Abstract
Purpose
The aim of the study was to investigate factors which may predispose patients to early neurological deterioration (END) and explore peripheral biomarkers for the prediction of END in cardiogenic cerebral embolism (CCE) patients.
Methods
Patients diagnosed with CCE within 24 hr of onset between January 2017 and January 2019 were included in this study. END was defined as an increase of ≥2 on the National Institutes of Health Stroke Scale (NIHSS) or the emergence of new neurological symptoms within 3 days of admission. Binary logistic regression was used to investigate the factors associated with END. Receiver operating characteristic (ROC) curves were then generated to determine the predictive value of the potential biomarkers and the optimal cutoff values.
Results
Of the 129 (male, 55.81%; mean age 71.85 ± 11.99 years) CCE patients, 55 patients with END were identified. Hemorrhage transformation (HT), coronary heart disease (CHD), diastolic blood pressure, cystatin C levels, NIHSS score, and platelet‐to‐lymphocyte ratio (PLR) at admission were independently associated with END. A peripheral cystatin C level ≥ 1.41 mg/L and a PLR ≥ 132.97 were predictive factors for END in CCE patients. The lymphocyte‐to‐monocyte ratio (LMR) was negatively independently associated with HT, and LMR < 2.31 may predict the occurrence of HT in patients with CCE.
Conclusions
Of the potential predisposing factors considered, increased cystatin C and PLR were associated with END within 3 days of CCE, and a decreased LMR may have predictive value for HT in CCE patients.
Keywords: acute ischemic stroke, biomarker, cardiogenic cerebral embolism, early neurological deterioration, hemorrhage transformation
Early neurological deterioration (END) in the early phase of acute ischemic stroke (AIS) generally leads to a marked increase in poor prognosis due to diverse mechanisms. Cystatin C levels (cutoff value 1.41 mg/L, AUC 0.635, 95% CI 0.537–0.733, p = .009) and PLR (cutoff value 132.97, AUC 0.619, 95% CI 0.518–0.719, p = .02) were predictive of END, with high specificity and sensitivity. Hemorrhage transformation (HT) was independently associated with END, the lymphocyte‐to‐monocyte ratio (LMR) was negatively independently associated with HT, and LMR < 2.31 may predict the occurrence of HT in patients with cardiogenic cerebral embolism (CCE).
1. INTRODUCTION
Cardiogenic cerebral embolism (CCE) accounts for 15%–20% of ischemic strokes (IS). Atrial fibrillation (AF), especially nonvalvular atrial fibrillation (NVAF), is the most common cause of the embolism, accounting for about 50% of cerebral embolisms (Doufekias et al., 2008). Yamanouchi et al. found that 28% of IS in autopsied elderly patients were CCE, 56% of which were caused by NVAF (Yamanouchi et al., 1997). AF is a common arrhythmia, afflicting about 2% of the world's population. In recent years, the incidence of AF has risen. It is expected that by 2050, the prevalence of AF will rise more than 2.5 times, possibly due to an aging population (Miyasaka et al., 2006). The average age of AF patients has also increased steadily, currently ranging from 75 to 85 years (Lip, 2013; Palacio & Hart, 2002). The mechanism underlying cerebral embolism in patients with AF is complex. The main mechanism of pathogenesis involves a change in the structure and function of the left atrial appendage (LAA), which leads to blood stasis and induction of LAA thrombosis(Yamaji et al., 2002). The embolus then falls off and embolizes the intracranial artery. In CCE, the embolus is often large and causes sudden occlusion of the large and medium intracranial vessels (Hong et al., 2013).
In our previous studies, we have found that patients with CCE often have accompanying early neurological deterioration (END). END in the early phase of acute ischemic stroke (AIS) generally leads to a marked increase in poor prognosis due to diverse mechanisms (Alawneh et al., 2009). However, the predisposing factors associated with END in AIS patients have not yet been fully elucidated and no unified conclusions have been drawn. Previous studies have suggested that many factors may be associated with END, including systolic blood pressure, neurological functional deficits at admission, hyperglycemia, fibrinogen, and hemorrhage transformation (HT; Chung et al., 2015; Dziedzic, 2008; Seners et al., 2014). Nevertheless, studies on the predisposing factors for END in CCE patients are quite rare. Studies have shown that the incidence of HT in AIS is about 4%–7%, and HT is associated with END (Seet & Rabinstein, 2012; Seners et al., 2015). However, the incidence of and predisposing factors for HT in patients with CCE are also unclear. The aim of this study was (a) to investigate the incidence of and predisposing factors for END; (b) to identify the incidence and predisposing factors for HT; and (c) to examine possible peripheral biomarkers for the prediction of END and HT in CCE patients.
2. METHODS
2.1. Study population
From January 2017 to January 2019, 129 consecutive patients with CCE were enrolled in the study at the Department of Neurology and Emergency Department of Shengjing Hospital affiliated with China Medical University. The inclusion criteria were as follows: (a) acute onset, confirmed by cranial CT or MRI, with AIS, and in line with the diagnostic criteria for CCE: Patients may be caused by emboli in the heart. (1) At least one related cardiac source of emboli must be determined; (2) clinical and neuroimaging findings are similar to LAA ischemic stroke; and (3) previous transient ischemic attack (TIA) or stroke in more than one vascular area or evidence of systemic embolism supports the clinical diagnosis of cardiogenic stroke (Quan et al., 2020; Sacco et al., 2013; “Stroke‐‐1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders,” 1989); (b) history of AF or confirmed during admission according to an electrocardiogram or dynamic electrocardiogram; and (c) age > 18 years old. The exclusion criteria were as follows: (a) patients with a severe consciousness disorder (Glasgow Coma Scale < 9) who could not cooperate with the examination; (b) echocardiography suggesting valvular heart disease or congenital heart disease; or (c) other serious systemic diseases such as infection, heart and lung failure, malignant tumor, or liver and kidney dysfunction. Ethical approval was obtained from the Shengjing hospital of China Medical University Ethics Committee before beginning the study.
2.2. Data collection
Demographic variables, traditional risk factors, and routine laboratory test results were collected using the hospital's electronic medical record system. All patients underwent MRI within 48 hr of admission. MRI examinations were performed with an Achieva 3.0 Tesla scanner (Philips Healthcare). The MRI protocol included T2‐weighted imaging (T2WI), T1‐weighted imaging (T1WI), fluid‐attenuated inversion recovery imaging (FLAIR), diffusion‐weighted imaging (DWI), and time‐of‐flight MRA. HT was defined as the presence of intracranial hemorrhage in the initial ischemic region, confirmed by CT or MRI (Hacke et al., 1998). The abnormal area of DWI was manually delineated on the MR sequence. In order to calculate the total DWI lesion volume of each patient, the DWI abnormal area was added and multiplied by the section thickness (mm) and the intersection gap (mm). Two neurologists, who were blinded to the clinical data, interpreted all the neuroimaging studies and reached consensus.
All patients in our study had a history of AF or were recently diagnosed with AF after admission. The existence of a history of AF indicated that the patient was clearly diagnosed by a cardiologist and had been received medication or ablation of AF before hospitalization prior to hospitalization in this study. The recent diagnosis of AF indicated that patients with no history of AF were diagnosed by a neurologist at admission based on electrocardiogram or Holter electrocardiogram (Kirchhof et al., 2016).
2.3. Definition of END
Stroke severity was evaluated according to the NIHSS upon admission and at 24 hr postadmission, and both assessments were performed by the same neurologist (Lee et al., 2017). END was defined as follows: (a) an increase of more than 2 points in the total NIHSS score compared to the score at admission; (b) an increase of more than 1 point in the NIHSS specificity subitems, namely level of consciousness (1a–1c) or motor capacity (5a–6b); or (c) new neurological deficits despite no change in the NIHSS score (Seners et al., 2014).
2.4. Treatment
According to the guidelines of the European Heart Association and the European Stroke Society in 2016, anticoagulant therapy was initiated 3 days after stroke onset, and anticoagulant therapy was initiated 2 weeks after onset in patients with severe stroke and a high risk of HT (Kirchhof et al., 2017). Warfarin was given at a dose of 2.5 mg/day, and prothrombin time (PT) and international normalized ratio (INR) were reexamined after 3 days of anticoagulation therapy. The warfarin dosage was then adjusted according to the INR results and was increased or decreased 0.625–1.25 mg each time until the INR was controlled at 2–3 (Tse et al., 2013).
2.5. Statistical analysis
The Mann–Whitney U test or Student's t test was used to compare non‐normally or normally distributed variables, respectively. Continuous variables are presented as mean ± standard deviation if normally distributed; otherwise, the median interquartile range (IQR) is presented. The categorical variables are expressed in terms of frequency and percentage, and the data were compared using a chi‐square test. The independent variables were analyzed by logistic regression analysis. The sensitivity and specificity of significant variables and the optimal cutoff values for predicting END and HT in CCE patients were determined by receiver operating characteristic (ROC) curves. Statistical analysis was performed using SPSS 22.0 software (SPSS Inc.). For all the statistics, p values below .05 were considered significant.
3. RESULTS
3.1. Sample characteristics
In total, 129 patients met the inclusion criteria and were included in this study. The patients had a mean age of 71.85 ± 11.99 years, and 55.81% were male. None of the enrolled patients received intravenous tPA (IVT) and endovascular treatment (EVT). The baseline clinical data and laboratory results are shown in Table 1.
TABLE 1.
Demographic, clinical, and laboratory data in cardiogenic embolism patients with and without END
| All (n = 129) | With END (n = 55) | Without END (n = 74) | p value | |
|---|---|---|---|---|
| Man, n (%) | 72 (55.81) | 26 (47.27) | 46 (62.16) | .092 |
| Age, (years, mean ± SD) | 71.85 ± 11.99 | 74.96 ± 12.30 | 69.53 ± 11.28 | .01 |
| Smoking, n (%) | 47 (36.43) | 16 (29.09) | 31 (41.89) | .135 |
| Alcohol drinking, n (%) | 41 (31.78) | 15 (27.27) | 26 (35.14) | .343 |
| Hypertension, n (%) | 83 (64.34) | 41 (74.55) | 42 (56.76) | .037 |
| Diabetes mellitus, n (%) | 35 (27.13) | 9 (16.36) | 26 (35.14) | .018 |
| Hyperlipemia, n (%) | 29 (22.48) | 15 (27.27) | 14 (18.92) | .261 |
| CHD, n (%) | 27 (20.93) | 20 (36.36) | 7 (9.46) | <.001 |
| History of stroke, n (%) | 32 (24.81) | 21 (38.18) | 11 (14.86) | .002 |
| HT, n (%) | 37 (28.68) | 23 (41.82) | 14 (18.92) | .004 |
| Epilepsy, n (%) | 4 (3.1) | 4 (7.27) | 0 (0) | .018 |
| NIHSS at admission | 6.90 ± 6.24 | 9.58 ± 6.47 | 4.91 ± 5.27 | <.001 |
| Systolic blood pressure (mmHg) | 146.39 ± 23.40 | 150.25 ± 23.75 | 143.51 ± 22.88 | .106 |
| Diastolic blood pressure (mmHg) | 86.47 ± 13.59 | 89.73 ± 12.57 | 84.05 ± 13.89 | .018 |
| Infarct volume (mm3) | 39.45 ± 77.80 | 50.15 ± 81.07 | 31.59 ± 74.90 | .189 |
| FBG (mmol/L) | 7.04 ± 4.35 | 7.34 ± 5.53 | 6.82 ± 3.23 | .504 |
| Cystatin C (mg/L) | 1.42 ± 0.62 | 1.61 ± 0.78 | 1.29 ± 0.41 | .003 |
| Urea (mmol/L) | 5.64 ± 2.58 | 5.99 ± 3.14 | 5.39 ± 2.06 | .194 |
| Creatinine (umol/L) | 81.20 ± 38.72 | 90.66 ± 53.09 | 74.16 ± 20.63 | .016 |
| Triglyceride (mmol/L) | 1.05 ± 0.51 | 0.96 ± 0.51 | 1.12 ± 0.50 | .069 |
| Total cholesterol (mmol/L) | 4.17 ± 0.99 | 3.98 ± 0.97 | 4.31 ± 0.99 | .065 |
| HDL (mmol/L) | 1.18 ± 0.36 | 1.15 ± 0.28 | 1.20 ± 0.40 | .370 |
| LDL (mmol/L) | 2.64 ± 0.93 | 2.52 ± 0.96 | 2.73 ± 0.90 | .192 |
| Hcy | 19.0 ± 7.47 | 19.86 ± 8.49 | 18.36 ± 6.59 | .262 |
| HbAlc (%) | 6.35 ± 1.64 | 6.32 ± 1.58 | 6.36 ± 1.70 | .891 |
| White blood cell count (109/L) | 8.59 ± 4.15 | 9.26 ± 5.25 | 8.10 ± 3.05 | .116 |
| Neutrophil count (109/L) | 6.18 ± 3.99 | 7.11 ± 4.99 | 5.49 ± 2.90 | .022 |
| Lymphocyte count (109/L) | 1.52 ± 0.63 | 1.36 ± 0.61 | 1.65 ± 0.62 | .008 |
| MCV | 94.24 ± 5.62 | 93.76 ± 4.56 | 94.59 ± 6.30 | .412 |
| MCH | 30.85 ± 2.57 | 30.57 ± 2.78 | 31.06 ± 2.39 | .286 |
| RDW | 14.03 ± 1.46 | 14.37 ± 1.66 | 13.77 ± 1.25 | .021 |
| Monocyte count (109/L) | 0.61 ± 0.30 | 0.64 ± 0.37 | 0.59 ± 0.24 | .280 |
| Platelet count (1012/L) | 187.48 ± 91.31 | 201.56 ± 129.06 | 177.01 ± 45.12 | .132 |
| Mean platelet volume | 10.41 ± 3.10 | 10.16 ± 3.04 | 10.59 ± 3.15 | .434 |
| Lymphocyte‐to‐monocyte ratio | 2.82 ± 1.44 | 2.40 ± 1.27 | 3.13 ± 1.50 | .004 |
| Neutrophil‐to‐lymphocyte ratio | 5.30 ± 4.89 | 6.83 ± 5.81 | 4.16 ± 3.73 | .002 |
| Platelet‐to‐lymphocyte ratio | 153.92 ± 126.15 | 193.16 ± 171.76 | 124.75 ± 63.70 | .002 |
Figures in parentheses are percentages, unless indicated otherwise
Abbreviations: CHD, coronary heart disease; Cys, C cystatin C; END, early neurological deterioration; FBG, fasting blood glucose; HbAlc, glycosylated hemoglobin; Hcy, homocysteine; HDL, high density lipoprotein; HT, hemorrhagic transformation; LDL, Low density lipoprotein; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; NIHSS, National institute of Health Stroke Scale; RDW, red cell distribution width.
3.2. Demographic, clinical, and laboratory data in cardiogenic embolism patients with and without END
Among the patients, 55 (42.64%) showed END within 72 hr of onset. We found that the patients with END were older (74.96 ± 12.30 vs. 69.53 ± 11.28; p = .01) and had a higher incidence of hypertension (74.55% vs. 56.76%; p = .037), a lower incidence of diabetes mellitus (16.36% vs. 35.14%; p = .018), a higher incidence of CHD (36.36% vs. 9.46%; p < .001), a higher incidence of stroke history (38.18% vs. 14.86%; p = .002), a higher incidence of HT (41.82% vs. 18.92%; p = .004), a higher incidence of epilepsy (7.27% vs. 0; p = .018), a higher NIHSS score at admission (9.58 ± 6.47 vs. 4.91 ± 5.27; p < .001), a higher diastolic blood pressure at admission (89.73 ± 12.57 vs. 84.05 ± 13.89; p = .018), a higher level of cystatin C (1.61 ± 0.78 vs. 1.29 ± 0.41; p = .003), a higher level of creatinine (90.66 ± 53.09 vs. 74.16 ± 20.63; p = .016), a higher neutrophil count (7.11 ± 4.99 vs. 5.49 ± 2.90; p = .022), a lower lymphocyte count (1.36 ± 0.61 vs. 1.65 ± 0.62; p = .008), a higher red cell distribution width (14.37 ± 1.66 vs. 13.77 ± 1.25; p = .021), a lower lymphocyte‐to‐monocyte ratio (LMR, 2.40 ± 1.27 vs. 3.13 ± 1.50; p = .004), a higher neutrophil‐to‐lymphocyte ratio (NLR, 6.83 ± 5.81 vs. 4.16 ± 3.73; p = .002), and a higher platelet‐to‐lymphocyte ratio (PLR, 193.16 ± 171.76 vs. 124.75 ± 63.70; p = .002) (Table 1).
Binary regression analysis was used to investigate the independent factors associated with END, and we found that cystatin C (OR 3.92, 95% CI 1.41–10.86, p = .009), HT (OR 4.87, 95% CI 1.60–14.85, p = .005), CHD (OR 10.37, 95% CI 2.48–43.31, p = .001), diastolic blood pressure at admission (OR 1.05, 95% CI 1.01–1.09, p = .007), NIHSS at admission (OR 1.05, 95% CI 1.01–1.09, p < .001), and PLR (OR 1.01, 95% CI 1.00–1.02, p = .006) were independently associated with END in CCE patients (Table 2).
TABLE 2.
Logistic regression analysis of factors associated with END
| OR | 95% CI | p value | |
|---|---|---|---|
| Cystatin C (mg/L) | 3.92 | 1.41–10.86 | .009 |
| Hemorrhage transformation | 4.87 | 1.60–14.85 | .005 |
| CHD | 10.37 | 2.48–43.31 | .001 |
| Diastolic pressure at admission | 1.05 | 1.01–1.09 | .007 |
| NIHSS at admission | 1.20 | 1.09–1.33 | <.001 |
| Platelet‐to‐lymphocyte ratio | 1.01 | 1.00–1.02 | .006 |
Abbreviations: CHD, coronary heart disease; Cys C, cystatin C; END, early neurological deterioration; NIHSS, National institute of Health Stroke Scale.
Next, ROC curve analysis was used to explore the potential value of peripheral biomarkers for the prediction of END. We found that cystatin C levels could predict END with a specificity of 75.7% and a sensitivity of 49.1% (cutoff value 1.41 mg/L, AUC 0.635, 95% CI 0.537–0.733, p = .009). PLR was also predictive of END, with a specificity of 73.0% and a sensitivity of 56.4% (cutoff value 132.97, AUC 0.619, 95% CI 0.518–0.719, p = .02) (Figure 1).
FIGURE 1.
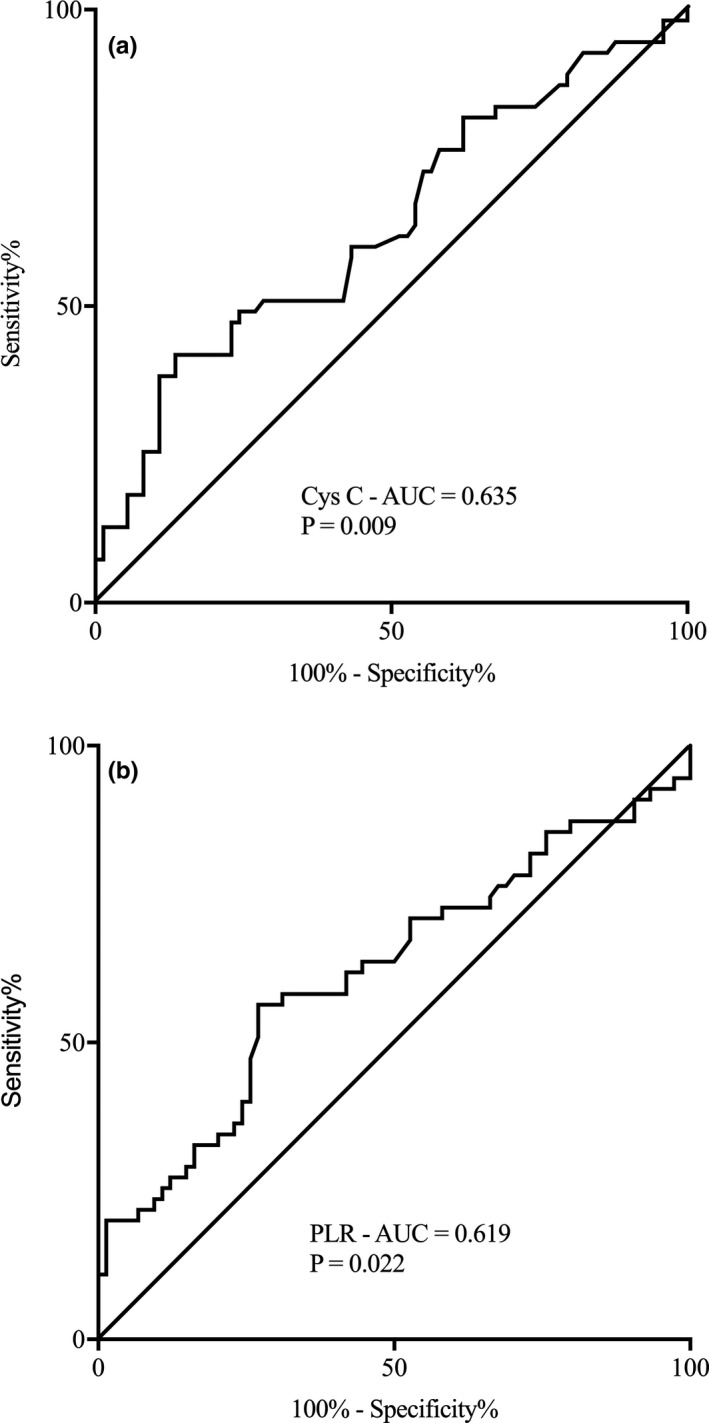
Receiver operating characteristic (ROC) curves for serum Cys C (a) and peripheral PLR (b) Cys C, cystatin C; AUC, area under the curve; END, early neurological deterioration; PLR, platelet‐to‐lymphocyte ratio; CCE, cardiogenic cerebral embolism
3.3. Demographic, clinical, and laboratory data in cardiogenic embolism patients with and without HT
Since HT is related to END in CCE patients, we determined which factors were associated with HT. We found that 37 (28.68%) patients had HT, and patients with HT had higher levels of cystatin C (1.60 ± 0.90 vs. 1.35 ± 0.45; p = .038), higher white blood cell counts (9.93 ± 5.50 vs. 5.61 ± 3.21; p = .010), higher neutrophil counts (7.60 ± 5.28 vs. 5.61 ± 3.21; p = .010), higher monocyte counts (0.71 ± 0.42 vs. 0.57 ± 0.23; p = .020), lower LMRs (2.35 ± 1.27 vs. 3.01 ± 1.47; p = .018), and higher NLRs (6.86 ± 5.76 vs. 4.67 ± 4.38; p = .021) (Table 3).
TABLE 3.
Demographic, clinical, and laboratory data in cardiogenic embolism patients with and without HT
| With HT (n = 37) | Without HT (n = 92) | p value | |
|---|---|---|---|
| Man, n (%) | 19 (51.35) | 53 (57.61) | .517 |
| Age, (years, mean ± SD) | 69.81 ± 13.57 | 72.66 ± 11.27 | .223 |
| Smoking, n (%) | 11 (29.73) | 36 (39.13) | .316 |
| Alcohol drinking, n (%) | 13 (35.14) | 28 (30.43) | .604 |
| Hypertension, n (%) | 25 (67.57) | 58 (63.04) | .628 |
| Diabetes mellitus, n (%) | 9 (24.32) | 26 (28.26) | .649 |
| Hyperlipemia, n (%) | 6 (16.22) | 23 (25.0) | .28 |
| CHD, n (%) | 8 (21.62) | 19 (20.65) | .903 |
| History of stroke, n (%) | 8 (21.62) | 24 (26.09) | .595 |
| Epilepsy, n (%) | 1 (2.70) | 3 (3.26) | .869 |
| NIHSS at admission | 8.24 ± 6.27 | 6.34 ± 6.18 | .121 |
| Systolic pressure (mmHg) | 149.27 ± 21.48 | 145.23 ± 24.15 | .377 |
| Diastolic pressure (mmHg) | 87.08 ± 12.73 | 86.23 ± 13.98 | .749 |
| Infarct volume | 59.92 ± 78.39 | 30.83 ± 76.36 | .056 |
| FBG (mmol/L) | 7.11 ± 6.21 | 7.02 ± 3.37 | .910 |
| Cystatin C (mg/L) | 1.60 ± 0.90 | 1.35 ± 0.45 | .038 |
| Urea (mmol/L) | 5.49 ± 3.32 | 5.71 ± 2.24 | .675 |
| Creatinine (umol/L) | 90.90 ± 58.40 | 77.30 ± 26.56 | .071 |
| Triglyceride (mmol/L) | 1.05 ± 0.55 | 1.05 ± 0.49 | .998 |
| Total cholesterol (mmol/L) | 4.09 ± 1.04 | 4.20 ± 0.98 | .541 |
| HDL (mmol/L) | 1.22 ± 0.39 | 1.16 ± 0.34 | .413 |
| LDL (mmol/L) | 2.58 ± 1.06 | 2.66 ± 0.87 | .659 |
| Hcy | 19.38 ± 8.06 | 18.85 ± 7.26 | .717 |
| HbAlc (%) | 6.58 ± 2.24 | 6.25 ± 1.33 | .297 |
| White blood cell count (109/L) | 9.93 ± 5.50 | 8.05 ± 3.36 | .020 |
| Neutrophil count (109/L) | 7.60 ± 5.28 | 5.61 ± 3.21 | .010 |
| Lymphocyte count (109/L) | 1.43 ± 0.65 | 1.56 ± 0.62 | .287 |
| MCV | 94.97 ± 4.38 | 93.94 ± 6.04 | .347 |
| MCH | 30.94 ± 3.21 | 30.82 ± 2.28 | .802 |
| RDW | 14.24 ± 1.57 | 13.94 ± 1.41 | .297 |
| Monocyte count (109/L) | 0.71 ± 0.42 | 0.57 ± 0.23 | .020 |
| Platelet count (1012/L) | 192.27 ± 115.16 | 185.55 ± 80.42 | .707 |
| Mean platelet volume | 10.58 ± 3.17 | 10.34 ± 3.08 | .687 |
| Lymphocyte‐to‐monocyte ratio | 2.35 ± 1.27 | 3.01 ± 1.47 | .018 |
| Neutrophil‐to‐lymphocyte ratio | 6.86 ± 5.76 | 4.67 ± 4.38 | .021 |
| Platelet‐to‐lymphocyte ratio | 172.41 ± 153.40 | 146.48 ± 113.49 | .293 |
Figures in parentheses are percentages, unless indicated otherwise.
Abbreviations: CHD, coronary heart disease; Cys C, cystatin C; END, early neurological deterioration; FBG, fasting blood glucose; HbAlc, glycosylated hemoglobin; Hcy, homocysteine; HDL, high density lipoprotein; HT, hemorrhagic transformation; LDL, Low density lipoprotein; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; NIHSS, National institute of Health Stroke Scale; RDW, red cell distribution width.
Logistic regression showed that LMR (OR 0.69, 95% CI 0.49–0.95, p = .022) was negatively independently associated with HT in the CCE patients (table not shown). Moreover, LMR could predict HT with a specificity of 62.2% and a sensitivity of 63.0% (cutoff value 2.31, AUC 0.640, 95% CI 0.533–0.747, p = .013) (Figure 2).
FIGURE 2.
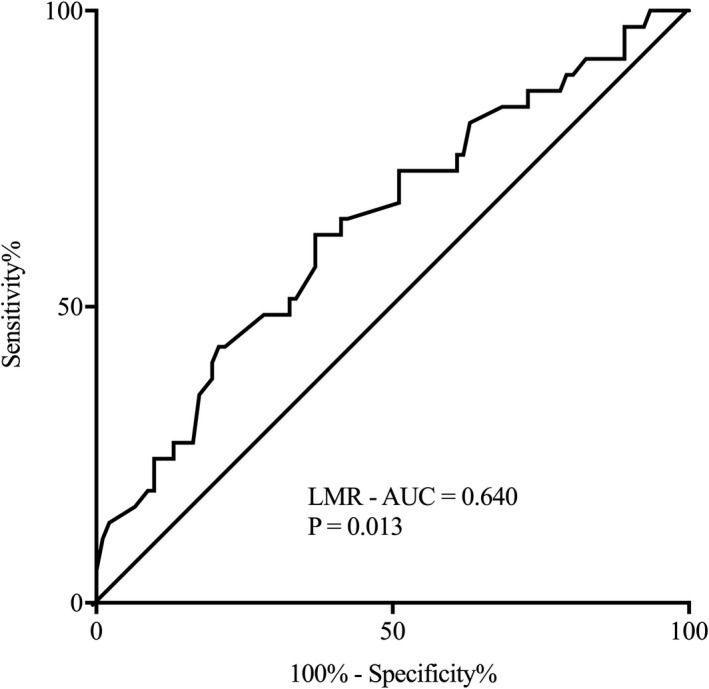
Receiver operating characteristic (ROC) curve for peripheral LMR. AUC, area under the curve; LMR, lymphocyte‐to‐monocyte ratio; CCE, cardiogenic cerebral embolism
4. DISCUSSION
In this study, we first determined the incidence of END in CCE patients and found that HT, CHD, diastolic blood pressure, NIHSS scores, cystatin C levels, and PLR at admission were independently associated with END. Furthermore, we found that cystatin C levels and PLR had value in predicting END. We also investigated factors related to HT in CCE patients and confirmed that LMR was negatively associated with HT and could also predict HT in CCE patients.
A large number of studies have confirmed that END is an independent risk factor for poor prognosis in stroke patients (Hui et al., 2018; Simonsen et al., 2016; You et al., 2019). Consequently, predictive factors for END are also of great interest. It has been reported that large artery atherosclerosis (Kim et al., 2016), initial glycemic variability (Hui et al., 2018), uric acid levels (Huang et al., 2018), mean platelet volumes (Oji et al., 2018), and fibrinogen levels (Lee et al., 2017) are associated with END. However, no unified conclusions regarding these risk factors have been reached, and no studies have been conducted on the predictive factors of END in CCE patients. Kim et al. recently reported that HT is an independent risk factor for END in AIS patients receiving endovascular thrombectomy (Kim et al., 2019). Gill et al. also found that severe HT was independently associated with worsening NIHSS scores in AIS patients receiving intravenous thrombolysis (Gill et al., 2016). These conclusions are consistent with our results. HT is independently associated with END, and the mechanism behind this correlation is complex. Usually, HT occurs in large artery atherosclerosis patients. Reperfusion injury may play a major role, and severe brain edema, damage to the blood–brain barrier, and excessive release of neurotoxic inflammatory mediators after HT may also be involved (Jeon et al., 2014; Juttler et al., 2014; Su et al., 2016). These patients also have higher NIHSS scores, which are consistent with our results. The incidence of AF in patients with CHD is significantly higher than that in healthy people, and CHD usually indicates more severe arteriosclerosis and is associated with poor prognosis in patients with AIS. Our results suggest that CHD is associated with END in patients with CCE (Drakopoulou et al., 2019; Ferrari & Fox, 2016; Wang et al., 2019). Blood pressure (BP) may contribute to the development of END and to poor outcomes by affecting cerebral perfusion (Toni et al., 1998). Other studies have also shown the importance of BP for prognosis after IS (Geeganage et al., 2011; Ko et al., 2010). Our study showed that increased diastolic blood pressure was associated with END in CCE patients, which is consistent with previous studies. Several studies have shown that cystatin C is associated with IS and could be considered a risk factor for AIS (Wang, Li, et al., 2019). Additionally, cystatin C is also independently associated with shorter survival in IS patients (Winovich et al., 2017). Kim et al. showed that cystatin C was a useful predictor of END in elderly patients with AIS (Kim et al., 2017). Our study confirmed that cystatin C is not only independently associated with END, but that cystatin C levels ≥ 1.41 mg/L can predict END in CCE patients. The PLR has been used as a convenient novel biomarker indicating inflammation, thrombosis, and plaque instability (Turkmen et al., 2013). Sung et al. reported that the PLR correlated with the severity of neurological impairment in patients after AIS (Sung et al., 2019). In addition, Zhang et al. recently reported that PLR could predict the severity of AIS and poor 30‐day outcomes (Zhang et al., 2019). In the present study, we further confirmed that PLR was independently related to END and identified the threshold value (PLR ≥ 132.97). PLR had a specificity of 73.0% and a sensitivity of 56.4% for predicting END. For END was a composite outcome that includes initial NIHSS, it was speculated he NIHSS was associated with END, and we did obtain that NIHSS was independently related to END at admission, which was consistent with the conclusion of Boulenoir et al. (2020).
As HT has not been reported to be associated with END in CCE patients, we further analyzed factors related to HT in this study. We found that LMR was negatively independently associated with HT, and LMR < 2.31 had a 63.0% sensitivity and 62.2% specificity for predicting HT. Ren et al. found that LMR on admission possessed good predictive value for AIS prognosis and that a lower LMR is closely related to AIS severity and poor prognosis (Ren et al., 2017). Park et al. reported that lower LMR on day 7 of AIS was associated with poorer prognosis at 3 months after stroke onset. LMR may be a useful biomarker for evaluating stroke‐induced immunosuppression (Park et al., 2018). Therefore, it is possible that decreased LMR may also suggest a poststroke inflammatory reaction in HT. Furthermore, this reaction may be involved in infarct evolution and may worsen the clinical outcome of patients with AIS.
The present study has a few limitations. First, it is a single‐center, retrospective study, and the sample size was small. Second, the PLR and LMR are dynamic indicators. We only recorded PLR and LMR at admission and did not remeasure them during the period of hospitalization. Third, we focused only on relatively early deterioration, and no long‐term follow‐up was conducted. Moreover, the sensitivity and specificity of cystatin C, PLR, or LMR are relatively low with all AUC less than 0.7, so their predictive values are of limited.
5. CONCLUSIONS
To the best of our knowledge, this is the first study to show that HT, CHD, diastolic blood pressure, cystatin C levels, NIHSS, and PLR at admission are independently associated with END. Furthermore, peripheral biomarkers, such as cystatin C and PLR, were predictive of END in CCE patients. LMR had a negative independent association with HT, and reduced LMR may have predictive value for HT. However, further studies with larger cohorts are needed to verify our findings.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
Lin Cong collected clinical data, performed follow‐up of patients, completed statistical analysis, and wrote the manuscript. Weining Ma designed the study and contributed to editing the manuscript. All authors read and approved the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.1985.
Cong L, Ma W. Early neurological deterioration in cardiogenic cerebral embolism due to nonvalvular atrial fibrillation: Predisposing factors and clinical implications. Brain Behav.2021;11:e01985 10.1002/brb3.1985
DATA AVAILABILITY STATEMENT
The data of our study will be available via connecting with Dr. Weining Ma (corresponding author).
REFERENCES
- Alawneh, J. A. , Moustafa, R. R. , & Baron, J. C. (2009). Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke, 40(6), 443–450. 10.1161/strokeaha.108.532465 [DOI] [PubMed] [Google Scholar]
- Boulenoir, N. , Turc, G. , Henon, H. , Laksiri, N. , Mounier‐Véhier, F. , Girard Buttaz, I. , Duong, D.‐L. , Papassin, J. , Yger, M. , Triquenot, A. , Lyoubi, A. , Ter Schiphorst, A. , Denier, C. , Baron, J.‐C. , Seners, P. , Ben Hassen, W. , Lapergue, B. , Lamy, C. , Lucas, L. , … Masson, M. (2020). Early neurological deterioration following thrombolysis for minor stroke with isolated internal carotid artery occlusion. European Journal of Neurology, 9, Advance online publication. 10.1111/ene.14541. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Chung, J.‐W. , Kim, N. , Kang, J. , Park, S. H. , Kim, W.‐J. , Ko, Y. , Park, J. H. , Lee, J. S. , Lee, J. , Yang, M. H. , Jang, M. S. , Oh, C. W. , Kwon, O.‐K. , Jung, C. K. , Kim, B. J. , Han, M.‐K. , Gorelick, P. B. , & Bae, H.‐J. (2015). Blood pressure variability and the development of early neurological deterioration following acute ischemic stroke. Journal of Hypertension, 33(10), 2099–2106. 10.1097/hjh.0000000000000675 [DOI] [PubMed] [Google Scholar]
- Doufekias, E. , Segal, A. Z. , & Kizer, J. R. (2008). Cardiogenic and aortogenic brain embolism. Journal of the American College of Cardiology, 51(11), 1049–1059. 10.1016/j.jacc.2007.11.053 [DOI] [PubMed] [Google Scholar]
- Drakopoulou, M. , Oikonomou, G. , Soulaidopoulos, S. , Toutouzas, K. , & Tousoulis, D. (2019). Management of patients with concomitant coronary and carotid artery disease. Expert Review of Cardiovascular Therapy, 17(8), 575–583. 10.1080/14779072.2019.1642106 [DOI] [PubMed] [Google Scholar]
- Dziedzic, T. (2008). Clinical significance of acute phase reaction in stroke patients. Frontiers in Bioscience, 13, 2922–2927. [DOI] [PubMed] [Google Scholar]
- Ferrari, R. , & Fox, K. (2016). Heart rate reduction in coronary artery disease and heart failure. Nature Reviews Cardiology, 13(8), 493–501. 10.1038/nrcardio.2016.84 [DOI] [PubMed] [Google Scholar]
- Geeganage, C. , Tracy, M. , England, T. , Sare, G. , Moulin, T. , Woimant, F. , Christensen, H. , De Deyn, P. P. , Leys, D. , O'Neill, D. , Ringelstein, E. B. , & Bath, P. M. W. (2011). Relationship between baseline blood pressure parameters (including mean pressure, pulse pressure, and variability) and early outcome after stroke: Data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST). Stroke, 42(2), 491–493. 10.1161/strokeaha.110.596163 [DOI] [PubMed] [Google Scholar]
- Gill, D. , Baheerathan, A. , Aravind, A. , Veltkamp, R. , & Kar, A. (2016). Severe hemorrhagic transformation after thrombolysis for acute ischemic stroke prevents early neurological improvement. Journal of Stroke and Cerebrovascular Diseases, 25(9), 2232–2236. 10.1016/j.jstrokecerebrovasdis.2016.04.020 [DOI] [PubMed] [Google Scholar]
- Hacke, W. , Kaste, M. , Fieschi, C. , von Kummer, R. , Davalos, A. , Meier, D. , Larrue, V. , Bluhmki, E. , Davis, S. , Donnan, G. , Schneider, D. , Diez‐Tejedor, E. , & Trouillas, P. (1998). Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet, 352(9136), 1245–1251. 10.1016/s0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- Hong, K.‐S. , Lee, J. , Bae, H.‐J. , Lee, J. S. , Kang, D.‐W. , Yu, K.‐H. , Han, M.‐K. , Cho, Y.‐J. , Song, P. , Park, J.‐M. , Oh, M.‐S. , Koo, J. , & Lee, B.‐C. (2013). Greater stroke severity predominates over all other factors for the worse outcome of cardioembolic stroke. Journal of Stroke and Cerebrovascular Diseases, 22(8), 373–380. 10.1016/j.jstrokecerebrovasdis.2013.04.008 [DOI] [PubMed] [Google Scholar]
- Huang, Z. X. , Wang, Q. Z. , Dai, Y. Y. , Lu, H. K. , Liang, X. Y. , Hu, H. , & Liu, X. T. (2018). Early neurological deterioration in acute ischemic stroke: A propensity score analysis. Journal of the Chinese Medical Association, 81(10), 865–870. 10.1016/j.jcma.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Hui, J. , Zhang, J. , Mao, X. , Li, Z. , Li, X. , Wang, F. , Wang, T. , Yuan, Q. , Wang, S. , Pu, M. , & Xi, G. (2018). The initial glycemic variability is associated with early neurological deterioration in diabetic patients with acute ischemic stroke. Neurological Sciences, 39(9), 1571–1577. 10.1007/s10072-018-3463-6 [DOI] [PubMed] [Google Scholar]
- Jeon, S. B. , Koh, Y. , Choi, H. A. , & Lee, K. (2014). Critical care for patients with massive ischemic stroke. Journal of Stroke, 16(3), 146–160. 10.5853/jos.2014.16.3.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüttler, E. , Unterberg, A. , Woitzik, J. , Bösel, J. , Amiri, H. , Sakowitz, O. W. , Gondan, M. , Schiller, P. , Limprecht, R. , Luntz, S. , Schneider, H. , Pinzer, T. , Hobohm, C. , Meixensberger, J. , & Hacke, W. (2014). Hemicraniectomy in older patients with extensive middle‐cerebral‐artery stroke. New England Journal of Medicine, 370(12), 1091–1100. 10.1056/NEJMoa1311367 [DOI] [PubMed] [Google Scholar]
- Kim, J.‐M. , Bae, J.‐H. , Park, K.‐Y. , Lee, W. J. , Byun, J. S. , Ahn, S.‐W. , Shin, H.‐W. , Han, S.‐H. , & Yoo, I.‐H. (2019). Incidence and mechanism of early neurological deterioration after endovascular thrombectomy. Journal of Neurology, 266(3), 609–615. 10.1007/s00415-018-09173-0 [DOI] [PubMed] [Google Scholar]
- Kim, J. M. , Moon, J. , Ahn, S. W. , Shin, H. W. , Jung, K. H. , & Park, K. Y. (2016). The etiologies of early neurological deterioration after thrombolysis and risk factors of ischemia progression. Journal of Stroke and Cerebrovascular Diseases, 25(2), 383–388. 10.1016/j.jstrokecerebrovasdis.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Kim, T. J. , Kang, M. K. , Jeong, H.‐G. , Kim, C. K. , Kim, Y. , Nam, K.‐W. , Mo, H. , An, S. J. , Ko, S.‐B. , & Yoon, B.‐W. (2017). Cystatin C is a useful predictor of early neurological deterioration following ischaemic stroke in elderly patients with normal renal function. European Stroke Journal, 2(1), 23–30. 10.1177/2396987316677197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof, P. , Benussi, S. , Kotecha, D. , Ahlsson, A. , Atar, D. , Casadei, B. , Castella, M. , Diener, H.‐C. , Heidbuchel, H. , Hendriks, J. , Hindricks, G. , Manolis, A. S. , Oldgren, J. , Popescu, B. A. , Schotten, U. , Van Putte, B. , Vardas, P. , Agewall, S. , Camm, J. , … Zeppenfeld, K. (2016). 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal, 37(38), 2893–2962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- Kirchhof, P. , Benussi, S. , Kotecha, D. , Ahlsson, A. , Atar, D. , Casadei, B. , Castellá, M. , Diener, H.‐C. , Heidbuchel, H. , Hendriks, J. , Hindricks, G. , Manolis, A. S. , Oldgren, J. , Alexandru Popescu, B. , Schotten, U. , Van Putte, B. , & Vardas, P. (2017). 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Revista Española de Cardiología (English Edition), 70(1), 50 10.1016/j.rec.2016.11.033 [DOI] [PubMed] [Google Scholar]
- Ko, Y. , Park, J. H. , Yang, M. H. , Ko, S.‐B. , Han, M.‐K. , Oh, C. W. , Lee, J. S. , Lee, J. , & Bae, H.‐J. (2010). The significance of blood pressure variability for the development of hemorrhagic transformation in acute ischemic stroke. Stroke, 41(11), 2512–2518. 10.1161/strokeaha.110.595561 [DOI] [PubMed] [Google Scholar]
- Lee, S. J. , Hong, J. M. , Lee, S. E. , Kang, D. R. , Ovbiagele, B. , Demchuk, A. M. , & Lee, J. S. (2017). Association of fibrinogen level with early neurological deterioration among acute ischemic stroke patients with diabetes. BMC Neurology, 17(1), 101 10.1186/s12883-017-0865-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip, G. Y. (2013). Recommendations for thromboprophylaxis in the 2012 focused update of the ESC guidelines on atrial fibrillation: A commentary. Journal of Thrombosis and Haemostasis, 11(4), 615–626. 10.1111/jth.12140 [DOI] [PubMed] [Google Scholar]
- Miyasaka, Y. , Barnes, M. E. , Gersh, B. J. , Cha, S. S. , Bailey, K. R. , Abhayaratna, W. P. , Seward, J. B. , & Tsang, T. S. M. (2006). Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation, 114(2), 119–125. 10.1161/circulationaha.105.595140 [DOI] [PubMed] [Google Scholar]
- Oji, S. , Tomohisa, D. , Hara, W. , Tajima, T. , Suzuki, M. , Saito, A. , Yoshida, N. , & Nomura, K. (2018). Mean platelet volume is associated with early neurological deterioration in patients with branch atheromatous disease: involvement of platelet activation. Journal of Stroke and Cerebrovascular Diseases, 27(6), 1624–1631. 10.1016/j.jstrokecerebrovasdis.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Palacio, S. , & Hart, R. G. (2002). Neurologic manifestations of cardiogenic embolism: An update. Neurologic Clinics, 20(1), 179–193. 10.1016/S0733-8619(03)00058-6 [DOI] [PubMed] [Google Scholar]
- Park, M. G. , Kim, M. K. , Chae, S. H. , Kim, H. K. , Han, J. , & Park, K. P. (2018). Lymphocyte‐to‐monocyte ratio on day 7 is associated with outcomes in acute ischemic stroke. Neurological Sciences, 39(2), 243–249. 10.1007/s10072-017-3163-7 [DOI] [PubMed] [Google Scholar]
- Quan, W. , Yang, X. , Li, Y. , Li, J. , Ye, W. , Zhang, O. , & Zhang, X. (2020). Left atrial size and risk of recurrent ischemic stroke in cardiogenic cerebral embolism. Brain and Behavior, 10(10), e01798 10.1002/brb3.1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, H. , Liu, X. , Wang, L. , & Gao, Y. (2017). Lymphocyte‐to‐monocyte ratio: A novel predictor of the prognosis of acute ischemic stroke. Journal of Stroke and Cerebrovascular Diseases, 26(11), 2595–2602. 10.1016/j.jstrokecerebrovasdis.2017.06.019 [DOI] [PubMed] [Google Scholar]
- Sacco, R. L. , Kasner, S. E. , Broderick, J. P. , Caplan, L. R. , Connors, J. J. B. , Culebras, A. , Elkind, M. S. V. , George, M. G. , Hamdan, A. D. , Higashida, R. T. , Hoh, B. L. , Janis, L. S. , Kase, C. S. , Kleindorfer, D. O. , Lee, J.‐M. , Moseley, M. E. , Peterson, E. D. , Turan, T. N. , Valderrama, A. L. , & Vinters, H. V. (2013). An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 44(7), 2064–2089. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet, R. C. , & Rabinstein, A. A. (2012). Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: A critical review of case definitions. Cerebrovascular Disease, 34(2), 106–114. 10.1159/000339675 [DOI] [PubMed] [Google Scholar]
- Seners, P. , Turc, G. , Oppenheim, C. , & Baron, J. C. (2015). Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: A systematic review with pathophysiological implications. Journal of Neurology, Neurosurgery and Psychiatry, 86(1), 87–94. 10.1136/jnnp-2014-308327 [DOI] [PubMed] [Google Scholar]
- Seners, P. , Turc, G. , Tisserand, M. , Legrand, L. , Labeyrie, M.‐A. , Calvet, D. , Meder, J.‐F. , Mas, J.‐L. , Oppenheim, C. , & Baron, J.‐C. (2014). Unexplained early neurological deterioration after intravenous thrombolysis: Incidence, predictors, and associated factors. Stroke, 45(7), 2004–2009. 10.1161/strokeaha.114.005426 [DOI] [PubMed] [Google Scholar]
- Simonsen, C. Z. , Schmitz, M. L. , Madsen, M. H. , Mikkelsen, I. K. , Chandra, R. V. , Leslie‐Mazwi, T. , & Andersen, G. (2016). Early neurological deterioration after thrombolysis: Clinical and imaging predictors. International Journal of Stroke, 11(7), 776–782. 10.1177/1747493016650454 [DOI] [PubMed] [Google Scholar]
- Stroke‐‐1989 . (1989). Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke, 20(10), 1407–1431. [DOI] [PubMed] [Google Scholar]
- Su, Y. , Fan, L. , Zhang, Y. , Zhang, Y. , Ye, H. , Gao, D. , Chen, W. , & Liu, G. (2016). Improved neurological outcome with mild hypothermia in surviving patients with massive cerebral hemispheric infarction. Stroke, 47(2), 457–463. 10.1161/strokeaha.115.009789 [DOI] [PubMed] [Google Scholar]
- Sung, P. H. , Chen, K. H. , Lin, H. S. , Chu, C. H. , Chiang, J. Y. , & Yip, H. K. (2019). The Correlation between severity of neurological impairment and left ventricular function in patients after acute ischemic stroke. Journal of Clinical Medicine, 8(2), 190 10.3390/jcm8020190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni, D. , Fiorelli, M. , Zanette, E. M. , Sacchetti, M. L. , Salerno, A. , Argentino, C. , Solaro, M. , & Fieschi, C. (1998). Early spontaneous improvement and deterioration of ischemic stroke patients. A serial study with transcranial Doppler ultrasonography. Stroke, 29(6), 1144–1148. 10.1161/01.STR.29.6.1144 [DOI] [PubMed] [Google Scholar]
- Tse, H.‐F. , Wang, Y.‐J. , Ahmed Ai‐Abdullah, M. , Pizarro‐Borromeo, A. B. , Chiang, C.‐E. , Krittayaphong, R. , Singh, B. , Vora, A. , Wang, C.‐X. , Zubaid, M. , Clemens, A. , Lim, P. , & Hu, D. (2013). Stroke prevention in atrial fibrillation–an Asian stroke perspective. Heart Rhythm, 10(7), 1082–1088. 10.1016/j.hrthm.2013.03.017 [DOI] [PubMed] [Google Scholar]
- Turkmen, K. , Erdur, F. M. , Ozcicek, F. , Ozcicek, A. , Akbas, E. M. , Ozbicer, A. , Demirtas, L. , Turk, S. , & Tonbul, H. Z. (2013). Platelet‐to‐lymphocyte ratio better predicts inflammation than neutrophil‐to‐lymphocyte ratio in end‐stage renal disease patients. Hemodialysis International, 17(3), 391–396. 10.1111/hdi.12040 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Li, W. , Yang, J. , Zhang, M. , Tian, C. , Ma, M. , & Zhang, Q. (2019). Association between Cystatin C and the risk of ischemic stroke: A systematic review and meta‐analysis. Journal of Molecular Neuroscience, 69(3), 444–449. 10.1007/s12031-019-01373-1 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Zhai, X. , Xue, M. , Cheng, W. , & Hu, H. (2019). Prognostic value of lipoprotein (a) level in patients with coronary artery disease: A meta‐analysis. Lipids in Health and Disease, 18(1), 150 10.1186/s12944-019-1092-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winovich, D. T. , Longstreth, W. T. , Arnold, A. M. , Varadhan, R. , Zeki Al Hazzouri, A. , Cushman, M. , Newman, A. B. , & Odden, M. C. (2017). Factors associated with ischemic stroke survival and recovery in older adults. Stroke, 48(7), 1818–1826. 10.1161/strokeaha.117.016726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji, K. , Fujimoto, S. , Yutani, C. , Hashimoto, T. , & Nakamura, S. (2002). Is the site of thrombus formation in the left atrial appendage associated with the risk of cerebral embolism? Cardiology, 97(2), 104–110. 10.1159/000057681 [DOI] [PubMed] [Google Scholar]
- Yamanouchi, H. , Nagura, H. , Mizutani, T. , Matsushita, S. , & Esaki, Y. (1997). Embolic brain infarction in nonrheumatic atrial fibrillation: A clinicopathologic study in the elderly. Neurology, 48(6), 1593–1597. 10.1212/wnl.48.6.1593 [DOI] [PubMed] [Google Scholar]
- You, W. , Li, Y. , Ouyang, J. , Li, H. , Yang, S. , Hu, Q. , & Zhong, J. (2019). Predictors of poor outcome in patients with minor ischemic stroke by using magnetic resonance imaging. Journal of Molecular Neuroscience, 69(3), 478–484. 10.1007/s12031-019-01379-9 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Jiang, L. , Yang, P. , & Zhang, Y. (2019). Comparison of lymphocyte count, neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in predicting the severity and the clinical outcomes of acute cerebral infarction patients. Clinical Laboratory, 65(7). 10.7754/Clin.Lab.2019.190102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of our study will be available via connecting with Dr. Weining Ma (corresponding author).


