Abstract
Aims.
To identify drug use typologies based on substances used and persistence of use over two time points, use a genetically-informed design to explore twin concordance of and genetic influence on the use typologies, and compare patterns of declined/discontinued (“desistant”) and persistent drug use on drug use correlates.
Design.
Latent class analysis was applied to data from a cross-sectional self-report survey on current and past drug use. Use characteristics, use disorder, and psychiatric problems were compared across classes.
Setting.
Computer-assisted telephone interview in respondents’ homes.
Participants.
A total of 3,785 individual twins and siblings (1,365 men, 2,420 women; Mage=32) from the Australian Twin Registry Cohort III.
Measurements.
A comprehensive interview assessed prior to past year and past year use of cannabis, stimulants, cocaine/crack, hallucinogens, opioids, sedatives, inhalants, dissociatives, and solvents; age of first use; opportunity to use; peer drug use; attention deficit/hyperactivity, conduct, antisocial personality, depressive, and substance use disorders; and suicidality.
Findings.
A five-class solution emerged: no/low use (50%), desistant cannabis use (23%), desistant party drug use (18%), persistent prescription drug misuse (4%), and persistent polydrug use (5%). Twin concordances were higher among monozygotic (ⱪ=.30–.35) than dizygotic pairs (same-sex ⱪ=.19–.20; opposite sex ⱪ=.07), and biometric modeling suggested that the persistent polydrug use class, in particular, was highly heritable (a2=.94). Conduct disorder (OR=2.40), antisocial personality disorder (OR=3.27), and suicidal ideation (OR=1.98) increased persistent polydrug use risk; depression (OR=2.38) and lifetime suicide attempt (OR=2.31) increased persistent prescription misuse risk. Relative to persistent prescription drug misuse, persistent polydrug use was associated with higher rates of cannabis and stimulant use disorder (OR=6.14–28.01), younger first substance use (OR=0.82–0.83), more drug use opportunity (OR=10.66–66.06), and more drug-using peers (OR=4.66–9.20).
Conclusions.
Unique patterns of desistant and persistent drug use are differentially heritable and differentially associated with risk factors, psychiatric symptoms, and substance use disorder outcomes.
Keywords: illicit drugs, polydrug use, quasi-longitudinal, latent class analysis, twin study
Longitudinal research on the initiation and persistence of drug use through the third decade of life suggests that there are distinct trajectories of use over time. For example, one study of substance use persistence and decline/discontinuation (“desistance”) identified four temporal typologies of substance use: consistent abstention, light alcohol use with rare illicit drug use, moderate alcohol use with experimental illicit drug use, and persistent heavy alcohol with heavy illicit drug use that continued into the 30s (1). There is also evidence that trajectories vary across different drugs. Though it is not uncommon for cannabis and cocaine use to persist into the late 20s, there is minimal initiation of illicit drug use after age 25; when drug use is initiated at this point, it is most often with cocaine or psychoactive prescription medications (2). However, focusing on the use of specific drugs in isolation overlooks potentially meaningful patterns of polysubstance use; despite this, many studies combine all non-cannabis illicit drugs into a single category (1, 3), include only a subset of more common drug types (4), or focus on a single drug type (e.g., illegal opioids, prescription drugs, club drugs) (5–7). A more useful approach might be to examine multiple addictive substances in a single multivariate model that would conceptualize their use as a complete pattern of interrelated behaviors.
A germane example comes from a latent class analysis (LCA) of data from a study of Australian twins that included a comprehensive inventory of lifetime use of illicit drugs: cannabis, stimulants, cocaine/crack, hallucinogen, opioids, sedatives, inhalants, and solvents (8). Five typologies of drug use were identified: “low use” (some cannabis use); “moderate use” (cannabis use, some stimulant and hallucinogen use); “party drug” (cannabis use, some cocaine/crack, stimulant, hallucinogen, and inhalant use); “opioid/sedative” (sedative and opioid use, some cannabis use); and “polydrug” (use of all drugs) (8). However, these typologies were defined by use measured at only a single timepoint. While valuable work on illicit drug use has been conducted using LCA (8), twin designs (9, 10) and longitudinal analysis (11), research on illicit drug use that marries the strengths of these designs is, to our knowledge, nearly nonexistent.
Present Study
The present study extends this previous research by using a novel twin sample to model the persistence of illicit drug use within an LCA framework (8) with the goals of 1) examining temporal characteristics of drug use patterns by including retrospective cross-sectional reports of prior to past year and past year drug use as indicators of latent classes, 2) comparing classes on important drug use correlates, 3) assessing heritable influences on class membership, and 4) contrasting desistant and persistent use classes. We hypothesized that the “low use” class, as defined in the previous LCA study, would represent a consistently low-using class, the “moderate use,” and “party drug” classes would represent desistant drug use trajectories (experimental use/“maturing out”) (1, 12), and “polydrug use” would represent a persistent trajectory. The trajectory of the opioid/sedative class is unclear, but is of particular interest and importance in light of the current opioid epidemic in the United States (US) and substantial risk for post-prescription opioid misuse (13). Though this study presents data from Australia, population-level trends in Australia have shown increases in opioid prescribing and related harms over the past two to three decades, including during the period of data collection for the present study (14); as such, these data may help inform research and policy regarding the US opioid epidemic.
Methods
Participants and Procedure
Participants were 3,298 individual twins and 487 non-twin siblings from the Australian Twin Registry (Mage=32.13 [SD=3.04], range=21–46 [94% between age 28 and 38]; 64% female) (15). The twin subsample included 169 monozygotic (MZ) male pairs, 396 MZ female pairs, 116 dizygotic (DZ) male pairs, 298 DZ female pairs, and 225 DZ opposite-sex pairs. Participants were surveyed via computer-assisted telephone interview in 2005–2009 (participation rate=76%) (15). Original data collection was approved by the Institutional Review Boards at Washington University and Berghofer QIMR; secondary analysis of these data was deemed exempt by the University of Missouri Institutional Review Board. Analyses were not pre-registered and results should be considered exploratory.
Measurements
Substance Use
The assessment of substance use was facilitated by providing participants with a booklet that contained nine lists of substances, with each list corresponding to a class of drug (i.e., cannabis, stimulants [amphetamines], cocaine/crack, hallucinogens, opioids, sedatives, inhalants, dissociatives, and solvents). For each list, participants were asked if they 1) ever had the opportunity to use and 2) had ever used any of the substances listed. Those who endorsed having used were asked their age at first use, the recency of their last use, and to identify the specific substances(s) on the list they had used. Over-the-counter and prescription medications with abuse potential were counted if taken not as directed or without a prescription. Responses were coded positive for “past use” if first use occurred prior to the past year. Responses were coded positive for “current use” if last use occurred within the past year. Lifetime use and age of first use of alcohol and nicotine were also assessed.
Perceived Peer Drug Use
Participants were queried regarding the proportion of their current male friends, female friends, and coworkers that have ever used illicit drugs. Responses were rated on a 1–100 percentile scale.
Substance Use and Mental Health Problems
Assessments of DSM-IV SUD and psychiatric disorders were from the Australian version of the Semi-Structured Assessment of the Genetics of Alcoholism (16). Diagnoses of lifetime SUDs were obtained by combining abuse and dependence diagnoses. Attention Deficit/Hyperactivity Disorder (ADHD), Conduct Disorder, and lifetime Antisocial Personality Disorder (ASPD), Major Depression, and suicidality (ideation, attempt) were also assessed (16–18).
Analytic Plan
Analyses were conducted in Mplus Version 8 (19). Missing data were minimal (2.4%) and handled using full information maximum likelihood estimation. First, a replication of the previous LCA model (8) was run to verify its replicability before expanding upon it. The model was successfully replicated (Supplemental Table S1). We then performed an additional check by rerunning the replication model with thresholds of 3 and 10 lifetime uses to confirm that the single-use threshold was not producing spurious results. The same class solution was produced.
The quasi-longitudinal model was built using past and current use of cannabis, stimulants, cocaine/crack, hallucinogens, opioids, sedatives, inhalants, dissociatives, and solvents as indicators of the latent classes. The purpose of this analysis was twofold: 1) to identify discrete classes of drug use trajectory based upon the specific types of drugs used (or not used) prior to the past year and in the past year, and, relatedly, 2) to identify “persistent” classes of drug use. Gender was included as a covariate in all models. Maximum likelihood ratio sandwich estimation was applied to adjust standard errors for familial nonindependence. Akaike’s Information Criterion (AIC), the Bayesian Information Criterion (BIC), and Lo-Mendell-Rubin adjusted likelihood ratio tests (LMR LRTs) assessed model fit (20–22). All external validators (demographics, age of first use, opportunity to use, perceived peer use, SUD, and psychiatric disorder) were modeled as auxiliary variables using the Bolck-Croon-Hagenaars (BCH) method (23, 24). This method outperforms other approaches for modeling distal outcomes, using a weighted multiple group analysis to avoid shifts in latent class that can occur when distal outcomes are included in the LCA model; it is also robust in cases in which the variance of an auxiliary variable differs substantially across classes (23). Though most often used to model continuous variables, the BCH method is also appropriate for modeling binary outcomes (23); the expected value can be interpreted as the proportion of 1s in the class (or the probability of a 1 rather than a 0 for a randomly selected class member) (25, 26). Descriptive statistics and tests of omnibus differences across classes were generated within the auxiliary model.
After class identification, three sets of contrasts were conducted using logistic regression to examine differences between latent classes. Multivariate models were built for each “cluster” of variables (i.e., demographics, age of first use, opportunity to use, perceived peer use, SUD, and psychiatric disorder). Two contrasts compared pairs of desistant and persistent use classes, and were selected a priori with the aim of identifying factors associated specifically with persistent use; desistant and persistent classes were matched for comparison based on relative similarity of drug use probabilities at the prior to past year time point to better isolate factors potentially related to persistence. The third contrast compared persistent use classes, and was identified after observing their divergent use probability patterns.
Twin Analyses
Twin similarity for latent class membership was estimated in SAS software version 9.4 (27). For each zygosity group, twin concordance for most probable class membership was evaluated using the kappa coefficient. Significant twin similarity would provide evidence for the validity of the latent classes, and greater MZ than DZ twin similarity would provide evidence for a genetic contribution to latent class membership.
These omnibus tests of twin similarity were followed with biometric modeling applied individually to each latent class. These analyses partitioned the variation in class membership liability into additive genetic, common environmental, and unique environmental influences. Models were fitted directly to the raw twin data by the method of robust weighted least squares; bias-corrected bootstrapped confidence intervals were estimated. These analyses were conducted in Mplus Version 8 (19).
Results
In line with past research, use of any one illicit drug increased odds of use of all others (28), supporting a multivariate approach (see Supplemental Table S2 for odds ratios and use rates). Cannabis and hallucinogens were typically initiated earliest (Mage=17.91, 19.95, respectively), while other drugs were initiated in the early- to mid-20s (Mage=20.67–24.89). Cocaine, opioids, and stimulants were, on average, the most recently used substances (M=4.89–4.98 years since last use), followed by dissociates and sedatives (M=5.33–5.64), and cannabis, hallucinogens, and inhalants (M=7.37–8.99). Among respondents who reported use in the past year, last use was, on average, 3–4 months prior (except hallucinogens [7 months]). Solvents were an exception, with first and last use occurring relatively early (Mage=14.96, 16.10).
Latent Class Solution
A 5-class solution best fit the data, producing the lowest AIC and BIC values (Table 1). The 6-class solution was the first with a highly nonsignificant LMR LRT, indicating that it was the largest viable solution and likely did not add information above and beyond the 5-class model (29). The 5-class solution is theoretically sound, replicating past findings (8) without identifying any overly small, difficult-to-define classes.
Table 1.
Model Fit Indices for Quasi-Longitudinal Model Class Solutions
| Information Criteria | LMR LRT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Likelihood ratio χ2 | χ2 p | χ2 df | AIC | BIC | SSBIC | Entropy | Value | p |
| 2-Class | 2432.80 | 1.00 | 262035 | 28508.21 | 28745.29 | 28624.54 | .91 | 6783.00 | <.0001 |
| 3-Class | 2023.62 | 1.00 | 262035 | 27661.27 | 28023.12 | 27838.83 | .85 | 881.59 | <.0001 |
| 4-Class | 1713.15 | 1.00 | 262024 | 27238.91 | 27725.53 | 27477.69 | .86 | 459.58 | <.0001 |
| 5-Class | 1584.32 | 1.00 | 262010 | 27036.35 | 27647.75 | 27336.36 | .76 | 241.09 | .0003 |
| 6-Class | 1413.16 | 1.00 | 261986 | 26946.03 | 27682.21 | 27307.26 | .77 | 129.54 | .34 |
Note. Bold indicates preferred solution; AIC=Akaike’s Information Criterion; BIC=Bayesian Information Criterion; SSBIC=sample-size adjusted Bayesian Information Criterion; LMR LRT=Lo-Mendell-Rubin adjusted likelihood ratio test; entropy is reported for descriptive purposes and should not be used for model selection.
Based upon the drug use endorsement probabilities (Supplemental Table S3), the classes were interpreted as follows: 1) no/low use (NL; 50%), with near-zero drug use overall (Figure 1a); 2) desistant cannabis use (DC; 23%), with near-universal past cannabis use, slightly elevated probability of past stimulant use, and near-zero current use (Figure 1b), 3) desistant party drug use (DP; 18%;), with near-universal past cannabis and stimulant use, and elevated probabilities of past cocaine/crack and hallucinogen use that were reduced to zero or near-zero for current use (except cannabis; Figure 1c), 4) persistent prescription drug misuse (PRX; 4%), with near-universal past prescription opioid misuse, and elevated probabilities of past cannabis use and current opioid use (Figure 1d), and 5) persistent polydrug use (PP; 5%), with elevated probabilities of past use for all substances except solvents, and sustained elevated probabilities of current cannabis, stimulant, and, more moderately, cocaine/crack use (Figure 1e). The validity of the latent classes was supported by their differences across a range of important drug use correlates (Table 2).
Figure 1.
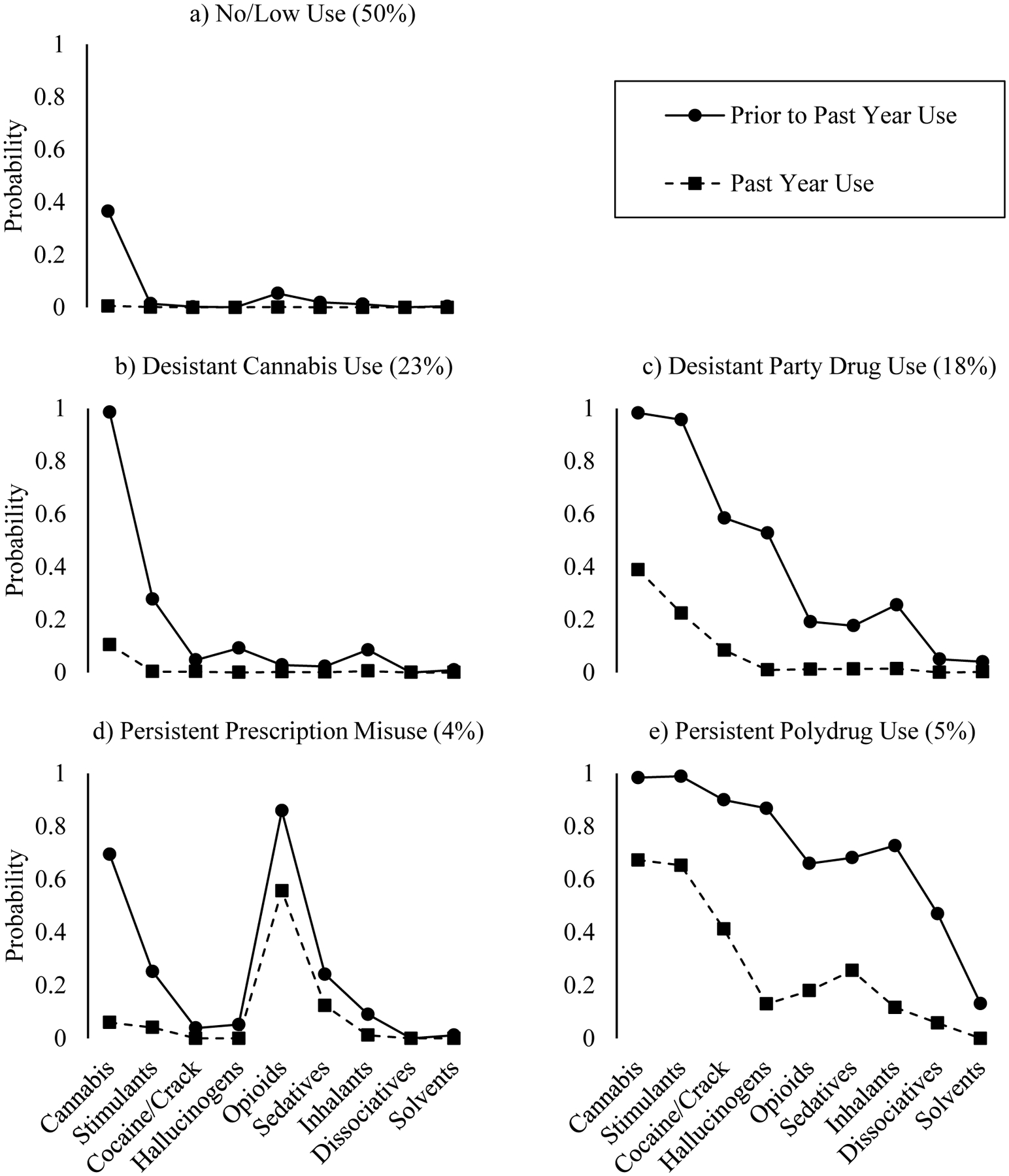
Drug Use Endorsement Probabilities by Latent Class
Table 2.
Characteristics of the five latent classes
| Desistant Classes | Persistent Classes | ||||||
|---|---|---|---|---|---|---|---|
| Full Sample | 1. No/Low Use | 2. Desistant Cannabis Use | 3. Desistant Party Drug Use | 4. Persistent Prescription Misuse | 5. Persistent Polydrug Use | Omnibus χ2 | |
| Demographics | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Full sample | 3785 (100) | 1901 (50) | 855 (23) | 681 (18) | 169 (4) | 179 (5) | - |
| Within men | 1365 (36) | 333 (24) | 572 (42) | 306 (22) | 37 (3) | 117 (9) | - |
| Within women | 2420 (64) | 1568 (65) | 283 (12) | 375 (16) | 132 (5) | 62 (3) | - |
| % Female | 64 | 82 | 33 | 55 | 78 | 35 | - |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| Age | 31.77 (0.05) | 32.31 (0.10)3 | 32.13 (0.15) | 31.63 (0.13)1 | 32.51 (0.28) | 31.81 (0.22) | 24.60* |
| Education | 13.24 (0.02) | 13.40 (0.04)5 | 13.08 (0.08) | 13.23 (0.07) | 13.14 (0.14) | 12.78 (0.13)1 | 29.08* |
| % Married | 57 | 6735 | 6135 | 361245 | 6035 | 121234 | 414.59* |
| Lifetime Drugs Used | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| 1.73 (0.03) | 0.52 (0.01)2345 | 1.56 (0.02)1345 | 3.87 (0.04)1245 | 2.49 (0.08)1235 | 6.66 (0.09)2345 | 5642.66* | |
| Age of First Use | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| Alcohol | 15.85 (0.04) | 16.87 (0.08)235 | 14.98 (0.10)145 | 14.97 (0.09)145 | 16.16 (0.24)235 | 13.94 (0.18)1234 | 473.3* |
| Nicotine | 14.27 (0.06) | 15.09 (0.14)235 | 13.90 (0.16)15 | 13.68 (0.16)1 | 14.59 (0.37)5 | 12.52 (0.29)124 | 96.14* |
| Cannabis | 17.91 (0.07) | 19.69 (0.33)235 | 18.15 (0.14)135 | 16.89 (0.13)125 | 18.15 (0.41)5 | 15.64 (0.24)1234 | 215.28* |
| Opportunity to Use | % | % | % | % | % | % | |
| Cannabis | 89 | 752345 | 100134 | 991245 | 891235 | 100134 | 468.90* |
| Stimulants | 46 | 102345 | 661345 | 98124 | 411235 | 99124 | 4095.32* |
| Cocaine/Crack | 27 | 5235 | 25135 | 781245 | 1435 | 951234 | 2620.21* |
| Hallucinogens | 27 | 32345 | 301345 | 701245 | 121235 | 951234 | 2329.30* |
| Opioids | 24 | 15345 | 12345 | 301245 | 95123 | 82123 | 840.22* |
| Sedatives | 14 | 5345 | 5345 | 22125 | 36125 | 841234 | 631.23* |
| Inhalants | 14 | 0235 | 17135 | 301245 | 1035 | 861234 | 874.00* |
| Dissociatives | 6 | <135 | 135 | 111245 | 035 | 681234 | 349.47* |
| Solvents | 6 | 1235 | 815 | 915 | 65 | 281234 | 102.95* |
| Peer Drug Use | % of Peers | % of Peers | % of Peers | % of Peers | % of Peers | % of Peers | |
| Male Friends | 45 | 202345 | 612345 | 741245 | 401235 | 902345 | 1627.87* |
| Female Friends | 33 | 152345 | 40135 | 621245 | 29135 | 762345 | 1134.38* |
| Coworkers | 9 | 635 | 935 | 1412 | 95 | 23124 | 131.21* |
| Lifetime Use Disorder | % | % | % | % | % | % | |
| Alcohol | 37 | 152345 | 7014 | 66145 | 471235 | 82134 | 736.21* |
| Nicotine | 32 | 152345 | 4715 | 571 | 4415 | 70124 | 472.00* |
| Cannabis | 16 | 02345 | 18135 | 451245 | 14135 | 741234 | 919.69* |
| Stimulants | 9 | 035 | 035 | 251245 | 335 | 731234 | 589.12* |
| Cocaine/Crack | 1 | 05 | 05 | 35 | 05 | 151234 | 46.99* |
| Hallucinogens | 2 | 05 | 05 | 35 | 05 | 41234 | 79.10* |
| Opioids | 2 | 05 | 05 | 35 | 35 | 191234 | 62.92* |
| Sedatives | 1 | 05 | 05 | <15 | 35 | 141234 | 41.22* |
| Inhalants | <1 | 0 | 0 | 0 | 0 | 2 | 6.06 |
| Dissociatives | <1 | 0 | 0 | 0 | 0 | <1 | 9.35 |
| Solvents | <1 | 0 | 0 | 0 | 0 | <1 | 2.01 |
| Psychiatric Symptoms | % | % | % | % | % | % | |
| ADHD | 4 | 235 | 5 | 61 | 8 | 111 | 33.01* |
| Conduct Disorder | 9 | <12345 | 1215 | 1715 | 1615 | 391234 | 288.14* |
| ASPD | 3 | 02345 | 315 | 415 | 81 | 23123 | 96.21* |
| Depressive Episode | 25 | 2245 | 2145 | 274 | 49123 | 3712 | 53.77* |
| Suicidal Ideation | 27 | 19345 | 2745 | 3215 | 4512 | 55123 | 115.27* |
| Suicide Attempt | 4 | 33 | 25 | 81 | 12 | 122 | 41.47* |
Note. Lifetime drugs used range=0–9; ADHD=attention deficit/hyperactivity disorder; ASPD=antisocial personality disorder; superscript denotes significant pairwise difference from correspondingly numbered class at Bonferroni-corrected p<.0002;
p<.0001.
Distinguishing Prescription Misuse and Illicit Drug Use
We examined specific substances within heterogenous drug categories that included both prescription and illicitly manufactured forms (i.e., opiates, stimulants). This substantiated that prescription misuse, rather than street drug use, characterized PRX: 92% of members had misused prescription opioids while only 4% had ever used heroin/opium. Conversely, PP, which also showed elevated probabilities for opioid use, evidenced higher rates of illicit opioid use (41%; χ2(1)=68.67, p<.0001), but lower rates of prescription opioid misuse (55%; χ2(1)=59.85, p<.0001) as compared to PRX. Opioid use disorder was more common among those who used illicit opioids than those who misused prescription opioids (χ2(1)=52.92, p<.0001), explaining higher observed rates of opioid use disorder in PP as compared to PRX despite high rates of opioid use in both classes (Table 2). Similarly, although both DP and PP displayed comparable rates of past stimulant use (96–99%; χ2(1)=1.45, p=.23; Figure 1c, e), they showed divergent patterns of current use (χ2(1)=134.90, p<.0001). Both classes evidenced high, though significantly different, rates of ecstasy use (DP=85%, PP=97%; χ2(1)=16.55, p<.0001) and prescription stimulant misuse (DP=76%, PP=96%; χ2(1)=36.94, p<.0001), but five times the proportion of PP members reported use of methamphetamine (DP=11%, PP=56%; χ2(1)=174.04, p<.0001). Respondents who used methamphetamine evidenced higher rates of stimulant use disorder than those who used ecstasy (χ2(1)=123.51, p<.0001) or misused prescription stimulants (χ2(1)=109.05, p<.0001). Of relevance, methamphetamine tends to be used more chronically and via routes that increase risk for addiction (e.g., injection), while ecstasy is typically used less frequently, restricted to more specific, youth-oriented contexts (e.g., clubs/raves), ingested orally, and has lower addictive potential (30–34).
Twin Similarity and Contributions of Genetic and Environmental Influences
The validity of the latent classes was supported by the twin similarity for latent class membership (see Supplemental Table S4 for cross-tabulations of twin similarity with concordances and relative risks) (35). Kappa coefficients were higher among MZ twins (men=0.30, p<.0001; women=.35, p<.0001) than among same-sex DZ twins, (men=0.20, p=.0005; women=0.19, p<.0001; χ2=6.33, df=1, p=.01), providing evidence for a genetic contribution to latent class membership. The estimate for opposite-sex DZ twins was relatively low, although also significant (k=.07, p=.02).
Biometric models (Figure 2, Supplemental Table S5) applied individually to each of the five latent classes revealed that there were significant genetic influences for DP and PP, with a substantially larger heritability for the more persistent class. Liability for both DC and PRX was primarily explained by unique environmental factors.
Figure 2.
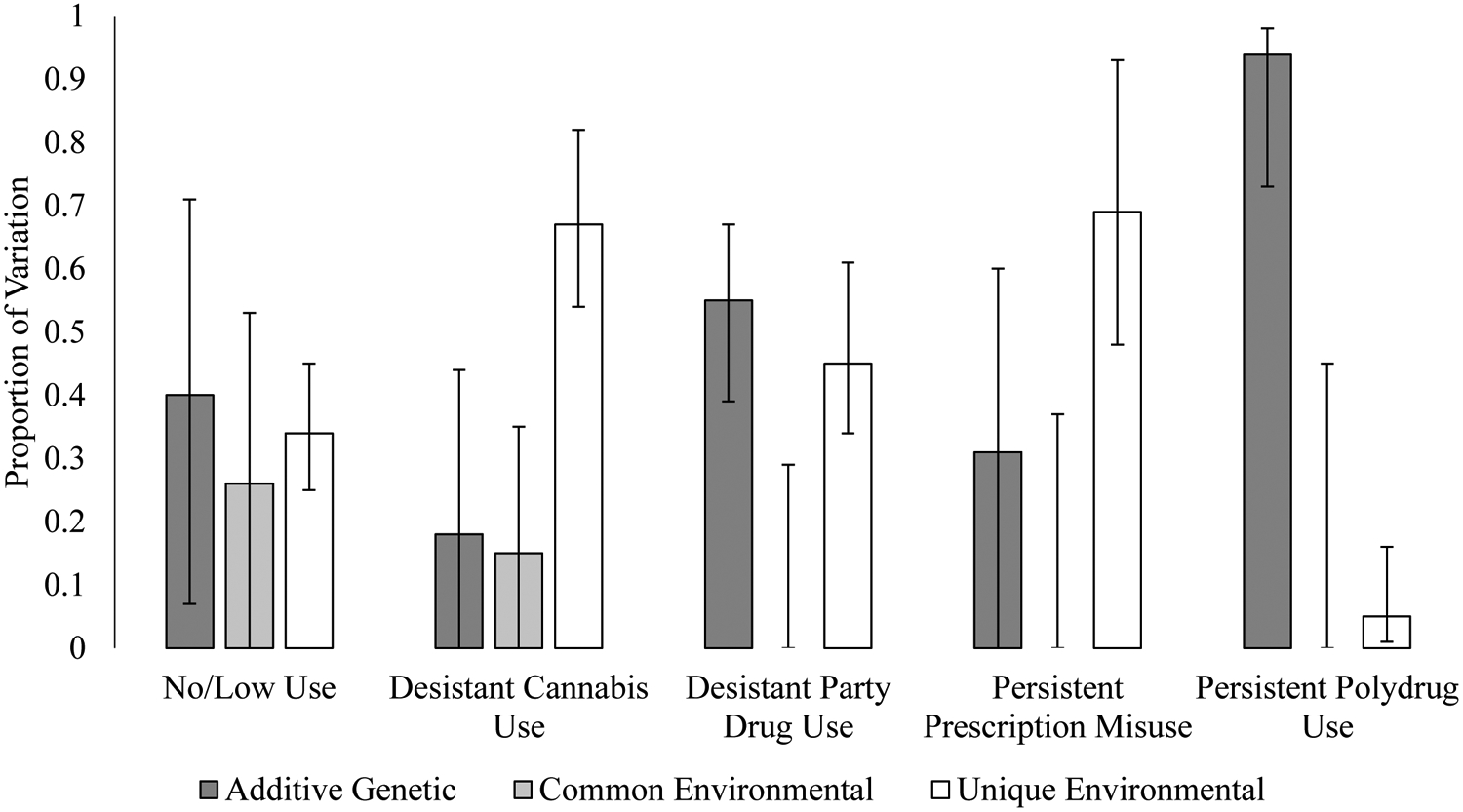
Proportion of Variation in Latent Class Membership Liability Attributable to Additive Genetic, Common Environmental, and Unique Environmental Factors
Note. Error bars represent 95% confidence intervals.
Latent Class Contrasts
Persistent Prescription Misuse vs. Desistant Cannabis Use
PRX and DC evidenced more similarities than might be expected (Table 3). While female gender decreased odds of DC (adjusted odds ratio [AOR]=0.14), these classes did not differ on other demographic characteristics or age of first alcohol, nicotine, or cannabis use. Opportunity to use cannabis decreased odds of PRX (AOR=0.04), as did opportunity to use stimulants (AOR=0.58); opportunity to use opioids and sedatives increased odds of PRX (AOR=39.61, 3.35). PRX evidenced fewer drug-using male friends (AOR=0.37) and lower odds of alcohol use disorder (AOR=0.68). Depression (AOR=2.38) and having attempted suicide (AOR=2.31) increased odds of PRX as compared to DC.
Table 3.
Latent Class Contrasts
| Contrast | |||
|---|---|---|---|
| Persistent Rx Misuse vs. Desistant Cannabis Use | Persistent Polydrug Use vs. Desistant Party Drug Use | Persistent Polydrug Use vs. Persistent Rx Misuse | |
| Reference Category | Desistant Cannabis Use | Desistant Party Drug Use | Persistent Rx Misuse |
| Demographics | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| Sex | 0.14 (0.09–0.20) | 2.07 (1.45–2.97) | 6.28 (3.61–10.91) |
| Marital status | 0.98 (0.68–1.43) | 3.48 (2.20–5.50) | 7.80 (4.34–14.04) |
| Education | 0.98 (0.86–1.11) | 0.86 (0.76–0.97)* | 0.86 (0.72–1.02) |
| Lifetime Drugs Used | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| 5.06 (3.84–6.66) | 7.34 (5.44–9.90) | 14.30 (6.95–29.43) | |
| Age of First Use | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| Alcohol | 1.01 (0.91–1.13) | 0.86 (0.78–0.95)** | 0.82 (0.71–0.95)* |
| Nicotine | 0.99 (0.93–1.05) | 0.97 (0.87–1.03) | 0.99 (0.91–1.06) |
| Cannabis | 0.99 (0.93–1.06) | 0.88 (0.82–0.96)** | 0.83 (0.75–0.92)** |
| Opportunity to Use | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| Cannabis | 0.04 (0.01–0.16) | - | - |
| Stimulants | 0.58 (0.36–0.94)* | 2.46 (0.51–11.93) | 16.28 (1.58–168.02)* |
| Cocaine/Crack | 0.66 (0.37–1.17) | 2.54 (1.26–5.14)* | 66.06 (13.54–322.19) |
| Hallucinogens | 0.34 (0.19–0.60)*** | 2.34 (1.17–4.67)* | 34.51 (8.26–144.27) |
| Opioids | 39.61 (23.29–67.39) | 3.08 (1.86–5.10) | 0.24 (0.06–0.98)* |
| Sedatives | 3.35 (1.86–6.01) | 4.26 (2.64–6.88) | 10.66 (2.59–43.84)** |
| Inhalants | 0.55 (0.28–1.07) | 4.29 (2.65–6.95) | 22.48 (6.13–82.45) |
| Dissociatives | 0.29 (0.06–1.57) | 5.53 (3.43–8.89) | - |
| Solvents | 0.47 (0.20–1.12) | 1.54 (0.85–2.77) | 1.69 (0.37–7.61) |
| Perceived Peer Drug Use | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| Male Friends | 0.37 (0.19–0.72)** | 7.15 (2.84–18.01) | 9.20 (6.98–57.31) |
| Female Friends | 1.45 (0.69–3.03) | 3.94 (2.30–6.75) | 4.66 (1.82–11.96)*** |
| Coworkers | 1.97 (0.64–6.03) | 2.78 (1.37–5.58)** | 6.68 (1.50–28.83)* |
| Lifetime Use Disorder | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| Alcohol | 0.68 (0.49–0.95)* | 1.15 (0.73–1.81) | 1.73 (0.89–3.38) |
| Nicotine | 1.16 (0.83–1.64) | 1.04 (0.69–1.57) | 1.21 (0.63–2.31) |
| Cannabis | 1.04 (0.65–1.66) | 1.62 (1.05–2.48)* | 6.14 (23.13–12.07) |
| Stimulants | - | 3.95 (2.64–5.89) | 28.01 (11.82–66.37) |
| Cocaine/Crack | - | 2.78 (1.37–5.61)** | - |
| Hallucinogens | - | 2.51 (1.37–4.60)** | - |
| Opioids | - | 2.55 (1.22–5.31)* | 2.96 (0.71–12.32) |
| Sedatives | - | 3.18 (1.27–7.97)* | 1.06 (0.22–5.23 ) |
| Psychiatric Symptoms | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| ADHD | 0.80 (0.38–1.70) | 1.00 (0.53–1.89) | 1.06 (0.46–2.44) |
| Conduct Disorder | 1.08 (0.60–1.92) | 1.50 (0.95–2.37) | 2.40 (1.25–4.62)** |
| ASPD | 1.85 (0.77–4.47) | 3.27 (1.76–6.10)*** | 1.75 (0.74–4.12) |
| Depressive Episode | 2.38 (1.62–3.48) | 0.99 (0.66–1.49) | 0.53 (0.32–0.87)* |
| Suicidal Ideation | 1.30 (0.87–1.94) | 1.98 (1.34–2.91)** | 1.60 (0.96–2.66) |
| Suicide Attempt | 2.31 (1.13–4.75)* | 0.78 (0.42–1.45) | 0.80 (0.37–1.74) |
Note. Predictor reference categories: sex=female, marital status=married, opportunity to use=no, use disorder=not present; psychiatric symptoms=not present; ADHD=attention deficit/hyperactivity disorder; ASPD=antisocial personality disorder; dash (–) indicates at least one cell N being too low to accurately estimate contrast (N=0–5); Bonferroni-corrected p=.0002; bold indicates significance, p≤.0001 unless noted;
p≤.0005,
p≤.005,
p<.05.
Persistent Polydrug Use vs. Desistant Party Drug Use
Compared to DP, PP members were more likely to be male (AOR=2.07), never married (AOR=3.48), and less educated (AOR=0.86; Table 3). Older age of first use of alcohol (AOR=0.86) and cannabis (AOR=0.88) decreased odds of PP as compared to DP. Opportunity to use all substances (except stimulants and solvents) increased odds of PP (AOR=2.34–5.53), as did reporting more male friends and coworkers who use drugs (AOR=2.78–7.15). PP also evidenced higher odds of all drug use disorders (AOR=1.62–3.95), ASPD (AOR=3.27), and suicidal ideation (AOR=1.98).
Persistent Polydrug Use vs. Persistent Prescription Misuse
Despite both evidencing patterns of persistent drug use, PRX and PP differed substantially in their patterns of problems and risk (Table 3). PP members were more likely to be male (AOR=6.28) and never married (AOR=7.80), report younger ages of first alcohol (AOR=0.82) and cannabis use (AOR=0.83), have more opportunity to use drugs (except opioids and solvents; AOR=10.66–66.06), and report more peers who use drugs (AOR=4.66–9.20). PP evidenced higher odds of cannabis and stimulant use disorders (AOR=6.14–28.01); rates of all illicit drug use disorders (except cannabis) were 5% or lower among PRX members, compared to as high as 72% in the PP class. Of the psychiatric conditions, conduct disorder increased odds of PP (AOR=2.40), while depression decreased odds of PP compared to PRX (AOR=0.53).
Discussion
As hypothesized, our quasi-longitudinal LCA identified two classes that represented desistence from previous drug involvement and two classes that represented persistent drug involvement. We also demonstrated that persistent polydrug use is more heritable than desistant drug use. A number of characteristics differentiated people who persisted in their use from those who desisted, and the two persistent typologies from each other. As anticipated, people who reported persistent polydrug use evidenced higher rates of lifetime SUD than did those who ultimately desisted in their drug use. Younger age of first alcohol and cannabis use emerged as a factor increasing odds of persistent polydrug use, even compared to respondents who ultimately desisted but reported a history of multi-drug use. The differences between the two persistent use classes highlight that prescription opioid availability fosters risk for misuse even in the face of numerous protective factors, such as older age of first substance use, limited opportunity to use substances, and few drug-using peers (36, 37). Conversely, the patterns characteristic of the PP class reinforce repeated findings that show younger age of first use, opportunity to use drugs, and having many drug-using peers to be associated with high-risk drug use and substance-related problems.
Psychiatric symptoms appear to be important risk factors for both forms of persistent use- specifically, antisociality (conduct disorder, ASPD) for polydrug use and depressive symptoms for prescription drug misuse. The rates of conduct disorder and ASPD were far lower among PRX members than PP members, linking antisociality more specifically to polydrug use, which itself confers risk for persistence via increased risk of SUD. Individuals with conduct disorder tend to initiate substance use at younger ages (38), which incurs risk for continued use; regular use in the teens and early 20s dramatically increases risk of substance use into mid-adulthood (2, 39). ASPD is by definition a persistent disorder that itself is robustly associated with drug use (40, 41); as such, the persistence of ASPD may partially explain the persistence of polydrug use (42, 43). With respect to PRX, the high rates of depression and suicidality are notable given bidirectional evidence that individuals with depressive symptoms are more likely to misuse prescribed opioid analgesics (44) and that use of opioid analgesics increases risk for depression (45). As such, screening for history of depression may be an important component of evaluating candidacy for opioid-based pain management approaches to minimize risk of prescription misuse.
The PRX class evidenced unique characteristics. It was the only class for which cannabis did not have the highest use probability as compared to the other drugs, its breadth of use was relatively small, and it appeared quite similar to the lower-using classes with respect to gender composition, use probability for non-prescription substances, risk factors for substance-related problems, and SUD prevalence. As has been observed over the years of the opioid epidemic, individuals who are non-drug seeking and/or drug naïve not infrequently receive legitimate opioid prescriptions and subsequently misuse opioids (13, 46). Most adults receive more opioid medication than is needed to manage their pain and keep the surplus at home, creating easy opportunity for misuse (47); a majority of adults who misuse prescription medication source the substance from their own prescription (48). This pattern is in line with findings that most individuals who initiate drug use in their mid- to late-20s use psychoactive prescription medications (2). This is particularly important in light of the ongoing opioid epidemic in the US. While it cannot fully explain the surge in heroin use and overdose deaths, opioid prescribing and misuse has been implicated in these increases; most people who use heroin initiated opioid use with prescription analgesics (though it should be noted that most people who misuse prescription opioids do not transition to heroin use) (37). Trends in opioid use over the past century capture a pattern of prescription misuse impacting groups historically not involved in opioid use, demonstrating that these drugs reach individuals who do not fit the “typical” profile of drug use risk (36). That is, opioid prescribing opens a door to opioid misuse to otherwise seemingly low-risk individuals who are not typically presented with opportunities to use drugs.
The PRX class was also predominately female, despite men generally engaging in drug use at higher rates than women (49). This is in line with findings that women are disproportionately represented in mid-20s prescription “downer” initiates (2, 50). Further, there is evidence that women misuse pain medication more than men and are more likely to become dependent upon nonmedically used prescription medications, such as opioid analgesics (49, 51, 52), and that men are more likely to abuse prescription stimulants than prescription opioids (53). The latter finding may explain men’s disproportional representation in the DP and PP classes and their relative absence in the PRX class. It is notable that rates of opioid use disorder were relatively low in the PRX class, potentially reflecting findings that women most often misuse prescription opioids in a “rule abiding” manner (e.g., misusing medication from one’s own prescription, ingesting via the intended route of administration, using at prescribed doses) (54–56). These patterns point toward a gender effect that should be pursued in future research.
Limitations
There are limitations of this study to note. First, it is unclear how these results from a sample of Australian adults will generalize to other countries. Laws surrounding the regulation of opioid analgesics have also changed since the time of data collection, potentially altering accessibility. Additionally, the “past use” time point represents a wider window than does the “current use” time point, and these data were retrospective, cross-sectional self-reports rather than truly longitudinal data. As such, it was not possible to use a longitudinally-oriented analytic approach, such as latent transition analysis (57). Regardless, as we sought to define our latent classes by behavior over time rather than observe changes in latent class over time, LCA provided an appropriate method. Despite these limitations, the present study leveraged multiple time points and twin data to provide a novel approach to understanding trajectories of illicit drug use among adults, laying a foundation for future longitudinal work in this area.
Conclusions
This study presents a successful replication of a past model of subtypes of drug users and its effective extension to a quasi-longitudinal analysis examining temporal patterns of drug use. The results presented here reflect that: 1) there are distinct, heritable types of drug use with unique substance preferences and temporal characteristics, 2) specific substances are more strongly associated with persistent use and SUD than other drugs in the same drug class, 3) exposure to prescription opioids among seemingly low-risk individuals may set in motion a pattern of persistent drug use despite the presence of protective factors, and 4) subtypes of drug users evidence differential levels of antisocial behavior that could represent a predisposition to or manifestation of their pattern of drug use. These findings illuminate factors associated with divergent trajectories of individuals who initiate drug use; that is, risks that contribute to some “desisting” while others persist in their use. Although much can be learned from a genetically-informative quasi-longitudinal study of polysubstance use, we look forward to future truly longitudinal, genetically-informed multivariate studies that can examine the initiation, persistence, and desistence of the full spectrum of illicit drugs (58).
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Drug Abuse (MTL, grant number R01DA18267) and the National Institute on Alcohol Abuse and Alcoholism (GFD, grant number T32AA013526).
Footnotes
The authors declare no conflict of interest.
References
- 1.Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: the effects of familial alcoholism and personality. Journal of abnormal psychology. 2004;113(4):483. [DOI] [PubMed] [Google Scholar]
- 2.Raveis VH, Kandel DB. Changes in drug behavior from the middle to the late twenties: initiation, persistence, and cessation of use. American journal of public health. 1987;77(5):607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derefinko KJ, Charnigo RJ, Peters JR, Adams ZW, Milich R, Lynam DR. Substance use trajectories from early adolescence through the transition to college. Journal of studies on alcohol and drugs. 2016;77(6):924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, et al. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US Veteran cohort. Drug and alcohol dependence. 2010;110(3):208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly BC, Rendina HJ, Vuolo M, Wells BE, Parsons JT. A typology of prescription drug misuse: A latent class approach to differences and harms. Drug and alcohol review. 2015;34(2):211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramo DE, Grov C, Delucchi K, Kelly BC, Parsons JT. Typology of club drug use among young adults recruited using time-space sampling. Drug Alcohol Depend. 2010;107(2–3):119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monga N, Rehm J, Fischer B, Brissette S, Bruneau J, El-Guebaly N, et al. Using latent class analysis (LCA) to analyze patterns of drug use in a population of illegal opioid users. Drug and alcohol dependence. 2007;88(1):1–8. [DOI] [PubMed] [Google Scholar]
- 8.Lynskey MT, Agrawal A, Bucholz KK, Nelson EC, Madden PA, Todorov AA, et al. Subtypes of illicit drug users: a latent class analysis of data from an Australian twin sample. Twin research and human genetics. 2006;9(4):523–30. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of general psychiatry. 2000;57(3):261–9. [DOI] [PubMed] [Google Scholar]
- 10.Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard twin study of substance abuse: what we have learned. Harvard review of psychiatry. 2001;9(6):267–79. [PubMed] [Google Scholar]
- 11.Meacham MC, Roesch SC, Strathdee SA, Gaines TL. Perceived treatment need and latent transitions in heroin and methamphetamine polydrug use among people who inject drugs in Tijuana, Mexico. Journal of psychoactive drugs. 2018;50(1):62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Malley PM. Maturing out of problematic alcohol use. Alcohol Research & Health. 2004;28(4):202. [Google Scholar]
- 13.Shah A Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use—United States, 2006–2015. MMWR Morbidity and Mortality Weekly Report. 2017;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanch B, Pearson SA, Haber PS. An overview of the patterns of prescription opioid use, costs and related harms in Australia. British journal of clinical pharmacology. 2014;78(5):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PA, Martin NG. An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Res Hum Genet. 2012;15(5):631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock V, Nurnberger J Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of studies on alcohol. 1994;55(2):149–58. [DOI] [PubMed] [Google Scholar]
- 17.Heath AC, Bucholz K, Madden P, Dinwiddie S, Slutske W, Bierut L, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological medicine. 1997;27(6):1381–96. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Statistical Manual of Mental Disorders (DSM-IV). American Psychiatric Pub; 1994. [Google Scholar]
- 19.Muthén LK, Muthen B. Mplus user’s guide: Statistical analysis with latent variables, user’s guide: Muthén & Muthén; 2017. [Google Scholar]
- 20.Asparouhov T, Muthén B. Using Mplus TECH11 and TECH14 to test the number of latent classes. Mplus Web Notes. 2012;14:22. [Google Scholar]
- 21.Parzen E, Tanabe K, Kitagawa G. Selected Papers of Hirotugu Akaike: Springer Science & Business Media; 2012. [Google Scholar]
- 22.Schwarz G Estimating the dimension of a model. The annals of statistics. 1978;6(2):461–4. [Google Scholar]
- 23.Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Using the BCH method in Mplus to estimate a distal outcome model and an arbitrary secondary model. Mplus Web Notes. 2020;21(6):1–22. [Google Scholar]
- 24.Bolck A, Croon M, Hagenaars J. Estimating latent structure models with categorical variables: One-step versus three-step estimators. Political Analysis. 2004;12(1):3–27. [Google Scholar]
- 25.Dziak JJ, Bray BC, Wagner AT. LCA_Distal_BCH SAS Macro Users’ Guide (Version 1.1). The Methodology Center, University Park, PA, Penn State. 2017. [Google Scholar]
- 26.Davis CN, Slutske WS, Martin NG, Agrawal A, Lynskey MT. Identifying subtypes of cannabis users based on simultaneous polysubstance use. Drug and alcohol dependence. 2019;205:107696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SAS Institute, Inc. Base SAS 9.4 procedures guide: SAS Institute; 2015. [Google Scholar]
- 28.Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, et al. Co-occurrence of Abuse of Different Drugs in Men. Arch Gen Psychiatry. 1998;55:967–72. [DOI] [PubMed] [Google Scholar]
- 29.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling: A multidisciplinary Journal. 2007;14(4):535–69. [Google Scholar]
- 30.Topp L, Hando J, Dillon P, Roche A, Solowij N. Ecstasy use in Australia: patterns of use and associated harm. Drug and alcohol dependence. 1999;55(1–2):105–15. [DOI] [PubMed] [Google Scholar]
- 31.Hammersley R, Ditton J, Smith I, Short E. Patterns of ecstasy use by drug users. British journal of criminology. 1999;39(4):625–47. [Google Scholar]
- 32.Degenhardt L, Barker B, Topp L. Patterns of ecstasy use in Australia: findings from a national household survey. Addiction. 2004;99(2):187–95. [DOI] [PubMed] [Google Scholar]
- 33.Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, et al. A comparison of patterns of methamphetamine and cocaine use. Journal of addictive diseases. 2001;21(1):35–44. [DOI] [PubMed] [Google Scholar]
- 34.Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of drugs of abuse-the case of methylenedioxy amphetamines (MDMA, ecstasy), and amphetamines. Dialogues in clinical neuroscience. 2009;11(3):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGue M When assessing twin concordance, use the probandwise not the pairwise rate. Schizophrenia bulletin. 1992;18(2):171–6. [DOI] [PubMed] [Google Scholar]
- 36.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA psychiatry. 2014;71(7):821–6. [DOI] [PubMed] [Google Scholar]
- 37.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. New England Journal of Medicine. 2016;374(2):154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown SA, Gleghorn A, Schuckit MA, Myers MG, Mott MA. Conduct disorder among adolescent alcohol and drug abusers. Journal of studies on alcohol. 1996;57(3):314–24. [DOI] [PubMed] [Google Scholar]
- 39.Kandel DB, Raveis VH. Cessation of illicit drug use in young adulthood. Archives of General Psychiatry. 1989;46(2):109–16. [DOI] [PubMed] [Google Scholar]
- 40.Van Den Bree MB, Svikis DS, Pickens RW. Genetic influences in antisocial personality and drug use disorders. Drug and Alcohol Dependence. 1998;49(3):177–87. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub; 2013. [Google Scholar]
- 42.Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Journal of Clinical Psychiatry. 2005;66(6):677–85. [DOI] [PubMed] [Google Scholar]
- 43.Brennan GM, Hyde LW, Baskin-Sommers AR. Antisocial pathways associated with substance use disorders: Characterizing etiological underpinnings and implications for treatment. Current Opinion in Behavioral Sciences. 2017;13:124–9. [Google Scholar]
- 44.Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. The Annals of Family Medicine. 2012;10(4):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scherrer JF, Svrakic DM, Freedland KE, Chrusciel T, Balasubramanian S, Bucholz KK, et al. Prescription opioid analgesics increase the risk of depression. Journal of general internal medicine. 2014;29(3):491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edlund MJ, Martin BC, Russo JE, Devries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic non-cancer pain: the role of opioid prescription. The Clinical journal of pain. 2014;30(7):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porucznik C, Sauer B, Johnson E, Crook J, Wrathall J, Anderson J, et al. Adult use of prescription opioid pain medications-Utah, 2008. Morbidity and Mortality Weekly Report. 2010;59(6):153–7. [PubMed] [Google Scholar]
- 48.Griesler PC, Hu M-C, Wall MM, Kandel DB. Medical Use and Misuse of Prescription Opioids in the US Adult Population: 2016–2017. American journal of public health. 2019;109(9):1258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SR. Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gender medicine. 2010;7(5):402–13. [DOI] [PubMed] [Google Scholar]
- 50.Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use, and discontinuation. American journal of public health. 1984;74(7):660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly BC, Wells BE, LeClair A, Tracy D, Parsons JT, Golub SA. Prevalence and correlates of prescription drug misuse among socially active young adults. International Journal of Drug Policy. 2013;24(4):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jamison RN, Butler SF, Budman SH, Edwards RR, Wasan AD. Gender differences in risk factors for aberrant prescription opioid use. The Journal of Pain. 2010;11(4):312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use, and diversion of abusable prescription drugs. Journal of American college health. 2006;54(5):269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: Findings from the National Survey on Drug Use and Health. Addictive behaviors. 2010;35(11):1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McHugh RK, DeVito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, et al. Gender differences in a clinical trial for prescription opioid dependence. Journal of substance abuse treatment. 2013;45(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. Journal of substance abuse treatment. 2015;48(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences: John Wiley & Sons; 2009. [Google Scholar]
- 58.Lisdahl KM, Sher KJ, Conway KP, Gonzalez R, Ewing SWF, Nixon SJ, et al. Adolescent brain cognitive development (ABCD) study: overview of substance use assessment methods. Developmental cognitive neuroscience. 2018;32:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


