [79, 80] |
| Hyperferritinaemia | 71 | [81] | 63–96 | [82, 83, 84] |
| Associated aPLs | ||||
| LA | 83 | [24] | 45–91 | [9, 10, 11] |
| aCL IgG | 81 | [24] | 4.7 | [12] |
| aCL IgM | 49 | [24] | 23 | [12] |
| Anti-β2-glycoprotein I IgG | 78 | [24] | 2.9 | [12] |
| Anti-β2-glycoprotein I IgM | 40 | [24] | 5.2 | [12] |
CAPS: catastrophic APS; COVID-19: coronavirus disease 2019.
Venous thromboembolism
The clinical features seen in COVID-19 respiratory failure mirror what would be expected of progressive in situ thrombosis in the lung [85, 86]. Observational studies revealed a very high incidence of pulmonary embolism in critically ill COVID-19 patients (87% in a cohort of 75 patients) [66], compared with previous reports of critically ill patients with influenza A virus subtype H1N1 and bacterial pneumonia [87]. Radiologically, perfusion defects seen on dual energy perfusion CT (DECT) scan and ventilation perfusion scan with proximal dilated vessels suggested in situ thrombosis [88, 89]. Indeed, autopsies consistently show diffuse microvascular thrombosis and marked right ventricular dilatation, suggesting acute pressure overload [90]. Likewise, several other studies reported a very high prevalence of pulmonary arterial thrombosis in autopsies of COVID-19 patients [42, 91–93].
These observations suggest that hypercoagulability is a unique pathophysiological mechanism in COVID-19, distinguishing it from other causes of ARDS. In addition, these manifestations appear to be shared with SARS, in whiche significant microvascular pulmonary embolism was noted in post-mortem studies [94, 95], suggesting that pulmonary embolism could be a common pathophysiological mechanism across beta-coronavirus infectious diseases. Interestingly, the prevalence of pulmonary embolism [67] and ARDS [2, 64] are similar to what have been described in the CAPS registry [24].
The incidence of deep vein thrombosis was reported to be ∼1% of all severely ill COVID-19 patients in initial studies [5, 66]. However, a recent Chinese study showed that ≤46.1% of a cohort of 143 patients had developed deep vein thrombosis [77], despite one-third of them receiving thromboprophylaxis with low molecular weight heparin. This is rather unusual given the low rate of venous thromboembolism in a Chinese population [96] and raises the possibility that COVID-19 infection might be contributory to this phenomenon. Moreover, a French study demonstrated a prevalence of 79% for deep venous thrombosis in COVID-19 patients within 48 h of intensive care unit admission despite thrombophylaxis [78]. Based on the studies described above, the rate of venous thromboembolism reported in COVID-19 patients appears to be similar to that of the CAPS registry.
Immunological features
Immunological studies have been informative in understanding the pathophysiology of COVID-19, although these studies focused primarily on patients with severe disease [65, 97]. Patients with lymphopenia demonstrated decreased peripheral blood T cells with increased plasma levels of pro-inflammatory cytokines, including IL-6, IL-10, G-CSF, monocyte chemoattractant protein 1, macrophage inflammatory protein 1 and TNF-α [65, 97]. Although these findings described above suggest similarities between COVID-19 and ARDS, there are differences in lymphocyte subsets between critically ill COVID-19 patients and ARDS patients. Small studies examining lymphocyte subsets in COVID-19 patients found lower CD4+ T cells in critically ill patients than in mildly affected patients [98, 99], which parallels the CD4+ T cell depletion observed in APS [100] and contrasts with CD4+ T cell expansion observed in ARDS attributable to other infections [101, 102].
Thrombocytopenia
Thrombocytopenia has been observed in patients with severe COVID-19 [79], many hypotheses for which have been suggested but none has been proved definitively [103]. This feature is similar to CAPS, where 60–70% of patients develop thrombocytopenia [22]. Given the incidence of high D-dimer levels with thrombocytopenia in critically ill COVID-19 patients, it is plausible that this is secondary to disseminated intravascular coagulopathy and likely to represent a pre-terminal event [80, 104–106]. However, the increased fibrinogen levels in critically ill patients contradict the diagnosis of disseminated intravascular coagulopathy [107] and are more consistent with thrombotic microangiopathy seen in CAPS patients [22, 108].
Hyperferritinaemia
A study of 99 COVID-19 patients revealed that the incidence of elevated ferritin levels was as high as 60% [82], which was confirmed by other groups [83, 84]. These observations formed the basis of the use of tocilizumab in an attempt to counteract this hyperinflammatory syndrome seen in severe COVID-19 [109]. Interestingly, hyperferritinaemia is a common pathological feature described in severe sepsis, macrophage activation syndrome, Still’s disease and CAPS [110]. A previous study reported that hyperferritinaemia was observed in about two-thirds of all patients with CAPS, correlating with venous thrombosis and skin necrosis [81]. Ferritin plays a pivotal role in many biological pathways. Besides being a circulating acute phase reactant, ferritin also induces expression of pro-inflammatory mediators, such as IL-1β, inducible nitric oxide synthase, intercellular adhesion molecule 1, inhibitor κBα and T cell immunoglobulin-domain and mucin-domain 2, by activating the nuclear factor-κB signalling cascade [111]. Conversely, increased pro-inflammatory cytokines can stimulate further synthesis of ferritin by macrophages [112], thereby resulting in a cytokine storm. The unusually high incidence of hyperferritinaemia in COVID-19 is similar to what is observed in the CAPS registry [22, 81].
Cardiac and cerebral manifestations
Myocardial infarctions and valvular vegetations have been observed in patients with SARS and COVID-19 [95, 113], suggesting a common pathophysiological pathway with APS [114]. Two studies demonstrated that cardiac enzymes were elevated, signifying cardiac injury, in ∼20–30% of COVID-19 patients [69, 70]. Moreover, a recent study reported close to 40% of localized ST-elevation myocardial infarctions with no culprit lesion, which raises the possibility of other mechanisms of cardiac injury aside from coronary artery disease [71]. Likewise, most APS patients with myocardial infarctions have normal coronary angiography findings [115], suggesting a potential role of aPLs in the disease pathophysiology. This is further corroborated by raised titres of aPLs in a small but significant proportion of myocardial infarction patients [116].
Cerebral manifestations have been reported in 36.4% of COVID-19 patients in one study [68]. The study showed that patients with severe COVID-19 have a high incidence of neurological manifestations, such as acute cerebrovascular disease and impaired consciousness, in addition to skeletal muscle injury. Additionally, a recent case series of three patients reported the presence of anti-cardiolipin IgA, anti-β2-glycoprotein I IgA and IgG with associated strokes [8]. In the CAPS registry, 56% of the reported cases developed cerebral manifestations, whereas microvascular thrombosis was observed in 48.9% of autopsies from this registry [22].
Renal manifestations
Approximately 3% [82, 117] to ∼7% [64] of COVID-19 patients in initial studies from China had acute renal injury. However, subsequent larger studies from the USA showed incidences ranging from 33.9% (n = 850) [62] to 36.6% (n = 5449) [63]. Approximately 90% of patients with severe COVID-19 requiring mechanical ventilation developed acute renal injury [63].
The pathophysiology of renal dysfunction in COVID-19 is unclear, and it is postulated to be attributable to direct cellular injury caused by SARS-CoV-2 or sepsis-related cytokine storm syndrome [118]. An autopsy series of 26 COVID-19 patients revealed the presence of spherical virus-like particles similar to coronavirus in the proximal tubular epithelium and podocytes, suggesting direct cytopathic effects of the SARS-CoV-2 [119]. Interestingly, the pattern of endothelial injury is consistent with the distribution of ACE2 expression. Three cases within this autopsy series also had fibrin thrombi within the glomerular capillary loops with evidence of glomerular ischaemia [119], which is similar to renal autopsy findings in CAPS [22, 24].
Liver dysfunction
Recent meta-analyses indicated elevated liver enzymes in ∼15–20% of COVID-19 patients [75, 76], with a higher incidence of liver injury in those with severe disease. The mechanism of liver injuries is thought to be attributable to the direct binding of SARS-CoV-2 to ACE2-expressing cholangiocytes and not to hepatocytes [120]. A recent autopsy series documented the presence of a central vein thrombosis with focal necrosis of the liver cells in a COVID-19 patient, suggesting a hypercoagulable state similar to that in CAPS [91].
Dermatological manifestations
Although dermatological manifestations of COVID-19 have not been well reported, there is emerging evidence of cutaneous manifestations in ∼20–30% of COVID-19 patients [72, 73], and some of these dermatological features are similar to those found in APS. A recent large, prospective study identified the presence of livedo reticularis in ∼6% of COVID-19 patients [74]. Likewise, a case series described acral ischaemia in young COVID-19 patients with mild or no symptoms [121]. Prominently, a significant time lapse was noted between the onset of COVID-19 symptoms and the onset of acral ischaemia, which suggests that the pathophysiological processes of acral ischaemia could be a secondary effect of COVID-19 rather than a consequence of viremia.
Obstetric manifestations of COVID-19 mirror APS
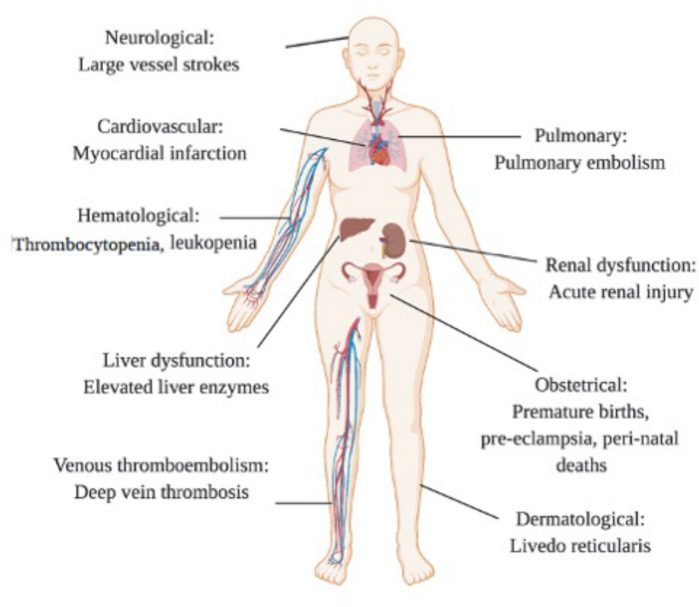
A systemic review revealed that preterm birth at <37 weeks of gestation occurred in 41.1% of pregnant women hospitalized for COVID-19, and the rate of perinatal death was 7% [122]. Moreover, it was noted that there were relatively increased rates of pre-eclampsia and cesarean delivery among these pregnant patients [122] The pathophysiological processes that explain these observations remain poorly understood. In a post-mortem study of the fetus in a COVID-19-positive primigravida patient with miscarriage, intervillous fibrin deposition and umbilical cord vasculitis were observed in the presence of detectable SARS-CoV-2 by PCR and absence of concomitant bacterial infection [123]. These pathological findings were initially attributed to viral infection, although other secondary causes, including LA, were not investigated. However, the role of viral infection in placental vessel thrombosis is controversial; a case series of 19 COVID-19 patients with miscarriages found the presence of vascular thrombosis in the absence of placental SARS-CoV-2 S protein or viral particles. The emergence of aPLs and the possibility of APS as a cause of miscarriages in pregnant COVID-19 patients warrant further investigation. Nonetheless, pregnancy morbidity, including premature births at <34 weeks of gestation owing to pre-eclampsia and the presence of placental insufficiency, in pregnant COVID-19 patients is significant because pregnancy morbidity is considered a clinical criterion for diagnosis of APS. This similarity between COVID-19 and APS further strengthens our argument that aPLs play a major role in the pathophysiology of COVID-19 (Fig. 2).
Fig. 2 .
Overlapping features of APS and COVID-19
There is a significant overlap of dermatological, haematological, obstetric, pulmonary and neurological manifestations of APS with COVID-19.
Therapeutic implications
Antithrombotic and fibrinolytic therapy
Recent updates on the clinical data from COVID-19 patients suggest that the use of anticoagulant reduces mortality [124]. Case reports of COVID-19 patients improving clinically after administration of systemic [125] or atomized plasminogen [126] support the inference that hypofibrinolysis is a major driver of the procoagulant state. Hence, thrombolytic therapy might be warranted in order to interrupt the coagulation cascade, restore homeostasis and prevent potential cardiorespiratory compromise. There are two ongoing prospective studies evaluating the role of thrombolytics in with patients severe COVID-19 and ARDS (Table 2).
Table 2.
Therapeutic implications of CAPS in COVID-19
| CAPS therapy | Potential role of target in COVID-19 | Drug type | Drug name | Number of studies on Clinical Trials.gov a |
|---|---|---|---|---|
| Anticoagulation | Heparin exhibits ant-inflammatory properties and might also prevent the binding of aPLs to their targets on cell surfaces | Heparin |
Enoxaparin Tinzaparin or UFH Heparin |
7 1 1 |
| Thrombolytic therapy | Fibrinolytic therapy | – | Alteplase | 2 |
| Glucocorticoids | Anti-inflammatory properties, inhibition of the pro-inflammatory transcription factors, such as nuclear factor-κB, in addition to reduction of aPL production |
Glucocorticoids: methylprednisolone prednisone |
Tocilizumab + methylprednisolone Siltuzimab + methylprednisolone Dexamethasone Prednisone/hydrocortisone Hydrocortisone Methyprednisolone |
3 5 2 6 |
| Plasma exchange | Removal of aPLs and cytokines, in addition to the restoration of natural anticoagulants with the use of fresh frozen plasma as a volume replacement | N/A | Plasma exchange | 1 |
| IVIG | Inhibits pathological autoantibody development, with subsequent reduction in aPL titres and downregulation of regulatory T cells during a cytokine storm | IVIG |
IVIG vs IST Human immunoglobulin Polyvalent immunoglobulin |
3 |
| CYC | Suppression of lymphoid tissues leads to a decreased level of aPLs and cytokine levels | – | N/A | N/A |
| Defibrotide | Exhibits haemostatic properties by increasing the release of prostacyclin and prostaglandin E2, reduces levels of leukotriene B4 and modulates platelet activity | – | Defibrotide | 2 |
| Eculizumab | Inhibits the cleavage of C5 to C5a and reduces the chemoattractant function and formation of the membrane attack complex | Anti-C5 mAb |
Eculizumab Ravulizumab |
3 2 |
| Anti-human complement factor C5a mAb | IFX-1 | 1 | ||
| Synthetic macrocyclic peptide inhibitor of the terminal complement protein C5 | Zilucoplan | 1 | ||
| Anti-C5a receptor antibody | Avdoralimab | 1 | ||
| N-Acetylcysteine | Inhibits reactive oxygen species-mediated thrombosis | Methylene Blue, vitamin C, N-acetyl cysteine |
MCN N-Acetylcysteine |
1 1 |
| Coenzyme Q10 | Inhibits aPL-mediated reactive oxygen species generation | N/A | N/A | N/A |
| Statins | Upregulate endothelial nitric oxide synthase and inhibit thrombosis in a mouse model of tissue factor-dependent APS | – |
Atorvastatin Simvastatin |
2 |
| HCQ | Inhibits anti-β2-glycoprotein I disruption of the annexin A5 shield and TLR7 activation in vitro and reduces thrombosis in APS mouse models | – | Combination therapy including HCQ | 90 |
The respective clinical trial numbers are provided in Supplementary Table S1, available at Rheumatology Advances in Practice online. COVID-19: coronavirus disease 2019; N/A: not assessed; IFX-1: Anti-C5a antibody(vilobelimab); IST: Immunosuppressive therapy; MCN: Methylene blue- vitamin C- N-acetyl Cysteine; TLR7: Toll-like receptor 7; UFH: Unfractionated heparin.
Glucocorticoids
Glucocorticoids are one of the commonest anti-inflammatory drugs used in the treatment for CAPS. Triple therapy with glucocorticoids, anticoagulation and plasma exchange, with or without IVIG, was associated with the lowest mortality rate compared with no treatment in the CAPS registry [127]. The use of glucocorticoids in patients with severe COVID-19 has recently been demonstrated to be effective in reducing mortality. The RECOVERY study, which randomized 2104 hospitalized COVID-19 patients to receive dexamethasone, found that the use of CSs reduced deaths by one-third in mechanically ventilated patients and by in one-fifth of patients receiving oxygen therapy [128]. Additional clinical trials are underway to evaluate the efficacy of this therapy.
Plasmapheresis and IVIG
The successful trial of one patient [129] with severe COVID-19 using plasmapheresis and IVIG in averting mechanical ventilation suggests that removing inflammatory cytokines, stabilizing endothelial membranes and resetting the coagulation cascade could halt disease progression and prevent mortality [130, 131]. This modality for therapy is recommended for CAPS, and it merits further investigation in patients with severe COVID-19 not responding to standard therapies.
Complement cascade inhibitors
There have been a few case reports on the benefit of eculizumab, an anti-C5 mAb, in the treatment of refractory CAPS [132–134]. Given the increasing evidence of the role of the complement pathway in the pathogenesis of COVID-19 [135], eculizumab appears to be a promising option for severe COVID-19. Several studies are underway to evaluate other complement inhibitors in COVID-19 (Table 2).
Antioxidant therapy
Antioxidant therapies, including N-acetylcysteine [136], statins [137–141] and coenzyme Q10 [48], are proposed as adjunctive therapy for APS based on a few observational and animal model studies. A recent retrospective study reported a possible association between statin pretreatment and reduced mortality rate in COVID-19 patients [142]. The parallels in the role of oxidative stress in CAPS and COVID-19 merit further investigation into the use of these agents to restore the redox balance.
HCQ
HCQ inhibits the disruption of annexin A5 by anti-β2-glycoprotein I antibodies and reduces Toll-like receptor 7 activation in vitro, reducing thrombosis in APS mouse models. HCQ has shown efficacy in preventing recurrent venous thromboembolism in a small, non-randomized prospective study in patients with APS [143]. An in vitro study demonstrated the antiviral properties of chloroquine to SARS-CoV-2 [144]. However, observational studies of the use of chloroquine and HCQ in COVID-19 patients reported contradictory results, with one study showing antiviral properties [145], whereas other studies reported the absence of such effects [146]. Additionally, two large retrospective studies found no clinical benefit of HCQ with or without azithromycin in COVID-19 patients [147, 148]. Perhaps, future randomized controlled trials evaluating the role of HCQ in COVID-19 patients with aPLs could shed light on the discrepancy in clinical efficacy and outcomes.
Defibrotide
Defibrotide exerts haemostatic properties by increasing the release of prostacyclin and prostaglandin E2, reduces the levels of leukotriene B4 and modulates platelet activity. It has been used off label in two patients with CAPS [20, 149], with a satisfactory outcome in one patient. There are at least two clinical trials investigating the use of this agent in COVID-19 patients.
Conclusions
We present a hypothetical model to elucidate the pathophysiology of APS in COVID-19. We also describe the clinicopathological similarities between CAPS and severe COVID-19 disease. In light of this, antithrombotic therapy would be the mainstay of treatment to minimize the progression of COVID-19. Effective screening strategies for aPLs in the early phase of COVID-19 are highly desirable in order to stratify patients into low- and high-risk groups. Preventive measures can be initiated in high-risk groups to reduce thromboembolic complications and mortality in COVID-19 patients. We acknowledge that the similarity of COVID-19 to APS is less striking when compared with CAPS. Given that CAPS is a multisystem disorder and is a relatively rare phenomenon in APS, we would like to highlight the possibility that these similarities could have occurred by chance. Thus, further research is needed to help us gain a better understanding COVID-19 pathophysiology and to develop effective strategies in the management of this disease.
Supplementary Material
Acknowledgements
We acknowledge Jennie Wong of Medical and Scientific Communication, Research Support Unit, National University Health System and Life Science Editors for proof-reading and editing this manuscript. All images in this manuscript were created with Biorender.com.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. World Health Organization. Coronavirus (COVID-19) events as they happen. World Health Organization, 2020. https://covid19.who.int/table. [Google Scholar]
- 2. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E. et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borges do Nascimento IJ, Cacic N, Abdulazeem HM. et al. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med 2020;9:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klok FA, Kruip MJHA, van der Meer NJM. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lodigiani C, Iapichino G, Carenzo L. et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdel-Wahab N, Talathi S, Lopez-Olivo MA, Suarez-Almazor ME.. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus 2018;27:572–83. [DOI] [PubMed] [Google Scholar]
- 7. Sun W, Wang BL, Liu BL. et al. Osteonecrosis in patients after severe acute respiratory syndrome (SARS): possible role of anticardiolipin antibodies. J Clin Rheumatol 2010;16:61–3. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Xiao M, Zhang S. et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowles L, Platton S, Yartey N. et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med 2020;383:288–90. [ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helms J, Tacquard C, Severac F. et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harzallah I, Debliquis A, Drénou B.. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost 2020;18:2064–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zuo Y, Estes SK, Ali RA. et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med 2020;12:eabd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giannakopoulos B, Krilis SA.. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013;368:1033–44. [DOI] [PubMed] [Google Scholar]
- 14. Miyakis S, Lockshin MD, Atsumi T. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 15. Galli M, Barbui T.. Antiphospholipid antibodies and thrombosis: strength of association. Hematol J 2003;4:180–6. [DOI] [PubMed] [Google Scholar]
- 16. Tripodi A, de Groot PG, Pengo V.. Antiphospholipid syndrome: laboratory detection, mechanisms of action and treatment. J Intern Med 2011;270:110–22. [ [DOI] [PubMed] [Google Scholar]
- 17. Reber G, Tincani A, Sanmarco M, de Moerloose P, Boffa MC.. Proposals for the measurement of anti-β2-glycoprotein I antibodies. Standardization group of the European Forum on Antiphospholipid Antibodies. J Thromb Haemost 2004;2:1860–2. [DOI] [PubMed] [Google Scholar]
- 18. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun 2000;15:145–51. [DOI] [PubMed] [Google Scholar]
- 19. Juby A, Davis P.. Prevalence and disease associations of certain autoantibodies in elderly patients. Clin Invest Med 1998;21:4–11. [PubMed] [Google Scholar]
- 20. Asherson RA, Cervera R, Piette J-C. et al. Catastrophic antiphospholipid syndrome. Medicine 2001;80:355–77. [DOI] [PubMed] [Google Scholar]
- 21. Asherson RA, Cervera R, de Groot PG. et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus 2003;12:530–4. [DOI] [PubMed] [Google Scholar]
- 22. Cervera R, Rodríguez-Pintó I, Espinosa G.. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: a comprehensive review. J Autoimmun 2018;92:1–11. [DOI] [PubMed] [Google Scholar]
- 23. Erkan D, Espinosa G, Cervera R.. Catastrophic antiphospholipid syndrome: updated diagnostic algorithms. Autoimmun Rev 2010;10:74–9. [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez-Pintó I, Moitinho M, Santacreu I. et al. Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun Rev 2016;15:1120–4. [DOI] [PubMed] [Google Scholar]
- 25. Weiss SR, Navas-Martin S.. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev 2005;69:635–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bosch BJ, van der Zee R, de Haan CAM, Rottier PJM.. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol 2003;77:8801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol 2016;3:237–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li W, Moore MJ, Vasilieva N. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coutard B, Valle C, de Lamballerie X. et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gharavi AE, Pierangeli SS.. Origin of antiphospholipid antibodies: induction of aPL by viral peptides. Lupus 1998;7:52–4. [DOI] [PubMed] [Google Scholar]
- 31. Amin NM. Antiphospholipid syndromes in infectious diseases. Hematol Oncol Clin N Am 2008;22:131–43. [DOI] [PubMed] [Google Scholar]
- 32. Buinitskaya Y, Gurinovich R, Wlodaver C, Kastsiuchenka S.. Highlights of COVID-19 pathogenesis. Insights Oxidat Damage 2020. doi: 10.6084/m9.figshare.12121575.v11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delgado-Roche L, Mesta F.. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res 2020;51:384–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. López-Pedrera C, Barbarroja N, Jimenez-Gomez Y. et al. Oxidative stress in the pathogenesis of atherothrombosis associated with anti-phospholipid syndrome and systemic lupus erythematosus: new therapeutic approaches. Rheumatology 2016;55:2096–108. [DOI] [PubMed] [Google Scholar]
- 35. Levine JS, Branch W, Rauch J.. The antiphospholipid syndrome. N Engl J Med 2002;346:752–63. [DOI] [PubMed] [Google Scholar]
- 36. Delgado Alves J, Ames PRJ, Donohue S. et al. Antibodies to high-density lipoprotein and β2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Arthritis Rheum 2002;46:2686–94. [DOI] [PubMed] [Google Scholar]
- 37. Charakida M, Besler C, Batuca JR. et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA 2009;302:1210–7. [DOI] [PubMed] [Google Scholar]
- 38. Passam FH, Rahgozar S, Qi M. et al. Beta 2 glycoprotein I is a substrate of thiol oxidoreductases. Blood 2010;116:1995–7. [DOI] [PubMed] [Google Scholar]
- 39. Fischetti F, Durigutto P, Pellis V. et al. Thrombus formation induced by antibodies to β2-glycoprotein I is complement dependent and requires a priming factor. Blood 2005;106:2340–6. [DOI] [PubMed] [Google Scholar]
- 40. Meroni PL, Borghi MO, Raschi E, Tedesco F.. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol 2011;7:330–9. [DOI] [PubMed] [Google Scholar]
- 41. Varga Z, Flammer AJ, Steiger P. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ackermann M, Verleden SE, Kuehnel M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amengual O, Atsumi T, Khamashta M, Hughes G.. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb Haemost 1998;79:276–81. [PubMed] [Google Scholar]
- 44. Edgington TS, Mackman N, Brand K, Ruf W.. The structural biology of expression and function of tissue factor. Thromb Haemost 1991;66:67–79. [PubMed] [Google Scholar]
- 45. Nemerson Y. Tissue factor and hemostasis. Blood 1988;71:1–8. [PubMed] [Google Scholar]
- 46. Vega-Ostertag M, Casper K, Swerlick R. et al. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum 2005;52:1545–54. [DOI] [PubMed] [Google Scholar]
- 47. Pierangeli S, Chen P, Raschi E. et al. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost 2008;34:236–50. [DOI] [PubMed] [Google Scholar]
- 48. Perez-Sanchez C, Ruiz-Limon P, Aguirre MA. et al. Mitochondrial dysfunction in antiphospholipid syndrome: implications in the pathogenesis of the disease and effects of coenzyme Q10 treatment. Blood 2012;119:5859–70. [DOI] [PubMed] [Google Scholar]
- 49. Alves JD, Grima B.. Oxidative stress in systemic lupus erythematosus and antiphospholipid syndrome: a gateway to atherosclerosis. Curr Rheumatol Rep 2003;5:383–90. [DOI] [PubMed] [Google Scholar]
- 50. Prinz N, Clemens N, Strand D. et al. Antiphospholipid antibodies induce translocation of TLR7 and TLR8 to the endosome in human monocytes and plasmacytoid dendritic cells. Blood 2011;118:2322–32. [DOI] [PubMed] [Google Scholar]
- 51. Espinola RG, Pierangeli SS, Ghara AE, Harris EN.. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb Haemost 2002;87:518–22. [PubMed] [Google Scholar]
- 52. Robbins DL, Leung S, Miller-Blair DJ, Ziboh V.. Effect of anticardiolipin/beta2-glycoprotein I complexes on production of thromboxane A2 by platelets from patients with the antiphospholipid syndrome. J Rheumatol 1998;25:51–6. [PubMed] [Google Scholar]
- 53. Hamberg M, Svensson J, Samuelsson B.. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA 1975;72:2994–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Laat B, Wu XX, Van Lummel M. et al. Correlation between antiphospholipid antibodies that recognize domain I of β2-glycoprotein I and a reduction in the anticoagulant activity of annexin A5. Blood 2007;109:1490–4. [DOI] [PubMed] [Google Scholar]
- 55. Huber R, Berendes R, Burger A, Luecke H, Karshikov A.. Annexin V-crystal structure and its implications on function. Behring Inst Mitt 1992;91:107–25. [PubMed] [Google Scholar]
- 56. Shin ML, Rus HG, Niculescu FI.. Membrane attack by complement. Assembly and biology of terminal complement complexes. Biomembranes: A Multi-Volume Treatise 1996;4:123–49. [Google Scholar]
- 57. Wetsel RA. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr Opin Immunol 1995;7:48–53. [DOI] [PubMed] [Google Scholar]
- 58. Kitchens MC. Thrombotic storm: when thrombosis begets thrombosis. Am J Med 1998;104:381–5. [DOI] [PubMed] [Google Scholar]
- 59. Ortel TL, Erkan D, Kitchens CS.. How I treat catastrophic thrombotic syndromes. Blood 2015;126:1285–93. [DOI] [PubMed] [Google Scholar]
- 60. Ortel TL, Kitchens CS, Erkan D. et al. Clinical causes and treatment of the thrombotic storm. Expert Rev Hematol 2012;5:653–9. [DOI] [PubMed] [Google Scholar]
- 61. Wright FL, Vogler TO, Moore EE. et al. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg 2020;231:193–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and Clinical Course of 1000 Patients with COVID-19 in New York: retrospective case series. BMJ 2020;369:m1996. [DOI] [PMC free article] [PubMed]
- 63. Hirsch JS, Ng JH, Ross DW. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020;98:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cao Y, Liu X, Xiong L, Cai K.. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol 2020;92:1449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Klok FA, Kruip MJHA, van der Meer NJM. et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020;191:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E.. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology 2020;296:E186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mao L, Wang M, Chen S. et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. Neurol 2020;77:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guo T, Fan Y, Chen M. et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shi S, Qin M, Shen B. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stefanini GG, Matteo M, Daniela T. et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation 2020;141:2113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 2020;34:e212–3. [DOI] [PubMed] [Google Scholar]
- 73. Tang K, Wang Y, Zhang H. et al. Cutaneous manifestations of the coronavirus disease 2019 (COVID-19): a brief review. Dermatol Ther 2020;33:e13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Galván Casas C, Català A, Carretero Hernández G. et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020;183:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sultan S, Altayar O, Siddique SM. et al. AGA Institute rapid review of the GI and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology 2020;159:320–34.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mao R, Qiu Y, He J-S. et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li Z, Xiaokai F, Danqing Z. et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation 2020;142:114–28. [DOI] [PubMed] [Google Scholar]
- 78. Nahum J, Morichau-Beauchant T, Daviaud F. et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19). JAMA Netw Open 2020;3:e2010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guan W, Ni Z, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Agmon-Levin N, Rosário C, Katz BSP. et al. Ferritin in the antiphospholipid syndrome and its catastrophic variant (cAPS). Lupus 2013;22:1327–35. [DOI] [PubMed] [Google Scholar]
- 82. Chen N, Zhou M, Dong X. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu C, Chen X, Cai Y. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gattinoni L, Chiumello D, Caironi P. et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020;46:1099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Creel-Bulos C, Hockstein M, Amin N. et al. Acute cor pulmonale in critically ill patients with Covid-19. N Engl J Med 2020;382:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Obi AT, Tignanelli CJ, Jacobs BN. et al. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord 2019;7:317–24. [DOI] [PubMed] [Google Scholar]
- 88. Oudkerk M, Büller HR, Kuijpers D. et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the national institute for public health of the Netherlands. Radiology 2020;297:E216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lang M, Som A, Mendoza DP. et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020;20:1365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fox SE, Akmatbekov A, Harbert JL. et al. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. The Lancet 2020;8:681–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lax SF, Skok K, Zechner P. et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med 2020;173:350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wichmann D, Sperhake J-P, Lütgehetmann M. et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020;173:1030. [DOI] [PubMed] [Google Scholar]
- 93. Schaller T, Hirschbühl K, Burkhardt K. et al. Postmortem examination of patients with COVID-19. JAMA 2020;323:2518–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xiang-hua Y, Le-min W, Ai-bin L. et al. Severe acute respiratory syndrome and venous thromboembolism in multiple organs. Am J Respir Crit Care Med 2010;182:436–7. [DOI] [PubMed] [Google Scholar]
- 95. Chong PY, Chui P, Ling AE. et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med 2004;128:195–204. [DOI] [PubMed] [Google Scholar]
- 96. Cheuk BLY, Cheung GCY, Cheng SWK.. Epidemiology of venous thromboembolism in a Chinese population. Br J Surg 2004;91:424–8. [DOI] [PubMed] [Google Scholar]
- 97. Zhou Y, Fu B, Zheng X. et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev 2020;nwaa041. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xu B, Fan C-Y, Wang A-L. et al. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect 2020;81:e51–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xu Z, Shi L, Wang Y. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Simonin L, Pasquier E, Leroyer C. et al. Lymphocyte disturbances in primary antiphospholipid syndrome and application to venous thromboembolism follow-up. Clin Rev Allergy Immunol 2017;53:14–27. [DOI] [PubMed] [Google Scholar]
- 101. Song M, Liu Y, Lu Z. et al. Prognostic factors for ARDS: clinical, physiological and atypical immunodeficiency. BMC Pulm Med 2020;20:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Risso K, Kumar G, Ticchioni M. et al. Early infectious acute respiratory distress syndrome is characterized by activation and proliferation of alveolar T-cells. Eur J Clin Microbiol Infect Dis 2015;34:1111–8. [DOI] [PubMed] [Google Scholar]
- 103. Amgalan A, Othman M.. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: unanswered questions. J Thromb Haemost 2020;18:1514–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lippi G, Plebani M, Henry BM.. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta 2020;506:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Henry BM, Santos de Oliveira MH, Benoit S, Plebani M, Lippi G.. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis Clin Chem Lab Med 2020;58:1021–8. [DOI] [PubMed] [Google Scholar]
- 106. Zhang L, Yan X, Fan Q. et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020;18:1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wright FL, Vogler TO, Moore EE. et al. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg 2020;231:193–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Espinosa G, Bucciarelli S, Cervera R. et al. Thrombotic microangiopathic haemolytic anaemia and antiphospholipid antibodies. Ann Rheum Dis 2004;63:730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Toniati P, Piva S, Cattalini M. et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y.. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med 2013;11:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ruddell RG, Hoang-Le D, Barwood JM. et al. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009;49:887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Torti FM, Torti SV.. Regulation of ferritin genes and protein. Blood 2002;99:3505–16. [DOI] [PubMed] [Google Scholar]
- 113. Bonow RO, Fonarow GC, O’Gara PT, Yancy CW.. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiology 2020;5:751–3. [DOI] [PubMed] [Google Scholar]
- 114. Turiel M, Muzzupappa S, Gottardi B. et al. Evaluation of cardiac abnormalities and embolic sources in primary antiphospholipid syndrome by transesophageal echocardiography. Lupus 2000;9:406–12. [DOI] [PubMed] [Google Scholar]
- 115. Gualtierotti R, Biggioggero M, Meroni PL.. Cutting-edge issues in coronary disease and the primary antiphospholipid syndrome. Clin Rev Allergy Immunol 2013;44:51–6. [DOI] [PubMed] [Google Scholar]
- 116. Adler Y, Finkelstein Y, Zandeman-Goddard G. et al. The presence of antiphospholipid antibodies in acute myocardial infarction. Lupus 1995;4:309–13. [DOI] [PubMed] [Google Scholar]
- 117. Ng JJ, Luo Y, Phua K, Choong AMTL.. Acute kidney injury in hospitalized patients with coronavirus disease 2019 (COVID-19): a meta-analysis. J Infect 2020;81:647–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Naicker S, Yang C-W, Hwang S-J. et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int 2020;97:824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020;98:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A. et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020; doi: 10.1101/2020.02.03.931766.
- 121. Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A. et al. Characterization of acute acro-ischemic lesions in non-hospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol 2020;83:e61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Di Mascio D, Khalil A, Saccone G. et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2:100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Baud D, Greub G, Favre G. et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020;323:2198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tang N, Bai H, Chen X. et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang J, Hajizadeh N, Moore EE. et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost 2020;18:1752–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu Y, Wang T, Guo C. et al. Plasminogen improves lung lesions and hypoxemia in patients with COVID-19. QJM 2020;113:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rodríguez-Pintó I, Espinosa G, Erkan D. et al. The effect of triple therapy on the mortality of catastrophic anti-phospholipid syndrome patients. Rheumatology 2018;57:1264–70. [DOI] [PubMed] [Google Scholar]
- 128. Horby P, Lim WS, Emberson J. et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020. https://www.nejm.org/doi/10.1056/NEJMoa2021436.
- 129. Shi H, Zhou C, He P. et al. Successful treatment of plasma exchange followed by intravenous immunogloblin in a critically ill patient with COVID-19. Int J Antimicrob Agents 2020;56:105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Busund R, Koukline V, Utrobin U, Nedashkovsky E.. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med 2002;28:1434–9. [DOI] [PubMed] [Google Scholar]
- 131. Keith P, Day M, Perkins L. et al. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care 2020;24:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kronbichler A, Frank R, Kirschfink M. et al. Efficacy of eculizumab in a patient with immunoadsorption- dependent catastrophic antiphospholipid syndrome: a case report. Med (Baltimore) 2014;93:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shapira I, Andrade D, Allen SL, Salmon JE.. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum 2012;64:2719–23. [DOI] [PubMed] [Google Scholar]
- 134. Zikos TA, Sokolove J, Ahuja N, Berube C.. Eculizumab induces sustained remission in a patient with refractory primary catastrophic antiphospholipid syndrome. J Clin Rheumatol 2015;21:311–3. [DOI] [PubMed] [Google Scholar]
- 135. Merad M, Martin JC.. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020;20:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kudaravalli J. Improvement in endothelial dysfunction in patients with systemic lupus erythematosus with N-acetylcysteine and atorvastatin. Indian J Pharmacol 2011;43:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Meroni PL, Raschi E, Testoni C. et al. Statins prevent endothelial cell activation induced by antiphospholipid (anti-β2-glycoprotein I) antibodies: effect on the proadhesive and proinflammatory phenotype. Arthritis Rheum 2001;44:2870–8. [DOI] [PubMed] [Google Scholar]
- 138. Ferrara DE, Liu X, Espinola RG. et al. Inhibition of the thrombogenic and inflammatory properties of antiphospholipid antibodies by fluvastatin in an in vivo animal model. Arthritis Rheum 2003;48:3272–9. [DOI] [PubMed] [Google Scholar]
- 139. Ferrara DE, Swerlick R, Casper K. et al. Fluvastatin inhibits up-regulation of tissue factor expression by antiphospholipid antibodies on endothelial cells. J Thromb Haemost 2004;2:1558–63. [DOI] [PubMed] [Google Scholar]
- 140. Martinez-Martinez L, Amigo MC, Orozco A.. Effect of rosuvastatin on VCAM-1 expression by HUVEC exposed to APS serum in an in vitro model. Clin Exp Rheumatol 2007;25:18–9.18021502 [Google Scholar]
- 141. López-Pedrera C, Ruiz-Limón P, Aguirre MÁ. et al. Global effects of fluvastatin on the prothrombotic status of patients with antiphospholipid syndrome. Ann Rheum Dis 2011;70:675–82. [DOI] [PubMed] [Google Scholar]
- 142. Zhang X-J, Qin J-J, Cheng X. et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab 2020;32:176–87.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Schmidt-Tanguy A, Voswinkel J, Henrion D. et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost 2013;11:1927–9. [DOI] [PubMed] [Google Scholar]
- 144. Wang M, Cao R, Zhang L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gautret P, Lagier J-C, Parola P. et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis 2020;34:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Molina JM, Delaugerre C, Le Goff J. et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect 2020;50:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Geleris J, Sun Y, Platt J. et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020;382:2411–8. [ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rosenberg ES, Dufort EM, Udo T. et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 2020;323:2493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cervera R, Espinosa G.. Update on the catastrophic antiphospholipid syndrome and the ‘CAPS Registry’. Semin Thromb Hemost 2012;38:333–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.