Abstract
Background
Identification of small pulmonary nodules is challenging in a limited intrathoracic field during minimally invasive video‐assisted thoracoscopic surgery (VATS), and preoperative localization is required. Various techniques have been reported with some failure and complications. Here, we compare the feasibility and safety between electromagnetic navigation bronchoscopic marking and computed tomography (CT)‐guided percutaneous marking using indocyanine green (ICG) and iopamidol.
Methods
A total of 47 patients with small‐sized pulmonary nodules, scheduled to undergo video‐assisted thoracoscopic limited resection, were enrolled in this study. A mixture of diluted ICG and iopamidol was injected into the lung parenchyma as a marker, using CT‐guided percutaneous or electromagnetic navigation bronchoscopic injection techniques and the results were examined and compared.
Results
A total of 35 and 12 patients underwent preoperative marking by percutaneous injection and electromagnetic navigation bronchoscopic injection, respectively, in which a marker was detected in 33/35 (94.3%) and 12/12 (100%) patients. No combination of these procedures was performed in any patient. All markers were successfully detected in three patients who underwent injection marking at two different lesion sites. Pneumothorax occurred in five patients (14%) in the percutaneous marking group, which was relieved in all patients without the necessity for chest tube drainage. No other complication was observed in this study.
Conclusions
Electromagnetic navigation bronchoscopic injection techniques using indocyanine green fluorescence plus iopamidol are safe and effective, and comparable with CT‐guided localization. Furthermore, a bronchoscopic approach enables marking of multiple lesion areas without increasing patient risk, especially for puncture‐related pneumothorax.
Key points
Significant findings of the study
Either computed tomography (CT)‐guided percutaneous or electromagnetic navigation bronchoscopic injection techniques can be used for preoperative marking of pulmonary nodules with indocyanine green (ICG) fluorescence.
What this study adds
Indocyanine green (ICG) is a safe and easily detectable fluorescent marker for video‐assisted thoracoscopic surgery (VATS).
A bronchoscopic injection approach enables marking of multiple lesion areas without increasing the risk of pneumothorax.
Keywords: CT‐guided percutaneous localization, electromagnetic navigation bronchoscopy, indocyanine green fluorescence (ICG), small pulmonary nodules, video‐assisted thoracoscopic surgery (VATS)
Computed tomography (CT)‐guided percutaneous or electromagnetic navigation bronchoscopic injection techniques can be used for preoperative marking of pulmonary nodules with indocyanine green (ICG) fluorescence. ICG is a safe and easily detectable fluorescent marker for video‐assisted thoracoscopic surgery (VATS). A bronchoscopic injection approach enables marking of multiple lesion areas without increasing the risk of pneumothorax.
Introduction
Lung cancer is one of the most prevalent lethal diseases worldwide and the leading cause of cancer‐related death. 1 Most patients have advanced disease at diagnosis, which is associated with poor prognosis. The five‐year overall survival has been reported to be 68%–92% for patients with stage I disease treated with radical resection, with a sharp decrease to 13%–36% and 0%–10% for patients with stage III and stage IV disease, respectively. 2 , 3 Therefore, early detection of lung cancer plays a critical role in overcoming this disease.
Surgical resection brings the best chance of cure for lung cancer. Lobectomy with lymph node dissection has been the standard surgical treatment for NSCLC, even in patients with stage IA NSCLC. According to the 1995 Lung Cancer Study Group (LCSG) trial, limited resection was associated with a five‐year overall survival of 42% compared with 63% in the lobectomy group (one‐sided P‐value = 0.088). Additionally, a significantly higher local recurrence rate (17% vs. 6%, P = 0.008) was seen in the limited resection than that in the lobectomy group. Similar results have been reported in patients with lung cancer. 4 Limited resection is not yet a standard treatment for lung cancer, however, since small lung nodules have become increasingly prevalent owing to the widespread availability of lung cancer screening with low‐dose computed tomography (CT), this raises the need for limited resection for the diagnosis and treatment of lung cancer. Despite the development in technology, the localization of small pulmonary nodules remains challenging in a thoracoscopic setting, since small nodules are invisible on the deflated lung under video‐assisted thoracic surgery (VATS), especially small pulmonary ground‐glass nodules (GGN) which may be far from the pleura. Therefore, many techniques have been developed to assist the localization of small pulmonary nodules using either a percutaneous or transbronchial approach. 5 However, no consensus has as yet been reached on a standard preoperative localization technique, and thoracic surgeons choose the method on the basis of complications, cost, technical difficulty, and availability of facilities.
Although CT‐guided percutaneous localization procedures are often the main method for localization of lung nodules, development of innovative localization techniques are required to improve the accuracy of localization for management of small pulmonary nodules in an era of minimally invasive surgery. Electromagnetic navigation bronchoscopy (ENB) is a relatively new navigation method which allows physicians to access peripheral pulmonary lesions by using an image‐guided flexible catheter and a dedicated navigation software system. Here, we compared the feasibility and detectability between CT‐guided percutaneous marking and electromagnetic navigation bronchoscopic marking to investigate a potential alternative for preoperative localization of small pulmonary nodules.
Methods
Patient population and study design
Patients who were scheduled for sublobar resection of a nonsolid, partly solid, or solid pulmonary nodule with a maximum nodule diameter <20 mm at Shantou Central Hospital and Lung Research Institute of Guangdong Provincial People's Hospital between January 2018 and December 2019 were included in this study. Patients were divided according to marking method (CT‐guided percutaneous marking or electromagnetic navigation bronchoscopic marking). Data used in this study were retrieved from patient medical records, which included the nodule size, CT characteristics (ground‐glass nodules [GGN], solid, nonsolid), operation method, lesion depth from pleura, location, pathological subtype (inflammatory pseudotumor, atypical adenomatous hyperplasia [AAH], adenocarcinoma in situ [AIS], microinvasive adenocarcinoma [MIA], infiltrating adenocarcinoma [IAC]). All patients provided their written informed consent to participate in the study. This study was approved by the Institutional Review Board of Shantou Central Hospital and Lung Research Institute of Guangdong Provincial People's Hospital.
VATS marking procedures
CT‐guided percutaneous marking
The patient was placed in a suitable position (prone, supine, or lateral), depending on the location and accessibility of the lesion. A preprocedural CT scan was performed using a radiopaque grid on the intended region of the chest wall skin. After local anesthesia using lidocaine, a needle filled with marking solution (indocyanine green [ICG]/iopamidol 0.3–0.5 mL, 0.125 mg/mL) was injected near the pulmonary nodule. Post‐procedural CT was performed to monitor the occurrence of late‐onset pneumothorax or air embolism. The procedure was done within 90 minutes to prevent pneumothorax. Closed chest drainage could be considered for controlling pneumothorax when necessary to reduce the risk from anesthesia‐related intubation. Figure 1 shows the procedure of CT‐guided percutaneous marking.
Figure 1.
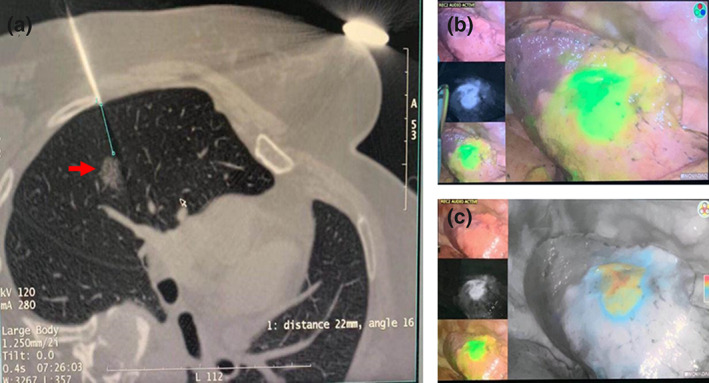
Computed tomography‐guided percutaneous procedure. (a) CT‐guided percutaneous marking with injection of a mixture (0.3 mL) of indocyanine green (ICG) and iopamidol; (b and c) The ICG fluorescence was detected by thoracoscopy under hybrid mode with both the infrared signal and the white light image together (b), and hybrid mode with both the infrared signal and the pseudo‐color enhanced white light image together (c).
Electromagnetic navigation‐guided bronchoscopic marking procedure
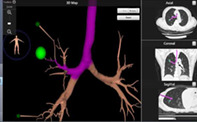
Virtual bronchoscopy navigation images were generated from thin‐section CT to estimate the surgical approach. Bronchoscopy was performed under general anesthesia, and the fiberscope was guided fluoroscopically to the estimated lesion site through the terminal bronchi (Fig 2). Lesions far from the terminal bronchus could be reached by blunt separation of lung parenchyma. When the fiberscope was close enough to the lesion site, marking solution (ICG/iopamidol 0.3–0.5 mL, 0.125 mg/mL) was injected. Subsequently, the anesthesiologist used an occluder or double‐lumen endotracheal tube for one lung ventilation depending on the patient's condition.
Figure 2.
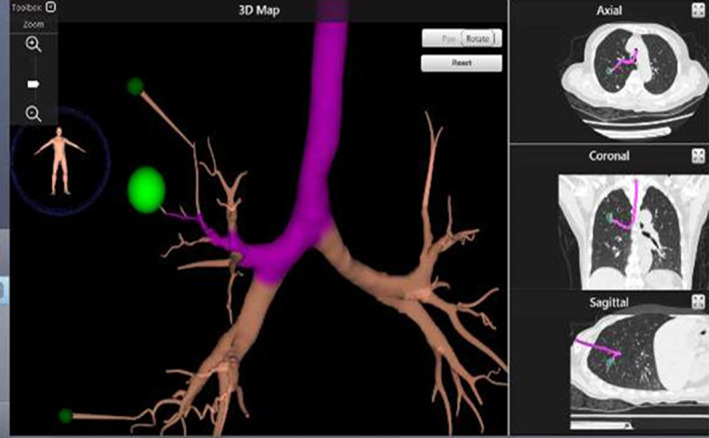
Electromagnetic navigation‐guided bronchoscopic marking procedure.
Wedge resection was conducted under fluoroscopy guidance, and the frozen section of the resected specimen was sent to pathology for intraoperative evaluation. Lobectomy and lymph node dissection were performed for patients with infiltrating adenocarcinoma.
Statistical analysis
The outcome assessed in this study was the success rate of dye localization, which was defined as the percentage of patients with successful identification of target lesions during the thoracoscopic procedure in all patients who underwent dye injection in each group. Data was analyzed using SPSS software (SPSS 21.0, Chicago, IL, USA). Enumeration data are presented as percentage and analyzed by χ2 test, and measurement data presented as mean ± standard deviation (SD) and analyzed by t‐test. A P‐value ≤0.05 was considered statistically significant.
Results
A total of 47 patients participated in this study, including 35 patients who had undergone percutaneous marking and 12 patients who had undergone bronchoscopic marking. No combination of these procedures was performed in any patient. The clinical characteristics and CT characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| CT‐guided percutaneous needle injection group (n = 35) | Bronchoscopic injection group (n = 12) | |
|---|---|---|
| Age, years (median, range) | 55 (40–79) | 56 (34–68) |
| Sex | 9 | 4 |
| Male | 26 | 8 |
| Female | ||
| Tumor size (mm, median, 95% CI) | 7 (3–20) | 11 (7–18) |
| CT characteristics | ||
| GGN | 21 | 11 |
| Solid | 14 | 4 |
| Depth from visceral pleura (cm, median, 95% CI) | 8.2 (1.1–38.1) | 12.5 (2.4–34.0) |
| Lesion location | ||
| Right superior lobe | 18 | 6 |
| Right middle lobe | 5 | 1 |
| Right inferior lobe | 2 | 3 |
| Left superior lobe | 7 | 3 |
| Left inferior lobe | 3 | 2 |
| Histological diagnosis of nodules | ||
| AAH | 1 | 1 |
| AIS | 12 | 3 |
| MIA | 7 | 3 |
| IAC | 13 | 4 |
| Metastatic | 1 | 0 |
| Benign | 1 | 4 |
AH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; GGN, ground‐glass density nodule; IAC, infiltrating adenocarcinoma; MIA, microinvasive adenocarcinoma.
The success rate was 94.3% (33/35) and 100% (12/12) in the percutaneous marking and bronchoscopic marking groups, respectively (Table 2). For two patients with an undetectable marker in the percutaneous group, one underwent anatomical wedge resection and the nodule was found in the resected tissues on fluoroscopy, and the second patient underwent lobectomy since the nodule was not detectable. The bronchoscopy group included three patients with two pulmonary nodules in the ipsilateral side of the lung. Ultimately, the target pulmonary nodules were successfully resected in all patients with a sufficient margin. Pneumothorax occurred in five patients (14%) in the percutaneous marking group, and this was relieved in all patients without the necessity for chest tube drainage. No other complication was observed in this study.
Table 2.
The success rates and complications of video‐assisted thoracoscopic surgery (VATS) marking
| CT‐guided percutaneous needle injection group (n = 35) | Bronchoscopic injection group (n = 12) | |
|---|---|---|
| Single marking | 35 | 9 |
| Double marking | 0 | 3 |
| Complication | ||
| Pneumothorax | 5/35 (14%) | 0/12 (0%) |
| Other | 0 | 0 |
Discussion
Sublobar resection is a less invasive type of thoracic surgery, which has recently been increasingly adopted to diagnose and treat small or faint lung nodules less than 2 cm in diameter that are clinically significant. However, localization of the target nodule is often challenging during VATS since these lesions are hardly visible or palpable. Furthermore, it can be difficult for a surgeon to determine the exact location of small nodules located 1 or 2 cm below the pleura. 6 Therefore, accurate localization is needed to enable thoracic surgeons to perform sublobar resections with enough margin in a short procedure time.
In the current study, favorable success rates were seen in both percutaneous marking and electromagnetic navigation bronchoscopic marking groups. We managed to reduce dye diffusion by limiting the injection volume of ICG to 0.3 mL, as well as adding iopamidol to increase the concentration of ICG. Based on these improvements, the success rate of localization reached 100%. However, ICG was reported to tend to diffuse in patients with poor pulmonary function, emphysema, or adjacent bullae. Electromagnetic navigation bronchoscopy was performed by a skilled endoscopist. We experienced one case with obscure localization which may have been attributed to the injection with insufficient ICG far from the pleura. We then heated the surface by electrocoagulation hook on the basis of the anatomical location to reveal the dye marking (Fig 3). The procedure was subsequently improved by increasing the volume of ICG at a closer injection site to the pleura. Bronchoscopic marking procedure is reported to be superior to other techniques in patients with multiple nodules because of less invasiveness and time saving. 7 , 8 Three patients with multiple unilateral nodules underwent bronchoscopic marking procedure in this study, all of which were successfully localized.
Figure 3.
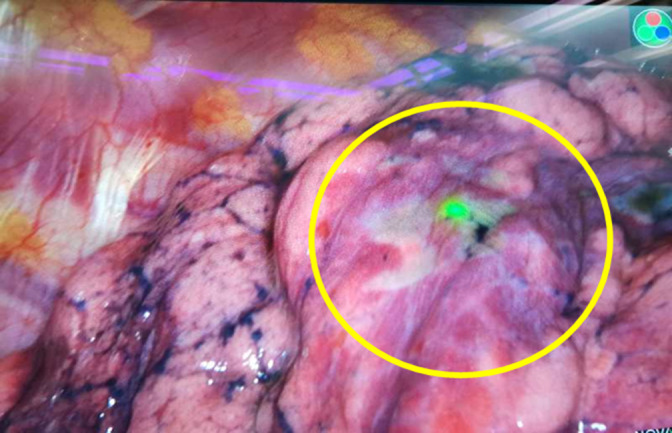
Detection of indocyanine green (ICG) fluorescence after burning the tissue surface.
Multiple methods have been developed to localize small lesions, which are mainly divided into percutaneous and transbronchial approaches. Both approaches have advantages and disadvantages. The CT‐guided percutaneous approach is a well‐established technique which can be easily performed with a success rate as high as 72% at a relatively lower cost. 9 , 10 Complications induced by pleural puncture is a major shortcoming of the percutaneous approach, which lead to difficulties in location for multiple lesions. A percutaneous approach is also associated with increased procedure time and risk since patients have to be moved from the CT room to the operating room. Moreover, the additional radiation exposure from repeated CT scanning should also be taken into consideration. Emerging evidence has shown the feasibility of the electromagnetic navigation‐guided technique in the management of lung nodules. 11 , 12 , 13 Electromagnetic navigation transbronchial localization allows localization of multiple lung nodules (>2 lung nodules) or bilateral lung lesions. 11 , 12 , 13 , 14 , 15 It is recommended for patients with emphysematous lung disease or COPD since it is associated with less complications such as pneumothorax and bronchial hemorrhage. 16 , 17 It also has the advantage of improved patient comfort as it is carried out under general anesthesia, and is a seamless procedure between localization and surgery without moving settings, therefore minimizing procedure time and risk. 18 , 19 The bronchoscopic approach facilitates entry into the regions that are not easily accessible by the percutaneous approach, such as nodules located near the costophrenic angle or under the scapula (such as the mediastinal side and the craniodorsal part obscured by the scapula). 7 , 8 The disadvantage is with respect to low cost‐effectiveness, it is more labor intensive, and places high demands on the operator's skill and experience, which makes it less available in most institutes. Furthermore, this technique is a relatively new method, which has only been performed in a small number of patients. Additional studies are needed to investigate the best practices, and planned surgical procedure such as nodule localization, resection type, and angle of approach. Therefore, we do not suggest the primary technique, and decision‐making should be dependent on the condition of the individual.
Many methods for localization of lesions have been reported in previous studies. Kleedehn et al. reported that hook‐wire needle localization is statistically equivalent to methylene blue injection in terms of success and complication rates, but complications such as pneumothorax and pulmonary hemorrhage remain major concerns; moreover, dislodgement of the wire may make the surgical procedure even more difficult. 20 The use of microcoils for localization of pulmonary nodules has been described more recently, and is a painless and convenient procedure. 21 Microcoils can be placed several days before surgery, making scheduling preoperative procedures and surgical resection easier. However, shortcomings of this approach include dislodgement with associated pneumothorax, intrapulmonary hemorrhage and pleural pain. The distance between the wire tip and the pleura should be greater than 30 mm, or the wire may fail to dock due to insufficient friction between the wire and pulmonary tissues. 21 Injection of methylene blue provides an effective, safe and inexpensive method for intrathoracic localization, while diffusion away from the nodule is a major limitation of this technique. ICG has been used medically for a variety of diseases since 1950s. It poses a low risk to patients with high specificity of fluorescent images on the basis of its high molecular weight, unstable fixation with albumin in the intravascular space, and rapid clearance by hepatobiliary extraction. 22 As a fluorophore, ICG absorbs and emits light at a wavelength of 820 nm which can be detected in the fluorescence camera with a near‐infrared (NIR)‐sensitive detector. 23 The use of NIR fluorescence imaging with ICG has gained popularity in many fields of VATS, such as localization of small pulmonary nodules, detection of the intersegmental plane, and segmental bronchography. 24 , 25 , 26 In addition, ICG is visible under any background with sufficient contrast, even in smoking‐related anthracosis. 27 In the present study, ICG was used for the visualization of targeted lesions, all of which were visible under fluoroscopy. Accurate localization of nodules is achieved by identifying the darkest field at modified mode when diffusion occurs. 28 Moreover, the ICG‐marked nodule can be localized in the resected tissues. Previous studies have reported the detection of skip metastasis of lymph node by assessment of the relationship between the anatomical segment of the primary tumor and the lymphatic flow using ICG in lung cancer. 29 No positive lymph node was found in our study, which could be explained by lack of lymph node metastasis or insufficient volume of injected ICG.
Our study had several potential limitations. The sample size was too small to draw a clinically significant difference despite a statistical significance. Our study was also limited by the constraints of the retrospective study design, which introduced selection bias. The electromagnetic navigation bronchoscopy guided technique was used in only about 30% patients since this technique has been introduced in clinical practice just for a few years and only a small subset of the total patients underwent preoperative localization by bronchoscopic marking in our institute. However, our study represents real‐world experience, and further large‐sample studies with direct comparison with other techniques are needed to establish the standard for marking pulmonary nodules.
Various localization techniques have been introduced into VATS using either a percutaneous or transbronchial approach; however, the best marking dye and approach remain uncertain due to the individual differences. Location, distance from pleura, nodule size, cost, patient and CT characteristics should be considered when decision‐making. In the current study, ICG marking provided an accurate and safe procedure for the preoperative localization of small pulmonary nodules. Accurate localization was achieved by both a CT‐guided percutaneous and electromagnetic navigation transbronchial approach, while the latter was associated with decreased risk of pneumothorax, and the feasibility of localization of multiple nodules.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was funded by Shantou Science and Technology Planning Project (Shanfuke [2017] No. 166‐10).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Goldstraw P, Chansky K, Crowley J et al The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 3. Sawabata N, Asamura H, Goya T et al Japanese lung cancer registry study: First prospective enrollment of a large number of surgical and nonsurgical cases in 2002. J Thorac Oncol 2010; 5: 1369–75. [DOI] [PubMed] [Google Scholar]
- 4. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non‐small cell lung cancer. Lung cancer study group. Ann Thorac Surg 1995; 60: 615–22 discussion 622‐3. [DOI] [PubMed] [Google Scholar]
- 5. Suzuki K, Nagai K, Yoshida J et al Video‐assisted thoracoscopic surgery for small indeterminate pulmonary nodules: Indications for preoperative marking. Chest 1999; 115: 563–8. [DOI] [PubMed] [Google Scholar]
- 6. Chen S, Zhou J, Zhang J et al Videoassisted thoracoscopic solitary pulmonary nodule resection after CT‐guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011; 25: 1723–9. [DOI] [PubMed] [Google Scholar]
- 7. Anayama T, Qiu J, Chan H et al Localization of pulmonary nodules using navigation bronchoscope and a near‐infrared fluorescence thoracoscope. Ann Thorac Surg 2015; 99: 224–30. [DOI] [PubMed] [Google Scholar]
- 8. Anayama T, Hirohashi K, Miyazaki R et al Near‐infrared dye marking for thoracoscopic resection of small‐sized pulmonary nodules: Comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg 2018; 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manhire A, Charig M, Clelland C et al Guidelines for radiologically guided lung biopsy. Thorax 2003; 58: 920–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta A, Suri JC, Bhattacharya D et al Comparison of diagnostic yield and safety profile of radial endobronchial ultrasound‐guided bronchoscopic lung biopsy with computed tomography‐guided percutaneous needle biopsy in evaluation of peripheral pulmonary lesions: A randomized controlled trial. Lung India 2018; 35: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marino KA, Sullivan JL, Weksler B. Electromagnetic navigation bronchoscopy for identifying lung nodules for thoracoscopic resection. Ann Thorac Surg 2016; 102: 454–7. [DOI] [PubMed] [Google Scholar]
- 12. Awais O, Reidy MR, Mehta K et al Electromagnetic navigation bronchoscopy‐guided dye marking for thoracoscopic resection of pulmonary nodules. Ann Thorac Surg 2016; 102: 223–9. [DOI] [PubMed] [Google Scholar]
- 13. Luo K, Lin Y, Lin X et al Localization of peripheral pulmonary lesions to aid surgical resection: A novel approach for electromagnetic navigation bronchoscopic dye marking. Eur J Cardiothorac Surg 2017; 52: 516–21. [DOI] [PubMed] [Google Scholar]
- 14. Long J, Petrov R, Haithcock B et al Electromagnetic transthoracic nodule localization for minimally invasive pulmonary resection. Ann Thorac Surg 2019; 108: 1528–34. [DOI] [PubMed] [Google Scholar]
- 15. Hsu PK, Chuang LC, Wu YC. Electromagnetic navigation‐guided preoperative localization of small malignant pulmonary tumors. Ann Thorac Surg 2020; 109: 1566–73. [DOI] [PubMed] [Google Scholar]
- 16. Eberhardt R, Morgan RK, Ernst A, Beyer T, Herth FJF. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigational bronchoscopy. Respiration 2010; 79: 54–60. [DOI] [PubMed] [Google Scholar]
- 17. Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: A systematic review and meta‐analysis. Respiration 2014; 87: 165–76. [DOI] [PubMed] [Google Scholar]
- 18. Bolton WD, Cochran T, Ben‐Or S et al Electromagnetic navigational bronchoscopy reduces the time required for localization and resection of lung nodules. Innovations (Phila) 2017; 12: 333–7. [DOI] [PubMed] [Google Scholar]
- 19. Hyun K, Park IK, Song JW, Park S, Kang CH, Kim YT. Electromagnetic navigation bronchoscopic dye marking for localization of small subsolid nodules: Retrospective observational study. Medicine (Baltimore) 2019; 98: e14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kleedehn M, Kim DH, Lee FT et al Preoperative pulmonary nodule localization: A comparison of methylene blue and hookwire techniques. AJR Am J Roentgenol 2016; 207: 1334–9. [DOI] [PubMed] [Google Scholar]
- 21. Finley RJ, Mayo JR, Grant K et al Preoperative computed tomography‐guided microcoil localization of small peripheral pulmonary nodules: A prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015; 149: 26–31. [DOI] [PubMed] [Google Scholar]
- 22. Desmettre T, Devoisselle JM, Mordon S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv Ophthalmol 2000; 45: 15–27. [DOI] [PubMed] [Google Scholar]
- 23. Matsui A, Tanaka E, Choi HS et al Real‐time intra‐operative near‐infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery 2010; 148: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okusanya OT, Holt D, Heitjan D et al Intraoperative near‐infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014; 98: 1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferrari‐Light D, Geraci TC, Sasankan P, Cerfolio RJ. The utility of near‐infrared fluorescence and indocyanine green during robotic pulmonary resection. Front Surg 2019; 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mun M, Okumura S, Nakao M, Matsuura Y, Nakagawa K. Indocyanine green fluorescence‐navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017; 3: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marshall M V, Rasmussen J C, Tan I‐C et al Near‐infrared fluorescence imaging in humans with Indocyanine green: A review and update. Open Surg Oncol J 2010; 2: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DSouza AV, Lin H, Henderson ER, Samkoe KS, Pogue BW. Review of fluorescence guided surgery systems: Identification of key performance capabilities beyond indocyanine green imaging. J Biomed Opt 2016; 21: 80901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mai K, Minamiya Y, Saito H et al Detection of pleural lymph flow using indocyanine green fluorescence imaging in non‐small cell lung cancer surgery: A preliminary study. Surg Today 2013; 43: 249–54. [DOI] [PubMed] [Google Scholar]


