Abstract
Background
This study compared a co‐ablation (CA) system, which is a novel ablation device, with an argon‐helium cryoablation (AHC) system. We aimed to compare the efficacy and safety of CA and AHC for the treatment of stage III–IV non‐small cell lung cancer (NSCLC).
Methods
We conducted a multicenter randomized controlled trial (RCT) to determine whether CA was noninferior to AHC. The primary efficacy endpoints were the iceball coverage rate (ICR) and the disease control rate (DCR) one month after treatment. Noninferiority was declared if the lower limit of two‐sided 95% confidence interval (CI) was less than 10%. The ICR and DCR were identified by logistic regression. Treatment safety was assessed.
Results
A total of 81 patients underwent randomization (41 assigned to the CA and 40 assigned to the AHC groups)and transthoracic ablation. The ICRs in the CA and AHC groups were 99.24% ± 2.18% and 98.66% ± 3.79%, respectively. Central lesions were associated with an increased risk of an incomplete ICR. The DCRs in the CA and AHC groups were 97.6% and 95%, respectively. A smaller lesion area in the CA group was significantly correlated with a better DCR. The rate of complications was 29.26% in the CA group and 30% in the AHC group. (P = 0.943). There was less probe usage per patient in the CA group.
Conclusions
We determined that CA is noninferior to AHC in terms of efficacy and safety for the treatment of stage III–IV NSCLC. A smaller lesion area in the CA group was significantly correlated with a better DCR.
Key points
CA was noninferior to AHC for stage III–IV NSCLC.
Keywords: Co‐ablation, cryoablation, non‐small cell lung cancer
Co‐ablation (CA) is noninferior to argon‐helium cryoablation (AHC) in terms of efficacy and safety for the treatment of stage III–IV NSCLC. A smaller lesion area in the CA group was significantly correlated with a better disease control rate (DCR).
Introduction
Lung cancer is a common cancer that accounts for 11.6% of all cases and has led to 18.4% of all cancer‐related deaths according to a report on the global burden of cancer worldwide in 2018. 1 Cryoablation has been previously shown to successfully treat lung cancer patients no longer deemed suitable for surgical resection. 2 , 3 Argon‐helium cryoablation (AHC) utilizes argon gas and helium gas for freezing and thawing, respectively. However, the high pressure of gas and inconvenience of helium make it difficult to promote AHC in local hospitals. A Chinese scientist named Liu Jing and his team invented a coablation (CA) system. This CA system is an internationally original multimodal tumor ablation device that integrates the advantages of cryoablation and high‐intensity thermal ablation by breaking through many technological bottlenecks. In the freezing mode, the temperature can be decreased as low as −196°C. Freezing causes the formation of ice crystals inside and outside cells, as well as cell dehydration, resulting in mechanical damage to cell membranes and organelles. In the warming mode, the temperature can be increased as high as 80°C. The alternation between freezing and warming produces substantial thermal stress, resulting in stronger mechanical damage. High temperature can effectively prevent bleeding in the puncture route as well as tumor implantation metastasis. CA has been proven to be effective in previous experiments. 4 , 5 , 6 To date, no reports have compared the clinical efficacy or safety of CA and AHC.We carried out a prospective, noninferiority, randomized, controlled trial (RCT) to compare the performance of CA and AHC in patients with stage III–IV non‐small cell lung cancer (NSCLC).
Methods
Trial design
A multicenter (four centers), noninferiority, parallel‐group RCT was conducted in China. Patients were randomly assigned to two groups, initially at a 1:1 ratio, to receive either CA or AHC. The RCT protocol was reviewed and approved by the Ethics Review Committee at each clinical trial center. All participants provided their written informed consent before enrollment. An independent endpoint committee assessed all endpoints according to prespecified criteria. The members of this committee were blinded to the treatment and other results of the patients.
Study participants
Patients were recruited from four centers. Eligible patients were adults aged 18–80 years who were diagnosed with stage III–IV NSCLC according to the seventh edition of the tumor‐node‐metastasis (TNM) classification 7 (having the following features: the diameter of the lesion was 2–5 cm, and the lesions could be measured and ablated). Other eligibility inclusion requirements included having a life expectancy >three months and being willing to complete the entire study.
The exclusion criteria included: (i) undergoing invasive treatment within one month; (ii) having undergone previous chemoradiotherapy within two months; (iii) being unable to lie flat, pregnant or lactating; (iv) participating in other clinical trials; (v) evidence of active infection; (vi) presence of psychiatric illness; (vii) uncontrolled coagulopathy or bleeding disorder; (viii) tumor associated with atelectasis or obstructive pneumonitis; and (ix) relapsing after other treatments within three months.
Interventions
Ablation procedures were performed under local anesthesia in an interventional suite with computed tomography (CT) image guidance. 81 patients with 83 lesions received transthoracic ablation. Under CT guidance, percutaneous puncture to the focus lesion was achieved with a probe in its planned route.
The HJY CHS 800001 Co‐ablation system (Hygea Medical Technology Co., Beijing, China) was used in the CA group; the equipped probe was 2.6 mm in diameter. Liquid nitrogen was delivered into the probe to decrease the probe temperature to as low as −196°C upon transition from a liquid to a gas phase in the probe. Alcohol steam was delivered to increase the probe temperature to as high as 80°C upon transition from a gas to a liguid phase in the probe. A cryocare surgical system (Endo‐Care, Irvine, California) equipped with a 2.4 mm diameter cryoprobe was used in the AHC group. High‐pressure argon and helium gas were used for freezing and thawing based on the Joule‐Thomson effect. The protocol was two freeze–thaw cycles (15–20 minutes freeze, five‐minutes thaw). Each procedure was monitored with noncontrast CT at 5–10 minutes intervals to visualize the evolving ablation zone.The number and orientation of the probes were based on the size and geometry of the tumors. The size of the iceball was recorded. After the probes were removed, CT images were obtained to identify any potential complications.
Outcomes
The primary endpoints were the iceball coverage rate (ICR) during the procedure and the disease control rate (DCR) one month after the procedure. The ICR was calculated as the largest axial area of iceball divided by the maximum axial area of the lesion. Follow‐up chest CT scans with contrast enhancement were carried out one month after the procedure. We assessed the DCR based on the New Response Evaluation Criteria in Solid Tumours. 8 The secondary endpoints were satisfaction with the device, tolerance to pain and quality of life (QOL). The pain response to ablation was assessed by the Numeric Rating Scale (NRS). QOL was evaluated by the QOL scale. Safety assessments included treatment‐related adverse events,which were recorded on a per treatment basis and were classified following the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute. 9 They were judged by the endpoint review committee to be causally related to the treatment. All follow‐up assessments were performed by study personnel who were unaware of the treatment assignments.
Sample size
Based on an expected incidence of the primary endpoint ICR/DCR of 98% at one month in the AHC group and with a noninferiority margin of 10%, we calculated that a sample size of 80 patients would be required for the trial to have 80% power to test the noninferiority of CA to AHC, with a two‐sided alpha level of 0.05 and an anticipated dropout rate of 20%. All sample size calculations were made by PASS 11 (NCSS, LLC. Kaysville, Utah, USA).
Randomization
The randomization sequence was created using SAS 9.3 (SAS Institute, Cary, NC, USA) statistical software and was stratified by center with a 2:2 allocation using random block sizes of 2, 4, and 6. Block randomization was generated by a computer random number list prepared by an investigator with no clinical involvement in the trial.
After patient acceptance and before admission to the center, the appropriate numbered envelope was opened at the central office; the card inside assigned the patient to the CA or AHC group, and this information was then given to the medical officer of the center.
Blinding
The patients and physicians allocated to the intervention group were aware of the allocated arm, but the outcome assessors and data analysts were blinded to the allocation.
Statistical analysis
Analysis of the primary endpoints was performed on both the full analysis set (FAS) and the per protocol set (PPS). Other analyses were based on the FAS. The FAS was defined according to the intention‐to‐treat principle,which included all patients who underwent randomization and their randomly assigned ablation procedure. The PPS consisted of patients who were treated and did not have a major protocol deviation. A major protocol deviation was defined as a deviation that confounded the efficacy end point.
Categorical variables were analyzed by the χ test or Fisher's exact test. Continuous variables were compared using the t‐test or Wilcoxon signed‐rank test. The primary endpoints (ICR and DCR) were tested for noninferiority. Noninferiority was declared if the lower limit of the two‐sided 95% confidence interval (CI) was less than 10%. Variables that were potentially predictive of an incomplete ICR were analyzed by binary logistic regression analysis. Factors that exhibited the DCR were analyzed by ordinal logistic regression analysis. The strength of associations was described by using the odds ratio (OR) and 95% CI. P‐values lower than 0.05 were considered statistically significant. Data were analyzed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Participant recruitment and flow
Between 2013 and 2015, 81 patients were recruited and randomly assigned to the CA (n = 41) or AHC group (n = 40). The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Fig 1. Patients were followed‐up for one month after the procedure.
Figure 1.
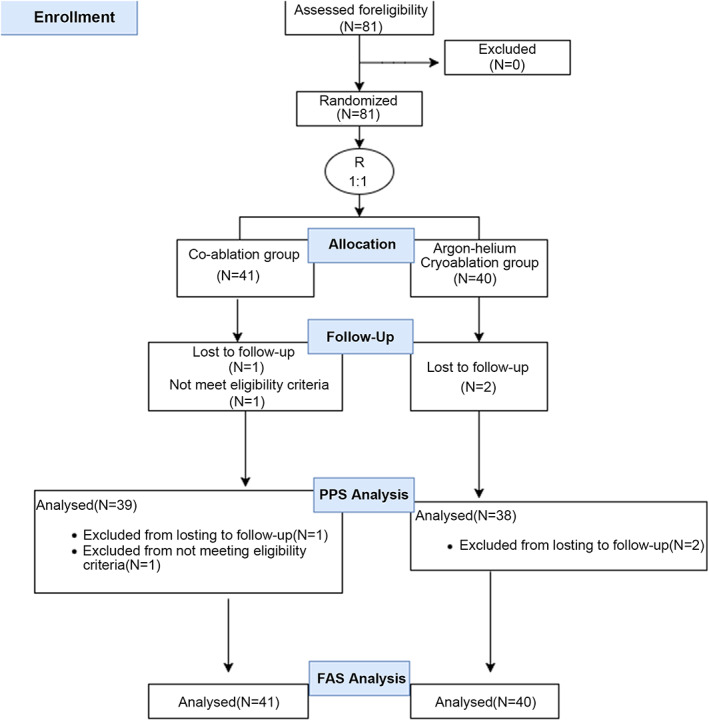
The CONSORT flow diagram.
Baseline data
The baseline data identified approximately 70.4% of patients as male and 29.6% as female,with 22.2% of patients having stage IIIa NSCLC, 17.3% of patients having stage IIIb, 59.3% of patients having stage IV, and 1.2% of patients having an unknown stage. The patient baseline characteristics were comparable between the two groups (Table 1).
Table 1.
Demographic and disease characteristics at baseline
| CA group (n = 41/lesion = 42) | AHC group (n = 40/lesion = 41) | P‐value | |
|---|---|---|---|
| Age‐year (mean ± SD) | 62.49 ± 9.74 | 64.73 ± 8.95 | 0.285 |
| Sex n (%) | 0.678 | ||
| Male | 28 (68.3%) | 29 (72.5%) | |
| Female | 13 (31.7%) | 11 (27.5%) | |
| Tumor location n (%) | 0.086 | ||
| Central | 20 (47.6%) | 12 (29.3%) | |
| Peripheral | 22 (52.4%) | 29 (70.7%) | |
| Pathology n (%) | 1.000 | ||
| Squamous | 11 (26.2%) | 12 (29.3%) | |
| Adenocarcinoma | 29 (69%) | 28 (68.3%) | |
| Large cell carcinoma | 1 (2.4%) | 0 (0%) | |
| Unknown | 1 (2.4%) | 1 (2.4%) | |
| Stage n (%) | 0.605 | ||
| IIIa | 7 (17.1%) | 11 (27.5%) | |
| IIIb | 7 (17.1%) | 7 (17.5%) | |
| IV | 26 (63.4%) | 22 (55%) | |
| NA | 1 (2.4%) | 0 (0%) | |
| Pulmonary resection history n (%) | 0.928 | ||
| Yes | 16 (39.0%) | 16 (40.0%) | |
| No | 25 (61.0%) | 24 (60.0%) | |
| Radiotherapy or chemotherapy history n (%) | 0.107 | ||
| Yes | 16 (39.0%) | 9 (22.5%) | |
| No | 25 (61.0%) | 31 (77.5%) | |
| Cardiovascular or pulmonary risk n (%) | 0.141 | ||
| Yes | 20 (48.8%) | 26 (65%) | |
| No | 21 (51.2%) | 14 (35%) | |
| Long axis of lesion cm (mean ± SD) | 4.01 ± 0.96 | 4.00 ± 1.10 | 0.966 |
| Short axis of lesion cm (mean ± SD) | 3.25 ± 1.17 | 3.22 ± 1.05 | 0.909 |
|
Largest area of the lesion cm2 (mean ± SD) |
14.34 ± 7.24 | 13.99 ± 7.87 | 0.835 |
Data analyzed
Data from 41 patients in the CA group and 40 patients in the AHC group were available in the FAS. One patient was lost to follow‐up in the CA group, and another patient did not meet the eligibility criteria. In the AHC group, two patients were lost to follow‐up. Thus, 39 patients in the CA group and 38 patients in the AHC group remained in the PPS. The primary and secondary outcomes in the FAS and PPS are detailed in Tables 2 and 3. The outcome of safety and other results are detailed in Table 4.
Table 2.
Results in the full analysis set (FAS)
| FAS | CA group (n = 41/lesion = 42) | AHC group (n = 40/lesion = 41) | 95% confidence interval | P‐value |
|---|---|---|---|---|
| Primary endpoint | ||||
| ICR | (99.24 ± 2.18)% | (98.66 ± 3.79)% | 0.394 | |
| Difference between groups | 10.58% | 9.24%–11.9% | ||
| DCR | 40 (97.6%) | 38 (95%) | ||
| Difference between groups | 3.3% | −4.7%–12.1% | ||
| Response rate n (%) | 0.463 | |||
| CR | 17 (41.5%) | 11 (27.5%) | ||
| PR | 22 (53.7%) | 24 (60%) | ||
| SD | 1 (2.4%) | 3 (7.5%) | ||
| PD | 1 (2.4%) | 2 (5%) | ||
| Secondary endpoint | ||||
| Satisfaction with the device | 100% | 100% | NA | |
| Pain score | 0.46 ± 0.87 | 0.68 ± 1.07 | 0.331 | |
| Difference in QOL before and after procedure | 13.47 ± 4.49 | 15.22 ± 5.05 | 0.130 | |
CR, complete response; DCR, disease control rate; ICR, iceball coverage rate; PD, progressive disease; PR, partial response; QOL, quality of life; SD, stable disease.
Table 3.
Results in the per protocol set (PPS)
| PPS | CA group (n = 39/lesion = 40) | AHC group (n = 38/lesion = 39) | 95% confidence interval | P‐value |
|---|---|---|---|---|
| Primary endpoint | ||||
| ICR | (99.38 ± 2.00)% | (98.59 ± 3.87)% | 0.264 | |
| Difference between groups | 10.79% | 9.39%–12.18% | ||
| DCR | 40 (100%) | 39 (100%) | ||
| Difference between groups | 0% | −9.1%–9.3% | ||
| Response rate n (%) | 0.125 | |||
| CR | 17 (43.6%) | 11 (28.9%) | ||
| PR | 22 (56.4%) | 24 (63.2%) | ||
| SD | 0 (0%) | 3 (7.9%) | ||
| PD | 0 (0%) | 0 (0%) | ||
CR, complete response; DCR, disease control rate; ICR, iceball coverage rate; PD, progressive disease; PR, partial response; SD, stable disease.
Table 4.
Complications and other results in the full analysis set (FAS)
| FAS | CA group (n = 41/lesion = 42) | AHC group (n = 40/lesion = 41) | P‐value |
|---|---|---|---|
| Complications n (%) | |||
| Overall | 12 (29.26%) | 12 (30%) | 0.943 |
| Pneumothorax | 4 (9.8%) | 5 (12.5%) | 0.737 |
| Pleural effusion | 2 (4.87%) | 3 (7.5%) | 0.675 |
| Hemoptysis | 6 (14.63%) | 2 (5%) | 0.264 |
| Pericardial effusion | 0 (0%) | 1 (2.5%) | 1.000 |
| Local bleeding at puncture sites | 0 (0%) | 1 (2.5%) | 1.000 |
| Other results | |||
| No of probes per patient (mean ± SD) | 1.85 ± 0.82 | 2.73 ± 1.15 | 0.000 |
Primary endpoints
The difference in the ICR between the two groups was 10.58% (95% CI: 9.24% to 11.9%) in the FAS. The difference in the ICR between the two groups was 10.79% (95% CI: 9.39% to 12.18%) in the PPS.
The difference in the DCR between the two groups was 3.3% (95% CI: −4.7% to 12.1%) in the FAS. The difference in the DCR between the two groups was 0% (95% CI: −9.1% to 9.3%) in the PPS.
The primary endpoints met the criteria confirming noninferiority for the CA and AHC groups. According to bivariate analysis, the central lesion was associated with an increased risk of an incomplete ICR (Table 5). According to univariate analysis, the CA group and a small lesion area were significantly associated with a better DCR (Table 6).
Table 5.
Binary logistic regression analysis of complete iceball coverage rate (ICR)
| Variable | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | SE | Wald | df | Significance | Exp (B) | Exp (B) 95% confidence interval |
| Tumor location | 1.365 | 0.662 | 4.256 | 1 | 0.039 | 3.917 | 1.071–14.329 |
Table 6.
Ordinal logistic regression analysis of the disease control rate (DCR)
| Variable | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | SE | Wald | df | Significance | Exp (B) | Exp (B) 95% confidence interval |
| Largest area of the lesion | 0.002 | 0.0007 | 8.506 | 1 | 0.004 | 1.002 | 1.001–1.003 |
| Group | 1.248 | 0.6225 | 3.978 | 1 | 0.046 | 3.483 | 1.002–11.869 |
Figure 2 shows the successful treatment of a lesion in the upper lobe of the left lung with a complete response (CR) to CA throughout the follow‐up period.
Figure 2.
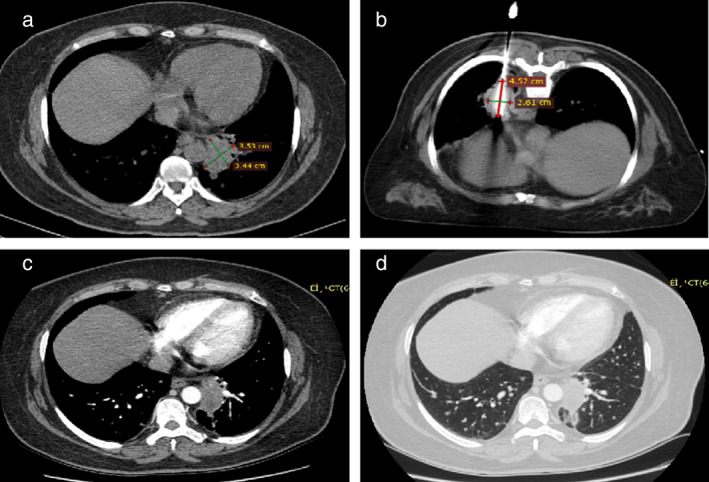
A 77‐year‐old woman with a 3.53 × 3.44 cm adenocarcinoma lesion received co‐ablation. (a) Initial computed tomographic (CT) scans before ablation show a lesion in the upper lobe of the left lung. (b) Co‐ablation was performed in the prone position using a probe. CT scan shows an iceball during co‐ablation. (c, d) Contrast CT scans one month after the procedure show no residual enhancement of the mass.
Secondary endpoints
The satisfaction rate with the application convenience of the two devices was 100%. There was no difference in pain scores between the CA and AHC groups during the procedure. The difference in QOL before and after the procedure was similar.
Safety
The occurrence of complications was similar between the CA and AHC groups: 29.26% (12/41) and 30% (12/40), respectively (P = 0.943). No serious adverse events were observed. Pneumothorax, pleural effusion, hemoptysis, pericardial effusion, and local bleeding at puncture sites similarly occurred in the CA and AHC groups.
Other results
The number of probes used per patient in the CA and AHC groups was 1.85 ± 0.82 and 2.73 ± 1.15, respectively (P = 0.000).
Discussion
Cryoablation has been previously shown to be successful in treating lung cancer. 10 , 11
In this study, coablation was compared with argon‐helium cryoablation in the treatment of stage III–IV NSCLC. No significant difference were observed in efficacy and safety. There was less probe usage per patient in the CA group. Central lesions were associated with an increased risk of an incomplete ICR. A smaller lesion area in the CA group was significantly correlated with a better DCR.
In this study, there was no significant difference in the ICR between the CA and AHC groups. The only significant predictor of an incomplete ICR was a central lesion. This is consistent with the study of Wang et al. 10 who found that tumor size and location were correlate with ice coverage. The ICR is a key factor directly related to cell death in cryoablation. The lethal zone can be achieved when the cryozone is located 5–10 mm beyond of the tumor. 12 To achieve higher iceball coverage, the following measures should be taken: (i) adjust the position of the probe after rewarming if the expected iceball coverage is not satisfactory; (ii) increase the number of probes, and (iii) adopt isolation methods to reduce the heat‐sink effect.
For stage III–IV NSCLC, the DCR in the CA group in both the FAS and PPS confirmed noninferiority for the AHC group,which was similar to the previously reported rate of 82%. 13 Gao et al. 14 reported a longer follow‐up period of three months with a local tumor progression rate of 13.6% (4/31) for stage IIIB/IV advanced NSCLC. For stage I lung cancer, local tumor progression occured in 3% of patients with a median follow‐up of 23 months. 3 Ablation is especially suitable for those middle‐aged and elderly patients who either refuse or cannot tolerate surgery because of poor PS, significant cardiovascular risk, poor pulmonary function, and/or comorbidities. It is an option after the failure of other treatments. As a part of comprehensive treatment, it can effectively reduce the tumor load and control tumor progression. In our study, the option of ablation was decided following discussion between a multiple disciplinary team (MDT) and according to the NCCN guidelines for NSCLC. Among the participants, 39.5% of the patients relapsed after surgery, 30.86% relapsed after radiotherapy or chemotherapy, 56.79% had significant cardiovascular risk or poor pulmonary function, and 3.7% had poor PS.
In our study, the CA group was a predictor of a better DCR. As the baseline characteristics of the patients were similar, fewer probes were used in the CA group, indicating that the probe used in the CA system is more powerful. This may be attributed to different cryogens and mechanisms between the two devices. The alternation of freezing and warming produces huge thermal stress, resulting in stronger mechanical damage. Liu and his colleagues invented the co‐ablation system. They investigated the efficacy of alternating cooling and heating in many tissues. 5 , 6 The differential scanning calorimetry (DSC) experiment demonstrated alternate cooling and heating damage resulted in physicial and component destruction. The benefits of co‐ablation may also contribute to the consequences of subsequent heating, resulting in a lower phase transition temperature. In the in vitro experiment, as the thaw cycle initiated, a sharp drop in transient strain and thermal shock rings were both observed in swine. Jing et al. 5 compared the performance of the liquid nitrogen and absolute ethanol‐based co‐ablation system with the argon and helium‐based cryoablation system. The cooling rates were similar, whereas the coablation system achieved a lower temperature resulting in a larger area of lethal zone. Shen et al. 15 reported that alternate cold and heat ablation (using ethanol and water as cooling and heating fluid) could result in severe vascular injury and a large area of tumor cell debris. Zhang et al.16 utilized both the cryoablation system and RFA to achieve the combined ablation goal. They compared the results of freezing alone and freezing‐heating in both ex vivo and in vivo experiments. The histopathology results showed that freezing‐heating induced a larger coagulative necrosis zone. The combined model could offer an inferior advantage based on the results of alternating cold and heat treatments. In our study, the co‐ablation system induced freezing and heating through a single probe,which overcame many technical difficulties and resulted in a major breakthrough. The study of different cryogens were also reported. Hewitt et al. 17 investigated the performance of cryotech LCS 3000 liquid nitrogen system and the CRYOcare argon gas‐based system. In a warm water bath (42°C), the diameter of iceball produced by the liquid nitrogen system was larger after five minutes of freezing.
Less area of the lesion was also significantly correlated with a better DCR in our study, which was similar to previous reports. McDevitt et al. 18 found that a tumor diameter >3 cm was associated with local progression. Yamauchi et al. 3 found that the existence of a thick vessel (diameter ≥ 3 mm) no more than 3 mm from the edge of the tumor was an independent factor associated with local progression. In contrast to radiofrequency ablation (RFA), cryoablation results in a lower risk of local and regional recurrence. 3 The proper reason may be the ability of cryoablation to visualize low‐density iceball under image guidance in order to reduce ablation omission. Georgiades et al. 19 reported that no viable cells were detected in the cryoablated region in the kidney of adult swine.
The doctors were completely satisfied with the performance and application of both devices. All patients tolerated local anesthesia well during the procedure. No ablation procedure was terminated due to pain. There was no significant difference in pain scores during the procedure between the two groups. The minimally invasive treatment has less impact on patient QOL. The recovery time in the hospital was short. In our study, the change in QOL between the two groups were similar. There was no significant difference in QOL before or after operation.
The main complications after the cryoablation were minor and patients were able to recover on their own. Wang et al. 10 reported pleural effusion in 14%, hemoptysis in 62% and nerve palsy in 0.5% cases. In contrast to earlier findings, however, in our study, the rate of adverse effects was found to be lower. Inoue et al. 20 reported a higher rate of complication after 193 cryoablation sessions in 117 patients, with pneumothorax in 61.7%, pleural effusion in 70.5%, and hemoptysis in 36.8%. In this study, a larger number of probes was a significant predictor of pneumothorax (P = 0.001), pleural effusion (P = 0.001) and hemoptysis (P < 0.001).
Nowadays, current developments are focusing on cryoimmunology, 21 , 22 which might play an important role in the future treatment of cancer. Some studies have found that combined ablation could generate a more powerful antitumor immune response, 23 , 24 and cryoablation combined with immunotherapy may significantly enhance antitumor efficacy. 25 Therefore, the strategy of combining coablation and immune therapy may be the focus in our future study.
In conclusion, CA is noninferior to AHC in terms of efficacy and safety for the treatment of stage III–IV NSCLC. A smaller lesion area in the CA group was significantly correlated with a better DCR.
Disclosure
The authors declare that they have no competing interests.
Trial registration: Chinese Clinical Trial Registry, ChiCTR2000034353.
Contributor Information
Huasong Feng, Email: fenghs99@163.com.
Kaiwen Hu, Email: drkaiwenhu@126.com.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (6): 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Inoue M, Nakatsuka S, Jinzaki M. Cryoablation of early‐stage primary lung cancer. Biomed Res Int 2014; 2014: 521691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamauchi Y, Izumi Y, Hashimoto K et al Percutaneous cryoablation for the treatment of medically inoperable stage I non‐small cell lung cancer. PLOS One 2012; 7 (3): e33223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan JF, Deng ZS, Liu J, Zhou YX. New modality for maximizing cryosurgical killing scope while minimizing mechanical incision trauma using combined freezing‐heating system. J Med Devices 2007; 1 (4): 264–71. [Google Scholar]
- 5. Liu Z, Yu G, Deng L. Minimally invasive probe system capable of performing both cryosurgery and hyperthermia treatment on target tumor in deep tissues. Minim Invasive Ther Allied Technol 2004; 13 (1): 47–57. [DOI] [PubMed] [Google Scholar]
- 6. Sun ZQ, Yang Y, Liu J. Alternative cooling and heating as a novel minimally invasive approach for treating obesity. Int J Therm Sci 2013; 64: 29–39. [Google Scholar]
- 7. Sobin LH, Gospodarowicz MK,Wittekind C. International Union against Cancer (UICC): TNM Classification of Malignant Tumours. 7th ed. Oxford: Wiley‐Blackwell; 2009. [Google Scholar]
- 8. Nishino M, Jackman DM, Hatabu H et al New response evaluation criteria in solid tumors (RECIST) guidelines for advanced non‐small cell lung cancer: Comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 2010; 195 (3): W221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Cancer Institute . Common terminology criteria for adverse events, version 4.03. 2010. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 5 March 2011.
- 10. Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: Initial experience with more than 200 procedures. Radiology 2005; 235 (1): 289–98. [DOI] [PubMed] [Google Scholar]
- 11. Kawamura M, Izumi Y, Tsukada N et al Percutaneous cryoablation of small pulmonary malignant tumors under computed tomographic guidance with local anesthesia for nonsurgical candidates. J Thorac Cardiovasc Surg 2006; 131 (5): 1007–13. [DOI] [PubMed] [Google Scholar]
- 12. Jones GC, Kehrer JD, Kahn J et al Primary treatment options for high‐risk/medically inoperable early stage NSCLC patients. Clin Lung Cancer 2015; 16 (6): 413–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pusceddu C, Sotgia B, Fele RM, Melis L. CT‐guided thin needles percutaneous cryoablation (PCA) in patients with primary and secondary lung tumors: A preliminary experience. Eur J Radiol 2013; 82 (5): e246–53. [DOI] [PubMed] [Google Scholar]
- 14. Gao W, Guo Z, Shu S, Xing W, Zhang W, Yang X. The application effect of percutaneous cryoablation for the stage IIIB/IV advanced non‐small‐cell lung cancer after the failure of chemoradiotherapy. Asian J Surg 2018; 41 (6): 530–6. [DOI] [PubMed] [Google Scholar]
- 15. Shen Y, Liu P, Zhang A, Xu L. Tumor microvasculature response to alternated cold and heat treatment. Conf Proc IEEE Eng Med Biol Soc 2005; 2005: 6797–800. [DOI] [PubMed] [Google Scholar]
- 16. Zhang K, Zou J, He K et al Study of enhanced radiofrequency heating by pre‐freezing tissue. Int J Hyperthermia 2018; 35 (1): 79–89. [DOI] [PubMed] [Google Scholar]
- 17. Hewitt PM, Zhao J, Akhter J, Morris DL. A comparative laboratory study of liquid nitrogen and argon gas cryosurgery systems. Cryobiology 1997; 35 (4): 303–8. [DOI] [PubMed] [Google Scholar]
- 18. McDevitt JL, Mouli SK, Nemcek AA, Lewandowski RJ, Salem R, Sato KT. Percutaneous cryoablation for the treatment of primary and metastatic lung tumors: identification of risk factors for recurrence and major complications. J Vasc Interv Radiol 2016; 27 (9): 1371–9. [DOI] [PubMed] [Google Scholar]
- 19. Georgiades C, Rodriguez R, Azene E et al Determination of the nonlethal margin inside the visible “ice‐ball” during percutaneous cryoablation of renal tissue. Cardiovasc Intervent Radiol 2013; 36 (3): 783–90. [DOI] [PubMed] [Google Scholar]
- 20. Inoue M, Nakatsuka S, Yashiro H et al Percutaneous cryoablation of lung tumors: Feasibility and safety. J Vasc Interv Radiol 2012; 23 (3): 295–302. [DOI] [PubMed] [Google Scholar]
- 21. Katzman D, Wu S, Sterman DH. Immunological aspects of cryoablation of non‐small cell lung cancer: A comprehensive review. J Thorac Oncol 2018; 13 (5): 624–35. [DOI] [PubMed] [Google Scholar]
- 22. Aarts BM, Klompenhouwer EG, Rice SL et al Cryoablation and immunotherapy: An overview of evidence on its synergy. Insights Imaging 2019; 10 (1): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu K, He K, Xue T, Liu P, Xu LX. The cryo‐thermal therapy‐induced IL‐6‐rich acute pro‐inflammatory response promoted DCs phenotypic maturation as the prerequisite to CD4(+) T cell differentiation. Int J Hyperthermia 2018; 34 (3): 261–72. [DOI] [PubMed] [Google Scholar]
- 24. Zhu J, Zhang Y, Zhang A, He K, Liu P, Xu LX. Cryo‐thermal therapy elicits potent anti‐tumor immunity by inducing extracellular Hsp70‐dependent MDSC differentiation. Sci Rep 2016; 6: 27136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen DS, Mellman I. Oncology meets immunology: The cancer‐immunity cycle. Immunity 2013; 39 (1): 1–10. [DOI] [PubMed] [Google Scholar]


