Abstract
Objective
Takayasu’s arteritis (TAK) is a granulomatous large‐vessel vasculitis primarily affecting the aorta and its proximal branches. TAK can be a difficult disease to diagnose and manage given the rarity of the disease as well as current limitations in biomarkers, imperfect imaging modalities, and few randomized controlled trials.
Methods
In developing the American College of Rheumatology/Vasculitis Foundation guideline for the management of TAK, we performed an extensive systematic literature review to guide our recommendations. We included RCTs first. When RCTs were not available, we included observational studies that reported on patient‐important outcomes for the intervention and comparison. When studies with comparative data were not available, we included case series that present patient‐important outcomes for either the intervention or the comparison.
Results
Three hundred forty‐seven articles were included for full review to answer 27 population, intervention, comparison, and outcome questions related to TAK. Ten studies were evaluated that addressed the use of glucocorticoids (GCs), non‐GC nonbiologic therapies, as well as biologics in treating TAK. A total of 33 studies, including 8 comparative studies, were included to determine the test accuracy of commonly available diagnostic tests for TAK.
Conclusion
This comprehensive systematic review synthesizes and evaluates the benefits and harms of different treatment options and the accuracy of commonly used tests for the management of TAK.
INTRODUCTION
Takayasu’s arteritis (TAK) is an idiopathic granulomatous large‐vessel vasculitis (LVV) that preferentially involves the aorta, its proximal branches, and the pulmonary arteries. Inflammation of the arterial wall may result in stenosis, occlusion, dilation, or aneurysm formation (1). The disease is more commonly diagnosed in young women of Asian descent, but occurs worldwide, with variable incidence rates ranging from 0.3 per million to 40 per million (2, 3, 4). Delay in diagnosis is common, as the clinical presentation can be nonspecific with a predominance of constitutional symptoms early in the disease course. The 1990 American College of Rheumatology Classification Criteria for TAK specify four clinical findings, including claudication of the extremities, decreased brachial artery pulse, blood pressure difference of more than 10 mm Hg, and bruits auscultated over the subclavian arteries or aorta (5). These clinical features are often later findings that manifest after inflammation and damage of the vessel wall has occurred. Difficulty in diagnosing TAK is confounded by insufficient biomarkers as well as the general lack of access to pathologic tissue. Because of these issues, clinicians tend to rely heavily on imaging modalities in diagnosing and monitoring patients with TAK. Guidance on effective management of TAK is often drawn from observational and retrospective studies. Given the rarity of the disease, there is a paucity of randomized controlled trials (RCTs) from which to extrapolate definitive therapeutic strategies. Active disease can result in significant morbidity, including strokes, aneurysm rupture, claudication, and renovascular hypertension.
The first aim of this systematic review is to compare the benefits and harms of different treatment options for patients with TAK. The literature reviewed includes RCTs and nonrandomized studies and presents the evidence and an assessment of its certainty for important outcomes. The second aim of this systematic review is to determine the accuracy of commonly available diagnostic tests for TAK. These reviews were used to inform evidence‐based recommendations on diagnostic and management strategies for TAK by the American College of Rheumatology (ACR)/Vasculitis Foundation (VF) Vasculitis Management Guidelines.
METHODS
Search strategy and data sources
An information specialist systematically searched the published English‐language literature, including OVID Medline, PubMed, Embase, and the Cochrane Library (including Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, and Health Technology Assessments) from the inception of each database through August 2018 to obtain direct evidence in patients with vasculitis relating to the population, intervention, comparison, and outcome (PICO) questions (Supplementary Appendix 1); the information specialist updated the searches conducted on August 2019. The methods team used DistillerSR software to identify duplicate records (https://distillercer.com/products/distillersr‐systematic‐reviewsoftware/). The search was specific to address the PICO questions asked for each vasculitis type. The ACR/VF Vasculitis Guideline Core Team developed 27 PICO questions for TAK that addressed relevant or commonly encountered patient diagnostic testing, treatment, and management scenarios (Supplementary Appendix 2).
Study selection
We included studies that would provide the highest‐certainty evidence. For questions addressing treatment options, we included RCTs first. When RCTs were not available, we included observational studies (cohort and case‐control studies) that reported on patient‐important outcomes for the intervention and comparison. When studies with comparative data were not available, we included case series that present patient‐important outcomes for either the intervention or the comparison. For questions addressing diagnosis, we included studies that report on diagnostic test accuracy (cohort studies, cross‐sectional studies).
Patients of any age presenting to inpatient or outpatient settings with suspected or confirmed TAK were eligible for inclusion. When studies addressed multiple vasculitis types, we included data when results were presented separately or when more than 80% of the population included was patients with TAK. Studies reporting outcomes comparatively for the intervention and comparison in the PICO question or reporting outcomes for either the intervention or the comparison were included. In case of diagnostic questions, when test accuracy results were presented comparatively for the index test and the comparator or for either the index test or the comparator, the studies were included.
Excluded studies were studies with irrelevant population, intervention, or outcome; studies that have no primary data, such as letters, opinion pieces, or commentaries; narrative reviews; systematic reviews; epidemiological studies that only include prevalence or incidence results; any study that had fewer than 10 patients or if a study had more than 10 patients but only fewer than 10 were vasculitis it was excluded; any study that addressed an organ‐limited vasculitis except renal limited; and any study about basic research in animals.
Screening and data extraction
Pairs of two independent reviewers conducted title and abstract screening and full‐text review in duplicate to identify eligible studies. Data extraction was also conducted independently and in duplicate, and conflicts were resolved by a third reviewer (MAK). Each pair of reviewers included at least one of five clinical experts (KB, ABD, KEJ, YCL, JMS). Data extracted included general study characteristics (authors, publication year, country, study design), duration of follow‐up, outcome data for the intervention and/or comparison, and diagnostic index test and reference standard, along with parameters to determine test accuracy (ie, sensitivity and specificity of the index test) when relevant.
Risk of bias and data synthesis
When direct comparative results were available from RCTs, reviewers entered the results into RevMan v.5.3 software (Cochrane, London, UK) (http://tech.cochrane.org/revman), which was used to calculate pooled effect estimates. Reviewers evaluated the risk of bias using the Cochrane risk of bias tool(http://handbook.cochrane.org/ ).
When direct comparative results were available from observational studies (cohort, case‐control studies), reviewers entered the results into RevMan v.5.3 software, which was used to calculate pooled effect estimates. Reviewers evaluated the risk of bias using a modified Newcastle‐Ottawa Quality Assessment Scale for observational studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
When comparative results were not available, reviewers abstracted data describing details of the population, interventions, and results into summary tables.
When test accuracy results were available, reviewers abstracted test accuracy information and used the QUADAS tool to assess the risk of bias in the included studies. When pooling was appropriate, the review team used Open Meta Analyst (MetaMorph Inc., Tennessee, USA) (http://www.cebm.brown.edu/openmeta/) to pool test accuracy results.
Two investigators familiar with the GRADEpro software (Evidence Prime, Ontario, Canada) (https://gradepro.org) (MAK, NMH) formulated a Grading of Recommendations Assessment, Development and Evaluation (GRADE) summary of findings table for each PICO question when direct comparative data or test accuracy results were available. The investigators used the GRADE framework to assess overall certainty by evaluating the evidence for each outcome on the following domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias.
Data analysis
For questions addressing treatment options, relative risks (eg, risk ratios [RRs] and odds ratios [ORs]) were calculated by pooling results from RCTs and from observational studies comparing treatments. When no direct comparisons between treatments within a study were available, the risk of an event (or proportion) in a study (eg, disease relapse) was calculated, and then the weighted proportions from each study were combined and presented in the outcome description section of the summary tables.
For questions addressing diagnosis tests, the accuracy estimates from individual studies were combined quantitatively (pooled) for each test using OpenMetaAnalyst (http://www.cebm.brown.edu/openmeta/). We conducted a bivariate analysis for pooling sensitivity and specificity for each of the test comparisons to account for variation within and between studies. Forest plots were created for each comparison. The Breslow‐Day test was used to measure the percentage of total variation across studies due to heterogeneity (I2); however, the results did not influence our judgment of the pooled estimates, as the literature has discouraged its use for test accuracy.
RESULTS
Description of studies
The initial search retrieved 13 800 nonduplicate studies of which 2596 were included for full‐text review. Following full‐text review, we found 1156 articles to be potentially eligible for data abstraction and inclusion in the systematic reviews for the seven different types of vasculitis. For this review, we considered 347 articles for data abstraction for TAK.
Medications in TAK
We reviewed 10 studies that evaluated glucocorticoids (GCs), non‐GC nonbiologic therapies, as well as biologic agents in treating TAK (see Supplementary Appendix 3 for single‐arm data).
Prednisone/GCs
One comparative observational study evaluated high‐dose GC (>30 mg prednisone daily) versus low‐dose GC (<30 mg prednisone daily) , the risk of relapse was higher with low‐dose GC (OR: 2.28; confidence interval [CI]: 0.98‐5.28, P = .047), low certainty evidence. The risk of serious adverse events was lower with low‐dose GC (Table 1) (6). Single‐arm studies looking at the use of GCs alone were evaluated from the placebo arms of two RCTs (7, 8). From the 33 patients across these studies, 61% to 67% of those treated with GCs alone had relapses. Serious adverse events were seen in 12 of 33 patients treated with GCs alone, including infections/infestations and gastrointestinal disorders, though there was high inconsistency in the results. In evaluating the use of prednisone in the perioperative period, we found one study that demonstrated very low to low certainty in the evidence that the use of perioperative GCs resulted in improved symptoms and a lower rate of complications (RR: 0.09; CI: 0.01‐0.66) and death (RR: 0.31; CI: 0.01‐7.16) (9).
Table 1.
Comparative studies on impact of high‐ versus low‐dose GC on disease‐related outcomes and treatment‐related adverse events
| Certainty Assessment | Number of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Low‐dose GCs (<30 mg/d) | High‐dose GCs (>30 mg/d) |
Relative (95% CI) |
Absolute (95% CI) |
|
| Relapse | |||||||||||
| 1 | Observational studies | Not serious | Not serious | Not serious | Serious a | Strong association | 20/39 (51.3%) | 18/57 (31.6%) |
OR 2.28 (0.98 to 5.28) |
197 more per 1000 (from 4 fewer to 393 more) |
⨁⨁◯◯ LOW |
| Death | |||||||||||
| 1 | Observational studies | Not serious | Not serious | Not serious | Very serious a | None | 2/39 (5.1%) | 3/57 (5.3%) |
OR 0.97 (0.15 to 6.11) |
1 fewer per 1000 (from 44 fewer to 201 more) |
⨁◯◯◯ VERY LOW |
| Serious adverse events | |||||||||||
| 1 | Observational studies | Not serious | Not serious | Not serious | Serious a | Strong association | 22/39 (56.4%) | 45/57 (78.9%) |
OR 0.35 (0.14 to 0.85) |
222 fewer per 1000 (from 445 fewer to 28 fewer) |
⨁⨁◯◯ LOW |
See Mutoh et al for reference.
Abbreviations: CI, confidence interval; GC, glucocorticoid; OR, odds ratio.
The lower versus upper limits of the CI would lead to different clinic decisions.
Nonglucocorticoid nonbiologic therapies
One comparative observational study evaluated the effect of using non‐GC nonbiologic therapy in TAK (10). The non‐GC nonbiologic therapies in this study included methotrexate, azathioprine, mycophenolate mofetil, cyclophosphamide, and leflunomide. It demonstrated that the 2‐year flare‐free survival was ~80% with biologics compared with 43% in patients on non‐GC nonbiologic therapies when adjusted for the number of treatment episodes per patient (P = .03). The clinical remission rate in another observational study with 6 months of follow‐up showed that remission was achieved in 71.7% of those treated with cyclophosphamide versus 75% of those treated with methotrexate. Imaging remained stable in 78% of those treated with cyclophosphamide compared with 83% of those treated with methotrexate. Wall enhancement at 6 months was reduced in the cyclophosphamide group (P = .032) but not in the methotrexate group (P = .433). Side effects were consistent with known profiles of these medications, with more infections in the cyclophosphamide group and more transaminitis in the methotrexate group (11).
Tumor necrosis factor inhibitors
Four studies were included for review on tumor necrosis factor inhibitor (TNFi) use in patients with TAK (12, 13, 14, 15). Three of these were retrospective observational studies, and one was an observational cohort study (12). Across the studies, 126 patients were included, and 90 achieved remission on TNFi for a total remission rate of 71%. The TNFi included in these studies were etanercept, infliximab, and adalimumab, and the majority of patients had failed other medications, including methotrexate, azathioprine, mycophenolate mofetil, and cyclophosphamide. In the study by Gudbrandsson et al, patients on TNFis had a higher sustained remission rate than patients on non‐GC nonbiologic medications (42% vs 20%, P = .03) (14). This was also seen in the study by Mekinian et al, which included 56 patients treated with TNFi (infliximab: n = 44; etanercept: n = 6; and adalimumab: n = 6), demonstrating a 3‐year relapse‐free survival of 91% in those treated with TNFi compared with 58.7% (43.3%‐79.7%) in those treated with non‐GC nonbiologic medications (13). In the study by Schmidt et al, 20 patients with refractory TAK were treated with TNFi (infliximab: n = 17; adalimumab: n = 2; etanercept: n = 1). Disease remission was achieved in 18 (90%), with sustained remission in 10 patients (50%) over 23 (interquartile range: 8.7‐38.9) months (15). In the study by Molloy et al, 25 patients with refractory TAK were treated with TNFi (infliximab: n = 21; etanercept: n = 9; 5 patients initially treated with etanercept subsequently switched to infliximab) for up to 7 years. Following TNFi therapy, remission was achieved in 15 patients, with complete discontinuation of prednisone, and an additional 7 patients successfully tapered prednisone to less than 10 mg/d. Major relapses occurred in four patients who initially achieved stable remission (12).
Three studies reported on adverse events, which included 18 adverse events in 94 patients; however, details of the events were not consistently reported (12, 13, 15).
Tocilizumab
One RCT (7) and one retrospective multicenter study (16) evaluated the role of tocilizumab (TCZ) in the management of patients with TAK. The retrospective study (16) showed a relapse rate of 6%, which was lower than the 34.6% relapse rate in non‐GC nonbiologics (P = .049). However, the RCT with 32 patients showed a relapse rate of 44% in the TCZ arm (7).
The study by Mekinian et al assessed patients with active TAK (National Institute of Health (NIH) score ≥2) treated with TCZ. Treatment response was defined as a NIH score less than 2 and prednisone dose less than 7.5 mg/d. Two‐thirds of patients had a treatment response with TCZ by 6 months (NIH score from 3 to 1, P < .0001, and daily prednisone from 15 to 5 mg/d, P < .001). Relapse was defined as active disease after a remission period and with change of the treatment regimen. At 24 months, the overall survival without TCZ failure was 0.72 (95% CI: 0.55‐0.95) (16). In the double‐blind RCT by Nakoaka (7), patients with TAK who relapsed in the previous 12 weeks were induced into remission with oral GC therapy and were randomly assigned to subcutaneous TCZ of 162 mg/wk (n = 18) or placebo (n = 18) along with a predefined oral GC taper. The primary end point was time‐to‐relapse of TAK (defined as ≥2 of the following: objective systemic symptoms, subjective systemic symptoms, elevated inflammation markers, and vascular signs and symptoms or ischemic symptoms). Relapses occurred in 8 TCZ and 11 placebo patients, with a hazards ratio of time‐to‐relapse of 0.41 (95.41% CI: 0.15‐1.10; P = .0596) in the intent‐to‐treat population and 0.34 (95.41% CI: 0.11‐1.00; P = .0345) in the per‐protocol set analysis. Although the primary end point was not met, the results favor TCZ over placebo in time‐to‐relapse of TAK, with no new safety signals. Six of the 18 patients in the TCZ plus glucocorticoid group had infections/infestations during the 56‐week trial (7). Across both studies, 3 of 46 patients on TCZ had serious adverse events (7, 16).
Abatacept
One randomized controlled trial evaluated the role of abatacept in sustaining remission in newly diagnosed or relapsing TAK (8). The trial included 34 patients, with 26 reaching week 12 randomization. The relapse‐free survival at 12 months was 22% for those on abatacept and 40% for those on placebo (P = .853). Treatment with abatacept compared with placebo was not associated with a longer median duration of remission (5.5 vs 5.7 months, P = .125). There was no difference in the frequency or severity of adverse events between treatment arms, including infection.
Test accuracy results: assessment and monitoring of disease
The use of catheter‐based arteriograms has been largely replaced by noninvasive imaging modalities including ultrasound (US), computerized tomography angiography (CTA), fluorodeoxyglucose‐positron emission tomography (FDG‐PET), and magnetic resonance imaging/magnetic resonance angiography (MRI/MRA). Our review identified eight studies that used a cohort or case‐control study design and evaluated FDG‐PET and MRI/MRA in diagnosis and monitoring patients with TAK. Combined, these modalities had a pooled sensitivity of 72% and specificity of 69% in diagnosis and monitoring of TAK. The certainty was very low mostly because of the risk of bias, imprecision and indirectness of the studies. We judged high risk of bias due to patient selection and that the results of the imaging modality being studied were interpreted with knowledge of the results of the standard reference. In addition, not all patients received a reference test (Table 2). A total of 33 studies evaluated noninvasive imaging modalities in assessing and monitoring disease activity in patients with TAK (see Supplementary Appendix 3 for single‐arm data).
Table 2.
Test accuracy studies of noninvasive imaging modalities
| Outcome | No. of Studies (No. of Patients) | Study Design | Factors that may Decrease Certainty of Evidence | Effect per 1000 Patients Tested | Test Accuracy CoE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | Pretest probability of 40% | Pretest probability of 50% | ||||
| True‐positives | 8 (180) | Cohort & case‐control type studies | Serious a | Serious b | Not serious c | Serious d | None | 286 (218‐337) | 358 (272‐421) |
⨁◯◯◯ VERY LOW |
| False‐negatives | 114 (63‐182) | 142 (79‐228) | ||||||||
| True‐negatives | 8 (193) | Cohort & case‐control type studies | Serious a | Serious b | Not serious c | Serious d | None | 416 (320‐490) | 347 (267‐408) |
⨁◯◯◯ VERY LOW |
| False‐positives | 184 (110‐280) | 153 (92‐233) | ||||||||
Sensitivity was 0.72 (95% CI: 0.54‐0.84) and specificity was 0.69 (95% CI: 0.53‐0.82). Prevalences were 40% and 50%. True‐positives are patients with disease activity; false‐negatives are patients incorrectly classified as not having disease activity; true‐negatives are patients without disease activity; and false‐negatives are patients incorrectly classified as having disease activity. See Webb et al, Grayson et al, Eshet et al, Quinn et al, John et al, Santhosh et al, Karapolat et al, Nguyen et al, for reference (25, 26, 27, 28, 29, 36, 37, 38).
Abbreviations: CI, confidence interval; COE, certainty of evidence.
Due to patient selection (some studies did not avoid inappropriate exclusions), the results of the index test were interpreted with knowledge of the results of the standard reference, and not all patients received a reference test.
Indirectly compares the interventions in which we are interested (invasive vs noninvasive) when applied to the populations in which we are interested.
The similarity of point estimates and overlap of CIs make inconsistency not serious.
Clinical action would differ if the upper versus the lower boundary of the CI represented the truth.
Test accuracy results: inflammatory markers
The utility of measuring erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP) in patients with TAK was evaluated in three single‐arm studies and showed a sensitivity and specificity of 75% and a low overall test accuracy (17, 18, 19). Although ESR and CRP are neither highly sensitive or specific for disease activity in TAK, they are still used in clinical practice to monitor disease activity and are incorporated into the NIH disease activity score as well as the Indian Takayasu Clinical Activity Score (ITAS) disease activity measurement (1, 20). Certain medications, such as TCZ, suppress the production of inflammatory markers, making them less reliable for tracking disease activity (21).
Test accuracy results: imaging modalities
Ultrasound
There were no comparative studies that evaluated US in TAK. A single‐arm study looking at US (22) demonstrated that contrast‐enhanced US grade correlated significantly with the NIH activity index (<0.001) and ITAS 2000 (P = .004) and that repeat US imaging was helpful in detecting progression of lesions (23). US of the carotids correlated with clinical disease activity and remission but did not reach statistical significance (24).
Figure 1.
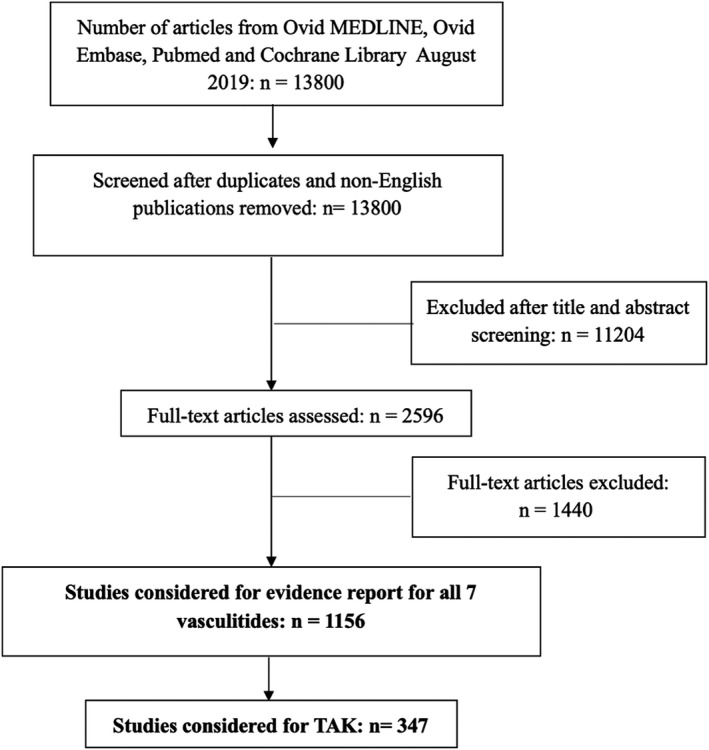
PRISMA flow diagram for included studies. TAK, Takayasu arteritis.
Magnetic resonance imaging/magnetic resonance angiography
Two comparative studies of MRI/MRA had a sensitivity of 40% to 44% when evaluating new arterial wall enhancement or interval appearance of anatomical changes compared with clinical symptoms and ESR/CRP in monitoring disease activity in patients with TAK (Figure 2) (25) (risk of bias assessment in Supplementary Appendix 4). When ITAS or ESR were used as a reference, the sensitivity of MRI/MRA ranged from 67% to 90% and specificity ranged from 65% to 85% (25, 26). In a study of patients with LVV (30 with TAK, 35 giant‐cell arteritis [GCA]) edema and wall thickness on MRA was associated with FDG‐PET activity. Clinical status was associated with disease activity by FDG‐PET but not MRA. In 51% of patients in clinical remission, both MRA and FDG‐PET demonstrated active disease (27).
Figure 2.
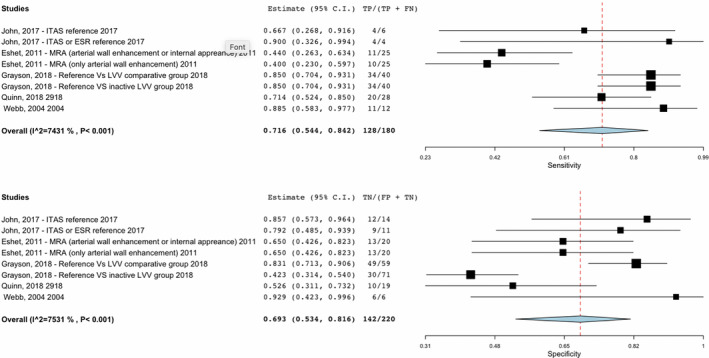
Sensitivity and specificity of studies on noninvasive imaging modalities in Takayasu arteritis (TAK).
fluorodeoxyglucose‐positron emission tomography/computed tomography
Six comparative studies evaluated the role of FDG‐PET/CT in diagnosing and monitoring TAK (Figure 2). A study evaluating FDG‐PET in 115 patients (30 GCA and 26 TAK in the LVV group compared with 35 dyslipidemia, 17 disease‐mimicking LVV, and 7 healthy controls in the comparator group) showed a sensitivity of 85% and a specificity of 83% to distinguish between patients with clinically active LVV and comparator subjects (clinically active disease defined by physician interpretation of history, examination, and laboratory assessments). FDG‐PET had a specificity of 42% in distinguishing patients with clinically active LVV and patients with LVV in clinical remission (defined as absence of any clinical symptoms directly attributable to vasculitis) (28). In the study by Quinn et al comparing MRA with FDG‐PET scan, clinical status was associated with disease activity by FDG‐PET. FDG‐PET in patients with TAK had a sensitivity of 71% but a specificity of 52% when compared with MRA (27). Another comparator study of 18 patients showed a sensitivity of 88% and specificity of 93% in use of FDG‐PET when compared with clinical disease activity including positive angiography for the initial assessment of active vasculitis in TAK (29).
A single‐arm study of 39 patients evaluating FDG‐PET had moderate test accuracy in diagnosing active disease in TAK, with a sensitivity of 91% and specificity of 92% using NIH criteria as the gold standard (includes assessment of ESR and CRP) (30). Across three single‐arm studies that included 41 patients with TAK, an increase in FDG uptake was observed in patients with TAK who had increased acute phase reactants (31, 32, 33).
DISCUSSION
This review presents pooled estimates of important patient outcomes, such as the ability to achieve remission, rate of flares or relapses, and adverse effects of therapy. We also report the test accuracy for commonly available diagnostic tests for TAK.
Medications in TAK
The certainty of evidence was low to very low for studies of GC use in patients with TAK, and there were no comparative studies evaluating non‐GC, nonbiologic, or biologic therapies in the treatment of TAK. Comparative data on GC supported the use of higher‐dose GC in the treatment of TAK, with lower relapse rates (7, 8). They also demonstrated the benefit of using GC during the perioperative period (9).
When evaluating the impact of therapeutic options in TAK, a significant limitation is the lack of standardized definitions for remission and relapse across studies. Evaluation of single‐arm studies and extrapolation from available studies demonstrated that non‐GC nonbiologic therapies, such as methotrexate, mycophenolate mofetil, or azathioprine, can be used to sustain remission in TAK (11, 34). No RCTs have been done evaluating TNFi in TAK; however, data across studies showed that ~70% of patients were able to achieve remission with TNFi (including infliximab, etanercept, and adalimumab) in patients who had previously failed non‐GC nonbiologic medications (12, 13, 14, 15) and that the rate of sustained remission was higher in those treated with TNFi compared with those treated with non‐GC nonbiologic medications (14). Abatacept and TCZ were each evaluated in RCTs that failed to meet their primary end point. However, TCZ can effectively be used to attain remission, with variable relapse rates reported, between 6% and 44% (7, 16). Although the primary outcome of time to relapse was not met, TCZ did show a numerically higher rate of relapse‐free survival (51%) compared with placebo (23%) and can thus be used in refractory disease (7). Abatacept was not able to demonstrate longer sustained remission compared with placebo in patients with TAK who had already achieved remission (8). Given the high relapse rate with GC monotherapy, data support the use of additional immunosuppressive agents in the induction and maintenance of remission in TAK. There were no randomized trials evaluating non‐GC nonbiologics compared with biologics in newly diagnosed disease. Based on the data, which shows that a subset of patients can achieve sustained clinical and radiographic remission with non‐GC nonbiologics, we recommend that patients should initially be treated with high‐dose GCs as well as a non‐GC nonbiologic therapy with escalation to TNFi in refractory or relapsing disease. In patients who fail TNFi, there is evidence to support the use of TCZ.
Inflammatory markers and imaging modalities in TAK
This review also aimed to evaluate the accuracy and reliability of imaging modalities in diagnosing and monitoring patients with TAK. Although there has been research evaluating potential biomarkers in TAK, in practice we tend to incorporate measurements of ESR and CRP as part of our assessment for active disease. Three single‐arm studies showed a sensitivity and specificity of 75% for elevations in ESR and CRP correlating with clinical disease activity and a low overall test accuracy (17, 18, 19). Because of the unreliability of lab parameters combined with the nonspecific disease manifestations of patients with active TAK, heavy reliance is placed on imaging modalities in both diagnosing and monitoring patients with TAK.
Invasive modalities, such as angiography, have been largely replaced by noninvasive options, including FDG‐PET scan, CTA, MRI/MRA, and US. The ideal imaging modality would allow for early detection of inflammation in the vessel wall and accurate morphology of the vessel, and it would correlate with clinical disease activity, demonstrate responsiveness to therapies, and be predictive of future vascular abnormalities. Each of the available noninvasive imaging modalities has specific advantages and disadvantages (Table 3). US is a low‐risk, inexpensive method of assessing blood vessels, but it cannot be used to visualize the thoracic aorta. A few single‐arm studies showed correlation between US and clinical disease activity measurements, such as the NIH criteria and ITAS 2000 (22, 23). There was no comparative data on the use of CTA in diagnosing or monitoring patients with TAK. Despite the high anatomic detail provided by CTA, the high radiation exposure combined with the young age of these patients who will need long‐term repeat interval monitoring discourages the use of CTA for monitoring disease activity if other modalities can be used.
Table 3.
Imaging modalities in Takayasu arteritis
| Modality | Invasiveness | Vessel Wall | Inflammation | Radiation | Considerations |
|---|---|---|---|---|---|
| Angiography | Invasive | High |
|
||
| CDUS | Noninvasive | ✔□ | ✔□ | None |
|
| CTA | Noninvasive | ✔□ | ✔□ | High |
|
| MRA | Noninvasive | ✔□ | ✔□ | None |
|
| PET/CT | Noninvasive | ✔□ | ✔□ | High |
|
Eight studies that were cohort or case‐control in design evaluated FDG‐PET and MRI/MRA in diagnosis and monitoring of patients with TAK and demonstrated a pooled sensitivity of 72% and specificity of 69%. Features suggesting active disease on MRI/MRA include new areas of wall thickening and enhancement, and depending on the comparator arm, the sensitivity ranges from 40% to 90% with a specificity of 65% to 85% (25, 26). Wall thickening on MRI/MRA may be due to active inflammation but may also reflect vessel wall damage or remodeling. The use of FDG‐PET scan in TAK is being highly investigated given its high sensitivity, which ranges from 70% to 90%, across multiple studies (27, 28, 29, 30) and correlation with clinically active disease (27). The ability to detect activity in the acute phase prior to edema and other vascular structural changes is promising; however, access and costs are significant limitations to the widespread use of FDG‐PET scan (35). Additionally, false‐positives can be seen in conditions such as atherosclerosis. Although FDG uptake in atherosclerosis often demonstrates discontinuous patchy uptake compared with the smooth linear uptake in TAK, there can be overlap (28). As noted in the study by Quinn et al, about half of patients in clinical remission were found to have active disease on MRI/MRA and PET (27). The responsiveness of radiographic features to therapy and the ability of imaging modalities to predict clinical relapses or outcomes needs continued study. Therapeutic decision making must take into consideration multiple modalities of assessing disease activity, including radiographic and clinical assessment.
Based on the available data from comparative and single‐arm studies, we recommend using regularly scheduled imaging in addition to checking ESR/CRP and repeated long‐term clinical evaluation of patients with TAK. Studies had variable intervals for how frequently imaging was performed or inflammatory markers were checked, so how often to obtain them is unknown. This results in a significant challenge in the monitoring of these patients, though it is reasonable to consider shorter intervals early in the disease course, and longer in patients with established, quiescent disease. Routine imaging in inactive disease is generally not recommended for children.
This review has several strengths. The comprehensive and systematic approach for identifying studies makes it unlikely that relevant studies were missed. Additionally, we assessed the certainty of evidence in this area and identified sources of bias. We note a few limitations in this comprehensive systematic review. We limited our review to the English language. Given the relative rarity of the disease, there were a limited number of RCTs or comparative data available to answer the posed questions. There is no gold standard diagnostic study for TAK, so in assessing test accuracy, comparator arms were variable.
This comprehensive systematic review synthesizes and evaluates the benefits and toxicities of different treatment options and accuracy of commonly used tests for the diagnosis of TAK. Estimates of benefits and toxicities as well as sensitivity and specificity from this review were used to model diagnostic and management strategies and inform evidence‐based recommendations for the ACR/VF Vasculitis Management Guideline.
AUTHOR CONTRIBUTIONS
ABD, KB, MAK, NMH, RAM contributed to drafting the report; ABD, KB, JMS, KEJ, YCL, AVF critically revised the report; AA, MM, SAC, CL, provided feedback on the included studies, and contributed to the critical revision of the report; all authors approved the final version of the manuscript
Study conception and design
Kalot, Husainat, Mustafa.
Acquisition of data
Dua, Kalot, Husainat, Byram, Springer, James, Lin, Villa‐Forte, Mustafa.
Analysis and interpretation of data
Dua, Kalot, Husainat, Byram, Springer, James, Lin, Villa‐Forte, Abril, Mehdrad, Langford, Chung, Mustafa
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
The systematic review team would like to acknowledge Amy Turner, Regina Parker, and Robin Lane for their assistance with administrative support, data management, and coordination of the project. The review team would also like to acknowledge the voting panel, expert panel, and patient panel members of the American College of Rheumatology Vasculitis Practice Clinical Guidelines 2020 for their review of the evidence and input during the guideline development process.
This systematic review was conducted to support the development of the American College of Rheumatology 2020 guidelines for diagnosis and management of vasculitis. The entire guideline development process was funded by the American College of Rheumatology. Through the Outcomes and Implementation Research Unit at the University of Kansas Medical Center, some researchers received salary or grant support, whereas others volunteered their time.
Drs. Dua and Kalot contributed equally to this work.
Dr. Dua has consulted for Chemocentryx, Novartis, Abbvie and GSK. Dr. Springer has been a site investigator for InflaRx. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med 1994;120:919–29. [DOI] [PubMed] [Google Scholar]
- 2. Watts R, Al‐Taiar A, Mooney J, Scott D, Macgregor A. The epidemiology of Takayasu arteritis in the UK. Rheumatology (Oxford) 2009;48:1008–11. [DOI] [PubMed] [Google Scholar]
- 3. Zaldivar Villon ML, de la Rocha JA, Espinoza LR. Takayasu arteritis: recent developments. Curr Rheumatol Rep 2019;21:45. [DOI] [PubMed] [Google Scholar]
- 4. Toshihiko N. Current status of large and small vessel vasculitis in Japan. Int J Cardiol 1996;54:S91–8. [DOI] [PubMed] [Google Scholar]
- 5. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed] [Google Scholar]
- 6. Mutoh T, Shirai T, Fujii H, Ishii T, Harigae H. Insufficient use of corticosteroids without immunosuppressants results in higher relapse rates in Takayasu arteritis. J Rheumatol 2020;47:255–63. [DOI] [PubMed] [Google Scholar]
- 7. Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double‐blind, placebo‐controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 2018;77:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A randomized, double‐blind trial of abatacept (CTLA‐4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017;69:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng T, Zhu S, Ou JF, Fang WG, Qiao ZY, Qi RD, et al. Treatment with corticosteroid and/or immunosuppressive agents before surgery can effectively improve the surgical outcome in patients with Takayasu's arteritis. J Invest Surg 2019;32:220–7. [DOI] [PubMed] [Google Scholar]
- 10. Aeschlimann FA, Eng SW, Sheikh S, Laxer RM, Hebert D, Noone D, et al. Childhood Takayasu arteritis: disease course and response to therapy. Arthritis Res Ther 2017;19:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun Y, Ma L, Ma L, Kong X, Chen H, Lv P, et al. Cyclophosphamide could be a better choice than methotrexate as induction treatment for patients with more severe Takayasu's arteritis. Rheumatol Int 2017;37:2019–26. [DOI] [PubMed] [Google Scholar]
- 12. Molloy ES, Langford CA, Clark TM, Gota CE, Hoffman GS. Anti‐tumour necrosis factor therapy in patients with refractory Takayasu arteritis: long‐term follow‐up. Ann Rheum Dis 2008;67:1567–9. [DOI] [PubMed] [Google Scholar]
- 13. Mekinian A, Comarmond C, Resche‐Rigon M, Mirault T, Kahn JE, Lambert M, et al. Efficacy of biological‐targeted treatments in Takayasu arteritis: multicenter, retrospective study of 49 patients. Circulation 2015;132:1693–700. [DOI] [PubMed] [Google Scholar]
- 14. Gudbrandsson B, Molberg Ø, Palm Ø. TNF inhibitors appear to inhibit disease progression and improve outcome in Takayasu arteritis; an observational, population‐based time trend study. Arthritis Res Ther 2017;19:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt J, Kermani TA, Bacani AK, Crowson CS, Matteson EL, Warrington KJ. Tumor necrosis factor inhibitors in patients with Takayasu arteritis: experience from a referral center with long‐term followup. Arthritis Care Res (Hoboken) 2012;64:1079–83. [DOI] [PubMed] [Google Scholar]
- 16. Mekinian A, Resche‐Rigon M, Comarmond C, Soriano A, Constans J, Alric L, et al. Efficacy of tocilizumab in Takayasu arteritis: multicenter retrospective study of 46 patients. J Autoimmun 2018;91:55–60. [DOI] [PubMed] [Google Scholar]
- 17. Comarmond C, Biard L, Lambert M, Mekinian A, Ferfar Y, Kahn JE, et al. Long‐term outcomes and prognostic factors of complications in Takayasu arteritis: a multicenter study of 318 patients. Circulation 2017;136:1114–22. [DOI] [PubMed] [Google Scholar]
- 18. Keşkek ŞÖ, Bozkırlı‐Ersözlü ED, Kozanoglu I, Yücel AE. High levels of circulating endothelial progenitor cells are associated with acrotism in patients with Takayasu arteritis. Med Princ Pract 2017;26:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Dang A, Lv N, Liu Q, Chen B. High‐sensitivity C‐reactive protein predicts adverse cardiovascular events in patients with Takayasu arteritis with coronary artery involvement. Clin Rheumatol 2016;35:679–84. [DOI] [PubMed] [Google Scholar]
- 20. Misra R, Danda D, Rajappa SM, Ghosh A, Gupta R, Mahendranath KM, et al. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010). Rheumatology (Oxford) 2013;52:1795–801. [DOI] [PubMed] [Google Scholar]
- 21. Naka T, Nishimoto N, Kishimoto T. The paradigm of IL‐6: from basic science to medicine. Arthritis Res 2002;4 Suppl 3:S233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Z, Zheng Z, Ding J, Li X, Zhao Y, Kang F, et al. Contrast‐enhanced ultrasonography for monitoring arterial inflammation in Takayasu arteritis. J Rheumatol 2019;46:616–22. [DOI] [PubMed] [Google Scholar]
- 23. Sun Y, Yip PK, Jeng JS, Hwang BS, Lin WH. Ultrasonographic study and long‐term follow‐up of Takayasu's arteritis. Stroke 1996;27:2178–82. [DOI] [PubMed] [Google Scholar]
- 24. Fan W, Zhu J, Li J, Zhang W, Li C. Ultrasound morphological changes in the carotid wall of Takayasu's arteritis: monitor of disease progression. Int Angiol 2016;35:586–92. [PubMed] [Google Scholar]
- 25. Eshet Y, Pauzner R, Goitein O, Langevitz P, Eshed I, Hoffmann C, et al. The limited role of MRI in long‐term follow‐up of patients with Takayasu's arteritis. Autoimmun Rev 2011;11:132–6. [DOI] [PubMed] [Google Scholar]
- 26. John RA, Keshava SN, Danda D. Correlating MRI with clinical evaluation in the assessment of disease activity of Takayasu's arteritis. Int J Rheum Dis 2017;20:882–6. [DOI] [PubMed] [Google Scholar]
- 27. Quinn KA, Ahlman MA, Malayeri AA, Marko J, Civelek AC, Rosenblum JS, et al. Comparison of magnetic resonance angiography and (18)F‐fluorodeoxyglucose positron emission tomography in large‐vessel vasculitis. Ann Rheum Dis 2018;77:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et al. (18) F‐fluorodeoxyglucose‐positron emission toAL‐Nahhasmography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol 2018;70:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Webb M, Chambers A, A, Mason JC, Maudlin L, Rahman L, et al. The role of 18F‐FDG PET in characterising disease activity in Takayasu arteritis. Eur J Nucl Med Mol Imaging 2004;31:627–34. [DOI] [PubMed] [Google Scholar]
- 30. Tezuka D, Haraguchi G, Ishihara T, Ohigashi H, Inagaki H, Suzuki J, et al. Role of FDG PET‐CT in Takayasu arteritis: sensitive detection of recurrences. JACC Cardiovasc Imaging 2012;5:422–9. [DOI] [PubMed] [Google Scholar]
- 31. Walter MA, Melzer RA, Schindler C, Müller‐Brand J, Tyndall A, Nitzsche EU. The value of [18F]FDG‐PET in the diagnosis of large‐vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging 2005;32:674–81. [DOI] [PubMed] [Google Scholar]
- 32. Alibaz‐Oner F, Dede F, Ones T, Turoglu HT, Direskeneli H. Patients with Takayasu's arteritis having persistent acute‐phase response usually have an increased major vessel uptake by 18F‐FDG‐PET/CT. Mod Rheumatol 2015;25:752–5. [DOI] [PubMed] [Google Scholar]
- 33. Banerjee S, Quinn KA, Gribbons KB, Rosenblum JS, Civelek AC, Novakovich E, et al. Effect of treatment on imaging, clinical, and serologic assessments of disease activity in large‐vessel vasculitis. J Rheumatol 2020;47:99–107. [DOI] [PubMed] [Google Scholar]
- 34. Goel R, Danda D, Joseph G, Ravindran R, Kumar S, Jayaseelan V, et al. Long‐term outcome of 251 patients with Takayasu arteritis on combination immunosuppressant therapy: single centre experience from a large tertiary care teaching hospital in Southern India. Semin Arthritis Rheuma 2018;47:718–26. [DOI] [PubMed] [Google Scholar]
- 35. Pelletier‐Galarneau M, Ruddy TD. PET/CT for diagnosis and management of large‐vessel vasculitis. Curr Cardiol Rep 2019;21:34. [DOI] [PubMed] [Google Scholar]
- 36. Santhosh S, Mittal BR, Gayana S, Bhattacharya A, Sharma A, Jain S. F‐18 FDG PET/CT in the evaluation of Takayasu arteritis: an experience from the tropics. J Nucl Cardiol 2014;21:993–1000. [DOI] [PubMed] [Google Scholar]
- 37. Karapolat I, Kalfa M, Keser G, Yalçin M, Inal V, Kumanlioğlu K, et al. Comparison of F18‐FDG PET/CT findings with current clinical disease status in patients with Takayasu's arteritis. Clini Exp Rheumatol, 2013;31(1 Suppl 75), S15–S21. [PubMed] [Google Scholar]
- 38. Nguyen AD, Crowhurst T, Lester S, Dobson R, Bartholomeusz D, Hill C. The utility of fluorine‐18‐fluorodeoxyglucose positron emission tomography in the diagnosis and monitoring of large vessel vasculitis: A South Australian retrospective audit. Int J Rheum Dis. 2019;22:1378–1382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


