Abstract
Purpose:
To provide clinicopathologic correlations for retrocorneal membranes associated with Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) failure.
Design:
Retrospective case series.
Methods:
The specimens and medical records of the patients diagnosed with clinically significant retrocorneal membranes associated with DSAEK failure at the Bascom Palmer Eye Institute or the University of Miami Veterans Hospital between October 2015 and March 2020, were reviewed for demographics, clinical presentation, comorbidities and surgeries performed. Histopathological analysis was performed on Hematoxylin and eosin and periodic acid-Schiff sections. Immunohistochemical studies were performed for smooth muscle actin (α-SMA), pancytokeratin and CK7. Immunofluorescence was performed for vimentin, N-cadherin, ROCK1, RhoA, ZEB1 and Snail.
Results:
A total of seven patients (3 males and 4 females) were identified to have a clinically significant retrocorneal membranes at the time of graft failure. The average age at the time of first DSAEK was 70 years (range: 55-85). All patients were pseudophakic and had a glaucoma drainage device in place; one had a history of failed DSAEK. Ranging from 0 to 47 months after surgery, a variably thick retrocorneal fibrous membrane was observed, eventually leading to graft failure. Four patients underwent subsequent penetrating keratoplasty and three underwent repeat DSAEK. On histopathologic evaluation, a pigmented fibrocellular tissue was identified along the posterior margin of the corneas and DSAEK buttons in all cases. Further characterization with immunohistochemistry and immunofluorescence demonstrated membranes to be negative for pancytokeratin and positive for α-SMA, vimentin, CK7, N-cadherin, ZEB1, Snail, ROCK1 and RhoA.
Conclusions:
Fibrocellular retrocorneal membrane proliferation may be associated with DSAEK failure in patients with previous glaucoma drainage device surgery. Our results demonstrate myofibroblastic differentiation and a lack of epithelial differentiation. Positivity for markers of an endothelial to mesenchymal transition indicates possible endothelial origin and could be the hallmark for future targeted pharmacotherapy.
Introduction
Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) has become one of the preferred techniques for patients with endothelial cell dysfunction and severe anterior segment disease.1,2 It has gained popularity due to the decreased risk of rejection and more predictable refractive outcomes when compared to penetrating keratoplasty.3 However, despite its popularity, complications occur that can lead to graft failure including graft detachment, allograft rejection, and infection. Histopathologic findings in failed DSAEK grafts vary and include endothelial attenuation, retained host Descemet’s, and/or fibrocellular membranes. These fibrocellular membranes can be found in the interface between the DSAEK graft and the host anterior stroma or posterior to the graft’s Descemet’s membrane; the latter of which are termed retrocorneal membranes.4,5
Retrocorneal membranes were first described in 1901 by Fuchs as a complication of penetrating keratoplasty (PKP) and were postulated to occur secondary to iritis.6 Further histopathologic studies demonstrated that up to 50% of failed PKP have tissue behind Descemet’s membrane, most often being fibrocellular in nature.6,7 Postulated causes of retrocorneal membranes include epithelial downgrowth, keratocyte ingrowth, and endothelial cell metaplasia.2,8,9 Most retrocorneal membranes are not appreciated clinically and are identified solely on histopathologic analysis.
Retrocorneal membranes have been described to a lesser extent in the setting of DSAEK, but a few studies have reported on retrocorneal membranes in DSAEK and Descemet Membrane Endothelial Keratoplasty (DMEK).10–12 In one retrospective study, histopathologic examination with light microscopy of failed grafts found retrocorneal fibrous membranes in 4 of 13 cases (31%) of early failed DSAEK grafts.2 One case report described a clinically significant retrocorneal membrane causing DSAEK failure 1 month after surgery in a 76 year-old female. In this case, peripheral anterior synechiae were noted with a retrocorneal membrane extending from the iris to the cornea, with loss of anterior chamber volume demonstrated on anterior segment optical coherence tomography (AS-OCT). Unfortunately, no histopathological analysis was performed as the patient did not undergo further surgery.10 The mechanisms underlying DSAEK-associated retrocorneal membranes are postulated to be similar to those found in PKP, with epithelial downgrowth, keratocyte ingrowth or/and fibrous metaplasia of the corneal endothelium as the main hypothetical contributors to the formation of these membranes.12,13,14
We identified a subset of patients who presented with clinically significant, progressive retrocorneal membranes which led to DSAEK failure. Given the limited data on the nature of retrocorneal membranes in DSAEK, the purpose of this study was to perform a clinicopathologic assessment and characterization of these membranes with the goal of identifying their source and providing a rationale for future targeted pharmacotherapy.
Materials and Methods
Study population:
Under approval of the institutional review boards of the University of Miami and the Miami Veterans Affairs Hospital, specimens and medical records of the patients diagnosed with clinically significant, progressive retrocorneal membranes associated with DSAEK failure at the Bascom Palmer Eye Institute and the University of Miami Veterans Hospital between October 2015 and March 2020 were reviewed. Patients were identified based on pathology records from the Florida Lions Ocular Pathology Laboratory at the Bascom Palmer Eye Institute. Data extracted from the clinical record included demographics, clinical presentation, comorbidities and surgeries performed.
Histologic analysis:
Three mechanisms have been proposed for retrocorneal membrane formation: epithelial downgrowth, keratocyte (fibroblastic) ingrowth, and/or fibrous metaplasia of the corneal endothelium.12,13,14 We thus chose our immunohistochemistry and immunofluorescence probes to evaluate each of these possibilities.
Epithelial downgrowth is characterized by 1 to 3 layers of stratified nonkeratinized squamous epithelium extending over the posterior cornea.15 These cells are cytokeratin positive16 and thus, we performed immunohistochemistry for pancytokeratin to evaluate an epithelial derivation of the membranes.
Unfortunately, there are no unique markers to discern stromal and endothelial origins, but immunoreactivity for a combination of markers can be highly suggestive of a cell of origin within these lineages. Keratocytes express CD34 under normal physiologic conditions, however, this marker is lost when cells are perturbed and thus this marker was not tested for in our membranes.14 Following injury, keratocytes can transform into either fibroblasts or myofibroblasts17, the latter of which leads to contraction and scarring as part of the normal wound healing process. Myofibroblasts express alpha-Smooth Muscle Actin (α-SMA), an element of the contractile unit in cells, which mediate pseudo-podia retraction,18,19 and Vimentin, an intermediate filament that strengthens and maintains the integrity of the myofibroblast cell body.20
However, as with keratocytes, endothelial cells can also undergo a transformation toward mesenchymal phenotypes, including myofibroblasts, and thus α-SMA and vimentin positivity alone cannot differentiate between a keratocyte21 or endothelial cell of origin.14 The process whereby an endothelial cell changes in phenotype towards a mesenchymal cell is termed Endothelial to Mesenchymal Tansition (EndoMT)17 and has been mostly described in vitro with cultured corneal endothelial cells (CECs).22–24 During this process, the endothelial cells change morphology and acquire a fibroblastic, spindle-like appearance.24
Numerous extracellular signals have been experimentally used to drive EndoMT, including Transforming Growth Factor-β (TGF-β). TGF-β stimulation leads to activation of the transcription factors Snail and ZEB125 which subsequently drive the phenotypic changes characteristic of EndoMT, such as disassembly of tight and gap junctions, and reversal of endothelial cell polarity. Molecularly, expression of E-cadherin25–27 is reduced during EndoMT, while N-cadherin is upregulated.28,29 ZEB1 activation also upregulates α-SMA and vimentin. 25,27,30 Another pathway activated by TGF-β is the GTPase RhoA31 and its downstream effector, ROCK1, 24,32 which are involved in actin cytoskeletal reorganization and mediate the extension of cell projections and stress fiber formation (contractile actin bundles).24,31,33 We thus evaluated the immunofluorescence of ZEB1, Snail, N-cadherin, RhoA and ROCK1 as evidence of an activated EndoMT process in the membranes. Finally, as CK7 has been described to be positive in diseased corneal endothelium, we performed CK7 staining to confirm endothelial metaplasia.14,34
Microscopic glass slides prepared from paraffin embedded tissue sections were stained with Hematoxylin and eosin and periodic acid-Schiff. Immunohistochemical studies were performed in the Immunohistochemistry Department of the University of Miami for pancytokeratin, CK7 and α-SMA. Immunohistochemistry was performed by incubating samples in α-SMA (Catalog #PA0943, Leica, Buffalo Grove, IL), CK7 (Catalog #PA0942, Leica, Buffalo Grove, IL), or pancytokeratin cocktail consisting of AE1/AE3 (1:200, Dako, Santa Clara, CA) Cam 5.2 (1:1500, Becton Dickinson, Franklin Lakes, NJ) and HMW (1:50, Dako, Santa Clara, CA) for 15 minutes. Post Primary for 8 minutes, polymer for 8 minutes, peroxide block for 8 minutes, red chromogen for 10 minutes and counterstained with hematoxylin for 10 minutes. Pretreatment with Epitope Retrieval Solution 1 (from Leica) low pH 6 was performed for 20 mins for CK7 and pancytokeratin.
For immunofluorescence analysis, paraffin-embedded sections were initially deparaffinized using xylene, followed by rehydration in serial alcohol dilutions. Afterwards, antigen retrieval with citrate buffer, permeabilization, and blocking was performed. The samples were incubated overnight with primary antibody [rabbit anti-ZEB1 primary antibody (1:50; Santa Cruz, Dallas, TX), rabbit anti-Snail primary antibody (1:50; Cell signaling, Danvers, MA), rabbit anti-N-cadherin primary antibody (1:100; Abcam, Cambridge, MA), mouse anti-vimentin primary antibody (1:100; Santa Cruz, Dallas, TX), mouse anti-RhoA primary antibody (1:50; Santa Cruz, Dallas, TX) or mouse anti-ROCK1 primary antibody (1:50; Santa Cruz, Dallas, TX)]. Samples were then washed three times in PBS and incubated for 2 hours in the appropriate secondary antibody [goat anti-rabbit AlexaFluor594, donkey anti-mouse AlexaFluor488 or donkey anti-mouse AlexaFluor594 secondary antibody (1:200, Abcam, Cambridge, MA)]. Samples were washed three times with PBS and counterstained with 1X PureBlu DAPI (BioRad, Hercules, CA) for 20 minutes, and mounted. Slides were then imaged using a laser scanning confocal microscope (Leica SP8, Leica microsystems, Buffalo Grove, IL).
Results
A total of seven patients were identified with clinically significant progressive retrocorneal membranes that eventually led to DSAEK graft failure. Three individuals were male and four were female (Table 1). The average age at time of initial DSAEK was 70 years (range 55-85). At the time of initial DSAEK, all patients were pseudophakic: one with anterior chamber intraocular lenses (IOL) and six with posterior chamber IOLs, four in bag and two scleral fixated. One patient had a history of previous DSAEK. All patients had a history of glaucoma, five had primary open angle glaucoma (POAG), one had neovascular glaucoma (NVG), and one had chronic angle-closure glaucoma (CACG). All patients had a history of glaucoma drainage device surgery. Five had one Baerveldt implant with the tube in the anterior chamber (AC), one had both a Baerveldt and Ahmed implant with the tubes in the AC, and one had a Molteno3 implant with the tube in the vitreous.
Table 1.
Demographics and characteristics of failed DSAEK caused by retrocorneal membranes.
| N | Sex | Age (years) | Race | Ethnicity | Preoperative diagnosis | Intraocular lens at time of DSAEK | Glaucoma implant at time of DSAEK | Time to graft failure (months) | Second procedure |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 55 | W | H | Failed DSAEK | PCIOL (Bag) | Baerveldt (tube in AC) | 3 | DSAEK |
| 2 | F | 66 | B | NH | Corneal edema | PCIOL (Bag) | Baerveldt (tube in AC) | 5 | PKP |
| 3 | M | 54 | B | NH | Corneal edema | PCIOL (Bag) | Baerveldt (tube in AC), CPC | 10 | PKP, removal of lens, shunt revision, vitrectomy |
| 4 | M | 69 | B | NH | Corneal edema | ACIOL | Baerveldt (tube in AC) | 12 | PKP, ACIOL removal + sutured PCIOL, shunt revision, vitrectomy |
| 5 | F | 78 | W | NH | Corneal edema | PCIOL (Scleral fixated) | Ahmed, Baerveldt (tubes in AC) | 7 | DSAEK |
| 6 | M | 81 | W | NH | Corneal edema | AKREOS Scleral sutured lens | Molteno3 (tube in vitreous) | 1 | PKP |
| 7 | F | 85 | W | H | Corneal edema | PCIOL (Bag) | Baerveldt (tube in AC) | 47 | DSAEK |
F=female; M=male; W=white; B=black; H=Hispanic; NH=Non-Hispanic; AC=anterior chamber; DSAEK= Descemet Stripping Automated Endothelial Keratoplasty; PKP=Penetrating keratoplasty
Initial postoperative visits showed a clear graft, with no signs of infection. However, between 0 to 47 months after DSAEK surgery, a variably thick retrocorneal fibrous membrane was observed which proliferated and eventually lead to graft failure. After graft failure, four patients underwent penetrating keratoplasty and three underwent repeat DSAEK. The membranes were removed during surgery and sent for histopathologic evaluation. During repeat DSAEK or PKP, two patients underwent concurrent procedures, including vitrectomy, tube repositioning, and IOL removal.
On histopathologic evaluation, a pigmented fibrocellular tissue was identified along the posterior margin of the corneas and DSAEK buttons in all cases on Hematoxylin-eosin (Figure 4A). Further characterization and immunohistochemical studies demonstrated all membranes to be negative for pancytokeratin (Figure 4B), and positive for α-SMA (Figure 4C). Four of the membranes demonstrated positivity for CK7 (Figure 4D) and three were noncontributory. Immunofluorescence showed all membranes to be positive for vimentin, N-cadherin, ZEB1, Snail, ROCK1 and RhoA (Figure 5).
Figure 4.
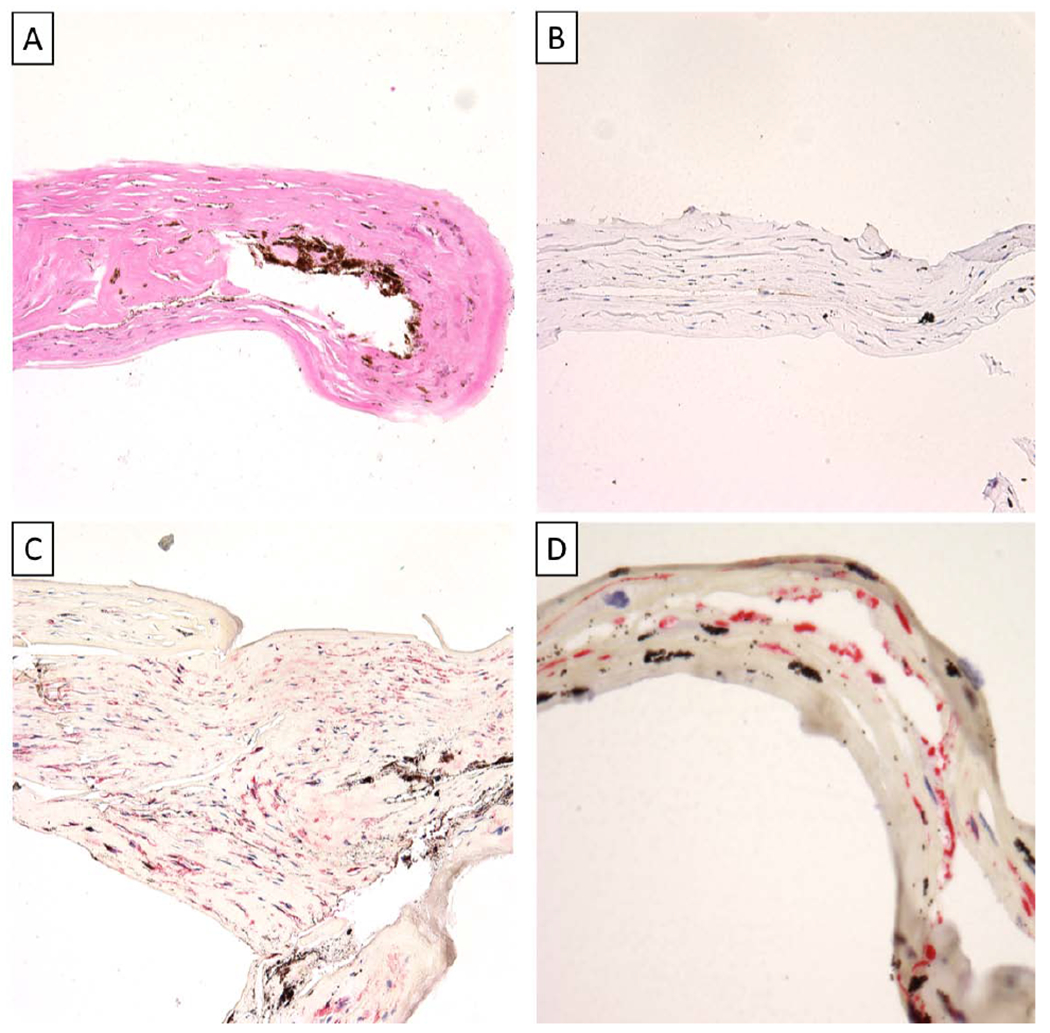
A) Pigmented fibrocellular tissue (Hematoxylin-eosin, Original magnification x 200) B) Pancytokeratin negative fibrocellular tissue (Pancytokeratin, Original magnification x 200) C) Fibrocellular tissue with myofibroblastic differentiation (Smooth muscle actin (SMA), Original magnification x 200) D) Cytokeratin 7 (CK7) positive fibrocellular tissue (CK7, Original magnification x 400)
Figure 5.
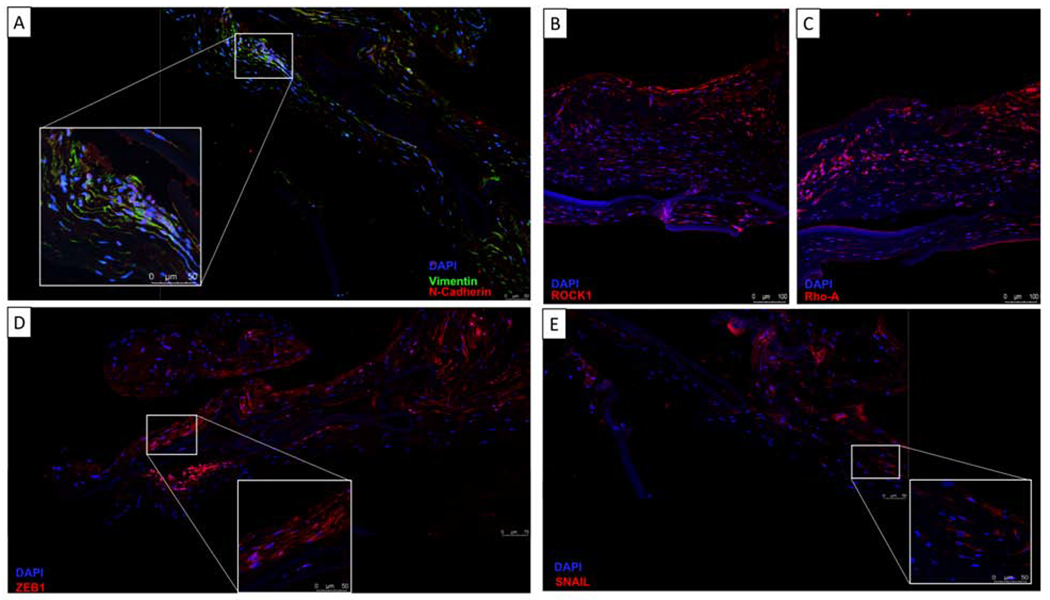
Retrocorneal membrane immunofluorescence on paraffin-embedded sections. Membranes demonstrate positivity for Vimentin (A), N-Cadherin (A), Rock1 (B), Rho-A (C), Zeb1(D) and Snail (E). All nuclei were counterstained with DAPI (blue).
Below are representative case narratives of three of the seven individuals.
Case 1:
A 56-year-old male with a history of cataract extraction (CE) with posterior intraocular lens (IOL) placement and POAG, treated with Baerveldt tube and cyclophotocoagulation, presented with corneal edema (Figure 1A). A DSAEK was performed and simultaneously, a second Baerveldt implant placed with both tubes positioned in the sulcus. After eight months, a dense fibrovascular membrane was noted that covered the anterior IOL and involved the inferior cornea and iris, with peripheral anterior synechiae (PAS) (Figure 1B). However, the central corneal was still clear. A Nd:YAG laser was used to open the anterior lens capsule in an attempt to clear the membrane. Within two months, the cornea became cloudy with progressive fibrosis that caused further contraction of the iris onto the cornea (Figure 1C). As such, a full vitrectomy was performed under a temporary keratoprosthesis, the tubes repositioned into the vitreous, the IOL, membranes, and involved iris removed, and the graft sewn into place. The graft was initially clear (Figure 1D–E) but eventually failed 2.5 years after PKP (Figure 1F) with no signs of recurrent membranes.
Figure 1.
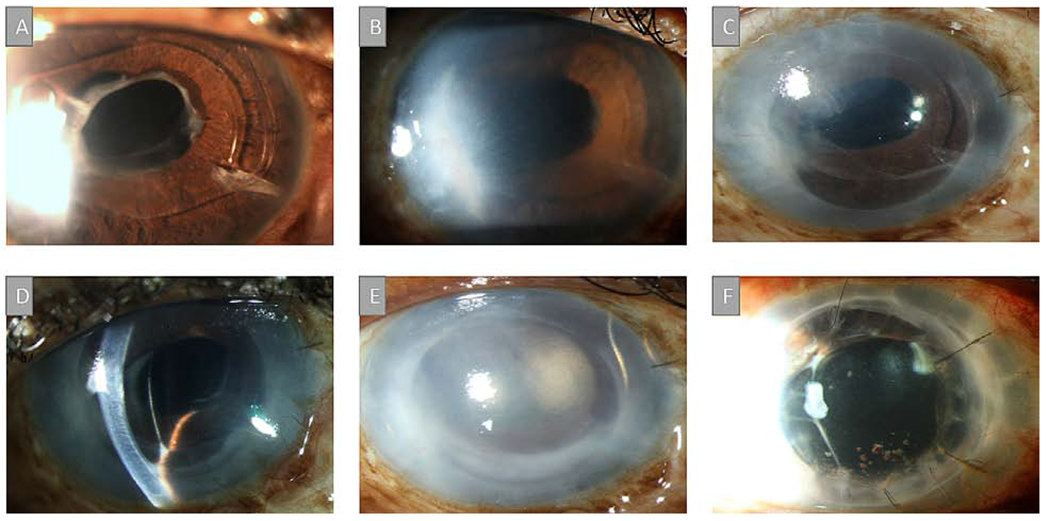
A) Slit-lamp picture demonstrating corneal edema in a 56-year-old male with a clinical history of primary open-angle glaucoma (POAG) treated with Baerveldt implant and cyclophotocoagulation. B) A Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) was performed and a dense fibrovascular membrane was noted eight months after DSAEK. C) Within two months, the cornea became cloudy with progressive fibrosis causing contraction of the iris onto the cornea. D-E) A penetrating keratoplasty was performed and tubes were repositioned into the vitreous. F) The graft eventually failed 2.5 years after penetrating keratoplasty (PKP) with no signs of recurrent membranes.
Case 2:
A 69-year-old male with a history of complex CE necessitating an anterior chamber IOL (ACIOL) and POAG treated with a Baerveldt drainage device and anterior chamber tube (Figure 2A) presented with corneal edema 17 months after cataract surgery (Figure 2B). Eight months later, a DSAEK was performed due to worsening edema. Three months after DSAEK, a fibrous membrane was noted on the ACIOL (Figure 2C) but the angle was still open. Six months after DSAEK, the angle was noted to be closed inferiorly with new PAS but the central cornea remained clear. (Figure 2D) However, one year after DSAEK, the cornea became opaque and the lens, iris and cornea were all contracted due to a proliferative membrane with 360-degree PAS. (Figure 2E) As such, a full vitrectomy was performed under a temporary keratoprosthesis, the ACIOL was removed, the glaucoma tube moved to the vitreous, the membranes peeled off the iris, a 3-piece acrylic lens sutured to the iris, and the graft sewn into place. One-year post-PKP, the corneal graft remained clear with no signs of recurrent membranes. (Figure 2F)
Figure 2.
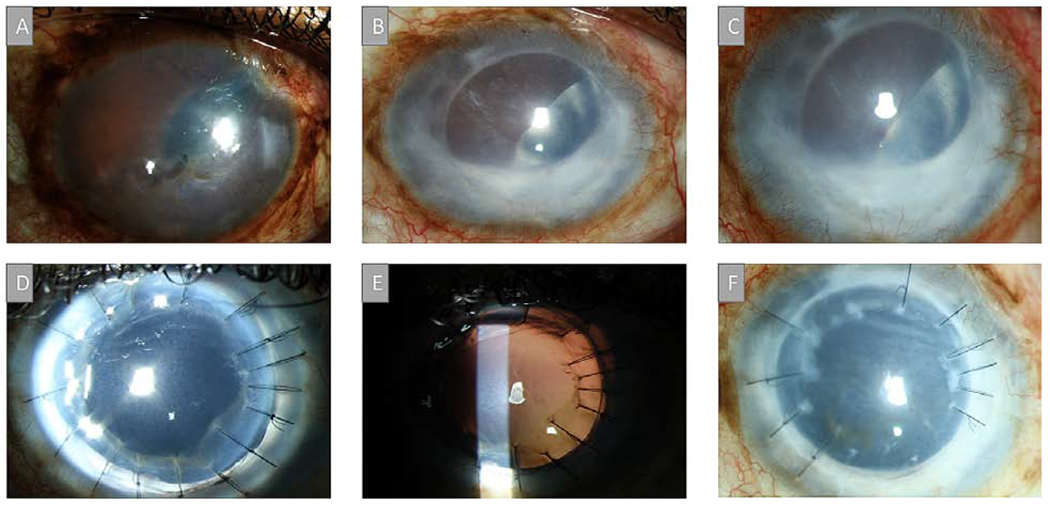
A) Slit-lamp picture of a 69-year-old male with a history of complex cataract extraction (CE), anterior chamber intraocular lens (ACIOL) and POAG treated with a Baerveldt drainage device and anterior chamber tube. B) Corneal edema was noted 17 months after cataract surgery. C) DSAEK was performed due to worsening edema and three months post-DSAEK, a fibrous membrane was noted. C) Six months after DSAEK, the angle was noted to be closed inferiorly with new PAS. D) One year after DSAEK, the cornea started to become opaque. E) The proliferation of the membrane eventually caused the lens, iris and cornea to contract together. F) One-year post-PKP, the corneal graft remained clear with no signs of recurrent membranes.
Case 3:
An 81-year-old man with a history of complex CE necessitating an ACIOL followed by retinal detachment repair developed iris neovascularization and underwent a Molteno implant with the tube placed in the vitreous cavity. One year later, corneal edema was noted (Figure 3A) and the ACIOL was removed and an intraocular lens was sutured to the sclera (Figure 3B). Eight months later, a DSAEK was performed and intraoperatively, thick membranes were found that connected the endothelium, angle, and iris superiorly. During their removal, bleeding occurred with residual blood in the anterior chamber at the time of graft placement (Figure 3C). The graft was initially attached but a month later, was found to be completely detached. (Figure 3D) Given our past experience with retrocorneal membranes as a poor prognostic sign for long term DSAEK survival, we choose to proceed with PKP instead of attempting to re-bubble the graft. As such, three months after DSAEK, a PKP was performed along with membrane and superior iris removal. (Figure 3E) Six month later, the graft remains clear with no recurrent membranes noted. (Figure 3F)
Figure 3.
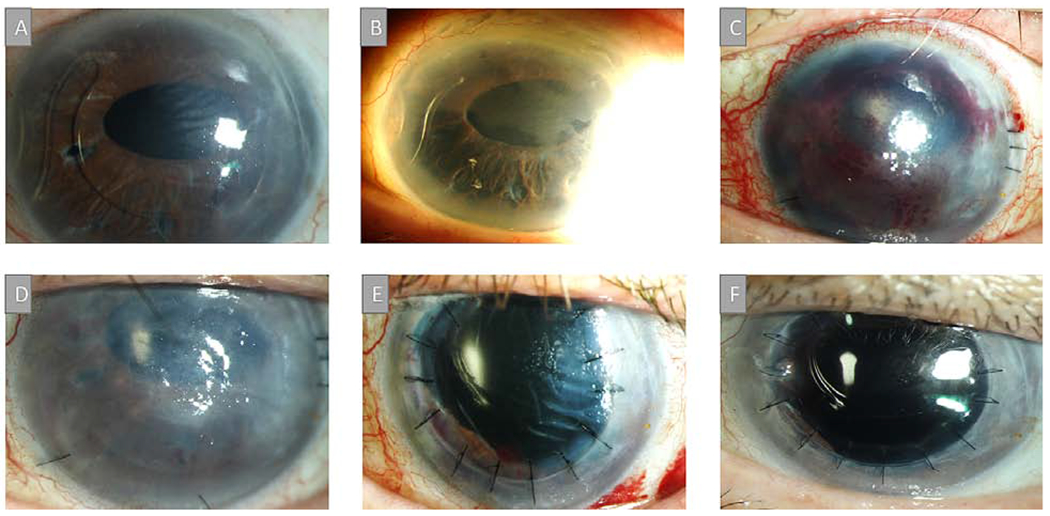
A) Slit-lamp picture of an 81-year-old man with a history of complex CE, ACIOL and Molteno implant with the tube placed in the vitreous cavity with corneal edema one year after surgery. B) ACIOL was removed and an intraocular lens was sutured to the sclera. C) Eight months later, a DSAEK was performed and a thick membrane that connected the iris, corneal endothelium and angle was removed. D) Graft detached one month later and recurrent membranes in the anterior chamber were noted. E) Three months after DSAEK, a PKP was performed along with membrane and superior iris removal. F) Graft remains clear with no recurrent membranes noted at six months follow up.
Discussion
Endothelial keratoplasty permits selective replacement of diseased corneal endothelium.35 This allows for earlier visual recovery, earlier refractive stability, more predictable postoperative refractive outcomes, avoidance of wound- and suture-related complications, shorter surgical time, easier post-operative follow up and reduced risk of intraoperative and late suprachoroidal hemorrhage.36 Despite the advantages of DSAEK, studies have reported a 5% frequency of graft failure (range, 0%-29%).36 The main causes of graft failure include graft dislocation, endothelial rejection, and primary graft failure.4,37 In our series, we identified a poorly described cause of graft failure, namely progressive anterior chamber membranes leading to formation of PAS and ultimately DSAEK failure.
Interestingly, all individuals had a history of prior surgery including placement of a glaucoma drainage device and cataract extraction. It is well known that eyes with glaucoma drainage devices have a worse prognosis after DSAEK than eyes without glaucoma drainage devices. Even when surgery is successful, grafts in eyes with glaucoma drainage devices typically fail within 3–5 years.38 Endothelial damage due to mechanical stress, increased blood-aqueous permeability to oxidative, apoptotic and inflammatory proteins, and nutritional depletion are thought to underlie this clinical finding.39
Regardless of etiology for failure, the most common histopathologic finding in failed DSAEK grafts is endothelial cell loss35,40, and this was seen in all of our specimens. Additionally, histopathological analysis with Hematoxylin and eosin demonstrated a pigmented fibrocellular membrane with elongated, spindle-shaped cells, that varied in thickness and cellularity on the posterior surface of the DSAEK in all cases. Some membranes were adhered to the button and some were detached. All membranes were positive for the same markers and thus all appear to be derived from the same cell of origin and/or pathologic process.
None of the specimens were positive for pancytokeratin and thus an epithelial origin could be ruled out. On the other hand, the membranes were all α-SMA and Vimentin positive, indicating a myofibroblastic and mesenchymal nature of these membranes. Previous studies on retrocorneal membranes have found similar results. In an interventional cases series, histopathologic analysis was performed on corneal buttons removed at the time of secondary PKP in 2 cases of primary graft failure after DMEK (PKP performed 6 months post-DMEK). H&E staining revealed a retrocorneal membrane composed of collagen and elongated fibroblast-like cells, which was positive for α-SMA.11 A similar finding was described in a series of eleven eyes with fibrous retrocorneal membranes associated with perforating injury and ulceration studied by light and electron microscopy. On histopathology, spindle-shaped cells consistent with myofibroblasts were identified, and electron microscopy showed the presence of fibroblasts and myofibroblasts. The retrocorneal membranes were positive for α-SMA and vimentin, indicating a myofibroblastic identity.21
Our novel contribution to the field is in the evaluation for markers of an EndoMT process in these membranes. In fact, all of the membranes in our cohort stained positive for Snail, ZEB1, N-cadherin, RhoA and Rock1. This suggests that the myofibroblastic membranes originate from remnant host endothelial cells that undergo a mesenchymal transformation. A similar study was performed by Jakobiec et al. on the histopathology of retrocorneal membranes of failed grafts (32 PKP, 6 DSAEK). Their group also stained for α-SMA, Vimentin and CK7 as a markers of endothelial origin.14 In their study, they identified five different membranes, including epithelial, keratocytic, endothelial metaplasia, indeterminate and mixed. The keratocytic membranes were thicker and were positive for α-SMA and Vimentin, while negative for CK7, whereas thinner membranes that were positive for α-SMA, Vimentin and CK7 were considered of endothelial origin 14 In comparison, our membranes of similar thickness to the membranes Jakobiec deemed to have a keratocytic origin.
Based on prior and current findings, we postulate that multiple mechanisms may contribute to the observed membranes including EndoMT and/or keratocytic fibrous downgrowth. However, the positivity for EndoMT markers and CK7 seen in our study leads us to postulate that EndoMT is an important contributor to membrane formation. Furthermore, as all retrocorneal membranes were clinically observed to start in the periphery and extend centrally, we hypothesize that the membranes originate from host cells, compromise the angle and iris and proliferate towards the center of the cornea, causing graft failure. Unfortunately, on histopathology, we cannot identify the exact location of the membranes in relation to the cornea as many membranes separate during specimen processing and thus their original position cannot be determined with certainty.
It is interesting that all individuals in our series had glaucoma drainage devices and we postulate that their presence may be a risk for retrocorneal membrane formation. That is because myofibroblastic differentiation can be driven by a range of molecules, including TGF-β, inflammatory cytokines, and oxidative stress proteins.41,42 It is well described that individuals with glaucoma drainage devices have increased pro-inflammatory cytokines and oxidative stress markers in their aqueous humor.39 The combination of an inflammatory milieu, coupled with the stress of DSAEK, exposure of stroma, and damage to adjacent endothelial cells via the Descemet stripping procedure, may constitute the ideal context for endothelial metaplasia and membrane formation. Iris injury and damage that may occur at time of DSAEK may have also contributed to increased anterior chamber cytokine levels and thus membrane formation.43 Additionally, we believe the iris contributed the pigment present in these membranes as a result of direct injury or via iridocorneal adhesion after membranes formation.
The findings of this study should be considered within the constraints of its limitations, which include a limited number of cases and defined histopathological markers. Despite these limitations, our findings set the ground for future targeted pharmacotherapy in retrocorneal membranes. The fact that our cells were positive for markers of EndoMT indicate that this pathway may be manipulated therapeutically. Animal models have demonstrated that a RhoA/ROCK1 pathway inhibitor, Y27632, promotes corneal endothelium cell (CEC) adhesion and preserves endothelial morphology.44,45 In a rabbit model, the corneal endothelium was mechanically scraped with a 20-gauge silicone needle and rabbit CECs (RCECs) were injected concomitantly with and without Y-27632. The inhibitor treated eyes presented with a monolayer hexagonal-shaped cells, whereas the eyes in which RCECs were injected without Y-27632 exhibited a stratified fibroblastic phenotype positive for α-SMA.44 The TGF-β pathway represents another potential therapeutic target in light of our findings. Studies have demonstrated that SB431542, an inhibitor of TGF-β pathways, halts the spontaneous occurrence of EndoMT in vitro.46 When human and primate CECs were cultured with SB431542, there was inhibition of morphologic changes to a fibroblastic phenotype, and endothelial cells were able to retain expression of the endothelial functional markers Na+/K+-ATPase and ZO-1.46 Similarly, Bone morphogenetic protein-7 (BMP-7) , a member of the TGF-β superfamily which is known to antagonize the effects of TGF-β1 mesenchymal transformations via a smad-dependent mechanism,47 not only inhibited EndoMT, but also reversed the process. The elongated, fibroblastic phenotype was reversed to the polygonal cell morphology and cells maintained functional marker expression in a BMP-7 concentration-dependent manner.46 Although promising, these therapies have not been studied in human corneas and have only been used in vitro and in animal studies. Further in vitro and animal studies are needed to elucidate their safety and potential in preventing or treating DSAEK associated membranes.
Highlights.
Fibrocellular retrocorneal membrane proliferation may be associated with DSAEK failure in patients with previous glaucoma drainage device surgery.
Retrocorneal membranes have myofibroblastic differentiation and a lack of epithelial differentiation.
Positivity for markers of an endothelial to mesenchymal transition indicates possible endothelial origin and could be the hallmark for future targeted pharmacotherapy.
Acknowledgements:
The authors are grateful to Cynthia Maza and Magdaly Rivas from the Florida Lions Ocular Pathology laboratory for helping with the preparation of the histopathology specimens, to Enrique Salero-coca, PhD for his collaboration with the antibodies and Jaime Martinez, MD for his assistance identifying these patients.
Funding and Support: This research was supported by the Florida Lions Eye Bank (Andrea Naranjo, Pedro Monsalve and Sander R. Dubovy), Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research I01 CX002015 (Dr. Galor), Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense GW190010 (Dr. Galor), R01EY026174 (Dr. Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant.
Appendix

Andrea Naranjo graduated as doctor in medicine from the University of the Andes in Colombia. She completed a Cornea and Ocular Surface Disease Research Fellowship, as well as an Ocular Pathology Research Fellowship at Bascom Palmer Eye Institute. She is currently completing her intern year in General Surgery at Stanford University and will continue her training as an Ophthalmology resident at Stanford University. During her time as research fellow, her main focus was on infectious keratitis, endothelial graft failure and ocular surface disease management.

Dr. Anat Galor is a cornea and uveitis trained specialist with a dual appointment at the Bascom Palmer Eye Institute, University of Miami and the Miami Veterans Affairs (VA) medical center. Dr. Galor completed an ophthalmology residency at the Cole Eye Cleveland Clinic, a uveitis fellowship at the Wilmer Eye Institute, and a cornea and external diseases fellowship at Bascom Palmer Eye Institute. Dr. Galor runs the ocular surface program at the Miami VA and has focused her research on understanding mechanisms underlying dry eye and ocular pain, including microbiome, nerve, and environmental contributors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banitt MR, Chopra V. Descemet’s stripping with automated endothelial keratoplasty and glaucoma. Curr Opin Ophthalmol. 2010;21(2):144–149. [DOI] [PubMed] [Google Scholar]
- 2.Alkatan H, Al-Rajhi A, Al-Shehri A, Khairi A. Histopathological findings of failed grafts following Descemet’s stripping automated endothelial keratoplasty (DSAEK). Saudi J Ophthalmol. 2012;26(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price FW Jr., Price MO. Descemet’s stripping with endothelial keratoplasty in 200 eyes: Early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32(3):411–418. [DOI] [PubMed] [Google Scholar]
- 4.Suh LH, Yoo SH, Deobhakta A, et al. Complications of Descemet’s stripping with automated endothelial keratoplasty: survey of 118 eyes at One Institute. Ophthalmology. 2008;115(9): 1517–1524. [DOI] [PubMed] [Google Scholar]
- 5.Kremer I, Rapuano CJ, Cohen EJ, Laibson PR, Eagle RC Jr., Retrocorneal fibrous membranes in failed corneal grafts. Am J Ophthalmol. 1993;115(4):478–483. [DOI] [PubMed] [Google Scholar]
- 6.Brown SI, Kitano S. Pathogenesis of the retrocorneal membrane. Arch Ophthalmol. 1966;75(4):518–525. [DOI] [PubMed] [Google Scholar]
- 7.Chi HH, Teng CC, Katzin HM. Histopathology of corneal endothelium. A study of 176 pathologic discs removed at keratoplasty. Am J Ophthalmol. 1962;53:215–235. [PubMed] [Google Scholar]
- 8.Mihail Z, Alina-Cristina S, Speranta S. Retrocorneal membranes after penetrating keratoplasty. Rom J Ophthalmol. 2015;59(4):230–234. [PMC free article] [PubMed] [Google Scholar]
- 9.Lifshitz T, Oshry T, Rosenthal G. Retrocorneal membrane after penetrating keratoplasty. Ophthalmic Surg Lasers. 2001;32(2):159–161. [PubMed] [Google Scholar]
- 10.Young AL, Tam PM, Lau TT, Cheng LL, Rao SK, Lam PT. Case of post Descemet stripping endothelial keratoplasty retrocorneal fibrous membrane. Clin Exp Ophthalmol. 2009;37(4):418–419. [DOI] [PubMed] [Google Scholar]
- 11.Heindl LM, Schlotzer-Schrehardt U, Cursiefen C, Bachmann BO, Hofmann-Rummelt C, Kruse FE. Myofibroblast metaplasia after descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2011;151 (6):1019–1023 e1012. [DOI] [PubMed] [Google Scholar]
- 12.Yum HR, Kim MS, Kim EC. Retrocorneal membrane after Descemet membrane endothelial keratoplasty. Cornea. 2013;32(9):1288–1290. [DOI] [PubMed] [Google Scholar]
- 13.Sherrard ES, Rycroft PV. Retrocorneal membranes. I. Their origin and structure. Br J Ophthalmol. 1967;51(6):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakobiec FA, Bhat P. Retrocorneal membranes: a comparative immunohistochemical analysis of keratocytic, endothelial, and epithelial origins. Am J Ophthalmol. 2010;150(2):230–242 e232. [DOI] [PubMed] [Google Scholar]
- 15.Weiner MJ, Trentacoste J, Pon DM, Albert DM. Epithelial downgrowth: a 30-year clinicopathological review. Br J Ophthalmol. 1989;73(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasher P, Muftuoglu O, Hsiao ML, Bowman RW, Hogan RN, Mootha VV. Epithelial downgrowth after descemet stripping automated endothelial keratoplasty. Cornea. 2009;28(6):708–711. [DOI] [PubMed] [Google Scholar]
- 17.Kovacic JC, Dimmeler S, Harvey RP, et al. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(2):190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63(1):21–29. [PubMed] [Google Scholar]
- 19.Myrna KE, Pot SA, Murphy CJ. Meet the corneal myofibroblast: the role of myofibroblast transformation in corneal wound healing and pathology. Vet Ophthalmol. 2009;12 Suppl 1:25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaurasia SS, Kaur H, de Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. 2009;89(2):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cockerham GC, Hidayat AA. Retrocorneal membrane with myofibroblasts after perforating injury: an immunohistochemical and ultrastructural study of 11 cases. Cornea. 1999;18(6):700–706. [DOI] [PubMed] [Google Scholar]
- 22.Engelmann K, Friedl P. Optimization of culture conditions for human corneal endothelial cells. In Vitro Cell Dev Biol. 1989;25(11):1065–1072. [DOI] [PubMed] [Google Scholar]
- 23.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179(3):1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy O, Leclerc VB, Bourget JM, Theriault M, Proulx S. Understanding the process of corneal endothelial morphological change in vitro. Invest Ophthalmol Vis Sci. 2015;56(2):1228–1237. [DOI] [PubMed] [Google Scholar]
- 25.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. [DOI] [PubMed] [Google Scholar]
- 26.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. [DOI] [PubMed] [Google Scholar]
- 27.Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121(Pt 20):3317–3324. [DOI] [PubMed] [Google Scholar]
- 28.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121(Pt 6):727–735. [DOI] [PubMed] [Google Scholar]
- 29.Welch-Reardon KM, Wu N, Hughes CC. A role for partial endothelial-mesenchymal transitions in angiogenesis? Arterioscler Thromb Vasc Biol. 2015;35(2):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Jung E, Heur M. Injury induces endothelial to mesenchymal transition in the mouse corneal endothelium in vivo via FGF2. Mol Vis. 2019;25:22–34. [PMC free article] [PubMed] [Google Scholar]
- 31.Godde NJ, Galea RC, Elsum IA, Humbert PO. Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions. J Mammary Gland Biol Neoplasia. 2010;15(2):149–168. [DOI] [PubMed] [Google Scholar]
- 32.Piera-Velazquez S, Jimenez SA. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol Rev. 2019;99(2):1281–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreis TE, Birchmeier W. Stress fiber sarcomeres of fibroblasts are contractile. Cell. 1980;22(2 Pt 2):555–561. [DOI] [PubMed] [Google Scholar]
- 34.Jirsova K, Merjava S, Martincova R, et al. Immunohistochemical characterization of cytokeratins in the abnormal corneal endothelium of posterior polymorphous corneal dystrophy patients. Exp Eye Res. 2007;84(4):680–686. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Randleman JB, Stulting RD, et al. Clinicopathologic findings in failed descemet stripping automated endothelial keratoplasty. Arch Ophthalmol. 2010; 128(8):973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818–1830. [DOI] [PubMed] [Google Scholar]
- 37.Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006;25(8):886–889. [DOI] [PubMed] [Google Scholar]
- 38.Anshu A, Price MO, Price FW. Descemet’s stripping endothelial keratoplasty: long-term graft survival and risk factors for failure in eyes with preexisting glaucoma. Ophthalmology. 2012;119(10):1982–1987. [DOI] [PubMed] [Google Scholar]
- 39.Anshu A, Price MO, Richardson MR, et al. Alterations in the aqueous humor proteome in patients with a glaucoma shunt device. Mol Vis. 2011;17:1891–1900. [PMC free article] [PubMed] [Google Scholar]
- 40.Suh LH, Dawson DG, Mutapcic L, et al. Histopathologic examination of failed grafts in descemet’s stripping with automated endothelial keratoplasty. Ophthalmology. 2009;116(4):603–608. [DOI] [PubMed] [Google Scholar]
- 41.Petroll WM, Jester JV, Bean JJ, Cavanagh HD. Myofibroblast transformation of cat corneal endothelium by transforming growth factor-beta1, -beta2, and -beta3. Invest Ophthalmol Vis Sci. 1998;39(11):2018–2032. [PubMed] [Google Scholar]
- 42.Nakano Y, Oyamada M, Dai P, Nakagami T, Kinoshita S, Takamatsu T. Connexin43 knockdown accelerates wound healing but inhibits mesenchymal transition after corneal endothelial injury in vivo. Invest Ophthalmol Vis Sci. 2008;49(1):93–104. [DOI] [PubMed] [Google Scholar]
- 43.Yazu H, Yamaguchi T, Aketa N, et al. Preoperative Aqueous Cytokine Levels are Associated With Endothelial Cell Loss After Descemet’s Stripping Automated Endothelial Keratoplasty. Invest Ophthalmol Vis Sci. 2018;59(2):612–620. [DOI] [PubMed] [Google Scholar]
- 44.Okumura N, Koizumi N, Ueno M, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181(1):268–277. [DOI] [PubMed] [Google Scholar]
- 45.Okumura N, Koizumi N, Kay EP, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013;54(4):2493–2502. [DOI] [PubMed] [Google Scholar]
- 46.Okumura N, Kay EP, Nakahara M, Hamuro J, Kinoshita S, Koizumi N. Inhibition of TGF-beta signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS One. 2013;8(2):e58000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9(7):964–968. [DOI] [PubMed] [Google Scholar]


