Abstract
Capmatinib is a MET tyrosine kinase inhibitor (TKI) that has recently been approved for the treatment of advanced non–small cell lung cancer (NSCLC) positive for skipping mutations of MET exon 14 (METex14). Drug‐induced interstitial lung disease (ILD) is a relatively rare, but potentially serious, side effect of TKIs administered for lung cancer treatment. Here we report a case of capmatinib‐induced ILD in a patient with NSCLC harboring a METex14 skipping mutation. Capmatinib should be immediately discontinued if ILD is suspected, and treatment with corticosteroid should be considered.
Keywords: Capmatinib, ILD, NSCLC, MET skipping mutation
Capmatinib is a MET tyrosine kinase inhibitor (TKI) that has recently been approved for the treatment of advanced non‐small cell lung cancer (NSCLC) positive for skipping mutations of MET exon 14 (METex14). Here we report a case of capmatinib‐induced ILD in a patient with NSCLC harboring a skipping mutation of METex14.
Introduction
Identification of biologically significant genetic alterations that lead to activation of oncogenes in cancer has the potential to provide therapeutic opportunities. Molecularly targeted therapy for lung adenocarcinoma harboring skipping mutations of METex14 has recently been introduced into clinical practice. 1 Two MET TKIs—capmatinib and tepotinib—have thus been approved for the treatment of advanced NSCLC in patients confirmed to be positive for such mutations. 2 , 3 METex14 alterations were initially identified in small cell lung cancer and NSCLC in 2003 and 2005, respectively. 4 Genetic alterations of METex14 have been detected in 4.3% of lung adenocarcinomas and in 3.0% of squamous cell lung cancers. 5 These alterations are targetable driver mutations similar to alterations of EGFR and ALK, 6 given that lung adenocarcinomas harboring METex14 alterations show a substantial clinical response to MET inhibition. 7 , 8 Drug‐induced ILD is a relatively rare but potentially serious side effect of TKIs administered for lung cancer treatment. 9 Here, we report a case of capmatinib‐induced ILD in a patient with NSCLC harboring a METex14 skipping mutation.
Case report
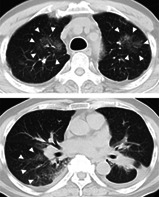
A 68‐year‐old male former smoker (50 pack years) of Japanese descent was diagnosed with stage IV lung adenocarcinoma (cT3N3M1c) with multiple pleural disseminations as well as a positive intra‐abdominal lymph node. He was taking amlodipine besilate as a routine medication, and there was no clinically apparent ILD at baseline. Analysis of biopsied tumor tissue with an Applied Biosystems 7500 real‐time PCR system revealed a METex14 skipping mutation. Given the lack of an approved MET‐targeted therapy, the patient participated in cohort 5b (treatment‐naïve) of a phase 2 trial, GEOMETRY mono‐1, and capmatinib was administered orally at a dose of 400 mg twice daily. After treatment for 29 days, he was admitted to our hospital with fever and dyspnea. His oxygen saturation was 90% on room air. No findings such as edema or jugular vein distention were noted. Laboratory tests revealed a B‐type natriuretic peptide level of 56.0 pg/mL (normal range; 0–20 pg/mL) and KL‐6 level of 531 U/mL (normal range; <500 U/mL). Sputum culture was negative. Chest computed tomography (CT) revealed extensive right consolidation with multiple ground‐glass opacities (GGOs) throughout both lungs, despite shrinkage of the primary lung lesion in the left lower lobe (Fig 1a,b). Capmatinib treatment was immediately discontinued, and empirical treatment with piperacillin and tazobactam was administered. After antibiotic treatment for four days, his fever remained and CT revealed progression of the GGOs in both upper lobes. We performed bronchoscopy to identify the underlying etiology of the pneumonitis, and cellular analysis of bronchoalveolar lavage fluid revealed a lymphocytic cellular pattern (Fig 1c). Hematoxylin‐eosin staining of a specimen of the right lung revealed mild infiltration of small round inflammatory cells and neutrophils, suggestive of alveolitis (Fig 1d). On the basis of these clinical features, we made a diagnosis of capmatinib‐induced ILD of grade 3. We initiated prednisolone treatment at a dose of 60 mg daily. The patient experienced gradual clinical improvement and was discharged on treatment with prednisolone at 20 mg/day for one month.
Figure 1.
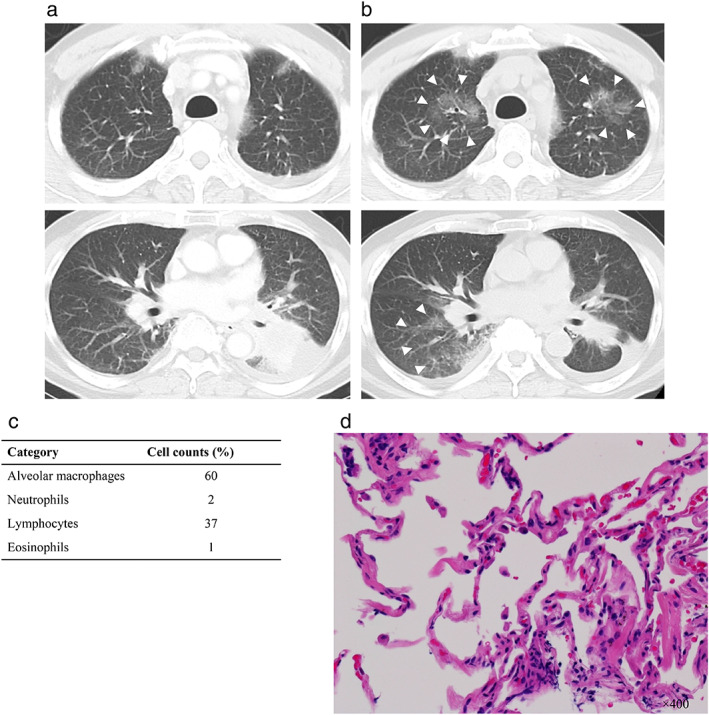
Computed tomography (CT) scan and histological findings indicative of capmatinib‐induced ILD. (a) CT scan showing the primary lung lesion in the left lower lobe before capmatinib treatment. (b) CT scan at 31 days after the initiation of capmatinib treatment showing extensive right consolidation with multiple ground‐glass opacities (GGOs) throughout both lungs (arrowheads), despite shrinkage of the primary lung lesion. (c) Cellular analysis of bronchoalveolar lavage fluid at 33 days after capmatinib initiation. (d) Hematoxylin‐eosin staining of a specimen of the right lung revealed mild infiltration of small round inflammatory cells and neutrophils at 33 days after capmatinib initiation.
Discussion
Capmatinib was approved by the U.S. Food and Drug Administration in May 2020 and by the Ministry of Health, Labor, and Welfare of Japan in June 2020 for the treatment of advanced NSCLC positive for METex14 skipping mutations. 2 Indeed, increasing evidence supports the use of MET‐TKIs in patients with NSCLC harboring such mutations. 1 The increasing application of next‐generation sequencing (NGS) to tumor and blood samples has resulted in the relatively frequent identification of METex14 mutations in individuals with NSCLC. 10 , 11 , 12 These mutations appear to be more frequent in older adults, with age also being a risk factor for ILD. 9 , 13 As far as we are aware, our report is the first to provide CT and histological evidence for capmatinib‐associated ILD as the first line treatment. Another case of capmatinib‐induced pneumonitis in a patient who had received prior pembrolizumab treatment has already reported. 14 Recent studies have implicated signaling by hepatocyte growth factor and its receptor (MET) in inhibition of tissue fibrosis. It remains unclear whether MET‐TKIs influence such an antifibrotic role, with the mechanism underlying the pulmonary toxicity of MET‐TKIs remaining incompletely understood. 15 In a clinical trial of tepotinib, a TKI in the same class as capmatinib, the development of ILD led to permanent drug discontinuation in two patients (1.3%). 3 A recent retrospective review of 1669 patients who received crizotinib, a multikinase TKI that targets MET, in four clinical trials (PROFILE 1001, 1005, 1007, and 1014) reported an overall incidence of ILD of 1.2%, although the incidence was higher at 3.7% in Japanese patients. 16 A higher incidence of drug‐induced lung injury has been noted in Japan than in other countries. 9 , 17 Treatment with corticosteroid should be considered for this condition as appropriate. 9 Although the patient in this report developed steroid‐sensitive ILD, it remains possible that MET‐TKIs may induce severe acute ILD. Physicians should thus recognize that capmatinib has the potential to cause acute interstitial pneumonia in some patients. The present case highlights the need to identify risk factors and the underlying etiology of ILD in order to develop effective strategies for monitoring of this condition in patients treated with MET‐TKIs.
Disclosure
MT reports honoraria from Boehringer Ingelheim, Chugai Pharmaceutical, AstraZeneca, Ono Pharmaceutical, Bristol‐Myers Squibb Company and Novartis during the conduct of the study.
KN reports grants and personal fees from AstraZeneca, grants and personal fees from Astellas Pharma Inc., grants and personal fees from MSD, grants, personal fees and other from Ono Pharmaceutical, grants and personal fees from Nippon Boehringer Ingelheim, grants and personal fees from Novartis Pharma, grants, personal fees and other from Pfizer Japan Inc., grants and personal fees from Bristol Myers Squibb Company, grants, personal fees and other from Eli Lilly Japan, grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Daiichi Sankyo, grants and personal fees from Merck Serono/Merck Biopharma, during the conduct of the study; personal fees from Clinical Trial, personal fees from MEDICUS SHUPPAN,Publishers, personal fees from Care Net, Inc., personal fees from Reno. Medical, personal fees and other from KYORIN Pharmaceutical, personal fees from Medical Review, personal fees from Roche Diagnostics, personal fees from Bayer Yakuhin, personal fees from Medical Mobile Communications, personal fees from 3H Clinical Trial, personal fees from Nichi‐Iko Pharmaceutical, grants, personal fees and other from Takeda Pharmaceutical, grants and personal fees from Taiho Pharmaceutical, grants and personal fees from SymBio Pharmaceuticals Limited., personal fees from NANZANDO, personal fees from YODOSHA, personal fees from Nikkei Business Publications, personal fees from Thermo Fisher Scientific, personal fees from Yomiuri Telecasting Corporation., personal fees from Nippon Kayaku, grants and personal fees from AbbVie Inc., grants from inVentiv Health Japan, grants from Icon Japan, grants from Gritsone Oncology, grants from Parexel International, grants from Kissei Pharmaceutical, grants from EPS Corporation., grants from Syneos Health., grants from Pfizer R&D Japan G.K., grants from A2 Healthcare, grants from Quintiles/IQVIA Services JAPAN, grants from EP‐CRSU, grants from Linical, grants from Eisai, grants from CMIC Shift Zero, grants from Kyowa Hakko Kirin, grants from Bayer Yakuhin, grants from EPS International, grants from Otsuka Pharmaceutical, outside the submitted work. The remaining authors state that they had no conflicts of interest.
Acknowledgments
The patient described in this report was treated in a capmatinib clinical trial (CINC280A2201, NCT02414139, GEOMETRY mono‐1) that was sponsored by Novartis Pharma.
References
- 1. Recondo G, Che J, Janne PA, Awad MM. Targeting MET dysregulation in cancer. Cancer Discov 2020; 10 (7): 922–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf J, Seto T, Han JY et al Capmatinib in MET exon 14‐mutated or MET‐amplified non‐small‐cell lung cancer. N Engl J Med 2020; 383 (10): 944–57. [DOI] [PubMed] [Google Scholar]
- 3. Paik PK, Felip E, Veillon R et al Tepotinib in non‐small‐cell lung cancer with MET exon 14 skipping mutations. N Engl J Med 2020; 383 (10): 931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: A coherent approach to targeted therapy. Nat Rev Cancer 2018; 18 (6): 341–58. [DOI] [PubMed] [Google Scholar]
- 5. Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in lung cancer: Will expectations finally be MET? J Thorac Oncol 2017; 12 (1): 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsuta K, Kozu Y, Mimae T et al C‐MET/phospho‐MET protein expression and MET gene copy number in non‐small cell lung carcinomas. J Thorac Oncol 2012; 7 (2): 331–9. [DOI] [PubMed] [Google Scholar]
- 7. Frampton GM, Ali SM, Rosenzweig M et al Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015; 5 (8): 850–9. [DOI] [PubMed] [Google Scholar]
- 8. Paik PK, Drilon A, Fan PD et al Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015; 5 (8): 842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kubo K, Azuma A, Kanazawa M et al Consensus statement for the diagnosis and treatment of drug‐induced lung injuries. Respir Investig 2013; 51 (4): 260–77. [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal C, Thompson JC, Black TA et al Clinical implications of plasma‐based genotyping with the delivery of personalized therapy in metastatic non‐small cell lung cancer. JAMA Oncol 2019; 5 (2): 173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalemkerian GP, Narula N, Kennedy EB et al Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol 2018; 36 (9): 911–9. [DOI] [PubMed] [Google Scholar]
- 12. Mosele F, Remon J, Mateo J et al Recommendations for the use of next‐generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann Oncol 2020; 31 (11): 1491–505. [DOI] [PubMed] [Google Scholar]
- 13. Awad MM, Oxnard GR, Jackman DM et al MET exon 14 mutations in non‐small‐cell lung cancer are associated with advanced age and stage‐dependent MET genomic amplification and c‐met overexpression. J Clin Oncol 2016; 34 (7): 721–30. [DOI] [PubMed] [Google Scholar]
- 14. El Husseini K, Chaabane N, Mansuet‐Lupo A, Leroy K, Revel M‐P, Wislez M. Capmatinib‐induced interstitial lung disease: A case report. Current Problems in Cancer: Case Reports 2020; 2: 100024. [Google Scholar]
- 15. Ghatak S, Bogatkevich GS, Atnelishvili I et al Overexpression of c‐Met and CD44v6 receptors contributes to autocrine TGF‐beta1 signaling in interstitial lung disease. J Biol Chem 2014; 289 (11): 7856–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoneda KY, Scranton JR, Cadogan MA et al Interstitial lung disease associated with crizotinib in patients with advanced non‐small cell lung cancer: Independent review of four PROFILE trials. Clin Lung Cancer 2017; 18 (5): 472–9. [DOI] [PubMed] [Google Scholar]
- 17. Kudoh S, Kato H, Nishiwaki Y et al Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case‐control study. Am J Respir Crit Care Med 2008; 177 (12): 1348–57. [DOI] [PubMed] [Google Scholar]


