Abstract
Introduction
This study explored the safety and tolerability features of donanemab (LY3002813) in patients with mild cognitive impairment due to Alzheimer's disease (AD) or mild to moderate AD dementia.
Methods
Patients with AD were enrolled into the single‐ascending dose phase and were administered a single, intravenous (IV) dose of donanemab (five dosing cohorts from 0.1 to 10 mg/kg) or placebo followed by a 12‐week follow‐up period for each dose level. After the follow‐up period, the same patients proceeded into the multiple‐ascending dose (MAD) phase (five cohorts) and were administered IV doses of donanemab (0.3 to 10 mg/kg) or placebo approximately once per month for up to four doses depending on the initial doses (only cohort 1 went from 0.1 mg/kg to a higher dose of 0.3 mg/kg during the MAD phase). This phase concluded with a 12‐week follow‐up period. The relative exposure assessment of an unblinded, single, subcutaneous 3‐mg/kg dose of donanemab in patients with AD was also performed, followed by a 12‐week follow‐up period. One cohort of healthy subjects received an unblinded, single, IV 1‐mg/kg dose of donanemab. These two cohorts did not continue to the MAD phase.
Results
Donanemab was generally well tolerated up to 10 mg/kg. After single‐dose administration from 0.1 to 3.0 mg/kg, the mean terminal elimination half‐life was ≈4 days, increasing to ≈10 days at 10 mg/kg. Only the 10‐mg/kg dose showed changes in amyloid positron emission tomography. Amyloid reduction of 40% to 50% was achieved. Approximately 90% of subjects developed anti‐drug antibodies at 3 months after a single intravenous dose.
Discussion
Intravenous donanemab 10 mg/kg can reduce amyloid deposits in AD despite having a shorter than expected half‐life.
Keywords: Alzheimer's disease, dementia, donanemab, mild to moderate, tolerability
1. BACKGROUND
Donanemab (LY3002813) is a humanized immunoglobulin G1 antibody directed at an N‐terminal pyroglutamate amyloid beta (Aβ) epitope that is present only in brain amyloid plaques, a defining pathological feature of Alzheimer's disease (AD). Donanemab was developed to remove existing amyloid plaques through microglial‐mediated clearance. Administration of the murine surrogate of donanemab in aged amyloid precursor protein transgenic mice resulted in dose‐dependent plaque lowering without microhemorrhage liability. 1
This single‐dose and multiple‐dose, placebo‐controlled, dose‐escalation study was the first assessment of the safety, tolerability, and pharmacokinetics of a wide‐dose range of donanemab in amyloid‐positive patients with mild cognitive impairment (MCI) due to AD or mild to moderate AD dementia. Based on donanemab's predicted efficacious dose and minimum anticipated biological effect level, the proposed dose range was 0.1 to 10 mg/kg. Florbetapir scans were performed at screening and after the last multiple‐ascending dose (MAD), separated by ≈7 months, to assess the pharmacodynamic effects of donanemab on cerebral amyloid. Serial magnetic resonance imaging (MRI) scans were performed for assessment of amyloid‐related imaging abnormalities (ARIA; eg, vasogenic edema, microhemorrhage).
The primary objective of this study was to explore the safety and tolerability features of single and multiple doses of donanemab in Japanese and non‐Japanese patients with MCI due to AD or mild to moderate AD dementia, to define an appropriate dose range for further clinical research. The diagnosis of MCI due to AD or mild to moderate AD were biomarker verified.
The secondary objectives were to assess the serum pharmacokinetics of single intravenous (IV) and subcutaneous (SC) doses, and multiple IV doses of donanemab in Japanese and non‐Japanese patients with MCI due to AD or mild to moderate AD dementia and to assess the effect of donanemab on brain plaque load using florbetapir imaging.
Other objectives included assessing the serum pharmacokinetics of a single IV dose of donanemab in healthy volunteers. We also evaluated changes in plasma, serum, and cerebrospinal fluid (CSF) biomarkers after multiple doses of donanemab, measured CSF donanemab levels after multiple IV doses of donanemab, and evaluated the immunogenicity of single and multiple doses of donanemab.
2. METHODS
2.1. Patient population
From May 3, 2013 to August 24, 2016, the study enrolled men or nonfertile women ≥50 years of age with evidence of memory impairment on the Free and Cued Selective Reminding Test with Immediate Recall (FCSRT; picture version), a Mini‐Mental State Examination (MMSE) score of 16 to 30, and a florbetapir positron emission tomography (PET) scan consistent with amyloid pathology. Patients with contraindication for MRI, presence of more than four microhemorrhages on MRI, or history or evidence on MRI of macrohemorrhage were excluded. Japanese patients were included in this study to facilitate inclusion of Japanese participants in subsequent studies. In addition, a cohort of healthy volunteers aged 18 to 40 was enrolled. These participants were required to have no more than four microhemorrhages on MRI; amyloid positivity was not assessed or required for this cohort. All participants provided informed consent.
HIGHLIGHTS
Donanemab was generally well tolerated up to 10 mg/kg.
After donanemab single‐dose administration from 0.1 to 3.0 mg/kg, the mean terminal elimination half‐life was ≈4 days, increasing to ≈10 days at 10 mg/kg.
Donanemab 10 mg/kg intravenous can reduce amyloid deposits in Alzheimer's disease despite having a shorter than expected half‐life.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources. Several anti‐amyloid antibodies have been reported in the literature, which have also demonstrated a reduction in the amyloid load in Alzheimer's disease (AD) patients using amyloid positron emission tomography imaging. These relevant publications are appropriately cited.
Interpretation: Our findings demonstrated that donanemab was generally well tolerated up to 10 mg/kg. Donanemab 10 mg/kg intravenous can reduce amyloid deposits in AD despite having a shorter than expected half‐life. The observed amyloid‐lowering effects of donanemab are consistent with clinical findings currently in the public domain.
Future directions: Future clinical studies with donanemab include: (1) A phase I study in progress to further explore donanemab's immunogenicity and observed reduction in amyloid load in AD patients. (2) A phase 2 study to investigate the impact of donanemab's amyloid‐lowering effect on cognitive and functional outcomes in AD patients.
2.2. Study design
The study was a subject‐ and investigator‐blind, randomized, placebo‐controlled, parallel‐group, single‐dose followed by a multiple‐dose, dose‐escalation study. The number of patients administered LY3002813 or placebo in each cohort was as follows: Cohort 1 (0.1 mg/kg IV)– 4 LY3002813: 2 placebo; Cohort 2 (0.3 mg/kg IV)–7 LY3002813: 2 placebo; Cohort 3 (1 mg/kg IV)–9 LY3002813: 2 placebo; Cohort 4 (3 mg/kg IV)–11 LY3002813: 3 placebo; Cohort 5 (10 mg/kg IV)–6 LY3002813: 3 placebo; Cohort 6 (3 mg/kg SC)–8 LY3002813; Cohort 7 (1 mg/kg IV in healthy volunteers)–6 LY3002813. The study was conducted with both Japanese and non‐Japanese subjects at sites across the United States and Japan. Patients with AD were enrolled into the single‐ascending dose (SAD) phase and were each administered a single IV dose of donanemab (five dosing cohorts from 0.1 to 10 mg/kg; Cohort 1: 0.1 mg/kg, Cohort 2: 0.3 mg /kg, Cohort 3: 1 mg/kg, Cohort 4: 3 mg/kg, Cohort 5: 10 mg/kg) or placebo followed by a 12‐week follow‐up period for each dose level (Figure 1). The starting dose of 0.1 mg/kg was below the predicted efficacious dose based on donanemab data from the transgenic mouse model. The top dose of 10 mg/kg allowed the predicted efficacious dose range to be examined while maintaining an appropriate margin of safety to animal toxicology observations. Sentinel dosing was conducted for Cohort 1, in which the first two patients were initially dosed, with one patient receiving donanemab and the other receiving placebo in a blinded manner. After ≈7 days and after safety data through 72 hours after dosing were evaluated, the remaining subjects were dosed at least 1 day apart. After the follow‐up period, the same patients proceeded into the MAD phase (five cohorts) and were administered IV doses of donanemab (0.3 to 10 mg/kg) or placebo approximately once per month for up to four doses depending on the initial doses (only Cohort 1 went from 0.1 mg/kg to a higher dose of 0.3 mg/kg during the MAD phase). This phase concluded with a 12‐week follow‐up period. The relative exposure assessment of an unblinded, single, SC 3‐mg/kg dose of donanemab in patients with AD was also performed, followed by a 12‐week follow‐up period. One cohort of healthy subjects received an unblinded, single, IV 1‐mg/kg dose of donanemab. These two cohorts did not continue to the MAD phase. Safety in the study was assessed at regular intervals by adverse events, MRI, electrocardiograms (ECGs), vital signs, safety laboratories (comprising clinical chemistry, haematology, urinalysis), physical/neurological examinations, and immunogenicity.
FIGURE 1.
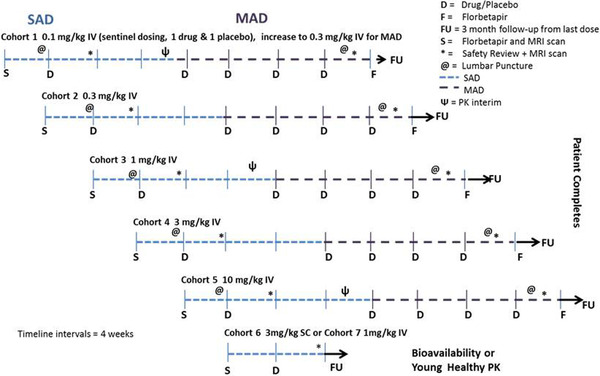
Overview of seamless single‐ascending dose phase followed by multiple‐ascending dose phase. Abbreviations: D, drug/placebo; F, florbetapir; FU, 3‐month follow‐up; IV, intravenous; MAD, multiple‐ascending dose; MRI, magnetic resonance imaging; PK, pharmacokinetic; S, Florbetapir and MRI scan; SAD, single‐ascending dose; SC, subcutaneous. The number of patients administered LY3002813 or placebo in each cohort was as follows: Cohort 1 (0.1 mg/kg IV)–4 LY3002813: 2 placebo; cohort 2 (0.3 mg/kg IV)–7 LY3002813: 2 placebo; cohort 3 (1 mg/kg IV)–9 LY3002813: 2 placebo; cohort 4 (3 mg/kg IV)–11 LY3002813: 3 placebo; cohort 5 (10 mg/kg IV)–6 LY3002813: 3 placebo; cohort 6 (3 mg /kg SC)–8 LY3002813; cohort 7 (1 mg/kg IV in healthy volunteers)–6 LY3002813
Florbetapir scans were performed at baseline and after the last multiple ascending dose, and up to 500 days after dosing. Standardized uptake value ratio (SUVR) with cerebellum reference region was calculated according to the method of Barthel et al. 2 Exploratory analyses were performed according to the SUVR method of Clark et al. 3 The latter SUVR values were converted to centiloid units. 4 The change in SUVR and centiloids was compared across dose cohorts.
Cognition was assessed by the Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐Cog‐14), the MMSE, and the FCSRT at screening or baseline and ≈8 months later.
2.3. Bioanalytical methods
Serum and CSF samples obtained during this study were analyzed for donanemab using a validated enzyme‐linked immunosorbent assay method at Covance Laboratories in Chantilly, Virginia, USA. The lower limit of quantification for the serum assay was 100 ng/mL and the upper limit of quantification was 5000 ng/mL. The inter‐assay accuracy (% relative error) during validation ranged from −1.0% to 5.8%. The inter‐assay precision (% relative standard deviation) during validation was ≤17.2%. For the CSF assay, the lower limit of quantification was 5 ng/mL, and the upper limit of quantification was 500 ng/mL. Samples above the limit of quantification were diluted to yield results within the calibrated range. The inter‐assay accuracy (% relative error) during validation ranged from 1.2% to 20.0%. The inter‐assay precision (% relative standard deviation) during validation ranged from 6.0% to 13.9%.
2.3.1. Pharmacokinetic and pharmacodynamic analyses
Serum pharmacokinetic parameter estimates for donanemab were calculated by standard noncompartmental methods of analysis using Phoenix WinNonlin Version 6.3 (Certara L.P., Raleigh, North Carolina, USA) from data collected during the SAD phase.
Parameters estimated after SC and IV administration included maximum observed drug concentration, area under the concentration versus time curve (AUC) from time zero to time infinity (AUC[0‐∞]), and terminal half‐life. Mean plasma concentration versus time profiles and summary statistics of pharmacokinetic parameter estimates by treatment group were generated. The CSF concentrations of donanemab from the lumbar punctures were compared to the serum concentrations to calculate CSF/serum penetration ratio.
Composite SUVR from florbetapir scans were analyzed to estimate change 3 in amyloid burden. Furthermore, those SUVR values were converted to the centiloid scale, a standardized methodology to quantify amyloid burden from PET scans. 4
2.3.2. Statistical analysis
The demographic variables and other baseline characteristics were summarized using standard descriptive statistics. The safety parameters were also summarized using standard descriptive statistics. Safety analyses were conducted for all enrolled patients, whether or not they completed all protocol requirements.
Immunogenecity evaluation was based on antibody formation, which was summarized over time. Mixed effect model was used with time and dose as fixed effects and the dependent variable as the change from baseline in antibody formulation after dosing of donanemab. Treatment‐emergent antidrug antibodies (TE‐ADAs) were defined as those with a titer two‐fold (one dilution) greater than the minimum required dilution if no ADAs were detected at baseline or those with a four‐fold (two dilutions) increase in titer compared to baseline if ADAs were detected at baseline. The minimum required dilution of the ADA assay was 1:10.
Pharmacodynamic analyses were conducted on the full analysis set, which included all data from all randomized patients receiving a dose of the investigational product according to the treatment the patients actually received. The pharmacodynamic measures included florbetapir scans in centiloid unit and were analyzed using an analysis of covariance model with fixed effects of treatment and baseline as covariate. Plasma Aβ1‐40 and Aβ1‐42 were also summarized using standard descriptive statistics.
The clinical outcomes, ADAS‐Cog, Clinical Dementia Rating–Sum of Boxes, MMSE, Alzheimer's Disease Cooperative Study/Mild Cognitive Impairment Activities of Daily Living scale, and the FCSRT, were analyzed using a mixed model for repeated measures with preinfusion cognitive measures as a baseline covariate and fixed effects of dose and time after infusion.
Statistical analyses were performed using SAS software.
3. RESULTS
3.1. Demographics
A total of 63 subjects, 30 male and 33 female, between the ages of 21 to 89 years old participated in this study (Table 1).
TABLE 1.
Summary of demographic and other baseline characteristics for all patients/subjects
| Severity of Alzheimer's a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dose | N | Mean age, years [SD] | Sex (M/F) | Japanese/non‐Japanese | Mean BMI,kg/m2 [SD] | Mean bodyweight,kg [SD] | MCI/mild, N (%) | Moderate, N (%) |
| Blinded | ||||||||
| Placebo (IV) | 12 | 76.8 [4.9] | 5/7 | 5/7 | 22.94 [4.76] | 63.07 [22.66] | 9 (75%) | 3 (25%) |
| 0.1 mg/kg donanemab (IV) | 4 | 71.3 [11.6] | 1/3 | 2/2 | 24.53 [2.80] | 58.93 [11.59] | 3 (75%) | 1 (25%) |
| 0.3 mg/kg donanemab (IV) | 7 | 75.0 [8.4] | 3/4 | 3/4 | 27.46 [8.73] | 72.87 [33.57] | 0 (0%) | 7 (100%) |
| 1 mg/kg donanemab (IV) | 9 | 74.7 [9.3] | 5/4 | 3/6 | 23.73 [4.14] | 63.91 [16.27] | 8 (88.9%) | 1 (11.1%) |
| 3 mg/kg donanemab (IV) | 11 | 73.0 [8.8] | 3/8 | 4/7 | 27.29 [7.12] | 71.17 [24.14] | 10 (90.9%) | 1 (9.1%) |
| 10 mg/kg donanemab (IV) | 6 | 72.3 [10.7] | 4/2 | 2/4 | 27.06 [7.93] | 72.57 [19.44] | 4 (66.7%) | 2 (33.3%) |
| Unblinded | ||||||||
| 1 mg/kg donanemab single‐dose (IV) HV | 6 | 25.8 [3.3] | 6/0 | 0/6 | 24.24 [2.87] | 79.55 [15.96] | N/A | N/A |
| 3 mg/kg donanemab (SC) | 8 | 74.5 [9.5] | 3/5 | 2/6 | 25.53 [5.00] | 67.88 [16.96] | 8 (100%) | 0 (0%) |
| All donanemab multiple dose | 37 | 73.5 [9.0] | 16/21 | 14/23 | 26.12 [6.53] | 68.63 [22.28] | 32 (86.5%) | 5 (13.5%) |
| All donanemab (AD) | 45 | 73.7 [9.0] | 19/26 | 16/29 | 26.01 [6.24] | 68.50 [21.26] | 40 (88.9%) | 5 (11.1%) |
| All donanemab | 51 b | 68.0 [17.7] | 25/26 | 16/35 | 25.81 [5.95] | 69.80 [20.88] | 40 (78.4%) | 5 (9.8%) |
| Overall | 63 b | 69.7 [16.4] | 30/33 | 21/42 | 25.26 [5.82] | 68.51 [21.21] | 49 (77.8%) | 8 (12.7%) |
Abbreviations: AD, Alzheimer's disease; BMI, body mass index; F, female; HV, healthy volunteers; IV, intravenous; M, male; MCI, mild cognitive impairment; N, number of subjects; SC, subcutaneous; SD, standard deviation.
Patients who had a pre‐existing diagnosis of MCI are included in the mild AD category.
Severity of Alzheimer's: N/A = 6.
3.2. Disposition
Of the 63 patients/subjects who received at least one dose of study drug, 58 completed the study, and five did not complete the study (one due to adverse event of infusion reaction, two due to sponsor decision [subject unable to complete follow‐up visits and adverse event], and two due to patient decision, both withdrawal of consent).
3.3. Single‐ascending dose phase—single‐dose pharmacokinetics
After a single dose, donanemab serum concentrations (Figure 2) followed a bi‐exponential decline after administration, with the terminal elimination phase generally starting ≈1 week after IV administration. The lower limit of quantification of 100 ng/mL of the donanemab assay prevented detection of concentrations during the terminal elimination phase after administration of the lower doses. Table 2 provides a summary of noncompartmental pharmacokinetic parameters across treatment groups. Based on comparison of the mean AUC(0‐∞) values for the 3‐mg/kg IV and SC groups, the bioavailability of the subcutaneous formulation was 60%.
FIGURE 2.
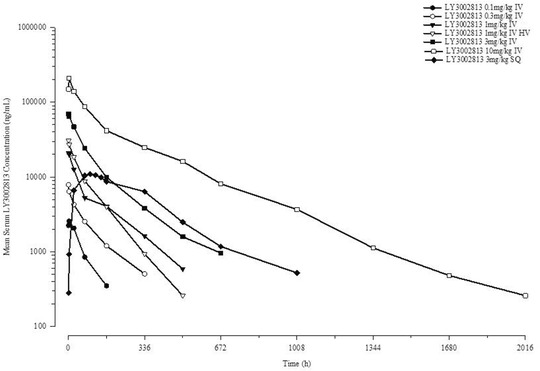
Donanemab (LY3002813) mean plasma concentration‐time profile after the administration of a single dose of donanemab in patients with mild cognitive impairment due to Alzheimer's disease or mild to moderate Alzheimer's disease dementia. HV, heathy volunteers; IV, intravenous; SC, subcutaneous
TABLE 2.
Donanemab noncompartmental pharmacokinetic parameters after a single dose of donanemab in patients with mild cognitive impairment due to Alzheimer's disease or mild to moderate Alzheimer's disease and healthy subjects
| Treatment group | |||||||
|---|---|---|---|---|---|---|---|
| PK parameters (geometric mean [CV%]) | 0.1 mg/kg IV | 0.3 mg/kg IV | 1 mg/kg IV | 1 mg/kg IV HV | 3 mg/kg IV | 3 mg/kg SC | 10 mg/kg IV |
| N | 4 | 7 | 9 | 6 | 11 | 8 | 6 |
| Cmax (μg/mL) | 2.90 (35%) | 5.99 (77%) | 21.7 (21%) | 31.5 (29%) | 71.6 (24%) | 12.0 (34%) | 218 (16%) |
| t1/2 (day) | 2.26 (37%) | 4.64 (63%) | 4.84 (58%) | 3.19 (24%) | 5.40 (55%) | 7.47 (40%) | 10.5 (50%) |
| AUC(0‐∞) (μg•day/mL) | 7.94 (32%) | 26.3 (33%) | 79.9 (26%) | 91.6 (17%) | 263 (19%) | 157 (41%) | 1140 (39%) |
Abbreviations: AUC(0‐∞), area under concentration versus time curve from zero to infinity; Cmax, maximum observed drug concentration; CV, coefficient of variation; HV, healthy volunteers; IV, intravenous; N, number of patients; SC, subcutaneous; t1/2, half‐life associated with the terminal rate constant.
The pharmacokinetic parameters were generally comparable between healthy volunteers and patients with AD and MCI after the administration of a single 1‐mg/kg dose of donanemab (Figure 2). Figures S1 and S2 in supporting information provide individual maximum observed drug concentration (Cmax) and AUC values, respectively, after single doses for patients with AD and MCI.
It was observed that donanemab had a significantly shorter half‐life at low doses compared to the highest dose. After single‐dose administration from 0.1 to 3.0 mg/kg, the mean terminal elimination half‐life was ≈4 days, increasing to ≈10 days (243 hours) at the 10‐mg/kg dose level.
While the metabolism of donanemab has not been assessed in this or animal studies, it is anticipated that donanemab's metabolism would be similar to other biotechnology‐derived products, which is degradation into small peptides and amino acids via lysosomal degradation after cell‐mediated endocytosis.
3.4. Multiple‐ascending dose phase—multiple‐dose pharmacokinetics serum
In the multiple‐dose phase, serum donanemab concentrations in most patients at dose levels ≤3 mg/kg were below the limits of detection 28 days after dosing; patients receiving 10 mg/kg had sustained quantifiable concentrations 28 days after dosing. Figure 3A compares trough concentrations on days 113, 141, and 169 with the concentrations observed on day 29 (ie, a similar time point after the first dose) as well as concentrations on day 85 (3 months after the first dose). Figure 3B compares donanemab concentrations measured at similar times 3 days after the first dose and 4 days after the last dose. For many patients, donanemab concentrations were numerically lower after multiple doses of donanemab than after the first, single dose. In contrast to the other dose levels, at the 10‐mg/kg dose level, donanemab concentrations were generally similar to those observed after single‐dose administration.
FIGURE 3.
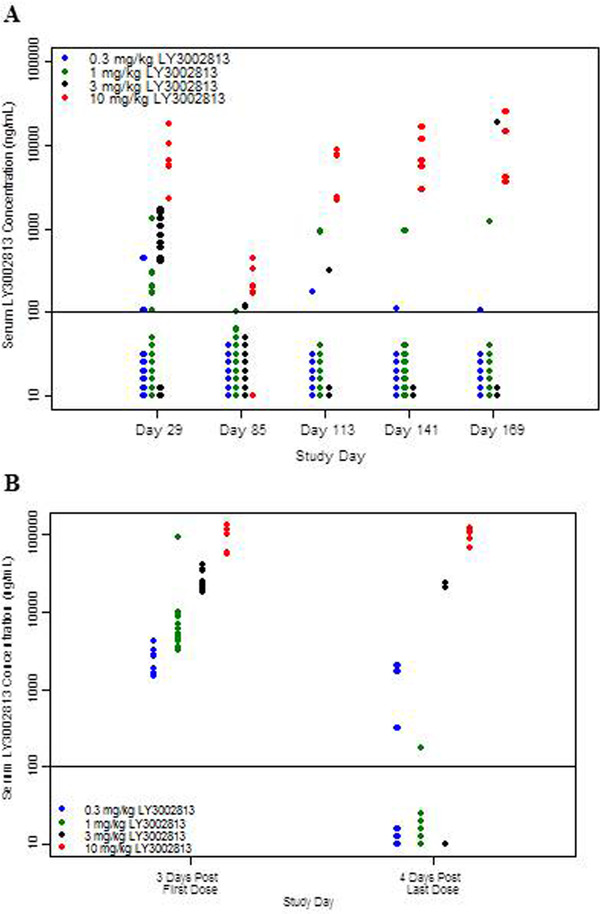
Donanemab (LY3002813) trough concentrations after single and multiple doses (A) and donanemab (LY3002813) concentrations ≈3 days after the first dose and 4 days after the last dose (B). In (A), the solid black line indicates the lower limit of quantification. Data points below the line indicate samples in which concentrations were determined to be below the limit of quantification. All samples were collected 28 days postdose, with the exception of day 85, which occurred after the first dose of donanemab (ie, 84 days after the first dose). All assayed samples are plotted unless the patient missed the previously scheduled dose. In (B), the solid black line indicates the lower limit of quantification. Data points below the line indicate samples in which concentrations were determined to be below the limit of quantification. All samples are plotted from patients receiving donanemab on study day 1 or day 169
3.5. Cerebrospinal fluid
Quantifiable levels of donanemab in CSF could be detected in eight subjects in total: one, three, and four subjects in the 1 mg/kg, 3 mg/kg, and 10 mg/kg cohorts, respectively. CSF donanemab concentration values from these subjects were 0.17% (range 0.04% to 0.64%) of the donanemab concentrations in serum collected at a similar time point as the CSF sample (≈4 days after the last dose of study drug).
3.6. Florbetapir positron emission tomography—standardized uptake value ratio and centiloid scale
Compared to placebo at 28 weeks, there was a statistically significant reduction in cerebral amyloid observed by PET at the highest dose of 10 mg/kg (P < 0.0002). The analysis showed a consistent reduction in cortical amyloid among patients receiving three to five doses of 10 mg/kg donanemab. The 10‐mg/kg IV arm had a mean SUVR change from baseline of −0.26 (standard deviation [SD] = 0.12), and a mean centiloid change from baseline of −44.4 (SD = 14.2), which corresponds to an average reduction of brain amyloid of 40% to 50%, compared to minimal change from baseline in the pooled placebo arms (Figure 4A). Figure 4B shows the baseline and follow‐up centiloid values over time for all subjects in the 10‐mg/kg donanemab and pooled placebo arms. No significant amyloid reduction was seen in the lower donanemab multiple dose levels (0.3 to 3 mg/kg). At the 10 mg/kg dose level, there was no clear relationship between serum donanemab exposure and reduction in amyloid.
FIGURE 4.
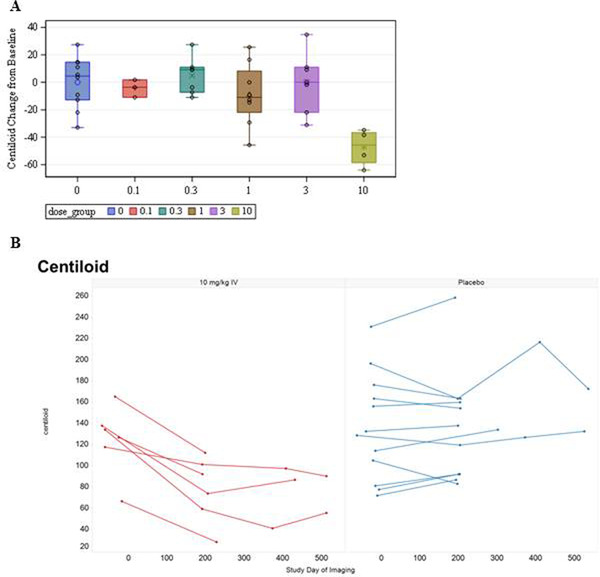
Centiloid change of florbetapir scans from baseline at 28 weeks for donanemab (A). Baseline and follow‐up centiloid values in 10 mg/kg donanemab and pooled placebo arms (B). IV, intravenous
3.7. Plasma Aβ
There were no meaningful changes in plasma Aβ1‐40 and Aβ1‐42 after administration of donanemab at all doses.
3.8. Immunogenicity
All five treatment groups randomized to repeated IV administration of donanemab exhibited distinctly higher antidrug antibody titers relative to the pooled placebo group, which exhibited virtually no TE‐ADAs. Overall, more than 90% of the patients with AD randomized to the study drug had TE‐ADAs 3 months after the first dose; titers tended to increase by the end of the multiple‐dose phase and persisted 3 months after the last dose (Figure 5).
FIGURE 5.
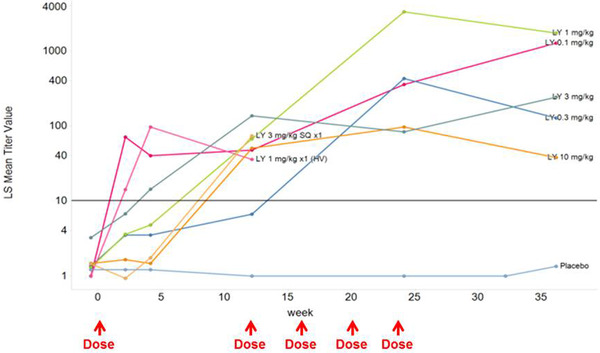
Mean antidrug antibodies titers by time for dose groups. HV, healthy volunteers; LS, least squares; LY, LY3002813 (donanemab); SC, subcutaneous
3.9. Safety
There were no deaths or drug‐related serious adverse events reported in the study. There were four serious adverse events not related to study drug, including hip fracture, cervical vertebral fracture, urinary tract infection, and noncardiac chest pain. The most common adverse event was mild to moderate infusion reactions and was experienced by 6 of 37 patients with AD who had IV dosing. Infusion reactions occurred in one patient in the 0.3‐mg/kg cohort, two patients in the 1.0‐mg/kg cohort, and three patients in the 3.0‐mg/kg cohort and were observed after two or three doses of donanemab. No infusion reactions occurred in the highest 10 mg/kg cohort. Three of the six subjects who experienced infusion reactions were pretreated with antihistamines or anti‐inflammatory medication prior to subsequent infusions. One patient discontinued from the study because of an infusion reaction. Infusion reactions began during the infusion or within 30 minutes of completing the infusion and lasted between 1 minute and 8 hours. Symptoms of infusion reactions included chills/shivering, flushing, asymptomatic hypotension, dyspnea, myalgia, rash, and fever.
There were two cases of ARIA‐microhemorrhage, which was asymptomatic and experienced by one patient in the 3‐mg/kg IV cohort and one patient in the 3‐mg/kg SC single‐dose cohort (Table 3). Both patients had microhemorrhages at baseline. There were no cases of ARIA‐edema. There were no significant findings on clinical labs, ECG, vital signs, physical examination, neurological exam, and Columbia‐Suicide Severity Rating Scale.
TABLE 3.
Treatment‐emergent adverse events
| Donanemab dose | ||||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | 0.1 to 0.3 mg/kg IV | 0.3 mg/kg IV | 1 mg/kg IV | 3 mg/kg IV | 10 mg/kg IV | 3 mg/kg SC single dose | 1 mg/kg IV single dose (healthy volunteers) | |
| N | 12 | 4 | 7 | 9 | 11 | 6 | 8 | 6 |
| AEs, n (%) | 9 (75%) | 3 (75%) | 4 (57.1%) | 6 (66.7%) | 7 (63.6%) | 2 (33.3%) | 4 (50%) | 3 (50%) |
| SAEs, n | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Discontinuation due to AE, n | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| ARIA‐E, n | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ARIA‐H, n | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Infusion reactions, n | 0 | 0 | 1 | 2 | 3 | 0 | NA | 0 |
Abbreviations: AE, adverse event; ARIA‐E, amyloid‐related imaging abnormalities–vasogenic edema; ARIA‐H, amyloid‐related imaging abnormalities–microhemorrhage; IV, intravenous; N, number of patients; SAE, serious adverse event; SC, subcutaneous.
3.10. Cognition
There were no significant changes in any of the cognitive measures with donanemab treatment, across all the dose groups.
3.11. Ethnicity
There were no apparent differences between Japanese and non‐Japanese subjects in any of the safety, pharmacokinetic, or pharmacodynamic measurements.
4. DISCUSSION
This study was a randomized, placebo‐controlled, parallel‐group, single‐dose followed by multiple‐dose, dose‐escalation study in patients with MCI due to AD or mild to moderate AD to assess the safety, tolerability, and pharmacokinetics of single and multiple IV doses of donanemab, an antibody that targets amyloid plaques in the brain.
Donanemab was generally well tolerated when administered up to 10 mg/kg. There were no drug‐related serious adverse events reported. The most common treatment‐emergent adverse event was mild to moderate infusion‐related reaction. While two occurrences of ARIA‐microhemorrhage were observed in the study, there was no dose dependency in incidence nor severity observed. In addition, there were no occurrences of ARIA‐edema seen. This is in contrast to other amyloid therapies, which have been associated with treatment‐emergent ARIA‐edema, where ARIA‐edema has been observed as early as 1 month after dosing. 5 Additional studies with donanemab at therapeutically relevant doses will need to be conducted to further understand the risk of ARIA‐edema with donanemab treatment.
In the SAD phase, donanemab had a significantly shorter half‐life than the ≈24‐day half‐life predicted based on data from nonclinical studies (a prediction that was consistent with the expected half‐life of an immunoglobulin G1 antibody 6 ). After single‐dose administration from 0.1 to 3.0 mg/kg, the mean terminal elimination half‐life was ≈4 days, increasing to ≈10 days (243 hours) at the 10‐mg/kg dose level. The reason for the shorter than predicted half‐life is unclear. To investigate whether the observed shorter than expected half‐life was the result of donanemab binding to its intended target, a cohort of healthy volunteers (given the subjects’ age it could be assumed that they had not accumulated amyloid plaque) was administered a single IV dose of 1.0 mg/kg donanemab. The pharmacokinetics of a single dose of 1.0 mg/kg IV donanemab in healthy volunteers was indistinguishable from the pharmacokinetics in patients with AD at the same dose level, suggesting that half‐life was not dependent on antigen binding.
More than 90% of the patients with AD had TE‐ADAs 3 months after the first dose at all dose groups; titers tended to increase by the end of the MAD phase and persisted 3 months after the last dose. It is possible that TE‐ADA may interact with donanemab and result in a shorter than predicted half‐life. This is supported by the increasing titers and lower concentrations of donanemab during the MAD phase. The mechanisms and clinical implications of the rapid clearance and immunogenicity are under investigation in nonclinical studies and a phase 1b study.
The results suggest that as few as three to five doses of donanemab 10 mg/kg IV can substantially reduce amyloid deposits in AD. The significant reduction in brain amyloid after treatment with 10 mg/kg donanemab is consistent with the amyloid‐lowering observed with other amyloid therapies like bapineuzumab and gantenerumab. 7 , 8 , 9 The lack of change in plasma Aβ1‐40 and Aβ1‐42 supports the mechanism of action of donanemab being centered on deposited amyloid plaque rather than soluble forms of Aβ.
No dose/exposure‐effect analysis could be conducted due to the possible impact of TE‐ADA on the pharmacokinetics of donanemab. Interestingly, patients in the 10 mg/kg dose group achieved similar serum concentrations after multiple doses of donanemab compared to serum concentrations achieved after the first dose, while also achieving significant reduction in brain amyloid, despite the development of TE‐ADA during the MAD dosing period. Overall, donanemab exposure up to the highest dose level of 10 mg/kg did not exceed the margin of safety for animal toxicology observations, but this is not surprising given the lower than expected half‐life observed. There were no changes in cognitive assessments associated with the reduction in brain amyloid after donanemab treatment. However, this may be expected given the small sample size and the limited duration of dosing. Larger therapeutic studies of sufficient duration in the future will assess the clinical relevance of the amyloid reduction seen with donanemab.
5. CONCLUSION
Donanemab was generally well tolerated when administered up to 10 mg/kg. After donanemab single‐dose administration from 0.1 to 3.0 mg/kg, the mean terminal elimination half‐life was ≈4 days, increasing to ≈10 days at 10 mg/kg. Donanemab 10 mg/kg intravenous can reduce amyloid deposits in AD despite having a shorter than expected half‐life.
CONFLICTS OF INTEREST
Authors are employees and minor stockholders of Eli Lilly and Company. Anne Hawdon is a former employee of Eli Lilly and Company.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This work was supported by Eli Lilly and Company, who provided financial support for the conduct of the study and preparation of the article; collection, analysis, and interpretation of data; writing of the report; and in the decision to submit the article for publication.
Lowe SL, Willis BA, Hawdon A, et al. Donanemab (LY3002813) dose‐escalation study in Alzheimer's disease. Alzheimer's Dement. 2021;7:e12112 10.1002/trc2.12112
REFERENCES
- 1. DeMattos RB, Lu J, Tang Y, et al. A plaque‐specifc antibody clears existing β‐amyloid plaques in Alzheimer's disease mice. Neuron 2012;76(5):908‐920. [DOI] [PubMed] [Google Scholar]
- 2. Barthel H, Gertz H‐J, Dresel S, et al. Florbetaben Study Group . Cerebral amyloid‐β PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 2011;10(5):424‐435. [DOI] [PubMed] [Google Scholar]
- 3. Clark CM, Schneider JA, Bedell BJ, et al. AV45‐A07 Study Group . Use of florbetapir‐PET for imaging beta‐amyloid pathology. JAMA 2011;305(3):275‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navitsky M, Joshi AD, Kennedy I, et al. Standardization of amyloid quantitation with florbetapir standardized uptake value ratios to the Centiloid scale. Alzheimers Dement 2018;14(12):1565‐1571. [DOI] [PubMed] [Google Scholar]
- 5. Carlson C, Siemers E, Hake A, et al. Amyloid‐related imaging abnormalities from trials of solanezumab for Alzheimer's disease. Alzheimers Dement 2016;2:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 2004;93(11):2645‐2668. [DOI] [PubMed] [Google Scholar]
- 7. Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 2009;73:2061‐2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rinne JO, Brooks DJ, Rossor MN, et al. 11C‐PiB PET assessment of change in fibrillar amyloid‐beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double‐blind, placebo‐controlled, ascending‐dose study. Lancet Neurol 2010;9:363‐372. [DOI] [PubMed] [Google Scholar]
- 9. Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti‐Aβ antibody demonstrates sustained cerebral amyloid‐β binding and elicits cell‐mediated removal of human amyloid‐β. J Alzheimers Dis 2012;28(1):49‐69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


