Abstract
Objectives:
This study aimed to evaluate the mucograft collagen matrix (CM) to increase keratinized tissue around teeth compared to free gingival graft (FGG).
Materials and Methods:
The present double-blind, randomized, controlled clinical trial studied 12 patients who had 2 mm or less keratinized gingiva bilaterally around mandibular premolars. The 6-month width of keratinized tissue, periodontal parameters (preoperatively and 1, 3, and 6 months postoperatively), color match, pain, and total surgical time were measured.
Results:
The mean dimensional change of keratinized gingiva 6 months postoperatively was 4.1±0.7 mm for FGG and 8±1.7 mm for CM. Periodontal parameters were not affected in the two groups. The CM group had a significantly lower pain, experienced less surgery time, and gained better aesthetics compared to the FGG group.
Conclusion:
CM appears to be a suitable substitute for FGG in procedures designed to increase keratinized tissue around teeth. It has remarkable benefits, such as acceptable keratinized tissue gain, less pain, less surgical chair time, and better aesthetics.
Keywords: Gingiva, Mucograft, Tissue Transplantation
INTRODUCTION
The width of keratinized gingiva differs in different individuals and even in different teeth [1]. The rationale of mucogingival therapy has been primarily based on the fact that a minimum width of gingiva is critical for maintaining gingival health and preventing gingival recession [2]. However, today, it is believed that gingival health can be maintained independently of its dimensions [3,4]. As a result, narrow gingiva alone cannot justify surgical intervention. However, gingival augmentation procedures may be indicated when patients experience discomfort during tooth brushing and/or chewing due to interference from a lining mucosa or when orthodontic tooth movement is planned and the buccal position of teeth can result in alveolar bone dehiscence. An increase of the gingiva may also be considered when subgingival restorations are placed in areas with a thin marginal tissue [5].
Various techniques have been invented for increasing the width of keratinized gingiva. Since the late 1960s, clinicians have corrected insufficient keratinized tissue and insufficient vestibules by placing autogenous free gingival grafts (FGGs), free connective tissue grafts, and surgically releasing the vestibular area (vestibuloplasty) [6]. One of the earliest and most common gingival augmentation procedures involves FGG in which the graft is harvested from the patient’s palate. This technique has more predictable results but it has some disadvantages. First, the palate is healed by secondary intention and requires a dressing for 10 to 14 days, which is uncomfortable for most patients [7]. Other disadvantages include the inability to harvest large grafts, high morbidity rates after surgery, and poor aesthetics due to differences in texture and color from adjacent areas [8]. In patients with difficult-to-control bleeding at the graft donor site, treatment of multiple sites would be a challenge [9].
Possible alternatives to FGGs are xenografts and allografts [10]. The Mucograft ® Collagen Matrix (CM; Geistlich Pharma AG, Wolhusen, Switzerland) is a resorbable, three-dimensional (3D) matrix that is designed specifically for soft tissue regeneration in the oral cavity. It is fabricated as a matrix of pure type I and III porcine collagen obtained with standardized and controlled manufacturing processes without cross-linking or chemical treatment [8].
Some previous studies have investigated the clinical outcome of CM for augmentation of insufficient keratinized tissue [11–13]. These studies have shown favorable clinical results; however, most of them have been conducted around dental implants and not around teeth. In most cases, the design of the studies was not split-mouth; therefore, patient-related factors were not the same in the control and test groups. As a result, we decided to compare CM and FGG for the augmentation of keratinized gingiva around teeth in a split-mouth study.
MATERIALS AND METHODS
Study design and participants:
The present double-blind, randomized, controlled clinical trial (IRCT registration number: IRCT2013052813501N1) was conducted according to the guidelines of the Helsinki Declaration of 1975 (revised, 2000). The Ethics Committee of the Dental Research Center of Tehran University of Medical Sciences approved the research protocol (Ethical code: 91-04-10-18791-78324).
The study population consisted of patients referring to the Department of Periodontics, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran, who had less than 2mm of attached keratinized gingiva bilaterally on the buccal aspect of the mandibular premolar teeth.
The inclusion criteria were: 1) age over 18 years, 2) good oral hygiene; O’Leary oral plaque index < 15%, 3) no bleeding on probing (BOP) based on the Ainamo and Bay index (1976), 4) the presence of an identifiable cementoenamel junction (CEJ), and 5) teeth in need of prosthetic or orthodontic treatment. Exclusion criteria were: 1) active carious lesions or restorations or crowns at the CEJ, 2) smoking, 3) systemic conditions precluding periodontal surgery, 4) systemic conditions affecting the periodontium; 5) high frenum pull, 6) history of mucogingival surgery in the area, and 7) pathologic movement of the involved teeth. The sample size was predicated based on obtaining 80% power for testing the primary study endpoint, which evaluated whether or not mucograft was inferior to FGG in the generation of keratinized tissue from the baseline to 6 months postoperatively.
This assumed a paired t-test of non-inferiority with a non-inferiority margin of 1.0 mm, a within-patient standard deviation (SD) of 1.0 mm, and a one-sided alpha of 0.05. Under these assumptions and according to the below formula, a sample size of 10 was required to power the primary endpoint [13]. To account for potential loss to follow-up, 12 patients were enrolled in the trial. The investigator blinded to the details of the study and surgical protocols carried out randomization of the patients and their assignment to intervention groups.
The patients were numbered according to when they had presented to the department. After the patients’ eligibility for enrollment in the study was confirmed, all the surgeries were done according to the patients’ numbers. Concealed allocation was performed by using sealed, coded envelopes that were opened just before surgery to determine the test (Mucograft ®) and control (FGG) groups. To allow for possible dropouts, 12 patients were recruited. One experienced surgeon, who was blinded to the randomization sequences, performed all surgeries.
Periodontal parameters:
Patients received oral hygiene instructions for two weeks before surgery, and professional scaling and root planing (SRP) was carried out. A calibrated postgraduate student, who was blinded to the study protocol, measured all clinical parameters before and after surgery. All measurements were made using a standard UNC-15 periodontal probe (Hu-Friedy, Chicago, IL, USA) on a surgical stent that was made for each patient.
To assess the safety and effectiveness of CM compared to FGG, clinical measures of periodontal health and healing were recorded. The measurements included: 1) O’Leary plaque index, 2) Loe and Silness gingival index, 2) width of keratinized tissue, 3) probing pocket depth (PPD), and 4) clinical attachment level (CAL).
Surgical procedures:
After local anesthesia, a partial-thickness dissection was accomplished to remove the mucosa while preserving the periosteum.
As first described by Bjorn [14], a coronal incision was made at the height of the existing mucosa, extending at least to the line angle of the adjacent teeth. Recipient sites were slightly larger than the CM and FGG grafts. The CM and FGG grafts were 10×20 mm2. Vertical incisions were made on the mesial and distal aspects of the CM and FGG sites, extending apically as far as the vestibules allowed. The mesial and distal incisions were then connected apically. Muscle fibers were removed with scissors, creating a clean periosteal bed.
The CM was placed dry (not pre-wet), and blood was allowed to soak into the matrix to form an initial stable clot. The FGG was harvested from the randomly assigned palate donor site. The CM and FGG test and control materials were placed in direct contact with the appropriate randomly assigned recipient bed and sutured in place with non-resorbable 4-0 Cytoplast™ PTFE suture’s monofilament construction that does not allow bacterial wicking into the surgical site. Five regions of the graft were sutured (mesio-cervical, mesio-apical, disto-cervical, disto-apical, and central). A surgical dressing was used to cover the surgical sites.
The duration of surgery was recorded for both CM and control FGG procedures (Fig. 1).
Fig. 1.
Gingival augmentation by free gingival graft (FGG) on the right side (a and b) and mucograft on the left side (c and d)
Two 400 mg ibuprofen tablets were given to the patients immediately after surgery, and they were asked to use additional ibuprofen only if they need to. They were also instructed to use a 0.2% chlorhexidine mouthwash twice daily for 6 weeks and take amoxicillin (500 mg) three times a day for 7 days.
The patients were recalled for hygiene reinstruction and prophylaxis biweekly for 12 weeks and then monthly until 6 months post-surgery. All study variables were measured again 1, 3, and 6 months postoperatively.
Evaluation of postoperative pain:
A visual analog scale (VAS) was used to evaluate postoperative pain and discomfort. The scale consisted of a 10cm line with a range from 0 (no pain/edema) to 10 (severe and intolerable pain/edema). The patients were instructed for the use of the scale and were asked to mark the severity of their pain on the scale 1–14 days after surgery.
Evaluation of aesthetic outcome and success:
Six months after surgery, three expert periodontists were asked to evaluate the aesthetic outcome according to their subjective concept. Color match, consistency, and surface texture of the newly formed tissue were considered for the evaluation of aesthetic outcomes. The initial size of the graft was the same in both FGG and CM groups (10×20 mm 2). The final size of the generated keratinized tissue relative to the initial dimensions was considered for the evaluation of success.
Data analysis:
For each clinical measurement, descriptive statistics such as mean, median, and SD were obtained.
The primary hypothesis of the study evaluates whether or not CM was inferior to FGG in the generation of keratinized tissue from the baseline to 6 months postoperatively. A paired t-test was used to test for non-inferiority, using a one-sided significance level of 0.05 and a noninferiority margin of 1.0 mm. For comparison of pain and keratinized tissue gain in each group, two-way repeated-measures analysis of variance (ANOVA) was used to determine the statistical significance defined as P<0.05. All statistical analyses were performed using SPSS 22 (IBM Corp., Armonk, NY, USA).
RESULTS
Twelve patients participated in this study (7 men and 5 women with an average age of 40±13.9 years), and 24 sites were examined. No patient developed significant complications, and normal healing was observed in both CM and FGG sites. Periodontal parameters that were assessed at the beginning and the follow-up sessions are listed below:
Periodontal pocket depth (PPD):
At the beginning of the study, on the FGG side, there were 5 patients with PPD=1 mm and 7 patients with PPD=0 mm. On the MC side, there were 4 patients with PPD=1 mm and 8 patients with PPD=0 mm. During the study, PPD did not change, except in one patient in whom the changes were similar on both sides and were not clinically or statically significant.
Plaque index (PI):
Two patients had PI=1 and 10 patients had PI=0 on the FGG side. On the other side, 3 patients had PI=1 and 9 patients had PI=0. Until the end of the study, all patients gained PI=0, except for one patient on the FGG side.
GI (Gingival Index):
Except for one patient with GI=1 from the beginning of the study, the rest of the patients had GI=0. At the end of the study, all patients had GI=0.
Clinical attachment level (CAL):
Patients had different CALs at the beginning of the study, which did not change until the end of the study. Since root coverage was not considered in this study, and on the other hand, a recession was not observed in patients, this factor was not significant, either statistically or clinically.
It can be concluded that the surgical procedure did not alter the periodontal parameters significantly in the two groups. The changes in the primary outcome of the study (keratinized tissue width) are shown in Table 1.
Table 1.
The mean keratinized tissue width (mm) in the free gingival graft and mucograft groups
| Group | Days | Mean | SD |
|---|---|---|---|
| Free Gingival Graft | BS | 1.25 | 0.62 |
| 30 | 9.92 | 0.9 | |
| 90 | 8.33 | 1.67 | |
| 180 | 8 | 1.71 | |
| Mucograft | BS | 1.25 | 0.62 |
| 30 | 7.58 | 0.9 | |
| 90 | 5.08 | 0.79 | |
| 180 | 4.17 | 0.72 |
SD: Standard Deviation; BS: Before surgery
The width of keratinized gingiva was evaluated using a UNC-15 probe in millimeters and recorded preoperatively and at intervals of 1, 3, and 6 months after surgery. Because one person performed both interventions, the obtained data were correlated, and two-way repeated-measures ANOVA was used. P-values less than 0.05 were considered significant. The difference in keratinized gingival width between two groups of FGG and CM was statistically significant, regardless of the intervention time. It should be noted, however, that in the CM group, the keratinized gingiva was less than that in the FGG group. The mean dimensional change of keratinized gingiva 6 months postoperatively was 4.1±0.7 mm for FGG and 8±1.7 mm for CM (Fig. 2 and 3).
Fig. 2.
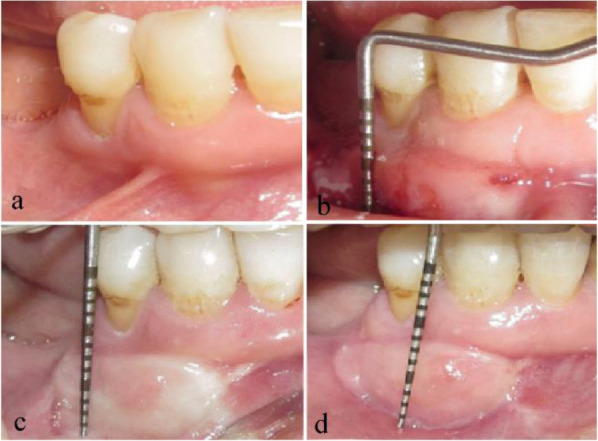
Free Gingival graft: (a) Before surgery. (b) 30 days after surgery. (c) 90 days after surgery. (d) 180 days after surgery
Fig. 3.
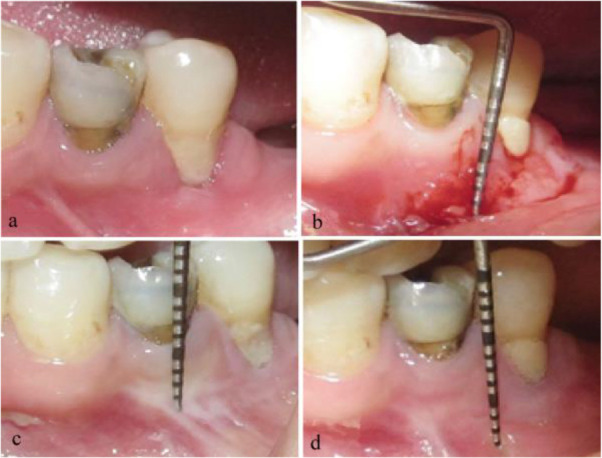
Mucograft: (a) Before surgery. (b) 30 days after surgery. (c) 90 days after surgery. (d) 180 days after surgery
Also, the difference in keratinized gingival width in each group was significant at different times after surgery, regardless of the type of surgery (FGG or CM; P<0.001; Fig. 4).
Fig. 4.
The mean keratinized gingival width at the baseline and the follow-ups in the free gingival graft and mucograft groups; CI: Confidence Interval
Pain:
The pain was evaluated using the VAS. The records of patients on days 1, 3, 5, and 7 after surgery are shown in Table 2. According to collected data, pain was significantly less in the CM group compared to the FGG group (P<0.001), and in both groups, pain significantly decreased over time.
Table 2.
The mean change of pain over time in the free gingival graft and mucograft groups on the visual analog scale (VAS)
| Group | Days | Mean VAS | SD |
|---|---|---|---|
| Free Gingival Graft | 1 | 7.75 | 1.22 |
| 3 | 7 | 1.41 | |
| 5 | 6.33 | 1.15 | |
| 7 | 4.67 | 1.15 | |
| Mucograft | 1 | 6.08 | 1.51 |
| 3 | 4.08 | 1.24 | |
| 5 | 2.83 | 1.40 | |
| 7 | 1.92 | 1.03 |
SD: Standard Deviation
Total surgery time:
The time of each surgery was recorded from the start of the first anesthesia injection to the last suture with a stopwatch in minutes.
The mean time of surgery was 47.75±3.5 minutes for the FGG group and 25.83±3.2 minutes for the CM group, which was significantly less in the CM group (P<0.001).
Aesthetics and success:
Regarding the success, FGG was more successful than CM (P=0.039, sign test), and regarding the aesthetics, color match, consistency, and surface texture of the CM method were better than that of the FGG (P=0.06, sign test).
DISCUSSION
Despite the controversy about the need for a certain amount of keratinized gingiva around teeth, gingival augmentation is required when patients have difficulty in removing dental plaque at the gingival margin, in cases of subgingival restoration, and for aesthetic reasons [15].
According to the literature, to date, FGG is the best method to treat inadequate keratinized tissue [16] but it still has some disadvantages and limitations. The major limitation is the need for a second surgical site as a donor, which is most commonly the hard palate [17]. The other limitation of FGG is aesthetic mismatch [18]. Because of these limitations, alternative techniques were introduced. Among the new methods, those that do not need a donor site and provide better aesthetic results are favored. Acellular dermal matrix (ADM) is “a connective tissue allograft generated by a decellularization process, which preserves the intact extracellular matrix of the skin” [19]. It is favored by some clinicians but may have great shrinkage after the healing period and is not completely incorporated histologically [20–22].
Wei et al [22] showed ADM to be less effective in generating attached tissue, compared to FGG, because of shrinkage and “inconsistent quality” of ADM-generated attached tissue. The study reported 3.2 mm and 6.2 mm of attached tissue with 71% versus 16% shrinkage for ADM and FGG, respectively [22]. The other method of keratinized gingival augmentation is tissue-engineered (TE) live-cell therapy, such as expanded allogenic gingival fibroblasts or allogenic keratinocyte/fibroblast bilayer constructs (BCTs). McGuire and Nunn [23] found 1 mm less keratinized tissue generated for a human fibroblast-derived dermal substitute compared to apically positioned flap/vestibuloplasty (APF/V) plus FGG (2.7 mm versus 3.9 mm of keratinized tissue width). In a multicenter study, McGuire et al [24] found 4.6 mm of keratinized tissue generated by APF/V plus FGG versus 3.2 mm for APF/V plus BCT. Today, it is generally believed that APF/V plus FGG can be expected to generate 4 mm of keratinized tissue, whereas graft substitutes, including TE constructs, appear to generate 3mm of keratinized tissue [9]. It should be noted that live-cell therapies cause immunologic complications and virus infection transmission.
The Geistlich Mucograft ® is a unique 3D CM designed for soft-tissue regeneration in the oral cavity. Some previous studies have examined CM but mostly around dental implants. Sanz et al [13] compared CM with free connective tissue graft around teeth with 1mm or less of keratinized tissue. The width of gained keratinized tissue was not significantly different between the two groups after 6 months (2.6 mm versus 2.5 mm) while CM had lower patient morbidity and less surgery time [13]. In 2011, Nevins et al [8] compared the use of a bilayer CM to an autogenous gingival graft (AGG) in the ability to increase the zone of keratinized attached gingiva. In this prospective split-mouth pilot case series, five patients with inadequate keratinized attached gingiva bilaterally in the posterior mandible were enrolled. There was a statistically significant increase in attached gingiva at all test (CM) and control (AGG) sites. They conclude that CM has lower morbidity and provides unlimited supply and patient satisfaction; therefore, it appears to be a suitable substitute for FGG in vestibuloplasty to increase keratinized tissue around teeth [8]. McGuire and Scheyer [25] published the long-term results in 2016 and stated that keratinized tissue width averaged >3 mm for both test and control sites five years postoperatively. PPD remained the same at all the time points [25]. The 6-month to 5-year changes for root coverage, keratinized tissue width, and PPD were not significantly different between the groups. Color match to surrounding tissues remained similar in both groups. There was a difference in tissue texture 6 months and 5 years postoperatively; sites of CM with coronally advanced flap were equally firm while sites of connective tissue grafts with coronally advanced flap were more firm. Patient satisfaction was high with no statistically significant difference between two treatment modalities at any time point [25]. Our study revealed the same outcomes at the 6-month follow-up. Schmitt et al [12] compared vestibuloplasty in edentulous lower jaws with dental implants using two layers of FGG in one group of patients and CM in another group. Both groups showed the same healing postoperatively. However, after 5 years, the FGG group showed more loss of keratinized tissue width. They also reported that the use of CM not only reduced the surgery time but also provided better aesthetics [12]. This was in line with the results of our study. In 2018, Menceva et al [11] performed FGG and CM surgery in patients with gingival recession and took a micro-punch biopsy of the grafted area and evaluated it histologically after 6 months. They reported a significant difference as the CM group showed more mature collagen tissue while the FGG group showed more fragmented collagen and elastic fibers [11].
According to the results of the present study, CM can be considered as a substitute for traditional soft tissue autogenous graft therapy. The control FGG generated significantly more keratinized tissue than did the test CM after 6 months (6.75 mm versus 2.92 mm). However, it is mostly accepted that 2.0 mm of keratinized tissue is sufficient [2,26]. It is unknown whether more keratinized tissue is necessarily better, a question for which the answer may never exactly be known.
The use of CM has remarkable advantages, which are shown in articles similar to the current one [13]. The time of surgery is much less when using CM, and patients experience less pain in this method. It also provides an unlimited source of tissue, and there is no significant difference in periodontal parameters using this xenograft. Nevertheless, there is a lack of data on the long-term stability of the CM results, and future studies with long-term follow-ups are suggested.
CONCLUSION
CM appears to be a suitable substitute for FGG in vestibuloplasty procedures designed to increase keratinized tissue around teeth. It has remarkable benefits, such as acceptable keratinized tissue gain, less pain, less surgical chair time, and suitable color match.
ACKNOWLEDGMENTS
The authors thank the Vice-Chancellery of Research of Tehran University of Medical Sciences for supporting this research (Grant no. 18791).
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared
REFERENCES
- 1.Shirmohammadi A, Faramarzie M, Lafzi A. A clinical evaluation of anatomic features of gingiva in dental students in tabriz, iran. J Dent Res Dent Clin Dent Prospects. 2008. Summer;2(3):90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang NP, Löe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972. Oct;43(10):623–7. [DOI] [PubMed] [Google Scholar]
- 3.Lindhe J, Nyman S. Alterations of the position of the marginal soft tissue following periodontal surgery. J Clin Periodontol. 1980. Dec;7(6):525–30. [DOI] [PubMed] [Google Scholar]
- 4.Freedman AL, Salkin LM, Stein MD, Green K. A 10-year longitudinal study of untreated mucogingival defects. J Periodontol. 1992. Feb;63(2):71–2. [DOI] [PubMed] [Google Scholar]
- 5.Chambrone L, Valenzuela FSG, Oliveira L. Rationale for Gingival Tissue Augmentation and Vestibuloplasty Around Teeth and Dental Implants. In: Nares S. Advances in Periodontal Surgery. A Clinical Guide to Techniques and Interdisciplinary Approaches. Springer; Nature Switzerland AG, 2020:157–176. [Google Scholar]
- 6.Thoma DS, Benić GI, Zwahlen M, Hämmerle CH, Jung RE. A systematic review assessing soft tissue augmentation techniques. Clin Oral Implants Res. 2009. Sep;20 Suppl 4:146–65. [DOI] [PubMed] [Google Scholar]
- 7.Edel A. The use of a free connective tissue graft to increase the width of attached gingiva. Oral Surg Oral Med Oral Pathol. 1975. Mar;39(3):341–6. [DOI] [PubMed] [Google Scholar]
- 8.Nevins M, Nevins ML, Kim SW, Schupbach P, Kim DM. The use of mucograft collagen matrix to augment the zone of keratinized tissue around teeth: a pilot study. Int J Periodontics Restorative Dent. 2011. Jul–Aug;31(4):367–73. [PubMed] [Google Scholar]
- 9.McGuire MK, Scheyer ET. Randomized, controlled clinical trial to evaluate a xenogeneic collagen matrix as an alternative to free gingival grafting for oral soft tissue augmentation. J Periodontol. 2014. Oct;85(10):1333–41. [DOI] [PubMed] [Google Scholar]
- 10.Dragan IF, Hotlzman LP, Karimbux NY, Morin RA, Bassir SH. Clinical Outcomes of Comparing Soft Tissue Alternatives to Free Gingival Graft: A Systematic Review and Meta-Analysis. J Evid Based Dent Pract. 2017. Dec;17(4):370–380.e3. [DOI] [PubMed] [Google Scholar]
- 11.Menceva Z, Dimitrovski O, Popovska M, Spasovski S, Spirov V, Petrushevska G. Free Gingival Graft versus Mucograft: Histological Evaluation. Open Access Maced J Med Sci. 2018. Mar 27;6(4):675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt CM, Moest T, Lutz R, Wehrhan F, Neukam FW, Schlegel KA. Long-term outcomes after vestibuloplasty with a porcine collagen matrix (Mucograft®) versus the free gingival graft: a comparative prospective clinical trial. Clin Oral Implants Res. 2016. Nov;27(11):e125–e133. [DOI] [PubMed] [Google Scholar]
- 13.Sanz M, Lorenzo R, Aranda JJ, Martin C, Orsini M. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: a randomized prospective clinical trial. J Clin Periodontol. 2009. Oct;36(10):868–76. [DOI] [PubMed] [Google Scholar]
- 14.Bjorn H. Free transplantation of gingiva propria. Swed Dent J. 1963;22:684–689. [Google Scholar]
- 15.Stetler KJ, Bissada NF. Significance of the width of keratinized gingiva on the periodontal status of teeth with submarginal restorations. J Periodontol. 1987. Oct;58(10):696–700. [DOI] [PubMed] [Google Scholar]
- 16.Deo SD, Shetty SK, Kulloli A, Chavan R, Dholakia P, Ligade S, et al. Efficacy of free gingival graft in the treatment of Miller Class I and Class II localized gingival recessions: A systematic review. J Indian Soc Periodontol. 2019. Mar–Apr;23(2):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keceli HG, Aylikci BU, Koseoglu S, Dolgun A. Evaluation of palatal donor site haemostasis and wound healing after free gingival graft surgery. J Clin Periodontol. 2015. Jun;42(6):582–9. [DOI] [PubMed] [Google Scholar]
- 18.Shah R, Thomas R, Mehta DS. Recent modifications of free gingival graft: A case series. Contemp Clin Dent. 2015. Jul–Sep;6(3):425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boháč M, Danišovič Ľ, Koller J, Dragúňová J, Varga I. What happens to an acellular dermal matrix after implantation in the human body? A histological and electron microscopic study. Eur J Histochem. 2018. Jan 22;62(1):2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei PC, Laurell L, Geivelis M, Lingen MW, Maddalozzo D. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 1. A clinical study. J Periodontol. 2000. Aug;71(8):1297–305. [DOI] [PubMed] [Google Scholar]
- 21.Basegmez C, Karabuda ZC, Demirel K, Yalcin S. The comparison of acellular dermal matrix allografts with free gingival grafts in the augmentation of peri-implant attached mucosa: a randomised controlled trial. Eur J Oral Implantol. 2013. Summer;6(2):145–52. [PubMed] [Google Scholar]
- 22.Wei PC, Laurell L, Lingen MW, Geivelis M. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 2. A histological comparative study. J Periodontol. 2002. Mar;73(3):257–65. [DOI] [PubMed] [Google Scholar]
- 23.McGuire MK, Nunn ME. Evaluation of the safety and efficacy of periodontal applications of a living tissue-engineered human fibroblast-derived dermal substitute. I. Comparison to the gingival autograft: a randomized controlled pilot study. J Periodontol. 2005. Jun;76(6):867–80. [DOI] [PubMed] [Google Scholar]
- 24.McGuire MK, Scheyer ET, Nevins ML, Neiva R, Cochran DL, Mellonig JT, et al. Living cellular construct for increasing the width of keratinized gingiva: results from a randomized, within-patient, controlled trial. J Periodontol. 2011. Oct;82(10):1414–23. [DOI] [PubMed] [Google Scholar]
- 25.McGuire MK, Scheyer ET. Long-Term Results Comparing Xenogeneic Collagen Matrix and Autogenous Connective Tissue Grafts With Coronally Advanced Flaps for Treatment of Dehiscence-Type Recession Defects. J Periodontol. 2016. Mar;87(3):221–7. [DOI] [PubMed] [Google Scholar]
- 26.Wennström J, Lindhe J, Nyman S. Role of keratinized gingiva for gingival health. Clinical and histologic study of normal and regenerated gingival tissue in dogs. J Clin Periodontol. 1981. Aug;8(4):311–28. [DOI] [PubMed] [Google Scholar]




