Abstract
The World Health Organization has described the 2019 Coronavirus disease caused by an influenza-like virus called SARS-CoV-2 as a pandemic. Millions of people worldwide are already infected by this virus, and severe infection causes hyper inflammation, thus disrupting lung function, exacerbating breath difficulties, and death. Various inflammatory mediators bio-synthesized through the arachidonic acid pathway play roles in developing cytokine storms, injuring virus-infected cells. Since pro-inflammatory eicosanoids, including prostaglandins, and leukotrienes, are key brokers for physiological processes such as inflammation, fever, allergy, and pain but, their function in COVID-19 is not well defined. This study addresses eicosanoid's crucial role through the arachidonic pathway in inflammatory cascading and recommends using bioactive lipids, NSAIDs, steroids, cell phospholipase A2 (cPLA2) inhibitors, and specialized pro-resolving mediators (SPMs) to treat COVID-19 disease. The role of soluble epoxide hydrolase inhibitors (SEHIs) in promoting the activity of epoxyeicosatrienoic acids (EETs) and 17-hydroxide-docosahexaenoic acid (17-HDHA) is also discussed. Additional research that assesses the eicosanoid profile in COVID-19 patients or preclinical models generates novel insights into coronavirus-host interaction and inflammation regulation.
Keywords: Arachidonic cascade, COVID-19, COX, NSAID
1. Introduction
SARS-CoV-2 causes the coronavirus disease 2019 (COVID-19), a highly contagious disease that was identified first in China in late 2019 [1,2]. SARS-CoV-2 is a positive RNA virus similar to the SARS and MERS viruses that causes severe acute respiratory syndrome emerged as a pandemic throughout the world. It is reported that SARS-CoV-2 shares about 80 % identity with SARS-CoV and is 96 % identical at the whole genome level to that of a bat coronavirus(CoV) [3]. Like other SARS- CoV, SARS-CoV-2 exploits Angiotensin-Converting Enzyme-2 (ACE-2) protein mainly and transmembrane protease serine-2 (TMPRSS2) as its entry receptors to infect the host. The SARS-CoV-2 spike was about 20 times higher in binding ACE-2 [4]. ACE-2 is a peptidase that transforms angiotensin I to angiotensin (1–9) and angiotensin II to angiotensin (1–7), peptide hormones that regulate vasoconstriction and blood pressure [5]. ACE-2 is a type I membrane protein expressed in the lungs, heart, kidneys, and intestine [6]. Spike glycoprotein (S protein) of SARS-CoV-2 cleaved into S1 and S2 subunit during the viral infection. S1 subunit, containing the receptor-binding domain, directly binds to the protease domain (PD) of ACE-2, whereas the S2 subunit is responsible for membrane fusion. When S1 binds to the host receptor ACE-2, another cleavage site on S2 expose and cleave by host proteases, a process that is critical for viral infection [6]. Since some CoV hijack ACE-2, decreased the expression of ACE-2 is associated with cardiovascular diseases, hampering angiotensin II cleavage and causing pathological changes due to angiotensin II type 1a receptor activation [7]. Vasoconstriction, hypoperfusion, hypoxia in the myocardium, and oxidative stress in endothelial cells also results from local increases in Ang II. So the increase in Ang II could also result in dysfunction of the heart's vascular endothelium via the induction of oxidative stress [8]. SARS-CoV-2 also seems related to a complex lung injury and worsening observed in COVID-19 [5].
Being a cytopathic virus, SARS-CoV-2 causes the injury or death of the infected cells and tissue associated with the cytokine storm. Cytokines are polypeptide signaling molecules responsible for various biological processes via cell surface receptors [9]. Different types of cytokines,i.e., pro-inflammatory (e.g.,IL-6, TNFα, MCP-1), anti-inflammatory (e.g.,IL-10), and immunomodulatory (e.g.,IL-2, IL-4),are recorded. In response to viral and microbial infection, the host cell releases cytokines for defensive action [10]. In contrast, the clinical study suggested that COVID-19 infected patients admitted in the intensive care unit have elevated plasma levels of different pro-inflammatory cytokines such as IL-2, IL-6, TNFα, MCP-1, and others [11]. A recent study depicted a specific and unsuitable inflammatory condition in SARS-CoV-2 infection [12].
The most potent mediators are endogenous lipids, responsible for all inflammation stages and active in modulating and fine-tuning its path and healing process. Indeed, lipids play a crucial role in many intercellular and intracellular systems' pathophysiological activities, being a significant component of cell membranes and potent energy sources [13]. Innate immune cells, including granulocytes and monocytes/macrophages, are recruited to damaged sites during tissue insults or infections and release bioactive lipids [14]. Polyunsaturated fatty acid and its metabolites play a critical role in the process of microbial or viral infection. The current study carried out a literature search for published materials on COVID 19 and the arachidonic acid (AA) pathway. This study gathered information available in this field, defined and discussed all findings on the eventual interaction between the AA cascades and COVID-19 pathophysiology.
2. Bioactive lipids at different stages of inflammation
Classical eicosanoids as lipid mediators are responsible for acute inflammation characterizing the cardinal signs: redness, heat, swelling, and pain [14]. These are highly pro-inflammatory and ignite the inflammatory cascade during inflammation to remove injurious stimuli. These eicosanoids actively terminate inflammation and drive full tissue homeostasis restoration by activating resolution signs: removal, relief, restoration, regeneration, and remission [15,16]. If there is something wrong due to impaired resolution, it turns into chronic inflammation, resulting in divergent tissue remodeling and organ dysfunction [17]. The inflammation outcome also depends on the other two families of bioactive lipids, i.e., lysoglycerophospholipids/sphingolipids, and the endocannabinoid system (eCBs), which are essential for triggering cell proliferation and tissue adaption [18,19]. Indeed, prostaglandins (PGs), particularly PGE2 and PGI2, appear to be involved in persistent inflammation leading to chronic inflammation through functioning as "cytokine amplifiers" [[20], [21], [22]]. PGs generally lead to chronic inflammation by five main mechanisms: (i) improvement of the cascade release of pro-inflammatory cytokines [23]; (ii) amplification of innate immune response to pathogen- and damage-associated molecular patterns (PAMPs and DAMPs) [24]; (iii) activation of specific pro-inflammatory subsets of T helper cells, e.g., TH1 and TH17 [25,26]; (iv) Recruitment of chronic inflammation immune cells (for example, macrophages, T cells, and B cells) by interacting with chemokines synergistically [21]; (v) The increase of pro-inflammatory genes induced by cytokines. In acute inflammation, leukotrienes (LTs) cause an influx of inflamed tissues along with prostanoids, edemas, and neutrophils [20]. However, LTs are also central in perpetuating inflammatory signals that lead to tissue damage in many chronic diseases.
3. Arachidonic acid (AA) cascade and COVID-19
AA is a polyunsaturated fatty acid released from the membrane phospholipid by phospholipase A2 (PLA2) during inflammatory action. There are 11 distinct classes of PLA2s categorized into Ca 21-dependent (secretory and cytosolic) and Ca 21-independent (intracellular, membrane-associated, cytosolic) categories [27]. AA regulates membrane fluidity, regulating cellular signaling relevant membrane proteins [28,29], and plays a fundamental role in maintaining cell and organelle integrity and vascular permeability [30]. AA as a substrate for different pathways metabolizes to synthesize different types of pro-inflammatory lipid mediators like prostaglandins (PGs), thromboxanes (TXA2) through the cyclooxygenase (COX) pathway, and leukotrienes (LTs) through the lipoxygenase (LOX) pathway [31]. The ability of AA to control infection with S. mansoni was demonstrated in Egyptian children. The chemotherapy efficacy of AA and praziquantel (PZQ) is similarly high in low-infection settings and similarly low in high-infection children living in high-endemic areas [32]. AA can selectively destroy tumor cells in vitro by emitting lipid peroxidation from the cell-surface membrane that can be blocked by vitamin E, uric acid, superoxide dismutase, and glutathione peroxidase [33].
3.1. Cyclooxygenase (COX) pathway
COX catalyzes arachidonic acid oxidation into prostaglandin H2, which then isomerizes to different prostanoids, such as PGE2 [34]. There are different PGs (PGE2, PGI2, PGD2, PGF2α), contributing to the inflammatory process. Immune system activation occurs following the virus infection, including the release of inflammatory media such as pro-inflaming cytokines (IL-6, IL-1 β, TNF-α) and eicosanoids (PGs and LTs) [35]. The second cyclooxygenase isoform (COX-2) is responsible for forming the PGs' central part responsible for pain and inflammation [36]. The schematic diagram of the cyclooxygenase (COX) pathway stimulated by SAR-CoV-2 infection is represented in Fig. 1 . Similarly, the SARS-CoV of the 2003 outbreak was increasing PG's development by binding to COX-2 [36].
Fig. 1.
Arachidonic acid cascade through the cyclooxygenase (COX) pathway stimulated by SARS-CoV-2 infection.
Several studies show that PGE2 raises viral pathogens in many viral infections such as CMV, RB, RSV, HSV, EV71, and CVB2 by interacting with viral transcriptions and translations [35]. Virus infection induces the accumulation of COX-2 mRNA, protein expression, and development of PGE2 in most cell types. Although the disease's overall subject is EBV, it destroys accumulating PGE2 and COX-2 mRNA in peripheral mononuclear blood cells [37]. Overall, the mechanisms by which viral infection activates COX-2 expression and the pathways by which COX-2 development products attenuate the host response to the infection, or the virus replicative life cycle have not yet been well explained. The alteration of PGE2 synthesis might be a mechanism formed by viruses to alter cells' biological functions, enabling increased replication and viral dissemination [38]. In pulmonary microvascular endothelial cells of human, PGE2 can play an essential upstream role in inflammation. It leads to increased COX-2 expression without affecting the COX-1 isoform and increasing IL-8 [39]. PGE2 was suggested to play a significant role in hyperinflammatory and immune responses to COVID-19 pathophysiology [35]; thus, PGE2 can assess in COVID-19 patients. The author also hypothesizes the host-based immune sensitivity to COVID-19 may be enhanced if PGE2 can reduce via human micro-prostaglandin E synthase-1 (mPGES-1).
Moreover, it can be a promising therapeutic strategy for preventing severe disease progression and death. There was an increased exposition of COX-2 and PGE2 in humans during acute inflammation than women showing gender variation [40]. Following these results, elevated PGE2 levels in males may be one of the main factors leading to a more significant infection of COVID-19 in males [35]. Additional animal studies have shown that levels of PGE2 are affected by age, which can also be an underline factor of older patients' hypersensitivity concerning children with COVID-19 [35,41]. Because of the greater risk of disease aggravation in the obese patient, PGE2 can also lead to insights in COVID-19 patients with comorbidities, such as obesity, as PGE2 increased in obese patients in various studies [42]. Besides, PGE2 can aid intravascular thrombosis [43], another challenge in patients with COVID-19. The free radical attack of AA generates Isoprostans in vivo. Again, the increase in intracellular ROS from mechanical damage stimulates time-sensitive PGE2 development through COX-2 activation. The phosphorylation of extracellular signal-regulated protein kinase (ERK) stimulates COX-2 [44]. These findings indicate ROS-induced ERK activation leading to COX-2 activation and PGE2 synthesis in human keratinocytes that leads to mechanical injury in the acute phase [44]. Sonlicromanol, a selective ROS-1 inhibitor, demonstrated radical scavenging function, preventing ROS-induced cell death in patients with acute respiratory distress syndrome (ARDS) in pulmonary and lung tissues. [45].
Another prostaglandin of importance for respiratory viruses is PGD2. In an experimental animal model infected with respiratory viruses, there was a higher production of PGD2 and a greater level noticed in older animals [46]. In the inflammatory pulmonary condition, PGD2 control different steps of T cells' response in mice with SARS-CoV and MERS-CoV [46]. Besides, PGD2 production increased in patients with severe syncytial virus (RSV) bronchiolitis, suggesting that prostaglandin D (DP) receptor agonists may be useful antivirals for viral bronchiolitis treatment [47]. This approach can consider as a crucial preventive step for asthma patients in general.
In particular, phospholipase A2, group IID (PLA2G2D), predominantly contributes to anti-inflammatory/pro-resolving lipid mediator expression [48]. Higher levels of PLA2G2D in middle-aged mice's lungs would disrupt lipid metabolism. The lipid mediators, other than PGD2, would also be generated at higher levels. Levels of Pla2g2d+/+ in the lungs were higher compared to Pla2g2d–/– mice, which indicated that differences in AA levels resulted in differences in downstream metabolite levels, some of which had anti-inflammatory function (PGD2 and PGE2) levels of 6-keto PGF1α, TXB2, PGF2α, PGD2 and PGE2 [[49], [50], [51]]. Anti-inflammatory PLA2G2D led to worse outcomes in SARS-CoV infected mice. Of course, mice with no expression of PLA2G2D transformed a homogeneously lethal infection into a nonlethal infection (>80 % survival). The increased Dendritic cell (DC) migration to draining lymph nodes has increased cell response and decreased lung harm. In the absence of a virus, a threat like SARS-CoV, the enhanced expression of PLA2G2D supports the host by increasing the amount of different immunosuppressive lipid mediators such as PGD2. However, the higher basal levels of PGD2 and other pro-resolving lipid mediators negatively affect the activation of a defensive innate immune response and, eventually, the adaptive immune response. Treatments, which increase the levels of lipid mediators for resolution, can have unwanted effects in the sense of infection. As a result, a single PLA2G2D inhibition is an attractive therapeutic approach to restoring older patients' immune systems with a severe infection [52].
3.2. Lipoxygenase pathway
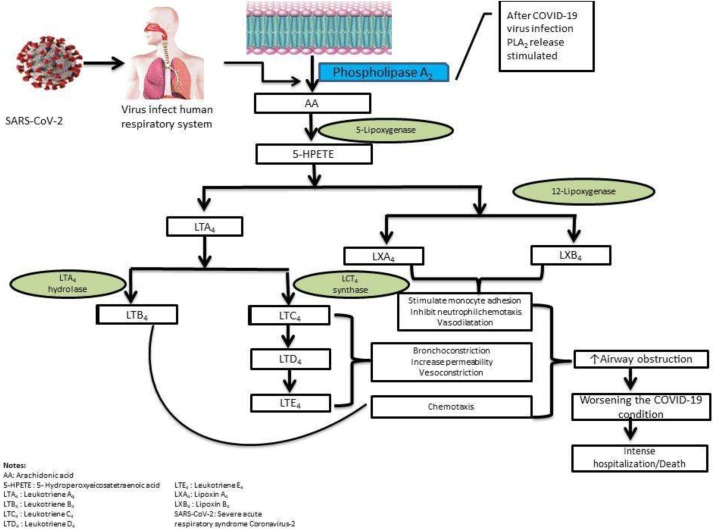
Besides COX-2 and PGE2, the 5-lipoxygenase (5-LOX) pathway is also involved in virus pathophysiology. The AA cascade stimulated by SARS-CoV-2 through the lipoxygenase pathway represents in Fig. 2 . Leukotrienes and lipoxins, which have pro- and anti-inflammatory activities, are produced through the 5-LOX pathway. These pro-inflammatory lipid mediators resolve inflammation, aid in wound healing, possess potent anti-inflammatory activity, remove the debris from the infection site and injury, and enhance microbial clearance [4]. The investigation of these agents' use recommends that these fatty acids' oral or intravenous administration may increase the recovery from these infections and potentially avoid these infections if present in appropriate concentrations in the immunocytes and body fluids (particularly in alveolar fluids) [4].
Fig. 2.
Arachidonic acid cascade through the lipoxygenase (LOX) pathway stimulated by SARS-CoV-2 infection.
Leukotrienes can cause hyperimmune and inflammation reactions with COVID-19 progression with high leukotriene levels, as previously identified by tracheal aspirates of ARDS patients [53]. The leukotriene B4 (LTB4) is one of the most powerfully known chemoattractants in neutrophils and subsets of lymphocytes via B leukotriene subtype 1 (BLT1) and G protein-coupled receptor (GPCR) signal [[54], [55], [56], [57]]. The 5-LOX enzyme inhibitor such as zileuton inhibits the synthesis of all downstream LTs. Again the cysteinyl leukotrienes 1 (CysLT1) receptor antagonist (montelukast) and the leukotriene receptor antagonists (LTRA) can be used for the treatment of mild-moderate asthma, allergic rhinitis, and inflammatory symptoms in the airway with a unique safety profile [[58], [59], [60], [61], [62], [63]]. It was predicted that Montelukast would bind to SARS-CoV-2 and can interrupt viral replication [64,65]. A regulated network of molecules for lipid signaling, including LTs and cytokines, orchestrates adequate leukocyte recruitment in inflammation situations [66]. Leukotrienes, in particular LTB4, inhibit viral replication of influenza in vivo [67]. The Influenza virus upregulates 5-LOX in the lungs of either infected animal models (mice) or humans [68]. LTB4-treated neutrophils had enhanced virucidal activity against the influenza virus, human CoV, and RSV [69]. PGE2 level reduces in COX-1−/− airways, whereas cysteinyl leukotrienes (cysLTs) level elevates in COX-2−/− airways following infection. A higher level of cysLTs observed in mice with the influenza virus increased the survival rate, reducing morbidity despite higher viral titers [70]. Lipoxin A4 was also reported to attenuate adipose inflammation, decreasing IL-6 and increasing IL-10 expression in aged mice [71]. In recent years, the healing effects of lipoxin A4 encapsulated in polylactic-co-glycolic acid microparticles in topical treatments for skin lesions of dorsal-rats have been considerable with its particular receptor on skin cells [72].
3.3. CYP and sEH pathway in arachidonic acid cascade
Pathway cytochrome P450 (CYP) is the prospective third branch of AA metabolism. AA can convert to hydroxyeicosatetraenoic acids (HETEs) by CYP hydroxylase enzymes. The essential metabolic product in the pathway is 20-HETE, the major vasoactive eicosanoid in the microcirculation [73]. It contributes to regulating the vascular tone in the brain and peripheral organs and promotes inflammatory cytokines and adhesion molecules [73]. The CYP epoxygenase enzymes generate epoxyeicosatrienoic acids (EETs), catalyzing arachidonic acid's epoxidation, resulting in four major isomeric EETs (e.g., 5,6-EET, 8,9-EET,11,12-EET, and 14,15-EET) [74,75]. EETs are essential molecules for signaling biological processes involving anti-inflammation and pain relief [74,76]. The enzyme hydrolysis easily converts these epoxy metabolites by the soluble epoxide hydrolase (sEH) as the main disposal pathway. After hydrolysis, sEH can convert into their corresponding biologically less active 1,2-diol molecules like dihydroxyeicosatrienoic acids (DHETs), dihydroxyeicosatetraenoic acids (DHEQs), dihydroxydocosapentaenoic acids (DiHDPAs) [77]. The use of suitable sEH inhibitors can prolong the anti-inflammatory activity of EETs and be used to treat lung and heart [78]. The overall biosynthetic pathway is represented in the Fig. 3 .
Fig. 3.
Arachidonic acid cascade through the cytochrome P450 (CYP) and soluble epoxide hydrolase (sEH) pathway stimulated by SARS-CoV-2 infection.
4. Arachidonic acid cascades and covid-19 management strategy
Several COVID-19 management strategies can develop by modifying specific inflammatory pathways of the AA cascade. Some COVID-19 management strategy through blocking or stimulating specific inflammatory pathways is outlined in the Fig. 4 .
Fig. 4.
COVID-19 management strategies through the AA cascade.
4.1. Bioactive lipids inactivating coronavirus (COVID-19)
Mechanisms by which invasive microorganisms are eliminated or neutralized involve the generation of unique immunocyte antibodies. It helps destroy microorganisms by producing different cytokines, reactive oxygen species (ROS), complement system activation, and other unique and non-specific ways of capturing and killing microorganisms with little or no damage to different tissues. Streptococci, which is present on normal human skin, is made ineffective by skin surface lipids. Staphylococci in the lung alveoli are killed in the highly unsaturated AA in the surface material, often outside alveolar macrophages [4]. AA has been proposed as an antiviral medication because it can inactivate the viral protein that encloses various viruses, including influenza virus, HIV, or SARS-CoV. The ability to induce leaching or lysis of the cells, inhibit respiratory activity, and decouple oxidative phosphorylation are some mechanisms that cause AA and other fatty acids' antimicrobial effect [4,79].
Consequently, alveolar macrophages, T and B cells, leukocytes, macrophages, and other cells release AA and other unsaturated fatty acids when affected by viruses, such as SARS-CoV-2, SARS, and MERS. They can inactivate the invading microorganisms and thus protect the lungs and other tissues [4]. A shortfall in AA can, therefore, make humans more vulnerable to SARS-CoV-2 [80].
Bioactive lipids derived from omega-3 fatty acids that can resolve inflammation are resolvins. Docosahexaenoic acid (DHA) and Eicosapentaenoic acid (EPA) are omega -3 fatty acids with resolving activity typically classified as D and E series resolvins [81]. Resolvins inhibit cytokine production and resolve the destructive inflammation by controlling leukocyte transportation and removing inflammatory mediators [16]. 17-hydroxy-docosahexaenoic acid (17-HDHA) is a precursor of D series resolvins and shows good analgesic activity in reducing pain from osteoarthritis and chronic inflammation [82].
4.2. NSAIDs and COXIBs
Non-steroidal anti-inflammatory drugs (NSAIDs) block the synthesis of inflammatory mediators by covalent inhibition of COX-1/2 [83], resulting in forestall of inflammation. Usually, NSAIDs treat acute inflammatory symptoms but practically inactive in chronic conditions, leading to the belief that prostanoids are much less active in chronic inflammatory pathologies [20].
When researching the seriousness of COVID-19 symptoms in asthma and hypertension patients, Fang et al. linked the SARS-CoV-2 to the downregulation of ACE-2, which instead is upregulated by Ibuprofen [84]. Indomethacin, generally prescribed for gout and arthritis treatment, inhibits the human SARS-CoV replication with a concentrated 1 mg/kg dose [85]. Several studies challenged NSAIDs and Ibuprofen's role in COVID-19 patients due to several side effects such as gastrointestinal, cardiovascular, nephrotoxicity, and so on. NSAIDs inhibit COX and inhibit the synthesis of anti-inflammatory AA mediators, particularly lipoxins and resolvins, resulting in inflammation resolution [86]. In March 2020, the French health authorities warned NSAIDs' severe side-effects in patients with COVID-19 [87]. In COVID-19, EMA reported no evidence to deteriorate NSAIDs and Ibuprofen [88]. Accordingly, there has been no new ibuprofen and deteriorating COVID-19 study by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) [89].
Besides, WHO proposes Ibuprofen's first treatment as an antipyretic agent and paracetamol for fever or pain, patients who take NSAIDs for different reasons should not stop using them to fear their COVID-19 risks [36]. Since NSAIDs may cause gastrointestinal side effects (ulcer, bleeding) or an increased risk of heart disease, explained by a disorder between AA mediators (prostacyclin and TX), a better controlling strategy for COVID-19 inflammation can be a selective inhibition of mPGES-1. Das et al. suggested that an intravenous or oral administration of AA can increase the resistance, facilitate the recovery, or even prevent SARS-CoV-2 [4]. Furthermore, we believe that dual COX-2 inhibitors / TP antagonists that offer anti-inflammatory effects and cardiovascular protection can be useful for SARS-CoV-2 patients.
4.3. Steroids and COVID-19
Patients with severe COVID-19 infections develop a cluster of respiratory complexes, leading to severe lung tissue damage resulting in inflammation and fluid retention [[90], [91], [92], [93]]. Unfortunately, no specific treatment against COVID-19 viral infection has been identified yet. However, several studies perform various drugs to combat this pandemic disease caused by SARS-CoV-2 [[94], [95], [96]]. Corticosteroids previously used to treat patients associated with identical syndromes as COVID-19 such as SARS-CoV [[97], [98], [99]], the MERS-CoV, influenza, viral pneumonia [[100], [101], [102], [103]], and now are also being used in critically ill patients with COVID-19 [[104], [105], [106]]. However, the role of corticosteroids in viral COVID-19 remains controversial. Several studies claimed that corticosteroids might be detrimental, causing slower clearance of viral RNA, and similar findings were also observed during the outbreaks of SARS [[107], [108], [109]]. The World Health Organization had now revealed an analysis that survival rates ameliorated in the case of severely ill COVID-19 patients when they were treated with dexamethasone and other corticosteroids, including hydrocortisone or methylprednisolone [110,111]. The University of Oxford carried out the recuperation test (the randomized assessment of Covid-19 therapy) to evaluate the beneficial use of dexamethasone in patients hospitalized with COVID-19 and reported efficacy in the case of severely ill patients [[112], [113], [114]].
Dexamethasone is a corticosteroid widely used to treat a range of infections. Its action mechanism is its ability to reduce inflammation and suppress immune reactions elicited during an infection [115,116]. Most people with COVID-19 have no signs of illness–they are asymptomatic–or have mild symptoms such as dry cough, mild fever, or loss of taste and smell. In a small minority, though the effects are much worse, with oxygen or ventilation, patients needing to help the lungs gain oxygen. These are the people that are effective in dexamethasone. In extreme cases, the body's immune system over-reacts to the virus and destroys the cells that carry it. It is called a cytokine storm, in which cells of the immune system release chemicals called cytokines that cause excessive inflammation. Dexamethasone works on the immune system to dampen the reaction and reduce the storm of the cytokine. Besides, it avoids massive inflammation seen in the lungs and hearts responsible for serious breathing irregularities in very sick patients [117,118].
A total of 2104 randomized patients got 6 mg dexamethasone once every day for ten days (by mouth or intravenous injection) compared to 4321 randomized patients who were given standard treatment alone. The 28-day mortality among those who received standard treatment alone was highest in those who needed ventilation (41 %), the intermediary in those who only needed oxygen (25 %), and the lowest in those who needed no respiratory intervention (13 %). The dexamethasone decreases the mortality rate for ventilated patients by one third and in other patients who are consuming only oxygen by one fifth (0.80[0.67 to 0.96]; p = 0.0021). Patients with no respiratory assistance were of no benefit (1.22 [0.86–1.75]; p = 0.14) [112,119].
4.4. Inhibition of cPLA2 impairs an early step of coronavirus replication in cell culture
The inactivation of cell phospholipase A2 (cPLA2) using a particular small-molecule inhibitor significantly decreases the synthesis of coronavirus RNA and, consequently, protein and infectious virus development. cPLA2 is inhibited and is the most common cause of viral ROs in the early stages of the viral replication cycle. The activity of cPLA2 has been reported to be necessary with HCoV-229E (genus Alpha-coronavirus) and MERS-CoV (genus Beta-coronavirus) replication. However, the cPLA2 inhibitor was also effective against SFV, a Togaviridae family member, showing that this phospholipase activity produces unique lipid compounds necessary to replicate phylogenetically distinct RNA viruses [120]. The precise role of cPLA2 in the production of fully functional ROs remains to be established.
There is also information that, regardless of its enzymatic activity, cPLA2 can alter the membrane phospholipid packaging via its hydrophobic C2 domain to induce the membrane bending needed for macrophages phagosome formation [121,122]. In addition to cPLA2 and related phospholipases, many other factors have been shown to induce membrane curvature in diverse biological systems. It was shown that phosphatidic acid (PA) species downregulate, whereas ceramide (Cer) and LPL species upregulate in HCoV-229E infected cells. PA is a crucial intermediate in the synthesis of glycerophospholipids and triacylglycerides and a vital lipid mediator involved in diverse cellular functions, including vesicular trafficking, cytoskeletal changes, and secretion membrane alterations [123,124]. The observed upregulation of bioactive Cer may indicate a cellular response to CoV replication or even a possible role of Cer in supporting coronaviral replication. Ceris is known to induce apoptosis and autophagy [125]. Both processes have been suggested to be involved in CoV replication and represent emerging fields of CoV research, with partially controversial information reported for different viral and cellular systems [120]. Together, these observations support the idea that LPLs generated by cPLA2 is functionally relevant components of ROs produced in coronavirus-infected cells. The inhibition of cPLA2 activity by Py-2 affect HCV replication in vitro [126]. The production of infectious virus progeny reduces, most probably, by reducing lipid droplets required for HCV particle formation [126,127]. As mentioned above, the replication of MERS-CoV (genus Beta-coronavirus) and SFV (family Togaviridae) was inhibited in the presence of Py-2, identifying cPLA2 as an essential host factor for RNA virus replication. The formation of ROs of CoVs and several other RNA viruses depends on specific LPLs produced by cellular cPLA2 activities. The selective inhibitory effects observed for members of only a few RNA virus families suggest very specific lipid requirements for these viruses and contradict the potential nonspecific/toxic effects responsible for the observed antiviral effects of Py-2 against corona alphaviruses [120].
5. Cytokine-directed therapy in COVID-19
5.1. Recombinant IFN as an antiviral treatment
One of the first defenses of the human body against RNA viruses like SARS-CoV-2 is the release of type I and III interferons (IFNs). It is worthy of mention that type I IFN (IFNα/β) receptors are ubiquitously expressed, so IFNα/β signaling can result in not only antiviral effects but also the activation of immune cells that potentially exacerbate pathogenesis. In contrast, type III IFN (also known as IFNλ) signals mainly in epithelial cells and a restricted pool of immune cells. Because type III IFNs have immunomodulatory functions, subsequent signaling could induce a potent antiviral effect without enhancing pathogenic inflammation [128,129]. One study of 40 patients with SARS-CoV-1 infection described unresolved elevated type I IFNs in those with poor outcomes [130]. Others report that exogenous type I IFN does not improve outcomes when given with ribavirin in patients with MERS-CoV infection [131], suggesting that the role of IFN as a therapeutic or prophylactic option may be a strain or species-specific [132]. In the case of RNA viruses such as SARS-CoV-2, these pathways are initiated through the engagement of pattern-recognition receptors (PRRs) by viral single-stranded RNA (ssRNA) and double-stranded RNA (dsRNA) via cytosolic RIG-I like receptors (RLRs) and extracellular and endosomal Toll-like receptors (TLRs). Upon PRR activation, downstream signaling cascades trigger the secretion of cytokines. Among these, type I/III IFNs are considered the most crucial antiviral defense. However, other cytokines, such as pro-inflammatory TNF-α and interleukin-1 (IL-1), IL-6, and IL-18, are also released. Together, they induce antiviral programs in target cells and potentiate the adaptive immune response. If present early and properly localized, IFN-I can effectively limit CoV infection [133,134]. Early evidence demonstrated that SARS-CoV-2 is sensitive to IFN-I/III pretreatment in vitro, perhaps to a greater degree than SARS-CoV-1 [11]. Likely, the IFN-induced transmembrane family (IFITM) proteins inhibit SARS-CoV-2 entry, as demonstrated for SARS-CoV-1 [135].
5.2. Cytokine blockade
Hyper inflammatory responses and elevated levels of inflammatory cytokines, including IL-6, -8, and -10, have been shown to correlate with COVID-19 severity [11]. This cytokine storm's drivers remain to be established, but they are likely triggered initially by a combination of viral PAMPs and host danger signals. The heterogeneous response between patients suggests other factors are involved, possibly including the SARS-CoV-2 receptor, ACE2 [136]. Specific subsets of CD4 T cells that express GM-CSF and IL-6 are more abundant in severe COVID-19 patients than in COVID-19 patients who do not require intensive care [137]. Other major pro-inflammatory cytokines (TNF-α, IFN-ɣ, IL-2) and chemokines (CCL2, CCL3, and CCL4) are elevated, which underscore a potentially pathogenic TH1/2 program in COVID-19 [138,139]. Therefore, biological agents targeting the expression of cytokines and specific cytokine antagonists, such as IL-6R monoclonal antibodies, TNF inhibitors, IL-1 antagonists, Janus kinase (JAK) inhibitors, and others, have been considered [140].
5.3. The S protein as a vaccine target
Since SARS-CoV-1 first emerged, the S protein has been favored as the most promising target for vaccine development to protect against CoV infection. This particular viral protein has essential roles in viral entry and stimulating the immune response during natural infection and in vaccination studies of both SARS-CoV-1 and MERS-CoV [11], which has also been confirmed in SARS-CoV-2 [141]. The S protein induces robust and protective humoral and cellular immunity, including the development of neutralizing antibodies (nAbs) and T cell-mediated immunity [142]. nAbs can protect against infection by blocking receptor binding and viral entry, shown with pseudovirus-based neutralization assays [143,144]. Studies of SARS-CoV-1 indicate that T cell response against the S protein correlated with nAb titers and dominated the T cell response after natural infection. Antiviral T cell reactions specific to the receptor-binding domain observed in individuals who recovered from COVID-19, further validating its promise as a vaccine target [143,145].
5.4. Therapeutic interventions with specialized pro-resolving mediators
Specialized pro-resolving mediators (SPMs), including four families, namely lipoxins, resolvins, proteins, and maresins, are bioactive lipid autacoids, which mediate the endogenous resolution stimulating a phagocytic macrophage of cellular debris and preventing the release of pro-inflammatory cytokines [[146], [147], [148]]. SPMs promote antiviral Blymphocytic activity in influenza [109]. SPM precursors, including 17-HDHA, have also been reported, which protect against primary influenza infection and promote adaptive immunity adjuvants [149,150]. Another course of action involving arachidonic acid-derived epoxyeicosatrienoic acids (EETs) also promotes inflammation resolution. Because soluble epoxide hydrolases (sEH) quickly metabolize EETs, the use of sEH inhibitors (SEHIs) may help stabilize EETs. Administration of SEHIs can prevent pulmonary inflammation and improve the pulmonary system's functions in animal models [151]. The NF-κB, the core of cytokine eicosanoid mediated storms, encourages pro-inflammatory cytokines and prostaglandin synthesis through COX downregulated by both SPMs, and SEHIs [146,152]. Notably, both SPMs and SEHIs can attenuate pathologic thrombosis and promote clot removal, which is now recognized as a critical pathology of COVID-19 infection [[153], [154], [155]]. SPMs and SEHIs are currently in clinical trials for other inflammatory diseases. They could rapidly translate for the management of COVID-19 by complementing current antiviral strategies via debris clearance and inflammatory cytokine suppression [156].
6. Conclusion
COVID-19 disease caused by the influenza virus is highly contagious and rapidly transmitted worldwide and has been declared by WHO as a pandemic. After insertion into the host alveolar epithelium, the SARS-CoV-2 virus can develop a severe inflammatory state that ultimately disrupts the alveolar function and develops the severe COVID-19 disease condition. The hyperinflammatory state develops through the hyperactivity of the biosynthetic pathway of synthesizing different inflammatory cytokines from AA. We can hypothesize different interventions for the treatment of COVID-19 disease by blocking or promoting different steps of the AA cascade. Some clinical studies on some NSAIDs and steroids found success in reducing the case severity and morbidity rate of COVID-19 disease. Developing specific small-molecule for inhibiting cPLA2 is another COVID-19 management strategy as it reduces viral RNA synthesis. SPM precursors, including 17-HDHA, and EETs, can consider managing the inflammatory condition in COVID-19 disease. Judging by the current study, further study can be started targeting specific molecules in the AA cascade to manage COVID-19 disease.
References
- 1.Chan J.F., Kok K., Zhu Z., Chu H., Kai-wang K., Yuan S., Yuen K., Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Di Jiang R., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das U.N. Can Bioactive Lipids Inactivate Coronavirus (COVID-19)? Arch. Med. Res. 2020;51:282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock J.O., Ortea I. Re-analysis of SARS-CoV-2-infected host cell proteomics time-course data by impact pathway analysis and network analysis: a potential link with inflammatory response. Aging (Albany. NY) 2020;12:11277–11286. doi: 10.18632/aging.103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science (80-.) 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crackower M.A., Sarao R., Oliveira-dos-Santos A.J., Da Costa J., Zhang L. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 9.Bartee E., McFadden G. Cytokine synergy: an underappreciated contributor to innate antiviral immunity. Cytokine. 2013;63:237–240. doi: 10.1016/j.cyto.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., Pia L., Risson E., Saffern M., Salomé B., Esai Selvan M., Spindler M.P., Tan J., van der Heide V., Gregory J.K., Alexandropoulos K., Bhardwaj N., Brown B.D., Greenbaum B., Gümüş Z.H., Homann D., Horowitz A., Kamphorst A.O., Curotto de Lafaille M.A., Mehandru S., Merad M., Samstein R.M., Agrawal M., Aleynick M., Belabed M., Brown M., Casanova-Acebes M., Catalan J., Centa M., Charap A., Chan A., Chen S.T., Chung J., Bozkus C.C., Cody E., Cossarini F., Dalla E., Fernandez N., Grout J., Ruan D.F., Hamon P., Humblin E., Jha D., Kodysh J., Leader A., Lin M., Lindblad K., Lozano-Ojalvo D., Lubitz G., Magen A., Mahmood Z., Martinez-Delgado G., Mateus-Tique J., Meritt E., Moon C., Noel J., O’Donnell T., Ota M., Plitt T., Pothula V., Redes J., Reyes Torres I., Roberto M., Sanchez-Paulete A.R., Shang J., Schanoski A.S., Suprun M., Tran M., Vaninov N., Wilk C.M., Aguirre-Ghiso J., Bogunovic D., Cho J., Faith J., Grasset E., Heeger P., Kenigsberg E., Krammer F., Laserson U. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiurchiù V., Leuti A., Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 15.Basil M.C., Levy B.D. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 18.El Alwani M., Wu B.X., Obeid L.M., Hannun Y.A. Bioactive sphingolipids in the modulation of the inflammatory response. Pharmacol. Ther. 2006;112:171–183. doi: 10.1016/j.pharmthera.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Chiurchiu V., Battistini L., Maccarrone M. Endocannabinoid signalling in innate and adaptive immunity. Immunology. 2015;144:352–364. doi: 10.1111/imm.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis E.A., Norris P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki T., Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 2012;33:304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Narumiya S., Furuyashiki T. Fever, inflammation, pain and beyond: prostanoid receptor research during these 25 years. FASEB J. 2011;25:813–818. doi: 10.1096/fj.11-0302ufm. [DOI] [PubMed] [Google Scholar]
- 23.Honda T., Segi-Nishida E., Miyachi Y., Narumiya S. Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J. Exp. Med. 2006;203:325–335. doi: 10.1084/jem.20051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata T., Narumiya S. Prostanoids as regulators of innate and adaptive immunity. Adv. Immunol. 2012:143–174. doi: 10.1016/B978-0-12-394300-2.00005-3. Academic Press Inc. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q., Muramoto K., Masaaki N., Ding Y., Yang H., MacKey M., Li W., Inoue Y., Ackermann K., Shirota H., Matsumoto I., Spyvee M., Schiller S., Sumida T., Gusovsky F., Lamphier M. A novel antagonist of the prostaglandin E 2 EP 4 receptor inhibits Th1 differentiation and Th17 expansion and is orally active in arthritis models. Br. J. Pharmacol. 2010;160:292–310. doi: 10.1111/j.1476-5381.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K., Sugimoto Y., Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat. Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 27.Ma Z., Turk J. The molecular biology of the group VIA Ca2+-independent phospholipase A2. Prog. Nucleic Acid Res. Mol. Biol. 2001;67:1–33. doi: 10.1016/S0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- 28.Brash A.R. Arachidonic acid as a bioactive molecule. J. Clin. Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pompéia C., Lopes L.R., Miyasaka C.K., Procópio J., Sannomiya P., Curi R. Effect of fatty acids on leukocyte function. Braz. J. Med. Biol. Res. 2000;33:1255–1268. doi: 10.1590/S0100-879X2000001100001. [DOI] [PubMed] [Google Scholar]
- 30.Beck R., Bertolino S., Abbot S.E., Aaronson P.I., Smirnov S.V. Modulation of arachidonic acid release and membrane fluidity by albumin in vascular smooth muscle and endothelial cells. Circ. Res. 1998;83:923–931. doi: 10.1161/01.RES.83.9.923. [DOI] [PubMed] [Google Scholar]
- 31.Steer S.A., Corbett J.A. The role and regulation of COX-2 during viral infection. Viral Immunol. 2003;16:447–460. doi: 10.1089/088282403771926283. [DOI] [PubMed] [Google Scholar]
- 32.Tallima H., El Ridi R. Arachidonic acid: physiological roles and potential health benefits – a review. J. Adv. Res. 2018;11:33–41. doi: 10.1016/j.jare.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das U.N. Tumoricidal action of cis-unsaturated fatty acids and their relationship to free radicals and lipid peroxidation. Cancer Lett. 1991;56:235–243. doi: 10.1016/0304-3835(91)90008-6. [DOI] [PubMed] [Google Scholar]
- 34.Spencer A.G., Woods J.W., Arakawa T., Singer I.I., Smith W.L. Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J. Biol. Chem. 1998;273:9886–9893. doi: 10.1074/jbc.273.16.9886. [DOI] [PubMed] [Google Scholar]
- 35.Smeitink J., van Maanen R., Beyrath J., Smeitink J.A., Khondrion M.B. 2020. Hypothesis: mPGES-1-derived Prostaglandin E2, a so Far Missing Link in COVID-19 Pathophysiology? [DOI] [Google Scholar]
- 36.FitzGerald G.A. Misguided drug advice for COVID-19. Science (80-.) 2020;367:1434. doi: 10.1126/science.abb8034. [DOI] [PubMed] [Google Scholar]
- 37.Savard M., Bélanger C., Tremblay M.J., Dumais N., Flamand L., Borgeat P., Gosselin J. EBV suppresses prostaglandin e 2 biosynthesis in human monocytes. J. Immunol. 2000;164:6467–6473. doi: 10.4049/jimmunol.164.12.6467. [DOI] [PubMed] [Google Scholar]
- 38.Janelle M.E., Gravel A., Gosselin J., Tremblay M.J., Flamand L. Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6: role for cyclic AMP-responsive element-binding protein and activator protein-1. J. Biol. Chem. 2002;277:30665–30674. doi: 10.1074/jbc.M203041200. [DOI] [PubMed] [Google Scholar]
- 39.Aso H., Ito S., Mori A., Morioka M., Suganuma N., Kondo M., Imaizumi K., Hasegawa Y. Prostaglandin e 2 enhances interleukin-8 production via ep4 receptor in human pulmonary microvascular endothelial cells. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2012;302:266–273. doi: 10.1152/ajplung.00248.2011. [DOI] [PubMed] [Google Scholar]
- 40.Pace S., Rossi A., Krauth V., Dehm F., Troisi F., Bilancia R., Weinigel C., Rummler S., Werz O., Sautebin L. Sex differences in prostaglandin biosynthesis in neutrophils during acute inflammation. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-03696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D., Mura C., Beharka A.A., Han S.N., Paulson K.E., Hwang D., Meydani S.N. Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am. J. Physiol. - Cell Physiol. 1998;275 doi: 10.1152/ajpcell.1998.275.3.c661. [DOI] [PubMed] [Google Scholar]
- 42.Fain J.N., Kanu A., Bahouth S.W., Cowan G.S.M., Hiler M.L., Leffler C.W. Comparison of PGE2, prostacyclin and leptin release by human adipocytes versus explants of adipose tissue in primary culture. Prostaglandins Leukot. Essent. Fat. Acids. 2002;67:467–473. doi: 10.1054/plef.2002.0430. [DOI] [PubMed] [Google Scholar]
- 43.Gross S., Tilly P., Hentsch D., Vonesch J.L., Fabre J.E. Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. J. Exp. Med. 2007;204:311–320. doi: 10.1084/jem.20061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y.P., Peng Y.B., Zhang Y.F., Wang Y., Yu W.R., Yao M., Fu X.J. Reactive oxygen species mediated prostaglandin E2 contributes to acute response of epithelial injury. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/4123854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beyrath J., Pellegrini M., Renkema H., Houben L., Pecheritsyna S., Van Zandvoort P., Van Den Broek P., Bekel A., Eftekhari P., Smeitink J.A.M. KH176 safeguards mitochondrial diseased cells from redox stress-induced cell death by interacting with the thioredoxin System/Peroxiredoxin enzyme machinery. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-24900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vijay R., Fehr A.R., Janowski A.M., Athmer J., Wheeler D.L., Grunewald M., Sompallae R., Kurup S.P., Meyerholz D.K., Sutterwala F.S., Narumiya S., Perlman S. Virus-induced inflammasome activation is suppressed by prostaglandin D2/DP1 signaling. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E5444–E54413. doi: 10.1073/pnas.1704099114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werder R.B., Lynch J.P., Simpson J.C., Zhang V., Hodge N.H., Poh M., Forbes-Blom E., Kulis C., Smythe M.L., Upham J.W., Spann K., Everard M.L., Phipps S. PGD2/DP2 receptor activation promotes severe viral bronchiolitis by suppressing IFN- production. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao0052. [DOI] [PubMed] [Google Scholar]
- 48.Miki Y., Yamamoto K., Taketomi Y., Sato H., Shimo K., Kobayashi T., Ishikawa Y., Ishii T., Nakanishi H., Ikeda K., Taguchi R., Kabashima K., Arita M., Arai H., Lambeau G., Bollinger J.M., Hara S., Gelb M.H., Murakami M. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators, J. Exp. Med. 2013;210:1217–1234. doi: 10.1084/jem.20121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murata T., Aritake K., Tsubosaka Y., Maruyama T., Nakagawa T., Hori M., Hirai H., Nakamura M., Narumiya S., Urade Y., Ozaki H. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5205–5210. doi: 10.1073/pnas.1218091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalinski P. Regulation of immune responses by prostaglandin e 2. J. Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vancheri C., Mastruzzo C., Sortino M.A., Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004;25:40–46. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Vijay R., Hua X., Meyerholz D.K., Miki Y., Yamamoto K., Gelb M., Murakami M., Perlman S. Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection. J. Exp. Med. 2015;212:1851–1868. doi: 10.1084/jem.20150632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sala A., Murphy R.C., Voelkel N.F. Direct airway injury results in elevated levels of sulfidopeptide leukotrienes, detectable in airway secretions. Prostaglandins. 1991;42:1–7. doi: 10.1016/0090-6980(91)90088-W. [DOI] [PubMed] [Google Scholar]
- 54.Ford-Hutchinson A.W., Bray M.A., Doig M.V., Shipley M.E., Smith M.J.H. Leukotriene B., a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 55.Bisgaard H., Helqvist S., Boudet L., Venge P., Dahl R., Søndergaard J. Chemotactic activity of LTB4 in man. Allergy. 1986;41:365–372. doi: 10.1111/j.1398-9995.1986.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 56.Tager A.M., Bromley S.K., Medoff B.D., Islam S.A., Bercury S.D., Friedrich E.B., Carafone A.D., Gerszten R.E., Luster A.D. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat. Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 57.Taube C., Miyahara N., Ott V., Swanson B., Takeda K., Loader J., Shultz L.D., Tager A.M., Luster A.D., Dakhama A., Gelfand E.W. The leukotriene B4 receptor (BLT1) is required for effector CD8 + t cell-mediated, mast cell-dependent airway hyperresponsiveness. J. Immunol. 2006;176:3157–3164. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- 58.Valentovic M. XPharm Compr. Pharmacol. Ref. Elsevier Inc.; 2007. Montelukast; pp. 1–4. [DOI] [Google Scholar]
- 59.Werz O., Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol. Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Valentovic M. XPharm Compr. Pharmacol. Ref. Elsevier Inc.; 2007. Zileuton; pp. 1–3. [DOI] [Google Scholar]
- 61.Funk C.D. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat. Rev. Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 62.Funk C.D., Ardakani A. A novel strategy to mitigate the hyperinflammatory response to COVID-19 by targeting leukotrienes. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones T.R., Labelle M., Belley M., Champion E., Charette L., Evans J., Ford-Hutchinson A.W., Gauthier J.Y., Lord A., Masson P., McAuliffe M., McFarlane C.S., Metters K.M., Pickett C., Piechuta H., Rochette C., Rodger I.W., Sawyer N., Young R.N. Pharmacology of montelukast sodium (Singulair(TM)), a potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 1995;73:191–201. doi: 10.1139/y95-028. [DOI] [PubMed] [Google Scholar]
- 64.Almerie M.Q., Kerrigan D.D. The association between obesity and poor outcome after COVID-19 indicates a potential therapeutic role for montelukast. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sadik C.D., Luster A.D. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J. Leukoc. Biol. 2012;91:207–215. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaudreault É., Gosselin J. Leukotriene B 4 induces release of antimicrobial peptides in lungs of virally infected mice. J. Immunol. 2008;180:6211–6221. doi: 10.4049/jimmunol.180.9.6211. [DOI] [PubMed] [Google Scholar]
- 68.Gentile D.A., Fireman P., Skoner D.P. Elevations of local leukotriene C4 levels during viral upper respiratory tract infections. Ann. Allergy Asthma Immunol. 2003;91:270–274. doi: 10.1016/S1081-1206(10)63529-6. [DOI] [PubMed] [Google Scholar]
- 69.Widegren H., Andersson M., Borgeat P., Flamand L., Johnston S., Greiff L. LTB4 increases nasal neutrophil activity and conditions neutrophils to exert antiviral effects. Respir. Med. 2011;105:997–1006. doi: 10.1016/j.rmed.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carey M.A., Bradbury J.A., Seubert J.M., Langenbach R., Zeldin D.C., Germolec D.R. Contrasting effects of Cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza a viral infection. J. Immunol. 2005;175:6878–6884. doi: 10.4049/jimmunol.175.10.6878. [DOI] [PubMed] [Google Scholar]
- 71.Börgeson E., McGillicuddy F.C., Harford K.A., Corrigan N., Higgins D.F., Maderna P., Roche H.M., Godson C. Lipoxin A4 attenuates adipose inflammation. FASEB J. 2012;26:4287–4294. doi: 10.1096/fj.12-208249. [DOI] [PubMed] [Google Scholar]
- 72.Reis M.B., Pereira P.A.T., Caetano G.F., Leite M.N., Galvão A.F., Paula-Silva F.W.G., Frade M.A.C., Faccioli L.H. Lipoxin A4 encapsulated in PLGA microparticles accelerates wound healing of skin ulcers. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishizuka T., Cheng J., Singh H., Vitto M.D., Manthati V.L., Falck J.R., Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-κB activation and the production of inflammatory cytokines in human endothelial cells, J. Pharmacol. Exp. Ther. 2008;324:103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 74.Wagner K., Vito S., Inceoglu B., Hammock B.D. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014;113–115:2–12. doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iliff J.J., Jia J., Nelson J., Goyagi T., Klaus J., Alkayed N.J. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010;91:68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang G., Kodani S., Hammock B.D. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 2014;53:108–123. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hashimoto K. Soluble epoxide hydrolase: a new therapeutic target for depression. Expert Opin. Ther. Targets. 2016;20:1149–1151. doi: 10.1080/14728222.2016.1226284. [DOI] [PubMed] [Google Scholar]
- 78.Duflot T., Roche C., Lamoureux F., Guerrot D., Bellien J. Design and discovery of soluble epoxide hydrolase inhibitors for the treatment of cardiovascular diseases. Expert Opin. Drug Discov. 2014;9:229–243. doi: 10.1517/17460441.2014.881354. [DOI] [PubMed] [Google Scholar]
- 79.Das U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J. Adv. Res. 2018;11:57–66. doi: 10.1016/j.jare.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoxha M. What about COVID-19 and arachidonic acid pathway? Eur. J. Clin. Pharmacol. 2020 doi: 10.1007/s00228-020-02941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwanke R.C., Marcon R., Bento A.F., Calixto J.B. EPA- and DHA-derived resolvins’ actions in inflammatory bowel disease. Eur. J. Pharmacol. 2016;785:156–164. doi: 10.1016/j.ejphar.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 82.Valdes A.M., Ravipati S., Menni C., Abhishek A., Metrustry S., Harris J., Nessa A., Williams F.M.K., Spector T.D., Doherty M., Chapman V., Barrett D.A. Association of the resolvin precursor 17-HDHA, but not D-or E-series resolvins, with heat pain sensitivity and osteoarthritis pain in humans. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-09516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vane J.R. Biomedicine: back to an aspirin a day? Science (80-.) 2002;296:474–475. doi: 10.1126/science.1071702. [DOI] [PubMed] [Google Scholar]
- 84.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu T., Gao X., Wu Z., Selinger D., Zhou Z. Indomethacin has a potent antiviral activity against SARS CoV-2 in vitro and canine coronavirus in vivo. BioRxiv. 2020 doi: 10.1101/2020.04.01.017624. 2020.04.01.017624. [DOI] [Google Scholar]
- 86.Voiriot G., Philippot Q., Elabbadi A., Elbim C., Chalumeau M., Fartoukh M. Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients. J. Clin. Med. 2019;8:786. doi: 10.3390/jcm8060786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.The Guardian; 2021. Anti-inflammatories May Aggravate Covid-19, France Advises | Coronavirus |.https://www.theguardian.com/world/2020/mar/14/anti-inflammatory-drugs-may-aggravate-coronavirus-infection (n.d.) (Accessed October 18, 2020) [Google Scholar]
- 88.European Medicines Agency; 2021. EMA Gives Advice on the Use of Non-steroidal Anti-inflammatories for COVID-19 |.https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19 (n.d.), (Accessed October 18, 2020) [Google Scholar]
- 89.2021. Ibuprofen Use and Coronavirus (COVID-19)https://www.gov.uk/government/news/ibuprofen-use-andcovid19coronavirus (n.d.), (accessed October 18, 2020) [Google Scholar]
- 90.McGonagle D., O’Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manjili R.H., Zarei M., Habibi M., Manjili M.H. COVID-19 as an acute inflammatory disease. J. Immunol. 2020;205:12–19. doi: 10.4049/jimmunol.2000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lingeswaran M., Goyal T., Ghosh R., Suri S., Mitra P., Misra S., Sharma P. Inflammation, Immunity and Immunogenetics in COVID-19: A Narrative Review. Indian J. Clin. Biochem. 2020;35:260–273. doi: 10.1007/s12291-020-00897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tu Y.F., Chien C.S., Yarmishyn A.A., Lin Y.Y., Luo Y.H., Lin Y.T., Lai W.Y., Yang D.M., Chou S.J., Yang Y.P., Wang M.L., Chiou S.H. A review of sars-cov-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tao Y., Tang L.V., Hu Y. Treatments in the COVID-19 pandemic: an update on clinical trials. Expert Opin. Emerg. Drugs. 2020;25:81–88. doi: 10.1080/14728214.2020.1773431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stockman L.J., Bellamy R., Garner P. SARS: Systematic review of treatment effects. PLoS Med. 2006;3:1525–1531. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Auyeung T.W., Lee J.S.W., Lai W.K., Choi C.H., Lee H.K., Lee J.S., Li P.C., Lok K.H., Ng Y.Y., Wong W.M., Yeung Y.M. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J. Infect. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ho J.C., Ooi G.C., Mok T.Y., Chan J.W., Hung I., Lam B., Wong P.C., Li P.C., Ho P.L., Lam W.K., Ng C.K., Ip M.S., Lai K.N., Chan-Yeung M., Tsang K.W. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2003;168:1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- 99.Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q., Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J. Infect. 2020;81:e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., Almotairi A., Al Khatib K., Alraddadi B., Shalhoub S., Abdulmomen A., Qushmaq I., Mady A., Mady O., Al-Aithan A.M., Al-Raddadi R., Ragab A., Balkhy H.H., Balkhy A., Deeb A.M., Al Mutairi H., Al-Dawood A., Merson L., Hayden F.G., Fowler R.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 102.Lansbury L.E., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Lim W.S. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated cochrane systematic review and meta-analysis. Crit. Care Med. 2020;48:E98–E106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 103.Siemieniuk R.A.C., Meade M.O., Alonso-Coello P., Briel M., Evaniew N., Prasad M., Alexander P.E., Fei Y., Vandvik P.O., Loeb M., Guyatt G.H. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and metaanalysis. Ann. Intern. Med. 2015;163:519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
- 104.2021. Treatment – COVID Reference.https://covidreference.com/treatment (n.d.)., (accessed October 18, 2020) [Google Scholar]
- 105.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Corral L., Bahamonde A., delas Revillas F.A., Gomez-Barquero J., Abadia-Otero J., Garcia-Ibarbia C., Mora V., Cerezo-hernandez A., Hernandez J.L., Lopez-Muniz G., Hernandez-Blanco F., Cifrian J.M., Olmos J.M., Carrascosa M., Farinas maria C., Riancho J.A., Investigators G. GLUCOCOVID: a controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. MedRxiv. 2020 doi: 10.1007/s00508-020-01805-8. https://www.medrxiv.org/content/10.1101/2020.06.17.20133579v1 2020.06.17.20133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee N., Chan P.K.S., Hui D.S.C., Rainer T.H., Wong E., Choi K.W., Lui G.C.Y., Wong B.C.K., Wong R.Y.K., Lam W.Y., Chu I.M.T., Lai R.W.M., Cockram C.S., Sung J.J.Y. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J. Infect. Dis. 2009;200:492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee N., Allen Chan K.C., Hui D.S., Ng E.K.O., Wu A., Chiu R.W.K., Wong V.W.S., Chan P.K.S., Wong K.T., Wong E., Cockram C.S., Tam J.S., Sung J.J.Y., Lo Y.M.D. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.2021. WHO Updates Guidance on Corticosteroids in Covid-19 Patients.https://www.pharmaceutical-technology.com/news/who-corticosteroids-covid-advice (n.d.)., (accessed October 18, 2020) [Google Scholar]
- 111.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., Cavalcanti A.B., Dequin P.F., Du B., Emberson J., Fisher D., Giraudeau B., Gordon A.C., Granholm A., Green C., Haynes R., Heming N., Higgins J.P.T., Horby P., Jüni P., Landray M.J., Le Gouge A., Leclerc M., Lim W.S., MacHado F.R., McArthur C., Meziani F., Møller M.H., Perner A., Petersen M.W., Savović J., Tomazini B., Veiga V.C., Webb S., Marshall J.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA - J. Am. Med. Assoc. 2020;324:E1–E12. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lammers T., Sofias A.M., van der Meel R., Schiffelers R., Storm G., Tacke F., Koschmieder S., Brümmendorf T.H., Kiessling F., Metselaar J.M. Dexamethasone nanomedicines for COVID-19. Nat. Nanotechnol. 2020;15:622–624. doi: 10.1038/s41565-020-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.2021. Corticosteroids | COVID-19 Treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/ (n.d.),(accessed October 18, 2020) [Google Scholar]
- 114.2021. Results — RECOVERY Trial.https://www.recoverytrial.net/results (n.d.), (accessed October 18, 2020) [Google Scholar]
- 115.2021. Dexamethasone - an Overview | ScienceDirect Topics.https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/dexamethasone (n.d.), (accessed October 19, 2020) [Google Scholar]
- 116.Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A., Martín-Rodríguez C., Díaz-Domínguez F.J., Serna-Grande P., Rivas R., Ferreres J., Belda J., Capilla L., Tallet A., Añón J.M., Fernández R.L., González-Martín J.M., Álvarez J., Añón J.M., Asensio M.J., Blanco J., Blasco M., Cachafeiro L., del Campo R., Carbonell J.A., Carbonell N., Cariñena A., Carriedo D., Chico M., Conesa L.A., Corpas R., Cuervo J., Díaz-Domínguez F.J., Domínguez-Antelo C., Fernández L., Fernández R.L., Gamboa E., González-Luengo R.I., González-Martín J.M., Ortiz Díaz-Miguel R., Pérez-González R., Prieto A.M., Prieto I., Rojas-Viguera L., Romera M.A., Sánchez-Ballesteros J., Segura J.M., Serrano A., Solano R., Soler J.A., Soro M. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 117.2021. Dexamethasone: What Is the Breakthrough Treatment for COVID-19?https://theconversation.com/dexamethasone-what-is-the-breakthrough-treatment-for-covid-19-140966 (n.d.). (accessed October 19, 2020) [Google Scholar]
- 118.2021. Biology of Dexamethasone: The First Lifesaving Drug for Covid-19 | by Shin Jie Yong | Microbial Instincts | Medium.https://medium.com/microbial-instincts/biology-of-dexamethasone-the-first-lifesaving-drug-for-covid-19-357ed9daaf7a (n.d.) (accessed October 19, 2020) [Google Scholar]
- 119.MJL RECOVERY Collaborative Group, Horby Peter, Lim Wei Shen, Emberson Jonathan R., Mafham Marion, Bell Jennifer L., Linsell Louise, Staplin Natalie, Brightling Christopher, Ustianowski Andrew, Elmahi Einas, Prudon Benjamin, Green Christopher, Felton Timothy. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Müller C., Hardt M., Schwudke D., Neuman B.W., Pleschka S., Ziebuhr J. Inhibition of cytosolic phospholipase A2α impairs an early step of coronavirus replication in cell culture. J. Virol. 2017;92 doi: 10.1128/jvi.01463-17. JVI.01463-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gallop J.L., McMahon H.T. BAR domains and membrane curvature: bringing your curves to the BAR. Biochem. Soc. Symp. 2005;72:223–231. doi: 10.1042/bss0720223. [DOI] [PubMed] [Google Scholar]
- 122.Zizza P., Iurisci C., Bonazzi M., Cossart P., Leslie C.C., Corda D., Mariggio S. Phospholipase A2IVα regulates phagocytosis independent of its enzymatic activity. J. Biol. Chem. 2012;287:16849–16859. doi: 10.1074/jbc.M111.309419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X., Devaiah S.P., Zhang W., Welti R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 124.Athenstaedt K., Daum G. The life cycle of neutral lipids: synthesis, storage and degradation. Cell. Mol. Life Sci. 2006;63:1355–1369. doi: 10.1007/s00018-006-6016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gault C.R., Obeid L.M., Hannun Y.A. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Menzel N., Fischl W., Hueging K., Bankwitz D., Frentzen A., Haid S., Gentzsch J., Kaderali L., Bartenschlager R., Pietschmann T. MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious Hepatitis C virus particles. PLoS Pathog. 2012;8:21. doi: 10.1371/journal.ppat.1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gubern A., Casas J., Barceló-Torns M., Barneda D., De La Rosa X., Masgrau R., Picatoste F., Balsinde J., Balboa M.A., Claro E. Group IVA phospholipase A2 is necessary for the biogenesis of lipid droplets. J. Biol. Chem. 2008;283:27369–27382. doi: 10.1074/jbc.M800696200. [DOI] [PubMed] [Google Scholar]
- 128.Andreakos E., Salagianni M., Galani I.E., Koltsida O. Interferon-λs: Front-line guardians of immunity and homeostasis in the respiratory tract. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Prokunina-Olsson L., Alphonse N., Dickenson R.E., Durbin J.E., Glenn J.S., Hartmann R., Kotenko S.V., Lazear H.M., O’Brien T.R., Odendall C., Onabajo O.O., Piontkivska H., Santer D.M., Reich N.C., Wack A., Zanoni I. COVID-19 and emerging viral infections: the case for interferon lambda. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M., Willey B.M., DeVries M.E., Fang Y., Seneviratne C., Bosinger S.E., Persad D., Wilkinson P., Greller L.D., Somogyi R., Humar A., Keshavjee S., Louie M., Loeb M.B., Brunton J., McGeer A.J., Kelvin D.J. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007;81:8692–8706. doi: 10.1128/jvi.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., Jose J., Alraddadi B., Almotairi A., Al Khatib K., Abdulmomen A., Qushmaq I., Sindi A.A., Mady A., Solaiman O., Al-Raddadi R., Maghrabi K., Ragab A., Mekhlafi G.A.A., Balkhy H.H., Al Harthy A., Kharaba A., Gramish J.A., Al-Aithan A.M., Al-Dawood A., Merson L., Hayden F.G., Fowler R. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin. Infect. Dis. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11 doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-Infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., Sompallae R., McCray P.B., Meyerholz D.K., Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang I.C., Bailey C.C., Weyer J.L., Radoshitzky S.R., Becker M.M., Chiang J.J., Brass A.L., Ahmed A.A., Chi X., Dong L., Longobardi L.E., Boltz D., Kuhn J.H., Elledge S.J., Bavari S., Denison M.R., Choe H., Farzan M. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hirano T., Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu Y., Feng Z., Li P., Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin. Chim. Acta. 2020;509:220–223. doi: 10.1016/j.cca.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV - A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ni L., Ye F., Cheng M.L., Feng Y., Deng Y.Q., Zhao H., Wei P., Ge J., Gou M., Li X., Sun L., Cao T., Wang P., Zhou C., Zhang R., Liang P., Guo H., Wang X., Qin C.F., Chen F., Dong C. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52:971–977. doi: 10.1016/j.immuni.2020.04.023. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M., Lu Q., Li X., Sun Q., Liu J., Fan C., Huang W., Xu M., Wang Y. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., Baysal E., Mangold M., Henze L., Lauster R., Mall M.A., Beyer K., Röhmel J., Voigt S., Schmitz J., Miltenyi S., Demuth I., Müller M.A., Hocke A., Witzenrath M., Suttorp N., Kern F., Reimer U., Wenschuh H., Drosten C., Corman V.M., Giesecke-Thiel C., Sander L.E., Thiel A. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 146.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Serhan C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31:1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dalli J. Does promoting resolution instead of inhibiting inflammation represent the new paradigm in treating infections? Mol. Aspects Med. 2017;58:12–20. doi: 10.1016/j.mam.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 149.Ramon S., Baker S.F., Sahler J.M., Kim N., Feldsott E.A., Serhan C.N., Martínez-Sobrido L., Topham D.J., Phipps R.P. The Specialized Proresolving Mediator 17-HDHA Enhances the Antibody-Mediated Immune Response against Influenza Virus: A New Class of Adjuvant? J. Immunol. 2014;193:6031–6040. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morita M., Kuba K., Ichikawa A., Nakayama M., Katahira J., Iwamoto R., Watanebe T., Sakabe S., Daidoji T., Nakamura S., Kadowaki A., Ohto T., Nakanishi H., Taguchi R., Nakaya T., Murakami M., Yoneda Y., Arai H., Kawaoka Y., Penninger J.M., Arita M., Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 151.Jiang J.X., Zhang S.J., Liu Y.N., Lin X.X., Sun Y.H., Shen H.J., Yan X.F., Xie Q.M. EETs alleviate ox-LDL-induced inflammation by inhibiting LOX-1 receptor expression in rat pulmonary arterial endothelial cells. Eur. J. Pharmacol. 2014;727:43–51. doi: 10.1016/j.ejphar.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 152.Schmelzer K.R., Kubala L., Newman J.W., Kim I.H., Eiserich J.P., Hammock B.D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cherpokova D., Jouvene C.C., Libreros S., DeRoo E.P., Chu L., De La Rosa X., Norris P.C., Wagner D.D., Serhan C.N. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood. 2019;134:1458–1468. doi: 10.1182/blood.2018886317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Imig J.D., Hammock B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2007575. E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Panigrahy D., Gilligan M.M., Huang S., Gartung A., Cortés-Puch I., Sime P.J., Phipps R.P., Serhan C.N., Hammock B.D. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020;39:337–340. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]