Abstract
Introduction
Recent developments suggest that insulin‐sensitizing agents used to treat type II diabetes (T2DM) may also prove useful in reducing the risk of Alzheimer's disease (AD). The objective of this study is to analyze the association between exenatide use among Medicare beneficiaries with T2DM and the incidence of AD.
Methods
We performed a retrospective cohort analysis on claims data from a 20% random sample of Medicare beneficiaries with T2DM from 2007 to 2013 (n = 342,608). We compared rates of incident AD between 2009 and 2013 according to exenatide use in 2007–2008, measured by the number of 30‐day‐equivalent fills. We adjusted for demographics, comorbidities, and use of other drugs. Unmeasured confounding was assessed with an instrumental variables approach.
Results
The sample was mostly female (65%), White (76%), and 74 years old on average. Exenatide users were more likely to be male (38% vs. 35%), White (87% vs. 76%), and younger (by 4.2 years) than non‐users. Each additional 30‐day‐equivalent claim was associated with a 2.4% relative reduction in incidence (odds ratio 0.976; 95% confidence interval 0.963–0.989; P < .001). There was no evidence of unmeasured confounding.
Discussion
Exenatide use is associated with a reduced incidence of AD among Medicare beneficiaries aged 65 years or older with T2DM. The association shown in this study warrants consideration by clinicians prescribing insulin sensitizing agents to patients.
Keywords: Alzheimer's disease incidence, diabetes, exenatide, prevention
1. INTRODUCTION
Alzheimer's disease (AD) is currently the leading cause of dementia worldwide. 1 It is estimated that AD affects 5.5 million people in the United States. 2 With an aging “baby boomer” population in the United States and life expectancy increasing in developed nations, the prevalence of AD is expected to more than double by 2050. 3 As the prevalence of the disease increases, so too will the costs incurred by the health‐care system and caregivers. In 2010, the total cost of informal and formal care for AD and other dementias in the United States was between $157 and $215 billion, and these costs will likely double by 2040. 4 These trends of growing prevalence and cost are poised to make AD a significant burden on patients and society in the upcoming years. 3
AD is a neurodegenerative disorder of unknown etiology. AD pathology is defined by two abnormal features in the brain: increased deposition of extracellular amyloid beta (Aβ) plaques and an accumulation of intraneuronal neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein. 5 A recent survey showed that 112 agents were in the AD treatment pipeline in 2018, the majority of which are disease‐modifying therapies and symptomatic cognitive enhancers. 6 In preclinical drug development studies, various compounds have successfully reduced neuroinflammation, prevented the development of NFTs, and cleared Aβ plaques in animal models of AD. 7
Despite these encouraging results, few drugs have offered clinical benefits to patients. Between 2002 and 2012, 244 compounds were studied in 413 AD clinical trials, with only one compound advancing to the Food and Drug Administration (FDA) for review and approval. 8 Presently, only five drugs are FDA‐approved to treat AD symptoms, 9 warranting more innovative approaches to develop novel and effective treatment strategies.
In the past few decades, a link has been established between type II diabetes (T2DM) and AD. Both diseases share aging as a primary risk factor as well as several pathophysiological features, such as insulin resistance, amyloid aggregation, inflammation, oxidative stress, and cognitive disturbances. 10 , 11 Epidemiological studies show that patients with T2DM are more likely to develop AD. 12 A growing body of research indicates resistance to insulin may underpin both conditions. 13 , 14 , 15
Insulin, alongside its regulatory role in the digestive system, is also expressed in the central nervous system. 16 In the brain, insulin promotes many of the functions that are impaired in AD, including learning, memory, inflammatory responses, Aβ clearance, and tau protein phosphorylation. 17 , 18 Levels of insulin and its receptors were markedly reduced in AD brains, demonstrating a strong similarity between brain insulin resistance in AD and muscle insulin resistance in T2DM. 19 , 20 Moreover, intranasal administration of insulin resulted in cognitive improvement in individuals with mild cognitive impairment (MCI) or AD. 20 The convincing relationship between T2DM and AD has provided the impetus to make use of existing insulin‐sensitizing agents as a potential treatment strategy for AD. The effects of such treatment have been analyzed. 21 , 22
Incretin mimetics are a class of medications that have been used to treat T2DM and have shown positive outcomes in preclinical studies of neurodegenerative diseases. 23 , 24 The two well‐known incretin peptides, glucagon‐like peptide‐1 (GLP‐1), and gastric inhibitory polypeptide (GIP), mainly function to regulate insulin secretion in the body. 25 Exenatide, a synthetic analogue of the incretin hormone GLP‐1, was the first incretin mimetic approved by the FDA to treat T2DM in the United States. Exenatide is capable of crossing the blood‐brain barrier, resisting rapid degradation, and binding to GLP‐1 receptors in the central nervous system (CNS), 26 stimulation of which has resulted in a neuroprotective response, indicated in vitro and in various animal models. 27 In mouse models of AD, exenatide administration protected against impairment of learning and memory, 28 , 29 , 30 defective insulin signaling associated with Aβ oligomers, 31 Aβ peptide accumulation, 27 and a reduction in cognitive ability. 32 A newly developed triple agonist compound that targets both GLP‐1 and GIP receptors, as well as glucagon receptors, was found to reduce levels of mitochondrial pro‐apoptotic signaling, protect against synaptic loss, promote neurogenesis, and decrease both Aβ accumulation and neuroinflammation in AD mouse models. 33 In humans, a trial of 38 patients who already had AD found that GLP‐1 stabilized glucose metabolism. 34 A small clinical trial of 21 patients that evaluated exenatide use in individuals with MCI/early AD has published mostly negative results regarding disease‐modifying effects with medication use. 35 Though this study was not able to address the effects of exenatide on disease onset, the study did find a decrease in Aβ42 plasma neuronal extracellular vesicles compared to baseline levels in exenatide users, suggesting a reduction in severity of brain amyloidosis. 35 Nonetheless, small N and early termination of the study reduce the power of the study and suggest the results should be interpreted with caution.
HIGHLIGHTS
Incretin mimetics used to treat diabetes may have neuroprotective effects.
We analyzed Alzheimer's disease (AD) incidence among Medicare beneficiaries with type 2 diabetes.
After adjustment, exenatide use was negatively associated with AD onset.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources. Potential neuroprotective effects of insulin‐sensitizing agents in general and incretin mimetics in particular have been hypothesized but are not well understood. There have been several recent publications addressing these effects, and the relevant literature is appropriately cited.
Interpretation: Our findings lend support to the hypothesis that exenatide use can be neuroprotective, in this case by delaying the onset of Alzheimer's disease among older Medicare beneficiaries with type 2 diabetes.
Future directions: While this study adjusted for a wide range of demographic, health, and socioeconomic factors, unmeasured confounding remains a concern for an observational analysis such as this one. Large trials assessing the neuroprotective effects of exenatide would strengthen the evidence base and could lead to better health in an aging population.
The neuroprotective effects of GLP‐1 agonists, particularly exenatide, and the established link between T2DM and AD lead us to hypothesize that exenatide use in T2DM patients will influence the potential onset of AD. Such evidence has recently emerged for other drug classes. 36 , 37 To our knowledge, no studies have used longitudinal data to identify an association between exenatide use and AD incidence in individuals with T2DM. We used administrative data from a 20% random sample of Medicare fee‐for‐service beneficiaries from 2007 to 2013 and identified patients with T2DM, and later AD, with corresponding diagnostic codes. We designated the exposure period to be between 2007 and 2008 and set the 2009 to 2013 as the outcome period. We performed logistic regression analyses on the onset of AD in exenatide users and non‐exenatide users with T2DM, while controlling for other drug use, comorbidities, age, sex, and race.
2. METHODS
2.1. Study design and population
We performed a retrospective analysis on a 20% random sample of Medicare fee‐for‐service beneficiaries based on medical and pharmacy claims data between 2002 and 2013. Individuals with T2DM were selected into our sample based on diagnoses in medical claims over 2002 to 2006. During our exposure window (2007–2008), exenatide fills in prescription drug claims were identified by National Drug Codes in First Databank. 38 In our outcome period (2009–2013), incident AD was identified based on medical claims, as described below. Multivariable analyses controlled for a variety of patient characteristics.
We identified patients with T2DM based on an ICD‐9 (International Classification of Diseases, Ninth Revision, Clinical Modification) diagnosis code of 250.x0 or 250.x2 within Part A or B claims (specifically, Inpatient, Hospital Outpatient, Skilled Nursing Facility, Carrier, and Home Health Agency Files) prior to the exposure period (2007–2008). Individuals in the sample were required to be continuously enrolled in Medicare Parts A and B over 2006 to 2013 as well as a standalone Part D prescription drug plan over 2007 to 2008. In addition, individuals had to be age 65 or older as of January 2007. Enrollment and demographic characteristics were obtained from the Master Beneficiary Summary Files (MBSF). Finally, individuals in the sample had not been diagnosed with AD prior to 2008, as indicated by two or more medical claims with a diagnosis code of 331.0. 37
2.2. Statistical analyses
We used multivariable logistic regression to evaluate the relationship between exenatide use and AD incidence. The outcome variable was any incidence of AD based on diagnoses in medical claims between 2009 and 2013. The exposure variable was the number of 30‐day‐equivalent fills (based on days supplied) of exenatide over 2007–2008, as measured from Prescription Drug Event Files.
We adjusted for age (defined by 5‐year bands) at the beginning of 2007, sex, race, a proxy for socioeconomic status (dual eligibility or receipt of a low‐income subsidy [LIS] during the exposure period), and the presence of 25 chronic comorbidities (all reported in the MBSF and measured using validated claims algorithms) prior to 2009. 39 We also adjusted for year of first diabetes diagnosis as well as fills of other diabetes drugs (specifically, metformin, insulin, DPP‐4 and thiazolidinediones, identified by NDCs) during 2007–2008, because exenatide is typically a second‐line treatment. 40 We also controlled for the use of other medications that may affect AD onset, such as statins, 36 antihypertensives 41 (beta blockers, angiotensin‐converting‐enzyme [ACE] inhibitors, angiotensin‐receptor blockers [ARBs], Ca2+ channel blockers, loop diuretics ,and thiazide‐like diuretics), and some asthma medications (β2AR agonists). 42 In sensitivity analysis, we adjusted for community characteristics (logarithm of median household income, percent of residents with at least a high‐school degree, percent of housing units that were owner‐occupied, and percent foreign‐born) by linking each beneficiary to zip code data from the 2007–2011 American Community Survey. 43
It is possible that unmeasured factors (such as unmeasured diabetes severity) affect exenatide use and AD incidence. To address potential confounding, we performed an additional instrumental variable (IV) analysis. 44 We used a binary indicator for whether an individual's primary prescribing physician (defined by the number of fills across all drugs, with ties broken randomly) ever prescribed exenatide to another beneficiary during 2007 and 2008. 45 For our IV estimation, we applied both probit and two‐stage residual inclusion (2SRI) methods. 46 The first stage was a linear regression of the potentially endogenous variable (30‐day‐equivalent claims) on the instrument and all patient characteristics. The second stages were probit and logistic models, respectively, of AD incidence on patient characteristics; the 2SRI model included the first‐stage residual. The IV method is valid only if the instrument is systematically unrelated to unmeasured determinants of AD onset. While this assumption cannot be directly tested, we explore whether the potential determinants in our regression are related to the instrument, based on standardized differences. 47 A lack of relationship with respect to measured characteristics would be consistent with the hypothesis that the instrument is also unrelated to unmeasured factors. 46
Additional analysis assessed whether the relationship between exenatide use and AD incidence was modified by sex, age, race/ethnicity, and metformin use. In sensitivity analysis, we excluded individuals with a diagnosis of non‐AD dementia prior to 2009. 48 We also compared mortality (measured using death dates from the MBSF) among non‐users, non‐intensive exenatide users, and intensive users (the latter two defined by median days supplied), because our primary sample requires that patients survive through 2013. Significance for all statistical analyses was determined by two‐sided hypothesis tests with a P‐value less than .05. All analyses were conducted using Stata version 14.2.
3. RESULTS
Between 2007 and 2013, the 20% Medicare database contained 9,260,965 beneficiaries. Our sample included a total of 342,608 beneficiaries who had had a T2DM diagnosis and were age 65 or older at the start of 2007 and who were continuously enrolled in Parts A and B from 2007 to 2013 as well as Part D during the exposure period (2007–2008); 4316 (1.26%) individuals filled a prescription for exenatide between 2007 and 2008 (Table 1). Individuals in our sample were mostly female (65%) and White (76%), and were 74 years old on average. In total, 27,000 individuals in the sample were diagnosed with AD from 2009 to 2103, resulting in a 5‐year AD incidence rate of 7.9%.
TABLE 1.
Descriptive statistics of study population by exenatide use
| Characteristic | Full sample (N = 342 426) | Non‐user (N = 338110 | Non‐intensive user (N = 2 186) | Intensive user (N = 2 130) | P value |
|---|---|---|---|---|---|
| Number of 30‐day exenatide claims | 0.118 (1.34) | — | 3.16 (1.97) | 15.7 (5.31) | — |
| Incident Alzheimer's disease diagnosis (%) | 7.88 | 7.92 | 5.31 | 4.13 | <0.001 |
| Mean age (years) | 74.2 (6.0) | 74.2 (6.0) | 70.6 (4.1) | 70.7 (4.3) | <0.001 |
| Male (%) | 35.4 | 35.4 | 37.1 | 38.0 | 0.010 |
| Race, White (%) | 76.3 | 76.2 | 85.8 | 87.6 | <0.001 |
| Incidence year of diabetes | |||||
| 2002 or earlier (%) | 41.9 | 41.7 | 53.8 | 55.0 | <0.001 |
| 2003 (%) | 14.6 | 14.6 | 13.8 | 13.1 | 0.060 |
| 2004 (%) | 14.5 | 14.6 | 13.4 | 13.0 | 0.028 |
| 2005 (%) | 15.5 | 15.5 | 12.9 | 13.6 | <0.001 |
| 2006 (%) | 13.4 | 13.5 | 6.0 | 5.4 | <0.001 |
| Dual eligible/received LIS (%) | 35.0 | 35.1 | 26.7 | 29.9 | <0.001 |
| Social economic status (zip level): | |||||
| Median household income (thousands), mean (SD) | 54.2 (22.5) | 13.548 | 55.3 (23.5) | 55.7 (22.8) | 0.001 |
| High school graduates (%), mean (SD) | 57.9 (12.1) | 35.102 | 58.5 (11.7) | 58.4 (12.3) | 0.009 |
| Foreign born (%), mean (SD) | 12.2 (13.7) | 12.2 (13.7) | 10.4 (11.7) | 10.8 (12.4) | <0.001 |
| Owner occupied housing (%), mean (SD) | 67.4 (16.7) | 67.4 (16.7) | 69.6 | 68.7 | <0.001 |
| Comorbidities (%): | |||||
| Acute myocardial infarction | 5.30 | 5.30 | 5.58 | 4.23 | 0.041 |
| Anemia | 60.2 | 60.2 | 60.2 | 57.9 | 0.107 |
| Asthma | 13.7 | 13.7 | 17.7 | 14.6 | <0.001 |
| Atrial fibrillation | 13.9 | 13.9 | 13.7 | 14.2 | 0.876 |
| Benign prostate hyperplasia | 17.0 | 17.0 | 16.3 | 16.2 | 0.395 |
| Cancer, breast | 5.78 | 5.79 | 5.44 | 5.07 | 0.253 |
| Cancer, colorectal | 3.39 | 3.40 | 2.65 | 2.58 | 0.006 |
| Cancer, endometrial | 1.03 | 1.03 | 1.05 | 0.94 | 0.904 |
| Cancer, lung | 0.88 | 0.89 | 0.41 | 0.85 | 0.003 |
| Cancer, prostate | 5.07 | 5.08 | 5.08 | 4.41 | 0.329 |
| Cataracts | 74.8 | 74.8 | 71.3 | 70.7 | <0.001 |
| Chronic kidney disease | 21.9 | 21.8 | 33.2 | 31.4 | <0.001 |
| Chronic obstructive pulmonary disease | 26.8 | 26.8 | 28.1 | 22.9 | <0.001 |
| Congestive heart failure | 34 | 33.9 | 39.8 | 38.7 | <0.001 |
| Depression | 26.6 | 26.5 | 33 | 27.7 | <0.001 |
| Glaucoma | 27.2 | 27.2 | 26 | 27.2 | 0.437 |
| Hip fracture | 2.60 | 2.62 | 1.42 | 1.03 | <0.001 |
| Hyperlipidemia | 90.4 | 90.4 | 97.3 | 96.5 | <0.001 |
| Hypertension | 93.8 | 93.8 | 98 | 97.6 | <0.001 |
| Hypothyroidism | 22.5 | 22.4 | 24.1 | 25.4 | 0.002 |
| Ischemic heart disease | 61.4 | 61.4 | 67.4 | 63.5 | <0.001 |
| Osteoporosis | 22.6 | 22.7 | 15.7 | 16 | <0.001 |
| Rheumatoid arthritis | 60.7 | 60.7 | 63.6 | 61.4 | 0.015 |
| Stroke/transient ischemic attack | 15.8 | 15.9 | 13.6 | 12.8 | <0.001 |
| Other drug use (%): | |||||
| ACE inhibitors | 47.8 | 47.7 | 58.3 | 56.1 | <0.001 |
| ARBs | 28.9 | 28.7 | 42.2 | 41.9 | <0.001 |
| β2AR agonists | 13.4 | 13.3 | 17.8 | 15.4 | <0.001 |
| Beta blockers | 47.9 | 47.8 | 56.2 | 52.5 | <0.001 |
| Ca2+ channel blockers | 36.3 | 36.2 | 38.8 | 37 | 0.038 |
| DPP‐4 inhibitors | 4.50 | 4.35 | 23.3 | 13.6 | <0.001 |
| Insulin | 37.7 | 37.1 | 82.9 | 76.4 | <0.001 |
| Loop diuretics | 23.4 | 23.3 | 40.8 | 34.2 | <0.001 |
| Metformin | 34.6 | 34.2 | 74 | 70.7 | <0.001 |
| Statins | 61.0 | 60.8 | 70.9 | 74.5 | <0.001 |
| Thiazide diuretics | 34.9 | 34.8 | 43.4 | 40.9 | <0.001 |
| Thiazolidinediones | 18.3 | 17.9 | 51.2 | 44.9 | <0.001 |
Note: Intensive use defined my median days supplied among users (210).
Abbreviations: ACE, angiotensin‐converting‐enzyme; ARB, angiotensin‐receptor blockers; LIS, low‐income subsidy; SD, standard deviation.
Table 1 further shows descriptive statistics among non‐users, intensive exenatide users, and non‐intensive use (the latter two defined by median days supplied of 210 among users.) Of non‐users, 7.92% developed AD, while incidence was lower among non‐intensive exenatide users (5.31%) and lowest among intensive users (4.13%; P < .001). Intensive and non‐intensive users were similar on average in age (70.6 and 70.7 years, respectively), while non‐users were significantly older (74.2 years). Other individual characteristics also differed according to use. For example, users had higher rates of chronic kidney disease at baseline (end of exposure window): 33.2% and 31.4% of non‐intensive and intensive users, respectively, suffered from this comorbidity, compared to 21.8% of non‐users (P < 0.001).
3.1. Multivariable analysis
The multivariable analysis confirms that exenatide use was associated with a lower onset rate of AD. On an unadjusted basis (Table 2), the odds ratio (OR) for a 30‐day‐equivalent fill of exenatide was 0.954 (95% confidence interval [CI], 0.94–0.967, P ≤ 0.0001). In the adjusted model, the OR was 0.976 (95% CI, 0.964–0.989; P = .0002), with each additional fill reducing the 5‐year AD incidence rate by 2.2% in relative terms. Figure 1 plots the predicted AD incidence rate according to the number of exenatide fills over 2007 to 2008. The predicted rate was 7.90% (95% CI 7.81%–7.99%) for non‐users, compared to 7.71% for users with 1 fill, 6.81% for users with the median number (7) of fills, 5.86% for 12 fills, and 4.20% for 24 fills.
TABLE 2.
Multivariate logistic regression of Alzheimer's disease incidence on exenatide use
| Specification | Unadjusted (N = 342,426) | Adjusted (N = 342,426) |
|---|---|---|
| Odds ratio (95% CI) | ||
| Constant | 0.086*** (0.085–0.087) | 0.243*** (0.145–0.407) |
| # of 30‐day claims | 0.954*** (0.941–0.967) | 0.976*** (0.963–0.989) |
| Age 70–74 | – | 1.423*** (1.362–1.487) |
| Age 75–79 | – | 2.207*** (2.112–2.306) |
| Age 80–84 | – | 3.157*** (3.014–3.307) |
| Age 85 and above | – | 3.765*** (3.566–3.975) |
| Male | – | 0.877*** (0.839–0.916) |
| Race, Black | – | 1.263*** (1.207–1.322) |
| Race, Asian | – | 0.804*** (0.749–0.863) |
| Race, Hispanic | – | 1.175*** (1.121–1.232) |
| Race, other | – | 0.868** (0.776–0.971) |
| Dual eligible/Received LIS | – | 1.376*** (1.336–1.417) |
| Social economic status (zip level): | ||
| Median household income (log) | – | 0.815*** (0.778–0.853) |
| High school graduates (%) | – | 0.648*** (0.562–0.748) |
| Foreign born (%) | – | 1.315*** (1.161–1.488) |
| Owner occupied housing (%) | – | 1.231*** (1.106–1.370) |
| Incidence year of diabetes | ||
| 2003 | – | 1.005 (0.966–1.046) |
| 2004 | – | 0.962* (0.923–1.003) |
| 2005 | – | 0.925*** (0.886–0.966) |
| 2006 | – | 0.898*** (0.858–0.939) |
| Comorbidities: | ||
| Acute myocardial infarction | – | 0.928*** (0.878–0.982) |
| Anemia | – | 1.180*** (1.145–1.216) |
| Asthma | – | 0.948*** (0.911–0.986) |
| Atrial fibrillation | – | 0.965* (0.930–1.001) |
| Benign prostate hyperplasia | – | 1.070*** (1.019–1.124) |
| Cancer, breast | – | 0.917*** (0.869–0.967) |
| Cancer, colorectal | – | 0.959 (0.897–1.026) |
| Cancer, endometrial | – | 0.908 (0.802–1.027) |
| Cancer, lung | – | 0.959 (0.840–1.094) |
| Cancer, prostate | – | 0.988 (0.926–1.055) |
| Cataracts | – | 1.01 (0.978–1.044) |
| Chronic kidney disease | – | 1.038** (1.007–1.071) |
| Chronic obstructive pulmonary disease | – | 1.107*** (1.074–1.142) |
| Congestive heart failure | – | 1.093*** (1.060–1.128) |
| Depression | – | 1.866*** (1.815–1.918) |
| Glaucoma | – | 1.009 (0.981–1.038) |
| Hip fracture | – | 1.274*** (1.198–1.356) |
| Hyperlipidemia | – | 0.906*** (0.865–0.949) |
| Hypertension | – | 1.099*** (1.029–1.174) |
| Hypothyroidism | – | 1.014 (0.984–1.045) |
| Ischemic heart disease | – | 1.119*** (1.084–1.155) |
| Osteoporosis | – | 1.125*** (1.091–1.160) |
| Rheumatoid arthritis | – | 1.107*** (1.075–1.140) |
| Stroke/transient ischemic attack | – | 1.515*** (1.469–1.561) |
| Other drug use (%): | ||
| ACE inhibitors | – | 1.006 (0.978–1.035) |
| ARBs | – | 0.914*** (0.885–0.943) |
| β2AR agonists | – | 0.961* (0.923–1.001) |
| Beta blockers | – | 0.908*** (0.883–0.934) |
| Ca2+ channel blockers | – | 0.958*** (0.932–0.985) |
| DPP‐4 inhibitors | – | 0.956 (0.895–1.021) |
| Insulin | – | 1.123*** (1.088–1.159) |
| Loop diuretics | – | 0.949*** (0.919–0.981) |
| Metformin | – | 0.963** (0.934–0.994) |
| Statins | – | 1.007 (0.978–1.036) |
| Thiazide diuretics | – | 0.913*** (0.887–0.941) |
| Thiazolidinediones | – | 0.944*** (0.910–0.980) |
| Pseudo R‐squared | 0.0003 | 0.071 |
Notes: Statistical significance is defined at the *10% level, **5% level, and ***1% level.
Abbreviations: ACE, angiotensin‐converting‐enzyme; ARB, angiotensin‐receptor blockers; CI, confidence interval; LIS, low‐income subsidy; SD, standard deviation.
FIGURE 1.
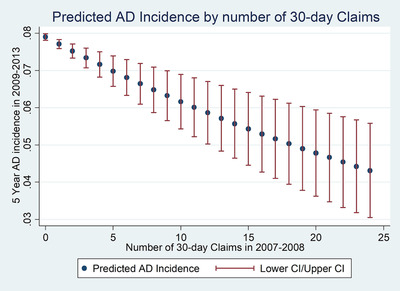
Predicted incidence of Alzheimer's disease by number of 30‐day claims. AD, Alzheimer's disease; CI, confidence interval
Observed differences in patient characteristics according to exenatide (Table 1) use raise concern about unmeasured confounding. To address this issue, we used whether a physician prescribed exenatide to another patient as an IV for the potentially confounded variable. A total of 338,183 individuals met the inclusion criteria for this analysis (vs. 342,426 in the preceding analysis). A regression of exenatide fills on physician prescribing (Table S1 in supporting information) indicates that an individual whose physician otherwise prescribed exenatide filled 0.249 more 30‐day‐equivalent claims (F = 569.8, P < .0001). In the IV analysis of use on incidence (both probit and 2SRI in Table 3), we were unable to reject the null hypothesis of no confounding, lending credibility to the primary results in Table 2. Observed patient characteristics were more similar with respect to the values of the instrument than with respect to use (Table S2 in supporting information). The average standardized difference (in terms of absolute value) was 0.047, below the common threshold of 0.10. By contrast, the average standardized difference was 0.193 for alternative use levels (maximum pairwise comparisons among users, non‐intensive, and intensive users).
TABLE 3.
Instrumental variable analyses of exenatide use
| Specification | Probit | 2SRI (logit outcome) |
|---|---|---|
| Constant | –0.848*** (–1.112–0.583) | 0.239*** (0.141–0.405) |
| # of 30‐day claims | 0.0320 (‐0.0404–0.104) | 1.052 (0.904‐1.223) |
| Age 70–74 | 0.168*** (0.147–0.190) | 1.428*** (1.363–1.496) |
| Age 75–79 | 0.390*** (0.368–0.413) | 2.224*** (2.116–2.337) |
| Age 80–84 | 0.582*** (0.557–0.608) | 3.199*** (3.030–3.377) |
| Age 85 and above | 0.680*** (0.651–0.709) | 3.812*** (3.585–4.052) |
| Male | –0.0567*** (–0.0779–0.0355) | 0.881*** (0.842–0.921) |
| Race, Black | 0.124*** (0.0994–0.148) | 1.273*** (1.213–1.336) |
| Race, Asian | –0.102*** (–0.138–0.0653) | 0.818*** (0.760–0.880) |
| Race, Hispanic | 0.0904*** (0.0651–0.116) | 1.189*** (1.131–1.250) |
| Race, other | –0.0596** (–0.115–0.00394) | 0.879** (0.784–0.985) |
| Dual eligible/received LIS | 0.153*** (0.138–0.169) | 1.350*** (1.309–1.392) |
| Social economic status (zip level): | ||
| Median household income (log) | –0.105*** (‐0.129–0.0814) | 0.816*** (0.778–0.855) |
| High school graduates (%) | ‐=0.215*** (‐0.288–0.142) | 0.662*** (0.572–0.766) |
| Foreign born (%) | 0.142*** (0.0781–0.207) | 1.326*** (1.169–1.504) |
| Owner occupied housing (%) | 0.109*** (0.0543–0.164) | 1.230*** (1.103–1.373) |
| Incidence year of diabetes | ||
| 2003 | 0.00449 (–0.0156–0.0246) | 1.006 (0.967–1.048) |
| 2004 | –0.0207* (–0.0417–0.000398) | 0.961* (0.921–1.002) |
| 2005 | –0.0364*** (–0.0578–0.0150) | 0.926*** (0.886–0.967) |
| 2006 | –0.0533*** (–0.0761–0.0304) | 0.900*** (0.859–0.942) |
| Comorbidities: | ||
| Acute myocardial infarction | –0.0347** (–0.0635–0.00582) | 0.934** (0.882–0.989) |
| Anemia | 0.0792*** (0.0642–0.0943) | 1.180*** (1.144–1.217) |
| Asthma | –0.0264** (–0.0466–0.00609) | 0.948*** (0.910–0.987) |
| Atrial fibrillation | –0.0160* (–0.0350–0.00299) | 0.967* (0.932–1.005) |
| Benign prostate hyperplasia | 0.0293** (0.00552–0.0532) | 1.067*** (1.015–1.122) |
| Cancer, breast | –0.0426*** (–0.0698–0.0154) | 0.918*** (0.869–0.969) |
| Cancer, colorectal | –0.0193 (–0.0535–0.0150) | 0.964 (0.901–1.032) |
| Cancer, endometrial | –0.0486 (–0.111–0.0142) | 0.910 (0.803–1.032) |
| Cancer, lung | –0.0179 (–0.0848–0.0490) | 0.958 (0.838–1.096) |
| Cancer, prostate | –0.00956 (–0.0418–0.0226) | 0.988 (0.924–1.055) |
| Cataracts | 0.00456 (–0.0118–0.0210) | 1.008 (0.975–1.043) |
| Chronic kidney disease | 0.0180** (0.00177–0.0343) | 1.036** (1.003–1.071) |
| Chronic obstructive pulmonary disease | 0.0535*** (0.0375–0.0694) | 1.111*** (1.076–1.147) |
| Congestive heart failure | 0.0462*** (0.0303–0.0620) | 1.095*** (1.061–1.131) |
| Depression | 0.323*** (0.309–0.337) | 1.872*** (1.821–1.926) |
| Glaucoma | 0.00414 (–0.0102–0.0185) | 1.007 (0.979–1.037) |
| Hip fracture | 0.136*** (0.102–0.170) | 1.270*** (1.192–1.353) |
| Hyperlipidemia | –0.0521*** (–0.0760–0.0283) | 0.898*** (0.857–0.942) |
| Hypertension | 0.0435*** (0.0110–0.0759) | 1.099*** (1.027–1.175) |
| Hypothyroidism | 0.00541 (–0.0101–0.0209) | 1.011 (0.980–1.042) |
| Ischemic heart disease | 0.0537*** (0.0381–0.0694) | 1.116*** (1.081–1.152) |
| Osteoporosis | 0.0607*** (0.0449–0.0766) | 1.122*** (1.087–1.158) |
| Rheumatoid arthritis | 0.0476*** (0.0328–0.0624) | 1.104*** (1.071–1.138) |
| Stroke/transient ischemic attack | 0.221*** (0.205–0.237) | 1.520*** (1.474–1.569) |
| Other drug use (%): | ||
| ACE inhibitors | 0.00171 (–0.0126–0.0160) | 1.004 (0.976–1.033) |
| ARBs | –0.0477*** (–0.0642—0.0312) | 0.909*** (0.879–0.939) |
| β2AR agonists | –0.0249** (–0.0455—0.00425) | 0.958** (0.919–0.998) |
| Beta blockers | –0.0482*** (–0.0623—0.0341) | 0.907*** (0.882–0.933) |
| Ca2+ channel blockers | –0.0198*** (–0.0337–0.00589) | 0.957*** (0.931–0.985) |
| DPP‐4 inhibitors | –0.0300* (–0.0636–0.00364) | 0.949 (0.885–1.017) |
| Insulin | 0.0531*** (0.0352–0.0710) | 1.112*** (1.073–1.153) |
| Loop siuretics | –0.0266*** (–0.0434—0.00982) | 0.945*** (0.913–0.978) |
| Metformin | –0.0226*** (–0.0395—0.00559) | 0.956*** (0.923–0.990) |
| Statins | 0.00241 (–0.0120–0.0168) | 1.006 (0.976–1.036) |
| Thiazide diuretics | –0.0460*** (–0.0608–0.0312) | 0.913*** (0.886–0.941) |
| Thiazolidinediones | –0.0335*** (–0.0536–0.0135) | 0.936*** (0.898–0.975) |
| Correlation between disturbances | –0.0574 (–0.1542–0.0394) | – |
| First‐stage residual | – | 0.928 (0.797–1.079) |
Notes: The instrumental variable is a dichotomous variable for patient's primary prescriber prescribing exenatide to someone else. First‐stage F‐test for weak IV F(1, 338 127) = 711.2, P < .0001. Second‐stage t‐test on endogeneity (P = .245 in IV Probit and P = .330 in 2SRI) leads us to fail to reject the null hypothesis of exogeneity; for IV probit, reported correlation is transformed as inverse hyperbolic tangent. Statistical significance is defined at the *10% level, **5% level, and ***1% level.
Abbreviations: 2SRI, two‐stage residual inclusion; ACE, angiotensin‐converting‐enzyme; ARB, angiotensin‐receptor blockers; CI, confidence interval; IV, instrumental variable; LIS, low‐income subsidy; SD, standard deviation.
Returning to conventional logistic regression, Figure S1 in supporting information plots the adjusted ORs and CIs by subgroups: sex, age, race/ethnicity, and metformin use. The association was similar in magnitude and more significant among female recipients (OR 0.976; 95% CI, 0.961–0.991; P = .0009). In addition, the relationship between use and incidence was not significant for patients age 85 and older.
In sensitivity analysis (Table S3 in supporting information), we excluded individuals with a diagnosis of non‐AD dementia prior to 2009. The relationship between use and incidence was nearly identical to that found in our main analysis (in particular, OR 0.977; 95% CI 0.963–0.992; P = .002). We also assessed mortality by use levels. Mortality was lower with greater use (39.3% for non‐users vs. 25.5% for non‐intensive users vs. 22.4% for intensive users, P < .001), and among those who died, the proportion with a prior AD diagnosis was similar across use levels (3.2% for non‐users vs. 3.1% for users).
4. DISCUSSION
The aim of this study was to investigate the link between exenatide use in T2DM patients and the incidence of AD. In mouse models of AD, exenatide has been demonstrated to have neuroprotective effects. Our claims‐based analysis indicates that exenatide use in individuals with T2DM is associated with a significantly reduced incidence of AD in a 5‐year follow‐up period. An instrumental variables model was used to assess unmeasured confounding and did not yield evidence of such confounding.
This association was strongest in magnitude among female patients. Women are disproportionately affected by the onset of AD and other dementias, 49 which may help to explain a stronger association within this demographic group. When distinguishing exenatide users by age, we found a similar association among 65‐ to 74‐year‐olds and 75‐ to 84‐year‐olds. For those age 85 and older, there was no association. This latter finding should be interpreted with caution, as a small sample size limited statistical power.
4.1. Possible mechanism
Administration of exenatide may delay AD onset by interacting with insulin signaling pathways. An ex vivo stimulation assay of AD brain tissue induced less activation of the PI3K/Akt signaling pathway than healthy brain tissue. 50 The group that conducted this study asserts that the most likely cause for reduced brain insulin signaling is severe dysfunction with the IRS‐1 protein, which interacts with the insulin receptor upstream, and with PI3K downstream. 50 Defects in this pathway have been shown to increase insulin resistance, thereby promoting states and components of AD including impaired Aβ clearance and altered tau phosphorylation status. 51
Considerable cross‐talk occurs between GLP‐1 and insulin signaling axes. 52 When exenatide crosses the blood‐brain barrier, it binds to GLP‐1 receptors in the CNS. Activation of GLP‐1 receptors via GLP‐1 agonists in the brains of both rats and mice have been shown to rescue the PI3K/Akt insulin signaling pathway. 53 , 54 Evidence suggests this may also occur in humans. Liraglutide, a GLP‐1 analog, was tested in a pilot clinical trial and was shown to prevent the decline of brain glucose metabolism in patients with AD after a 6‐month treatment, though there were not significant cognitive differences when compared to the placebo group. 55
Currently, a large clinical trial is underway to more extensively investigate the effects of liraglutide on AD (NCT01843075). 56 While RCTs and observational studies of liraglutide and other antidiabetic medications have been conducted, the evidence base is limited and needs further development. 34 , 57 Information on dysfunctional proteins at the transduction level would also prove beneficial in the production of new compounds that target insulin signaling pathways in AD.
4.2. Limitations
Our study has several limitations. First, our observational database lacks clinical details of diabetic patients such as laboratory test results or clinical measures of diabetic severity. T2DM has been linked to AD, and exenatide is typically used as a second‐line treatment for diabetes. Therefore, users may have a higher risk of AD if they have more severe diabetes than non‐users. We controlled for comorbidities and other diabetes medications to mitigate this potential bias and analyzed the relationship among metformin users who are plausibly more homogeneous in terms of diabetes severity than the sample as a whole. In addition, our instrumental variable analysis did not produce evidence of confounding.
Additionally, drug use from claims data is subject to measurement errors if patients are non‐adherent or if patients used exenatide beyond the 2007 to 2008 period. Measurement error tends to bias our findings toward the null of no relationship between exenatide use and AD incidence. On the other hand, mortality was higher among less‐intensive users. Finally, our findings may not generalize to other populations. This study considered older individuals enrolled in fee‐for‐service Medicare. The relationship found here may not hold for younger individuals with T2D, or for the minority of Medicare beneficiaries enrolled in managed‐care plans.
5. CONCLUSIONS
Our study identified a significant association between exenatide use and reduced AD incidence in a large and fairly representative sample of older T2DM patients. This finding underscores the possibility that certain diabetes treatments may reduce AD risk. The potential neuroprotective effects of exenatide merit further investigation.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
Julie Zissimopoulos's effort on this study was supported by the National Institute on Aging (R01AG055401). This funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication. The authors have no competing interests to declare. David Krane, BA, provided excellent editorial assistance, and was compensated for his effort.
Zhou B, Zissimopoulos J, Nadeem H, Crane MA, Goldman D, Romley JA. Association between exenatide use and incidence of Alzheimer's disease. Alzheimer's Dement. 2021;7:e12139 10.1002/trc2.12139
REFERENCES
- 1. Qiu C, Kivipelto M, von StraussE. Epidemiology of Alzheimer's disease: occurence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11:111‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hebert LE, Jennifer Weuve S, Paul Scherr SA, Denis Evans SA. Alzheimer disease in the United States (2010‐2050) estimated using the 2010 census. Neurology. 2013;80:1778‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zissimopoulos J, Crimmins E, St Clair P. The value of delaying Alzheimer's disease onset. Forum Health Econ Policy. 2014;18(1):25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;14368(4):1326‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alzheimer A. Ueber eine eigenartige erkrankung der hirnrinde. Z Gesamte Neurol Psychiatr. 1907;18:177‐179. [Google Scholar]
- 6. Cummings J, Lee G, Ritter A, Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimer's Dement Transl Res Clin Interv. 2018;4:195‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavanaugh SE, Pippin JJ, Barnard ND. Animal models of Alzheimer disease: historical pitfalls and a path forward. ALTEX. 2014;31(3):279‐302. [DOI] [PubMed] [Google Scholar]
- 8. Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug‐development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(37). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alzheimer's Association . 2017. FDA‐Approved treatments for Alzheimer's.
- 10. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer's disease and decline in cognitive function. Arch Neurol. 2004;61(5):661. [DOI] [PubMed] [Google Scholar]
- 11. Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer's disease: shared pathology and treatment?. Br J Pharmacol. 2011;71(3):365‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53(9):1937‐1942. [DOI] [PubMed] [Google Scholar]
- 13. Umegaki H. Therapeutic potential of antidiabetic medications in the treatment of cognitive dysfunction and dementia. Drugs Aging. 2016;33(6):399‐409. [DOI] [PubMed] [Google Scholar]
- 14. Götz J, Ittner LM, Lim Y‐A. Common features between diabetes mellitus and Alzheimer's disease. Cell Mol Life Sci. 2009;66(8):1321‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Craft S. The role of metabolic disorders in Alzheimer's disease and vascular dementia. Arch Neurol. 2009;66(3):300‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169‐178. [DOI] [PubMed] [Google Scholar]
- 17. Correia SC, Santos RX, Carvalho C, et al. Insulin signaling, glucose metabolism and mitochondria: major players in Alzheimer's disease and diabetes interrelation. Brain Res. 2012;1441:64‐78. [DOI] [PubMed] [Google Scholar]
- 18. Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res. 2012;2012:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin‐like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8(3):247‐268. [DOI] [PubMed] [Google Scholar]
- 20. Abdul‐Ghani MA, Defronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:76279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Avgerinos KI, Kalaitzidis G, Malli A, Kalaitzoglou D, Myserlis PG, Lioutas V‐A. Intranasal insulin in Alzheimer's dementia or mild cognitive impairment: a systematic review. J Neurol. 2018;265(7):1497‐1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Claxton A, Baker LD, Hanson A, et al. Long‐acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early‐stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44(3):897‐906. [DOI] [PubMed] [Google Scholar]
- 23. Hansen KB, Vilsbøll T, Knop FK. Incretin mimetics: a novel therapeutic option for patients with type 2 diabetes ‐ a review. Diabetes Metab Syndr Obes. 2010;3:155‐163. [PMC free article] [PubMed] [Google Scholar]
- 24. Li L, Li Y, Hölscher C. Incretin‐based therapy for type 2 diabetes mellitus is promising for treating neurodegenerative diseases. Rev Neurosci. 2016;27(7):689‐711. [DOI] [PubMed] [Google Scholar]
- 25. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1(1‐2):8‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kastin AJ, Akerstrom V. Entry of exendin‐4 into brain is rapid but may be limited at high doses. Int J Obes. 2003;27(3):313‐318. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Duffy KB, Ottinger MA, et al. GLP‐1 receptor stimulation reduces amyloid‐β peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19(4):1205‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen S, Liu A, An F, Yao W, Gao X. Amelioration of neurodegenerative changes in cellular and rat models of diabetes‐related Alzheimer's disease by exendin‐4. AGE. 2012;34(5):1211‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Wang L, Xu Y, Yu Q, Li L, Guo Y. Intranasal administration of Exendin‐4 antagonizes Ab31–35‐ induced disruption of circadian rhythm and impairment of learning and memory. Aging Clin Exp Res. 2016;28:1259‐1266. [DOI] [PubMed] [Google Scholar]
- 30. Jia X‐T, Zhang G, Liu Z, et al. Exendin‐4, a glucagon‐like peptide 1 receptor agonist, protects against amyloid‐β peptide‐induced impairment of spatial learning and memory in rats. Physiol Behav. 2016;159:72‐79. [DOI] [PubMed] [Google Scholar]
- 31. Bomfim TR, Forny‐Germano L, Sathler LB, et al. An anti‐diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease‐ associated Aβ oligomers. J Clin Invest. 2012;122(4):1339‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bomba M, Ciavardelli D, Silvestri E, et al. Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1‐KI mice but has no effects in 3xTg‐AD animals. Cell Death Dis. 2013;4(5):e612‐e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tai J, Liu W, Li Y, Li L, Hölscher C. Neuroprotective effects of a triple GLP‐1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer's disease. Brain Res. 2018;1678:64‐74. [DOI] [PubMed] [Google Scholar]
- 34. Gejl M, Gjedde A, Egefjord L, et al. Alzheimer's disease, 6‐month treatment with GLP‐1 analog prevents decline of brain glucose metabolism: randomized, placebo‐controlled, double‐blind clinical trial. Front Aging Neurosci. 2016;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mullins RJ, Mustapic M, Chia CW, et al. A Pilot Study of Exenatide Actions in Alzheimer's Disease. Curr Alzheimer Res. 2019;16(8):741‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 2017;74(2):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barthold D, Joyce G, Wharton W, Kehoe P, Zissimopoulos J. The association of multiple anti‐hypertensive medication classes with Alzheimer's disease incidence across sex, race, and ethnicity. PLoS One. 2018;13(11):e0206705 10.1371/journal.pone.0206705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hernandez I, Good CB, Cutler DM, Gellad WF, Parekh N, Shrank WH. The contribution of new product entry versus existing product inflation in the rising costs of drugs. Health Aff. 2019;38(1):76‐83. 10.1377/hlthaff.2018.05147. [DOI] [PubMed] [Google Scholar]
- 39. Chronic Conditions Data Warehouse. User Documentation. https://www2.ccwdata.org/web/guest/user-documentation. Accessed December 10, 2019.
- 40. American Diabetes Association . Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42(Suppl 1):S90‐S102. [DOI] [PubMed] [Google Scholar]
- 41. Wharton W, Goldstein FC, Zhao L, Steenland K, Levey AI, Hajjar I. Modulation of renin‐angiotensin system may slow conversion from mild cognitive impairment to Alzheimer's disease. J Am Geriatr Soc. 2015;63(9):1749‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chai G, Wang Y, Yasheng A, Zhao P. Beta 2‐adrenergic receptor activation enhances neurogenesis in Alzheimer′s disease mice. Neural Regen Res. 2016;11(10):1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Community Survey, authors. https://www.census.gov/programs-surveys/acs
- 44. McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA. 1994;272(11):859‐866. [PubMed] [Google Scholar]
- 45. Chen Y, Briesacher BA. Use of instrumental variable in prescription drug research with observational data: a systematic review. J Clin Epidemiol. 2011;64(6):687‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Terza JV, Basu A, Rathouz PJ. Two‐stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28(25):3083‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thunell J, Ferido P, Zissimopoulos J. Measuring Alzheimer's disease and other dementias in diverse populations using medicare claims data. J Alzheimers Dis. 2019;72(1):29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mazure CM, Swendsen J. Sex differences in Alzheimer's disease and other dementias. Lancet Neurol. 2016;15(5):451‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Talbot K, Trojanowski JQ, Arnold SE, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF‐1 resistance, IRS‐1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Talbot K. Brain insulin resistance in Alzheimer's disease and its potential treatment with GLP‐1 analogs. Neurodegener Dis Manag. 2014;4(1):31‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jones A, Kulozik P, Ostertag A, Herzig S. Common pathological processes and transcriptional pathways in Alzheimer's disease and type 2 diabetes. J Alzheimer's Dis. 2009;16(4):787‐808. [DOI] [PubMed] [Google Scholar]
- 53. Kimura R, Okouchi M, Fujioka H, et al. Glucagon‐like peptide‐1 (GLP‐1) protects against methylglyoxal‐induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience. 2009;162(4):1212‐1219. [DOI] [PubMed] [Google Scholar]
- 54. Xu W, Yang Y, Yuan G, Zhu W, Ma D, Hu S. Exendin‐4, a glucagon‐like peptide‐1 receptor agonist, reduces Alzheimer disease‐associated tau hyperphosphorylation in the hippocampus of rats with type 2 diabetes. J Investig Med. 2015;63(2):267‐272. [DOI] [PubMed] [Google Scholar]
- 55. McClean PL, Hölscher C. Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer's disease. Neuropharmacology. 2014;86:241‐258. [DOI] [PubMed] [Google Scholar]
- 56. ClinicalTrials.gov . Evaluating Liraglutide in Alzheimer's disease (ELAD). (2014). https://clinicaltrials.gov/ct2/ (Identification No. NCT01843075)
- 57. Cao B, Rosenblat JD, Brietzke E, et al. Comparative efficacy and acceptability of antidiabetic agents for Alzheimer's disease and mild cognitive impairment: a systematic review and network meta‐analysis. Diabetes, Obes Metab. 2018;20(10):2467‐2471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


