Abstract
Background
To determine whether combining brachytherapy with immunotherapy is safe in prostate cancer (PCa) and provides synergistic effects, we performed a Phase I/II trial on the feasibility, safety, and benefit of concurrent delivery of anti-PD-1 (nivolumab) with high-dose-rate (HDR) brachytherapy and androgen deprivation therapy (ADT) in patients with Grade Group 5 (GG5) PCa.
Methods
Eligible patients were aged 18 years or older with diagnosis of GG5 PCa. Patients received ADT, nivolumab every two weeks for four cycles, with two cycles prior to first HDR, and two more cycles prior to second HDR, followed by external beam radiotherapy. The primary endpoint was to determine safety and feasibility. This Phase I/II trial is registered with ClinicalTrials.gov (NCT03543189).
Results
Between September 2018 and June 2019, six patients were enrolled for the Phase I safety lead-in with a minimum observation period of 3 months after nivolumab administration. Overall, nivolumab was well tolerated in combination with ADT and HDR treatment. One patient experienced a grade 3 dose-limiting toxicity (elevated Alanine aminotransferase and Aspartate aminotransferase) after the second cycle of nivolumab. Three patients (50%) demonstrated early response with no residual tumor detected in ≥4 of 6 cores on biopsy post-nivolumab (4 cycles) and 1-month post–HDR. Increase in CD8+ and FOXP3+/CD4+ T cells in tissues, and CD4+ effector T cells in peripheral blood were observed in early responders.
Conclusion
Combination of nivolumab with ADT and HDR is well tolerated and associated with evidence of increased immune infiltration and antitumor activity.
Introduction
Immunotherapy with checkpoint inhibitors has revolutionized the treatment paradigm of many malignancies over the past five years [1–4]. However, immune checkpoint inhibitors (ICIs) have not demonstrated comparable rates of efficacy in prostate cancer (PCa) [5]. Early evidence demonstrated that there may be dramatic responses to ICIs in select populations, in combination with androgen receptor targeted agents [6].
Evidence from two randomized trials (TROG 96.01, DFCI 95096) has demonstrated a significant reduction of PCa specific mortality among men with Gleason grade 4 disease treated with radiation and androgen deprivation therapy (ADT), whereas this finding was not observed in men with Gleason grade 5 lesions [7–9]. These data suggest that a novel therapeutic approach is necessary to improve clinical outcomes in men with poorly or undifferentiated Grade Group 5 (GG5) disease (i.e., Gleason score 9 or 10) [9].
Preclinical studies and clinical observations have demonstrated that radiation modulates both the tumor microenvironment and the tumor itself to become an immunostimulatory milieu [10–13]. Preclinical studies suggested high radiation dose 5–20 Gy may have stronger immune-stimulatory effects [14, 15]. High-dose-rate (HDR) brachytherapy over a very short period of time could potentially enhance the immune-stimulatory effects of radiation while minimizing the off-target immune-suppressive effects on peripheral immune cells that occurs with conventional daily radiotherapy. There is evidence that ADT may augment an antitumor immune response by enhancing lymphopoiesis and mitigating tolerance to PCa antigens [16–18] and an active immune microenvironment is present within PCa and can be potentially augmented by ADT [19–22].
Considering the well documented evidence of radiation and ADT as immune modulators, we expect that the combination of ADT, ICIs, and radiation will likely improve outcomes for patients with GG5 PCa. Given this rationale, we initiated a Phase I/II clinical trial of combining nivolumab with HDR and ADT plus external beam radiotherapy (EBRT) for patients with GG5 PCa. The feasibility, safety, toxicity, and exploratory analysis from the Phase I portion are presented here.
Patients and methods
Study design
This is a Phase I/II study investigating the efficacy and safety of the combination of nivolumab with ADT and Iridium 192 HDR plus EBRT for patients with GG5 PCa (Fig. 1a). In this portion of the study, 6–9 patients with GG5 PCa were programmed for enrollment as a safety lead-in and treated on the same schedule as outlined in the protocol with a safety observation period of 12 weeks after initiation of nivolumab. Patients were enrolled sequentially and treated at one dose level of nivolumab (240 mg). If 3 or more patients developed unacceptable toxicity during any portion of the safety lead-in phase, then the study would be discontinued. After the 12-week safety observation period, toxicity was assessed on an ongoing basis for all patients until study completion. Currently, patients have approximately 1 year follow up since completion of treatments. This Phase I/II trial is registered with ClinicalTrials.gov (NCT03543189).
Fig. 1. Study design and systematically coordinated tissue biopsy system.
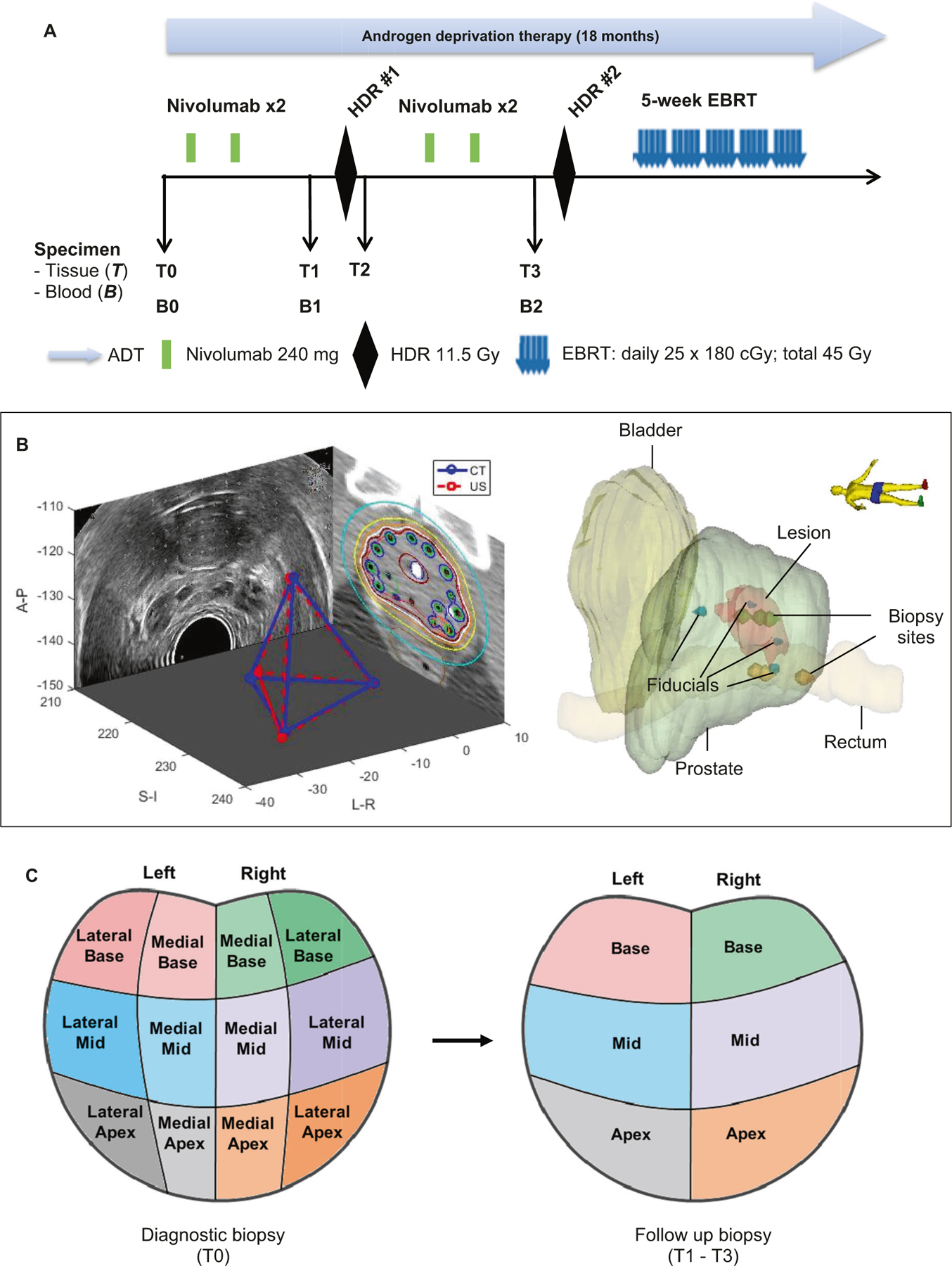
a Study schema. ADT: Androgen deprivation therapy. EBRT: External beam radiation therapy. HDR: High dose brachytherapy. Nivo: nivolumab. B0/T0: Baseline blood and diagnostic biopsy. B1/T1: Specimen collected after two cycles of nivolumab, and prior to HDR#1 brachytherapy. T2: Specimen collection within 2 h post HDR#1. B2/T3: Specimen collection after 4 cycles of nivolumab and four weeks after HDR#1, but prior to HDR#2. b Tissue collection schema. A carefully designed coordinates system was used to determine the location of the 6-core biopsy sites (left apex, left mid, left base, right apex, right mid, and right base). Four fiducial markers were placed into the apex, base, left mid and right mid gland. Biopsies were performed under ultrasound guidance by combining the prostate template grid, the ultrasound transducer stepper, and the location of the fiducial marker. Mirada’s multimodality registration was used to rigidly register the MRI image to the planning CT using local registration. The biopsy sites were documented for follow-up biopsy reference. c Prostate biopsy locations. Left panel: T0 is diagnostic biopsy prior to treatment; Right panel: T1 is post-nivolumab (2 Cycles) and Pre high dose radiation (HDR-Brachytherapy) biopsy; T2 is within 2 h post-HDR brachytherapy, T3 is post-nivolumab (4 cycles) and 1 month post-HDR brachytherapy.
Participants
Eligible patients were ≥18 years of age with pathologically proven diagnosis of PCa undergoing their first line of treatment for GG5 disease [23] with >30% of cores involved), any PSA or T-stage, and/or oligometastatic disease being treated with curative intent (≤3 sites of distant metastasis, and/or positive lymph nodes confined to the pelvis). PD-L1 status was not required.
Study agents
Patients received intravenous nivolumab at a dose of 240 mg administered intravenously every 2 weeks for 4 doses, with 2 cycles prior to first HDR (HDR#1), and 2 more cycles prior to second HDR (HDR#2). HDR dose was 11.5 Gy for each treatment. EBRT began two weeks after HDR was completed and was administered at a dose of 40–50 Gy at 1.8–2 Gy per fraction. All patients were started on Flomax approximately 2 weeks prior to the initiation of the HDR treatment.
All study patients had neoadjuvant, concurrent and adjuvant ADT utilizing an LHRH agent with or without an anti-androgen, which was applied as described in a previous study [24]. Initiation of ADT had to begin a minimum of four weeks and a maximum of 6 months prior to the first infusion of nivolumab. The duration of ADT for all patients on study is planned for 18 months.
Toxicity evaluation
This study utilized CTCAE version 5.0 for toxicity and event reporting. Nivolumab dose limiting toxicities (DLTs) were observed after each cycle. DLTs were observed after each 4-week cycle of nivolumab administration up to 12 weeks of observation. After the 12-week safety observation period, toxicity was assessed on an ongoing basis for all patients until study completion. A DLT was defined as any of the following events: (1) grade 3 or 4 immune-related toxicities, including dermatitis, hepatitis, thyroiditis, colitis, and pneumonitis; (2) grade 3 or 4 neutropenia; (3) others as listed in Supplementary Information. Overall toxicities of the combination regimen were assessed ever week during on-treatment-visit and subsequent follow-up using CTCAE 5.0.
Tumor samples collection
Prostate tissue samples were collected for translational research as follows (Fig. 1b, c): at baseline from diagnostic biopsy (T0), pre-HDR#1 (T1), post-HDR#1 (T2, approximately 2 h after HDR#1), and pre-HDR#2 (T3). For baseline prostate tissue (T0), the standard 12-core biopsy tumor tissue was requested. Immediately prior to HDR#1 brachytherapy catheter placement, four fiducial-markers were placed into the apex, base, left-mid, and right-mid region within the prostate and subsequently, 6-core biopsies were obtained as demonstrated in Fig. 1b. A carefully designed coordinates system was used to document the exact location of the 6-core biopsy sites (left apex, left mid, left base, right apex, right mid, and right base) by combining the prostate template grid, the ultrasound transducer stepper, and the location of the four fiducial markers (Fig. 1b). Two additional planned biopsies (post-HDR#1 and pre-HDR#2) were mapped to the same geographic locations as close as possible to the biopsy sites of pre-HDR#1 using the fiducial markers as reference points within the tetrahedron coordinates system (Fig. 1c). Mirada’s multimodality registration was used to rigidly register the MRI prostate image as the region of interest to the planning CT using local registration. MRI to CT registration algorithm in Mirada uses mutual information to match the images. Pathologically early responders were defined as “no tumor” in ≥4 of the 6-cores on prostate biopsy at T3 (post- 4 cycles of nivolumab and 1-month post -HDR). Otherwise, cases were grouped as late responders with significant residual positive cores at T3.
Statistical methodology
The primary objective of the Phase I portion was to evaluate the feasibility, safety, and toxicity profile. If a DLT was observed in 3 or more of the 6 patients, the combination therapy would be determined to be unsafe to conduct the Phase II portion of the trial, and the trial would be discontinued.
Results
Patients and treatments
Between September 2018 through June 2019, six patients with GG5 PCa were enrolled in the Phase I portion. Patient and tumor characteristics are shown in Supplementary Table S1. The median age was 65.5 years with PSA ranging from 3.4 to 17.8. One patient had oligometastatic disease (metastases to the right acetabulum and T12 vertebral body).
Five patients received ADT, nivolumab, HDR, and EBRT, as planned per protocol. One patient experienced a grade 3 DLT after the second cycle of nivolumab. This patient discontinued nivolumab treatment, per protocol, and went on to complete HDR and EBRT as planned.
Treatment-related toxicities
Nivolumab was well-tolerated in combination with HDR, ADT and EBRT. One of six patients experienced a protocol-defined grade 3 DLT due to elevated ALT and AST after the second cycle of nivolumab. Detailed common adverse events attributed to each treatment modality are summarized in Table 1. One patient experienced acute grade 3 adverse events within the 12-week surveillance period after nivolumab administration. Nivolumab associated acute grade 3 adverse events included autoimmune-related hepatitis and autoimmune-related ocular myasthenia gravis in patient PT-02. The other grade 3 toxicity was attributed to ADT with a prolonged QT in patient PT-01. No grade 4 or 5 event was observed. No long-term grade 3 or higher adverse events were observed within the 6–12 months follow-up.
Table 1.
GI, GU and other selected adverse events.
| Subject | Baseline | 12 weeks post nivolumab |
3 months post treatment |
6–12 months post treatment |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nivo | RT | ADT | Nivo | RT | ADT | Nivo | RT | ADT | ||
| PT-01 | G1: hot flashes, ED | None | G1: Dysuria, hematuria, urethral pain; G2: Urinary frequency | G2: Muscle weakness; G3: QT prolongation | None | G1: Urinary frequency, urinary incontinence, dysuria | G1: Arthralgia, hot flashes | None | G1: Urinary frequency, urinary incontinence | G1: Arthralgia, hot flashes, gynecomastia |
| PT-02 | G1: Testosterone deficiency, lipase increase | G2: Muscle weakness, sinus tachycardia; G3: Autoimmune hepatitis with elevated AST and ALT, autoimmune ocular myasthenia gravis | G1: Dysuria, rectal pain, urinary urgency; G2: Urinary frequency | G1: Diarrhea, hot flashes; G2: Muscle weakness | G1: HTN | G1: Urinary frequency, dysuria, urinary urgency | G1: Hot flashes, anemia; G2: Arthralgia | G1: HTN | G1: Urinary frequency | G1: Hot flashes, anemia; G2: Arthralgia |
| PT-03 | G1: ED, elevated AST and ALT; G2: Hot flashes, urinary incontinence | G1: Muscle Weakness | G1: Dysuria, muscle Weakness, fatigue; G2: Urinary frequency, urinary incontinence | G1: Muscle weakness, fatigue; G2: Hot flashes | None | G1: Hematuria, urinary frequency; G2: Urinary incontinence | G1: Hot flashes, arthralgia | None | G1: Urinary frequency, dysuria; G2: Urinary incontinence | G1: Hot flashes, arthralgia |
| PT-04 | G1: Hot flashes | G1: elevated ALT | G1: Fatigue, dysuria; G2: Urinary frequency | G1: Hot flashes, fatigue, anemia | G1: Blood bilirubin increased, creatinine increased | G1: Urinary frequency, urinary urgency, dysuria | G1: Blood bilirubin increased, creatinine increased, anemia; G2: Hot flashes | G1: Blood bilirubin increased | G1: Urinary frequency, urinary urgency, dysuria | G1: Blood bilirubin increased; G2: Hot flashes |
| PT-05 | G1: Hot flashes | G1: Muscle Weakness, lethargy | G1: Muscle Weakness, lethargy; G2: Urinary retention, urinary frequency | G1: Hot flashes, gynecomastia, muscle Weakness, lethargy | None | G2: Urinary frequency | G1: Hot flashes, gynecomastia | None | G1: Urinary frequency | G1: Hot flashes, gynecomastia |
| PT-06 | G1: Hot flashes, urinary urgency, dysuria | G1: Muscle Cramp, anemia, white blood cell count decreased | G1: Urinary urgency, dysuria, urinary incontinence; G2: Urinary frequency | G1: Hot flashes, muscle Cramp, anemia, white blood cell count decreased | G1: Anemia, white blood cell count decreased | G1: Urinary frequency | G1: Hot flashes, anemia, white blood cell count decreased | G1: Anemia, white blood cell count decreased | G1: Urinary frequency | G1: Hot flashes, anemia, white blood cell count decreased |
GI gastrointestinal, GU genitourinary, ALT alanine aminotransferase, AST aspartate aminotransferase, ED erectile dysfunction, HTN hypertension, Nivo Nivolumab, ADT androgen deprivation therapy, RT radiation therapy, G1 Grade 1, G2 Grade 2, G3 Grade 3.
Immune-related AEs (irAEs) localized in the pelvic region, especially colitis, proctitis, cystitis, urethritis, and dermatitis were of special interest given the combination of anti-PD-1 and HDR plus EBRT. No grade ≥3 of those irAEs of interest was observed in the safety observation period of 12 weeks after initiation of nivolumab and the ongoing follow-up period after completion of EBRT (range 6–12 months).
Antitumor activity and patterns of response to treatment
All patients showed significant biochemical response with PSA decrease at 1 to 3 months follow up after the completion of EBRT (Supplementary Table S2). Interestingly, following the trend of PSA response during the treatment course, we observed some temporary bounce of PSA post nivolumab administration, which might suggest inflammatory response in the tumor microenvironment primed by nivolumab (Supplementary Fig. 1). Pathological responses were assessed by analyzing coordinated fiducial marker-guided 6-core serial biopsies, which were obtained at three different time points: pre-HDR#1 (T1), post-HDR#1 (T2), and pre-HDR#2 (T3). Percentage of positive cores were averaged for the samples from the time points T1 and T2 since the interval of biopsy between T1 and T2 was approximately 2 h (Fig. 1a–c). Three patients (PT-01, PT-02, and PT-04) showed a continuing decrease in the percentage of positive cores at T1–T2 and T3 (Fig. 2). These three patients were considered as pathologically early responders. Specifically, PT-01 had 1 positive residual core out of 6 cores at T3; PT-02 and PT-04 were negative on repeat biopsy at T3 (Supplementary Table S2 and Fig. 2). The other three patients (PT-03, PT-05, and PT-06) represented late responders with significant residual positive cores at T3.
Fig. 2. Timing of pathologic response to treatment.
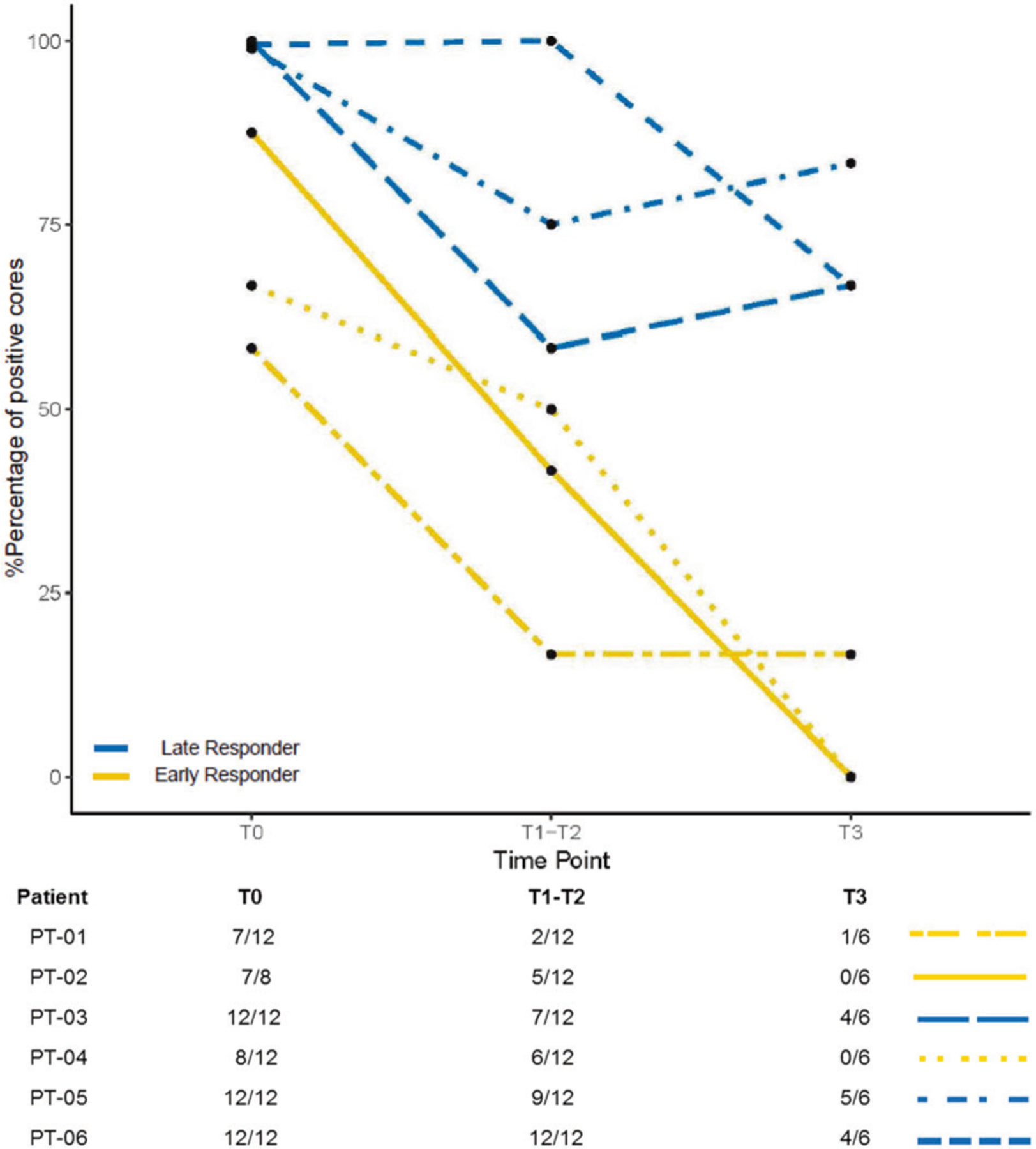
Pathological responses were assessed through analyzing serial 6-core biopsies, which were obtained at three different time points: pre-HDR#1 (T1), post-HDR#1 (T2), and pre-HDR#2 (T3). Percentage of positive cores were averaged for the samples from the time points T1 and T2 since the interval of biopsy between T1 and T2 was approximately 2 h. Number of positive cores over number of core biopsies for each patient at different time point was listed.
Exploratory analysis of immune profile
PD-L1 expression and immune cell infiltrate may affect the ability to respond to nivolumab for early vs. late responders. To explore this, biopsy samples were analyzed for PD-L1 expression, infiltrates of CD8+ T cells, CD4+ T cells and FOXP3+ Treg cells. PD-L1 expression was increased in both groups at T1 and T3 from baseline biopsy (T0) (Fig. 3a, left panel). On the other hand, although the number of CD8+ T cells increased at T1 in both early and late responders, there was a subsequent decrease in CD8+ T cell infiltrates at T3 for late responder patients (Fig. 3a, mid panel). Additionally, CD4+ T cells also showed enhanced levels at T1 compared with T0 for both groups, but their expression continued to increase at T3 only for the early responder patients (Fig. 3a, right panel). Figure 3b shows the multiplex immunostaining for two cases: PT-01 (early responder) and PT-03 (late responder). In an attempt to evaluate the temporal correlation between the tumor immune microenvironment and the peripheral blood of patients on nivolumab, flow cytometric analysis was performed at time points B0, B1, and B2, corresponding to tissue samples at time-points T0, T1, and T3, respectively. By comparing early responder (PT-01) with late responder (PT-03) we observe increasing CD4+ effector T cells with time progression for early response (5.95%, 7.48%, and 11% at B0, B1 and B2 respectively) (Fig. 4a), whereas CD4+ effector T cells levels remained unchanged for late response (6.16%, 5.48%, and 5.80% at B0, B1 and B2 respectively) (Fig. 4b). Lineages of CD4+ effector T cells will be further assessed in our phase II portion of the study.
Fig. 3. Detection of human CD8 (yellow), CD4 (green), PCK (cian), FOXP3 (red), Ki67 (magenta) and PD-L1 (orange) in FFPE prostate cancer biopsies by IHC-IF.
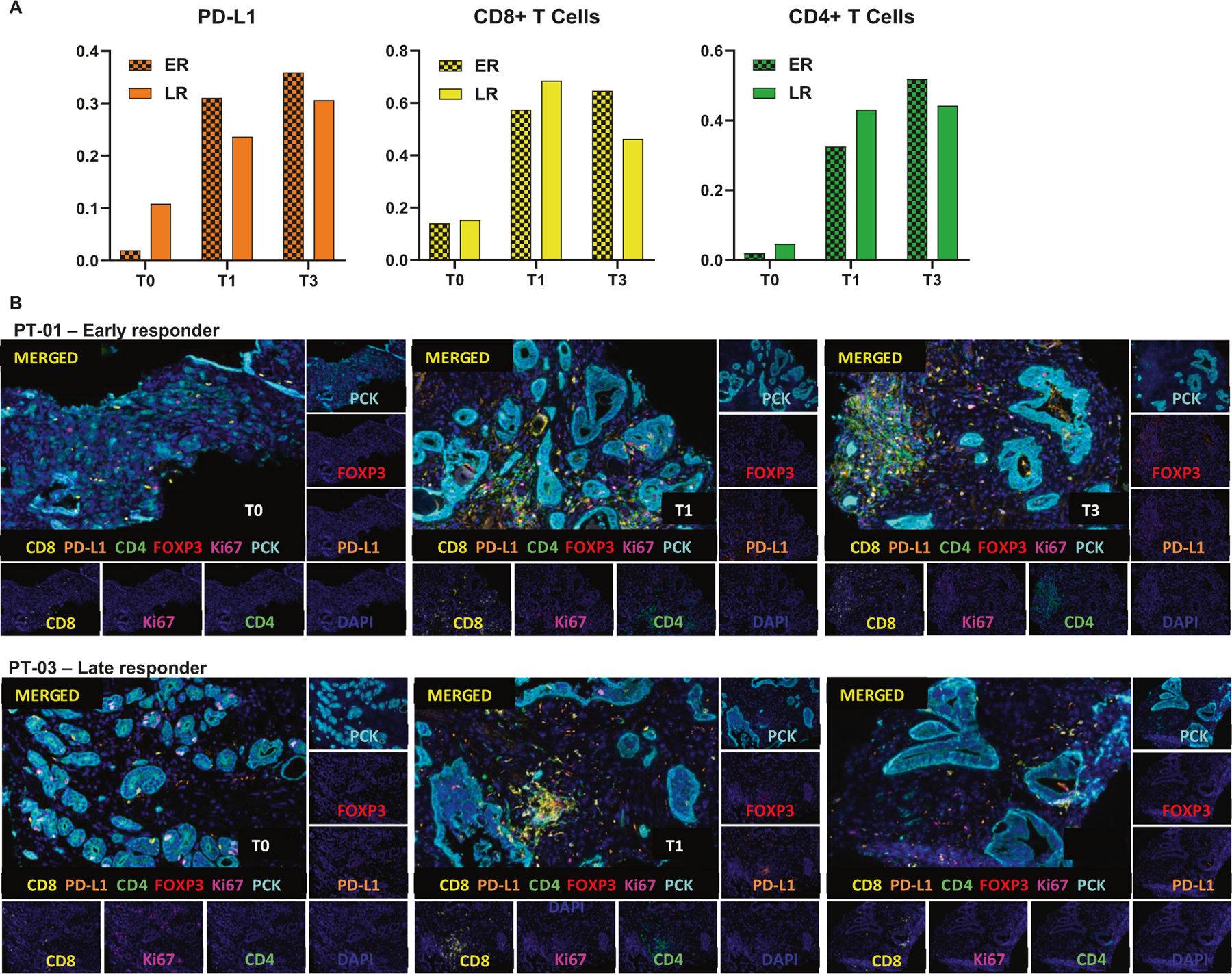
a Quantification of PD-L1 (left), CD8+ (middle) and CD4+ (right) in multiplex images of early responders (ER) and late responders (LR) patients; Three patients per group. b Representative Multiplex IHC images of patients 1 and 3 (PT-01 and PT-03) at T0 (Diagnostic biopsy), T1 (Specimen collected after two cycles of nivolumab, and prior to HDR#1) and T3 (Specimen collected after 4 cycles of nivolumab and four weeks after HDR#1, but prior to HDR#2).
Fig. 4. Quantification of CD4+ and CD8+ T cells in patients peripheral blood samples.
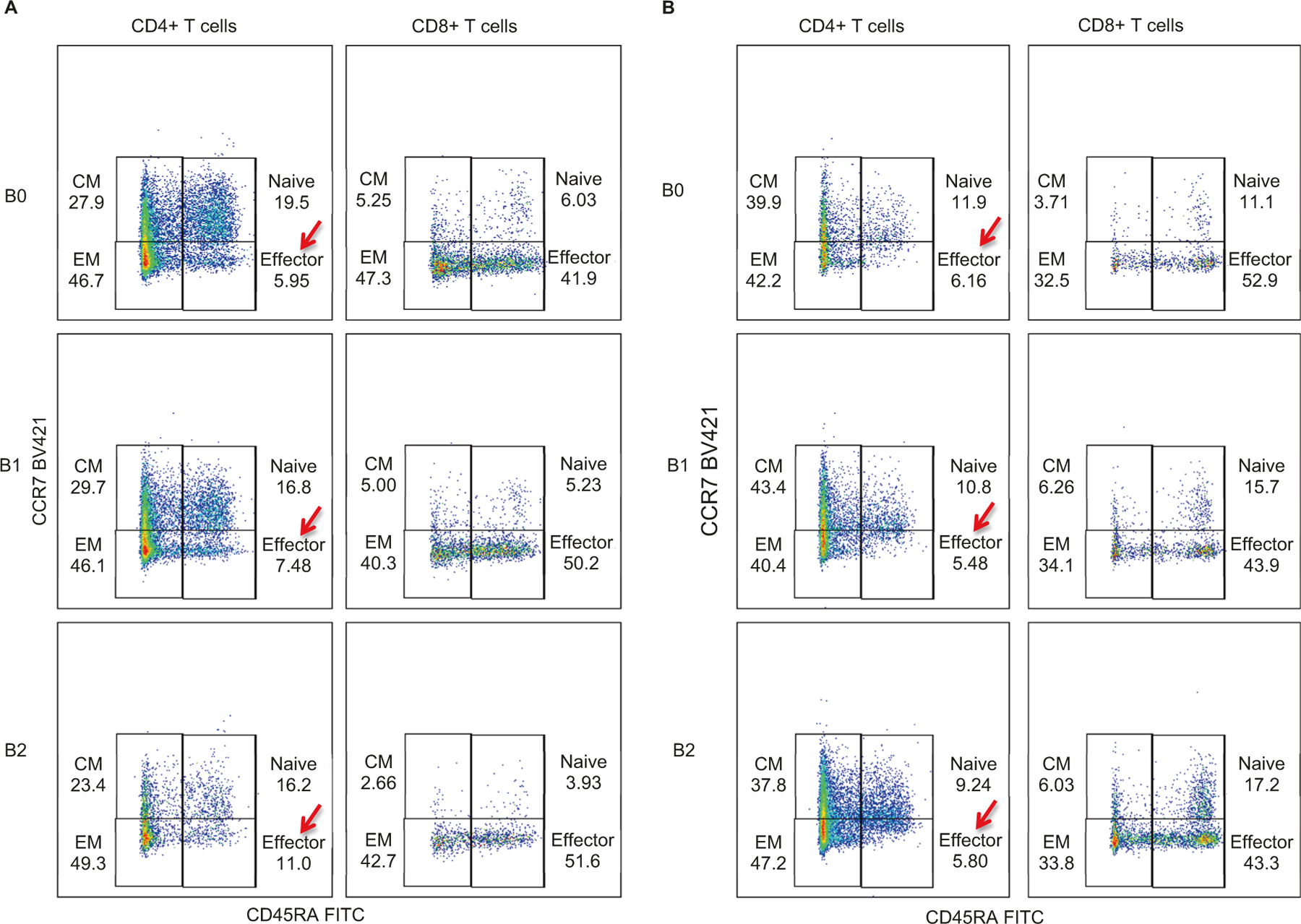
a Quantification of CD4+ and CD8+ T cells in blood samples of early responder patient PT-01 at different stages: B0 (Baseline blood), B1 (Blood collected after two cycles of nivolumab, and prior to HDR#1) and B2 (Specimen collected after 4 cycles of nivolumab and four weeks after HDR#1, but prior to HDR#2). b Quantification of CD4+ and CD8+ T cells in blood samples of late responder patient PT-03.
Discussion
The combination therapy of nivolumab administered concurrently with HDR and ADT, followed by standard EBRT in patients with GG5 PCa was well tolerated. While the combination of HDR and EBRT is associated with higher risk of proctitis [25] and anti-PD-1 immunotherapy is associated with irAEs such as colitis [26, 27], these adverse events appear to be less frequent and the toxicities are milder in PCa patients treated with the combination of ADT, nivolumab, and HDR/EBRT. Among six patients treated with this combination, one patient experienced grade 3 autoimmune-mediated ocular myasthenia gravis and grade 3 protocol-defined DLT (elevated AST and ALT). No other grade 3 gastrointestinal and genitourinary adverse events were observed. There were no grade 4 or 5 adverse events in the Phase I portion of this trial. A 16% (1/6) toxicity rate of ≥grade 3 is consistent with reports from other studies with single-agent PD-1 therapy [5, 6]. These findings suggested that the combination of ADT, anti-PD-1, HDR and EBRT is a safe and feasible approach in treating GG5 PCa. The Phase II portion of the study is actively enrolling patients as planned, per protocol.
Immunotherapy with ICIs has demonstrated durable responses and improved overall survival in multiple disease sites [28]. Immunologically hot tumors with significant immune cell infiltrate, elevated PD-L1, and high tumor mutation burden or neoantigen tend to have a better response to immunotherapy [29]. However, PCa has been shown to be a “cold” tumor with minimal immune cell infiltrates [30]. The clinical findings in multiple prospective trials using ICIs in metastatic PCa have been less prominent [31–33]. To enhance response or overcome resistance to immunotherapy, strategies using ICIs in combination with other immune-activating approaches, such as radiation and cancer vaccines, are actively being explored [34]. Our study utilizing the combination of ADT, anti-PD-1, and HDR may potentially offer a novel and personalized treatment approach in the management of high risk PCa.
Understanding the prostate tumor microenvironment and the interaction of ADT, ICIs, and RT (HDR and/or EBRT) will help to develop novel therapeutic strategies and select patients who may benefit from this treatment. While this Phase I study was not designed to evaluate the efficacy, there was preliminary evidence of potentially synergic antitumor activity with this combination, with a great biochemical response with PSA nadir in 1 to 3 months post treatment, which is much faster than historically reported median time to nadir of 6 months (range = 0.93–108 months) [35]. In addition, as we know ADT will drive the PSA down. However, the temporary PSA bounce after nivolumab administration for some patients is quite intriguing. Since prostate inflammation is known to induce PSA production, we speculate that the temporarily rising PSA might be secondary to underlying inflammatory immune response induced in the tumor microenvironment by nivolumab. That being said, the prostate might have been primed to a “hot” immune microenvironment. Indeed, we did observe an increase of infiltrate of immune cells in serial biopsy samples. Certainly, one of the limitations for this study is that there is no control arm (ADT+ HDR + EBRT alone) to directly attribute the early treatment responses to the effect of nivolumab. However, given that this is phase I proof-of-concept study, this question will be addressed in our future expanded clinical trial.
Further, significant tumor regression from serial biopsy findings was observed in three patients. The observed antitumor activity of this combination therapy is most likely developed through direct tumoricidal effects from HDR and enhanced by the combination of ADT and nivolumab since strong immune infiltrates were observed in the tissues as demonstrated from the serial coordinated biopsy samples. In the early responders, increasing CD4+ effector T cells in peripheral blood were also observed. Analysis of the serial biopsies and peripheral blood may provide additional insights into specific antitumor mechanisms resulting from PD-1 blockade. While this kind of analysis needs to be performed on a larger number of patients, in light of the change of immune cells in tumor samples, it is interesting to speculate that the combination of ADT, RT and ICI may cause a redistribution of immune cells from the blood into tumor and tissue sites. These findings provide novel insights into PCa tumor microenvironment in the setting of pre- and post-treatment with the combination of ADT, anti-PD-1, and HDR and their synergic antitumor mechanisms. This will be further assessed in a larger cohort of patients from the ongoing Phase II study.
We observed increased PD-L1 expression and immune infiltrates in both early and late responders in the serial biopsy samples. However, this may not fully explain the immune response in the PCa microenvironment. Recently, Verma et al. [36] found that patients with poor response to anti-PD-1 therapy had elevated dysfunctional PD-1+CD38hi CD8+ T cells in the tumor microenvironment, compared to responders. PD-1+CD38hi CD8+ T cells could be a biomarker for early response to immunotherapy. Further analysis of this subset of dysfunctional CD8+ T cells in PCa will be performed in the Phase II portion of this trial.
There are some limitations in this study, including a small sample size, potential sampling variation in the sequential biopsies, and the lack of a control group without nivolumab. Given these limitations, this phase I study is designed to report on safety for the use of nivolumab in men with very high-risk PCa as well as provides a novel proof-of-principle approach for combining nivolumab with HDR with serial biopsies. The findings on efficacy will be further validated in the phase II study. Despite these limitations, the safety and toxicity profile in this Phase I study is encouraging. As suggested by our preliminary analysis, the combination of ADT, anti-PD-1, and HDR demonstrated synergic immune activation and antitumor activity. This Phase I/II trial targeting GG5 PCa with a novel combination regimen may provide more insights into patient selection and development of new therapeutic approaches. In the phase II study, we will further assess acute and long-term toxicity, time to PSA nadir, two-year biochemical relapse free survival, patient-reported outcomes including gastrointestinal and genitourinary symptoms, molecular characterization of tumor mutation burden and microsatellite instability, immune profiling and identification of potential blood biomarkers related with immune response.
Supplementary Material
Acknowledgements
The authors acknowledge Moffitt Cancer Center CLIA Tissue Imaging Lab for Multiplex Immune Panel Procedure and Quantitative Image Analysis.
Funded by: Bristol Myers Squibb
Role of the funding source:
Bristol Myers Squibb (BMS) had no role in the study design, data collection, analysis, and interpretation, or writing of the study.
Footnotes
Clinical trial: This Phase I/II trial is registered with ClinicalTrials.gov (NCT03543189).
Supplementary information The online version of this article (https://doi.org/10.1038/s41391–020-0254-y) contains supplementary material, which is available to authorized users.
Conflict of interest J.Z. reports honorarium from AstraZeneca, Merck, Sanofi and Bayer for speaker programs and advisory boards. R.J. serves as an advisor for Pfizer and a speaker for Dava Oncology. R.L. is on Clinical trial protocol committee for Cold Genesys and BMS, and serves as a scientific advisor/consultant for BMS and Ferring. S.K. reports research funds from BMS and Astra Zeneca. The remaining authors declare that they have no conflict of interest.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl J Med 2017;376:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016;7: 52810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 2008;299:289–95. [DOI] [PubMed] [Google Scholar]
- 8.Denham JW, Steigler A, Lamb DS, Joseph D, Turner S, Matthews J, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 2011;12:451–9. [DOI] [PubMed] [Google Scholar]
- 9.D’Amico AV. Is Gleason grade 5 prostate cancer resistant to conventional androgen deprivation therapy? Eur Urol 2016;69:761–3. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 2004;64:4328–37. [DOI] [PubMed] [Google Scholar]
- 11.Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys 2016;95:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gameiro SR, Ardiani A, Kwilas A, Hodge JW. Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. Oncoimmunology 2014;3:e28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RB, Baniel CC, Sriramaneni RN, Bradley K, Markovina S, Morris ZS. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities. Brachytherapy 2018;17:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Therapeutic Adv Med Oncol 2018;10:1758834017742575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 2009;69:571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, et al. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol 2004;173:6098–108. [DOI] [PubMed] [Google Scholar]
- 18.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci: a J virtual Libr 2007;12:4957–71. [DOI] [PubMed] [Google Scholar]
- 19.Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology 1999;53:260–6. [DOI] [PubMed] [Google Scholar]
- 20.George DJ, Nabhan C, DeVries T, Whitmore JB, Gomella LG. Survival outcomes of sipuleucel-T phase III studies: impact of control-arm cross-over to salvage immunotherapy. Cancer Immunol Res 2015;3:1063–9. [DOI] [PubMed] [Google Scholar]
- 21.Small EJ, Lance RS, Gardner TA, Karsh LI, Fong L, McCoy C, et al. A randomized phase II trial of sipuleucel-T with concurrent versus sequential abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer. Clin Cancer Res 2015;21:3862–9. [DOI] [PubMed] [Google Scholar]
- 22.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA 2001;98:14565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol 2016;69:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabid A, Carrier N, Martin AG, Bahary JP, Lemaire C, Vass S, et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized phase III trial. Eur Urol 2018;74:432–41. [DOI] [PubMed] [Google Scholar]
- 25.Matta R, Chapple CR, Fisch M, Heidenreich A, Herschorn S, Kodama RT, et al. Pelvic complications after prostate cancer radiation therapy and their management: an international collaborative narrative review. Eur Urol 2019;75:464–76. [DOI] [PubMed] [Google Scholar]
- 26.Som A, Mandaliya R, Alsaadi D, Farshidpour M, Charabaty A, Malhotra N, et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases 2019;7:405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer 2018;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology 2014;28:39–48. [PubMed] [Google Scholar]
- 29.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: facts and hopes. Clin Cancer Res 2017;23:6764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol: Off J Am Soc Clin Oncol 2017;35:40–7. [DOI] [PubMed] [Google Scholar]
- 32.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 Study. J Clin Oncol 2019;38:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorbet MJ, Ranjan A. Cancer immunotherapy with immunoadjuvants, nanoparticles, and checkpoint inhibitors: recent progress and challenges in treatment and tracking response to immunotherapy. Pharmacol Ther 2019;207:107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geara FB, Bulbul M, Khauli RB, Andraos TY, Abboud M, Al Mousa A, et al. Nadir PSA is a strong predictor of treatment outcome in intermediate and high risk localized prostate cancer patients treated by definitive external beam radiotherapy and androgen deprivation. Radiat Oncol 2017;12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma V, Shrimali RK, Ahmad S, Dai W, Wang H, Lu S, et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1(+)CD38(hi) cells and anti-PD-1 resistance. Nat Immunol 2019;20:1231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


