Abstract
Objective
Eosinophilic granulomatosis with polyangiitis (EGPA) is part of a group of vasculitides commonly referred to as antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV), in addition to granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and renal‐limited vasculitis. Patients with EGPA characteristically have asthma and marked peripheral eosinophilia with only approximately 30% to 35% of patients being myeloperoxidase (MPO)‐ANCA positive, distinguishing it from other forms of AAV (1,2). The aim of this systematic review is to support the development of the American College of Rheumatology/Vasculitis Foundation guideline for the management of EGPA.
Methods
A systematic review was conducted of the literature for seven forms of primary systemic vasculitis (GPA, MPA, EGPA, polyarteritis nodosa, Kawasaki disease, giant cell arteritis, and Takayasu arteritis). The search was done for articles in English using Ovid Medline, PubMed, Embase, and the Cochrane Library. Articles were screened for suitability in addressing population/patients, intervention, comparator, and outcomes (PICO) questions, with studies presenting the highest level of evidence given preference. Two independent reviewers conducted a title/abstract screen and full‐text review for each eligible study.
Results
The initial search, conducted in August 2019, included 13 800 articles, of which 2596 full‐text articles were reviewed. There were 190 articles (addressing 34 PICO questions) reporting on the diagnosis and management of EGPA.
Conclusion
This comprehensive systematic review synthesizes and evaluates the accuracy of commonly used tests for EGPA as well as benefits and toxicities of different treatment options.
INTRODUCTION
Eosinophilic granulomatosis with polyangiitis (EGPA) is part of a group of vasculitides commonly referred to as antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV), in addition to granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and renal‐limited vasculitis. Patients with EGPA characteristically have asthma and marked peripheral eosinophilia, distinguishing it from other forms of AAV. Approximately 30% to 35% of patients with EGPA will be myeloperoxidase (MPO)‐ANCA positive(1, 2, 3). Diagnosis is based on a combination of characteristic clinical features, laboratory tests, and/or biopsy findings. Although no validated diagnostic criteria have been developed, the 1984 Lanham criteria, 1990 American College of Rheumatology (ACR) classification criteria, and 2012 revised Chapel Hill Consensus Conference nomenclature help define EGPA for the purposes of clinical trials (4, 5, 6). The annual incidence ranges from 1 to 3 per 1 000 000, whereas the prevalence ranges from 11 to 45 per 1 000 000 (7). Because of the rare nature of the disease, treatment studies in EGPA have often included other forms of AAV and/or other forms of primary necrotizing arteritis, such as polyarteritis nodosa (PAN).
The first aim of this systematic review is to search and compare the benefits and harms of different treatment options for patients with EGPA. It includes randomized controlled trials (RCTs) and nonrandomized studies and presents the evidence and an assessment of its certainty for important outcomes. The second aim of this systematic review is to determine the accuracy of commonly available tests for EGPA, which can be used to inform a combined strategy for diagnosis. These reviews were used to inform evidence‐based recommendations on diagnostic and management strategies for EGPA by the ACR/Vasculitis Foundation (VF) Vasculitis Management Guidelines.
MATERIALS AND METHODS
Search strategy and data sources
An information specialist performed systematic searches of the published English‐language literature, including Ovid Medline, PubMed, Embase, and the Cochrane Library (including Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, and Health Technology Assessments) from the inception of each database through August 2018 to obtain direct evidence in vasculitis patient populations relating to vasculitis questions (Supplementary Appendix 1). The information specialist updated the searches conducted on August 2019. The methods team used DistillerSR software to identify duplicate records (https://distillercer.com/products/distillersr‐systematic‐reviewsoftware/). The search was specific to address the population/patients, intervention, comparator, and outcomes (PICO) questions asked for each vasculitis type. The ACR/VF Vasculitis Guideline Core Team developed 34 PICO questions for EGPA that addressed relevant or commonly encountered patient diagnostic, treatment, and management scenarios (Supplementary Appendix 2).
Study selection
We included studies that provided the highest certainty evidence. For questions addressing treatment options, we included RCTs first. When RCTs were not available, we included observational studies (cohort and case–control studies) that reported on patient‐important outcomes for the intervention and comparison. When studies with comparative data were not available, we included case series that presented patient‐important outcomes for either the intervention or the comparison. For questions addressing diagnostic testing, we included studies that reported on diagnostic test accuracy (cohort studies and cross‐sectional studies) for EGPA.
Adult patients (18 years of age or older) presenting to inpatient or outpatient settings with suspected or confirmed EGPA were eligible for inclusion. When studies addressed multiple vasculitis types, we included data when results were presented separately or when more than 80% of the population included was patients with EGPA.
Studies reporting outcomes that compared the intervention and the comparator specified in the PICO question or reporting outcomes for either the intervention or the comparator were included. For questions regarding diagnostic testing, studies that presented test accuracy results for the intervention and comparator were included.
We excluded studies that included an irrelevant population, intervention, or outcome; studies that had no primary data, including letters, opinion pieces, and commentaries; narrative reviews; systematic reviews; epidemiological studies that only included prevalence or incidence results; any study that had fewer than 10 patients with vasculitis; any study that addressed an organ‐limited vasculitis except for renal‐limited vasculitis; and any study focusing on basic research in animals.
Screening and data extraction
Two independent reviewers conducted title and abstract screening and full‐text review in duplicate to identify eligible studies. Data extraction was also conducted independently and in duplicate, and conflicts were resolved by a third reviewer (MAK). Each pair of reviewers included at least one of five clinical experts (KB, AD, KEJ, YCCL, and JMS). Extracted data included general study characteristics (authors, publication year, country, and study design), duration of follow‐up, outcome data for the intervention and/or comparator, diagnostic index test, and reference standard, along with parameters to determine test accuracy (ie, sensitivity and specificity of the index test) when relevant.
Risk of bias and data synthesis
When direct comparisons of PICO comparators were available from RCTs, reviewers entered the results into RevMan version 5.3 software (http://tech.cochrane.org/revman), which was used to calculate pooled effect estimates. Reviewers evaluated the risk of bias using the Cochrane risk of bias tool (http://handbook.cochrane.org/).
When direct comparative results were available from observational studies (cohort and case–control studies), reviewers entered the results into RevMan version 5.3 software, which was used to calculate pooled effect estimates. Reviewers evaluated the risk of bias using a modified New‐Castle Ottawa scale for observational studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
When comparative results were not available, reviewers abstracted data describing details of the population, interventions, and results into summary tables.
When test accuracy results were available, reviewers abstracted test accuracy information and used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)‐2 tool to assess the risk of bias in the included studies. When pooling was appropriate, the review team used Open Meta Analyst (http://www.cebm.brown.edu/openmeta/) to pool test accuracy results.
Two investigators familiar with the GRADEpro software (https://gradepro.org) (MAK and NH) formulated Grading of Recommendations Assessment, Development, and Evaluation (GRADE) summary of findings tables for each PICO question when direct comparative data or test accuracy results were available. The investigators used the GRADE framework to assess overall certainty by evaluating the evidence for each outcome on the following domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias. There were two PICO questions with direct comparative data, which are shown in Tables 1 and 2.
Table 1.
Impact of using rituximab vs. cyclophosphamide for remission induction on disease‐related outcomes and treatment‐related adverse events, in patients with active severe EGPA
| Certainty Assessment | Patients, n (%) | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, n | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | RTX | CYC | Relative (95% CI) | Absolute (95% CI) | |
| Relapse within 30 months | |||||||||||
| 1 | Observational studies | Not serious | Not serious | Not serious | Very serious a | None | 4/14 (28.6) | 6/14 (42.9) | OR 0.53 (0.11 to 2.56) | 144 fewer per 1000 (352 fewer to 229 more) | Very low |
| Adverse events (hypogammaglobulinemia) | |||||||||||
| 1 | Observational studies | Not serious | Not serious | Not serious | Very serious a | None | 3/14 (21.4) | 4/14 (28.6) | OR 0.68 (0.12 to 3.83) | 72 fewer per 1000 (240 fewer to 319 more) | Very low |
CI, confidence interval; CYC, cyclophosphamide; EGPA, eosinophilic granulomatosis with polyangiitis; RTX, rituximab.
Clinical action would differ if the upper versus the lower boundary of the CI represented the truth, leading to very serious imprecision due to very small number of events and patients in the included study. See reference 29.
Table 2.
Impact of initiating treatment with azathioprine and glucocorticoids vs glucocorticoids alone on disease‐related outcomes and treatment‐related adverse events in patients with active nonsevere EGPA
| Certainty Assessment | Patients, n (%) | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, n | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Azathioprine + Glucocorticoids | Glucocorticoids Alone | Relative (95% CI) | Absolute (95% CI) | |
| Remission induction failures and relapses at Month 24 | |||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 12/25 (48.0) | 12/26 (46.2) | OR 1.08 (0.36 to 3.24) | 19 more per 1000 (226 fewer to 274 more) | Low |
| Initial remission | |||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 25/25 (100.0) | 25/26 (96.2) | OR 3.00 (0.12 to 77.17) | 25 more per 1000 (212 fewer to 38 more) | Low |
| Major relapses month 24 b | |||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 4/25 (16.0) | 3/24 (12.5) | OR 1.33 (0.27 to 6.70) | 35 more per 1000 (88 fewer to 364 more) | Low |
| Minor relapses month 24 c | |||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 7/25 (28.0) | 7/24 (29.2) | OR 0.94 (0.27 to 3.26) | 13 fewer per 1000 (192 fewer to 281 more) | Low |
| Asthma/rhinosinusitis exacerbation | |||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very serious a | None | 6/25 (24.0) | 5/26 (19.2) | OR 1.33 (0.35 to 5.06) | 48 more per 1000 (115 fewer to 354 more) | Low |
CI, confidence interval; EGPA, eosinophilic granulomatosis with polyangiitis; OR, odds ratio.
Clinical action would differ if the upper vs the lower boundary of the CI represented the truth, leading to very serious imprecision due to very small number of events and patients in the included study.
Major relapse was defined as the recurrence or new onset of potentially organ‐ or life‐threatening disease activity that cannot be treated with glucocorticoid intensification alone and requires further therapeutic escalation.
Minor relapse was defined as the recurrence or new onset of manifestations that are not potentially organ or life threatening. See reference 36.
Data analysis
For questions addressing treatment options, relative risks (eg, risk ratios [RRs] or odds ratios [ORs]) were calculated by pooling results from RCTs and from observational studies comparing treatments. When no direct comparisons between treatments within a study were available, the risk of an event (or proportion) in a study (eg, disease relapse) was calculated and then the weighted proportions from each study were combined and presented in the outcome description section of the summary tables.
For questions addressing diagnostic tests, the accuracy estimates from individual studies were combined quantitatively (pooled) for each test using OpenMetaAnalyst (http://www.cebm.brown.edu/openmeta/). We conducted a bivariate analysis by pooling sensitivity and specificity for each of the test comparisons to account for variation within and between studies. Forest plots were created for each comparison. The Breslow‐Day test was used to measure the percentage of total variation across studies due to heterogeneity (I2); however the results did not influence our judgment about heterogeneity of the pooled estimates due to methodological literature discouraging its use for test accuracy meta‐analysis.
When statistical pooling was not feasible, we summarized results qualitativly.
The systemic review was performed in accordance with the Preferred Reporting Items for Systemic Reviews and Meta‐Analysis (PRISMA) guidelines .
RESULTS
Description of studies
The initial search retrieved 13 800 nonduplicate studies, of which 2596 were included for full‐text review. Following full‐text review, we found 1156 articles to be potentially eligible for data abstraction and inclusion in the systematic reviews of the seven different types of vasculitis. For this review, we considered 190 articles for data abstraction for EGPA. Reasons for exclusion from the full‐text review included ineligible study design, irrelevant study population and/or intervention, sample size of less than 10 patients, and unacceptable reference standard or index test (Figure 1). Most of the evidence was of very low certainty due to risk of bias and imprecision.
Figure 1.
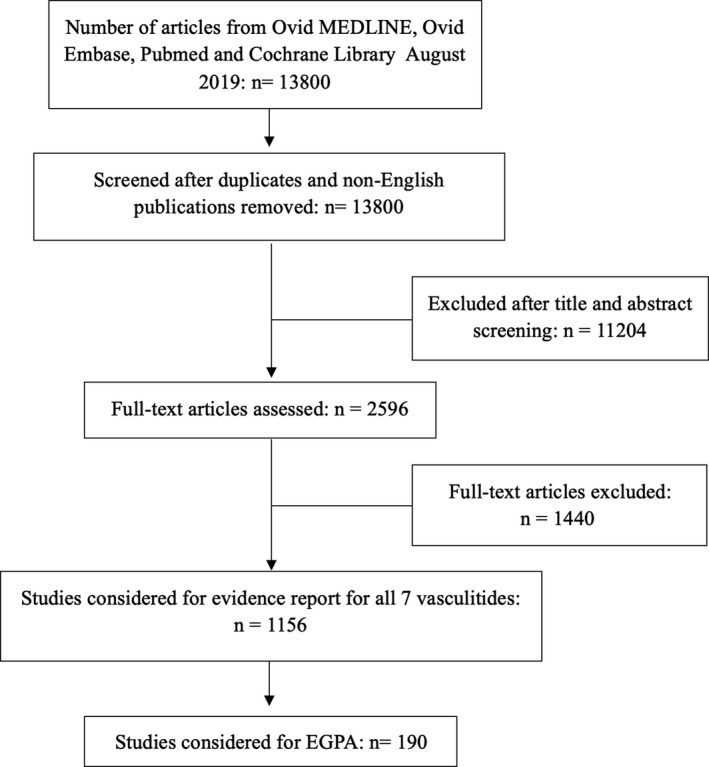
PRISMA flow diagram of the systematic review.
Prognosis: Five‐Factor Score
In 1996, published data from multiple prospective trials including a combination of patients with PAN (n = 260, likely including patients with MPA) and EGPA (n = 82) demonstrated five dominant risk factors for mortality known as the Five‐Factor Score (FFS). These include 1) proteinuria of more than 1 g/day (RR = 3.6), 2) renal insufficiency with creatinine of more than 1.58 mg/dl (RR = 1.86), 3) gastrointestinal involvement (RR = 2.83), 4) central nervous system involvement (RR = 1.76), and 5) cardiomyopathy (RR = 2.18). Each manifestation is scored with 1 point, with a significant rise in the 5‐year mortality rates with higher scores (12% with FFS = 0, 26% with FFS = 1 [P < 0.005 compared with 0], and 46% with FFS > 2 [P < 0.0001 compared with 0]) (8). Multiple prospective and retrospective studies have confirmed cardiac involvement to be one of the most important risk factors for mortality specifically in patients with EGPA (standardized mortality ratio = 3.06) (8, 9, 10). Stratifying treatment decisions in patients with EGPA based on the 1996 FFS, with cyclophosphamide (CYC) given to those with an FFS of 1 or more, resulted in a 7‐year survival of 90% regardless of baseline severity (11). The 1996 FFS has also been found to be predictive of relapse at 2 years in patients with EGPA, with an FFS of 1 or more associated with a higher relapse risk (69% vs 7% with FFS of 0; RR = 28.6; 95% confidence interval [CI] 2.89‐283.06; P = 0.001) (12). The 2009 revised FFS continued to show prognostic value for EGPA but was derived from a broader population of patients, including those with GPA, MPA, and PAN (13). The 2009 FFS includes 1) age greater than 65 years, 2) cardiac symptoms, 3) gastrointestinal involvement, 4) renal insufficiency (stabilizing peak creatinine ≥150 μmol/L) and 5) absence of ear, nose, or throat symptoms. Using the 2009 revised FFS cardiac insufficiency again showed a markedly higher risk of mortality in EGPA (hazard ratio [HR] = 2.8; 95% CI 1.2‐5.9; P = 0.02). In addition, ear, nose, or throat involvement was found to be associated with a lower risk of mortality (HR = 0.3; 95% CI 0.15‐0.9; P = 0.03). A subsequent study has shown that the 2009 FFS has a better prognostic accuracy compared with the 1996 FFS in AAV, and patients with a 2009 FFS of 2 or more treated with CYC benefitted from a prolonged survival (14).
Cardiac imaging
Patients with EGPA who are MPO‐ANCA negative have been recognized to be at higher risk of cardiac involvement in several cohorts (1, 2). Eleven studies evaluating cardiac imaging in EGPA were reviewed, including three case–control trials, six retrospective case series, and two prospective case series (15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25). Using multiple modalities for confirmation, including electrocardiogram, 24‐hour Holter monitoring, echocardiography, and cardiac magnetic resonance imaging (MRI), the prevalence of cardiac changes in participants with EGPA in remission is estimated to be 62% to 90% (18, 20, 21). During active disease, late gadolinium enhancement by cardiac MRI was seen in 50% to 65% of patients with EGPA. Late gadolinium enhancement on cardiac MRI has a sensitivity of 83% (95% CI 59‐93%) and specificity of 56% (95% CI 37‐73%) for cardiac involvement in EGPA (24). In one study, in patients with known cardiac MRI abnormalities, echocardiography had a sensitivity of 83% and a specificity of 80% (21).
Treatment
Glucocorticoid monotherapy for nonsevere disease (FFS = 0)
In one multicenter, prospective trial by Ribi et al, 72 participants with EGPA with active, nonsevere disease (FFS = 0) were given a standardized glucocorticoid regimen (26). Patients were allowed to receive one intravenous infusion of methylprednisolone (15 mg/kg) at the start of therapy. All participants received 1 mg/kg/day of oral prednisone for 3 weeks, which was then tapered by 5 mg every 10 days to 0.5 mg/kg/day, then by 2.5 mg every 10 days until down to 15 mg/day, and then by 1 mg every 10 days to the minimal effective dose or off. Forty percent of participants were ANCA positive. Mean follow‐up was 56.2 ± 31.7 months. Five patients (7%) failed to respond, whereas 25 (35%) relapsed (17 major and eight minor). Disease‐free survival was 100% at 1 year and 54% at 5 years. General survival was 100% at 1 year and 97% at 5 years. The most common overall adverse events were subclinical osteoporosis (14% of participants), infection requiring hospitalization (11% of participants), osteoporotic fractures (10% of participants), arterial hypertension (10% of participants), and thromboembolic events (10% of participants).
Refractory/relapsing disease after glucocorticoid monotherapy
In the study by Ribi et al (26), patients with persistent vasculitis manifestations requiring at least 20 mg/day of prednisone and relapsing patients were randomized to receive 6 months of azathioprine (AZA) (2 mg/kg/day) or 6 pulses of CYC (600 mg/m2 every 2 weeks for 1 month, then monthly thereafter). Of the patients randomized, 50% (5/10) receiving CYC and 78% (7/9) receiving AZA achieved remission. Subsequent relapses were 20% (1/5) in the CYC group and 43% (3/7) in the AZA group.
CYC for severe disease (FFS ≥ 1)
Two prospective trials included participants with EGPA (n = 62) with active disease and an FFS of 1 or more treated with intravenous CYC (27, 28). One study randomized patients to receive either 6 or 12 intravenous infusions of CYC (0.6 mg/m2/dose every 2 weeks for 1 month, then every 4 weeks) (28). Overall, 90% of patients treated with intravenous CYC were able to achieve remission, with another 5% achieving partial remission and no differences seen between the six‐pulse and 12‐pulse regimens. Of those achieving remission (n = 56), relapses occurred in 64% of participants over a follow‐up period of 3 to 8 years with variable maintenance strategies after CYC therapy. There was a higher number of overall relapses at 8 years follow‐up (without maintenance therapy after CYC) between the six‐pulse and 12‐pulse regimens during CYC therapy (78 vs 52%; OR = 3.32; 95% CI 0.94‐11.76; P = 0.031). However, there were no differences in major relapses (43 vs 32%; OR = 1.63; 95% CI 0.50‐5.31; P = 0.21). Severe adverse events were seen in 50% to 71% of participants, with no differences found between six‐pulse and 12‐pulse regimens. In a mixed population of patients more than 65 years old with systemic necrotizing vasculitis (including EGPA), a fixed low‐dose intravenous CYC regimen (maximum of six 500‐mg pulses every 2‐3 weeks) compared with a conventional CYC regimen (500‐mg/m2 pulses every 2‐3 weeks until remission) was associated with fewer severe adverse events (60% vs 78%; OR = 0.42; 95% CI 0.18‐1.00; P = 0.024), most frequently infections, at 3 years (27).
Rituximab for induction of remission for severe (FFS ≥ 1) and nonsevere disease (FFS = 0)
There was only one comparative trial evaluating rituximab (RTX) use in EGPA. In one retrospective, single‐center study, patients with EGPA received induction therapy with either RTX (1000 mg twice; n = 14) or CYC (median cumulative dose of 5.63 g; interquartile range [IQR] 4.31‐12.68) (29) (Table 1). Patients in the CYC group were age‐ and sex‐matched to the RTX group. Most patients (86%) in the RTX group received RTX because of refractory or relapsing disease. There were three patients in the RTX group who received additional doses of RTX (1000 mg twice every 6 months for 18‐30 months) for remission maintenance. Complete remission was achieved in five patients in the RTX group compared with four patients in the CYC group (OR = 1.39; 95% CI 0.28‐6.84; P = 0.404). Among those treated with RTX, there was a trend toward more ANCA‐positive patients achieving remission (5/11 [45%]) compared with ANCA‐negative patients (4/17 [23%]), which was consistent with other retrospective studies (30, 31, 32). The median daily prednisone dose in the RTX‐treated patients decreased from 22.5 mg (IQR 13.75‐32.5) to 5 mg (IQR 5‐7.5) at 12 months (P < 0.0001). All but one patient in each group received another maintenance therapy (MTX, AZA, mycophenolate, cyclosporine, or leflunomide). After a median observation period of 36 months, there were four relapses in the RTX group (three minor and one major) and six relapses in the CYC group (five minor and one major) (OR = 0.53; 95% CI 0.11‐2.56; P = 0.216).
Mepolizumab for relapsing/refractory disease
One multicenter, double‐blinded RCT compared the addition of mepolizumab (300 mg subcutaneous monthly for 48 weeks; n = 68) with the addition of placebo (n = 68) to current therapy in participants with refractory or relapsing EGPA (33). Remission was defined as a Birmingham Vasculitis Activity Score of 0 and no more than 4 mg daily of prednisone; however, active asthma symptoms were considered a feature of relapse. Only 10% of participants were ANCA positive at baseline. In the mepolizumab group, there was a greater proportion of patients able to sustain remission for at least 24 weeks (28% vs 3%; OR = 5.91; 95% CI 2.68‐13.03; P < 0.001) and at remission at both 36 and 48 weeks (32% vs 3%; OR = 16.74; 95% CI 3.61‐77.56; P < 0.001). Relapses at 52 weeks were less common in the mepolizumab group (56% vs 82%; HR = 0.32; 95% CI 0.21‐0.50; P < 0.001). In the mepolizumab group, there were significantly fewer flares involving vasculitis features (43% vs 65%; OR = 0.41; 95% CI 0.20‐0.81; P = 0.0053) and flares involving active asthma features (37% vs 60%, OR = 0.38; 95% CI 0.19‐0.77; P = 0.0033). The average doses of glucocorticoids (GCs) (prednisolone or prednisone) were lower in the mepolizumab group between Weeks 48 through 52 (OR = 0.20; 95% CI 0.09‐0.41; P < 0.001). The number of participants with severe adverse events was similar between mepolizumab and placebo groups (18 vs 26%; OR = 0.60; 95% CI 0.26‐1.36; P = 0.11).
Methotrexate for induction therapy in nonsevere disease and maintenance therapy
One open‐label, single‐center prospective trial evaluated methotrexate (MTX) (0.3 mg/kg/week intravenous infusions) for induction of remission in 11 ANCA‐negative patients with nonsevere EGPA (34). Most patients were able to achieve either a complete (n = 6) or partial (n = 2) remission, with a median time to remission of 5 months (range 2‐12).
In two open‐label, single‐center prospective trials, MTX (0.3 mg/kg/week intravenous or oral) was used for maintenance of remission after induction therapy with either CYC or MTX (34, 35). Out of a total of 40 patients with EGPA, 14 (35%) experienced a relapse by 24 to 48 months; however, MTX was discontinued at 12 months in one trial and after patients were off prednisone and in complete remission in the other. In one of these studies (34), patients with EGPA were randomized to either MTX (n = 17) or CYC (n = 13) (1.5 mg/kg/day oral) as maintenance therapy. There were no significant differences in relapse rates at 24 months between the MTX and CYC groups (18% vs 23%; OR = 0.71; 95% CI 0.12‐4.30; P = 0.36); however, given the small number of patients, the study was likely underpowered for this subgroup analysis.
AZA for induction of remission in nonsevere disease (FFS = 0)
One prospective, double‐blind, RCT evaluated the efficacy of the addition of AZA (2 mg/kg/day titrated to 3 mg/kg/day for insufficient response) to placebo for induction therapy in patients with EGPA, MPA, and PAN with an FFS of 0 (36) (Table 2). All participants were treated with a prespecified course of GCs, which was tapered off over 6 months. Participants were treated with AZA for 1 year. A total of 51 participants with EGPA were included (25 in the AZA group and 26 in the placebo group). Initial remission was achieved in 100% of participants with EGPA treated with AZA and 96% of patients with EGPA in the placebo group. At 24 months, in the participants with EGPA, there were no differences in the number of major relapses (16% with AZA vs 13% with placebo; OR = 1.33; 95% CI 0.27‐6.70; P = 0.363), overall relapses (48% with AZA vs 42% with placebo; OR = 1.29; 95% CI 0.42‐4.00; P = 0.328), or asthma/rhinosinusitis exacerbations (24% with AZA vs 19% with placebo; OR = 1.33; 95% CI 0.35‐5.06; P = 0.340). However, the study was not powered to detect specific outcome differences among the participants wit EGPA. Among all participants (ie, those with EGPA, MPA, and PAN), there were no differences in the number of participants with at least one severe adverse event (48% with AZA vs 47% with placebo; OR = 1.04; 95% CI 0.46‐2.32; P = 0.466), but there were significantly more severe treatment‐related adverse events with AZA (17% with AZA vs 6% with placebo; OR = 3.23; 95% CI 0.80‐13.02; P = 0.050).
Prophylaxis for pneumocystis jirovecii pneumonia
One retrospective study evaluated the risk of Pneumocystis jirovecii pneumonia (PJP) in patients receiving high‐dose GCs (≥ 30 mg/day prednisone or equivalent) for more than 4 consecutive weeks with and without trimethoprim/sulfamethoxazole (T/S) prophylaxis (either 160 mg/800 mg three times weekly or 80 mg/400 mg daily) (37). This study included patients with multiple different rheumatic diseases (n = 1092), including 50 with EGPA, Among the various rheumatic diseases, the incidence of PJP was highest in patients with GPA and MPA (12.14 per 100 person‐years; 95% CI 3.94‐28.33). Use of T/S significantly reduced both the 1‐year incidence of PJP (adjusted HR = 0.07; 95% CI 0.01‐0.53; P = 0.010) and PJP‐related mortality (adjusted HR = 0.08; 95% CI 0.0006‐0.71; P = 0.019) in a postmatched population. The number needed to treat to prevent one episode of PJP was 52 (95% CI 22‐124) and the number needed to harm for serious adverse drug reactions was 131 (95% CI 55‐∞).
Leukotriene inhibitors
Leukotriene inhibitors are commonly used for the treatment of asthma; however, it is not clear whether leukotriene inhibitors should be continued after the onset of EGPA. One case‐crossover design evaluated 78 patients with EGPA, 26% of whom were exposed to montelukast (38). Within 3 months of starting montelukast, the OR of developing EGPA was 4.5 (95% CI 1.5‐13.9). However, the study was confounded by the fact that montelukast is used at later stages of escalation therapy for asthma and can allow withdrawal of glucocorticoid therapy. A nested case–control study was performed in a US population of patients with asthma on three or more asthma medications. They found 47 possible or definite cases of EGPA and 4700 age‐and gender‐matched control subjects (39). Although the crude association between leukotriene inhibitors and EGPA remained strong (OR = 4.0; 95% CI 1.49‐10.60), when controlled for oral GC, inhaled GC, and number of asthma drug categories dispensed, the association was lost (OR = 1.32; 95% CI = 0.44‐3.96).
DISCUSSION
This review presents pooled estimates of patient‐important outcomes and test accuracy assessments for commonly available treatments and tests used for EGPA.
Derived from a heterogeneous population of patients with systemic necrotizing vasculitis, the FFS has shown clinical significance in both assessing prognosis and making treatment decisions in EGPA, with scores of 1 or greater being treated more aggressively. The FFS and other studies have consistently demonstrated cardiac involvement, typically manifesting as myocarditis, to be an especially prominent risk factor for mortality. Although the importance of early recognition and aggressive treatment of patients with cardiac involvement is recognized, the modality and frequency of screening is not clear. Although comparative studies are warranted comparing cardiac screening modalities, echocardiography is a safe and inexpensive means to identify cardiac features known to be associated with mortality in this population.
There are few randomized controlled treatment trials in EGPA, and when available, many have a limited number of patients with EGPA, with the primary outcomes typically derived from a mixed population that includes other forms of systemic necrotizing vasculitis leading to uncertain conclusions due to very low certainty in the effect estimates. Treatment of patients with good prognostic scores (ie, FFS = 0) with glucocorticoid monotherapy initially is associated with a high survival rate, but relapses, many of which are major, are common. The addition of AZA or MTX has not demonstrated clear benefit; however, the limited number of patients and lack of comparative trials limit the quality of the evidence. In those with active severe disease (FFS ≥ 1), CYC is effective at induction of remission; however, relapse rates remain high after discontinuation of CYC, strengthening support for the use of maintenance therapies after CYC induction. In retrospective trials with small sample sizes, RTX holds promise as an induction agent, especially in MPO‐ANCA–positive patients; however, results from prospective comparative trials are not yet available. Mepolizumab has been shown to be effective as an induction agent in relapsing or refractory disease; however, the randomized trial in which this agent was examined included a small number of ANCA‐positive patients, and patients with severe manifestations, including cardiac involvement, were excluded leading to very low certainty in the effect estimates. In patients receiving high‐dose prednisone, use of T/S effectively prevents of PJP. Continued use of T/S while on lower doses of prednisone in combination with other immunosuppressive drugs can be considered on an individualized basis. Although there has been concern about leukotriene inhibitors causing EGPA, there is insufficient evidence to support a causal relationship.
This review has several strengths. The comprehensive and systematic approach for identifying studies makes it unlikely that relevant studies were missed. Additionally, we assessed the certainty of evidence in this area and identified sources of bias. We note a few limitations in this comprehensive systematic review. We limited our review by English language. Multiple studies included patients with other forms of primary systemic necrotizing vasculitis (eg, other forms of AAV and PAN) and outcome data limited to patients with EGPA were not always available. In addition, because of the rarity of the disease, few randomized trials were identified.
In conclusion, this comprehensive systematic review synthesizes and evaluates the benefits and toxicities of different treatment options and the utility of commonly applied assessment tools in EGPA. Estimates of benefits and toxicities as well as sensitivity and specificity from this review were used to develop recommendations for the use of diagnostic tests and management strategies for the ACR/VF Vasculitis Management Guideline.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Reem A. Mustafa, Mohamad Kalot and Nedaa Husainat had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Kalot, Husainat, Mustafa.
Acquisition of data
Springer, Kalot, Husainat, Byram, Dua, James, Turbunbaev, Villa‐Forte, Maz, Mustafa.
Analysis and interpretation of data
Springer, Kalot, Husainat, Byram, Dua, James, Lin, Turbunbaev,Villa‐Forte, Abril, Maz, Mustafa, Chung.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The systematic review team would like to acknowledge Amy Turner, Regina Parker, Robin Lane for their assistance with administrative support, data management and coordination of the project. The review team would also like to acknowledge the panel members of the ACR Vasculitis Practice Clinical Guidelines 2020 for their review of the evidence and input during the guideline development process.
This systematic review was conducted to support the development of the American College of Rheumatology (ACR) 2020 guidelines for diagnosis and management of Vasculitis. The entire guideline development process was funded by the ACR. Through the Outcomes and Implementation Research Unit at University of Kansas Medical Center, some researchers received salary or grant support.
1Jason M. Springer, MD, MS, Kevin W. Byram, MD: Vanderbilt University Medical Center, Nashville; 2Mohamad A. Kalot, MD: State University of New York at Buffalo; 3Nedaa M. Husainat, MD: St. Mary's Hospital, St. Louis, Missouri; 4Anisha B. Dua, MD, MPH: Northwestern University Feinberg School of Medicine, Chicago, Illinois; 5Karen E. James, MD, MSCE: University of Utah Health, Salt Lake City; 6Yih Chang Lin, MD: University of South Florida, Tampa; 7Marat Turgunbaev, MD, MPH: American College of Rheumatology; 8Alexandra Villa‐Forte, MD, MPH, Carol Langford, MD, MHS: Cleveland Clinic, Cleveland, Ohio; 9Andy Abril, MD: Mayo Clinic, Jacksonville, Florida; 10Mehrdad Maz, MD, Reem A. Mustafa, MD, MPH, PhD: University of Kansas Medical Center, Kansas City; 11Sharon A. Chung, MD, MAS: University of California, San Francisco Medical Center.
Drs. Springer and Kalot contributed equally to this work.
Dr. Springer has served as site investigator for ChemoCentryx and InfaRx. No other disclosures relevant to this article were reported.
This work was funded by the American College of Rheumatology. Reem A Mustafa at the Outcomes and Implementation Research Unit received grant support.
REFERENCES
- 1. Sinico RA, di Toma L, Maggiore U, Bottero P, Radice A, Tosoni C, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg‐Strauss syndrome. Arthritis Rheum 2005;52:2926–35. [DOI] [PubMed] [Google Scholar]
- 2. Sable‐Fourtassou R, Cohen P, Mahr A, Pagnoux C, Mouthon L, Jayne D, et al. Antineutrophil cytoplasmic antibodies and the Churg‐Strauss syndrome. Ann Intern Med 2005;143:632–8. [DOI] [PubMed] [Google Scholar]
- 3. Moiseev S, Bossuyt X, Arimura Y, Blockmans D, Csernok E, Damoiseasux J, et al. International consensus on ANCA testing in eosinophilic granulomatosis with polyangiitis. Am J Respir Crit Care Med 2020. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4. Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg‐Strauss syndrome. Medicine (Baltimore) 1984;63:65–81. [DOI] [PubMed] [Google Scholar]
- 5. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg‐Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094–100. [DOI] [PubMed] [Google Scholar]
- 6. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Ntatsaki E, Watts RA, Scott DG. Epidemiology of ANCA‐associated vasculitis. Rheum Dis Clin North Am 2010;36:447–61. [DOI] [PubMed] [Google Scholar]
- 8. Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, et al. Prognostic factors in polyarteritis nodosa and Churg‐Strauss syndrome: a prospective study in 342 patients. Medicine (Baltimore) 1996;75:17–28. [DOI] [PubMed] [Google Scholar]
- 9. Bourgarit A, le Toumelin P, Pagnoux C, Cohen P, Mahr A, le Guern V, et al. Deaths occurring during the first year after treatment onset for polyarteritis nodosa, microscopic polyangiitis, and Churg‐Strauss syndrome: a retrospective analysis of causes and factors predictive of mortality based on 595 patients. Medicine (Baltimore) 2005;84:323–30. [DOI] [PubMed] [Google Scholar]
- 10. Moosig F, Bremer JP, Hellmich B, Holle JU, Holl‐Ulrich K, Laudien M, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg‐Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis 2013;72:1011–7. [DOI] [PubMed] [Google Scholar]
- 11. Samson M, Puechal X, Devilliers H, Ribi C, Cohen P, Stern M, et al. Long‐term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg‐Strauss syndrome) enrolled in two prospective trials. J Autoimmun 2013;43:60–9. [DOI] [PubMed] [Google Scholar]
- 12. Kim DS, Song JJ, Park Y‐B, Lee S‐W. Five factor score of more than 1 is associated with relapse during the first 2 year‐follow up in patients with eosinophilic granulomatosis with polyangiitis. Int J Rheum Dis 2017;20:1261–8. [DOI] [PubMed] [Google Scholar]
- 13. Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Toumelin PL, et al. The Five‐Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) chort. Medicine (Baltimore) 2011;90:19–27. [DOI] [PubMed] [Google Scholar]
- 14. Solans‐Laqué R, Rodriguez‐Carballeira M, Rios‐Blanco JJ, Fraile G, Saez‐Comet L, Martinez‐Zapico A, et al. Comparison of the Birmingham Vasculitis Activity Score and the Five‐Factor Score to assess survival in antineutrophil cytoplasmic antibody‐associated vasculitis: a study of 550 patients from Spain (REVAS Registry). Arthritis Care Res (Hoboken) 2020;72:1001–10. [DOI] [PubMed] [Google Scholar]
- 15. Cereda AF, Pedrotti P, de Capitani L, Giannattasio C, Roghi A. Comprehensive evaluation of cardiac involvement in eosinophilic granulomatosis with polyangiitis (EGPA) with cardiac magnetic resonance. Eur J Intern Med 2017;39:51–6. [DOI] [PubMed] [Google Scholar]
- 16. Fijolek J, Wiatr E, Gawryluk D, Nowicka U, Martusewicz‐Boros MM, Kober J, et al. The significance of cardiac magnetic resonance imaging in detection and monitoring of the treatment efficacy of heart involvement in eosinophilic granulomatosis with polyangiitis patients. Sarcoidosis Vasc Diffuse Lung Dis 2016;33:51–8. [PubMed] [Google Scholar]
- 17. Yune S, Choi D‐C, Lee B‐J, Lee J‐Y, Jeon E‐S, Kim SM, et al. Detecting cardiac involvement with magnetic resonance in patients with active eosinophilic granulomatosis with polyangiitis. Int J Cardiovasc Imaging 2016;32 Suppl 1:155–62. [DOI] [PubMed] [Google Scholar]
- 18. Hazebroek MR, Kemna MJ, Schalla S, Sanders‐van Wijk S, Gerretsen SC, Dennert R, et al. Prevalence and prognostic relevance of cardiac involvement in ANCA‐associated vasculitis: eosinophilic granulomatosis with polyangiitis and granulomatosis with polyangiitis. Int J Cardiol 2015;199:170–9. [DOI] [PubMed] [Google Scholar]
- 19. Mavrogeni S, Karabela G, Gialafos E, Stavropoulos E, Spiliotis G, Katsifis G, et al. Cardiac involvement in ANCA (+) and ANCA (‐) Churg‐Strauss syndrome evaluated by cardiovascular magnetic resonance. Inflamm Allergy Drug Targets 2013;12:322–7. [DOI] [PubMed] [Google Scholar]
- 20. Szczeklik W, Miszalski‐Jamka T, Mastalerz L, Sokolowska B, Dropinski J, Banys R, et al. Multimodality assessment of cardiac involvement in Churg‐Strauss syndrome patients in clinical remission. Circ J 2011;75:649–55. [DOI] [PubMed] [Google Scholar]
- 21. Dennert RM, van Paassen P, Schalla S, Kuznetsova T, Alzand BS, Staessen JA, et al. Cardiac involvement in Churg‐Strauss syndrome. Arthritis Rheum 2010;62:627–34. [DOI] [PubMed] [Google Scholar]
- 22. Marmursztejn J, Vignaux O, Cohen P, Guilpain P, Pagnoux C, Gouya H, et al. Impact of cardiac magnetic resonance imaging for assessment of Churg‐Strauss syndrome: a cross‐sectional study in 20 patients. Clin Exp Rheumatol 2009;27 Suppl 52:S70–6. [PubMed] [Google Scholar]
- 23. Neumann T, Manger B, Schmid M, Kroegel C, Hansch A, Kaiser WA, et al. Cardiac involvement in Churg‐Strauss syndrome: impact of endomyocarditis. Medicine (Baltimore) 2009;88:236–43. [DOI] [PubMed] [Google Scholar]
- 24. Wassmuth R, Gobel U, Natusch A, Schneider W, Kettritz R, Dietz R, et al. Cardiovascular magnetic resonance imaging detects cardiac involvement in Churg‐Strauss syndrome. J Card Fail 2008;14:856–60. [DOI] [PubMed] [Google Scholar]
- 25. Dunogue B, Terrier B, Cohen P, Marmursztejn J, Legmann P, Mouthon L, et al. Impact of cardiac magnetic resonance imaging on eosinophilic granulomatosis with polyangiitis outcomes: a long‐term retrospective study on 42 patients. Autoimmun Rev 2015;14:774–80. [DOI] [PubMed] [Google Scholar]
- 26. Ribi C, Cohen P, Pagnoux C, Mahr A, Arene JP, Lauque D, et al. Treatment of Churg‐Strauss syndrome without poor‐prognosis factors: a multicenter, prospective, randomized, open‐label study of seventy‐two patients. Arthritis Rheum 2008;58:586–94. [DOI] [PubMed] [Google Scholar]
- 27. Pagnoux C, Quemeneur T, Ninet J, Diot E, Kyndt X, de Wazieres B, et al. Treatment of systemic necrotizing vasculitides in patients aged sixty‐five years or older: results of a multicenter, open‐label, randomized controlled trial of corticosteroid and cyclophosphamide‐based induction therapy. Arthritis Rheumatol 2015;67:1117–27. [DOI] [PubMed] [Google Scholar]
- 28. Cohen P, Pagnoux C, Mahr A, Arene JP, Mouthon L, le Guern V, et al. Churg‐Strauss syndrome with poor‐prognosis factors: a prospective multicenter trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in forty‐eight patients. Arthritis Rheum 2007;57:686–93. [DOI] [PubMed] [Google Scholar]
- 29. Thiel J, Troilo A, Salzer U, Schleyer T, Halmschlag K, Rizzi M, et al. Rituximab as induction therapy in eosinophilic granulomatosis with polyangiitis refractory to conventional immunosuppressive treatment: a 36‐month follow‐up analysis. J Allergy Clin Immunol Pract 2017;5:1556–63. [DOI] [PubMed] [Google Scholar]
- 30. Mohammad AJ, Hot A, Arndt F, Moosig F, Guerry MJ, Amudala N, et al. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg‐Strauss). Ann Rheum Dis 2016;75:396–401. [DOI] [PubMed] [Google Scholar]
- 31. Teixeira V, Mohammad AJ, Jones RB, Smith R, Jayne D. Efficacy and safety of rituximab in the treatment of eosinophilic granulomatosis with polyangiitis. RMD Open 2019;5:e000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emmi G, Rossi GM, Urban ML, Silvestri E, Prisco D, Goldoni M, et al. Scheduled rituximab maintenance reduces relapse rate in eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis 2018;77:952–4. [DOI] [PubMed] [Google Scholar]
- 33. Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017;376:1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Metzler C, Hellmich B, Gause A, Gross WL, de Groot K. Churg Strauss syndrome–successful induction of remission with methotrexate and unexpected high cardiac and pulmonary relapse ratio during maintenance treatment. Clin Exp Rheumatol 2004;22 Suppl 36:S52–61. [PubMed] [Google Scholar]
- 35. Maritati F, Alberici F, Oliva E, Urban ML, Palmisano A, Santarsia F, et al. Methotrexate versus cyclophosphamide for remission maintenance in ANCA‐associated vasculitis: a randomised trial. PLoS One 2017;12:e0185880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Puechal X, Pagnoux C, Baron G, Quemeneur T, Neel A, Agard C, et al. Adding azathioprine to remission‐induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (churg‐strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognosis factors: a randomized, controlled trial. Arthritis Rheumatol 2017;69:2175–86. [DOI] [PubMed] [Google Scholar]
- 37. Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim‐sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high‐dose glucocorticoids. Ann Rheum Dis 2018;77:644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hauser T, Mahr A, Metzler C, Coste J, Sommerstein R, Gross WL, et al. The leucotriene receptor antagonist montelukast and the risk of Churg‐Strauss syndrome: a case‐crossover study. Thorax 2008;63:677–82. [DOI] [PubMed] [Google Scholar]
- 39. Harrold LR, Patterson MK, Andrade SE, Dube T, Go AS, Buist AS, et al. Asthma drug use and the development of Churg‐Strauss syndrome (CSS). Pharmacoepidemiol Drug Saf 2007;16:620–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


