Abstract
Background
Small nonfunctional pancreatic neuroendocrine tumors (NF-PNETs) ≤2 cm have variable biological features, and there is no gold standard treatment for their management. The present study aimed to evaluate the risk of malignancy of small NF-PNETs and their outcomes following curative resection.
Methods
Patients with NF-PNETs undergoing surgical resection at the First Affiliated Hospital, College of Medicine, Zhejiang University, between 2012 and 2017 were included. Clinicopathological characteristics, perioperative results, and prognosis were retrospectively analyzed.
Results
A total of 73 patients were identified, including 28 with small NF-PNETs and 45 large PNETs; 32.1% of NF-PNETs ≤2 cm underwent a parenchyma-sparing pancreas surgery, which was >6.7% in large NF-PNETs. No statistically significant differences in perioperative results, postoperative complications, and long-term outcomes were found between small tumors undergoing standard and parenchyma-sparing pancreatectomy. Eighteen small tumors (64.3%) developed a perioperative complication, with a clinically significant pancreatic fistula rate of 25%; however, only 2 patient needed reintervention. Small NF-PNETs in 3 patients were malignant. Multivariate logistic regression showed that grade ≥3 and lymphovascular invasion were independently related to malignancy in NF-PNETs.
Conclusions
Small NF-PNETs (≤2 cm) are not immune from potential malignancy. Surgical resection may be considered for small tumors and can provide favorable postoperative and long-term outcomes. Parenchyma-sparing pancreatectomy may be an alternative surgery for selected small local NF-PNETs.
Keywords: Nonfunctional pancreatic neuroendocrine tumors (NF-PNETs), malignancy, observation, parenchyma-sparing pancreatectomy
Introduction
Pancreatic neuroendocrine tumors (PNETs) are a group of endocrine tumors originating from the islet cells of the pancreas and wide heterogeneity, including variable biologic behavior and clinicopathological features (1,2). PNETs are very rare and account for less than 5% of all pancreatic tumors (3-5). However, it has been reported that there has been a significant increase in their incidence over the past several decades, particularly for early/localized PNETs, which is mainly due to the early utility of various advanced imaging tools (6-8). Depending on the presence of clinical syndromes caused by hormone hypersecretion, PNETs are generally divided into the functional type and non-functional type (2). Most PNETs are non-functional tumors, which comprise about 65–90% of all PNETs and are more aggressive than functional tumors (3,5,7).
Unlike functional PNETs that present with hypersecretion with almost all requiring surgical resection (1), nonfunctional PNETs (NF-PNETs) usually have no apparent symptoms or have non-specific symptoms; there is no gold standard treatment for their management. It is now generally recognized that NF-PNETs >2 cm is the main indication for surgical resection (9,10). However, whether surgery could provide more benefits than non-surgical treatment for NF-PNETs ≤2 cm remains a controversial issue. Recommendations for the treatment of small NF-PNETs were different across guidelines (1,11,12). There are currently two contrasting views regarding the risk of malignancy of NF-PNETs ≤2 cm. These are as follows: (I) small NF-PNETs are usually biologically indolent with rare, aggressive features, and long-term surveillance should be adapted as the primary option (13-15); and (II) a non-negligible risk of malignancy can be observed even in small NF-PNETs; therefore, aggressive surgical treatment is necessary to improve the prognosis (16-18). The primary aim of our study was to evaluate the risk of malignancy of small NF-PNETs ≤2 cm, and to explore further the effect of surgical resection based on our single-center experience. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-582).
Methods
Date collection
Medical records, radiologic reports, and pathologic results of all patients with PNETs at the First Affiliated Hospital of Zhejiang University between January 2012 and December 2017 were reviewed retrospectively. Patients who underwent curative resection and had pathologically confirmed NF-PNETs were included in our study. The exclusion criteria were as follows: (I) patients with incomplete medical data; (II) patients diagnosed with inherited diseases, including, but not limited to, multiple neuroendocrine neoplasia types 1 (MEN1) and Von Hippel-Lindau; (III) patients with a functional PNET neoplasm; (IV) patients who had undergone an R2 resection; and (V) recurrence of a preoperatively resected PNET. Eligible patients were divided into two groups according to their tumor sizes, the small tumor group (≤2 cm) and the large tumor group (>2 cm). All pathological reports were reviewed carefully and available slides were revised by an experienced pathologist. Tumor size was determined by the maximum diameter of the tumor in the pathological report. All tumors were classified according to the World Health Organization 2017 Grading of Recommendations Assessment, Development and Evaluation criteria, and the European Neuroendocrine Tumor Society (ENETS) classification system. Malignant signs of NF-PNETs were defined as the presence of tumor recurrence or nodal/distant metastases (synchronous or metachronous). The study was conducted in accordance with the Declaration of Helsinki as revised in 2013. This study was approved by Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. IIT20200277A) and all patient information was anonymous. Individual consent for this retrospective analysis was waived.
Surgery procedures and complications
Curative surgery was defined as R0 (absence of residual tumor under a microscope) or R1 (presence of residual tumor under a microscope) resection. For patients with resectable metastasis or invasive disease at the time of diagnosis, curative intent surgery contained simultaneous resection of the primary tumor and all metastases or invasion lesions. The surgical procedure included standard surgery such as pancreaticoduodenectomy (Whipple), distal pancreatectomy with or without splenectomy, total pancreatectomy and parenchyma-sparing pancreatectomy, which contained enucleation and central pancreatectomy. Clavien-Dindo classification was used to stratify postoperative comorbidities. Perioperative mortality was defined as in-hospital or within 60-day death. Long-term complications contain new-onset diabetes mellitus or worsen of diabetes mellitus, and pancreatic exocrine insufficiency.
Follow-up and survival
Follow-up data were obtained from postoperative outpatient visits records or telephone contact. The last follow-up was terminated in January 2020. Patients lost to follow-up were censored at the date of the last contact. Recurrence of NF-PNETs was defined as the presence of a local lesion or nodal/distant metastasis and determined by biopsy pathological findings and imaging. Disease-free survival (DFS) was calculated as the time between surgery and recurrence or metastasis or final follow-up. Overall survival (OS) was defined as the time between surgery and death or the last follow-up. The cause of death was investigated and defined if this was related to NF-PNETs.
Statistical analysis
Continuous variables with normal distribution were expressed as means ± standard deviations; medians and ranges were described used for non-normally distributed variables. Categorical variables were expressed as frequencies and percentages. The comparison of characteristics between two independent samples was performed using χ2-test or Fisher’s exact test for categorical data, Student’s t-test, or the Mann-Whitney U-test for continuous variables. Survival analysis was calculated using the Kaplan-Meier method and compared by the log-rank test. A stepwise binary logistic regression model was used to evaluate significant predictors associated with malignancy. Two-sided P value <0.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS Statistic version 22.0 (IBM, Chicago, IL, USA).
Results
Clinical and demographic characteristics
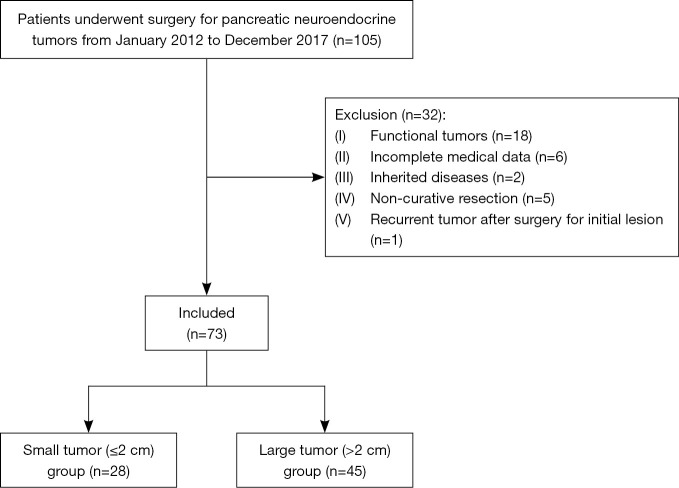
A total of 73 patients with NF-PNETs who underwent curative-intent surgery were included in our study (Figure 1). Patients’ baseline characteristics are presented in Table 1. Twenty-eight small tumors had a median size of 1.2 cm, with the minimum dimension being 0.7 cm. Forty-five patients (57.8% women, n=26) were in large tumor group (>2 cm). More than half of the patients (52.1%) did not have symptoms. Tumors located in the body/tail accounted for 65.8% (n=48) of all patients, while only 25 (34.2%) were located in the head/neck. There were no significant differences associated with age, sex, symptoms, and tumor site between the small and large tumors.
Figure 1.
Flow chart of the patient selection process.
Table 1. Clinical features and histopathology of patients with nonfunctional pancreatic neuroendocrine tumors.
| Variables | Total (n=73) | ≤2 cm (n=28) | >2 cm (n=45) | P value |
|---|---|---|---|---|
| Age, years, mean [SD] | 56 [11] | 59 [10] | 55 [10] | 0.090 |
| Gender | 0.725 | |||
| Male | 32 (43.8) | 13 (46.4) | 19 (42.2) | |
| Female | 41 (56.2) | 15 (53.6) | 26 (57.8) | |
| Symptom | 0.838 | |||
| No | 38 (52.1) | 15 (53.6) | 23 (51.1) | |
| Yes | 35 (47.9) | 13 (46.4) | 22 (48.9) | |
| Abdominal pain/discomfort | 28 (38.4) | 9 (32.1) | 19 (42.2) | 0.389 |
| Jaundice | 4 (5.5) | 1 (3.6) | 3 (6.7) | 0.971 |
| Weight loss | 3 (4.1) | 1 (3.6) | 2 (4.4) | 1.000 |
| Fatigue | 2 (2.7) | 2 (7.1) | 0 (0.0) | 0.144 |
| Site | 0.765 | |||
| Head/neck | 25 (34.2) | 9 (32.1) | 16 (35.6) | |
| Body/tail | 48 (65.8) | 19 (67.9) | 29 (64.4) | |
| Ki-67, median [range] | 5 [1–50] | 1 [1–30] | 7 [1–50] | <0.001 |
| WHO grade | <0.001 | |||
| G1 | 27 (37.0) | 19 (67.9) | 8 (17.8) | |
| G2 | 38 (52.1) | 6 (21.4) | 32 (71.1) | |
| G3 | 7 (9.6) | 3 (10.7) | 4 (8.9) | |
| NEC | 1 (1.4) | 0 (0.0) | 1 (2.2) | |
| ENETS staging | 0.001 | |||
| I–II | 51 (69.9) | 26 (92.9) | 25 (55.6) | |
| III–IV | 22 (30.1) | 2 (7.1) | 20 (44.4) | |
| Lymph node status | 0.680 | |||
| Positive | 7 (9.6) | 1 (3.6) | 6 (13.3) | |
| Negative | 31 (42.5) | 9 (32.1) | 22 (48.9) | |
| Unknown | 35 (47.9) | 18 (64.3) | 17 (37.8) | |
| Lymphovascular invasion | 0.003 | |||
| Present | 22 (30.1) | 3 (10.7) | 20 (44.4) | |
| Absent | 51 (69.9) | 25 (89.3) | 25 (55.6) | |
| Perineural invasion | 0.862 | |||
| Present | 6 (8.2) | 3 (10.7) | 3 (6.7) | |
| Absent | 67 (91.8) | 25 (89.3) | 42 (93.3) | |
| Distant metastasis before surgery | 0.149 | |||
| Yes | 5 (6.8) | 0 (0.0) | 5 (88.9) | |
| No | 68 (93.2) | 28 (100.0) | 40 (11.1) | |
| Adjuvant therapy | 1.000 | |||
| Yes | 6 (8.2) | 2 (7.1) | 4 (8.9) | |
| No | 67 (91.8) | 26 (92.9) | 41 (91.9) | |
| Recurrence | 0.053 | |||
| Yes | 15 (20.5) | 2 (7.1) | 13 (28.9) | |
| No | 58 (79.5) | 26 (92.9) | 32 (71.1) | |
| Malignancy | 0.057 | |||
| Yes | 18 (24.7) | 3 (10.7) | 15 (33.3) | |
| No | 55 (75.3) | 25 (89.3) | 30 (66.7) | |
| Follow-up, months, mean (SD) | 49.6 (24.5) | 52.5 (22.8) | 47.8 (25.5) | 0.428 |
| Death | 4 (5.5) | 0 (0.0) | 4 (8.9) | 0.291 |
Numbers in brackets represent percentage frequency if not otherwise specified. SD, standard deviation.
Surgical results
Surgery procedures and postoperative complications are shown in Table 2. Parenchyma-sparing pancreatectomy was more frequently preferred to treat the small PNETs rather than the large PNETs (32.1% vs. 6.7%, P=0.011). The majority of the patients (76.7%, n=56) underwent open surgery, whereas 17 patients (23.3%) underwent laparoscopy. Of the 28 patients with small NF-PNETs, 18 (64.3%) developed a perioperative complication, and 2 (11.1%) required reintervention. The rate of pancreatic fistula grade B/C was 25%. Worsening glucose control and exocrine insufficiency were observed in 5 (17.9%) and 6 (21.4%) cases, respectively. There were no statistically significant differences in perioperative results, postoperative complications, and long-term outcomes between small tumors with and without parenchyma-sparing pancreatectomy (Table 3).
Table 2. Surgical procedures and complications of nonfunctional pancreatic neuroendocrine tumors that underwent curative resection.
| Variable | Total (n=73) | ≤2 cm (n=28) | >2 cm (n=45) | P value |
|---|---|---|---|---|
| Surgery procedure | 0.021 | |||
| Whipple | 18 (24.7) | 4 (14.3) | 14 (31.1) | |
| DP | 17 (23.3) | 9 (32.1) | 8 (17.8) | |
| DP+ splenectomy | 25 (34.2) | 6 (21.4) | 19 (42.2) | |
| Central resection | 5 (6.8) | 4 (14.3) | 1 (2.2) | |
| Enucleation | 7 (9.6) | 5 (17.9) | 2 (4.4) | |
| TP+ splenectomy | 1 (1.4) | 0 (0.0) | 1 (2.2) | |
| Surgery type | 0.011 | |||
| Standard surgery | 61 (83.6) | 19 (67.9) | 42 (93.3) | |
| Parenchyma-sparing pancreatectomy | 12 (16.4) | 9 (32.1) | 3 (6.7) | |
| Approach | ||||
| Open surgery | 56 (76.7) | 19 (67.9) | 37 (82.2) | 0.158 |
| Laparoscopic surgery | 17 (23.3) | 9 (32.1) | 8 (17.8) | |
| Operative time, min, mean [SD] | 322 [143] | 342 [145] | 309 [143] | 0.336 |
| Blood loss, mL, median [range] | 200 [25–2,000] | 200 [25–300] | 200 [50–2,000] | 0.108 |
| Surgical margin | ||||
| R0 | 71 (97.3) | 27 (96.4) | 44 (97.8) | 1.000 |
| R1 | 2 (2.7) | 1 (3.6) | 1 (2.2) | |
| Length of stay, days, median [range] | 16 [5, 71] | 13 [6, 71] | 14 [5, 44] | 0.682 |
| Complications | 0.357 | |||
| No | 31 (42.5) | 10 (35.7) | 21 (46.7) | |
| Yes | 42 (57.5) | 18 (64.3) | 24 (53.3) | |
| Severity | 0.661 | |||
| Clavien-Dindo ≤II | 65 (81.0) | 26 (92.9) | 39 (86.7) | |
| Clavien-Dindo ≥III | 8 (19.0) | 2 (7.1) | 6 (13.3) | |
| POPF | 28 (38.4) | 14 (50.0) | 14 (31.1) | 0.107 |
| POPF grade B/C | 11 (15.1) | 7 (25.0) | 4 (8.9) | 0.125 |
| DGE | 4 (5.5) | 1 (3.6) | 3 (6.7) | 0.971 |
| Abdominal collection | 8 (10.9) | 3 (10.7) | 5 (11.1) | 1.000 |
| Abdominal bleeding | 1 (1.4) | 0 (0.0) | 1 (2.2) | 1.000 |
| Biliary leakage | 1 (1.4) | 0 (0.0) | 1 (2.2) | 1.000 |
| Chylous leakage | 2 (2.7) | 2 (7.1) | 0 (0.0) | 0.144 |
| Endocrine insufficiency | 12 (16.4) | 5 (17.9) | 7 (15.6) | 0.796 |
| Exocrine insufficiency | 17 (23.3) | 6 (21.4) | 11 (24.4) | 0.767 |
Numbers in brackets represent percentage frequency if not otherwise specified. DP, distal pancreatectomy; TP, total pancreatectomy; SD, standard deviation; POPF, postoperative pancreatic fistula; DGE, delayed gastric emptying.
Table 3. Comparison of parenchyma-preserving pancreatectomy and standard surgery for small nonfunctional pancreatic neuroendocrine tumors.
| Factors | Parenchyma-preserving pancreatectomy (n=9) | Standard surgery (n=19) | P value |
|---|---|---|---|
| Operation time, min, mean (SD) | 288.3 (169.4) | 368.0 (128.6) | 0.179 |
| Surgery approach | 0.195 | ||
| Open | 8 (88.9) | 11 (57.9) | |
| Minimal invasive | 1 (11.1) | 8 (42.1) | |
| Blood loss, mL, median (range) | 147.2 (25.0–250.0) | 186.6 (50.0–300.0) | 0.315 |
| Length of stay, days, median [range] | 17 [6–71] | 20 [6–71] | 0.844 |
| Surgical margin | 0.321 | ||
| R0 | 8 (88.9) | 19 (100.0) | |
| R1 | 1 (11.1) | 0 (0.0) | |
| Complications, n (%) | 0.677 | ||
| No | 4 (44.4) | 6 (31.6) | |
| Yes | 5 (55.6) | 13 (68.4) | |
| Severity (Clavien-Dindo grade) | 1.000 | ||
| CD ≤2 | 9 (100.0) | 17 (89.5) | |
| CD ≥3 | 0 (0.0) | 2 (10.5) | |
| POPF | 5 (55.6) | 9 (47.4) | 1.000 |
| POPF grade B/C | 5 (55.6) | 4 (21.1) | 0.097 |
| Endocrine insufficiency | 0 (0.0) | 5 (26.3) | 0.144 |
| Exocrine insufficiency | 2 (22.2) | 4 (21.1) | 1.000 |
| Recurrence | 0 (0.0) | 2 (10.5) | 1.000 |
| Disease-related death | 0 (0.0) | 0 (0.0) | 1.000 |
Numbers in brackets represent percentage frequency if not otherwise specified. SD, standard deviation; POPF, postoperative pancreatic fistula.
Pathological results
Postoperative pathological results are summarized in Table 1. Small tumors were more likely to have a better differentiation grade than large tumors, with 67.9% being G1 (n=19), compared to 17.8% (n=8) of large NF-PNETs. However, the rate of grade ≥3 [NET-G3 and neuroendocrine carcinoma (NEC)] was 10.7% in small tumors, which was similar to the large tumors (11.1%). The Ki-67 index was apparently lower in small NF-PNETs than in large NF-PNETs (P<0.001). Large tumors (>2 cm) had a greater proportion of ENETS stage III–IV, which tended to show frequent infiltrating or metastatic diseases, whereas small tumors tended to be local with early staging (ENETS staging I–II, 92.9% vs. 55.6%, P=0.001). One tumor with a diameter of 1.5 cm was found to have positive lymph nodes after a careful pathological examination. A statistically significant difference in lymphovascular invasion were noted between the 2 groups; the lymphovascular invasion was more frequent in NF-PNETs >2 cm (P=0.003).
Long-term outcomes
After a mean follow-up period of 49.6±24.5 months (range, 1–94 months), 6 cases were lost to follow-up. A total of 15 patients had tumor recurrence or metastasis after curative-intent surgery with a median time to recurrence of 13 months; 2 were small NF-PNETs, and the remaining 13 were large tumors (Table 1). The liver was the most common recurrence site of recurrence (n=11), followed by lymph nodes (n=2). In total, four patients developed disease-related deaths, all of them were large tumors. There were no deaths due to other causes.
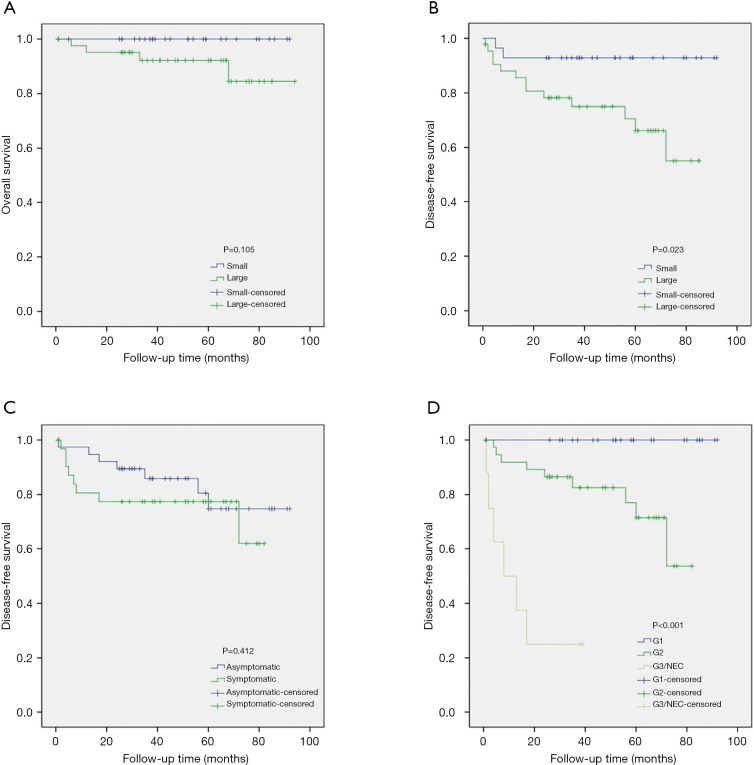
The difference in OS between the small and large tumor groups was not significant (P=0.105) (Figure 2A), while small NF-PNETs had better significantly better DFS than large NF-PNETs (P=0.023) (Figure 2B). For the 35 patients with symptoms, DFS did not differ from those who were asymptomatic (P=0.412) (Figure 2C). There were statistically significant differences in DFS in patients with tumors of different pathological grades (P<0.001) (Figure 2D).
Figure 2.
Kaplan-Meier curves of overall survival (OS) and disease-free survival (DFS) in patients with nonfunctional pancreatic neuroendocrine tumors. Patients with small tumors had similar OS (A), but better DFS than those with large tumors (B). DFS did not differ between patients with symptoms and those without (C). There were statistically significant differences in DFS in patients with tumors of different pathological grades (D).
Risk of malignancy of the NF-PNETs
A total of three patients with small NF-PNETs (10.7%) had symptoms of malignancy; 2 had a postoperative recurrence, and 1 had synchronous lymph node metastasis at the time of diagnosis. Baseline characteristics of 18 patients with NF-PNETs showing malignant signs are shown in Table 4. Variables associated with the presence of malignancy were assessed by univariate and multivariate logistic regression analysis (Table 5). Univariate analysis indicated that sex, tumor grade, lymphovascular invasion, perineural invasion and tumor size were significantly related to malignancy in NF-PNETs that underwent curative resection. These factors were included in a stepwise multivariate logistic regression model, whereas the presence of malignancy signs was only found to be associated with tumor grade and lymphovascular invasion.
Table 4. Baseline characteristics of 18 patients with symptoms of malignancy of nonfunctional pancreatic neuroendocrine tumors.
| Variable | Total (n=18) | ≤2 cm (n=3) | >2 cm (n=15) |
|---|---|---|---|
| Age, years, mean [SD] | 58 [8] | 62 [9] | 57 [8] |
| Gender | |||
| Male | 12 (66.7) | 3 (100.0) | 9 (60.0) |
| Female | 6 (33.3) | 0 (0.0) | 6 (40.0) |
| Symptom | |||
| Yes | 10 (55.6) | 2 (66.7) | 8 (53.3) |
| No | 8 (44.4) | 1 (33.3) | 7 (46.7) |
| Site | |||
| Head/tail | 8 (44.4) | 1 (33.3) | 7 (46.7) |
| Body/tail | 10 (55.6) | 2 (66.7) | 8 (53.3) |
| WHO grade | |||
| G1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| G2 | 11 (61.1) | 1 (33.3) | 10 (66.7) |
| G3 | 6 (33.3) | 2 (66.7) | 4 (26.7) |
| NEC | 1 (5.6) | 0 (0.0) | 1 (6.7) |
| Lymphovascular invasion | |||
| Present | 13 (72.2) | 2 (66.7) | 11 (73.3) |
| Absent | 5 (27.8) | 1 (33.3) | 4 (26.7) |
| Perineural invasion | |||
| Present | 4 (22.2) | 2 (66.7) | 2 (13.3) |
| Absent | 14 (77.8) | 1 (33.3) | 13 (86.7) |
| Size, cm, mean (SD) | 4.4 (2.2) | 1.8 (0.3) | 4.9 (2.0) |
| Surgery type | |||
| Standard surgery | 17 (94.4) | 3 (100.0) | 14 (93.3) |
| Parenchyma-sparing surgery | 1 (5.6) | 0 (0.0) | 1 (6.7) |
| Surgery approach | |||
| Open | 16 (88.9) | 2 (66.7) | 14(93.3) |
| Laparoscopically | 2 (11.1) | 1 (33.3) | 1 (6.7) |
| Surgical margin | |||
| R0 | 18 (100.0) | 3 (100.0) | 15 (100.0) |
| R1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Death | 4 (22.2) | 0 (0.0) | 4 (26.7) |
| Follow-up, months, mean (SD) | 46.6 (28.1) | 36.3 (30.1) | 48.6 (28.4) |
Numbers in brackets represent percentage frequency if not otherwise specified. SD, standard deviation.
Table 5. Univariate and multivariate logistic regression analyses of the risk factors associated with malignancy in patients with nonfunctional pancreatic neuroendocrine tumors.
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, years | 1.020 (0.968–1.074) | 0.462 | – | – | |
| ≤60 | Reference | – | – | – | |
| > 60 | 1.644 (0.555–4.876) | 0.370 | – | – | |
| Sex | |||||
| Male | Reference | – | – | – | |
| Female | 0.286 (0.093–0.879) | 0.029 | – | – | |
| Symptom | |||||
| No | Reference | – | – | – | |
| Yes | 1.615 (0.553–4.715) | 0.381 | – | – | |
| Tumor site | |||||
| Head/neck | Reference | – | – | – | |
| Body/tail | 0.559 (0.188–1.666) | 0.297 | – | – | |
| Grade | |||||
| G1/2 | Reference | – | Reference | – | |
| ≥G3 | 34.364 (3.833–308.064) | 0.002 | 39.160 (3.360–456.417) | 0.003 | |
| Lymphovascular invasion | |||||
| Absent | Reference | – | Reference | – | |
| Present | 11.700 (3.392–40.361) | <0.001 | 12.815 (2.962–55.449) | 0.001 | |
| Perineural invasion | |||||
| Absent | Reference | – | – | – | |
| Present | 7.571 (1.256–45.651) | 0.027 | – | – | |
| Surgery type | |||||
| Standard surgery | Reference | – | – | – | |
| Parenchyma-sparing surgery | 0.235 (0.028–1.965) | 0.181 | – | – | |
| Surgery approach | |||||
| Open | Reference | – | – | – | |
| Laparoscopic | 0.333 (0.068–1.627) | 0.174 | – | – | |
| Size, cm | |||||
| ≤2 | Reference | – | – | – | |
| >2 | 4.167 (1.082–16.048) | 0.038 | – | – | |
OR, odds ratio; CI, confidence interval.
Discussion
The definition of malignancy in NF-PNETs varied among different studies. Boninsegna et al. defined malignant NF-PNETs as tumors that had extra-pancreatic invasion or metastasis (19), whereas Regenet et al. define these as the presence of synchronous or metachronous metastases, including nodal and hepatic metastases (20). In addition to metastasis, histopathological grade ≥2 or 3 was also added to this definition (14,21). Given that a considerable proportion of patients with tumors ≥G2 had no recurrence or metastasis, tumor grade is probably just a clinicopathological predictor of malignancy. For this reason, malignancy in NF-PNETs was defined as the existence of tumor recurrence or nodal/distant metastasis (synchronous or metachronous) in the present study.
Primary tumor size is associated with clinical T-stages criteria according to the ENETS/American Joint Committee on Cancer (AJCC) TNM classification staging system. Significant differences in outcome comparing T-stage has been shown in many previously published papers and 2 cm has been widely adopted as the cutoff point in determining the biologic features of NF-PNETs (22). For small (≤2 cm) NF-PNET, there remains controversy over their biological behaviors. Kurita et al. performed a retrospective analysis of 23 patients with small sporadic NF-PNETs who were observed and found no tumor progression or nodal/distant metastases (23). A systematic review conducted by Sallinen et al. revealed that only 22% of 344 patients with sporadic small PNETs developed tumor growth and no patient developed nodal or distant metastasis (24). However, some reports have indicated that small tumors can recur or metastasize, and as tumor size was not significantly associated with the prognosis of NF-PNETs (17,25,26). Consistent with the previous reports, 3 of 28 small tumors in our study recurred and metastasized. Despite an obvious difference in DFS between small tumors and large tumors (P=0.023), there were no statistically significant differences in the rates of malignancy (P=0.057). Further, tumor size was not significantly correlated with the risk of malignancy in the multivariate analysis. The smallest tumor size with malignancy was 1.5 cm in our study, similar to those reported previously (18,27). Alternative tumor size cutoff points have been shown to effectively discriminate between benign and malignant NF-PNETs (20,23,28). However, some previously published studies have indicated that even very small PNETs (<0.5 cm) could pose a significant risk of nodal or distant metastasis (8,26), indicating that NF-PNETs of all sizes should be considered potentially malignant.
Controversy exists regarding the choice between active resection and conservative observation for patients with small NF-PNETs. ENETS guidelines recommend surgical resection for patients with small NF-PNETs, and observation for young patients who have small NF-PNETs <2 cm affected by MEN1 syndrome or those who have a severe comorbidity and are ineligible for surgery (11). However, the National Comprehensive Cancer Network and the North American Neuroendocrine Tumor Society suggest observation for smaller than 1 cm in size, low-grade, incidentally discovered NF-PNETs (1,12). Recently, published studies with large sample size have indicated that the resection of PNETs ≤2 cm is associated with better survival than observation, and surgery result in significantly better survival in patients with PNETs 1–2 cm but not those with PNETs <1 cm (29,30). From a radical cure perspective, surgical resection should be considered first, if tumors can be completely resected. Nevertheless, surgeons must carefully consider the potential benefits and complications of surgery before the procedure.
In our series, although the overall perioperative comorbidity of small NF-PNETs was high at 64.3%, only a small proportion of them had serious complications (11.1%); however, there were no mortalities. The rate of clinically relevant postoperative pancreatic fistulas was 25%, which was in agreement with the rates reported by other authors (28,31). However, although postoperative complications were common, perioperative mortality rates were very low for surgical resection of small PNETs, ranging from 0% to 3.6% in the literature (31,32), which compares favorably with the reported 6–10% rates of in-hospital mortality after pancreatectomy at the national level (28). Also, regarding long-term pancreatic function, the rates of postoperative endocrine and exocrine pancreatic insufficiency in the present study were similar to those reported by others (23,33). According to previously published studies, the recurrence rate of small NF-PNETs that underwent curative resection ranged from 5.4% to 11% (18,20,28), consistent with our study (7.1%). Taken together, patients with small NF-PNETs can be considered for surgical management, which is considered the only possible cure for NF-PNETs; surgical resection can provide a very high cure rate for small NF-PNETs, with good postoperative and long-term outcomes. And prospective randomized clinical trials are need to confirm whether surgical resection can provide survival benefit for small NF-PNETs of different tumor size groups.
In the present study, the small tumor group had a higher rate of clinically important pancreatic fistula than the large tumor group, but the difference was not significant (P=0.125). One plausible explanation for this is that patients with small NF-PNETs were likely to undergo parenchyma-sparing resection than those with large NF-PNETs (32.1% vs. 6.7%, P=0.011). It has been shown that parenchyma-sparing surgery has a higher rate of pancreatic fistula than standard pancreatic resection (1,34), which could be because enucleation provides a close resection margin to the main pancreatic duct, and central pancreatectomy has two pancreatic stumps (10). Parenchyma-sparing procedures tend to have a shorter operative time, lesser blood loss but higher postoperative morbidity compared to standard pancreatic resection (4,10). However, the increased risk of postoperative morbidity, particularly pancreatic fistula, was not found to be associated with a higher in-hospital mortality rate (34,35). As Table 3 shown, parenchyma-sparing resection for small NF-PNETs provided comparable operative results, complications and long-term outcomes than standard surgery, which is in line with previous studies (31,35). Notably, among 18 patients with malignant NF-PNETs, only one patient who developed late liver metastasis underwent parenchyma-sparing resection. However, neither tumor size nor surgery type indicated a close association with malignancy in the multivariate analysis. This difference could be because large tumors comprised most of the study sample (n=45, 61.6%), and had more frequent lymphovascular invasion than small NF-PNETs (P=0.003), making it more common to perform a standard pancreatic resection. According to these results, parenchyma-sparing resection could be used as an alternative for small local NF-PNETs in the absence of adjacent infiltration or metastasis. In contrast, for those large, regional invasion or metastasis, standard surgery should be the primary procedure.
The present study had several limitations. First, the study was inherently limited by its retrospective nature, which did not include patients that accepted non-operative management, and we could not assess the natural history of small NF-PNETs and determine the benefits of surgery. Second, the relatively small sample size from a single center may have affected the results and limited further subgroup analyses on small NF-PNETs of different tumor sizes. Third, the overall median follow-up period was relatively short (49.6±24.5 months); together with relatively higher disease-specific survival, it was difficult to determine the prognostic factors affecting OS. To better assess the risk of malignancy of small NF-PNETs and to evaluate the impact of surgical intervention on small NF-PNETs of different tumor size groups, multicenter studies with large sample size, and prospective randomized trials are needed.
Despite its limitations, the current study showed that small NF-PNETs are not immune from potential malignancy compared to NF-PNETs >2 cm, and surgical resection may be considered and can present favorable postoperative and long-term outcomes for small tumors. Parenchyma-sparing pancreatectomy may be an alternative for selected small local NF-PNETs. Further research is needed to confirm whether surgery is beneficial for small NF-PNETs of different tumor size groups than nonsurgical management.
Acknowledgments
Funding: This work was supported by the National Natural Sciences Foundation of China (grant numbers 81970543, 81570591); and Research Unit Project of Chinese Academy of Medical Sciences (grant number 2019-I2M-5-030).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki as revised in 2013. This study was approved by Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (NO.: IIT20200277A) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-582
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-582
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-582
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-582). The authors have no conflicts of interest to declare.
References
- 1.Howe JR, Merchant NB, Conrad C, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020;49:1-33. 10.1097/MPA.0000000000001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135:1469-92. 10.1053/j.gastro.2008.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franko J, Feng W, Yip L, et al. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg 2010;14:541-8. 10.1007/s11605-009-1115-0 [DOI] [PubMed] [Google Scholar]
- 4.Amin S, Kim MK. Islet Cell Tumors of the Pancreas. Gastroenterol Clin North Am 2016;45:83-100. 10.1016/j.gtc.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 5.Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727-33. 10.1093/annonc/mdn351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 2015;50:58-64. 10.1007/s00535-014-0934-2 [DOI] [PubMed] [Google Scholar]
- 8.Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol 2013;20:2815-21. 10.1245/s10434-013-3005-7 [DOI] [PubMed] [Google Scholar]
- 9.Tsoli M, Chatzellis E, Koumarianou A, et al. Current best practice in the management of neuroendocrine tumors. Ther Adv Endocrinol Metab 2018;10:2042018818804698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hain E, Sindayigaya R, Fawaz J, et al. Surgical management of pancreatic neuroendocrine tumors: an introduction. Expert Rev Anticancer Ther 2019;19:1089-100. 10.1080/14737140.2019.1703677 [DOI] [PubMed] [Google Scholar]
- 11.Partelli S, Bartsch DK, Capdevila J, et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017;105:255-65. 10.1159/000464292 [DOI] [PubMed] [Google Scholar]
- 12.Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw 2018;16:693-702. 10.6004/jnccn.2018.0056 [DOI] [PubMed] [Google Scholar]
- 13.Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 2013;98:4784-9. 10.1210/jc.2013-2604 [DOI] [PubMed] [Google Scholar]
- 14.Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011;150:75-82. 10.1016/j.surg.2011.02.022 [DOI] [PubMed] [Google Scholar]
- 15.Sadot E, Reidy-Lagunes DL, Tang LH, et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann Surg Oncol 2016;23:1361-70. 10.1245/s10434-015-4986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg 2011;146:534-8. 10.1001/archsurg.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherenfant J, Stocker SJ, Gage MK, et al. Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery 2013;154:785-91; discussion 791-3. 10.1016/j.surg.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Dong DH, Zhang XF, Poultsides G, et al. Impact of tumor size and nodal status on recurrence of nonfunctional pancreatic neuroendocrine tumors ≤2 cm after curative resection: A multi-institutional study of 392 cases. J Surg Oncol 2019;120:1071-9. 10.1002/jso.25716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boninsegna L, Panzuto F, Partelli S, et al. Malignant pancreatic neuroendocrine tumour: lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer 2012;48:1608-15. 10.1016/j.ejca.2011.10.030 [DOI] [PubMed] [Google Scholar]
- 20.Regenet N, Carrere N, Boulanger G, et al. Is the 2-cm size cutoff relevant for small nonfunctioning pancreatic neuroendocrine tumors: A French multicenter study. Surgery 2016;159:901-7. 10.1016/j.surg.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 21.Ricci C, Taffurelli G, Campana D, et al. Is surgery the best treatment for sporadic small (≤2 cm) non-functioning pancreatic neuroendocrine tumours? A single centre experience. Pancreatology 2017;17:471-7. 10.1016/j.pan.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 22.Bu J, Youn S, Kwon W, et al. Prognostic factors of non-functioning pancreatic neuroendocrine tumor revisited: The value of WHO 2010 classification. Ann Hepatobiliary Pancreat Surg 2018;22:66-74. 10.14701/ahbps.2018.22.1.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurita Y, Hara K, Kuwahara T, et al. Comparison of prognosis between observation and surgical resection groups with small sporadic non-functional pancreatic neuroendocrine neoplasms without distant metastasis. J Gastroenterol 2020;55:543-52. 10.1007/s00535-019-01655-w [DOI] [PubMed] [Google Scholar]
- 24.Sallinen V, Le Large TYS, Galeev S, et al. Surveillance strategy for small asymptomatic non-functional pancreatic neuroendocrine tumors - a systematic review and meta-analysis. HPB (Oxford) 2017;19:310-20. 10.1016/j.hpb.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 25.Song L, Zhai X, Yu S, et al. Clinical analysis of 547 patients with neuroendocrine tumors in a Chinese population: A single-center study. Cancer Med 2019;8:3729-37. 10.1002/cam4.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gratian L, Pura J, Dinan M, et al. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol 2014;21:3515-21. 10.1245/s10434-014-3769-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishi Y, Shimada K, Nara S, et al. Basing treatment strategy for non-functional pancreatic neuroendocrine tumors on tumor size. Ann Surg Oncol 2014;21:2882-8. 10.1245/s10434-014-3701-y [DOI] [PubMed] [Google Scholar]
- 28.Sallinen VJ, Le Large TYS, Tieftrunk E, et al. Prognosis of sporadic resected small (≤2 cm) nonfunctional pancreatic neuroendocrine tumors - a multi-institutional study. HPB (Oxford) 2018;20:251-9. 10.1016/j.hpb.2017.08.034 [DOI] [PubMed] [Google Scholar]
- 29.Chivukula SV, Tierney JF, Hertl M, et al. Operative resection in early stage pancreatic neuroendocrine tumors in the United States: Are we over- or undertreating patients? Surgery 2020;167:180-6. 10.1016/j.surg.2019.04.061 [DOI] [PubMed] [Google Scholar]
- 30.Assi HA, Mukherjee S, Kunz PL, et al. Surgery Versus Surveillance for Well-Differentiated, Nonfunctional Pancreatic Neuroendocrine Tumors: An 11-Year Analysis of the National Cancer Database. Oncologist 2020;25:e276-83. 10.1634/theoncologist.2019-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mintziras I, Keck T, Werner J, et al. Implementation of Current ENETS Guidelines for Surgery of Small (≤2 cm) Pancreatic Neuroendocrine Neoplasms in the German Surgical Community: An Analysis of the Prospective DGAV StuDoQ|Pancreas Registry. World J Surg 2019;43:175-82. 10.1007/s00268-018-4751-2 [DOI] [PubMed] [Google Scholar]
- 32.Guo J, Zhao J, Bi X, et al. Should surgery be conducted for small nonfunctioning pancreatic neuroendocrine tumors: a systemic review. Oncotarget 2017;8:35368-75. 10.18632/oncotarget.15685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreasi V, Partelli S, Capurso G, et al. Long-Term Pancreatic Functional Impairment after Surgery for Neuroendocrine Neoplasms. J Clin Med 2019;8:1611. 10.3390/jcm8101611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chua TC, Yang TX, Gill AJ, et al. Systematic Review and Meta-Analysis of Enucleation Versus Standardized Resection for Small Pancreatic Lesions. Ann Surg Oncol 2016;23:592-9. 10.1245/s10434-015-4826-3 [DOI] [PubMed] [Google Scholar]
- 35.Hüttner FJ, Koessler-Ebs J, Hackert T, et al. Meta-analysis of surgical outcome after enucleation versus standard resection for pancreatic neoplasms. Br J Surg 2015;102:1026-36. 10.1002/bjs.9819 [DOI] [PubMed] [Google Scholar]