Abstract
Aedes aegypti saliva facilitates blood meal acquisition through pharmacologically active compounds that prevent host hemostasis. Among these salivary proteins are the D7s, which are highly abundant and have been shown to act as scavengers of biogenic amines and eicosanoids. In this work, we performed comparative structural modeling, characterized the binding capabilities, and assessed the physiological functions of the Ae. aegypti salivary protein AeD7L2 compared to the well-characterized AeD7L1. AeD7L1 and AeD7L2 show different binding affinities to several biogenic amines and biolipids involved in host hemostasis. Interestingly, AeD7L2 tightly binds U-46619, the stable analog of thromboxane A2 (KD = 69.4 nm), which is an important platelet aggregation mediator, while AeD7L1 shows no binding. We tested the ability of these proteins to interfere with the three branches of hemostasis: vasoconstriction, platelet aggregation, and blood coagulation. Pressure myography experiments showed these two proteins reversed isolated resistance artery vasoconstriction induced by either norepinephrine or U-46619. These proteins also inhibited platelet aggregation induced by low doses of collagen or U-46619. However, D7 long proteins did not affect blood coagulation. The different ligand specificity and affinities of AeD7L1 and AeD7L2 matched our experimental observations from studying their effects on vasoconstriction and platelet aggregation, which confirm their role in preventing host hemostasis. This work highlights the complex yet highly specific biological activities of mosquito salivary proteins and serves as another example of the sophisticated biology underlying arthropod blood feeding.
Keywords: arthropods, platelet aggregation, salivary glands, vascular biology, vasodilators
Introduction
Aedes aegypti (Diptera: Culicidae) is a widely distributed mosquito that transmits several deadly pathogens, including yellow fever, dengue, Zika, and chikungunya viruses [1]. Hematophagous arthropods need to acquire vertebrate blood for egg development. In their search for blood, adult female mosquitoes introduce their proboscis into the host skin, causing damage to tissues and blood vessels [2], rapidly triggering a hemostatic response from the vertebrate host. Hemostasis, the host defense response that controls blood loss following a blood vessel injury, is a complex and redundant, interlinked biological response. It mainly consists of vasoconstriction, which reduces blood flow at the wound site, platelet aggregation, which forms a soft plug to prevent blood loss, and blood clotting, which tightly secures the hemostatic clot over the injured site [3]. The ability of the mosquito to overcome host hemostasis will define its success or failure in completing a blood meal [4,5]. First, mosquitoes pierce host skin and release salivary secretions into the dermis while searching for capillaries. Mosquito saliva contains active chemical compounds that counteract host hemostasis [3]. D7 proteins are one of the most abundant protein families across blood feeding arthropods, including mosquitoes, sand flies, and black flies [6]. D7 proteins belong to the odorant-binding protein superfamily that has a multigene organization due to gene duplication processes and is widespread in insects. In the evolution of hematophagy in the dipterans, members of this family were recruited to the salivary glands thanks to their ability to bind small molecules bearing hydrophobic chemical groups, to bind small molecules found in blood that are involved in hemostasis [3,6]. D7 proteins from different species have been functionally characterized as scavengers of host hemostasis mediators [7–13]. The binding capacities of several D7 proteins have been determined by isothermal titration calorimetry (ITC), showing a high affinity for biogenic amines or biolipids such as leukotrienes and/or U-46619, the stable analog of thromboxane A2 (TxA2) [7–10,13]. The stoichiometry of the binding is 1:1, one molecule of salivary protein per molecule of host mediator, which explains why high concentrations of these proteins are present in the saliva [6–10]. Several D7 protein structures have been solved in both the presence and absence of ligands [7,9–11]. Calorimetry and X-ray crystallography studies together have helped us to understand the nature of ligand binding and the amino acid residues involved in this binding. Mosquito D7 proteins come in two forms, the long D7 forms and truncated versions called short D7, which retain certain functionality [6]. In Anopheles gambiae, short forms are the most abundantly expressed and bind biogenic amines, while D7 long forms from Anopheles stephensi and intermediate forms from sand flies bind TxA2 and leukotrienes [7,8,10]. Aedes aegypti has two long D7 forms and four short forms [6,14]. However, only one of the two long form D7s, AeD7L (named AeD7L1, VectorBase ID AAEL006424) has been functionally characterized. AeD7L1 exhibits a multifunctional mechanism of ligand binding: The N-terminal domain binds cysteinyl leukotrienes, while the C-terminal domain shows high affinity to biogenic amines such as norepinephrine, serotonin, and histamine [9]. Although mosquito D7 proteins only share about 30% identity at the amino acid level [6], many of them share the same function [7–9]. It is assumed that conserved residues at binding sites imply similar binding; however, this is not always the case, as reflected in the Culex quinquefasciatus D7L1 binding activity [13]. Of the two long D7 proteins present in C. quinquefasciatus saliva, one, CxD7L2, shows the same classical binding as AeD7L1 [9]. CxD7L1, however, does not bind biogenic amines or leukotrienes. It has novel ADP and ATP binding, a feature which arrests these platelet aggregation mediators and helps C. quinquefasciatus mosquitoes to blood feed on mammals, where ADP is an important mediator of platelet aggregation [13]. Given the variation in binding affinities across D7 proteins, and in an effort to identify new functions, we aim to characterize the D7 long form AeD7L2, one of the most abundant secreted salivary proteins from Ae. aegypti. In this work, we compared the binding ability of AeD7L2 with previously published data from AeD7L1 [8,9]. We generated a 3D structural model of AeD7L2, which, alongside the ITC data, improved our understanding of its protein-ligand binding properties. Moreover, we described the interference of both AeD7L proteins with host vasoconstriction and platelet aggregation ex vivo and showed that these proteins had no effect on blood coagulation, as tested in PT and aPTT assays in vitro. We showed that protein binding parameters obtained by ITC reaffirm the inhibition of hemostasis we observed in ex vivo experiments. The results presented in this work show that both Aedes D7 long salivary proteins are potent molecules that help the mosquito overcome host hemostasis at the bite site, facilitating successful blood meal acquisition.
Results and Discussion
Gene expression analysis and protein characterization
AeD7L1 and AeD7L2 are among the most abundant D7 long proteins in the salivary glands of female Ae. aegypti mosquitoes [14]. In this paper, AeD7L1 corresponds to VectorBase ID AAEL006424, GenBank ID: AAA29347, NCBI Reference Sequence: XP_001657779, and PDB ID: 3DY9, whereas AeD7L2 is annotated in VectorBase as AAEL006417, GenBank ID: AAL16049, and NCBI Reference Sequence: XP_001657778. In 1991, James et al. [15] characterized the first insect D7 protein, AeD7L1, and showed transcript enrichment in female salivary glands. Through qPCR experiments, we confirmed that the expression of AeD7L1 and AeD7L2 genes is specific to the head and thorax of the adult female mosquitoes, where the salivary glands are located. There is a slight accumulation of AeD7L1 transcripts in female pupae that precedes overexpression in adults, which we believe indicates preparation for female adult blood feeding after emergence (Fig. 1A,B). Gene expression of AeD7L1 is much higher than AeD7L2, as observed by previous transcriptomic analysis of Ae. aegypti salivary glands [14] and in situ hybridization of RNA [16]. Although D7 proteins are traditionally assumed to be expressed in the salivary glands [14–16], a D7 protein absent in mosquito salivary glands was recently identified within Ae. aegypti hemolymph [17] and has different binding capabilities and function from salivary D7s [17,18].
Fig. 1.

Characterization of Aedes aegypti salivary proteins AeD7L1 and AeD7L2. Gene expression analysis of AeD7L1 (A) and AeD7L2 (B) transcripts in different stages of Ae. aegypti mosquitoes. Relative abundance was expressed as the fold change using the 40S ribosomal protein S7 as the housekeeping gene. Larvae stage (reference sample), male pupae, female pupae, and heads and thoraxes (H + T) from male adult and female adult mosquitoes were analyzed separately. Bars represent standard deviation. Two biologicalreplicates were tested. All samples were analyzed in technicaltriplicates. Purification of AeD7L1 (C) and AeD7L2 (D) proteins by size-exclusion chromatography using a Superdex 200 Increase 10/300 GL column. (E) Coomassie-stained NuPAGE Novex 4–12% Bis-Tris gel electrophoresis of the recombinant proteins AeD7L1 and AeD7L2 (5 μg) and Ae. aegypti salivary gland extract (SGE, 10 μg). SeeBlue Plus2 Prestained was used as the protein standard. (F) Western blot showing recognition of recombinant proteins AeD7L1 and AeD7L2 (50 ng) and other protein bands from the salivary gland extract (1.7 μg) by purified IgG recovered from the serum of a rabbit immunized with Ae. aegypti salivary gland extract.
Recombinant AeD7L1 and AeD7L2 proteins were produced for functional and biochemical characterization studies (Fig. 1C,D). The identities of purified recombinant proteins were confirmed by N-terminal sequencing. Both purified recombinant proteins migrated as single bands on Coomassie-stained precast polyacrylamide gels, and their apparent molecular weight (MW) in the gel corresponds to the predicted MWs: 36.9 kDa and 38.63 kDa for AeD7L1 and AeD7L2, respectively (Fig. 1E). Immunogenicity of both proteins in their recombinant forms was maintained, as they were recognized by purified IgG antibodies from a rabbit immunized against Ae. Aegypti salivary gland extract (SGE). IgG antibodies that recognized AeD7L1 were more abundant than antibodies against AeD7L2 (Fig. 1F), which may be a result of higher abundance of AeD7L1 in the salivary glands [14]. Another explanation might be a higher immunogenicity of this protein [20,21]. Having two proteins with similar binding capabilities and different immunogenicity might be a strategy for the mosquito to deviate the host immune response to one molecule while preserving the needed activity with a similar molecule, as similarly suggested for sand fly yellow proteins [22].
Ligand binding properties of Aedes aegypti D7L2
D7 salivary proteins help in mosquito blood feeding by binding and sequestering different mediators involved in the host’s hemostatic process [7–9,11]. Through isothermal calorimetry, we analyzed the binding capabilities of AeD7L2 and compared them with the ones previously known for AeD7L1 [8,9]. AeD7L2 binding capabilities are consistent with those described for salivary D7s in other blood feeding arthropods. AeD7L2 binds biogenic amines and biolipids (Table 1) with a calculated stoichiometry ratio close to 1 : 1 (one molecule of protein binding one molecule of ligand), as previously described for other salivary D7 proteins [7–10] and as predicted by our structural model. In addition, AeD7L2 displays a high binding affinity for serotonin, similar to that of AeD7L1-serotonin (Table 1, Fig. 2A). It also binds tryptamine, a natural vasoconstrictor [23] structurally similar to serotonin (5-hydroxytryptamine) (Table 1, Fig. S1A). Although both AeD7L1 and AeD7L2 bind histamine and norepinephrine, AeD7L2 displays a significantly lower affinity for both ligands when compared with AeD7L1 (Table 1, Fig. 2B, C). Interestingly, AeD7L2 has lost its ability to bind epinephrine (Table 1, Fig. S1B). Biogenic amines play important physiological roles in host hemostasis. Serotonin and histamine increase vascular permeability and induce host sensations of pain and itch [24]. Serotonin also acts as a weak platelet aggregation agonist. Platelets store serotonin at very high concentrations in their dense granules (at 65 mm) and secrete it upon activation. Resting plasma serotonin concentrations (around 10 nm) can rapidly increase to 10 μm when platelets become activated at the site of thrombus formation or inflammation [25]. The catecholamines norepinephrine, epinephrine, and tryptamine stimulate vasoconstriction [3,23]. The direct vascular permeability actions of histamine appear to involve both H1 and H2 receptors [26]. Given the KD values between histamine and the H1 receptor are in the nm range [27], AeD7L1 would be a good competitor for scavenging histamine (KD = 140 nm, Table 1). Arthropod salivary protein binding of biogenic amines has been previously reported in the literature, highlighting the importance of removing these mediators at the bite site [12]. The D7 multigene family has evolved through gene duplication and functional divergence, resulting in an increased dosage of the transcript and protein levels as well as binding specialization [3,8,11,28,29]. Due to the nonenzymatic, nonreceptor mediated way of antagonizing hemostasis, large amounts of D7 salivary proteins would be needed at the bite site. As proposed before, D7 proteins would presumably have to achieve a concentration of 0.2–2 μm (the normal receptor saturating concentration of endogenous physiologic mediators such as histamine, serotonin, or ADP) [8]. Scavenging of host biogenic amines in Ae. aegypti is dependent on the long D7 proteins, which are more abundant than short D7s [8,9,14]. Moreover, the function of short D7 proteins in Ae. aegypti has not yet been uncovered, and it has been suggested that they act as pseudogenes [8].
Table 1.
Thermodynamic parameters of AeD7L1 and AeD7L2 proteins by isothermal calorimetry analysis
| Protein | ΔH, kcal·mol−1 ± SE | TΔS, kcal·mol−1 | KD (nm) |
|---|---|---|---|
| Serotonin | |||
| AeD7L1 | −11.7 ± 0.21 | 1.330 | 0.39 ± 0.565 |
| AeD7L2 | −22.7 ± 0.513 | −10.500 | 1.68 ± 0.486 |
| Histamine | |||
| AeD7L1 | −9.5 ± 0.68 | 0.003 | 140 ± 47 |
| AeD7L2 | −30.4 ± 13.6 | −22.3 | 1330 ± 1140 |
| Tryptamine | |||
| AeD7L1 | ND | ND | ND |
| AeD7L2 | −14.7 ± 1.56 | −5.76 | 367 ± 103 |
| Epinephrine | |||
| AeD7L1 | −11.1 ± 0.23 | −1.45 | 102 ± 13 |
| AeD7L2 | NB | NB | NB |
| Norepinephrine | |||
| AeD7L1 | −16.2 ± 0.21 | −3.85 | 0.119 ± 0.05 |
| AeD7L2 | −16.3 ± 0.982 | −6.62 | 110 ± 25.6 |
| U-46619 | |||
| AeD7L1 | NB | NB | NB |
| AeD7L2 | −13.7 ± 0.657 | −3.73 | 69.4 ± 23.7 |
| Leukotriene C4 | |||
| AeD7L1 | −11.8 ± 0.3 | −1.7 | 57.4 ± 8.2 |
| AeD7L2 | −2.29 ± 0.23 | 5.030 | 5270 ± 2270 |
| Leukotriene D4 | |||
| AeD7L1 | −13.3 ± 0.3 | −3.2 | 54.4 ± 7.9 |
| AeD7L2 | −8.07 ± 0.373 | 0.563 | 597 ± 105 |
| Leukotriene E4 | |||
| AeD7L1 | −11.2 ± 0.4 | −1.2 | 60.2 ± 10.5 |
| AeD7L2 | −5.97 ± 2.71 | 1.950 | 1930 ± 1590 |
| Leukotriene B4 | |||
| AeD7L1 | −7.7 ± 0.6 | −1.8 | 140 ± 29 |
| AeD7L2 | −2.53 ± 0.568 | 5.930 | 769 ± 888 |
Fig. 2.
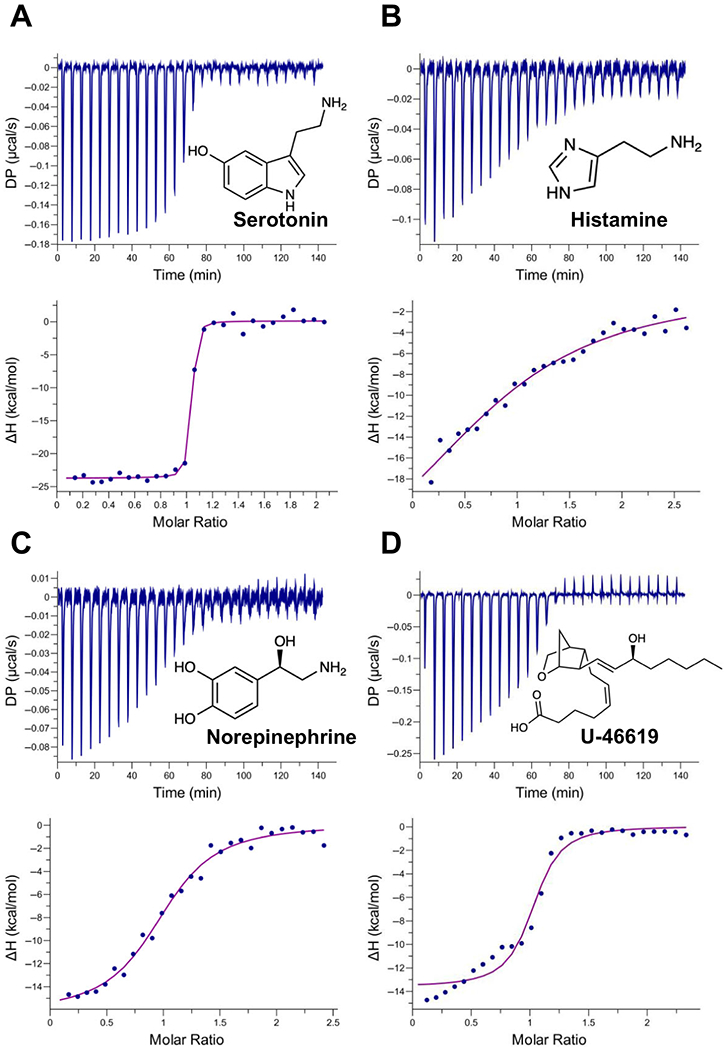
Binding of AeD7L2 to biogenic amines and U-46619 by isothermaltitration calorimetry. Binding experiments were performed on a VP-ITC microcalorimeter. Assays were performed at 30 °C. The upper curve in each panel shows the measured heat for each injection, while the lower graph shows the enthalpies for each injection and the fit to a single-site binding modelfor calculation of thermodynamic parameters. Panels A–D show binding of AeD7L2 to biogenic amines: serotonin (A), histamine (B), norepinephrine (C), and U-46619 (D). Titration curves are representative of at least two measurements. The insets show the names and chemical formulas for these compounds.
We also tested bioactive lipids known to be targeted by other members of mosquito D7 long forms. Leukotriene B4 (LTB4) functions as a potent chemotactic agent of polymorphonuclear cells [30] and cysteinyl leukotrienes C4 (LTC4), D4 (LTD4), and E4 (LTE4), which are involved in allergic reactions and sustain inflammatory reactions. They are potent activators of vasoconstriction, edema formation, and postcapillary venule leakage [30,31]. Cysteinyl LT receptors CysLT1 and CysLT2 are distributed on different immune cells and tissues, including the microvasculature of the skin. CysLT receptors bind LTC4 and LTD4 with high affinity (KD ~ 1–10 nm) while both receptors bind LTE4, the less bioactive and the most stable of the cysteinyl LTs, with lower affinity (KD ~ 100 nm) [32]. Given the high affinity of AeD7L1 to LT, it might compete with the CysLT receptors for the ligand at the mosquito bite site. However, AeD7L2 binds to LTB4, LTC4, LTD4, and LTE4 with lower affinity than does AeD7L1 (Table 1, Fig. S1C–F). Further experiments are needed to investigate the role of AeD7L2 in scavenging LT under physiological conditions. Interestingly, AeD7L2 tightly binds U-46619, the stable analog of thromboxane A2 (Fig. 2D), a relevant agonist of platelet aggregation and vasoconstriction. In contrast, AeD7L1 lacks this binding activity. This newly described activity of a salivary D7 supplements the anti-platelet aggregation and vasodilation activities of other Ae. aegypti salivary proteins.
AeD7L2 protein structure modeling
To generate a model of this protein’s structure, we used I-TASSER, a software that identifies structure templates from the PDB library (https://www.rcsb.org/). The model is based on the template of AeD7L1 (PDB ID: 3DY9). The model shows a C-score of 0.29, a confidence score for estimating the quality of predicted models that ranges from −5 to 2. Although these two proteins share 39% identity at the amino acid level (BLASTp: Score: 549, E: 5e-73, method: compositional matrix adjusted, 58% positives, 4% gaps, Fig. 3A), superposition of the AeD7L2 model with AeD7L1 solved structure (PDB ID: 3DY9) showed that both AeD7L proteins are structurally similar (Fig. 3B). I-TASSER software predicted 17 helixes and a positive binding to serotonin, LTE4, and U-46619 based on the presence of conserved residues involved in the corresponding binding sites. The functional prediction is based on structural similarities with other D7 salivary proteins with known binding properties. Serotonin binding was predicted based on its similarity to An. gambiae AngaD7R4 (PDB ID: 2QEH), whereas predictions of LTE4 or U-46619 binding were based on the An. stephensi AnStD7L1 structure (PDB ID: 3NHT). Our ITC experiments confirmed that AeD7L2 binds serotonin and U-46619 with high affinity and LTE4 with low affinity (Table 1), validating the structure model. In the case of U-46619, a tyrosine residue is known to stabilize the binding of the TxA2 mimetic (U-46619) in An. stephensi [7]. This residue is present in AeD7L2 (Tyr-52) but is absent in AeD7L1 (Fig. 3A), which may explain the difference observed in binding to U-46619 (Table 1). Structural studies indicate that AeD7L1 protein can simultaneously bind biogenic amines and cysteinyl leukotrienes [9]. We showed that AeD7L2 binds biogenic amines and biolipids through ITC studies (Table 1, Fig. 2 and Fig. S1). According to our predicted structure model, AeD7L2 might bind biolipids through its N-terminal domain and biogenic amines through its C-terminal domain (Fig. 3C,D), in a similar manner of AeD7L1 [9]. Protein multifunctionality allows D7 proteins to simultaneously remove two hemostatic agonists from the bite site and has been considered an adaptation of Ae. aegypti and C. quinquefasciatus salivary proteins to facilitate blood feeding [9,13].
Fig. 3.

Sequence alignment of AeD7 long salivary proteins and structure model of AeD7L2. (A) Comparison of Aedes D7 long salivary proteins: AeD7L1 (PDB ID: 3DY9) and AeD7L2 (VectorBase ID: AAEL006417, GenBank ID: AAL16049, or NCBI Reference Sequence: XP_001657778). Sequences without a signal peptide were aligned with Clustal Omega and refined using BoxShade server. Black background shading represents identical amino acids, while gray shading shows similar amino acids. The black arrows indicate predicted amino acids involved in U-46619 binding of AeD7L2 based on the similarity with AnStD7L1 (PDB ID: 3NHT). Red asterisks highlight predicted amino acids involved in serotonin binding for AeD7L2, based on similarity of the model structure with AngaD7R4 (PDB ID: 2QEH). Boxed Tyr-52 is predicted to be involved in TxA2 binding. (B) Superposition of AeD7L1 (PDB ID: 3DY9, shown in gray) and protein structure model of AeD7L2, represented in cyan, shows a similar overall helix structure. (C) Ribbon representation of AeD7L2 protein structure model shows the predicted docking of serotonin in its C-terminal domain binding pocket. (D) Ribbon representation of AeD7L2 protein structure model with U-46619 in its N-terminal domain predicted binding pocket. Amino acid residues of AeD7L2 predicted to be involved in binding are represented as sticks. Serotonin and U-46619 are colored in magenta. The model of the protein structure of AeD7L2 was predicted with I-TASSER software and the structural figures were produced using UCSF Chimera.
AeD7L1 and AeD7L2 interfere with vasoreactivity and platelet aggregation without compromising blood coagulation
During blood feeding, female mosquitoes insert their mouthparts into the host skin and feed directly from capillaries or from the hemorrhagic pool that they create by lacerating blood vessels [33,34]. The tissue injury caused when a mosquito inserts its mouthparts in the skin triggers vertebrate host hemostasis. Salivary proteins are secreted from the mosquito’s salivary channel during mosquito probing and are deposited at the bite site. Pharmacologically potent salivary proteins help the mosquito overcome the three branches of hemostasis for successful blood feeding. Primary hemostasis consists of early vasoconstriction and platelet aggregation which, in turn, triggers blood clotting, a result of secondary hemostasis, to ultimately prevent blood loss. D7 proteins counteract host hemostasis by scavenging host mediators. Here, we compared the activities of AeD7L1 and AeD7L2 in preventing the three branches of hemostasis in ex vivo experiments.
AeD7L1 and AeD7L2 act as vasodilators
To determine the role of Ae. aegypti D7 long salivary proteins in vascular reactivity, we measured their effect on isolated mesenteric arteries from mice. Blood vessel vasoconstriction was initiated by the addition of potent agonists including norepinephrine (NE) and U-46619. Once a stable vasoconstriction was achieved, D7 proteins were added to the working chamber (Fig. 4A,B). AeD7L1 and AeD7L2 showed strong vasodilating activity, relaxing the blood vessel by scavenging different vasoconstriction agonists. When NE was used as an agonist, the blood vessel showed a 37% reduction in the artery inner diameter (Fig. 4C). Norepinephrine binds to α1 and α2 adrenoreceptors located on vascular smooth muscle. These receptors are linked to G proteins that activate smooth muscle contraction through signal transduction pathways [35]. When AeD7L1 was added in equimolar concentration to NE, a 97.3% recovery of the resting blood vessel inner diameter was observed (Fig. 4C, Video S1). AeD7L1 tightly binds norepinephrine (KD = 0.119 nm [9], Table 1); thus, as NE was bound by the salivary protein, vessel constriction was reverted. AeD7L2 binds norepinephrine with approximately 1000-fold lower affinity (KD = 110 nm; Table 1, Fig. 2C) resulting in a lower degree of inhibition of vascular constriction (Fig. 4C, Video S2). Current information on the affinity of NE to the adrenoreceptors of capillaries is scarce. However, given the degree of constriction inhibition by AeD7L2, we speculate that the affinity of NE to the adrenoreceptors might be close to the affinity to AeD7L2, which agrees with published data in rabbit aorta where the KD of NE to alpha receptors is 339 nm [36].Thromboxane A2 (TxA2), a lipid mediator originating from arachidonic acid metabolism through the cyclooxygenase pathway, is a powerful constrictor of vascular smooth muscle and an agonist of platelet aggregation. It is released from platelets and smooth muscle during inflammation and injury. Thromboxane A2 (TxA2) specifically binds the thromboxane/endoperoxide (TP) receptor, a G protein-coupled receptor, which leads to phospholipase C activation, release of inositol triphosphate, and an increase in the intracellular Ca2+ levels, thereby triggering the smooth muscle contraction [37]. The stable analog of TxA2, U-46619, caused a rapid and sustained contraction of mesenteric arteries, observed as a 73% reduction in the arterial inner diameter (Fig. 4D). AeD7L1 did not have any role in inhibiting vasoconstriction caused by U-46619 (Fig. 4D, Supplementary Video S3), a finding consistent with the lack of binding of AeD7L1 to U-46619 [9]. AeD7L2, however, reverted U-46619-induced vasoconstriction, observed as a recovery of 47.8% of the arterial inner diameter (Fig. 4D, Video S4). This recovery was not as immediate as the effect of AeD7L1 on NE-induced vasoconstriction. AeD7L2 binds U-46619 with a KD of 69.4 nm (Table 1, Fig. 2D), while the inhibitory constant of U-46619 to TP receptor is 67 nm [38]. Given the similarity of the affinity of U-46619 to AeD7L2 and to the TP receptor, upon addition of AeD7L2, the protein starts sequestering molecules of U-46619 that were bound to its receptor until the equilibrium eventually is reached. To better understand the effect of these salivary proteins on vasodilation, we used equimolar concentrations of agonists and salivary proteins, in keeping with the 1:1 stoichiometry determined in our ITC studies (Table 1).
Fig. 4.

Effect of Aedes aegypti salivary proteins AeD7L1 and AeD7L2 as vasodilators. (A) Experimental setup. Mesenteric arteries isolated from mice were cannulated, pressurized and placed in the myograph chamber. (B) Experimental scheme: Vessel diameter was measured continuously prior to the agonist application (1 μm of NE or U-46619) and for 5 min following the addition of any agonist (baseline), and during and after the addition of 1 μm final concentration of either AeD7L1 or AeD7L2 to the myograph chamber. (C) The inner diameter of mouse isolated mesenteric arteries at baseline (black bar), after the addition of 1 μm of norepinephrine (magenta bar) and following the addition of the recombinant protein AeD7L1 or AeD7L2. (D) The inner diameter of mouse isolated mesenteric arteries at baseline (black bar), after the addition of 1 μm of U-46619 (purple bar) and following the addition of the recombinant protein AeD7L1 or AeD7L2. For each vessel, two measurements of the diameter were averaged (red arrows). For analysis, stable inner diameter values measured during baseline, vasoconstriction, or after the addition of the proteins were used. Results are expressed as the mean of the arterial inner diameter of the biological replicates ± SEM. Results from at least two arteries are represented for each group (four arteries for all experimental groups except when norepinephrine was used as an agonist and AeD7L1 was tested, that only data from two arteries was obtained). Vasoconstriction by NE or U-46619 was indicated as a reduction of diameter taking the baseline value as 100%. Vasodilation achieved by the presence of AeD7L1 or AeD7L2 was expressed as recovery, considering the vasoconstricted diameter as 0% and baseline value as 100%. Snapshots of the myography recordings are shown below each data bar.
Aedes aegypti D7 long proteins inhibit platelet aggregation in vitro
Because Ae. aegypti D7 proteins bind platelet aggregation agonists such as the TxA2 analog U-46619 or serotonin; we studied the role of these proteins in platelet aggregation inhibition in ex vivo experiments. At low concentrations of collagen (1 μg·mL−1), we observed the classical collagen induction trace. There is a delay in platelet shape change due to the release of secondary mediators, resulting in an initial decrease of light transmittance. Upon the action of the secondary mediators, platelets aggregate, as indicated by an increase in light transmittance. Both AeD7L1 and AeD7L2 inhibited platelet aggregation triggered by a low dose of collagen (Fig. 5A). AeD7L1 delayed platelet shape change and the resulting magnitude of aggregation was lower than the control, while AeD7L2 abrogated platelet aggregation. In a similar trend, AeD7L1 showed almost no effect on platelet aggregation induced by U-46619, whereas AeD7L2 entirely prevented aggregation (Fig. 5B). Exposure of circulating platelets to collagen from the subendothelial matrix or thrombin leads to the formation of a platelet monolayer that supports subsequent adhesion of activated platelets to one another [3,39]. At high concentrations (~10 μg·mL−1), collagen acts as a strong agonist of the GPVI receptor on platelet surface, which induces platelet aggregation in an independent manner of ADP or TXA2 secretion [39]. However, at low concentrations of collagen, TxA2 plays an important role in the extension and amplification step of the platelet plug formation. Using low concentrations of collagen, our experiments strongly indicate that the potent inhibitory effect on platelet aggregation observed for AeD7L2 is due to its tight binding to U-46619 and presumably TxA2. In the case of AeD7L1, the high affinity binding of this protein to serotonin [8], a known potentiator of platelet agonists such as ADP or collagen [40], may be responsible for the anti-platelet aggregation effect observed when low concentrations of collagen were used.
Fig. 5.

Effect of Aedes aegypti salivary proteins AeD7L1 and AeD7L2 on platelet aggregation. Platelet-rich plasma was preincubated with 1 μm of salivary proteins for 60s before the addition of the platelet aggregation agonists, indicated by an arrow. Platelet aggregation was measured by light transmittance over 6 min. Technical duplicates were run, and SD bars are represented in the figure. (A) Aedes aegypti D7 salivary proteins inhibit collagen-induced platelet aggregation. AeD7L1 shows a delay in platelet shape change and a lower aggregation magnitude. AeD7L2 abolishes collagen-induced platelet aggregation. (B) Platelet aggregation induced by U-46619, the stable analog of Thromboxane A2, was slightly reduced by the presence of AeD7L1 and completely abrogated by AeD7L2. PBS buffer was used as a control (blue). AeD7L1 and AeD7L2 groups are shown in green and magenta, respectively.
AeD7L1 and AeD7L2 do not interfere with blood clotting
Secondary hemostasis consists of the activation of blood clotting. At sites of vessel injury, bleeding is minimized by the formation of a hemostatic plug consisting of platelets and fibrin. Two pathways, intrinsic and extrinsic, originally independent, converge at a specific point, leading to fibrin activation. These fibrin subunits have an affinity for each other and combine into fibrin strands that bind platelets together, stabilizing the platelet plug [41]. Recalcification assay measures the activity of the entire clotting cascade [42]. A dose-dependent delay in the recalcification time of normal human plasma was observed in the presence of salivary gland extracts of female Ae. aegypti mosquitoes (Fig. 6). However, none of the recombinant salivary D7 proteins resulted in a delay of the recalcification time. Salivary gland extracts inhibited both the extrinsic and intrinsic coagulation pathways as they significantly increased prothrombin time and activated partial thromboplastin time (Fig. S2A,B). As expected, a lack of anticoagulant effect of AeD7L1 and AeD7L2 was observed in both the extrinsic and intrinsic pathways (Fig. S2A,B). Therefore, the anticoagulant effect present in the salivary glands could not be attributed to Ae. aegypti salivary D7 long proteins alone. The saliva of Aedes spp. is enriched in salivary serine protease inhibitors, or serpins [14,16], that inhibit factor Xa and are responsible for the anticoagulant effect [43,44]. D7 proteins from Culex or Aedes act by inhibiting vasoconstriction and platelet aggregation but do not affect coagulation [3]. On the other hand, hamadarin, a member of the short D7 proteins from An. stephensi, binds both factor XII and high molecular weight kininogen, and interferes with the activation of the plasma contact system [45]. Anopheles gambiae D7r1, another member of short D7 protein family, and ortholog of hamadarin, also inhibits blood coagulation [8].
Fig. 6.

Anticlotting activity of Aedes aegypti salivary gland extracts and AeD7L1 and AeD7L2 salivary proteins. Recalcification time of citrated normal human plasma in the absence (control) or presence of either Ae. aegypti SGE or the recombinant salivary proteins AeD7L1 and AeD7L2. Recalcification or clotting time was determined as the time to reach 0.025 absorption units at 650 nm (onset time to O.D. = 0.025). Exact onset times for Ae. aegypti SGE equivalent to 5 and 2.5 salivary glands could not be determined because coagulation did not occur in these samples during the experimental time of one hour. For plotting and analysis purposes, the maximum experimental time was designated (3600 s). The results are expressed as the mean ± SEM of technical triplicates. Results were analyzed by one-way ANOVA, using the control group as the reference. Statistical differences were set at P < 0.05 (*P < 0.05, ***P < 0.001, ****P < 0.0001).
In conclusion, we have characterized the mechanisms of action and the physiological functions of the D7 long form proteins from Ae. aegypti. We determined that AeD7L2 has similar binding capabilities as AeD7L1, with certain specific features that make it a better inhibitor of platelet aggregation than the latter. AeD7L2 has a high binding affinity for U-46619, which AeD7L1 lacks. The physiological relevance of AeD7L1 and AeD7L2 lies in their inhibition of vasoconstriction and platelet aggregation. These proteins do not, however, show any effect on blood coagulation. Although both AeD7L1 and AeD7L2 play a role in preventing primary hemostasis, their molecular mechanisms of action differ, which can be explained by their binding properties. Importantly, this work reinforces the relevance of the D7 family of salivary proteins, which are among the most abundant proteins in mosquito saliva, and have become a hot research topic, given their role in altering arbovirus infection and dissemination [46–49].
Experimental procedures
Ethics statement
Public Health Service Animal Welfare Assurance #A4149-01 guidelines were followed according to the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) Animal Office of Animal Care and Use (OACU). These studies were carried out according to the NIAID-NIH animal study protocol (ASP) approved by the NIH Office of Animal Care and Use Committee (OACUC), with approvals ID ASP-LMVR-3 and ASP-LMVR-21E.
Mosquito rearing and salivary gland dissection
Aedes aegypti (Liverpool strain) mosquitoes were reared at the Laboratory of Malaria and Vector Research, NIAID, NIH, under standard conditions (28 °C, 80% humidity, with a 12-h light/dark cycle and maintained with 10% Karo syrup solution). Salivary glands from 5- to 7-day old mosquitoes were dissected in PBS (137 mm NaCl, 2.7 mm KCl, 4.3 mm Na2HPO4, and 1.4 mm KH2PO4, pH 7.4, Teknova, Hollister, CA, USA). Salivary gland extract was obtained by disrupting the gland walls by sonication (Branson Sonifier 450, Danbury, CT, USA) and cleared by centrifugation (13 500 g for 5 min at 4 °C) [50].
AeD7L1 and AeD7L2 gene expression pattern
Aedes aegypti larvae and male and female pupae, sorted according to size, were collected and kept in Trizol reagent (Life Technologies, Carlsbad, CA, USA). Additionally, male and female adults were dissected, and the head and thorax were separated from abdomens, and kept in Trizol. In all cases, each sample consisted of 10 specimens. Total RNA was isolated with Trizol reagent following the manufacturer’s instructions (Life Technologies). cDNA was obtained with the QuantiTect Reverse Transcriptase Kit (Qiagen, Valencia, CA, USA), from 1 μg of starting RNA. The Nanodrop ND-1000 spectrophotometer was used to determine all concentrations and OD260/280 ratios of nucleic acids. For qPCR, specific primers to target AeD7L1 and AeD7L2 genes were designed (AeD7L1-F: 5’-AAGAAGCAATCCTACTTCG-3’, AeD7L1-R: 5’-TCATCTAAGACAGTGTATTGC-3’, AeD7L2-F: 5’-GAATTAGGAAGTATCAGATGG-3’, AeD7L2-R: 5’-AGTTCATTCTTGCTTGTCATG-3’). Briefly, in a final volume of 20 μL, the reaction mix was prepared with 2X SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA), 300 nm of each primer, and 100 ng of cDNA template. Two biological replicates were tested. All samples were analyzed in technical triplicates and nontemplate controls were included in all qPCR experiments as negative controls. qPCR data were manually examined and analyzed by the ΔΔCt method. ΔCt values were obtained by normalizing the data against Ae. aegypti 40S ribosomal protein S7 transcript (AAEL009496-RA; AeS7-F: 5’-GGGACAAATCGGCCAGGCTATC-3’ and AeS7-R: 5’-TCGTGGACGCTTCTGCTTGTTG-3’) as the reference gene. Larvae stage samples were chosen as controls for the ΔΔCt values. Relative abundance of genes of interest was calculated as 2−ΔΔCt.
Protein purification and 3D structure prediction of AeD7L2
AeD7L1 recombinant protein was produced as previously described [9]. Briefly, supernatant from transfected freestyle human embryonic kidney cells (HEK293F) with AeD7L1-VR2001 plasmid was submitted to an initial step of size-exclusion chromatography, followed by ion exchange, and a final analytical size-exclusion chromatography. Similarly, AeD7L2 mature cDNA sequence was codon optimized for a eukaryotic cell expression system and synthesized by BioBasic Inc. One Shot® TOP10 chemically competent Escherichia coli (Invitrogen, Carlsbad, CA, USA) were transformed with VR2001 vector containing the AeD7L2 sequence followed by a 6xHis-tag and stop codon (Vical Incorporated, San Diego, CA, USA) [51]. Plasmid DNA was prepared using the EndoFree plasmid MEGA prep kit (Qiagen). FreeStyle 293-F mammalian cells were transfected with sterile plasmid DNA at the SAIC Advance Research Facility (Frederick, MD, USA), and supernatants were collected 72 h after transfection. Recombinant proteins were purified from supernatants by affinity chromatography using Nickel-charged HiTrap Chelating HP columns and followed by size-exclusion chromatography in a Superdex 200 10/300 GL column (GE Healthcare Life Science, Piscataway, NJ, USA). A model of the protein structure of AeD7L2 was predicted with I-TASSER software [52] using the mature amino acid sequence of AeD7L2. All structural figures were produced in UCSF Chimera (Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, USA)[53].
Western blot
A rabbit was immunized with 1 mg of Ae. aegypti salivary gland extract, according to the standard protocol of Noble Life Science facilities. Total IgG was purified by affinity chromatography using a 5-mL HiTrap protein A HP column following manufacturer’s instructions (GE Healthcare Life Science). Purified IgG protein concentration was determined by Nanodrop ND-1000 spectrophotometer.
Western blot was carried out as described elsewhere [19]. Briefly, the content of half Ae. aegypti SG and 50 ng of either AeD7L1 or AeD7L2 were separated by 4-20 NuPAGE. Proteins were transferred to a nitrocellulose membrane (iBlot, Invitrogen) that was blocked overnight at 4 °C with blocking buffer: 0.05% Tween 20, 25 mm Tris/HCl (pH 8.0), 150 mm NaCl containing 5% (w/v) powdered nonfat milk. Purified IgG antibodies raised against Ae. aegypti salivary gland extract were diluted in blocking buffer (1 μg·mL) and incubated for 90 min. Goat anti-rabbit conjugated to alkaline phosphatase (Sigma, St. Louis, MO, USA) diluted in blocking buffer (1 : 10 000) was incubated for one hour. BCIP/NBT (Promega, Madison, WI, USA) was used as a substrate.
Isothermal titration calorimetry
Binding experiments were performed using a MicroCal VP-ITC (Malvern Panalytical, Westborough, MA, USA). Thermodynamic binding parameters of AeD7L1 have been already described [8,9]. AeD7L2 protein and ligands (serotonin, histamine, epinephrine, norepinephrine, and tryptamine) were diluted to 2 and 20 μm, respectively, in 20 mm Tris/HCl, 150 mm NaCl, pH 7.4 (TBS). For lipids ligands the solvent was evaporated under a stream of nitrogen and further dissolved in TBS, vortexed, and sonicated for 10 min (Branson 1510) to ensure dissolution. When lipid ligands were used, protein and ligands and were prepared at 5 and 50 μm, respectively. Protein and ligand samples were degassed using a MicroCal ThermoVac (Malvern Panalytical) prior to loading. Injections of 10 μL of ligand were added to the protein samples contained in the calorimeter cell at 300-s intervals. Assays were performed at 30 °C, and measured heats were converted to enthalpies and analyzed by fitting to a single-site binding model using the MicroCal PEAQ-ITC analysis software (Malvern Panalytical). Because N values were close to 1, as previously described for other salivary D7 proteins [7–10] and as predicted by the structure model the ITC data were refitted with a fixed N = 1 value. At least two different sets of experiments were carried out with similar results. ChemDraw software (Perkin Elmer, Waltham, MA, USA) was used for drawing the chemical structures of the ligands.
Blood vessel vasoconstriction studies
Euthanasia and collection of murine mesenteric arteries
While deeply anesthetized by inhalational isoflurane (furane 4%), each mouse was euthanized by bilateral pneumothorectomy, after which the small intestine was removed from the abdomen and placed in cold modified Krebs-HEPES buffer (KH buffer, pH 7.4; 4 °C). The KH buffer had the following composition 130 mm NaCl, 4.7 mm KCl, 1.18 mm KH2PO4, 1.17 mm MgSO4-7H2O, 14.9 mm NaHCO3, 5.5 mm Dextrose, 0.026 mm CaNa2 Versenate (EDTA), 1.6 mm CaCl2, and 10 mm HEPES. Third-order arteries were dissected from the mesentery of the small intestine and placed into an isolated microvessel chamber (Danish Myo Technologies, Hinnerup, Denmark) filled with KH buffer at 37 °C. Each mesenteric artery was subsequently doubly cannulated within this heated chamber (37 °C) that allowed the lumen and exterior of the vessel to be perfused and superfused, respectively, with KH buffer from separate reservoirs. The perfusing buffer reservoir was pressurized with room air.
Measurements of vascular reactivity in isolated mesenteric arteries
The heated microvessel chambers described above were placed on a 10× video microscope (Danish Myo Technologies). Each mesenteric artery was allowed to equilibrate for 60 min at 80% of mouse mean arterial pressure to approximate in vivo perfusion pressure. Any vessel that did not demonstrate significant vasoconstriction to 1 μm KCl at the equilibration pressure was discarded. Following equilibration, the vasoconstrictor reactivity of each artery was assessed in response to 1 μm norepinephrine or 1 μm U-46619. Vessel diameter was measured by digital caliper micrometer measurement of the video microscope using DMT MyoVIEW software (Danish Myo Technologies). Vessel diameter was measured continuously prior to agonist application and for 5 min following agonist dosage. The vascular response to NE or U-46619 was calculated from the maximum constricted diameter to each agonist and then plotted as percentage of vessel baseline diameter. The vessel chambers were washed 3X with KH buffer between agonist applications and allowed to equilibrate to baseline diameter. The constriction measurements to NE or U-46619 were then repeated in the presence of AeD7L1 and AeD7L2 (1 μm final concentration) following incubation time of (2 min) with either AeD7L1 or AeD7L2. Two vessels from two different mice were used for experiments with each agonist. For each vessel, two measurements of the diameter were averaged. After addition of the salivary protein, recovery of blood vessel dimeter was calculated as the response change taking the agonist constriction as 0% and the baseline as 100%.
Platelet aggregation assays
Platelet-rich plasma (PRP) was obtained from normal healthy donors on the NCI IRB-approved NIH protocol 99-CC-0168, ‘Collection and Distribution of Blood Components from Healthy Donors for In Vitro Research Use’. Research blood donors provide written informed consent, and platelets were de-identified prior to distribution. Briefly, one hundred μL of PRP was 1:3 diluted in HEPES-Tyrode’s buffer to a concentration of approximately 250 000 platelets·μL−1 [13]. Platelet aggregation was measured in a Chrono-Log aggregometer (Chrono-Log Corporation, Havertown, PA, USA) with stirring at 1200 rpm at 37 °C. Recombinant proteins (1 μm) were incubated for 1 min, and PRP samples were prestirred in the aggregometer for 1 min to monitor pre-aggregation effects. Aggregation was induced by using 1 μg·mL−1 (final concentration) of native collagen type I fibrils from equine tendons (Chrono-Log Corporation) or 0.7 μm U-46619 (Cayman Chemicals, Ann Arbor, MI, USA). Technical duplicates were performed. At least two different sets of experiments were carried out with similar results.
Human plasma recalcification assay
To measure recalcification time of human plasma, 30 μL of normal human citrated plasma (Diagnostica Stago, Mount Olive, NJ, USA) was mixed with equal volumes of either test samples or 10 mm HEPES, 150 mm NaCl, and pH 7.3 in a 96-well flat-bottom plate and incubated for 2 min at 37 °C. The coagulation cascade was triggered by adding 30 μL of prewarmed 25 mm CaCl2 to each mixture. The increase in turbidity at 650 nm was monitored in a VersaMax plate reader (Molecular Devices, San Jose, CA, USA) with measurements taken at 10-sec intervals. Recalcification or clotting time was determined as the time to reach 0.025 absorption units (onset time to O.D. = 0.025). Technical triplicates were performed.
Prothrombin time (PT) and activated partial thromboplastin time (aPTT)
Anticoagulant properties of Ae. aegypti SGE and recombinant proteins AeD7L1 and AeD7L2 were tested by PT and aPTT using a Start4 Hemostasis Analyzer (Diagnostica Stago). Experimental samples (5 pairs of Ae. aegypti salivary glands and recombinant D7 proteins) were diluted in 10 mm HEPES, 150 mm NaCl, and pH 7.3, while buffer alone was used as a negative control. Normal human reference plasma (50 μL) (Diagnostica Stago) was incubated with 5 μL of either HEPES buffer (control) or sample, and incubated at 37 °C for 2 min. For PT, 100 μL of Neoplastine CI Plus reagent (Diagnostica Stago) was added and the clotting time was recorded under stirring conditions. For aPTT, 50 μL of aPTT reagent was added to the mixture of protein and plasma and incubated for 3 min at 37 °C. Clotting was triggered by the addition of 50 μL of prewarmed 25 mm CaCl2. All samples were assayed in triplicates. Results were analyzed by one-way ANOVA, and statistical differences were set at P < 0.05.
Supplementary Material
Fig. S1. Binding of AeD7L2 to other biogenic amines and leukotrienes by isothermal titration calorimetry.
Fig. S2. Prothrombin time and activated partial thromboplastin time were not affected by AeD7L1 and AeD7L2.
Video S1. AeD7L1 reverts blood vessel vasoconstriction caused by norepinephrine.
Video S2. AeD7L2 prevents blood vessel vasoconstriction caused by norepinephrine at a lower degree than AeD7L1.
Video S3. AeD7L1 does not prevent blood vessel vasoconstriction caused by the stable analog of thromboxane A2 (U-46619).
Video S4. AeD7L2 partially reverts blood vessel vasoconstriction caused by the stable analog of thromboxane A2 (U-46619).
Acknowledgements
We thank Andre Laughinghouse, Kevin Lee, and Yonas Gebremicale for excellent mosquito rearing. Van My Pham for excellent salivary gland dissections. We thank Brian Martin (Research and Technology Branch, NIAID) for his help with the N-terminal sequencing. The authors thank Brigit Shea Sullivan, NIH Library Editing Service, for manuscript editing assistance. This research was supported by the Division of Intramural Research Program of the NIH/NIAID (AI001246-01).
Abbreviations
- ADP
adenosine 5′-diphosphate
- Ae.
Aedes
- An.
Anopheles
- aPTT
activated partial thromboplastin time
- ATP
adenosine 5′-triphosphate
- C.
Culex
- cDNA
complementary DNA
- HEK293
human embryonic kidney cells
- ITC
isothermal titration calorimetry
- LT
leukotriene
- MW
molecular weight
- NE
norepinephrine
- PT
prothrombin time
- SGE
salivary gland extract
- TP receptor
thromboxane/endoperoxide receptor
- TxA2
thromboxane A2
Footnotes
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Souza-Neto JA, Powell JR & Bonizzoni M (2019) Aedes aegypti vector competence studies: a review. Infect Genet Evol 67, 191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribeiro JM, Rossignol PA & Spielman A (1984) Role of mosquito saliva in blood vessel location. J Exp Biol 108, 1–7. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro JMC & Arca B (2009) From Sialomes to the Sialoverse: an insight into salivary potion of blood-feeding insects. Adv In Insect Phys 37, 59–118. [Google Scholar]
- 4.Arca B & Ribeiro JM (2018) Saliva of hematophagous insects: a multifaceted toolkit. Curr Opin Insect Sci 29, 102–109. [DOI] [PubMed] [Google Scholar]
- 5.Chagas AC, Ramirez JL, Jasinskiene N, James AA, Ribeiro JM, Marinotti O & Calvo E (2014) Collagen-binding protein, Aegyptin, regulates probing time and blood feeding success in the dengue vector mosquito, Aedes aegypti. Proc Natl Acad Sci U S A 111, 6946–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenzuela JG, Charlab R, Gonzalez EC, de Miranda-Santos IK, Marinotti O, Francischetti IM & Ribeiro JM (2002) The D7 family of salivary proteins in blood sucking diptera. Insect Mol Biol 11, 149–155. [DOI] [PubMed] [Google Scholar]
- 7.Alvarenga PH, Francischetti IM, Calvo E, Sa-Nunes A, Ribeiro JM & Andersen JF (2010) The function and three-dimensional structure of a thromboxane A2/cysteinyl leukotriene-binding protein from the saliva of a mosquito vector of the malaria parasite. PLoS Biol 8, e1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvo E, Mans BJ, Andersen JF & Ribeiro JM (2006) Function and evolution of a mosquito salivary protein family. J Biol Chem 281, 1935–1942. [DOI] [PubMed] [Google Scholar]
- 9.Calvo E, Mans BJ, Ribeiro JM & Andersen JF (2009) Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci U S A 106, 3728–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jablonka W, Kim IH, Alvarenga PH, Valenzuela JG, Ribeiro JMC & Andersen JF (2019) Functional and structural similarities of D7 proteins in the independently-evolved salivary secretions of sand flies and mosquitoes. Sci Rep 9, 5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mans BJ, Calvo E, Ribeiro JM & Andersen JF (2007) The crystal structure of D7r4, a salivary biogenic amine-binding protein from the malaria mosquito Anopheles gambiae. J Biol Chem 282, 36626–36633. [DOI] [PubMed] [Google Scholar]
- 12.Andersen JF & Ribeiro JMC. (2017) Chapter 4 – salivary Kratagonists: scavengers of host physiological effectors during blood feeding. In Arthropod Vector: Controller of Disease Transmission, Volume 2 (Wikel SK, Aksoy S & Dimopoulos G eds), pp. 51–63. Academic Press, Cambridge, MA. [Google Scholar]
- 13.Martin-Martin I, Paige A, Valenzuela Leon PC, Gittis AG, Kern O, Bonilla B, Chagas AC, Ganesan S, Smith LB, Garboczi DN & et al. (2020) ADP binding by the Culex quinquefasciatus mosquito D7 salivary protein enhances blood feeding on mammals. Nat Commun 11, 2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro JM, Martin-Martin I, Arca B & Calvo E (2016) A deep insight into the Sialome of male and female Aedes aegypti Mosquitoes. PLoS One 11, e0151400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James AA, Blackmer K, Marinotti O, Ghosn CR & Racioppi JV (1991) Isolation and characterization of the gene expressing the major salivary gland protein of the female mosquito, Aedes aegypti. Mol Biochem Parasitol 44, 245–253. [DOI] [PubMed] [Google Scholar]
- 16.Juhn J, Naeem-Ullah U, Maciel Guedes BA, Majid A, Coleman J, Paolucci Pimenta PF, Akram W, James AA & Marinotti O (2011) Spatial mapping of gene expression in the salivary glands of the dengue vector mosquito, Aedes aegypti. Parasit Vectors 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim IH, Pham V, Jablonka W, Goodman WG, Ribeiro JMC & Andersen JF (2017) A mosquito hemolymph odorant-binding protein family member specifically binds juvenile hormone. J Biol Chem 292, 15329–15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim IH, Castillo JC, Aryan A, Martin-Martin I, Nouzova M, Noriega FG, Barletta ABF, Calvo E, Adelman ZN, Ribeiro JMC et al. (2020) A mosquito juvenile hormone binding protein (mJHBP) regulates the activation of innate immune defenses and hemocyte development. PLoS Pathog 16, e1008288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Martin I, Chagas AC, Guimaraes-Costa AB, Amo L, Oliveira F, Moore IN, DeSouza-Vieira TS, Sanchez EE, Suntravat M, Valenzuela JG et al. (2018) Immunity to LuloHya and Lundep, the salivary spreading factors from Lutzomyia longipalpis, protects against Leishmania major infection. PLoS Pathog 14, e1007006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng Z, Estelle F & Simons R (2007) Mosquito allergy and mosquito salivary allergens. Protein Pept Lett 14, 975–981. [DOI] [PubMed] [Google Scholar]
- 21.Peng Z, Xu W, Lam H, Cheng L, James AA & Simons FE (2006) A new recombinant mosquito salivary allergen, rAed a 2: allergenicity, clinical relevance, and cross-reactivity. Allergy 61, 485–490. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Oliveira F, Chang BW, Collin N, Gomes R, Teixeira C, Reynoso D, Pham VM, Elnaiem DE, Kamhawi S et al. (2011) Structure and function of a “yellow” protein from saliva of the sand fly Lutzomyia longipalpis that confers protective immunity against Leishmania major infection. J Biol Chem 286, 32383–32393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kousar S, Anjuma S, Jaleel F, Khana J & Naseema S (2017) Biomedical significance of tryptamine: a review. J Pharmacovigil 5, 1–6. [Google Scholar]
- 24.Van Nueten JM, Janssens WJ & Vanhoutte PM (1985) Serotonin and vascular reactivity. Pharmacol Res Commun. 17, 585–608. [DOI] [PubMed] [Google Scholar]
- 25.Herr N, Bode C & Duerschmied D (2017) The effects of serotonin in immune cells. Front Cardiovasc Med 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greaves MW & Davies MG (1982) Histamine receptors in human skin: indirect evidence. Bri J Dermatol 107 (Suppl 23), 101–105. [DOI] [PubMed] [Google Scholar]
- 27.Sahid M, Tripathi T, Sobia F, Moin S, Siddiqui M & Khan RA (2009) Histamine, histamine receptors, and their role in immunomodulation: an updated systematic review. Open Immunol J 2, 9–41. [Google Scholar]
- 28.Mans BJ (2011) Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J Innate Immun 3, 41–51. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro JM, Mans BJ & Arca B (2010) An insight into the sialome of blood-feeding Nematocera. Insect Biochem Mol Biol 40, 767–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piper PJ (1984) Formation and actions of leukotrienes. Physiol Rev 64, 744–761. [DOI] [PubMed] [Google Scholar]
- 31.Peters-Golden M, Gleason MM & Togias A (2006) Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 36, 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyce JA (2005) Eicosanoid mediators of mast cells: receptors, regulation of synthesis, and pathobiologic implications. Chem Immunol Allergy 87, 59–79. [DOI] [PubMed] [Google Scholar]
- 33.Lavoipierre MM (1965) Feeding mechanism of blood-sucking arthropods. Nature 208, 302–303. [DOI] [PubMed] [Google Scholar]
- 34.Choumet V, Attout T, Chartier L, Khun H, Sautereau J, Robbe-Vincent A, Brey P, Huerre M & Bain O (2012) Visualizing non infectious and infectious Anopheles gambiae blood feedings in naive and saliva-immunized mice. PLoS One 7, e50464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langer SZ & Hicks PE (1984) Alpha-adrenoreceptor subtypes in blood vessels: physiology and pharmacology. J Cardiovasc Pharmacol 6 (Suppl 4), S547–S558. [DOI] [PubMed] [Google Scholar]
- 36.Besse JC & Furchgott RF (1976) Dissociation constants and relative efficacies of agonists acting on alpha adrenergic receptors in rabbit aorta. J Pharmacol Exp Ther 197, 66–78. [PubMed] [Google Scholar]
- 37.Bolla M, You D, Loufrani L, Levy BI, Levy-Toledano S, Habib A & Henrion D (2004) Cyclooxygenase involvement in thromboxane-dependent contraction in rat mesenteric resistance arteries. Hypertens 43, 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narumiya S, Sugimoto Y & Ushikubi F (1999) Prostanoid receptors: structures, properties, and functions. Physiol Rev 79, 1193–226. [DOI] [PubMed] [Google Scholar]
- 39.Andrews RK & Berndt MC (2004) Platelet physiology and thrombosis. Thromb Res 114, 447–453. [DOI] [PubMed] [Google Scholar]
- 40.Andersen JF, Francischetti IM, Valenzuela JG, Schuck P & Ribeiro JM (2003) Inhibition of hemostasis by a high affinity biogenic amine-binding protein from the saliva of a blood-feeding insect. J Biol Chem 278, 4611–4617. [DOI] [PubMed] [Google Scholar]
- 41.Smith SA, Travers RJ & Morrissey JH (2015) How it all starts: Initiation of the clotting cascade. Crit Rev Biochem Mol Biol 50, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valenzuela JG, Guimaraes JA & Ribeiro JM (1996) A novel inhibitor of factor X activation from the salivary glands of the bed bug Cimex lectularius. Exp Parasitol 83, 184–190. [DOI] [PubMed] [Google Scholar]
- 43.Stark KR & James AA (1998) Isolation and characterization of the gene encoding a novel factor Xa-directed anticoagulant from the yellow fever mosquito, Aedes aegypti. J Bio Chem 273, 20802–20809. [DOI] [PubMed] [Google Scholar]
- 44.Calvo E, Mizurini DM, Sa-Nunes A, Ribeiro JM, Andersen JF, Mans BJ, Monteiro RQ, Kotsyfakis M & Francischetti IM (2011) Alboserpin, a factor Xa inhibitor from the mosquito vector of yellow fever, binds heparin and membrane phospholipids and exhibits antithrombotic activity. J Bio Chem. 286, 27998–28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isawa H, Yuda M, Orito Y & Chinzei Y (2002) A mosquito salivary protein inhibits activation of the plasma contact system by binding to factor XII and high molecular weight kininogen. J Bio Chem 277, 27651–27658. [DOI] [PubMed] [Google Scholar]
- 46.Conway MJ, Londono-Renteria B, Troupin A, Watson AM, Klimstra WB, Fikrig E & Colpitts TM (2016) Aedes aegypti D7 saliva protein inhibits dengue virus infection. PLoS Negl Trop Dis 10, e0004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Girard YA, Mayhew GF, Fuchs JF, Li H, Schneider BS, McGee CE, Rocheleau TA, Helmy H, Christensen BM, Higgs S & et al. (2010) Transcriptome changes in Culex quinquefasciatus (Diptera: Culicidae) salivary glands during West Nile virus infection. J Med Entomol 47, 421–435. [DOI] [PubMed] [Google Scholar]
- 48.Reagan KL, Machain-Williams C, Wang T & Blair CD (2012) Immunization of mice with recombinant mosquito salivary protein D7 enhances mortality from subsequent West Nile virus infection via mosquito bite. PLoS Negl Trop Dis 6, e1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD & Bernard KA (2011) Mosquito saliva causes enhancement of West Nile virus infection in mice. J Virol 85, 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribeiro JM & Nussenzveig RH (1993) The salivary catechol oxidase/peroxidase activities of the mosquito Anopheles albimanus. J Exp Biol 179, 273–287. [DOI] [PubMed] [Google Scholar]
- 51.Chagas AC, McPhie P, San H, Narum D, Reiter K, Tokomasu F, Brayner FA, Alves LC, Ribeiro JMC & Calvo E (2014) Simplagrin, a platelet aggregation inhibitor from Simulium nigrimanum salivary glands specifically binds to the Von Willebrand factor receptor in collagen and inhibits carotid thrombus formation in vivo. PLoS Negl Trop Dis 8, e2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Yan R, Roy A, Xu D, Poisson J & Zhang Y (2015) The I-TASSER Suite: protein structure and function prediction. Nat Methods 12, 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC & Ferrin TE (2004) UCSF Chimera – a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Binding of AeD7L2 to other biogenic amines and leukotrienes by isothermal titration calorimetry.
Fig. S2. Prothrombin time and activated partial thromboplastin time were not affected by AeD7L1 and AeD7L2.
Video S1. AeD7L1 reverts blood vessel vasoconstriction caused by norepinephrine.
Video S2. AeD7L2 prevents blood vessel vasoconstriction caused by norepinephrine at a lower degree than AeD7L1.
Video S3. AeD7L1 does not prevent blood vessel vasoconstriction caused by the stable analog of thromboxane A2 (U-46619).
Video S4. AeD7L2 partially reverts blood vessel vasoconstriction caused by the stable analog of thromboxane A2 (U-46619).


