Abstract
Introduction
Herpesviruses, including Herpes simplex virus type 1 (HSV1) and varicella zoster‐virus (VZV), have been implicated in Alzheimer's disease (AD) development. Likewise, antiviral treatment has been suggested to protect against dementia development in herpes‐infected individuals.
Methods
The study enrolled 265,172 subjects aged ≥ 50 years, with diagnoses of VZV or HSV, or prescribed antiviral drugs between 31 December 2005 and 31 December 2017. Controls were matched in a 1:1 ratio by sex and birth year.
Results
Antiviral treatment was associated with decreased risk of dementia (adjusted hazard ratio [HR] 0.89, 95% confidence interval [CI] 0.86 to 0.92), while herpes infection without antiviral drugs increased the risk of dementia (adjusted HR 1.50, 95% CI 1.29 to 1.74).
Discussion
Antiviral treatment was associated with a reduced long‐term risk of dementia among individuals with overt signs of herpes infection. This is consistent with earlier findings indicating that herpesviruses are involved in the pathogenesis of AD.
Keywords: Alzheimer's disease, antiviral agents, dementia, herpes simplex, herpes zoster, retrospective cohort study, varicella zoster
1. BACKGROUND
There is growing evidence to support the link between herpes infections and Alzheimer's disease (AD). 1 , 2 , 3 Targeting herpesviruses with specific antiviral agents could provide new AD treatment possibilities if a preventive effect is confirmed.
Herpes simplex virus type 1 (HSV1) is the herpesvirus most strongly associated with AD. 1 , 2 , 3 Several population‐based cohort studies have demonstrated an increased risk of AD development for carriers of HSV1, 4 , 5 , 6 particularly in combination with genetic risk factors including apolipoprotein E ε4 (APOE ε4). 3 , 7 , 8 , 9 Both in vivo and in vitro, inoculation with HSV1 among other pathogens causes AD‐related changes with amyloid deposition. 10 , 11 , 12 , 13 , 14 Another neurotropic member of the Herpesviridae family implicated in dementia development is varicella zoster virus (VZV). 15 , 16 , 17 , 18 Reactivation of VZV causes shingles, that is, herpes zoster. Herpes zoster ophthalmicus is a subtype of the herpes zoster infection that affects the ophthalmic division of the trigeminal nerve. VZV infection of the central nervous system (CNS) has previously been linked to long‐term cognitive decline. 19 , 20 Recent data suggest that herpes zoster ophthalmicus and herpes zoster infection are associated with a 3.0‐ and 1.1‐fold increased risk of dementia development. 15 , 16 , 18 It has been hypothesized that in the event of herpes zoster ophthalmicus, the virus more frequently spreads to the brain, thereby explaining a stronger association with dementia development. 21
Herpes infections are commonly treated with acyclovir or its prodrug valacyclovir. Acyclovir is a nucleoside analogue that interferes with viral DNA replication and inhibits viral proliferation in infested cells. Interestingly, according to recent registry‐based studies, the risk of developing dementia with both symptomatic HSV and herpes zoster infection appears to be reduced after antiviral treatment (hazard ratio [HR] 0.09, 0.55, and 0.76, respectively). 15 , 18 , 22 The protective effect of antiviral drugs showed a dose‐response relationship in which longer duration of treatment (>30 days) appeared to have a greater preventive impact on dementia incidence compared to a shorter regime. 22
Thus, previous findings have indicated a potential protective role of antiviral treatment against dementia development 15 , 18 , 22 and these results need to be corroborated in other large‐scale cohorts. The aim of this study was to investigate whether specific antiviral treatment targeting herpesviruses and herpes infection with VZV and HSV, in absence of treatment, affects the risk of subsequent dementia in a large registry‐based cohort in Sweden.
2. METHODS
2.1. Ethical approval
The National Board of Health and Welfare in Sweden and the Regional Ethical Review Board in Umeå Sweden approved the study (Dnr 2013‐86‐31M). In accordance with Swedish legislation, the requirement for informed consent was waived by both institutions.
2.2. Research setting
This study took place in Sweden, a country of ≈10 million inhabitants, with 21 regions providing health care. The vast majority of health‐care services in Sweden are tax funded and include both hospitals and outpatient clinics.
RESEARCH IN CONTEXT
Systematic review: We searched PubMed for relevant articles. Previous reports have indicated that antiviral treatment might protect against dementia development in herpes‐infected individuals. In addition, the herpesviruses herpes simplex virus type 1 (HSV1) and varicella zoster virus (VZV) might contribute to Alzheimer's disease (AD) pathogenesis. However, more studies are warranted to further investigate the potential protective role of antiviral treatment in dementia.
Interpretation: Our findings together with previous reports suggest that antiviral treatment might reduce long‐term risk of dementia. In contrast, untreated herpes infection increases the subsequent dementia risk.
Future directions: Further epidemiological as well as clinical trials are needed to establish the effect of antiviral treatment for reducing the risk of AD development.
HIGHLIGHTS
Antiviral treatment was associated with decreased risk of dementia.
There is higher dementia risk with untreated herpes simplex and varicella zoster infection.
Herpesviruses might be involved in Alzheimer's disease pathogenesis.
2.3. Study cohort
We conducted a matched cohort study based on pre‐existing nation registries, of 265,172 individuals aged 50 years and older at inclusion. Inclusion criteria were having received diagnoses of HSV or VZV infection or having been prescribed antiviral drugs between 31 December 2005 and 31 December 2017. All available cases meeting the inclusion criteria were selected. Controls without herpes diagnoses and antiviral drugs were matched randomly in a 1:1 ratio by sex and year of birth from the whole Swedish population.
2.3.1. Databases
Data of viral diagnoses and antiviral treatment were collected from two nationwide databases: the National Patient Register (NPR) and the Swedish Prescribed Drug Register (SPDR). 23 , 24 , 25 Both SPDR and NPR are managed by the National Board of Health and Welfare in Sweden and hold data on age (in years), sex, geographical information, dates of admission and discharge, and drug prescription date. The registers are protected by secrecy according to Swedish legislation but can be disclosed upon reasonable request for research purposes. To protect patient privacy, data were anonymized by removal of the personal identity numbers from the data set.
The SPDR was established in July 2005 and includes all prescriptions dispensed by Swedish pharmacies and contains information on number of fillings and package size. 26 Anatomical Therapeutic Chemical (ATC) codes were used to identify drugs in the SPDR.
The NPR includes in‐ and outpatient diagnoses in specialist clinics. 27 The inpatient register has had full coverage since 1987 while registration of outpatient diagnoses began in 2001. Information concerning both main and secondary diagnoses is available. However, diagnoses made in primary health‐care centers are not included in the NPR. Diagnoses were registered according to the International Classification of Diseases‐10 (ICD‐10).
Education level and vital status were retrieved from Statistics Sweden and the Swedish Cause of Death Register, respectively.
2.4. HSV, VZV infections and antiviral treatment
HSV diagnoses were identified by ICD‐10 codes for genital (A60.x) and non‐genital HSV (B00.x)—ie, A60.0‐A60.1 and A60.9, and B00.0–B00.9, B00.1A–B00.1B, B00.1W, and B00.1X. Cases of VZV diagnosis included ICD‐10 codes for varicella (B01.x) and herpes zoster (B02.x)—ie, B01.0–B01.2 and B01.8–B01.9, and B02.0–B02.3 and B02.7–B02.9). The term of symptomatic HSV/VZV infection was defined as ≥1 in‐ or outpatient diagnosis of either HSV or VZV. HSV and VZV diagnoses in Sweden are usually based on physical exams with the presence of typical rashes and distribution. In more difficult cases or atypical presentation, polymerase chain reaction (PCR) or serological testing can be used, but it is not a routine procedure in clinical practice. Unfortunately, NPR does not hold information of serological testing or PCR results.
Antiviral treatment denotes individuals who were prescribed any of the following medications: acyclovir, cidofovir, famcyclovir, gancyclovir, valacyclovir, or valgancyclovir with ATC codes J05AB01, J05AB12, J05AB09, J05AB06, J05AB11, and J05AB14, respectively (Table 1).
TABLE 1.
Baseline characteristics
| Herpes diagnosis, no antiviral treatment and controls without antiviral treatment and herpes diagnosis | Herpes diagnosis, with antiviral treatment and controls without antiviral treatment and herpes diagnosis | Antiviral treatment irrespective of diagnosis and controls without antiviral treatment and herpes diagnosis | ||||
|---|---|---|---|---|---|---|
| Herpes infection, no antiviral treatment, n = 9933 | Controls, n = 9933 | Herpes infection, with antiviral treatment, n = 29,593 | Controls, n = 29,593 | Antiviral treatment | Controls, n = 255,239 | |
| Age, mean ± SD, y | 75 (± 11) | 75 (± 11) | 72 (± 10) | 72 (± 10) | 70 (± 10) | 70 (± 10) |
| Sex, female, (%) | 57.9 | 57.9 | 58.7 | 58.7 | 61.4 | 61.4 |
| Educational level, (%) | ||||||
| Missing a | 1.9 | 2.3 | 1.2 | 1.2 | 1.0 | 1.6 |
| Primary school | 42.9 | 41.2 | 36.3 | 38.1 | 32.3 | 35.4 |
| Secondary school | 36.9 | 36.6 | 24.7 | 38.1 | 38.7 | 39.4 |
| University | 18.9 | 19.9 | 24.7 | 22.0 | 28.0 | 23.6 |
| Antiviral treatment, (%) | ||||||
| Acyclovir (J05AB01) | 33.4 | 42.6 | ||||
| Gancyclovir (J05AB06) | <0.01 | <0.01 | ||||
| Famcyclovir (J05AB09) | 0.2 | 0.1 | ||||
| Valacyclovir (J05AB11) | 66.1 | 52.8 | ||||
| Cidofovir (J05AB12) | 0.0 | <0.01 | ||||
| Valgancyclovir (J05AB14) | 0.3 | 0.7 | ||||
| Antiviral prescriptions, n (%) | ||||||
| One | 27,449 (92.8) | 240,676 (94.3) | ||||
| Two | 1888 (6.4) | 12,584 (4.9) | ||||
| Three | 170 (0.6) | 1307 (0.5) | ||||
| Four or more | 86 (0.3) | 672 (0.3) | ||||
| Herpes diagnoses, (%) b , c | ||||||
| HSV diagnoses | 43.5 | 22.0 | ||||
| VZV diagnoses | 57.6 | 81.3 | ||||
| Incidence rate for dementia per 1000 person‐years | 12.9 | 10.2 | 8.5 | 9.4 | 6.6 | 7.4 |
| Incident dementia cases, (%) | 5.7 | 5.4 | 4.4 | 5.1 | 3.5 | 4.1 |
| Follow‐up time, mean, y | 4.4 (±3.5) | 5.3 (± 3.4) | 5.2 (±3.4) | 5.4 (±3.4) | 5.4 (±3.5) | 5.5 (±3.5) |
| Follow‐up time, median, y and range | 3.8 (0–12) | 4.9 (0–12) | 4.8 (0–12) | 5.1 (0–12) | 5.0 (0–12) | 5.2 (0–12) |
| Medical conditions, (%) b | ||||||
| Alcohol intoxication | 1.3 | 0.6 | 0.8 | 0.9 | 0.7 | 1.0 |
| Chronic obstructive pulmonary disease | 2.4 | 1.1 | 2.0 | 1.1 | 1.3 | 1.0 |
| Congestive heart failure | 3.7 | 2.0 | 2.3 | 1.6 | 1.5 | 1.3 |
| Myocardial infarction | 4.4 | 3.3 | 3.8 | 2.8 | 2.7 | 2.4 |
| Parkinson's disease | 0.3 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 |
| Stroke | 4.2 | 3.3 | 2.6 | 2.7 | 2.2 | 2.2 |
| Drug prescription | ||||||
| Antidepressants | 10.6 | 9.0 | 9.9 | 8.6 | 10.2 | 8.9 |
| Antidiabetics | 9.7 | 6.3 | 7.6 | 7.1 | 6.3 | 6.5 |
| Antihypertensive | 38.9 | 33.8 | 33.9 | 30.3 | 29.5 | 27.9 |
| Antipsychotics | 1.9 | 1.8 | 1.4 | 1.7 | 1.5 | 1.8 |
Abbreviations: HSV, herpes simplex virus; N, number; SD, standard deviation; VZV, varicella zoster virus; y, years.
Missing values were not imputed and were thus excluded from the Cox regression models.
Medical conditions at baseline are coded dichotomously and include diagnoses from specialist clinics.
Some of which had diagnoses of both VZV and HSV.
2.5. Outcome—dementia diagnoses and anti‐dementia drugs
Dementia status was defined as diagnoses of AD, vascular dementia, or unspecified dementia, registered by ICD‐10 codes F00.x, F01.x, F03.x in the NPR, or the prescription of anti‐dementia drugs, acetylcholinesterase inhibitors (donepezil, rivastigmine, or galantamine) or memantine in the SPDR (ATC codes N06DA02, N06DA03, N06DA04, N06DX01) during the study period. Individuals with any form of dementia or who had been prescribed anti‐dementia drugs at, or prior to the time of, inclusion, were excluded from both groups.
2.6. Statistical analyses
The index date was defined as the first date of diagnosis of HSV or VZV infection, or the first antiviral treatment date. Controls were assigned the same index date as their corresponding case. Follow‐up time was terminated by either diagnosis of dementia, prescription of anti‐dementia drugs, death, or on 31 December 2017, whichever occurred first.
Multivariable stratified Cox proportional hazard regression models were used to investigate the associations between herpes diagnoses with and without antiviral treatment and dementia incidence in the matched sample. 28 Separate analyses were performed for subjects with (1) herpes diagnoses without antiviral treatment, (2) herpes diagnoses with antiviral treatment, and finally (3) antiviral treatment irrespective of herpes diagnosis, many of whom did not have a registered diagnosis of herpes infection (ie, unknown indication, because the prescription was issued at a primary care center and the diagnoses therefore were not included in the NPR). The proportional hazard assumption was tested by evaluation of unadjusted Kaplan‐Meier curves for all groups and by plotting scaled Schoenfeld residuals for each variable against follow‐up time. We assessed that the variables were time‐independent before performing the Cox model analysis.
All multivariable analyses were adjusted for baseline comorbidity and educational level (primary, secondary, and university). We attempted to control for potential confounding by including pre‐exposure variables associated with the outcome and the exposure based on previous knowledge in the multivariable models, that is, variables for comorbidity status, prescription of drugs, and educational level. Variables for comorbidity status were coded dichotomously and included alcohol intoxication, chronic obstructive pulmonary disorder, congestive heart failure, myocardial infarction, Parkinson's disease, stroke (hemorrhagic and ischemic), and prescription of drugs (antidepressants, antidiabetics, antihypertensives, or antipsychotics). All diagnoses were based on ICD‐10 codes and were retrieved from the NPR. Thus, they only comprised diagnoses made in specialist clinics. The variables for prescribed drugs were used to account for conditions often managed in primary care settings and included antidepressants (ATC code N06A), drugs used in diabetes (ATC code A10), antihypertensives (ATC codes C02: antihypertensive drugs, C03: diuretic drugs, C07: beta‐blocking agents, C08: calcium‐blocking agents, and C09: agents acting on the renin‐angiotensin system), and antipsychotics (ATC code N05A, excluding N05AN, ie, lithium).
Separate models were fitted to compare the most common antiviral agents: acyclovir and valacyclovir. To distinguish the individual effect of HSV from VZV on incident dementia, subgroup analyses of HSV and VZV diagnoses were performed.
Additional Cox regression analyses were performed to compare dementia incidence between subjects with herpes diagnoses who were prescribed antiviral treatment, with those subjects with herpes diagnoses who were not prescribed antiviral treatment (ie, treated and untreated herpes). Because these analyses were not stratified, age and sex were included in the regression models to adjust for potential confounding. Kaplan‐Meier curves were constructed in R version 3.4.3 using the “ggplot2” package to visualize the incidence of dementia in the different subgroups.
SPSS Statistics version 24 (IBM Corporation, Armonk, NY, USA) was used for statistical analyses. A two‐tailed P‐value < .05 and 95% confidence interval not covering one were interpreted as significant.
3. RESULTS
The matched cohort study followed 265,172 subjects with herpes diagnoses and antiviral treatment, and the same number of controls (Table 1). Diagnoses of VZV infections were more common than diagnoses of HSV infections (4323 and 5722 with no antiviral treatment, respectively, and 24,045 and 6510 with antiviral treatment, respectively, some of which had diagnoses of both HSV and VZV, Table 1). The most commonly prescribed antiviral drug was valacyclovir, followed by acyclovir (Table 1).
Individuals with herpes diagnoses who did not receive antiviral treatment had higher incidence rates of dementia compared to their controls (12.9 and 10.2 per 1000 person‐years, respectively, Table 1). In contrast, herpes‐diagnosed subjects who received antiviral treatment had lower incidence rates of dementia than their controls (8.5 and 9.4 per 1000 person‐years, respectively, Table 1). Last, the dementia incidence rates of antiviral users irrespective of diagnosis and their controls were 6.6 and 7.4 per 1000 person‐years, respectively (Table 1).
Herpes diagnosis without antiviral treatment was associated with an increased risk of subsequent dementia (HR 1.50, 95% confidence interval [CI]: 1.29 to 1.74; Table 2) while antiviral treatment irrespective of diagnosis was associated with a decreased risk (HR 0.89, 95% CI 0.86 to 0.92). Among herpes‐diagnosed individuals treated with antiviral drugs, the HR for dementia was 0.90 (95% CI 0.82 to 0.98; Table 2). For the comparison of herpes diagnoses with and without antiviral treatment, antiviral drugs were associated with decreased dementia risk (HR 0.75, 95% CI 0.68 to 0.83; Table 2). Figure 1 shows the cumulative incidence of dementia for subjects with herpes diagnoses who did not receive antiviral treatment compared to those with herpes diagnoses who received antiviral treatment, and their respective controls. The cumulative incidence of dementia seems to increase gradually over time in the treated and untreated group (Figure 1). Tables S1 and S2 in supporting information include additional data with the results divided by sex and the correlation of all individual variables with the exposure (ie, antiviral treatment or herpes diagnoses) and the outcome (ie, dementia).
TABLE 2.
Cox proportional hazard regression models with associations between dementia incidence and herpes infection with and without antiviral treatment compared to controls without antiviral treatment or herpes diagnosis
| Unadjusted hazard ratio, 95% confidence interval, (P value) | Adjusted hazard ratio, 95% confidence interval, (P value) |
|---|---|
| Herpes diagnosis, no antiviral treatment, compared to controls | |
| 1.43, 1.24–1.65 (<.001) | 1.50, 1.29–1.74 (<.001) a |
| Herpes diagnosis, with antiviral treatment, compared to controls | |
| 0.91, 0.83–0.98, (.02) | 0.90, 0.82–0.98 (.015) a |
| Antiviral treatment, irrespective of diagnosis, compared to controls | |
| 0.89, 0.86–0.92 (<.001) | 0.89, 0.86–0.92 (<.001) a |
| Herpes diagnosis, with antiviral treatment compared to those without antiviral treatment b | |
| 0.66, 0.60–0.73 (<.001) | 0.75, 0.68–0.83 (<.001) c |
Hazard ratios adjusted for baseline comorbidity (alcohol intoxication; chronic obstructive pulmonary disease; congestive heart failure; myocardial infarction; Parkinson's disease; stroke; and the use of antidepressant, antidiabetic, antihypertensive, and antipsychotics drugs) and educational level.
The comparison groups in this model are not matched by sex and age.
Hazard ratios adjusted for sex, age, baseline comorbidity (alcohol intoxication; chronic obstructive pulmonary disease; congestive heart failure; myocardial infarction; Parkinson's disease; stroke; and the use of antidepressant, antidiabetic, antihypertensive, and antipsychotics drugs) and educational level. For this analysis, sex and age were also included in the model because the comparison groups were not matched.
FIGURE 1.
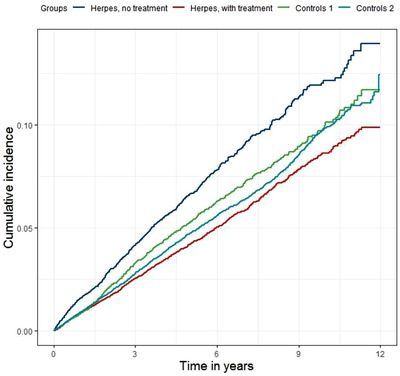
Kaplan‐Meier curve of the cumulative incidence of dementia in different groups. “Controls 1” denote controls of the “Herpes, no treatment” group. “Controls 2” denote controls of the “Herpes, with treatment” group. The follow‐up differentiated between subjects, but censors were not drawn in the diagram due to the high number of individuals censored at each time point
No differences in dementia incidence were observed comparing acyclovir to valacyclovir (data not shown). In further subgroup analyses, individuals with untreated VZV and HSV infection, respectively, had overall adjusted HRs of 1.61 (95% CI 1.33 to 1.95, P < .001) and 1.38 (95% CI 1.07 to 1.79, P = .014) for incident dementia, compared to their respective controls.
4. DISCUSSION
In this large matched cohort study, specific antiviral treatment targeting herpesviruses was associated with an 11% risk reduction of dementia. In contrast, having received a herpes diagnosis without antiviral treatment was associated with a 50% increased risk of dementia compared to controls. These results are in line with previous register‐based studies from Taiwan and South Korea indicating the potential protective role of antiviral treatment in dementia. 15 , 18 , 22 Our findings, together with previous reports, could have potential clinical implications in which physicians should be even more active in treating symptoms of herpes reactivations with antiviral drugs to possibly reduce the long‐term risk of dementia.
The decreased risk of dementia associated with antiviral treatment in the present study (HR 0.75) is consistent with the recent work by Tzeng et al., 22 Chen et al., 15 and Bae et al. 18 In the study by Tzeng et al., a risk reduction (HR 0.009) was found, 22 while Bae et al. reported a HR of 0.76, 18 and Chen et al. a HR of 0.55, compared to untreated herpes infection. 15 Different definitions of herpes infection could partially explain the difference in effect size between the studies. Herpes infection was defined as ≥3 symptomatic reactivations in the study by Tzeng et al., compared to at least one diagnosis in both the present study, and the studies by Bae et al. and Chen et al. It seems plausible that individuals with more frequent reactivations, if the reactivations are left untreated, may be at higher risk of dementia. It should also be noted that many dementia cases in the study by Tzeng et al. occurred during the very first year, but only in the untreated group, which could indicate reverse causality.
In this study, the group diagnosed with VZV infection appeared to have a slightly higher risk of dementia compared to the group diagnosed with HSV infection (HRs 1.61 and 1.38, respectively). This result was quite unexpected as HSV1 is the pathogen most frequently associated with AD in the literature. 1 , 3 Further classification of both VZV and HSV infection into subtypes (herpes zoster ophthalmicus, herpes zoster, and varicella, and non‐genital and genital HSV) may be informative when interpreting the results, but this subtype classification was not possible within our material. In population‐based cohorts, herpes zoster ophthalmicus infections rather than other herpes zoster infections have been reported to increase the risk of dementia. 15 , 16 It has been theorized that in herpes zoster ophthalmicus infections, the virus is more likely to enter the CNS compared to more peripherally located herpes zoster infections. 21 The potential involvement of VZV in AD development has been less investigated than in HSV1 and it is not completely clear how VZV contributes to the development of AD. VZV has previously been linked to vascular disease and proposed to be a risk factor for ischemic stroke. 29 For this reason, VZV could possibly increase the risk of vascular dementia instead of AD. The fact that almost everyone carries VZV makes an investigation in prospective epidemiological studies less straightforward compared to HSV1. 30 Also, VZV‐DNA in post mortem brain tissue specimens have not yet been unambiguously established. 31 , 32 Nonetheless, in three geographically diverse and independent epidemiological materials, the association between VZV and increased risk of dementia was demonstrated, and the role of VZV in dementia development warrants further investigation.
The protective effect of antiviral treatment in dementia development is not known and our observational study cannot determine the underlying mechanism. Notably, there are other human herpesviruses possibly involved in the pathogenesis of AD, 33 , 34 and any antiviral treatment targeting herpesviruses is not specific to HSV or VZV.
The control group in this study comprised both seropositive and seronegative individuals as the seropositivity status of the subjects is unknown, especially considering that approximately 70% of the population could be expected to carry HSV1 and > 95% VZV. 30 It is reasonable to assume that the controls probably have better immunological resilience to herpes infections with fewer episodes of symptomatic reactivations because they have not received a herpes diagnosis or been subjected to specialist medical care for this. Importantly, the herpes diagnoses primarily reflect symptomatic reactivation or primary infection with overt signs. Thus, the individuals with herpes diagnoses constitute a subgroup of those carrying the pathogen.
Genetic data for the study participants were not available. This is a limitation because HSV1 infection has been shown to be associated with increased AD risk, particularly in genetically predisposed individuals. 3 , 7 , 8 , 9 , 35 Conversely, individuals carrying the APOE ε4 allele are also more susceptible to HSV infections. 35 , 36 , 37 Another limitation of our study is that herpes diagnoses were only reported from specialist clinics. This could introduce a selection bias in which the cohort with herpes diagnoses may be more fragile than the average person with herpes, and also shift the proportion of herpes diagnosis subtypes compared to a primary care setting. Although we had access to many potential confounders, there is likely residual confounding that could influence the associations found. All these limitations must be taken into account when interpreting the results. A further limitation is that some dementia diagnoses are never detected by the NPR, and that subtypes of dementia (eg, AD, vascular dementia, and unspecified dementia) are often misclassified. 38 For this reason, we did not divide the material between different dementia subtypes. Also, the dementia diagnoses are primarily clinical and not based on post mortem examinations. Hence, our results may not be restricted for AD because mixed forms are probably more common than pure AD. 39 We chose to include individuals prescribed with anti‐dementia drugs to increase the sensitivity of register dementia diagnoses. The proportion of AD patients in Sweden treated with anti‐dementia drugs ranges from 40% to 85% in different reports. 40 , 41 , 42
There are other limitations with our study, including that subtype classification of VZV and HSV infection was not possible in our data set. Furthermore, we did not take different treatment regimens into account, as SPDR does not hold data on treatment duration and exactly defined daily doses. We chose to compare cases without antiviral treatment to those prescribed antiviral drugs at least once. Hence, we did not study any dosage‐ or duration‐related effects, which may be important in cases of irregular prescriptions or interrupted treatment. The standard dosage for treatment of herpes zoster reactivations according to Swedish clinical guidelines is valacyclovir 1000 mg three times a day for 7 days or acyclovir 800 mg five times a day for 7 days. This is the most common indication and, acyclovir and valacyclovir are the most commonly used antiviral drugs in Sweden.
The strengths of our investigation are the large number of subjects included in this nationwide study on one hand, and that we excluded those subjects already suffering from dementia at baseline on the other.
5. CONCLUSION
In this nationwide matched cohort study, antiviral treatment status was associated with a reduced risk of dementia, while untreated herpes infection was associated with an increased risk. These results are consistent with previous findings indicating a potential protective role of antiviral treatment against dementia development, as well as the suggestion that herpesviruses are involved in the pathogenesis of AD. However, a causal relationship among herpesvirus, antiviral treatment, and dementia cannot be established from these observations.
FUNDING INFORMATION
The study was financially supported by grants from Västerbotten County Council, the Knut and Alice Wallenberg Foundation, Umeå University, the Kempe Foundations, the Swedish Medical Associations, the Swedish Dementia Association, Erik and Anne‐Marie Detlof foundation, the Trolle‐Wachtmeister Foundation, the Dementia Fund in Västerbotten, the Swedish Alzheimer Fund, the Stohne Foundation, the Bergvall Foundation, and Umeå University Foundation for Medical Research.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Supporting information
Supporting Information
Lopatko Lindman K, Hemmingsson E‐S, Weidung B, et al. Herpesvirus infections, antiviral treatment, and the risk of dementia—a registry‐based cohort study in Sweden. Alzheimer's Dement. 2021;7:e12119 10.1002/trc2.12119
REFERENCES
- 1. Itzhaki RF, Lathe R, Balin BJ, et al. Microbes and Alzheimer's Disease. J Alzheimers Dis. 2016;51:979‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harris SA, Harris EA. Herpes simplex virus Type 1 and other pathogens are key causative factors in sporadic Alzheimer's disease. J Alzheimers Dis. 2015;48:319‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steel AJ, Eslick GD. Herpes viruses increase the risk of Alzheimer's disease: a meta‐analysis. J Alzheimers Dis. 2015;47:351‐364. [DOI] [PubMed] [Google Scholar]
- 4. Lovheim H, Gilthorpe J, Adolfsson R, Nilsson LG, Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer's disease. Alzheimer's Dement. 2015;11:593‐599. [DOI] [PubMed] [Google Scholar]
- 5. Lovheim H, Gilthorpe J, Johansson A, Eriksson S, Hallmans G, Elgh F. Herpes simplex infection and the risk of Alzheimer's disease: a nested case‐control study. Alzheimer's Dement. 2015;11:587‐592. [DOI] [PubMed] [Google Scholar]
- 6. Letenneur L, Peres K, Fleury H, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population‐based cohort study. PloS One. 2008;3:e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lovheim H, Norman T, Weidung B, et al. Herpes simplex virus, APOEvarepsilon4, and cognitive decline in old age: results from the betula cohort study. J Alzheimers Dis. 2019;67:211‐220. [DOI] [PubMed] [Google Scholar]
- 8. Lopatko Lindman K, Weidung B, Olsson J, et al. A genetic signature including apolipoprotein Eε4 potentiates the risk of herpes simplex–associated Alzheimer's disease. Alzheimer's Dement. 2019;5:697‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linard M, Letenneur L, Garrigue I, Doize A, Dartigues JF, Helmer C. Interaction between APOE4 and herpes simplex virus type 1 in Alzheimer's disease. Alzheimer's Dement. 2020;16:200‐208. [DOI] [PubMed] [Google Scholar]
- 10. Cairns DM, Rouleau N, Parker RN, Walsh KG, Gehrke L, Kaplan DL. A 3D human brain‐like tissue model of herpes‐induced Alzheimer's disease. Sci Adv. 2020;6:eaay8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar DK, Choi SH, Washicosky KJ, et al. Amyloid‐beta peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eimer W, Vijaya Kumar D, Washicosky KJ, et al. Antiviral activities of amyloid‐β (Aβ) against the AD‐linked virus HSV1. Unpublished abstract. Soc Neurosci. 2016;2016. [Google Scholar]
- 13. De Chiara G, Piacentini R, Fabiani M, et al. Recurrent herpes simplex virus‐1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog. 2019;15:e1007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bearer EL. HSV, axonal transport and Alzheimer's disease: in vitro and in vivo evidence for causal relationships. Future Virol. 2012;7:885‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen VC, Wu SI, Huang KY, et al. Herpes Zoster and dementia: a nationwide population‐based cohort study. J Clin Psychiatry. 2018;79. [DOI] [PubMed] [Google Scholar]
- 16. Tsai MC, Cheng WL, Sheu JJ, et al. Increased risk of dementia following herpes zoster ophthalmicus. PloS One. 2017;12:e0188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bubak AN, Como CN, Coughlan CM, et al. Varicella zoster virus infection of primary human spinal astrocytes produces intracellular amylin, amyloid‐beta, and an amyloidogenic extracellular environment. J Infect Dis. 2019;221(7):1088‐1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bae S, Yun SC, Kim MC, et al. Association of herpes zoster with dementia and effect of antiviral therapy on dementia: a population‐based cohort study. Eur Arch Psychiatry Clin Neurosci. 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19. Grahn A, Nilsson S, Nordlund A, Linden T, Studahl M. Cognitive impairment 3 years after neurological Varicella‐zoster virus infection: a long‐term case control study. J Neurol. 2013;260:2761‐2769. [DOI] [PubMed] [Google Scholar]
- 20. Persson A, Bergstrom T, Lindh M, Namvar L, Studahl M. Varicella‐zoster virus CNS disease—viral load, clinical manifestations and sequels. J Clin Virol. 2009;46:249‐253. [DOI] [PubMed] [Google Scholar]
- 21. Itzhaki RF, Lathe R. Herpes viruses and senile dementia: first population evidence for a causal link. J Alzheimers Dis. 2018;64:363‐366. [DOI] [PubMed] [Google Scholar]
- 22. Tzeng NS, Chung CH, Lin FH, et al. Anti‐herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections‐a nationwide, population‐based cohort study in Taiwan. Neurotherapeutics. 2018;15:417‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register/. Accsessed 15 July 2020. Stockholm, Sweden. Published 25 April 2019. Last updated 20 May 2019. [Google Scholar]
- 24. https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/lakemedelsregistret/. Accsessed 15 July 2020. Stockholm, Sweden. Published 7 November 2018. Last updated 9 July 2020. [Google Scholar]
- 25. https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/. Accsessed 15 July 2020. Stockholm, Sweden. Published 14 May 2019. Last updated 27 May 2019. [Google Scholar]
- 26. Wettermark B, Hammar N, Fored CM, et al. The new Swedish prescribed drug register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726‐735. [DOI] [PubMed] [Google Scholar]
- 27. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Measures to assess the prognostic ability of the stratified Cox proportional hazards model. Stat Med. 2009;28:389‐411. [DOI] [PubMed] [Google Scholar]
- 29. Lian Y, Zhu Y, Tang F, Yang B, Duan R. Herpes zoster and the risk of ischemic and hemorrhagic stroke: a systematic review and meta‐analysis. PloS One. 2017;12:e0171182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olsson J, Kok E, Adolfsson R, Lovheim H, Elgh F. Herpes virus seroepidemiology in the adult Swedish population. Immunity Ageing. 2017;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin WR, Casas I, Wilcock GK, Itzhaki RF. Neurotropic viruses and Alzheimer's disease: a search for varicella zoster virus DNA by the polymerase chain reaction. J Neurol Neurosurg Psychiatry. 1997;62:586‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hemling N, Roytta M, Rinne J, et al. Herpesviruses in brains in Alzheimer's and Parkinson's diseases. Ann Neurol. 2003;54:267‐271. [DOI] [PubMed] [Google Scholar]
- 33. Readhead B, Haure‐Mirande JV, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64‐82.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lovheim H, Olsson J, Weidung B, et al. Interaction between cytomegalovirus and herpes simplex virus type 1 associated with the risk of Alzheimer's disease development. J Alzheimers Dis. 2017;61(3):939‐945. [DOI] [PubMed] [Google Scholar]
- 35. Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet. 1997;349:241‐244. [DOI] [PubMed] [Google Scholar]
- 36. Koelle DM, Magaret A, Warren T, Schellenberg GD, Wald A. APOE genotype is associated with oral herpetic lesions but not genital or oral herpes simplex virus shedding. Sex Transm Infect. 2010;86:202‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuhlmann I, Minihane AM, Huebbe P, Nebel A, Rimbach G. Apolipoprotein E genotype and hepatitis C, HIV and herpes simplex disease risk: a literature review. Lipids Health Dis. 2010;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rizzuto D, Feldman AL, Karlsson IK, Dahl Aslan AK, Gatz M, Pedersen NL. Detection of Dementia cases in two swedish health registers: a validation study. J Alzheimers Dis. 2018;61:1301‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sonnen JA, Santa Cruz K, Hemmy LS, et al. Ecology of the aging human brain. Arch Neurol. 2011;68:1049‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Religa D, Fereshtehnejad SM, Cermakova P, et al. SveDem, the Swedish Dementia Registry–a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PloS One. 2015;10:e0116538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jedenius E, Johnell K, Fastbom J, Stromqvist J, Winblad B, Andreasen N. Dementia management programme in a community setting and the use of psychotropic drugs in the elderly population. Scand J Prim Health Care. 2011;29:181‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zahirovic I, Torisson G, Wattmo C, Londos E. Psychotropic and anti‐dementia treatment in elderly persons with clinical signs of dementia with Lewy bodies: a cross‐sectional study in 40 nursing homes in Sweden. BMC Geriatr. 2018;18:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


