Abstract
The coronavirus disease 2019 (COVID-19) has now rapidly spread around the world, causing an outbreak of acute infectious pneumonia. To develop effective and safe therapies for the prevention and treatment of COVID-19 has become the major global public health concern. Traditional medicine (TM)/herbal medicines (HMs) have been used to treat multiple epidemics in human history, which brings hope for the fight against COVID-19 in some areas. For example, in China, India, and South Korea with traditional medication history and theory, the governments issued a series of guidelines to support TM/HMs in the medication of COVID-19. In contrast, other countries e.g. North American and European governments are typically silent on these practices, unless to warn of possible harm and overselling. Such difference is due to the discrepancy in culture, history and philosophical views of health care and medication, as well as unharmonized policies and standards in the regulation and legalization of TM/HMs among different areas. Herein, we reviewed the responses and scientific researches from seven selected countries on the policies and legalization of TM/HMs to treat COVID-19, and also analyzed the major challenges and concerns to utilize the traditional knowledge and resource.
Abbreviations: AYUSH, Ayurveda, Yoga and Naturopathy, Unani, Siddha, Sowa Rigpa and Homeopathy; CDC, Centre for Disease Control; COVID-19, corona virus disease 2019; EU, European Union; HMs, herbal medicine; MHRA, Medicines and Healthcare products Regulatory Agency; PIs, pattern identifications; SARS, severe acute respiratory syndrome; SARS-CoV, severe acute respiratory syndrome corona virus; TCM, traditional Chinese medicine; TGA, Therapeutic Goods Administration; TKM, traditional Korean medicine; TM, traditional medicine; TURs, traditional use registrations; WEU-MAs, well-established use marketing authorizations; WHO, The World Health Organization
Keywords: COVID-19, Traditional medicine, Herbal medicines, Policy, Legalization
Graphical Abstract
1. Introduction
As a newly identified strain of coronavirus that causes illness ranging from symptoms similar to the common cold to fatal cases in human, coronavirus disease 2019 (COVID-19) has rapidly become the pandemic responsible for the current global health crisis. As of 21 December 2020, there have been 75,110,651confirmed cases of COVID-19, including 1,680,395 deaths in 216 countries or territories, reported to the World Health Organization (WHO). The majority of cases were reported in the USA, followed by India, Brazil, and Russian Federation [1].
Currently, several small molecule drugs are being tested for their efficacy on COVID-19 infection, some of which have reached clinical trials, while others are still in preclinical phase [2]. Velkury (remdesivir), the first and only drug approved by the U.S. Food and Drug Administration (FDA) for COVID-19 in the USA, was shown to shorten the recovery time of patients from 15 days to 10 days based on three randomized controlled trials [3]. But according to a WHO Solidarity trial, remdesivir and three other drugs appeared to have little or no effect on hospitalized COVID-19 patients, as indicated by overall mortality, initiation of ventilation and duration of hospital stay [4]. Besides the low accessibility, the price of getting remdesivir treatment ranging from US$2340 to US$3120 for a five-day course was also considered to be too high for patients worldwide. Since COVID-19 is not a rare disease, the development of other more scalable, affordable and equitable treatments is still of great importance.
On the other hand, traditional medicine (TM)/herbal medicines (HMs) have a long history and played an indispensable role in the prevention and treatment of several epidemic diseases. In 2003, a traditional Chinese medicine (TCM) was used to treat the severe acute respiratory syndrome (SARS) and showed significant beneficial effect [5]. The use of HMs in malaria ultimately led to the discovery of artemisinin, an herbal extract from Artemisia annua Linn. used as part of the standard treatment worldwide for Plasmodium falciparum Welch malaria [6]. Existing evidences showed that compared to the treatment of western medicine, the integration of TM and western medicine (hereinafter referred to as “Integrated Medicine”) for COVID-19 may have better effects [7], [8]. Due to the effectiveness, safety profile and relatively lower medication cost, TM/HMs therapies are still an alternative cost-effective strategy against COVID-19 in many countries or regions.
In the meantime, the perspectives on the use of TM/HMs to prevent and treat COVID-19 are inconsistent or even polarized worldwide. This study, therefore, aims to provide an overview of responses and policies related to TM/HMs for COVID-19 in selected countries. Besides, the current challenges and efforts to better utilize TM/HM in the therapy of COVID-19 have been discussed. The outcome of the study is expected to provide clues to understand the current status and possibilities of using TM/HMs for COVID-19, and also be of relevance to regulatory policy decision makers, stakeholders and industry members who wish to better realize the related current regulatory frameworks in the jurisdictions covered.
2. Methods
2.1. Country selection
The databases, i.e., Web of Science Core Collection, MEDLINE, Current Contents Connect, and SCIELO Citation Index were searched with the topic of “traditional medicine COVID-19″ or “herbal medicine COVID-19″ for the screening of countries involved. Data published prior to December 2 2020 were collected. Research domains of Science Technology, Social Sciences and Arts Humanities were included. All document types such as articles, reviews, clinical trials, and case reports were investigated. Data in languages other than English were excluded to ensure the accessibility of information and the further confirmation.
For the first screening, countries with the screened data records more than 2% of the total records were selected for the following analysis. Then, countries with authorized guidelines for the therapy of COVID-19 with TM/HMs were determined to be the ones responding to the prevention and treatment of COVID-19 with TM/HM. Next, countries in other continents were determined to be the typical examples for the individual analysis.
2.2. Data collection and analysis of individual country
Besides data mentioned in 2.1., the official law documents for the registration of HMs in the national drug regulatory authority and related guidelines for COVID-19 in selected countries were identified from the following websites: GOV.CN; National Health Commission of China; National Administration of Traditional Chinese Medicine, China; US Federal Food and Drug Administration; Ministry of Ayurveda, Yoga and Naturopathy, Unani, Siddha, Sowa Rigpa and Homeopathy (AYUSH), Government of India; GOV.UK; Medicines and Healthcare Products Regulatory Agency, UK; Therapeutic Goods Administration, Australian Government Department of Health; Ministry of Health and Medical Education, Iran; Korea Food and Drug Administration; Federal Institute for Drugs and Medical Devices, Germany; Italian Ministry of Health; European Medicines Agency; Guidelines International Network; and Evidence Aid. The keywords combination, such as “COVID” AND “guideline” OR “guide” AND “traditional medicine” OR “herbal medicine”, and the combination of “herbal medicine” OR “herbal drug” OR “herbal product” AND “registration” were used to search related documents. The inclusion and exclusion criteria used for the data screening in this part are presented in Table 1.
Table 1.
Inclusion and exclusion criteria used for the data screening and analysis.
| Inclusion criteria | Exclusion criteria |
|---|---|
| English and/or languages accessible | Languages not accessible |
| Guidelines for COVID-19 involving the use of TM/HMs | Guidelines for COVID-19 excluding the use of TM/HMs |
| HMs used for treating/curing purposes or supporting/improving body functions | HMs that do not have a therapeutic effect and are used as additives, flavors or cosmetics |
| HMs for human use only | HMs for animal use |
2.3. Data analysis
Data selected were extracted into a Microsoft Excel spreadsheet, analyzed for similarities and differences across the countries within each category, and presented in a comparison table. GraphPad Prism Version 7.0 for Windows software (Graphpad Software Inc., San Diego, CA, USA) was used for the analysis of data. The terminology used to describe “herbal medicines” in one country is different in another country; therefore, to maintain the consistency in the comparison procedure, where appropriate, the authors have defined “HMs” as “herbal crud drugs, prepared slices of herbal crude drugs, and herbal preparations used for maintaining health and to prevent, alleviate, or cure disease”.
3. Results and discussion
3.1. Country determination
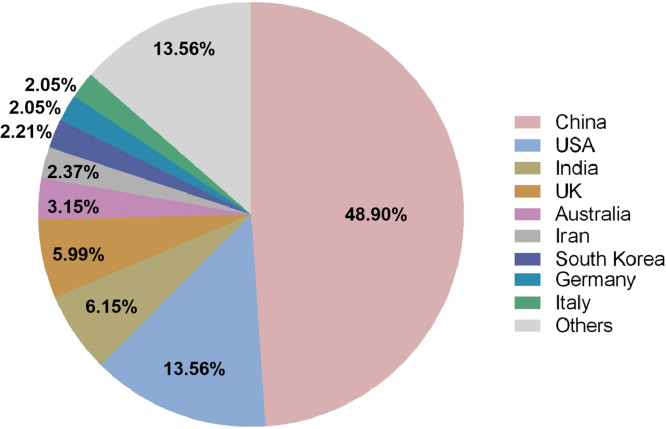
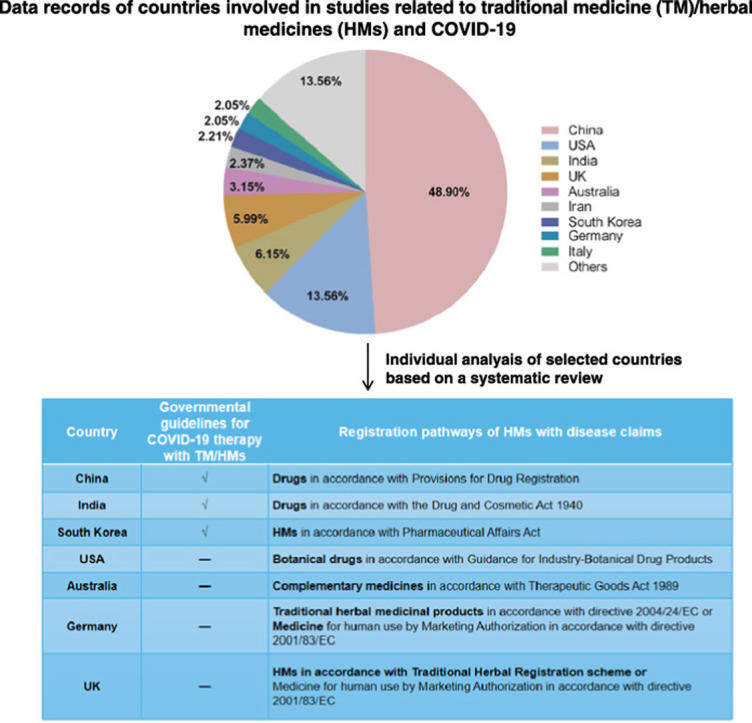
A total of 634 records of TM/HMs related to COVID-19 were found. Among them, 48.90% of the data were contributed by institutes in China, following 13.56% of USA, 6.15% of India, 5.99% of UK and 3.15% of Australia. Other countries with the data contribution higher than 2% were Iran, South Korea, Germany, and Italy ( Fig. 1). The data from the above nine countries contributed 86.44% of the total records, indicating their relatively high involvement in this topic. Therefore, the subsequent screening of related guidelines for COVID-19 with the use of TM/HM of the selected nine countries were performed.
Fig. 1.
Percentages of number of publications of different countries involved in research related to traditional medicine/herbal medicines and COVID-19 from databases of Web of Science Core Collection, MEDLINE, Current Contents Connect, and SCIELO Citation Index (accessed 2 December 2020).
According to the results, there are currently 6 versions of national diagnosis and treatment guidelines in China, 6 governmental guidelines for AYUSH practitioners in India, and 4 Korean medicine-professional associations-issued Korean guidelines which involve the application of TM/HMs for the prevention and treatment of COVID-19 ( Table 2). Therefore, a more detailed introduction of responses from China, India and South Korea to the prevention and treatment of COVID-19 with TM/HMs has been described in the following section.
Table 2.
National authorized guidelines for the prevention and treatment of COVID-19 with traditional medicine (TM)/herbal medicines (HMs) in China, India and South Korea.
| Country | TM system | Guideline | Involvement of TM |
Recommendation of HMs |
|||
|---|---|---|---|---|---|---|---|
| Prevention/ | Diagnosis & treatment | Single herb | Herbal formulae | Registered herbal preparation | |||
| immune enhancement | |||||||
| China | Traditional Chinese medicine | Guideline for the Diagnosis and Treatment of COVID-19 (Trial 3rd edition)[9] | – | √ | – | √ | – |
| Guideline for the Diagnosis and Treatment of COVID-19 (Trial 4th edition)[10] | – | √ | – | √ | √ | ||
| Guideline for the Diagnosis and Treatment of COVID-19 (Trial 5th edition)[11] | – | √ | – | √ | √ | ||
| Guideline for the Diagnosis and Treatment of COVID-19 (Trial 6th edition)[12] | – | √ | – | √ | √ | ||
| Guideline for the Diagnosis and Treatment of COVID-19 (Trial 7th edition)[13] | – | √ | – | √ | √ | ||
| Guideline for the Diagnosis and Treatment of COVID-19 (Trial 8th edition)[14] | – | √ | – | √ | √ | ||
| India | Ayurveda | Guidelines for Ayurveda Practitioners for COVID 19[15] | √ | √ | √ | √ | √ |
| Yoga | Guidelines for Yoga Practitioners for COVID 19[16] | √ | – | – | – | – | |
| Unani | Guidelines for Unani Practitioners for COVID 19[17] | √ | √ | √ | √ | √ | |
| Siddha | Guidelines for Siddha Practitioners for COVID 19[18] | √ | √ | √ | √ | √ | |
| Homoeopathic | Guidelines for Homoeopathic Practitioners for COVID 19[19] | √ | √ | √ | – | – | |
| Naturopathy | Guidelines for Naturopathy Practitioners for COVID 19[20] | √ | √ | √ | – | – | |
| South Korea | Traditional Korean medicine | COVID-19 Korean medicine clinical guidance (1st edition)[21] | – | √ | – | √ | √ |
| COVID-19 Korean medicine clinical guidance (2nd edition)[22] | – | √ | – | √ | √ | ||
| A consensus guideline of herbal medicine for coronavirus disease 2019[23] | – | √ | – | √ | √ | ||
| Guidance on COVID-19 Telemedicine of Korean Medicine[24] | √ | √ | – | √ | √ | ||
For the other countries, TM and HMs have not been included in their published guidelines. To better evaluate their related policies and legislation status, representative countries from different continents other than Asia, were selected from the nine prior indicated countries for the individual analysis, i.e. USA, Australia, Germany and UK. All the four countries have established registration systems for HMs and are major western countries where TM and HMs are used [25]. USA is the main market for the pharmaceutical industry in which approximately 20,000 kinds of HMs are available with an estimated value of US $62 billion, which the WHO expected will be increased to US $5 trillion by 2050 [25], [26]. In Australia, the practice of TM has been regulated on a national level since 2012, which paved a way for the integration of TM and prescription of HMs into the local mainstream healthcare system there [27]. For countries within the European Union (EU), the regulation of HMs falls within the scope of European Directive 2004/24/EC, which obligates each herbal medicinal product to be granted in the market. Among those EU countries, Germany has a very well-established HM registration system that predates EU legislation and approximately 70% of German physicians have confidence in prescribing HMs to their patients [28]. In contrast, UK introduced a system for HMs relatively recent based on the EU Directive, but also with some differences in the implementation of Directives [29], [30]. Therefore, Germany and UK are both chosen as representative countries of Europe.
3.2. The use of TM/HMs for COVID-19 based on guidelines in selected countries
3.2.1. China
TCM has a long history and played an indispensable role in the prevention and treatment of epidemic diseases in China. As an integral part of health services in China, TCM has been recommended in the official diagnosis and treatment guideline of COVID-19 since the beginning of the epidemic outbreak [9]. From the third to the eighth edition of the official guidelines [9], [10], [11], [12], [13], [14], TCM prescriptions and therapies have been included and constantly improved in different stages of prevention, critical illness and recovery of COVID-19, which showed good therapeutic effect [31] (Table 2). With the government-issued national guidelines as a reference, 17 provinces, 4 municipalities and 4 autonomous regions in mainland China officially issued traditional medicine-related guidelines for the prevention and treatment of COVID-19. All the guidelines were devised by local health authorities according to regional characteristics and local prevalence of COVID-19 and have been continuously updated since the first issuance [32].
In China, the Chinese government credits its swift turnaround to an ‘‘integrative’’ method through which patients received TCM plus biomedicine [33]. To prioritize the registration management of new drugs for COVID-19, including TCM, the State Administration for Market Regulation released a guideline on drug registration management on March 30, 2020 [34]. According to the guideline, the registrations of TCM are categorized as innovative TCM, modified TCM, compound preparation of classical prescription, compound preparation of the same prescription and so on. For the normal procedure of drug registration, the applicant must provide enough research data of the applied drug, including results of pharmacy, pharmacology, toxicology, clinical trials, quality standards, verification of production process in commercial scale and preparation for the inspection. Meanwhile, special registration with faster procedure can be applied for novel drugs meeting the following requirements: 1) drugs used to prevent and treat a severe disease with clinic evidences, which cannot be treated effectively by other drugs or therapies; 2) drugs in urgent need for public health, which show therapeutic effect in the clinic; 3) vaccines in response to the critical public health emergencies, of which the evaluated benefits are higher than the risks taken. Until now, three TCM products have been approved to treat patients with COVID-19 by the National Medical Products Administration of China [35]. These are Jinghua Qinggan Granules, Lianhua Qingwen Granules & Capsules, and Xue Bi Jing Injection.
3.2.2. India
Traditional Indian medicine is one of the oldest care systems to treat various diseases in human history. The Indian government has legalized traditional medicine systems, namely, Ayurveda, Yoga and Naturopathy, Unani, Siddha, Sowa Rigpa and Homeopathy, which are regulated by an independent Ministry of AYUSH. To respond the crisis of COVID-19, each of these systems published corresponding guidelines for the practitioners based on their own theory, standard, regulation policy, and research council [36] (Table 2). For example, in Unani medicine, epidemics are thought to occur if some contagion or certain harmful substances stay in air and water. Based on the modes of transmission and infection of COVID-19, general measures of isolation, quarantine, distancing and sanitization have been emphasized. Another traditional medicine system, Ayurveda, pays a higher emphasis on building strength of mind and body to cope with various stressors [37]. Since the COVID-19 crisis has led to high levels of psychological distress and induced significant impact on mental health, people with poor immune function and mental health conditions are more susceptible to viral respiratory tract infections [38]. Therefore, the Ayurvedic practitioners, through the Government of India, have suggested ten measures to help boost the immunity against COVID-19 through a possible and potential psychoneuroimmune mechanism. These measures include the consumption of warm water and herbal decoctions, oral rinsing with oil, and steam inhalation described in Ayurveda for respiratory illnesses [39].
Until now, several initiatives have been taken to utilize the vast potential of TM/HMs in the pandemic of COVID-19. The AYUSH systems of medicines issued an “Advisory on Coronavirus”. The advisory suggests “Homoeopathy for Prevention of Coronavirus Infections” and “Unani Medicines useful in the symptomatic management of Coronavirus infection”. The advisory listed several “Preventive Management Steps” as per Ayurvedic practices, and several “Unani Medicines useful in the symptomatic management of Coronavirus infection”. The recommended formulations include HMs of Sunthi (Zingiber officinale Roscoe.), Lavanga (Syzygium aromaticum Merr. & L. M. Perry) and Maricha (Piper nigrum Linn.) [40].
3.2.3. South Korea
Traditional Korean medicine (TKM) originates from prehistoric times and shares some similarities with TCM. Similar to the treating principle of TCM, TKM identifies the pathogenesis of COVID-19 to be the dampness obstructing the lung for the mild stage and toxin blocking the lung for the severe stage [32]. In South Korea, the Association of Korean Medicine and the Korean Association of Traditional Pulmonary Medicine each issued the traditional medicine guidelines on the prevention and treatment of COVID-19 at the end of February 2020 [41]. All the guidelines were drafted by clinical experts who were also regularly updated by both associations to provide clinical guidance for the prevention and treatment of COVID-19 in South Korea (Table 2).
According to the TKM guidelines on the prevention and treatment of COVID-19 issued in February 2020, 4 pattern identifications (PIs) and 15 herbal formulae for the mild stage, 3 PIs and 3 herbal formulae for the severe stage, and 2 PIs and 2 herbal formulae for the recovery stage are suggested. In addition, the Association of Korean Medicine established a telemedicine center for treating COVID-19. After reviewing the medical records of patients receiving telemedicine care for about a month from March 2020, 30% of the total herbal formulae advised was Qingfei Paidu Decoction [24], which has been recommended by both Chinese and Korean guidelines. This formula was reported to increase the immune system and reduce inflammation by interfering with viral infection-related pathways and cancer-related pathways when treating COVID-19 [42].
In Korea, at least 69% of the population has experienced TKM [43]. In recent years, TKM has become part of a national health system and its use has expanded. For the therapy against COVID-19, many of the herbal formulas used in the guidelines are within the health insurance system of South Korea [44]. TKM telemedicine care was able to continue treatment without interruption even if the patient’s residence was changed, which showed advantages in terms of efficient use of traditional Korean medical resources and continuous treatment of patients. Thus, by integrating traditional and modern approaches, TKM constitutes an exemplary case of a national health system that encompasses complementary and alternative approaches to prevent and treat COVID-19.
3.3. Policies and registration for TM/HMs in other selected countries
3.3.1. USA
The USA is the major market for the pharmaceutical industry in which approximately 20,000 HMs are available [45] with an estimated value of $62 billion. Most of the herbal products in USA are permitted to be marketed as foods or dietary supplements, which are used by acupuncturists/herbalists based on a one-to-one practitioner-client consultation [46]. Currently, multiple organizations such as the University of Arizona Andrew Weil Center for Integrative Medicine, the American Nutrition Association, and the American Association of Naturopathic Physicians have responded to COVID-19 by developing resource sites with respectful caveats and then linked professionals and members of the public to potentially useful supportive practices and natural agents cited by the existing suggestive science. Some practitioners of TM in USA also tend to practice philosophically more closely to the Chinese and Indian government perspective, by giving therapies which might have value in preventing, supporting, complementing, or rehabilitating COVID-19 [47]. However, the TM/HMs used currently can not be claimed to treat COVID-19 due to the characters of HMs and regulations.
First of all, since herbal products are mostly marketed as food supplements in USA, the health claims for medical uses are not permitted on the label of these products [48]. Otherwise, the marketer/manufacturer will be considered as marketing non-approved drugs. Secondly, if a herbal product is intended for use as part of a disease treatment regimen, e.g. for COVID-19, and intended to label such disease claims, the product will be regarded as a drug and requires assessing and registration under FDA’s Guidance for Industry-Botanical Drug Products [49] prior to marketing ( Table 3). This classifies the product under the regulations of botanical drug and the same rigorous requirements and standards as for conventional drugs apply, including the evidence of clinical studies, and assurance of quality and consistency of the product. Thirdly, even though FDA recognized previous human use of a herbal product and foreign clinical trial data as part of the evaluation [49], the lack of tradition use and well-designed clinical trials of HMs used in other countries now make it unlikely to approve HMs for the treatment of COVID-19 in a short term. As long as medicinal herbs lack data of the quality, efficacy, safety, and intellectual property protection, or the ethical committees do not allow to prove them, it is unlikely that there is much intention by pharmaceutical companies to invest money in the drug development based on HMs, like Western companies do, with legal patents.
Table 3.
Regulatory authorities and registration pathways of traditional herbal medicines (HMs) in selected countries.
| Country | Regulatory authority | Registration pathways of HMs with disease claims |
|---|---|---|
| China | National Medical Products Administration; National Administration of Traditional Chinese Medicine | Drugs in accordance with Provisions for Drug Registration |
| India | Ministry of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy | Drugs in accordance with the Drug and Cosmetic Act 1940 |
| South Korea | Korea Food and Drug Administration | Herbal medicines in accordance with Pharmaceutical Affairs Act |
| USA | Federal Food and Drug Administration | Botanical drugs in accordance with Guidance for Industry-Botanical Drug Products |
| Australia | Therapeutic Goods Administration | Complementary medicines in accordance with Therapeutic Goods Act 1989 |
| Germany | Federal Institute for Drugs and Medical Devices; European Medicines Agency | Traditional herbal medicinal products in accordance with directive 2004/24/EC or |
| Medicine for human use by Marketing Authorization in accordance with directive 2001/83/EC | ||
| UK | Medicines and Healthcare products Regulatory Agency | HMs in accordance with Traditional Herbal Registration scheme or |
| Medicine for human use by Marketing Authorization in accordance with directive 2001/83/EC |
3.3.2. Australia
HMs are referred to as complementary medicines in Australia, which are regulated under the legislation of therapeutics goods according to the Therapeutic Goods Act 1989 [50]. The Therapeutic Goods Administration (TGA) is responsible for the provision administration of the Act. Only therapeutic goods which have been evaluated by the TGA can be included in the database of the Australian Register of Therapeutic Goods prior to their supply.
Based on the level of risk, HMs are entered in the Australian Register of Therapeutic Goods as either Listed (of lower level of risk) or Registered (of higher level of risk) products. A particular HM may be associated with low or high risk depending on the toxicity of ingredients, proposed dosage, appropriateness of the indications and claims for self-diagnosis and management and the potential for adverse reactions. HMs which are assessed to be of higher risk must be individually evaluated for safety, quality and efficacy before they are released into the market. Since COVID-19 is considered a “serious form” of a medical condition, any products, including HMs, that claim to be used therapeutically in relation to COVID-19 are required to be expressly pre-approved by the TGA. Therefore, a complete procedures including licensing of manufacturers with the principles of Good Manufacturing Practice, pre-market assessment of products, and post-market regulatory activity should be performed for the inspection and audit by the TGA.
From what has been mentioned above, therapeutic goods, including TM/HMs products not expressly pre-approved by the TGA, cannot make any outright or implied reference to use as the therapeutic agents for COVID-19 in advertising to either the public or health professionals. Similar as the situation in the US, each procedure to prove the safety, quality and efficacy of HMs for the therapy of COVID-19 would take great investment, efforts and rather long time, which makes the legalization of such therapies based on TM/HMs hard.
3.3.3. Germany and UK
Germany has the most advanced herbal medicine processing technology in Europe, possessing the single largest HMs market and a longstanding culture and practice of TM [51]. As part of the EU, Germany is obligated to certain legislative acts concerning the use of HMs which have been issued for the union as a whole, which can be an example for 27 member states in EU. The main regulatory body of EU is the European Medicines Agency and Germany also has its own regulatory agency e.g. the Federal Institute for Drugs and Medical Devices [52]. The European Medicines Agency Community monograph is used as the safety and efficacy reference material and assessment standard by applicants and National Competent Authorities, which plays a supportive function in the marketing authorization or registration procedure in member states [53]. Traditional HMs have to be licensed under related EU and national regulations before circulated in the market. The major routes for the market approval of HMs include the full marketing authorization, well-established use marketing authorizations (WEU-MAs) and traditional use registrations (TURs). Generally, it was difficult for many HMs to meet all the requirements for the full marketing authorization, particularly in relation to efficacy, as are required under Directive 2001/83/EC [54]. Until now, only three new HMs, i.e. Veregen, Sativex and Episalvan, have obtained the full marketing authorizations in the EU, for which the results of non-clinical studies and clinical trials are needed [55]. Whereas for WEU-MAs and TURs, the bibliographic evidence on acceptable or recognized efficacy and safety of the target HM product can be accepted. As per the Traditional Herbal Medicinal Product Directive (2004/24/EC), the results of general toxicological and pharmacological tests or the results of clinical trials are no longer the essential requirement for well-established-use HMs that have at least 10 years of medicinal use in the EU, and for traditional-use HMs if the medicinal use of at least 30 years, including 15 years in the EU, can be documented and the therapeutic indication is considered safe for use without the supervision of a physician [56].
In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) is the regulator of medicines placed on the market. HMs must be registered through traditional herbal registration before marketing in the UK. During the current transition period, the UK and EU still share major common requirements and procedures for the registration of HMs [52]. Similar as the requirement of TURs in EU, applicants are required to produce bibliographic or expert evidences of traditional use of the target HM product. The HMs for registration should have been in medical use throughout a period of at least 30 years preceding the date of application and at least 15 of the 30 years use must relate to the EU [57]. Besides, a dossier demonstrating that the target product meets the requirements of quality, safety and patient information as per the Traditional Herbal Registration Scheme must be submitted to the MHRA. A slight difference from EU is that herbal practitioners in the UK do not need a license to supply HMs they create on the premises to patients following one-to-one consultations [52]. Due to the upcoming end of the transition period after Brexit, there would be some new rules related to the regulation of traditional HMs in the UK from 1 January 2021. For example, the MHRA may be able to accept the 15 years of traditional evidence from a wider range of countries in addition to countries in the European Economic Area [58].
According to the statistic report given by the European Medicines Agency in 2017, there are 286 WEU-MAs and 285 TURs for HMs which had been granted in Germany, and 1 WEU-MAs and 348 TURs granted in the UK [59]. One thing should be noticed that the indications for both WEU-MAs and TURs are limited to mild complaints (e.g. a cold). All severe indications such as diabetes, cancer and virus infections are excluded. Based on this perspective, the route for authorizing HMs to treat COVID-19 should be full marketing authorization but not via WEU-MAs or TURs [52], which requires analytical, pharmaco-toxicological tests and clinical trials and is likely too expensive for most TM/HMs manufacturers in both Germany and UK [57].
3.4. General discussion and conclusion
Due to the resource-poor healthcare system in many countries, the persistent shortages of personnel and equipment may not be able to sustain an emergency response on the scale required for the management of COVID-19 pandemic. Hence, the early control and prevention, less resource-intensive management and cost-effective therapies would be of great importance. TM/HMs, as an integral part of health services in Asian countries for thousands of years, showed an irreplaceable role in the fight against COVID-19 in such areas. On the contrary, the general view from western countries on the use of TM/HMs for therapies of COVID-19 is more inclined to warn or even object. One document from the French Agency for Food, Environmental and Occupational Health & Safety [60] discourages patients from using HMs by declaring that herbal supplements with an activity on the immune system could amplify the inflammation response and consequently worsen COVID-19. Such hypothesis in the association between inflammatory cytokines and poor clinical outcomes in patients might be misleading. Because many HMs capable of eliciting a nonspecific stimulation of the immune system exhibit simultaneously the anti-inflammatory, antiviral and antioxidant effects beneficial for the treatment of COVID-19. For example, Curcuma longa L., a typical traditional Chinese HM containing immunostimulant compounds, has also anti-cancer and antifibrotic activities, which makes it a possible candidate for post-COVID-19 patients with pulmonary fibrosis [61]. Meanwhile, it needs to be emphasized that any self-prescribed use of HMs should be avoided. Supervised TM prescriptions according to individual patient’s characters and illness developing stage are necessary to minimize the possible risk of adverse interactions between HMs and other drugs.
Although the variation in culture, medication and regulation among different countries makes it difficult to take the maximum advantages of TM/HMs to treat COVID-19 currently, some efforts can still be considered. Firstly, for TM/HMs therapies observed to show significant preventive/therapeutic effect in the clinic, rational clinical trials designed with strict and scientific standards should be performed before giving a conclusive claim. Results from initial pharmacological tests such as cell assays or simple animal experiments might be supportive for the action and mechanism elucidation of specific treatments, but are still far away from an authoritative judgement. Secondly, the development of more adaptive regulations conforming to the urgent needs of effective therapies would be necessary nowadays. For example, for a new disease, it’s unrealistic to provide data of more than ten-years medication for the market approval. Some “green channels” could be considered to be built for the application of TM/HMs therapies with confirmed therapeutic effects. Thirdly, more harmonized standards on the quality control of HMs are essential to mitigate the delays in the commercialization of beneficial products across countries. Until now, European Pharmacopoeia TCM working party has elaborated about 80 TCM herbal monographs. And there are ISO standards of more than 14 single HMs which have been published or under developed [62]. These standards provide important references to guarantee the quality consistence and safety of HMs with potential use to treat COVID-19 in the international market. Finally, some compounds derived from medicinal herbs can be interesting targets for further investigation and drug development. For instance, glycyrrhizin, a saponin from licorice, has been demonstrated recently to show an interesting anti-SARS-CoV activity [63]. Huynh et al. [64] found that rutin, a key component in the recommended herbal preparation of Lianhua Qingwen for COVID-19 in China, could be bound inside the main protease’s active site of SARS-CoV-2 and possibly inhibit its biological functions. After the structure optimization of rutin, it possesses much stronger binding affinities to the virus, shedding light on future structure-based designs of high-potent inhibitors for SARS-CoV-2. These investigations would be helpful not only for finding new strategies to limit other outbreaks of the disease, but also for relieving fears and psychological distress spread across the population during this pandemic.
Recently, the Regional Expert Committee on Traditional Medicine for COVID-19 formed by WHO, the Africa Centre for Disease Control and Prevention and the African Union Commission for Social Affairs has endorsed a protocol for phase III clinical trials of HMs for COVID-19 [65]. When in front of the common fight against an epidemic threatening all human beings, an impartial view and concerted efforts from different parties may make it more promising in finding the cure for the disease. Providing scientific evidences with multidisciplinary knowledge with international joint efforts supported by governments and stakeholders is a key for bridging traditional and western medicines.
Author contributions
YX supervised this research. YX and MW prepared the manuscript. MG and HC provided the data and information. BVD and FVH revised the manuscript.
Acknowledgement
This work was supported by National Natural Science Foundation of China (81660661), Fund of Yunnan Quality and Technology Supervision Bureau (KKPT202126008), Chinese National Science and Technology Major Project for “Significant New Drugs Development” (2019ZX09201005-006-004) and Wang Mei Expert Workstation of Yunnan Province (201905AF150001).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.World Health Organization (WHO). WHO Director-General’s opening remarks at the media briefing on COVID-19, 2020 https://covid19.who.int/, (Accessed 21 December 2020).
- 2.Sanders J.M., Monogue M.L., Jodlowski T.Z., et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 3.M. Parsey, An open letter from Merdad Parsey, Chief Medical Officer, Gilead Sciences, 2020. https://stories.gilead.com/articles/open-letter-from-merdad-parsey, (Accessed 22 October 2020).
- 4.World Health Organization (WHO). “Solidarity” Clinical trial for COVID-19 treatments, 2020. https://www.who.int/solidarity-clinical-trial-for-covid-19-treatments, (Accessed 10 November 2020).
- 5.Liu J.P., Manheimer E., Shi Y., Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J. Altern. Complement. Med. 2004;10:1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 6.Tu Y. Artemisinin – a gift from traditional Chinese medicine to the world (Nobel lecture) Angew. Chem. Int. Ed. 2016;55:10210–10226. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- 7.Lu R., Wang W., Li X. Clinical observation on 63 cases of suspected cases of new coronavirus pneumonia treated by Chinese medicine Lianhua Qingwen. J. Tradit. Chin. Med. 2020;61:655–659. [Google Scholar]
- 8.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z., Shuxiang H., Jun D., Xiaobo L., Xiaotao H., Lin W., Nanshan Z., Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission of the People’s Republic of China, Guideline for the Diagnosis and Treatment of COVID-19 (Trial 3rd edition), 2020. http://www.nhc.gov.cn/yzygj/s7653p/202001/f492c9153ea9437bb587ce2ffcbee1fa.shtml, (Accessed 28 October 2020).
- 10.National Health Commission of the People’s Republic of China, Guideline for the Diagnosis and Treatment of COVID-19 (Trial 4th edition), 2020. http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67.shtml, (Accessed 2 December 2020).
- 11.National Health Commission of the People’s Republic of China, Guideline for the Diagnosis and Treatment of COVID-19 (Trial 5th edition), 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml, (Accessed 2 December 2020).
- 12.National Health Commission of the People’s Republic of China, Guideline for the Diagnosis and Treatment of COVID-19 (Trial 6th edition), 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml, (Accessed 28 October 2020).
- 13.National Health Commission of the People’s Republic of China, Guideline for the Diagnosis and Treatment of COVID-19 (Trial 7th edition), 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml, (Accessed 2 December 2020).
- 14.National Health Commission of the People’s Republic of China, Guideline for the Diagnosis and Treatment of COVID-19 (Trial 8th edition), 2020. http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a.shtml, (Accessed 2 December 2020).
- 15.Government of India, Ministry of AYUSH. Guidelines for Ayurveda Practioners for COVID 19, 2020. https://www.ayush.gov.in/docs/ayurved-guidlines.pdf, (Accessed 2 December 2020).
- 16.Government of India, Ministry of AYUSH. Guidelines for Yoga Practioners for COVID 19, 2020. https://www.ayush.gov.in/docs/yoga-guidelines.pdf, (Accessed 2 December 2020).
- 17.Government of India, Ministry of AYUSH. Guidelines for Unani Practioners for COVID 19, 2020. https://www.ayush.gov.in/docs/unani-guidelines.pdf, (Accessed 2 December 2020).
- 18.Government of India, Ministry of AYUSH. Guidelines for Siddha Practioners for COVID 19, 2020. https://www.ayush.gov.in/docs/siddha-guidelines.pdf, (Accessed 2 December 2020).
- 19.Government of India, Ministry of AYUSH. Guidelines for Homoeopathic Practioners for COVID 19, 2020. https://www.ayush.gov.in/docs/homeopathy-guidelines.pdf, (Accessed 2 December 2020).
- 20.Government of India, Ministry of AYUSH. Guidelines for Naturopathy Practioners for COVID 19, 2020. https://www.ayush.gov.in/docs/naturopathy-guidelines.pdf, (Accessed 2 December 2020).
- 21.Korean Medicine Convergence Research Information Center. The Association of Korean Medicine COVID-19 Korean medicine clinical guidance (1st edition), 2020. https://www.kmcric.com/news/newspaper/view/41660, (Accessed 2 December 2020).
- 22.Korean Medicine Convergence ResearchInformation Center. The Association of Korean Medicine COVID-19 Korean medicine clinical guidance (2nd edition), 2020. https://www.kmcric.com/news/newspaper/view/41842, (Accessed 2 December 2020).
- 23.Lee B.J., Lee J.A., Kim K.I., Choi J.Y., Jung H.J. A consensus guideline of herbal medicine for coronavirus disease 2019. Integr. Med. Res. 2020;9 doi: 10.1016/j.imr.2020.100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D., Chu H., Min B.K., Moon Y., Park S., Kim K., Park S.H., Kim Y.D., Song M., Choi Gh, Lee E. Telemedicine Center of Korean Medicine for treating patients with COVID-19: a retrospective analysis. Integr. Med. Res. 2020;9(3) doi: 10.1016/j.imr.2020.100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alostad A.H., Steinke D.T., Schafheutle E.I., et al. International comparison of five herbal medicine registration systems to inform regulation development: United Kingdom, Germany, United States of America. Pharmacol. Res. 2018;32(1):32–49. doi: 10.1007/s40290-018-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajazuddin S.S. Legal regulations of complementary and alternative medicines in different countries. Pharmcol. Rev. 2012;6(12):154–160. doi: 10.4103/0973-7847.99950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J., Loyeung B., Zaslawski C., Liang Fr, Li Wh. Comparison of traditional Chinese medicine education between mainland China and Australia-a case study. J. Integr. Med. 2016;14(4):291–296. doi: 10.1016/S2095-4964(16)60259-5. [DOI] [PubMed] [Google Scholar]
- 28.Joos S., Glassen K., Musselmann B. Herbal medicine in primary healthcare in Germany: the patient’s perspective. Evid. Based Complement. Altern. Med. 2012;2012:1–10. doi: 10.1155/2012/294638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alostad A.H., Steinke D.T., Schafheutle E.I. Herbal medicine classification: policy recommendations. Front. Med. 2020;7 doi: 10.3389/fmed.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramadoss M.S.K., Koumaravelou K. Regulatory compliance of herbal medicines – a review. Int. J. Res. Pharm. Sci. 2019;10(4):3127–3135. [Google Scholar]
- 31.Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19) Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ang L., Lee H.W., Choi J.Y., Zhang J., Lee M.S. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr. Med. Res. 2020;9 doi: 10.1016/j.imr.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., Tian J. Efficacy and safety of integrated traditional Chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.State Administration for Market Regulation. Provisions for Drug Registration, 2020. http://gkml.samr.gov.cn/nsjg/fgs/202003/t20200330_313670.html, (Accessed 30 March 2020).
- 35.National Medical Products Administration of China. 2020. http://www.nmpa.gov.cn/WS04/CL2417/378459.html, (Accessed 28 October 2020).
- 36.Chaturvedi S., Kumar N., Tillu G., et al. AYUSH, modern medicine and the Covid-19 pandemic, Indian. J. Med. Ethics. 2020:1–4. doi: 10.20529/IJME.2020.058. [DOI] [PubMed] [Google Scholar]
- 37.Golechha M. Time to realise the true potential of ayurveda against COVID-19. Brain Behav. Immun. 2020;87:130–131. doi: 10.1016/j.bbi.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajkumar R.P. COVID-19 and mental health: a review of the existing literature. Asian J. Psychiatry. 2020;52 doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajkumar R.P. Ayurveda and COVID-19: where psychoneuroimmunology and the meaning response meet. Brain Behav. Immun. 2020;87:8–9. doi: 10.1016/j.bbi.2020.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Press Information Bureau, Government of India, Advisory for Corona virus. Homoeopathy for Prevention of Corona virus Infections, Unani Medicines useful in symptomatic management of Corona Virus infection, 2020. https://pib.gov.in/PressReleasePage.aspx?PRID=1600895, (Accessed 28 March 2020).
- 41.Association of Korean Medicine News. Announcement of the first version of Oriental Medicine Clinical Practice Guideline by the National University Network of Traditional Medicine Department of Internal Medicine 2020, http://akomnews.com/bbs/board.php?bo table=news&wr id=38324, (Accessed 6 March 2020).
- 42.Zhao J., Tian S.S., Yang J., et al. Investigating the mechanism of Qing-Fei-Pai-Du-Tang for the treatment of novel coronavirus pneumonia by network pharmacology. Chin. Tradit. Herb. Drugs. 2020;51(04):829–835. [Google Scholar]
- 43.Korea Food and Drug Administration. The Study for Activation and Development of Herbal Adverse Reaction Reporting System, 2007. (Accessed 28 October 2020).
- 44.Acupuncture Today. Official COVID-19 Clinical Guidelines from the Association of Korean Medicine (Pt. 1), 2020. https://www.acupuncturetoday.com/print_friendly.php?pr_file_name=http%3A%2F%2Fwww.acupuncturetoday.com%2Fmpacms%2Fat%2Farticle.php%3Fid%3D33883%26no_paginat%3Dtrue%26p_friendly%3Dtrue, (Accessed 2 December 2020).
- 45.Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation-grand rounds at University of California, San Francisco medical center. J. Gen. Intern. Med. 2008;23(6):854–859. doi: 10.1007/s11606-008-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan T.P., Deal G., Koo H.L., Rees D., Sun H., Chen S., Dou J.H., Makarov V.G., Pozharitskaya O.N., Shikov A.N., Kim Y.S., Huang Y.T., Chang Y.S., Jia W., Dias A., Wong V.C., Chan K. Future development of global regulations of Chinese herbal products. J. Ethnopharmacol. 2012;140(3):568–586. doi: 10.1016/j.jep.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 47.Weeks J. Call to action: announcing the traditional, complementary and integrative health and medicine COVID-19 support registry. J. Altern. Complement. Med. 2020;26(4):256–258. doi: 10.1089/acm.2020.29083.jjw. [DOI] [PubMed] [Google Scholar]
- 48.Food and Drug Administration (FDA). Guidance for Industry: substantiation for Dietary Supplement Claims Made Under Section 403 (r) (6) of the Federal Food, Drug, and Cosmetic Act, 2009. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-substantiation-dietary-supplement-claims-made-under-section-403r-6-federal-food, (Accessed 2 December 2020).
- 49.Food and Drug Administration (FDA). Guidance for Industry-Botanical Drug Products, 2016. https://www.fda.gov/media/93113/download, (Accessed 2 December 2020).
- 50.Commonwealth of Australia. Therapeutic Goods Act 1989 as amended, 2001,
- 51.Fleischer T., Su Y.C., Lin S.J.S. How do government regulations influence the ability to practice Chinese herbal medicine in western countries. J. Ethnopharmacol. 2017;196:104–109. doi: 10.1016/j.jep.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 52.Medicines and Healthcare products Regulatory Agency. Apply for a traditional herbal registration (THR), 2014. https://www.gov.uk/guidance/apply-for-a-traditional-herbal-registration-thr, (Accessed 2 December 2020).
- 53.Peschel W. The use of community herbal monographs to facilitate registrations and authorisations of herbal medicinal products in the European Union 2004–2012. J. Ethnopharmacol. 2014;158:471–486. doi: 10.1016/j.jep.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Verma N. Current regulatory challenges and approaches in the registration of herbal drugs in Europe. Clin. Res. Regul. Aff. 2016;33:9–24. doi: 10.3109/10601333.2016.1130717. [DOI] [Google Scholar]
- 55.European Medicines Agency. Marketing Authorisation, 2015. https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation, (Accessed 2 December 2020).
- 56.The European Parliament and the Council of the European union. Directive 2004/24/EC of the European Parliament and of the Council, 2012. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:136:0085:0090:en:PDF, (Accessed 2 December 2020).
- 57.Medicines and Healthcare products Regulatory Agency. Briefing note: sources of evidence of traditional use under the proposed Directive on Traditional Herbal Medicinal Products, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/274562/MHRA-guidance-on-the-evidence-of-traditional-use.pdf, (Accessed 2 December 2020).
- 58.Medicines and Healthcare products Regulatory Agency. Guidance on new provisions for traditional herbal medicinal products and homoeopathic medicinal products from 1 January 2021, 2020. https://www.gov.uk/guidance/guidance-on-new-provisions-for-traditional-herbal-medicinal-products-and-homoeopathic-medicinal-products-from-1-january-2021, (Accessed 2 December 2020).
- 59.European Medicines Agency. Uptake of the Traditional Use Registration Scheme and Implementation of the Provisions of Directive 2004/24/EC in EU Member States (revision 7), 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2011/05/WC500106706.pdf, (Accessed 2 December 2020).
- 60.French Agency for Food, Environmental and Occupational Health & Safety. ANSES Warns Against Taking Food Supplements That Could Lower the Body’s Immune Response, 2020. https://www.anses.fr/fr/system/fifiles/NUT2020SA0045.pdf, (Accessed 28 May 2020).
- 61.Catanzaro M., Corsini E., Rosini M., Racchi M., Lanni C. Immunomodulators inspired by nature: a review on curcumin and echinacea. Molecules. 2018;23(11) doi: 10.3390/molecules23112778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi Y.H., Huang Y.F., Wang W.Y., Yang L., Zhou H., Sang Z. Analysis on the current quality standards of Chinese materia Medica used in COVID-19 prevention and treatment. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin an active component of liquorice roots and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huynh T., Wang H.R., Luan B.Q. Structure-based lead optimization of herbal medicine rutin for inhibiting SARS-CoV-2’s main protease. Phys. Chem. Chem. Phys. 2020;22(43):25335–25343. doi: 10.1039/d0cp03867a. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization (WHO). Expert Panel Endorses Protocol for COVID-19 Herbal Medicine Clinical Trials, 2020. https://www.afro.who.int/news/expert-panel-endorses-protocol-covid-19-herbal-medicine-clinical-trials, (Accessed 2 December 2020).