Abstract
Background:
Fibromyalgia is defined by idiopathic, chronic, widespread musculoskeletal pain. In adults with fibromyalgia, meta-analysis of lower-leg skin biopsy demonstrated 45% pooled prevalence of abnormally low epidermal neurite density (END). END <5th centile of the normal distribution is the consensus diagnostic threshold for small-fiber neuropathy. However, the clinical significance of END findings in fibromyalgia is unknown. The prevalence of small fiber pathology has not yet been studied in juvenile fibromyalgia.
Methods:
We screened 21 patients aged 13-20y with fibromyalgia diagnosed by pediatric rheumatologists. Fifteen meeting the American College of Rheumatology criteria (modified for juvenile fibromyalgia) underwent lower-leg measurements of END and completed validated questionnaires assessing pain, functional disability, and dysautonomia symptoms. The primary outcome was proportion of fibromyalgia patients with END <5th centile of age/gender/race-based laboratory norms. Cases were systematically matched by ethnicity, race, sex, and age to a group of previously biopsied healthy adolescents with selection blinded to biopsy results. All 23 controls matching demographic criteria were included.
Results:
Among biopsied JFM patients, 53% (8/15) had END <5th centile versus 4% (1/23) of healthy controls (p<0.001). Mean patient END was 273/mm2 skin surface (95% confidence interval: 198-389) versus 413 (95% CI: 359-467; p<0.001). As expected, fibromyalgia patients reported more functional disability, dysautonomia, and pain than healthy controls.
Conclusion:
Abnormal END reduction is common in adolescents with fibromyalgia, with similar prevalence to adults with fibromyalgia. More studies are needed to fully characterize the significance of low END in fibromyalgia and to elucidate the clinical implications of these findings.
Keywords: fibromyalgia, adolescent, nerve fibers/pathology, epidermis/innervation
Introduction:
Juvenile fibromyalgia (JFM) is characterized by persistent, widespread musculoskeletal pain, fatigue, and other somatic symptoms (1). It affects 2-6% of school-aged children, predominantly adolescent females (2). Patients often report impaired physical, school, social and emotional functioning (3). The causes of fibromyalgia are unknown, and the relative contributions of central versus peripheral neurological abnormalities are debated. Brain imaging studies show altered regional blood flow and grey-matter volume, changed activation and connectivity of pain-processing regions (4), and altered levels of several neurotransmitters (5). However, no primary brain pathology has been identified as causal, and similar abnormalities are seen in various acute and chronic pain conditions. These central findings may be non-specific responses to changed peripheral input, pain, deconditioning, mood, or medications (6).
Small-fiber neuropathy (SFN) is a peripheral polyneuropathy that like fibromyalgia, causes chronic widespread pain, exertional intolerance, gastrointestinal symptoms and chronic headache (7, 8). Common causes in mature adults include diabetes, chemotherapy, inflammation, and dysimmunity, with genetic variants and other toxins being rare causes (8). SFN is characterized by electrical hyperactivity and distal degeneration of the peripheral neurons that mediate pain, heat and cold sensation, and internal autonomic functions. Electrophysiologists report excess, spontaneous, and prolonged firing of C and Aδ pain-fibers in adult fibromyalgia and SFN (9, 10).
Measuring epidermal neurite density (END) within PGP9.5-immunolabelled lower-leg skin biopsies has become standard for confirming suspected cases of SFN (8). Systematic review of early skin-biopsy studies of adult fibromyalgia generated 45% pooled prevalence of pathological biopsies (95% CI:32-59%) (11). A subsequent study of 117 skin-biopsied adults with fibromyalgia identified correlations between severity of multiple fibromyalgia symptoms and extent of cutaneous denervation, and documented abnormal proximal as well as distal skin biopsies in some patients (10). Nerve conduction studies have also identified correlations between low medial plantar and sural nerve amplitudes and low END in adult fibromyalgia (12). Brain imaging studies show reduced functional connectivity between anterior cingulate, amygdala and precuneus in SFN, with severity paralleling skin denervation, supporting the hypothesis that SFN can produce post-synaptic brain effects similar to those reported in fibromyalgia (13). Given the increasing importance of skin-biopsies in adult fibromyalgia, we undertook the first study to examine END in juvenile fibromyalgia.
Patients and Methods:
Fibromyalgia patients aged 13-20y were recruited at Rutgers-Robert Wood Johnson and Columbia University Medical Centers between December 2016-November 2018. Inclusion for screening required clinical diagnosis by a pediatric rheumatologist. Patients with disorders associated with small fiber neuropathy were excluded. This study was approved by the institutional review boards of the participating centers (Rutgers IRB Pro20160000631, Columbia IRB AAAR3311). Participants over 18 provided consent, those under 18 provided assent plus parental permission.
Participants who did not meet the 2010 American College of Rheumatology (ACR) fibromyalgia diagnostic criteria (modified for juvenile fibromyalgia) were excluded (1). They completed validated surveys of pain, functional disability, and dysautonomic symptoms. Self-reported pain during the prior week was assessed by 0-10 numeric rating scale (0=none; 10=worst pain). We administered the Functional Disability Inventory (FDI), a 60-point self-report scale with higher scores indicating more disability (14). Dysautonomia symptoms were assessed by Composite Autonomic Symptom Score 31 (COMPASS-31) whose 0-100 range encompasses orthostatic, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor symptoms with higher scores indicating worse symptoms (15). COMPASS-31 is validated in adolescent as well as adult SFN (16). Symptom duration was calculated as months between symptom onset and skin biopsy.
Participants underwent 3mm skin punch biopsy from the standard site above the lateral malleolus. Biopsies mailed in Zamboni's fixative to Massachusetts General Hospital (MGH) clinically accredited neuropathology lab were processed, PGP9.5 immunolabeled, and analyzed according to clinical standards. MGH is one of very few diagnostic labs with skin biopsy data from healthy children and adolescents, providing pediatric normative values. All biopsies were blindly evaluated by the same morphometrist. MGH reports ENDs as per mm2 skin surface area to control for section thickness variability between labs; the correction factor is 20 for comparing to reports from labs that use 50 μm sections but report linear ENDs.
We constructed the control group by screening the 65 previously biopsied healthy controls between 13-20y. Thirteen were excluded for unobtainable ethnicity. The remaining 52 were evaluated for inclusion using a non-biased algorithm that matched race, ethnicity, sex and age of the JFM cohort. Matching was blind to all other results including skin-biopsies. To match race and ethnicity, every eligible Black, Hispanic and Asian control was included, then all other males excluded to match the 95% female JFM group, which yielded 23 healthy controls. With MGH IRB approval (1999P009042/MGH), all 23 healthy controls were re-contacted and invited to complete the study questionnaires.
The primary outcome was END<5th centile of age/sex/race-based norms. Survey question outcomes were secondary. After verifying normality, categorical outcomes were summarized by proportions and continuous outcomes by means. Fisher exact test compared the prevalence of abnormal results of categorical variables, Student’s T-test compared means of continuous variables, and Wilcoxon rank sum test compared non-normal variables.
Results:
Twenty-one adolescents with clinical JFM completed the surveys, and the 18 meeting ACR diagnostic criteria were offered skin biopsies. Fifteen underwent uncomplicated biopsies, 3 declined. The JFM and control samples were well matched by sex (7% vs. 9% male; p=0.99), age (17.2 vs. 17.9; p=0.13), ethnicity (4 Hispanic vs. 3 Hispanic; p=0.4), and race (2 Black vs. 3 Black; p=0.99; 1 Asian vs. 0 Asian). Ten of the 23 healthy controls completed surveys, 13 did not respond.
Fifty-three percent (8/15; 95% CI: 26%-79%) of JFM participants had END <5th centile of the age/gender/race-based normal distribution versus 4% (1/23, 95% CI: 0%-22%) of healthy controls (p<0.01; Figure 1). Mean END among participants with fibromyalgia was 273/mm2 skin surface (95% CI: 198-389) versus 413 (95% CI: 359-467) for controls (p<0.001). JFM participants had markedly higher median pain scores (7 vs. 1), FDI (33 vs. 1), and COMPASS-31 (49 vs. 14) than healthy controls (Table 1) and no controls had scores consistent with fibromyalgia. There were no evident differences between JFM participants with END above vs. below the 5th centile with respect to median age (17 vs. 17 years), symptom duration (26 vs. 21 months), pain scores (7 vs. 7), FDI (33 vs. 33), or COMPASS-31 scores (43 vs 53).
Figure 1.
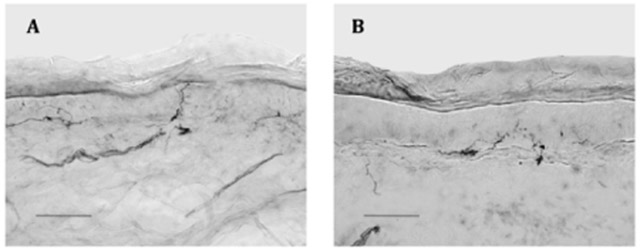
Representative skin biopsies from 10 cm above the lateral malleolus, bright-field, 40 x. Scale bar =100μm; arrows demonstrate epidermal nerve fibers
A. Healthy control: Biopsy from 17.9-year-old Caucasian female with epidermal neurite density (END) of 500/mm2 skin surface area; at the 64.3 percentile of the age, sex, and race-adjusted normal distribution.
B. Juvenile fibromyalgia patient: Biopsy from a 17.8-year-old Caucasian patient with END (242/mm2 skin surface area) at the 4.7 centile of predicted.
Table 1:
Patient characteristics, skin biopsy data, and survey data.
| Patient Characteristics | JFM patients (n=15) |
Healthy controls (n=23) |
P value |
|---|---|---|---|
| Age, y, median (IQR) | 17.2 (15.7 - 18.2) | 17.9 (16.3 - 19.3) | 0.13 |
| Female sex, n (%) | 14 (93) | 21 (91) | 0.99 |
| Hispanic, n (%) | 4 (26) | 3 (13) | 0.4 |
| Black, n (%) | 2 (13) | 3 (13) | 0.99 |
| Asian, n (%) | 1 (7) | 0 | |
| Skin Biopsy Data | |||
| END < 5th centile, n (%) | 8 (53) | 1 (4) | <0.01 |
| Mean END (neurite/mm2) ± SD | 273 ± 149 | 413 ± 132 | <0.001 |
| Surveys | JFM patients (n=15) |
Healthy Controls (n=10) | |
| Pain Score (median, IQR) | 7 (6-8) | 1 (0-2) | <0.001 |
| FDI (median, IQR) | 33 (26-39) | 1 (0-4) | <0.001 |
| COMPASS-31 (median, IQR) | |||
| Total | 49 (41-59) | 14 (3-19) | <0.001 |
| Orthostatic Intolerance | 24 (22-32) | 12 (0-19) | <0.001 |
| Vasomotor | 0 (0-3) | 0 (0-0) | 0.08 |
| Secretomotor | 6 (4-10) | 0 (0-0) | <0.001 |
| Gastrointestinal | 12 (8-16) | 1 (0-3) | 0.001 |
| Bladder | 1 (0-2) | 0 (0-0) | 0.03 |
| Pupilomotor | 3 (2-3) | 0 (0-0) | <0.001 |
JFM: juvenile fibromyalgia; y: years; END: epidermal neurite density; FDI: functional disability inventory; COMPASS-31: Composite Autonomic Symptom Score 31; IQR: interquartile range.
Discussion:
This case-control study provides the first prevalence data for small fiber pathology in adolescents with fibromyalgia, with the 53% prevalence of abnormally low END comparable to the 45% pooled prevalence in adult patients (11). Juvenile and adult fibromyalgia share many clinical characteristics, and most adolescents and young adults continues to experience symptoms into adulthood, suggesting a common spectrum of disease.
These objective neuropathologic findings may have potential for guiding treatment and measuring post-treatment axonal regeneration (8). Some investigators hypothesize that undiagnosed SFN is a cause of fibromyalgia symptoms(10), whereas others believe peripheral changes in fibromyalgia to be epiphenomena of centralized chronic pain (17). Many patients with SFN have presentations that do not resemble fibromyalgia, including exclusively distal or autonomic symptoms, and small-fiber pathology can develop in other neurodegenerative conditions, suggesting that decreased END in patients with fibromyalgia could be an incidental, non-specific finding (18).
A strength of our study is the strict inclusion criteria requiring clinical diagnosis of JFM plus best-available diagnostic criteria (1). Our results corroborate previous reports of abnormal skin biopsies and autonomic function testing in youth with more loosely defined chronic widespread pain, in whom JFM diagnoses were not captured (19, 20). Given barriers to recruiting healthy children for research requiring invasive procedures, another strength of our study is the availability of comparator biopsies from demographically matched healthy controls. This approach contrasts with a recent publication that used a convenience control sample of younger children undergoing skin biopsies for other chronic neuromuscular diseases (20).
Our study's main limitation was lack of neurologists’ evaluations to determine which JFM patients met criteria for SFN. Questionnaires and other metrics for diagnosing and tracking SFN should be validated in children, along with forthcoming research diagnostic criteria for adult SFN (7, 16). Also, our small sample may not have fully represented the JFM population, providing an inexact population prevalence. Controls were recruited at different times and from a different geographic region than JFM participants, though they were demographically matched. Another limitation is that the healthy controls’ participation in surveys was limited, and their surveys were completed at different times than skin biopsies. The survey results from controls, however, reflected values expected from healthy youth. Finally, the small sample size limited our ability to compare JFM patients with vs. without abnormal END. Despite this, we still observed striking differences in END between cases and controls that were highly consistent with studies of adults with fibromyalgia.
In summary, our study offers the first description of epidermal neurite density measurements in juvenile fibromyalgia. Our data demonstrate abnormally low epidermal neurite density in half of juvenile fibromyalgia participants, in marked contrast to the expected 5% prevalence in healthy adolescents. These findings parallel those from adult fibromyalgia patients. More research is needed to fully characterize the significance of epidermal neurite density findings in fibromyalgia and to elucidate the clinical implications of these findings.
Acknowledgments
Sources of support: Supported in part by a CARRA-Arthritis Foundation Grant (AB), and the National Institutes of Health (R01NS093653, K24NS059892, K23AR070286) and the Department of Defense (GW093049) and the Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Footnotes
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Contributor Information
Alexis Boneparth, Department of Pediatrics, Columbia University Medical Center, New York, NY, USA.
Shan Chen, Department of Neurology, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
Daniel B. Horton, Department of Pediatrics, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA, and Rutgers Center for Pharmacoepidemiology and Treatment Science, Institute for Health, Health Care Policy, and Aging Research, New Brunswick, NJ, US and Department of Biostatistics and Epidemiology, Rutgers School of Public Health, Piscataway, NJ, USA.
L. Nandini Moorthy, Department of Pediatrics, Division of Rheumatology, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
Ian Farquhar, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Heather M. Downs, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Hang Lee, Biostatistics Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Anne Louise Oaklander, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA, and Department of Pathology (Neuropathology), Massachusetts General Hospital, Boston, MA, USA.
References:
- 1.Ting TV, Barnett K, Lynch-Jordan A, Whitacre C, Henrickson M, Kashikar-Zuck S. 2010 American College of Rheumatology Adult Fibromyalgia Criteria for Use in an Adolescent Female Population with Juvenile Fibromyalgia. J Pediatr 2016;169:181–7 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashikar-Zuck S, Ting TV. Juvenile fibromyalgia: current status of research and future developments. Nat Rev Rheumatol 2014;10:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashikar-Zuck S, Parkins IS, Ting TV, Verkamp E, Lynch-Jordan A, Passo M, et al. Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology (Oxford, England) 2010;49:2204–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 2014;44:68–75 [DOI] [PubMed] [Google Scholar]
- 5.Pyke TL, Osmotherly PG, Baines S. Measuring glutamate levels in the brains of fibromyalgia patients and a potential role for glutamate in the pathophysiology of fibromyalgia symptoms: A systematic review. Clin J Pain 2017;33:944–54 [DOI] [PubMed] [Google Scholar]
- 6.Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain 2011;152:1966–75 [DOI] [PubMed] [Google Scholar]
- 7.Lodahl M, Treister R, Oaklander AL. Specific symptoms may discriminate between fibromyalgia patients with vs without objective test evidence of small-fiber polyneuropathy. Pain Rep 2018;3:e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oaklander AL, Nolano M. Scientific advances in and clinical approaches to small-fiber polyneuropathy: A review. JAMA Neurol 2019;76:1240–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, et al. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 2013;75:196–208 [DOI] [PubMed] [Google Scholar]
- 10.Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol 2019;86:504–16 [DOI] [PubMed] [Google Scholar]
- 11.Grayston R, Czanner G, Elhadd K, Goebel A, Frank B, Uceyler N, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: Implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum 2019;48:933–40 [DOI] [PubMed] [Google Scholar]
- 12.Lawson VH, Grewal J, Hackshaw KV, Mongiovi PC, Stino AM. Fibromyalgia syndrome and small fiber, early or mild sensory polyneuropathy. Muscle Nerve 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh PC, Tseng MT, Chao CC, Lin YH, Tseng WY, Liu KH, et al. Imaging signatures of altered brain responses in small-fiber neuropathy: reduced functional connectivity of the limbic system after peripheral nerve degeneration. Pain 2015;156:904–16 [DOI] [PubMed] [Google Scholar]
- 14.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, et al. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain 2011;152:1600–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 2012;87:1196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treister R, Lodahl M, Lang M, Tworoger SS, Sawilowsky S, Oaklander AL. Initial development and validation of a patient-reported symptom survey for small-fiber polyneuropathy. J Pain 2017;18:556–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clauw DJ. What is the meaning of "small fiber neuropathy" in fibromyalgia? Pain 2015;156:2115–6 [DOI] [PubMed] [Google Scholar]
- 18.Dalla Bella E, Lombardi R, Porretta-Serapiglia C, Ciano C, Gellera C, Pensato V, et al. Amyotrophic lateral sclerosis causes small fiber pathology. Eur J Neurol 2016;23:416–20 [DOI] [PubMed] [Google Scholar]
- 19.Oaklander AL, Klein MM. Evidence of small-fiber polyneuropathy in unexplained, juvenile-onset, widespread pain syndromes. Pediatrics 2013;131:e1091–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorlach J, Amsel D, Kolbel H, Grzybowsky M, Rutsch F, Schlierbach H, et al. Diagnostic utility of small fiber analysis in skin biopsies from children with chronic pain. Muscle Nerve 2020;61:173–81 [DOI] [PubMed] [Google Scholar]


