Abstract
Particles extracted from yeast cell walls are naturally occurring immunomodulators with significant therapeutic applications. Their biological function has been thought to be a consequence of the overall chemical composition. In contrast, here we achieve direct nanoscale visualization of the compositional and structural heterogeneity of yeast cell wall particles and demonstrate that such nanoscale heterogeneity directly influences the receptor function of immune cells. By combining peak force infrared (PFIR) microscopy with super-resolution fluorescence microscopy, we achieve simultaneous chemical, topographical, and mechanical mapping of cell wall particles extracted from the yeast Saccharomyces cerevisiae with ≈6 nm resolution. We show that polysaccharides (β-glucan and chitin) and proteins are organized in specific nonuniform structures, and their heterogeneous spatial organization leads to heterogeneous recruitment of receptors on immune cell membranes. Our findings indicate that the biological function of yeast cell wall particles depends on not only their overall composition but also the nanoscale distribution of the different cell wall components.
Graphical Abstract

Yeast cell wall particles are a group of naturally occurring immunomodulators.1,2 Derived from the cell walls of yeast microbes, these hollow microparticles retain the cross-linked polysaccharide layer and other key cell wall components of their source. These key components bind to immune receptors. Therefore, they have long been used as adjuvants to enhance immune responses in cancer therapy, vaccination, and infectious disease treatments.3-6 The spherical hollow interiors of yeast cell wall particles have also been exploited as a means of vaccine and drug delivery.3,4 Despite the long history of using yeast cell wall particles for therapeutic purposes, many aspects of their mechanism of action remain unclear. In particular, we do not understand how the structural organization of the cell wall relates to its immune function.
The immunomodulatory effects of individual components isolated from the yeast cell wall have been identified and characterized by an extensive body of previous work. The most abundant types of polysaccharide in the cell walls of yeast and other fungi and bacteria are β-glucans. They have been found to be potent immune modulators that bind to several innate immune receptors.7,8 Later studies, however, revealed that not all β-glucans are biologically active. The immune function of β-glucans has been found, largely, to depend specifically on features of their higher-degree molecular architecture, such as the length of the polymers and their degree of branching.9,10 This finding highlights the complexity of the structure–function relationships of yeast cell wall particles and the challenge involved in understanding them. It is quite possible that the biological function of these cell wall components depends on much more than just their individual chemical identities taken separately. It may also depend critically on how the different components are assembled and organized over the cell wall. It is not possible to investigate this possibility with conventional chemical and enzymatic analyses that have been used in previous studies.
A variety of microscopy techniques have allowed the structures on cell wall particles and intact microbes to be directly visualized. Surface features as small as the fibril structures of the polymers on yeast cell wall particles have been resolved by using scanning electron microscopy (SEM) and transmission electron microscopy (TEM).11,12 Atomic force microscopy (AFM) enables simultaneous measurement of the surface structure and mechanical properties of the microbial cell wall.13-17 However, these microscopy techniques have a major drawback: they do not reveal the chemical identity of the cell wall structures being imaged. To identify components that form a specific feature of the cell wall structure, one has to use tedious indirect approaches. In some studies, AFM tips were chemically modified with specific functional groups such that the tip–sample adhesion force was used to infer charge groups on the surface of microbes and the presence of polysaccharides.17-22 However, this approach yields only indirect compositional information. In some other studies, AFM microscopy images were matched with lower-resolution optical images of antibody-labeled samples, so that the nanoscale structure of the cell wall can be correlated with the identity of specific chemical components.23 This approach suffers from drawbacks such as the limited spatial resolution of optical microscopy. It also cannot chemically identify unknown cell wall components.
In this study, we achieve simultaneous chemical, structural, and mechanical mapping of yeast cell wall particles with ~6 nm resolution using peak force infrared (PFIR) microscopy. PFIR microscopy is a spectroscopic scanning probe technique based on the detection of infrared (IR) light-induced photothermal expansion. PFIR allows simultaneous mechanical and chemical measurements of samples at high spatial resolution without extrinsic labels.24 PFIR microscopy has been used previously to discriminate nanoscale domains having different chemical compositions in nonbiological samples, such as block copolymer thin films, oil shales, and aerosol particles.24-27 This study represents the first use of PFIR microscopy to reveal chemical compositions through correlative imaging for biological samples. The yeast cell wall particles used in this study are zymosan particles, a boiled and trypsin-treated cell wall extract from Saccharomyces cerevisiae. With PFIR microscopy, we revealed the heterogeneous nanoscale distribution of β-glucans, chitin, and proteins on the zymosan particle surface. β-Glucans are enriched on the cell wall and form a meshlike skeletal structure over the particle surface but are slightly depleted from the inside of bud scars, which are structures left from the fracture of the yeast cell wall during division.28 In contrast, chitin and proteins are mostly concentrated in the bud scars, with chitin on the bud scar rim and proteins inside. Combining the PFIR results with super-resolution structured illumination microscopy (SIM) imaging, we further demonstrated that the heterogeneous distribution of cell wall components leads to heterogeneous clustering and organization of receptor Dectin-1 on membranes during cell uptake of the zymosan particles by immune cells. Thus, combining two complimentary super-resolution techniques with different strengths, we revealed the chemical heterogeneity on the surfaces of zymosan particles and its correlation with their biological functions. While it is known that the composition of yeast cell walls is not uniform, our results here provide nanoscale visualization of the compositional and structural heterogeneity over the surface of cell wall particles and illustrate the immunological significance of the spatial organization of molecules.
RESULTS AND DISCUSSION
Chemical Heterogeneity of the Surfaces of Zymosan Particles.
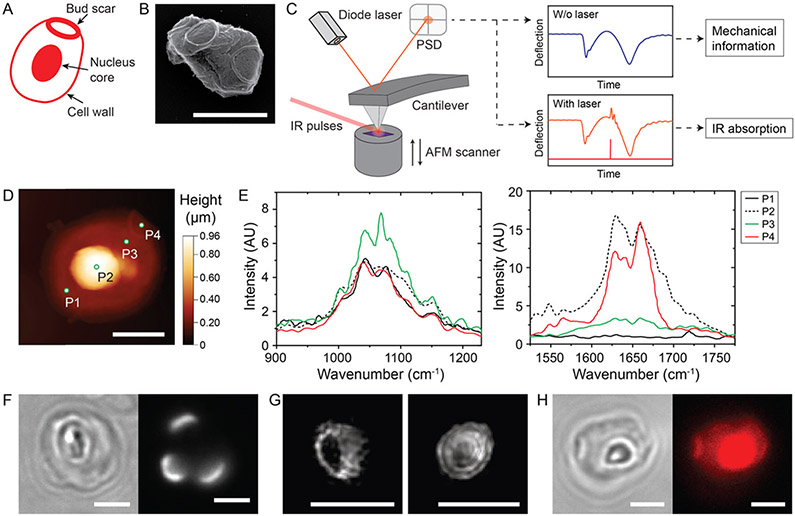
We first examined the morphology of zymosan particles created from yeast cell walls, after critical point drying, using SEM. As schematically illustrated in Figure 1A and shown in the SEM image in Figure 1B, zymosan particles typically exhibit an ellipsoidal shape. Many of them have one or more ringlike structures that protrude slightly above the particle surface. These structures are bud scars, formed from the fracture of the yeast cell wall during division.28
Figure 1.
Identification of chemical heterogeneity on the surface of zymosan particles. (A) Schematic illustration showing the structure features (cell wall, bud scar, and nucleus core) of a zymosan particle. (B) SEM image of a zymosan particle. (C) Schematic illustration of the signal generation of PFIR microscopy. An AFM is operated in the peak force tapping mode, synchronized external laser pulses are focused to the tip and the sample region, and the deflection of the cantilever (force curve) is monitored in real time through a built-in position-sensitive detector (PSD). Mechanical information and IR absorption signal are obtained from the regular force curve (blue) and the force curve with a laser pulse (red). (D) Topography image of a zymosan particle. Dots marked as P1–P4 indicate the four spots where IR signals were measured. (E) PFIR spectral scans at the four different positions marked as P1–P4 in (D). (F) Bright-field and fluorescence images of a zymosan particle labeled with Alexa Fluor 568-tagged wheat germ agglutinin (WGA). (G) Maximum projection of three-dimensional (3-D) SIM images of bud scars on WGA-labeled zymosan particles. (H) Bright-field and fluorescence images of an Alexa Fluor 568-labeled zymosan particle. Scale bars in all images: 2 μm.
We next investigated the chemical composition of zymosan particles using PFIR microscopy. In the instrument setup (schematic illustration in Figure 1C), the AFM is operated in peak force tapping mode to make controlled dynamic contact with the sample, yielding the mechanical signal. Meanwhile, an IR pulse is synchronized with every other peak force tapping cycle. The IR light vibrationally excites the sample and produces a rapid photothermal expansion. The photothermal expansion is detected as additional vertical deflections of the AFM cantilever. This PFIR signal is extracted through real-time data acquisition and analysis. Details of the PFIR microscopy technique have been described previously.24 To prepare samples for PFIR microscopy, zymosan particles were dried in the air on silicon wafers. Unlike the ellipsoidal shape observed in SEM, zymosan particles during the air-drying process became flattened to disklike shapes of only 200–300 nm height (Figures S1 and 2A), but bud scars on particles remained obvious. The air-drying process is commonly used in preparation of microbe samples for Fourier transform infrared (FTIR) and AFM studies and is not expected to influence the chemical composition of the zymosan particles.29-31 A large core of approximately 1.5 μm diameter at the center of all dried particles also became noticeable (Figure 1D). This large core is likely the nucleus of the original S. cerevisiae, based on its morphology and the previous reports that the cores inside zymosan particles can be heavily labeled with nuclear stains.32,33
Figure 2.
Simultaneous mapping of structural, mechanical, and chemical images of zymosan particles. (A) SEM image of a zymosan particle after air drying. (B–F) Corresponding topography (B), PFIR signal at 1030 cm−1 (C) and 1630 cm−1 (D), Young’s modulus (E), and adhesion (F) images of the same zymosan particle. (G, H) The line scan profiles of height, PFIR intensities at 1030 and 1630 cm−1, Young’s modulus, and adhesion along the dash line indicated in (B). (I) Schematic illustration showing the component distribution on zymosan particles. Scale bars in all images: 2 μm.
To investigate the chemical composition of structurally distinct locations on zymosan particles, we measured IR spectra at four positions over the surface of each particle. Two measurement spots were chosen in areas away from the bud scar and the core. The third one was on the surface above the nucleus core, and the fourth was in the bud scar area (Figure 1D). These four spots were chosen consistently on all zymosan particles in our series of measurements. IR signals were scanned in two distinct frequency ranges of 900–1250 and 1525–1775 cm−1, which cover regions of the infrared spectrum absorbed by polysaccharides and proteins, respectively. The metallic coated AFM tip locally enhances the infrared field underneath the apex of the tip over a range of ~10 nm. In the 900–1250 cm−1 frequency range, spectra from all four test locations on zymosan particles show a strong absorption intensity between 1000 and 1150 cm−1, which indicates an abundance of polysaccharide on the cell wall. Specifically, the prominent IR peaks at around 1075 and 1150 cm−1 correspond to β-(1 → 3) glucans, and the band at around 1045 cm−1 corresponds to mannans.34 The less intense IR peaks around 1000 cm−1 likely reflect the absorption of β-(1 → 6) and β-(1 → 4) glucans. This result, in agreement with previous findings, indicates that the β-glucans are the major constituent of the entire zymosan surface with β-(1 → 3) glucan as the dominant component compared to β-(1 → 6) and β-(1 → 4) glucans. However, IR absorption in the 1525–1775 cm−1 range shows significant heterogeneity among the different spots on zymosan particles. Little absorption in this range was observed for surface areas away from the bud scar and core, as indicated by signals from positions P1 and P3 in Figure 1D. In contrast, spectra obtained at positions near the core and bud scar, indicated as P2 and P4, respectively, in Figure 1D, show strong absorption at 1657 and 1628 cm−1 and to a lesser extent 1548 cm−1. The IR bands at 1657 and 1628 cm−1 correspond to the C═O bond stretch vibrations in amide I, and that at 1548 cm−1 corresponds to the N–H bending and C–N stretching in amide II. Two components of the yeast cell wall can possibly contribute to these amide absorption bands. One includes proteins and polypeptides.35,36 The other is chitin, a long-chain polymer of N-acetyl-D-glucosamine linked by a β-glycosidic bond that provides rigidity to the fungal cell wall.37 The IR bands of proteins and chitins overlap in the 1550–1650 cm−1 range and therefore cannot be identified separately. However, we observed that the spectra from the regions over the core (P2) and the bud scar (P4) show different amide I:II intensity ratios. This suggests that even if proteins and chitins are both present in different areas on the same zymosan particles, their abundance varies locally. Regarding the IR spectra obtained from the zymosan area near the nucleus core, it is important to point out that the IR absorption likely includes signals from both the cell wall and the core underneath if the nucleus core is positioned within the proximity of 60–70 nm from the tip, which is the measuring depth of PFIR. However, because the evanescence field intensity decays exponentially from the tip, the signal contribution from the cell wall is expected to dominate that from the nucleus core.
To distinguish chitin and proteins and further confirm their heterogeneous distribution on zymosan particles, we used fluorescence labels specific to either cell wall component. We first labeled zymosan particles with fluorescently tagged wheat germ agglutinin (WGA), which binds specifically to chitin with high affinity.38,39 Using super-resolution SIM, we observed that the rim of the bud scars was intensely labeled by WGA compared to the remaining areas on zymosan particles and appeared as rings in fluorescence images (Figure 1F). This observation indicates that the rims of bud scars are enriched with chitin, which is in agreement with previous reports that chitin is concentrated in the division septum in the neck between a mother cell and its daughter bud.38,40 Interestingly, we observed two types of ring morphology of chitin at bud scars. About half of the zymosan particles examined showed a single chitin ring, whereas the other half showed double rings (Figure 1G). We next labeled zymosan particles with Alexa Fluor 568 succinimidyl ester, which reacts specifically with primary amines in proteins. We found that both the nucleus core and bud scars of zymosan particles were strongly labeled with no noticeable difference in fluorescence intensity (Figure 1H). In contrast, other areas of zymosan particles were only slightly labeled. Because primary amines are present in proteins but not in chitin, this result confirms that proteins are mostly concentrated on the entire bud scars and the nucleus core of zymosan particles. The observation of protein enrichment in bud scars is consistent with previous reports that proteins are recruited to bud scars during yeast budding.41,42
Simultaneous Mapping of the Structural, Mechanical, and Chemical Heterogeneity of Zymosan Particles.
To reveal the nanoscale heterogeneity of the chemical composition on zymosan particles, we next mapped the IR absorption over the entire particle surface at frequencies of 1030 and 1630 cm−1 (Figure 2C,D). It is evident that the main surface of zymosan particles contains mostly β-glucans and proteins at a lower level, which is consistent with our spectral scan results shown in Figure 1E. At the rims of bud scars, both 1030 and 1630 cm−1 absorbances are strong, indicating local enrichment of β-glucans, chitin, and proteins. In contrast, β-glucan is relatively depleted inside the bud scar, as shown by the weaker 1030 cm−1 absorbance compared to other areas on the zymosan particles (Figure 2G). Interestingly, we also observed some nanodomains on the surfaces of the particles with strong absorption intensity at 1630 cm−1 (Figure 2D and more examples in Figure S2). The nanodomains are sparsely distributed and exhibit no clear pattern of arrangement on zymosan particles. While previous studies have reported that proteins on yeast cell surfaces may aggregate and form nanodomains,21,22 it is unclear what caused the nanodomains in our observations. Along with IR signal mapping, we also simultaneously measured the topography, Young’s modulus, and adhesion of the sample zymosan particles. Our topography scan shows that the rims of bud scars protrude ≈100 nm from the surfaces of zymosan particles and that the height of the nucleus core is ≈500 nm. In modulus and adhesion mapping, we found that the rim of bud scars has slightly lower Young’s modulus and adhesion strength than other areas on the zymosan particles (Figure 2H). This is possibly a result of the high concentration of chitin at the rim of the bud scar.12,38 The fringe pattern in the adhesion mapping (Figure 2F) was possibly caused by the interference of the AFM feedback laser.43 The PFIR and fluorescence imaging results together demonstrate the heterogeneous composition on the zymosan particle surfaces. As schematically illustrated in Figure 2I, β-glucans are abundant over the entire zymosan particle surface, chitin is enriched only at the rim of the bud scars, and proteins are concentrated over the bud scars and the nucleus cores.
Nanoscale Meshwork Structure on the Surfaces of Zymosan Particles.
We next sought to resolve the nanoscale structures and chemical heterogeneity of the surfaces of zymosan particles around the bud scars. As for the measurements shown in Figure 1D, we first measured the IR absorption in two frequency ranges of 900–1250 and 1520–1780 cm−1 at seven different locations on the bud scar. Three positions (P1, P2, and P3) were chosen on the rim of the bud scar, two (P4 and P5) on the inner edge of the rim, and two (P6 and P7) inside the bud scar. IR spectra from all seven different positions show similar band positions but different absorption intensities (Figure 3A). Notably, the IR peak intensities around 1657, 1628, and 1548 cm−1, which correspond to amide I and II absorption, were different even for positions that were supposedly on the same structure of the bud scar. Positions P6 and P7 are examples. Given that the spatial resolution of PFIR microscopy is ~6 nm (Figure S3), such a difference in absorption intensities prompted us to ask if the distribution of chitin and proteins is nonuniform on a length scale comparable to our imaging resolution. To answer this question, we focused on the areas around bud scars and performed high-resolution simultaneous mapping of IR adsorptions at 1030 and 1630 cm−1 together with topography, Young’s modulus, and adhesion scanning. We observed that β-glucans (1030 cm−1 adsorption), chitin, and proteins (1630 cm−1 absorption) are all organized in a dense mesh network on the zymosan surface (Figures 3B and S2). Overlaid PFIR images of 1030 and 1630 cm−1 both inside and outside the bud scars show the colocalization of the different cell wall components despite their varied concentrations in different areas (Figure 3C,D). The mesh skeletal structure was clearly outlined using NeuronJ image analysis that traces high-intensity image features. The mesh size, estimated from the peak-to-peak distance in the line scan plots, is ≈50 nm (Figure 3D). The modulus and adhesion mapping also show clearly the nanoscale mesh structure, indicating that the nanofibrils made of cell wall components have different mechanical properties than the voids in the mesh. It is also evident that the rim of the bud scars overall exhibits slightly lower Young’s modulus and adhesion strength than the inside of the bud scars, which is likely a result of the high concentration of chitin.
Figure 3.
Nanoscale mechanical and chemical mapping of zymosan bud scars. (A) Topography image of a zymosan bud scar and PFIR spectral scans at seven different positions marked as P1–P7. Scale bar: 500 nm. (B) Topography, PFIR signal at 1030 and 1630 cm−1, Young’s modulus, and adhesion images of a bud scar. Scale bars: 500 nm. (C, D) Zoom-in images of merged PFIR signals (red for 1030 cm−1 and green for 1630 cm−1), tracked mesh skeletal structure, and line scan profiles of PFIR intensities inside the bud scar (C) and outside the bud scar (D), areas outlined in the white boxes in (B). Scale bars: 100 nm.
Chemical and Structural Heterogeneity of Zymosan Particles Results in Heterogeneous Recruitment of Immune Receptors on Phagosomes.
Zymosan particles activate several membrane receptors of macrophages. In particular, the β-glucans activate Dectin-1 and the lipopeptides bind to Toll-like receptor (TLR) 1/2.44 After ligand–receptor recognition, the zymosan particles are engulfed into intracellular vacuoles known as phagosomes. We hypothesized that the heterogeneous distribution of cell wall components on zymosan particles dictates the arrangements of the corresponding membrane receptors on the phagosome membrane. To test this hypothesis, we used zymosan particles to stimulate RAW264.7 macrophages expressing GFP-Dectin-1 receptors and examined the spatial distribution of GFP-Dectin-1 receptors on phagosomes after particle engulfment. As shown in the Supporting Information video, macrophage cells extended cell membranes to engulf zymosan particles into phagosomes. At the beginning of the uptake process, a high concentration of Dectin-1 was found in the cell membrane that was wrapped around zymosan particles, indicating the recruitment of Dectin-1 upon binding to the β-glucan on the particles. As the engulfment progressed, Dectin-1 was recruited to the entire phagosome membrane as discrete nanoclusters. Due to the spatial resolution limit of optical microscopy, we were unable to resolve single Dectin-1 receptors inside each nanocluster. However, from live cell imaging and 3-D super-resolution images of fixed cell samples, we observed that the Dectin-1 nanoclusters were concentrated over the area outside of bud scars and particularly near the rims of bud scars in a ringlike accumulation pattern but depleted from the inside of bud scars (Figure 4). This result agrees with our findings from PFIR scans that β-glucans are abundant over the zymosan particle surface except inside bud scars (Figure 2C). It is evident that the heterogeneous distribution of β-glucans on zymosan particles leads to heterogeneous spatial distribution of the Dectin-1 receptors on phagosome membranes, which is expected to affect the signaling outcome of cells.
Figure 4.
Heterogeneous recruitment of Dectin-1 receptors on phagosomes containing zymosan particles. Fluorescently labeled zymosan particles (red) were internalized by RAW264.7 macrophages expressing GFP-Dectin-1 (green). (A) Schematic illustration of phagosomes encapsulating zymosan particles inside a macrophage cell. Three-dimensional (3-D) SIM images (B) and constructed 3-D surface models (C) show the distribution of GFP-Dectin-1 on the membrane of phagosomes encapsulating zymosan particles. Scale bars in all images: 1 μm.
CONCLUSIONS
Zymosan particles are derived from the cell walls of the yeast S. cerevisiae. In this study, we demonstrated that the surfaces of these particles have a chemically heterogeneous structure. Further, by examining how these particles interact with macrophages, we found evidence that this heterogenous surface structure is biologically significant. By combining PFIR with super-resolution fluorescence microscopy, we determined how the three main chemical components of these cell walls (β-glucans, chitin, and proteins) are organized. We found that β-glucans are abundant over the cell wall but are present at lesser abundance inside the bud scars. They form a meshlike skeletal structure with a mesh size of ≈50 nm. While proteins colocalize with β-glucans across this network, they are present at significantly higher concentrations over entire bud scars and in the nucleus core. In contrast, chitin is mostly concentrated on the rims of the bud scars, distributed as either single or double rings. Macrophages engulf zymosan particles and encapsulate them into intracellular vacuoles to initiate immune responses. This process involves the binding of β-glucans to their immune receptor, Dectin-1. We made the important finding that the inhomogeneous distribution of β-glucans over the surfaces of zymosan particles results in the accumulation of Dectin-1 receptors on phagosomes outside and near the rim of bud scars but not in areas inside the bud scars. Based on our previous finding that macrophage inflammatory responses depend on the spacing between Dectin-1 and other membrane receptors,45 our results here indicate that heterogeneous spatial organization of the yeast cell wall components has a direct impact on the immune responses that they trigger. While the immune effects of individually isolated yeast cell wall compounds are well established, our findings provide new insights into the mechanism of action of yeast cell wall particles at both the molecular and whole-particle levels.
This study showcases the capacity of combining PFIR microscopy with super-resolution fluorescence imaging in resolving the compositions and organization of biological systems that are heterogeneous on the nanoscale. PFIR microscopy enables simultaneous chemical, topographical, and mechanical mapping of yeast cell wall particles without extrinsic labels. A high spatial resolution (≈6 nm), far better than the optical diffraction limit, was achieved in chemical imaging with infrared spectroscopic capabilities. PFIR microscopy also enables correlative imaging with mechanical mapping modalities of AFM at a comparable and high spatial resolution. The yeast cell wall particles used in this study were imaged in dried condition, but our results here provide an important foundation for the further development of PFIR microscopy toward imaging biological samples, such as the cell wall of microbes, in their native environment. Of course, PFIR, like any other IR technique, has its limitation in identifying complex chemical compositions that have overlapping IR bands. An integral approach of combining PFIR with other complimentary techniques, such as super-resolution fluorescence imaging, as demonstrated in this study, is expected to be powerful in providing an understanding of the complex structures and architectures of biological systems.
MATERIALS AND METHODS
Materials.
Zymosan particles were purchased from InvivoGen (San Diego, CA). Wheat germ agglutinin (WGA, L-1020-10) was purchased from Vector Laboratories (Burlingame, CA). Formaldehyde (PFA) was purchased from Avantor (Radnor, PA). Alexa Fluor 568 succinimidyl ester was purchased from ThermoFisher Scientific (Waltham, MA). Dulbecco’s modified Eagle’s medium (DMEM, 10-013-CV), L-glutamine (25-005-CI), penicillin–streptomycin (30-002-CI), and fetal bovine serum (FBS, 35-010-CV) were purchased from Corning (Corning, NY). GFP-Dectin-1-expressing RAW264.7 macrophages were originally provided by Prof. David Underhill at Cedars-Sinai Medical Center and cultured in DMEM supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% FBS.
SEM Sample Preparation and Imaging.
Twelve millimeter coverslips were etched in piranha solution (3:1 mixture of sulfuric acid and 30% hydrogen peroxide) at room temperature for 15 min, cleaned with deionized (DI) water, and then incubated with 0.01% poly-L-lysine overnight. After rinsing in DI water, the coverslips were placed into a 24-well plate. Zymosan particles were added and incubated on the coverslips for 3 h at room temperature to ensure particle adhesion. Samples were sequentially dehydrated by 30, 50, 75, 90, and 95% ethanol on ice for 5 min each time and 100% ethanol for 5 min, 3 times. Samples were dried using a Leica CPD030 Critical Point Dryer and then sputter-coated with a 10 nm thick film of Pd/Rh alloy using a Denton Vacuum Sputter Coater. Coated samples were imaged using an FEI Quanta 600F SEM with 30 kV voltage, high pressure, and 5–6 mm working distance.
PFIR Measurement and Sample Preparation.
A home-built PFIR setup (schematically shown in Figure 1C) was used to obtain topography, IR absorption, Young’s modulus, and adhesion images. The technique details and detection mechanism of PFIR microscopy were described in the previous publications.24 Briefly, the setup includes an AFM (Multimode 8 with Nanoscope V controller, Bruker Nano), a quantum cascade laser (MIRcat, Daylight Solutions), and data acquisition devices (PXI-5122, National Instruments). A parabolic mirror with a numerical aperture of 0.25 was used to focus IR beam onto the AFM tip apex. Customized LabVIEW programs were used to extract IR absorption signals in real time. Platinum-coated AFM tips (HQ: NSC14Pt, MikroMasch) were used in all measurements. Modulus and adhesion signals were usually obtained simultaneously without laser illumination to avoid any interference from laser-induced mechanical effects. The detection mechanism of PFIR determines that the intensity in IR images at the scanning position is proportional to the intensity of local IR absorptions. Thus, if the laser is tuned to a characteristic frequency of a certain type of molecule, the spatial distribution of this molecule can be mapped with indications to their relative local abundance. PFIR spectra were obtained by sweeping the laser frequency when keeping the tip position fixed at any point of interest on the sample. To prepare samples for PFIR measurements, 20 μL of aqueous solution containing zymosan particles at 20 μg/mL concentration was dropped on silicon wafers and air-dried before measurement. To estimate the signal-to-background and signal-to-noise ratios of the spectroscopy, we used the PFIR images at 1030 and 1630 cm−1 shown in Figure 2C,D and obtained average background intensities from the areas outlined by the white boxes and average signal intensities from the areas outlined by black boxes (Figure S4). The statistical root mean square (rms) of the signal intensities was calculated as the noise. The estimated signal-to-background ratio is 3.1 for the 1030 cm−1 image and 2.0 for the 1630 cm−1 image. The estimated signal-to-noise ratio is 4.5 for the 1030 cm−1 image and 185 for the 1630 cm−1 image. The mesh skeletal structure in the overlaid PFIR images of 1030 and 1630 cm−1 was analyzed and traced using an ImageJ plugin, NeuronJ.
Fluorescence Labeling and Imaging.
WGA or zymosan particles were resuspended in 0.1 M NaHCO3 and labeled with Alexa Fluor 568 succinimidyl ester by following the protocol recommended by the manufacturer. Zymosan particles were labeled with WGA by incubating with 10 μg/mL Alexa Fluor 568-tagged WGA in PBS buffer at room temperature for 2.5 h. Fluorescently labeled particles were incubated with GFP-Dectin-1-expressing macrophages for 5 min at 37 °C and fixed with 2% PFA on ice for 5 min. Super-resolution SIM images were acquired using a DeltaVision OMX SR imaging system equipped with an Olympus Plan Apo 60×/1.42 PSF objective and a pco. edge sCMOS camera. SIM images were reconstructed using SoftWorx software. Image analysis was performed using ImageJ and Imaris.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Yi Yi at the Nanoscale Characterization Center at Indiana University, Dr. James Power at the Light Microscopy Imaging Center (LMIC), and Dr. Barry Stein at the Electron Microscopy Center for assistance with instrument use. The 3D SIM microscope at LMIC was acquired under the National Institutes of Health (NIH) award NIH1-S10OD024988-01. The research reported in this publication was supported by the National Institute of General Medical Sciences of NIH under Award Number R35GM124918 (to Y.Y.), National Science Foundation CHE 1847765 (to X.G.X.), and Beckman Young Investigator Award from the Arnold and Mabel Beckman Foundation (to X.G.X.). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.langmuir.0c00627.
SEM images and PFIR topography images showing disklike shapes of zymosan particles (Figure S1); nanoscale mechanical and chemical images of a zymosan bud scar (Figure S2); estimation of the spatial resolution of PFIR mapping (Figure S3); estimation of the signal-to-noise ratio and signal-to-background ratio for PFIR measurement (Figure S4) (PDF)
Macrophage cells extended cell membranes to engulf zymosan particles into phagosomes (AVI)
The authors declare no competing financial interest.
Contributor Information
Wenqian Li, Department of Chemistry, Indiana University, Bloomington, Indiana 47405, United States.
Haomin Wang, Department of Chemistry, Lehigh University, Bethlehem, Pennsylvania 18015, United States.
Xiaoji G. Xu, Department of Chemistry, Lehigh University, Bethlehem, Pennsylvania 18015, United States.
Yan Yu, Department of Chemistry, Indiana University, Bloomington, Indiana 47405, United States.
REFERENCES
- (1).Pan Y; Li X; Kang T; Meng H; Chen Z; Yang L; Wu Y; Wei Y; Gou M Efficient Delivery of Antigen to DCs Using Yeast-Derived Microparticles. Sci. Rep 2015, 5, No. 10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Novak M; Vetvicka V B-Glucans, History, and the Present: Immunomodulatory Aspects and Mechanisms of Action. J. Immunotoxicol 2008, 5, 47–57. [DOI] [PubMed] [Google Scholar]
- (3).De Smet R; Allais L; Cuvelier CA Recent Advances in Oral Vaccine Development: Yeast-Derived B-Glucan Particles. Hum. Vaccines Immunother 2014, 10, 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).De Smet R; Demoor T; Verschuere S; Dullaers M; Ostroff GR; Leclercq G; Allais L; Pilette C; Dierendonck M; De Geest BG; Cuvelier CA B-Glucan Microparticles are Good Candidates for Mucosal Antigen Delivery in Oral Vaccination. J. Controlled Release 2013, 172, 671–678. [DOI] [PubMed] [Google Scholar]
- (5).Abou Elazab MF; Inoue Y; Kamei H; Horiuchi H; Furusawa S Zymosan A Enhances Humoral Immune Responses to Soluble Protein in Chickens. J. Vet. Med. Sci 2017, 79, 1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yan J; Allendorf DJ; Brandley B Yeast Whole Glucan Particle (WGP) B-Glucan in Conjugation with Antitumor Monoclonal Antibodies to Treat Cancer. Expert Opin. Biol. Ther 2005, 5, 691–702. [DOI] [PubMed] [Google Scholar]
- (7).Volman JJ; Ramakers JD; Plat J Dietary Modulation of Immune Function by B-Glucans. Physiol. Behav 2008, 94, 276–284. [DOI] [PubMed] [Google Scholar]
- (8).Stier H; Ebbeskotte V; Gruenwald J Immune-Modulatory Effects of Dietary Yeast Beta-1,3/1,6-D-Glucan. Nutr. J 2014, 13, No. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bohn JA; BeMiller JN (1→3)-B-D-Glucans as Biological Response Modifiers: A Review of Structure-Functional Activity Relationships. Carbohydr. Polym 1995, 28, 3–14. [Google Scholar]
- (10).Synytsya A; Noväk M Structural Diversity of Fungal Glucans. Carbohydr. Polym 2013, 92, 792–809. [DOI] [PubMed] [Google Scholar]
- (11).Osumi M The Ultrastructure of Yeast: Cell Wall Formation. Micron 1998, 29, 207–233. [DOI] [PubMed] [Google Scholar]
- (12).Cabib E; Bowers B Chitin and Yeast Budding. Localization of Chitin in Yeast Bud Scars. J. Biol. Chem 1971, 246, 152–159. [PubMed] [Google Scholar]
- (13).Dufrene YF Atomic Force Microscopy in Microbiology: New Structural and Functional Insights into the Microbial Cell Surface. mBio 2014, 5, No. e01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dague E; Gilbert Y; Verbelen C; Andre G; Alsteens D; Dufrene YF Towards A Nanoscale View of Fungal Surfaces. Yeast 2007, 24, 229–237. [DOI] [PubMed] [Google Scholar]
- (15).Dague E; Alsteens D; Latge JP; Dufrene YF High-Resolution Cell Surface Dynamics of Germinating Aspergillus fumigatus Conidia. Biophys. J 2008, 94, 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Alsteens D; Verbelen C; Dague E; Raze D; Baulard AR; Dufrene YF Organization of the Mycobacterial Cell Wall: A Nanoscale View. Pfluegers Arch. Eur. J. Appl Physiol 2008, 456, 117–125. [DOI] [PubMed] [Google Scholar]
- (17).Alsteens D; Dague E; Verbelen C; Andre G; Dupres V; Dufrene YF Nanoscale Imaging of Microbial Pathogens Using Atomic Force Microscopy. Wiley Interdiscip. Rev.: Nanomed. Nano-biotechnol 2009, 1, 168–180. [DOI] [PubMed] [Google Scholar]
- (18).Ahimou F; Denis FA; Touhami A; Dufrene YF Probing Microbial Cell Surface Charges by Atomic Force Microscopy. Langmuir 2002, 18, 9937–9941. [Google Scholar]
- (19).Alsteens D; Dupres V; Mc Evoy K; Wildling L; Gruber HJ; Dufrene YF Structure, Cell Wall Elasticity and Polysaccharide Properties of Living Yeast Cells, as Probed by AFM. Nanotechnology 2008, 19, No. 384005. [DOI] [PubMed] [Google Scholar]
- (20).Müller DJ; Helenius J; Alsteens D; Dufrene YF Force Probing Surfaces of Living Cells to Molecular Resolution. Nat. Chem. Biol 2009, 5, 383–390. [DOI] [PubMed] [Google Scholar]
- (21).Formosa C; Schiavone M; Boisrame A; Richard ML; Duval RE; Dague E Multiparametric Imaging of Adhesive Nanodomains at the Surface of Candida albicans by Atomic Force Microscopy. Nanomedicine 2015, 11, 57–65. [DOI] [PubMed] [Google Scholar]
- (22).Alsteens D; Garcia MC; Lipke PN; Dufrene YF Force-Induced Formation and Propagation of Adhesion Nanodomains in Living Fungal Cells. Proc. Natl. Acad. Sci. U.S.A 2010, 107, 20744–20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gilbert Y; Deghorain M; Wang L; Xu B; Pollheimer PD; Gruber HJ; Errington J; Hallet B; Haulot X; Verbelen C; Hols P; Dufrene YF Single-Molecule Force Spectroscopy and Imaging of the Vancomycin/D-Ala-D-Ala Interaction. Nano Lett. 2007, 7, 796–801. [DOI] [PubMed] [Google Scholar]
- (24).Wang L; Wang H; Wagner M; Yan Y; Jakob DS; Xu XG Nanoscale Simultaneous Chemical and Mechanical Imaging via Peak Force Infrared Microscopy. Sci. Adv 2017, 3, No. e1700255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wang L; Huang D; Chan CK; Li YJ; Xu XG Nanoscale Spectroscopic and Mechanical Characterization of Individual Aerosol Particles using Peak Force Infrared Microscopy. Chem. Commun 2017, 53, 7397–7400. [DOI] [PubMed] [Google Scholar]
- (26).Wang H; Wang L; Jakob DS; Xu XG Tomographic and Multimodal Scattering-Type Scanning Near-Field Optical Microscopy with Peak Force Tapping Mode. Nat. Commun 2018, 9, No. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Jakob DS; Wang L; Wang H; Xu XG Spectro-Mechanical Characterizations of Kerogen Heterogeneity and Mechanical Properties of Source Rocks at 6 nm Spatial Resolution. Anal. Chem 2019, 91, 8883–8890. [DOI] [PubMed] [Google Scholar]
- (28).Talens LT; Miller MW; Miranda M Electron Micrograph Study of the Asci and Ascospores of Metschnikowia Kamienski. J. Bacteriol 1973, 115, 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Hayhurst EJ; Kailas L; Hobbs JK; Foster SJ Cell Wall Peptidoglycan Architecture in Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A 2008, 105, 14603–14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Turner RD; Hurd AF; Cadby A; Hobbs JK; Foster SJ Cell Wall Elongation Mode in Gram-negative Bacteria Is Determined by Peptidoglycan Architecture. Nat. Commun 2013, 4, No. 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wang L; Jakob DS; Wang H; Apostolos A; Pires MM; Xu XG Generalized Heterodyne Configurations for Photoinduced Force Microscopy. Anal. Chem 2019, 91, 13251–13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Jawhara S; Habib K; Maggiotto F; Pignede G; Vandekerckove P; Maes E; Dubuquoy L; Fontaine T; Guerardel Y; Poulain D Modulation of Intestinal Inflammation by Yeasts and Cell Wall Extracts: Strain Dependence and Unexpected Anti-Inflammatory Role of Glucan Fractions. PLoS One 2012, 7, No. e40648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lombard Y; Giaimis J; Makaya-Kumba M; Fonteneau P; Poindron P A New Method for Studying the Binding and Ingestion of Zymosan Particles by Macrophages. J. Immunol. Methods 1994, 174, 155–165. [DOI] [PubMed] [Google Scholar]
- (34).Galichet A; Sockalingum GD; Belarbi A; Manfait M FTIR Spectroscopic Analysis of Saccharomyces cerevisiae Cell Walls: Study of An Anomalous Strain Exhibiting A Pink-Colored Cell Phenotype. FEMS Microbiol. Lett 2001, 197, 179–186. [DOI] [PubMed] [Google Scholar]
- (35).Higgins HG; Stewart CM; Harrington KJ Infrared Spectra of Cellulose and Related Polysaccharides. J. Polym. Sci 1961, 51, 59–84. [Google Scholar]
- (36).Singh BR Basic Aspects of the Technique and Applications of Infrared Spectroscopy of Peptides and Proteins In Infrared Analysis of Peptides and Proteins;Tabouret G, Ed.; American Chemical Society: Washington, 1999; pp 2–37. [Google Scholar]
- (37).Negrea P; Caunii A; Sarac I; Butnariu M The Study of Infrared Spectrum of Chitin and Chitosan Extract as Potential Sources of Biomass. Dig. J. Nanomater. Bios 2015, 10, 1129–1138. [Google Scholar]
- (38).Powell CD; Quain DE; Smart KA Chitin Scar Breaks in Aged Saccharomyces cerevisiae. Microbiology 2003, 149, 3129–3137. [DOI] [PubMed] [Google Scholar]
- (39).Chaudhari RD; Stenson JD; Overton TW; Thomas CR Effect of Bud Scars on the Mechanical Properties of Saccharomyces cerevisiae Cell Walls. Chem. Eng. Sci 2012, 84, 188–196. [Google Scholar]
- (40).Orlean P Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Sumita T; Yoko-o T; Shimma Y.-c.; Jigami Y. Comparison of Cell Wall Localization among Pir Family Proteins and Functional Dissection of the Region Required for Cell Wall Binding and Bud Scar Recruitment of Pir1p. Eukaryotic Cell 2005, 4, 1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Rolli E; Ragni E; Calderon J; Porello S; Fascio U; Popolo L Immobilization of the Glycosylphosphatidylinositol-Anchored Gas1 Protein into the Chitin Ring and Septum is Required for Proper Morphogenesis in Yeast. Mol. Biol. Cell 2009, 20, 4856–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Méndez-Vilas A; González-Martin ML; Nuevo MJ Optical Interference Artifacts in Contact Atomic Force Microscopy Images. Ultramicroscopy 2002, 92, 243–250. [DOI] [PubMed] [Google Scholar]
- (44).Goodridge HS; Underhill DM Fungal Recognition by TLR2 and Dectin-1 In Handbook of Experimental Pharmacology;- Bauer S; Hartmann G, Eds.; Springer-Verlag: Berlin Heidelberg, 2008; pp 87–109. [DOI] [PubMed] [Google Scholar]
- (45).Li W; Yan J; Yu Y Geometrical Reorganization of Dectin-1 and TLR2 on Single Phagosomes Alters Their Synergistic Immune Signaling. Proc. Natl. Acad. Sci U.S.A 2019, 116, 25106–25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.