Abstract
Introduction
The Dutch Brain Research Registry aims to facilitate online recruitment of participants for brain disease studies.
Methods
Registrants were primarily recruited through an online social media campaign. The registration process included a short questionnaire, which was subsequently used in the prescreening process to match participants to studies.
Results
In the first 18 months, 17,218 registrants signed up (58±11 years old, 78% female). Out of 34,696 study invitations that were sent, 36% were accepted by registrants, of which 50% to 84% were finally enrolled, resulting in 10,661 participants in 28 studies. Compared to non‐participants, study participants were more often older, male, more highly educated, retired or unemployed, non‐smoking, healthier, and more often had a family member with dementia.
Discussion
The Dutch Brain Research Registry facilitates effective matching of participants to brain disease studies. Participant factors related to study enrollment may reflect facilitators or barriers for participation, which is useful for improving recruitment strategies.
Keywords: Alzheimer's disease, clinical trials, dementia, preclinical Alzheimer's disease, pre‐screening, recruitment, register, registry, secondary prevention trials
1. BACKGROUND
Alzheimer's disease (AD) and other dementias are a major threat to the health and well‐being of our aging population. 1 The number of people suffering from dementia worldwide is currently estimated at 36 million and this number is expected to triple by 2050. 2 Without curative or disease‐modifying drugs currently available, major efforts are put into clinical studies including drug trials. However, difficulty in participant recruitment is a significant barrier for drug development. 3 There is a major and increasing mismatch between the limited number of participants available and the high number of subjects required for clinical studies. This mismatch has resulted in prolonged recruitment periods, insufficient participant numbers, underpowered studies, and excessive costs. 4 In addition, specialized clinical facilities see patients in more advanced disease stages, while prodromal and preclinical disease phases are increasingly the focus of clinical trials and because this is a very narrow population, larger sample sizes are likely to be needed. Therefore, alternative recruitment strategies are needed to meet the demands for recruitment of participants for current and future clinical studies.
One approach to address the urgent need for participants in clinical studies is a registry of potential participants who are interested in contributing to research. In the Netherlands, we set up a nationwide, online registry for recruitment of participants for a broad spectrum of brain disease studies with the aim to accelerate the search for solutions to brain diseases such as AD (Dutch Brain Research Registry, or Hersenonderzoek.nl in Dutch). Here, we present the first results of our Dutch Brain Research Registry on participant recruitment and enrollment in studies. Additionally, our aim was to investigate which demographic, social, and health‐related factors were related to study participation.
2. METHODS
The Medical Ethics Review Committee of the VU University Medical Center reviewed the study and provided a waiver for ethical approval. Launch of the registry was on September 21, 2017 (World Alzheimer's Day). Data presented in the current study was collected from September 2017 (launch) until March 2019.
2.1. Registry structure
The primary structure of our registry includes (1) the project website (www.hersenonderzoek.nl), (2) management software, and (3) database. The website provides visitors the opportunity to sign up or log in to their personal portal for registered participants. Furthermore, it provides information for a lay audience on currently recruiting studies, study results, information on participation and instructions, and information about the registry for potential investigators and (inter)national colleagues (https://hersenonderzoek.nl/for-researchers/). The management software was a Software as a Service (SaaS) solution provided by the Brain Health Registry (BHR) of the University of California San Francisco, which facilitates participant registration, matching of participants with studies, invitation of eligible participants, and online portals for participants and investigator, as described in more detail by Weiner et al. 5 The software and database were securely stored on a Microsoft Azure server located in the Netherlands, with a back‐up in Ireland. BHR was responsible for the functional and technical management of the SaaS through a service level agreement. The executive team of Hersenonderzoek.nl consists of a project manager, community manager, and database manager.
RESEARCH IN CONTEXT
Systematic review: Participant recruitment registries are a promising response to the crisis in study recruitment. To date, several registries have emerged globally aiming to accelerate study participant recruitment with differences including geographical area, size, focus, and population.
Interpretation: We demonstrated the feasibility to recruit study participants via an online registry for a variety of studies with a high enrollment rate. In addition, several participant factors were related to study enrollment, which may be a reflection of facilitators and barriers for participation.
Future directions: Comparison of results from different recruitment registries provides better understanding of the effectiveness of registries to accelerate participant recruitment and are useful for improving recruitment strategies.
2.2. Use of the registry by registrants and investigators: user journeys
End users of the registry are registrants and investigators. User journeys of registrants and investigators in the Dutch Brain Research Registry are shown in Figure 1 and described in seven steps, with steps for registrants only (1–3), investigators only (4–6), and shared steps (7–8).
FIGURE 1.
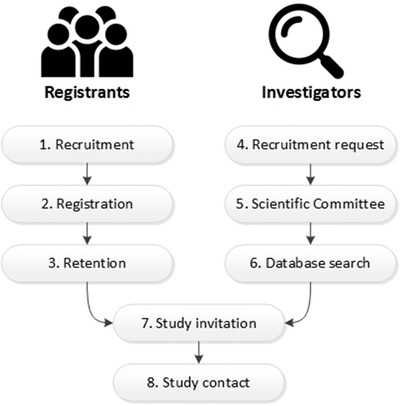
Overview of the registry journeys for registrants (left) and investigators (right)
2.2.1. Registrant journey
Step 1: Recruitment. In collaboration with an (online) content marketing agency we developed a recruitment campaign through Facebook targeting people living in the Netherlands ages 50 to 70 years. The campaign consisted of advertisements with facts about AD, brain quizzes and puzzles, and short informative videos called “one‐minute academies” (see Figure 2 and www.facebook.com/hersenonderzoek.nl). In the week of the launch, we published two press releases in collaboration with a communications agency, which were also disseminated by Alzheimer Nederland (Dutch Alzheimer's Society) and Hersenstichting (Dutch Brain Foundation). During the week of the official launch on September 21, 2017, we exhibited at a senior fair (50PlusFair) and distributed informative participant brochures.
FIGURE 2.
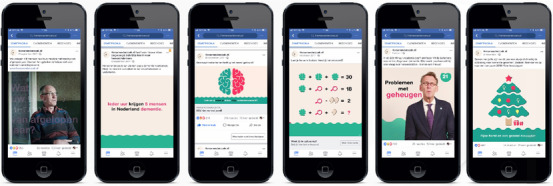
Facebook advertisement campaign for participant recruitment, including (from left to right) awareness video, brain fact pinned posts, brain quizzes, puzzles, one‐minute academy videos, and weekly free posts
Step 2: Registration. Dutch‐speaking persons ages 18 years and older can sign up through our dedicated registry website (www.hersenonderzoek.nl). Participants give online informed consent to be invited for neuroscience studies. The registration process consists of an electronic informed consent procedure (as described by Weiner et al. 5 ) and a short (10‐minute) registration questionnaire to collect contact and medical information, including geographical information, presence of memory complaints, medical history, medication and substance use (see Appendix). After registration, registrants alternate between step 3 (Retention) and step 6 (Study invitation)
Step 3: Retention. Our retention strategy aims to keep registrants actively engaged with the registry and motivated for upcoming studies. We keep registrants informed on (inter)national neuroscience news, new upcoming studies, and results of studies through a monthly newsletter, website, our Facebook page, and communication channels of our partner organizations. In addition, we actively search for (online) studies that can invite a substantial number of participants, if not the entire registry, so that we can send out invitations for studies to each registrant at least once a year.
2.2.2. Investigator journey
Step 4: Recruitment request. Investigators can apply for participant recruitment by e‐mail. The Dutch Brain Research Registry team requests a copy of the institutional review board approval letter and a data application form, which provides a description of the desired participants for that particular study.
Step 5: Scientific Committee. An independent Scientific Committee assesses whether the data application aligns with the goals and characteristics of the registrant cohort of the Dutch Brain Research Registry. When the data request is approved, the investigator signs a Data Use Agreement to ensure appropriate use of the provided participant data.
Step 6: Database search. Our database manager performs the search for eligible registrants by matching participant registration information with study in‐ and exclusion criteria.
2.2.3. Registrant and investigator journey
Step 7: Study invitation. Study invitations are sent to eligible registrants by e‐mail (participant status “invited,” see Table 2). They can reply (participant status “responded”) by using dedicated buttons in the e‐mail to accept or decline the invitation. When accepted, participant status is changed to “interested.” If no reply is received within 1 week, an automatic reminder e‐mail is sent.
TABLE 2.
Overview of referrals to studies
| Design | Study description | Invited | Responded | Interested | Enrolled |
|---|---|---|---|---|---|
| Online (n=9) | Survey on knowledge and attitudes toward dementia risk reduction in CN (Lifestyle) | 10,276 | 4388 | 3466 | 3466 |
| Online test battery on daily functioning and cognition in cognitively normal population (Think&Do) | 8380 | 8359 | 4386 | 3204 | |
| Effect of age and sex on navigation skills (Navigation) | 5839 | 2665 | 2159 | 2159 | |
| Screening for music‐based intervention (Music) | 2613 | 1344 | 1002 | 1002 | |
| Validity of online social behavior application in CN, SCD, and AD (BeHapp) | 2828 | 1479 | 443 | 317 | |
| Information provision regarding diagnostic testing in AD patient caregivers (ABIDE‐Delphi) 15 | 977 | 617 | 141 | 31 | |
| RCT on best‐practice advice for PET disclosure (ABIDE Simulation) | 701 | 319 | 231 | 231 | |
| Study on feasibility of application‐based lifestyle intervention strategy in SCD (Euro‐SCD) | 77 | 64 | 48 | 36 | |
| Study on validity of application‐based lifestyle intervention strategy in SCD (HelloBrain) | 135 | 82 | 63 | 55 | |
| Subtotal | 31,826 | 19,317 (61%) | 11,939 (38%) | 10,501 (33%) | |
| Site visit, observational (n=14) | Multicenter, pan‐European, longitudinal cohort study in adults without dementia (EPAD) 16 | 663 | 405 | 234 | 104 |
| Multicenter, pan‐European study on patient engagement strategies in non‐demented elderly (MOPEAD) 17 | 362 | 225 | 145 | 107 | |
| Study on predictors of music‐cued precision in CN (Moving to music) | 248 | 91 | 51 | 33 | |
| Study on cognitive correlates of connectivity in CN (MuMoBrain) | 213 | 90 | 36 | 17 | |
| Study on movement and cognition in CN (MIB) | 212 | 108 | 45 | 19 | |
| Multicenter study on the biological construct of social withdrawal in AD and schizophrenia (PRISM) 18 | 188 | 86 | 21 | 10 | |
| Longitudinal cohort study in adults with SCD (SCIENCE) 19 | 153 | 92 | 46 | 31 | |
| Study on insomnia and affect in isomnia patients and CN (Sleep) | 111 | 48 | 9 | 1 | |
| Study on brain networks and tau pathology in CN, SCD, MCI, and AD (MANTA) | 44 | 15 | 13 | 6 | |
| MRI study in OCD patients and CN (OCD) | 20 | 6 | 5 | 3 | |
| Study on development of a cognitive measure for progression in SCD, MCI, and dementia (CatchCog) 20 | 19 | 13 | 8 | 0 | |
| Remote Assessment of Disease and Relapse in MDD (RADAR‐CNS) 21 | 15 | 8 | 5 | 1 | |
| Effect of light on emotional processing in PD, MDD, and CN (Light) | 11 | 6 | 6 | 0 | |
| Study on resilience to dementia in elderly aged ≥90 (EMIF AD 90+) 22 | 10 | 6 | 5 | 1 | |
| Subtotal | 2269 | 1199 (53%) | 583 (26%) | 333 (15%) | |
| Intervention (n=5) | RCT of exercise intervention in vascular cognitive disorders (Excersion‐VCI) 23 | 170 | 98 | 46 | 18 |
| RCT of treatment in anxiety‐ or MDD patients (MOTAR) 24 | 167 | 96 | 15 | 8 | |
| Study on effectiveness navigation rehabilitation training in people with acquired brain injury and CN (Wayfinder) | 156 | 90 | 24 | 3 | |
| RCT of exercise intervention in CN (Move) | 91 | 62 | 35 | 26 | |
| RCT of cognitive training in PD (Cogtips) 25 | 17 | 14 | 10 | 3 | |
| Subtotal | 601 | 360 (60%) | 115 (19%) | 58 (10%) | |
| Total | 34696 | 20876 (60%) | 12637 (36%) | 10661 (31%) |
Note: Data are presented as n or n (%).
Abbreviations: AD, Alzheimer's disease; CN, cognitively normal adults; MCI, mild cognitive impairment; MDD; major depression disorder; PD, Parkinson's disease; SCD, subjective cognitive decline; RCT, randomized controlled trial.
Step 8: Study contact. Contact information of interested registrants is securely provided to the investigator though the investigator portal. The investigator contacts interested registrants by phone or e‐mail for further prescreening and finally enrollment (participant status “enrolled”).
2.3. Statistical analysis
To investigate whether demographic, social, and health‐related factors are related to study participation, we compared the registrant profiles of study participants to those who were invited but did not participate (ie, declined the study invitation or due to prescreen failure reasons). Nineteen demographic and medical variables (corresponding to the registration questionnaire, see Appendix) were used as independent variables and “enrolled in study” as the dependent variable. Analyses were performed for each study separately and restricted to those studies with sufficient numbers of events (enrolled participants), we took as a rule of thumb that at least 10 participants for each variable should be available. As a result, we analyzed five studies with more than 190 enrolled participants.
First, to find the optimal set of predictors for enrollment in each study, we performed multivariable logistic regression analyses with backward stepwise selection and a P value greater than 0.10 for removal of variables (Model 1). Dichotomized independent factors include age (based on median as ≤58 years or >58 years); sex; education (low vs. high 6 ); self‐rated health (good to excellent vs. moderate to poor); employment (employed vs. retired/unemployed); first‐degree family member with mild cognitive impairment (MCI) or dementia; subjective cognitive decline 7 (positive answer to the question “Do you have memory problems?”); medication use; smoking; and self‐reported (history of) hypertension, high cholesterol, diabetes, heart disease, stroke, cancer, psychiatric disease, neurological disease, and specifically MCI or dementia.
Next, factors that were significant determinants in three or more studies (P < .010) were identified as consistent predictors. To compare the odds ratios (ORs) of these consistent predictors between studies, factors were entered simultaneously in a multivariable logistic regression model for each study (Model 2). Logistic regression analyses were performed in IBM SPSS Statistics version 24.
3. RESULTS
3.1. Participant recruitment
Publication of two press releases at the registry launch date resulted in two articles in national magazines, 11 articles in online media, two radio interviews (local and national), and three publications in newsletters from project partners (patient organizations and a health‐care insurance company). In the week of the launch, we were present with an exhibition stand at the “50Plus Beurs,” a fair aimed at people ages 50 years and older, which attracted a total of 105,000 visitors. Our Facebook recruitment campaign ran from September 21, 2017 until March 2018 and had a total reach of more than 1,200,000 unique people. This resulted in 160,000 clicks to our landing page (www.hersenonderzoek.nl) and more than 10,000 successful registrations. A short follow‐up advertisement campaign from January until February 2019 had a total reach of 97,000 unique people, resulting in 27,500 clicks and 5300 registrations. Details on the advertisement campaign can be found at www.facebook.com/hersenonderzoek.nl. In the first year, our landing page was visited 322,000 times, with more than 209,000 unique visitors. The majority of them were directly referred via Facebook advertisement (>70%).
3.2. Registrant characteristics
From September 2017 until March 2019, 17,218 people registered. They were 18 to 90 years of age, and 72% were female (Table 1). A total of 4805 (28%) reported subjective cognitive decline and 433 (3%) had a formal diagnosis of MCI or dementia. A first‐degree family history of MCI or dementia was present in 32% of registrants. History of cardiovascular, neurological, and psychiatric disease was reported by, respectively, 12%, 5%, and 21% of participants. Over the period of 18 months, 79 (0.005%) participants withdrew their consent.
TABLE 1.
Demographics of Hersenonderzoek.nl cohort
| Total | 17,218 |
| Age (years) | 58 ± 11 |
| Female | 13,353 (78%) |
| Education (range 1–7) a | 4.7 ± 0.9 |
| Employed | |
| Full‐ or part‐time | 9238 (54%) |
| Not working or retired, other | 6846 (46%) |
| Self‐rated health (range 1–5) | |
| Good to excellent | 13,422 (84%) |
| Moderate to poor | 2716 (16%) |
| First‐degree family member with MCI or dementia | 5005 (29%) |
| Smoking | 2016 (13%) |
| Marital status | |
| Married or cohabiting | 10,507 (61%) |
| Single, divorced, or widow(er), other | 5622 (30%) |
| Subjective cognitive decline | 4805 (28%) |
| Medical history | |
| Hypertension | 4469 (26%) |
| High cholesterol | 3269 (19%) |
| Diabetes | 1024 (6%) |
| Stroke | 731 (4%) |
| Cancer | 1444 (8%) |
| Cardiovascular disease | 1664 (10%) |
| Psychiatric disease | 3544 (21%) |
| Neurological disease | 822 (5%) |
| Diagnosis MCI or dementia | 433 (3%) |
| Medication use | 8768 (51%) |
Notes: Data are presented as n (%) or mean±SD. aAccording to Verhage, 6 ranging from 1 to 7 (low to highly educated).
Abbreviations: MCI, mild cognitive impairment; SD, standard deviation.
3.3. Study invitation and participation
The Dutch Brain Research Registry was used by investigators from several cities throughout the Netherlands, including Groningen (University of Groningen, University medical center Groningen), Leiden (Leiden University), Utrecht (Utrecht University), and Amsterdam (VU University, VU University medical center, Amsterdam UMC, Netherlands Institute for Neuroscience). We sent out invitations for a total of 28 studies, including 9 online observational studies, 14 observational studies with visits at the study site, and 5 non‐pharmacological intervention studies (see Table 3).
TABLE 3.
Backward stepwise selection results of multivariate logistic regression models for the association between participant characteristics and study participation (Model 1). The first seven predictors were significant in three or more studies (P < 0.1) and therefore identified as consistent predictors that were used for further analyses (Model 2, Figure 3)
| Lifestyle | Think&Do | Navigation | Music | BeHapp | |
|---|---|---|---|---|---|
| Older age | 1.37 (1.23–1.54)* | 1.70 (1.51–1.92)* | 1.15 (1.00–1.33) † | 1.46 (1.18–1.81)* | – |
| Male | – | – | 1.18 (1.03–1.35)* | 1.22 (1.02–1.46)* | 1.33 (1.03–1.72)* |
| Higher educated | 1.34 (1.21–1.48)* | 1.36 (1.22–1.51)* | 1.17 (1.03–1.33)* | 1.36 (1.13–1.64)* | – |
| Retired or not working | 1.17 (1.0–1.31)* | 1.21 (1.07–1.37)* | 1.15 (0.99–1.33) † | – | – |
| Better self‐rated health | 1.30 (1.12–1.50)* | 1.24 (1.06–1.44)* | 1.24 (1.03–1.50)* | 1.32 (1.00–1.72)* | 1.61 (0.97–2.69) † |
| First‐degree family member with dementia | 1.17 (1.06–1.29)* | 1.23 (1.11–1.36)* | 1.26 (1.10–1.45)* | 1.23 (1.02–1.47)* | – |
| Not smoking | 1.63 (1.40–1.90)* | 1.69 (1.43–2.00)* | 1.49 (1.23–1.79)* | 1.70 (1.23–2.36)* | 1.75 (1.05–2.91)* |
| Married or cohabiting | 1.14 (1.03–1.27)* | – | – | – | – |
| Subjective cognitive decline | – | – | 0.78 (0.67–0.91)* | – | – |
| Hypertension | – | 0.89 (0.79–1.00)* | – | – | – |
| High cholesterol | – | 1.13 (1.00–1.29) † | – | – | – |
| Diabetes | 0.79 (0.65–0.97)* | 0.80 (0.64–0.99)* | – | – | – |
| Heart disease | – | – | – | – | – |
| Stroke | 1.28 (1.01–1.61)* | – | – | – | – |
| Cancer | – | – | – | 1.30 (0.98–1.74) † | – |
| Neurological disease | – | – | – | – | n.a. |
| Diagnosis of MCI or dementia | 0.74 (0.52–1.06) † | n.a. | 1.35 (0.97–1.89) † | – | n.a. |
| Psychiatric disease | 1.36 (1.21–1.54)* | – | – | – | n.a. |
| Medication use | – | – | – | – | – |
Notes: n.a.; Participant characteristic variable was used as exclusion criteria for the particular study and therefore no data was available.
P < 0.05;.
P < 0.1.
Abbreviation: MCI, mild cognitive impairment.
All registrants received at least one invitation for a study; 26% received two invitations, 32% received three invitations, 24% received four invitations, and 13% received > 4 invitations. Out of 34,696 sent invitations, we received 20,876 (60%) responses, of which 12637 (36%) were positive (ie, accepted the invitation). Registrants expressed highest interest for online studies (38% interested), followed by observational studies with site visits (26%) and lowest for intervention studies (19%).
Of all accepted invitations, 10,661 (84%) resulted in a study enrollment (representing n = 4359 unique study participants). When we compared the number of accepted study invitations to the number of study enrollments, we found highest enrollment rates for online studies (86%), followed by observational studies with site visits (62%), and intervention studies (50%), the latter often due to additional prescreening by the investigator after invitation.
Out of the 28 studies serviced, nine studies (32%) recruited n=1–10 study participants, nine studies (32%) recruited n=11–100 study participants, four studies (14%) recruited n=100–189 study participants, and five studies (18%) recruited >190 study participants. For two studies (4%), we were not able to recruit participants as we could not find eligible participants (Parkinson's disease patients not using medication) or due to recruitment time restrictions.
3.4. Study participant characteristics
To investigate whether demographic, social, and health‐related factors are related to participation in studies, we compared characteristics of study participants (participants with status “enrolled”) to non‐participants (participants who were invited but did not accept the study invitation) in five studies (Lifestyle, Think&Do, Navigation, Music, and BeHapp; see Table 2 and Figure 3) that met criteria for analyses.
FIGURE 3.
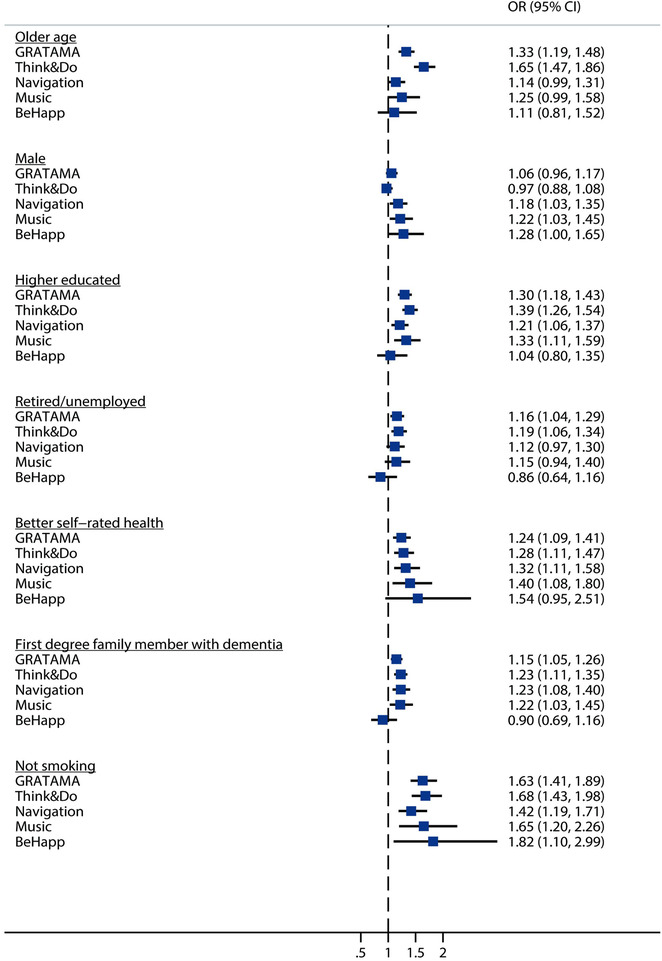
Odds ratios of consistent participant factors (as determined by Model 1) for study participation as simultaneously analyzed per study in multivariate logistic regression analyses (Model 2). Results are visualized per factor. Participants may be enrolled in more than one study. CI, confidence interval; OR, odds ratio
First, multivariate logistic regression analyses with backward stepwise selection for the association between participant factors and study participation (Model 1, Table 3) identified seven determinants that were significant predictors in three or more studies. These consistent predictors for study participation were age, sex, education, employment, smoking, self‐reported health, and presence of first‐degree family member with dementia.
Next, simultaneous analyses of these seven consistent factors (as determined in Model 1) in multivariate logistic regression analyses (Model 2) showed that over studies, study participants were older (ORs ranged from 1.11–1.33), more often male (ORs = 0.97–1.28), more highly educated (ORs = 1.04–1.39), more often retired or unemployed (ORs = 0.86–1.19), not smoking (ORs = 1.42–1.82), reporting better health (ORs = 1.24–1.54), and having a first‐degree family member with dementia (ORs = 0.90–1.23) compared to those who were not enrolled in studies (Figure 3).
4. DISCUSSION
The Dutch Brain Research Registry is now an established online, nationwide registry of individuals interested in participating in neuroscience studies in the Netherlands. Launch of the Dutch Brain Research Registry in the Netherlands provided proof of concept for the feasibility of an online platform for participant recruitment. Our findings demonstrate that an online registry is an effective and efficient way to recruit study participants for a variety of neuroscience studies, including observational as well as intervention studies, with a high enrollment rate. Older age, male sex, higher education, not working or retired, not smoking, better health, and presence of a first‐degree family member with dementia were consistent predictors for study participation.
In its first year, the Dutch Brain Research Registry primarily focused on recruitment of people to build up the database. By far the most effective recruitment strategy was the Facebook advertisement campaign, which was targeted at people above the age of 50 years. Registrants frequently report a first‐degree family member with MCI or dementia (32%) and relatively often experience cognitive decline (29%), but rarely report a formal diagnosis of dementia (3%). These findings confirm that our registry is very suitable for studies searching for middle‐to‐late age cognitively normal elderly.
The high efficiency of our recruitment campaign shows the great interest in brain research in the general population and underlines that our registry is an effective approach to involve the general population in clinical brain disease studies.
In addition to requests for recruitment of cognitively normal adults, we also received several requests for recruitment of participants with cognitive impairment or a brain disease diagnosis; often patients with prodromal AD or mild AD dementia, but also Parkinson's disease, obsessive‐compulsive disorder, schizophrenia, and depression. To date, these people were less effectively targeted by our general campaign. Therefore, we will shift focus toward recruitment of (prodromal) AD patients using content marketing, including search engine optimization (SEO) and search engine advertising (SEA), and through care professionals in memory clinics.
Our findings indicate that older age, male sex, higher education, and not working (59% were retired) were consistent predictors for study participation. In line with earlier studies, males more often participated than females. 8 , 9 Furthermore, those who participated were less likely to be a current smoker and had better self‐rated health, 9 which may be a reflection of their interest in health‐related research. We also found that study participants more often had a family member with MCI or dementia. Having a relative with a brain disorder may be another intrinsic motivation for participation. 8 , 10 , 11 At the same time, this characteristic is more often associated with pre‐clinical disease, and therefore often used as prescreening criteria for studies. These findings are useful for improving recruitment strategies targeting these participant populations.
It is important to note that the current findings are limited to participation in (five) online studies. It can be expected that motivations for participation may be different for other types of studies, or studies—including intervention studies and clinical trials in particular—of other health‐related topics. More insight in participant characteristics and motivations may help to improve recruitment strategies and participation experience, which may eventually contribute to faster and more efficient participant recruitment. In 2019, a global collaborative of the Dutch Brain Research Registry together with six other digital participant recruitment platforms for dementia research (TrialMatch, Alzheimer's Prevention Registry, GeneMatch, 12 and Brain Health Registry 5 [USA]; Join Dementia Research [UK]; and StepUp for Dementia Research [Australia]) was established to generate new insights and evidence that can help further enhance participant recruitment and potentially support other similar global initiatives. This collaboration also provides the opportunity to investigate predictors for study enrollment in pooled analyses.
A major facilitator for the success of our registry was the use of the SaaS solution for online management of our registry provided by the BHR, which provided a jump start to the set‐up and launch of the Dutch Brain Research Registry. This SaaS solution, called Ebisu, was developed by the IT specialists of the BHR team and is currently used by BHR. 5 Second, a major factor contributing to the success of our recruitment campaign is the high internet accessibility (mobile and/or at home) in the Netherlands. Overall, 98% of the population ages 16 to 65 years and 84% of the those ages 65 to 75 years have internet access in the Netherlands. Furthermore, Dutch elderly have become increasingly active on social media and Facebook in particular, with 77% of those ages 40 to 64 years and 67% of those between 65 and 79 years. 13 The use of this medium potentially provides access to a more diverse, heterogeneous group of people with multiple/intersecting identities and characteristics compared to traditional recruitment channels, contributing to a broader inclusion of people from minority and marginalized groups. 14
Currently, ongoing recruitment campaigns ensure a continuous influx of new registrants to meet the demands for the growing number of recruiting studies. Additionally, we run recruitment campaigns focusing on (prodromal) AD patients specifically. Focus groups and online surveys among participants will give a better understanding of participant motivations and experiences to optimize our services. Finally, we are developing a business model for long‐term (financial) sustainability of the platform. This will enable us to effectively and efficiently facilitate participant recruitment for an increasing number of studies with a broader participant population (including patients) and ensure long‐term sustainability of our services.
In conclusion, the Dutch Brain Research Registry facilitates effective matching of potential participants to brain disease studies. Our findings demonstrate the feasibility to recruit participants via an online registry for a large number of studies with a high enrollment rate.
CONFLICTS OF INTEREST
Dr. Weiner receives support for his work from the following funding sources: NIH: 5U19AG024904‐14; 1R01AG053798‐01A1; R01 MH098062; U24 AG057437‐01; 1U2CA060426‐01; 1R01AG058676‐01A1; and 1RF1AG059009‐01, DOD: W81XWH‐15‐2‐0070; 0W81XWH‐12‐2‐0012; W81XWH‐14‐1‐0462; W81XWH‐13‐1‐0259, PCORI: PPRN‐1501‐26817, California Dept. of Public Health: 16‐10054, U. Michigan: 18‐PAF01312, Siemens: 444951‐54249, Biogen: 174552, Hillblom Foundation: 2015‐A‐011‐NET, Alzheimer’s Association: BHR‐16‐459161; The State of California: 18‐109929. He also receives support from Johnson & Johnson, Kevin and Connie Shanahan, GE, VUmc, Australian Catholic University (HBI‐BHR), The Stroke Foundation, Fidelity Charitable, and the Veterans Administration. Dr. Weiner is a full time Professor for the University of California San Francisco (UCSF), and Principal Investigator of many projects with the above grant funding. Dr. Weiner has served on Advisory Boards for Cerecin/Accera, Alzheon, Inc., Nestle/Nestec, PCORI/PPRN, Dolby Family Ventures, National Institute on Aging (NIA), Boston University Alzheimer’s Disease and CTE Center, MIRIADE at VUmc for Amsterdam UMC, Cytox, Indiana University, Acumen, Brain Health Registry and ADNI. He serves on the Editorial Boards for Alzheimer’s & Dementia, TMRI and MRI. He has provided consulting and/or acted as a speaker/lecturer to Cerecin/Accera, Inc., Alzheimer’s Drug Discovery Foundation (ADDF), BioClinica, The Buck Institute for Research on Aging, FUJIFILM‐Toyama Chemical (Japan), Garfield Weston, Baird Equity Capital, University of Southern California (USC), T3D Therapeutics, Cytox, and Japanese Organization for Medical Device Development, Inc. (JOMDD). He holds stock options with Alzheon, Inc., Alzeca, and Anven. D. Prins is consultant to Boehringer Ingelheim and Aribio. He is co‐PI of a study with Fuji Film Toyama Chemical. He serves on the DSMB of Abbvie’s M15‐566 trial. He is CEO and co‐owner of the Brain Research Center, The Netherlands. All other authors report no financial disclosures or conflicts of interest.
ACKNOWLEDGMENTS
Hersenonderzoek.nl is supported by ZonMw‐Memorabel (project no 73305095003; a project in the context of the Dutch Deltaplan Dementie), Gieskes‐Strijbis Foundation, Alzheimer Nederland (Dutch Alzheimer's Society), and Hersenstichting (Dutch Brain Foundation).
Codebook Dutch Brain Research Registry
| Question | Variable | Description | Values |
|---|---|---|---|
| 1 | Name | Surname | [text] |
| 2 | Firstname | First name or initials | [text] |
| 3 | Gender | Gender |
|
| 4 | Birthdate | Date of birth | [date] |
| 5 | Address | Address | [text] |
| 6 | Zipcode | Zipcode | [text] |
| 7 | City | City | [text] |
| 8 | Phone | Telephone number | [text] |
| 9 | E‐mail | E‐mail address | [text] |
| 10 | Signature | Signature (type your name) | [text] |
| 11 | Source | How did you hear about us? |
|
| 12 | Length | What is your height? | [text] cm |
| 13 | Weight | How much do you weigh? | [text] kg |
| 14 | Education |
What is your highest level of education? Comparable to…. |
|
| 15 | Employment | Are you employed (paid labor)? |
|
| 16 | Marital status | What is your marital status? |
|
| 17 | Housing | In what kind of residence do you live? |
|
| 18 | Health | How would you describe your health in general? |
|
| 19 | Complaints | Do you have memory complaints? |
|
| 20 | Worries | Do you worry about these memory complaints? |
|
| 21 | Family diagnosis | Have your parents or siblings ever had a diagnosis of dementia? |
|
| 22 | Family diagnosis = yes | What diagnosis do your parents or siblings have? |
|
| 23 | Medical | Could you indicate for the following diseases if you have them at the moment or have had them in the past? | |
| 24 | Medical | Hypertension (high blood pressure) |
|
| 25 | Medical | High cholesterol |
|
| 26 | Medical | Diabetes |
|
| 27 | Medical | Cardiovascular diseases |
|
| 28 | Cardiovascular diseases = yes | What kind of cardiovascular disease do you have/have you experienced? Several answers possible. |
|
| 29 | Medical | Stroke or CVA |
|
| 30 | Stroke or CVA = yes | What kind of stroke or CVA do you have/have you experienced? Several answers possible . → |
|
| 31 | Medical | Cancer |
|
| 32 | Cancer = yes | What type of cancer do you have/have you experienced? | [text] |
| 33 | Cancer = yes | Did you experience this in the last 5 years? |
2. no 1. yes |
| 34 | Medical | Epilepsy, MS, Parkinson's disease or another neurological condition (other than Alzheimer's disease or other dementia) |
|
| 35 | Neurological condition = yes | Which neurological conditions do you have / have you experienced? Several answers possible. |
|
| 36 | AD diagnosis = yes | What specific diagnosis do you have? |
|
| 37 | Medical | Depression or another psychiatric condition |
|
| 38 | Psychiatric condition = yes | What psychiatric conditions do you have/have you experienced? Several answers possible. |
|
| 39 | Medication | Do you use medication at the moment? |
|
| 40 |
Medication = yes |
Do you use any of the following medication at the moment? |
|
|
|||
|
|||
| 41 | Smoking | Do you smoke at the moment or have you smoked in the past? |
|
Abbreviations: CVA, cerebrovascular accident; MCI, mild cognitive impairment.
Zwan MD, van der Flier WM, Cleutjens S, et al. Dutch Brain Research Registry for study participant recruitment: Design and first results. Alzheimer's Dement. 2021;7:e12132 10.1002/trc2.12132
REFERENCES
- 1. Scheltens P, Blennow K, Breteler MM, et al. Alzheimer's disease. Lancet. 2016;388:505‐517. [DOI] [PubMed] [Google Scholar]
- 2. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112‐21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer's and dementia: an action plan for solutions. Alzheimers Dement. 2016;12:1113‐1115. [DOI] [PubMed] [Google Scholar]
- 4. Aisen P, Touchon J, Andrieu S, et al. Registries and Cohorts to Accelerate Early Phase Alzheimer's Trials. A Report from the E.U./U.S. Clinical Trials in Alzheimer's Disease Task Force. J Prev Alzheimers Dis. 2016;3:68‐74. [DOI] [PubMed] [Google Scholar]
- 5. Weiner MW, Nosheny R, Camacho M, et al. The Brain Health Registry: an internet‐based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 2018;14:1063‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verhage F, Intelligentie en leeftijd: onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar. Assen: Van Gorcum; 1964. [Google Scholar]
- 7. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19:271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vermunt L, Muniz‐Terrera G, Ter Meulen L, et al. Prescreening for European Prevention of Alzheimer Dementia (EPAD) trial‐ready cohort: impact of AD risk factors and recruitment settings. Alzheimers Res Ther. 2020;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. James SN, Lane CA, Parker TD, et al. Using a birth cohort to study brain health and preclinical dementia: recruitment and participation rates in Insight 46. BMC Res Notes. 2018;11:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molinuevo JL, Gramunt N, Gispert JD, et al. The ALFA project: a research platform to identify early pathophysiological features of Alzheimer's disease. Alzheimers Dement (N Y). 2016;2:82‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin Registry for Alzheimer's Prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2018;10:130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langbaum JB, Karlawish J, Roberts JS, et al. GeneMatch: a novel recruitment registry using at‐home APOE genotyping to enhance referrals to Alzheimer's prevention studies. Alzheimers Dement. 2019;15:515‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Statistics Netherlands . Internet; toegang, gebruik en faciliteiten; 2012–2019. 08‐10‐2019. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/83429NED/table?dl=3AC09
- 14. Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: a systematic review. Alzheimers Dement (N Y). 2019;5:751‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fruijtier AD, Visser LNC, van Maurik IS, et al. ABIDE Delphi study: topics to discuss in diagnostic consultations in memory clinics. Alzheimers Res Ther. 2019;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solomon A, Kivipelto M, Molinuevo JL, Tom B, Ritchie CW. European Prevention of Alzheimer's Dementia Longitudinal Cohort Study (EPAD LCS): study protocol. BMJ Open. 2019;8:e021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez‐Gomez O, Rodrigo A, Iradier F, et al. The MOPEAD project: advancing patient engagement for the detection of “hidden” undiagnosed cases of Alzheimer's disease in the community. Alzheimers Dement. 2019;15:828‐839. [DOI] [PubMed] [Google Scholar]
- 18. Bilderbeck AC, Penninx B, Arango C, et al. Overview of the clinical implementation of a study exploring social withdrawal in patients with schizophrenia and Alzheimer's disease. Neurosci Biobehav Rev. 2019;97:87‐93. [DOI] [PubMed] [Google Scholar]
- 19. Slot RER, Verfaillie SCJ, Overbeek JM, et al. Subjective Cognitive Impairment Cohort (SCIENCe): study design and first results. Alzheimers Res Ther. 2018;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jutten RJ, Harrison J, de Jong FJ, et al. A composite measure of cognitive and functional progression in Alzheimer's disease: design of the Capturing Changes in Cognition study. Alzheimers Dement (N Y). 2017;3:130‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matcham F, Barattieri di San Pietro C, Bulgari V, de Girolamo G, Dobson R, Eriksson H, et al. Remote assessment of disease and relapse in major depressive disorder (RADAR‐MDD): a multi‐centre prospective cohort study protocol. BMC Psychiatry. 2019;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Legdeur N, Badissi M, Carter SF, et al. Resilience to cognitive impairment in the oldest‐old: design of the EMIF‐AD 90+ study. BMC Geriatr. 2018;18:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leeuwis AE, Hooghiemstra AM, Amier R, et al. Design of the ExCersion‐VCI study: the effect of aerobic exercise on cerebral perfusion in patients with vascular cognitive impairment. Alzheimers Dement (N Y). 2017;3:157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lever‐van Milligen BA, Verhoeven JE, Schmaal L, et al. The impact of depression and anxiety treatment on biological aging and metabolic stress: study protocol of the MOod treatment with antidepressants or running (MOTAR) study. BMC Psychiatry. 2019;19:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Balkom TD, Berendse HW, van der Werf YD, et al. COGTIPS: a double‐blind randomized active controlled trial protocol to study the effect of home‐based, online cognitive training on cognition and brain networks in Parkinson's disease. BMC Neurol. 2019;19:179. [DOI] [PMC free article] [PubMed] [Google Scholar]


