Abstract
Two contrasting guar varieties [Cyamopsis tetragonoloba (L.) Taub.], RGC-1002—a better drought tolerant than RGC-936—a comparatively sensitive variety, were subjected to 8 days of water stress. The physiological characteristics of gas exchange, pigments and the spectral properties of the leaves were monitored and assessed. The guar variety, RGC-1002 exhibited higher relative water content (RWC) and biomass accumulation in water stress condition as compared to RGC-936. The RGC-1002 variety also showed a higher rate of photosynthesis, stomatal conductance, and lesser reduction in chlorophyll content as compared to RGC-936 variety. On the 8th day of drought, the leaf anthocyanin levels were also higher in RGC-1002 than RGC-936. Increased levels of anthocyanin result in decrease in absorption of light, and an increase in reflectance, and transmittance. The scattering coefficient of leaf spectra was significantly increased in RGC-936 than in RGC-1002 leaves; this is due to an increase in the size of intercellular air spaces and shrinkage of cells as a result of water loss. The leaf reflectance was also observed to be significantly increased in RGC-936 than in RGC-1002 leaves. The physiological and leaf optical observations substantiate that RGC-1002 was better adapted to water stress than RGC-936 variety.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02674-2.
Keywords: Anthocyanin, Carbon assimilation, Light absorbance coefficient, Light scattering coefficient
Introduction
Guar (Cyamopsis tetragonoloba (L).Taub.) is a drought-tolerant, multi-purpose annual arid legume crop, cultivated mainly during the rainy season, with seed sowing during June–July and is harvested up to October. In India, the major guar producing areas are the arid and semi-arid regions of Rajasthan, Gujarat and Haryana. Economically important gum is extracted from guar seeds, while the pods are used as a vegetable (Verma et al. 2013; Meftahizadeh et al. 2019).
Drought interferes with the photosynthetic process which restraints physiological processes involved in plant growth and development (Singh et al. 2014). Consequently, stomatal conductance and transpiration rate are also affected (Ouyang et al. 2017), and results in a restricted total leaf area and plant growth (Aguirrezabal et al. 2006; Tapia et al. 2016). Plants adapt several mechanisms to withstand environmental stresses such as drought (Ahmed et al. 2020). One of the properties of leaves to combat water stress is to adjust their optical attributes by maintaining a balance between light reflectance, absorbance and transmittance (Wong and Gamon 2015).
Reflectance and absorbance are the most informative optical properties of the leaf. Measurement of these optical properties is of immense use in determining the biochemistry of leaves (Curran et al. 2001; Gitelson and Solovchenko 2018). It is also a valuable tool to obtain information concerning photosynthetic efficiency. The alterations in reflectance in the visible spectrum region due to stress condition result from the sensitivity of light-harvesting pigments such as chlorophyll and carotenoids to metabolic disturbances. Research studies have shown the relationship of these leaf pigments with spectral reflectance or its derived indices (Carter and Knapp 2001; Garriga et al. 2014; Katsoulas et al. 2016; Lobos et al. 2019). Spectral indices are developed using ratios of wavelengths and they are very sensitive to a particular type of pigment (Croft et al. 2014). The spectral band wavelength near 700 nm monitors plant stress and helps in estimating leaf chlorophyll concentration (Carter and Knapp 2001). Chlorophyll absorbs strongly in the red spectral region, with maximum at 660 and 680 nm of visible wavelengths, and shows maximum reflectance at 550 nm in the green wavelength region (Beamish et al. 2017).
Several researchers have also developed the potential of using leaf reflectance for measuring the plant water status (Ma et al. 2019). Relationships between leaf water status, gas exchange parameters and spectral reflectance measurements amongst several crop plants have been studied in peanut and wheat (Peñuelas and Inoue 1999), soybean (Inamullah and Isoda 2005) and cotton leaves (Singh et al. 2014; Zhang et al. 2018). The vegetative indices calculated from reflectance data of distinct spectral bands are influenced by optical properties of leaves and physical properties of pigments. Numerous spectral indices have been proposed for the estimation of leaf pigment. Vegetation Indices are combinations of surface reflectance at two or more wavelengths designed to highlight a particular property of vegetation. Among different vegetative indices, normalized difference vegetation index (NDVI) is the most simple and commonly used index. It correlates with absorbed photosynthetically active radiation and photosynthetic capacity (Tan et al. 2013). Photochemical reflectance index (PRI) that is derived from spectral reflectance data can detect photosynthetic efficiency of the leaves and the entire ecosystem (Middleton et al. 2016). The PRI measures xanthophyll cycle activity and the cycle pigments play an active role in the energy dissipation process. It is also been reported that PRI is related to carotenoid chlorophyll ratio in green leaves (Middleton et al. 2016). Spectral indices such as NDVI and PRI can be used to predict leaf biochemistry along with some leaf characteristics and physiological characteristics (Silva-Perez et al. 2017).
Our study aimed to evaluate the responses of two contrasting guar varieties to changes in leaf reflectance spectroscopy in water-stressed conditions. How do these varieties respond photosynthetically to drought? Can leaf spectral measurements be used for screening large populations to review the physiological status of the plants, rapidly and in a non-invasive way? This can be useful to plan the irrigation regimes during cultivation.
Materials and methods
Plant material and growth conditions
Seeds of guar [Cyamopsis tetragonoloba (L.) Taub.] varieties, namely RGC-936 a drought sensitive, and the other tolerant, RGC-1002 (Unpublished work from our Lab.), were sown in 10.0-L pots filled with garden soil, sandy loam soil composed of 17% clay, 12% silt and 71% sand mixed with organic manure, pH 7.56 (Thapa et al. 2018). The plants were allowed to germinate in a plant growth chamber (PGR-15, Conviron, Controlled Environments Ltd., Canada). The photosynthetic active radiation (PAR) between 5:30 and 11:30 h was gradually increased from 50 to 1400 µmol m−2 s−1. Later on, it was decreased gradually in a similar way with a 14-h light/10-h dark cycle. The temperature was maintained between 25 and 35 °C during the dark and light periods, respectively. Relative humidity varied between 50 and 60%. Forty-day-old plants were used for the study.
Experimental design and measurements of plant biomass
A set of five plants, each for watered-control and drought, were used. The plants under control condition were watered about 500 mL daily to maintain relative water content (RWC) of approximately 90% and above. The potted plants were subjected to drought for 8 days by withholding water. The morphological features, physiological attributes, pigment concentration and spectral reflectance studies were assessed in 4th and 8th days of drought treatment on water-stressed plants along with the watered or the control plants. Third fully matured leaf from the top of the plant was selected for each measurement. The biomass of root, stem and leaves was recorded after properly surface cleaning the tissues and drying them in oven at 70 °C.
Measurements of relative water content
Five leaf samples were excised from control, 4th and 8th days of desiccation early in the morning before the onset of the photoperiod. Fresh weight of excised leaves was recorded immediately. These leaves were then immersed in water to attain full turgidity at room temperature for 6 h. Turgid weight (TW) of leaves was recorded and then dried at 70 °C in an oven to a constant weight and the dry weight (DW) was noted. The relative water content (RWC) of the leaves was calculated as RWC (%) = (FW – DW/TW – DW) × 100) (Barrs and Weatherley 1962).
Gas exchange measurements
The gas exchange parameters of net photosynthesis (PN), rate of transpiration (E) and rate of stomatal conductance (gs) were monitored with portable photosynthesis system (LI-6400, LI-COR, Lincoln, NE, Nebraska, USA). All the measurements were performed at PPFD of 1000 µmol m−2 s−1, CO2 level inside the leaf cuvette was maintained at 400 µmol (CO2) mol−1, the temperature of the leaf was at 30 °C and the vapour pressure deficit (VPD) level was less than 3 kPa. The ratio of PN to E was considered as the photosynthetic water use efficiency (WUE). All the gas exchange measurements and water relation parameters were measured between 8:00 and 11:00 h (Singh et al. 2014).
Pigments estimation
Approximately 20–25 mg of leaf discs, 1.5 cm2 were harvested by identical-machine punches from five independent plants, each for watered and water-stressed. The samples were kept immediately in 2 mL of Eppendorf tube with 1 mL of 80% (v/v) acetone. After this, the samples were kept in dark for 12 h at room temperature. Then absorbance of the extract was recorded at 470, 663 and 646 nm. Chlorophylls and total carotenoid were calculated using the formulae.
Chlorophyll a (Ca) = 12.21A663 − 2.81A646, Chlorophyll b (Cb) = 20.13A646 − 5.03A663, and Total Carotenoid = (1000A470 − 3.27Ca − 104Cb)/198 (Wellburn 1994).
Anthocyanin was estimated with 30–35 mg of leaf discs extracted with 1% acidified methanol (1.0% HCl, v/v). The absorbance of the extract was recorded at 530 and 650 nm. The corrected anthocyanin absorbance value for chlorophyll interference was calculated as, A530 − (0.288 × A650). Total anthocyanin content was calculated using corrected anthocyanin absorbance and the molar absorbance coefficient for anthocyanin at 530 nm as 30,000 L mol−1 cm−1 (Murray and Hackett 1991).
Leaf spectral measurements
Changes in leaf surface reflectance and transmittance spectra of the guar leaf were measured using Spectroradiometer (LI-1800, LI-COR, Lincoln, NE, Nebraska, USA). The third leaf from the top was selected for the spectral studies. Spectra were determined within the wavelength range from 400 to 1100 nm. Both reflectance and transmittance were measured as a ratio with white reference data. Adaxial reflectance spectra of the leaves were measured against barium sulfate as a standard with a spectral sampling resolution of 2 nm.
Spectral indices
The different reflectance indices were calculated from the derived spectral reflectance curves as follows, photochemical reflectance index, PRI = (R531 – R570)/(R531 + R570), (Gamon et al. 1992), normalized difference vegetation index, NDVI = (R750 – R705)/(R750 + R705) and modified red edge normalized difference vegetation index mNDVI = (R750 – R705)/(R750 + R705 – 2R445), (Sims and Gamon 2002) and plant senescence reflectance index, PSRI = (R680 – R500)/(R750), (Merzlyak et al. 1999). The cold hard band (CHB) is found to correlate with the formation of chlorophyll–xanthophyll–protein complex(es) which protects the plant against freezing (Gilmore and Ball 2000). The cold hard band was calculated as, CHB = (R750 – R710)/(R750 + R710), (Lovelock and Robinson 2002). In these equations, R denotes reflectance and the subscripts refer to specific spectral wavelength. The spectral indices provide information about the functional and pigment changes in the leaves during drought. These narrowband greenness vegetation indexes are a combination of reflectance measurements sensitive to the combined effects of foliage chlorophyll concentration, canopy leaf area, etc. These are designed to provide a measure of the overall amount and quality of photosynthetic tissue in vegetation which is essential for understanding the state of vegetation.
The linear relation for the analyses of reflectance spectra at wavelengths 696, 727, 731 and 770 nm and chlorophyll pigments is explained by the fact that the ratio of reflectance spectrum responds to changes in chlorophyll concentrations (Blackburn 1998).
Calculation of scattering and absorbance coefficients
The scattering coefficient (s) and absorbance coefficient (k) were calculated using the reflectance data between 500 and 750 nm according to Cordón and Lagorio (2007). The wavelength of weak absorption (approx. 750 nm) is larger for scattering, due to higher optical pathlength of the wavelength. The absorption coefficient widened during senescence due to the degradation of chlorophyll and the formation of some light-absorbing compounds in the green–yellow region of the electromagnetic spectrum. As leaves senesce, tannins (brown pigments) are formed and absorption over the range from 500 to 750 nm increases. The former is associated with the fraction of light scattered and the latter represents the fraction of light absorbed by the leaf surface; these were calculated as follows:
where a and b are optical constants calculated using diffused transmittance (t) and reflectance (r) of the leaf.
The symbol (Δ) is defined as Δ2 = (1 + r + t) (1 + r − t) (1 − r + t) (1 − r − t). These calculated optical parameters for the leaves are based on the application of the Kubelka–Munk theory (Cordón and Lagorio 2007).
Data analysis
All the graphs were plotted using Sigma Plot 11.0 (Systat Software, Inc, Richmond, CA, USA) and correlation coefficients were done using Microsoft Excel. t-test was performed to study the level of significance between watered and water-stressed samples at each time point for all physiological and vegetative indices studies (Figs. 1, 2 and 4). The level of significance are shown as *P < 0.05, **P < 0.001, and ***P < 0.0001. All data are shown as means ± standard deviation (SD) from measurements made on leaves of five different plants. A principal component analysis (PCA) was performed based on the correlation matrix, to identify correlations among the measured parameters (PAST version 3.0).
Fig. 1.
The gas exchange parameters of net photosynthesis rate, PN (a), stomatal conductance, gs (b), transpiration, E (c), and water use efficiency, WUE (d) in watered (Control), 4th day and 8th day of water stress in guar plants. Data represent the means ± SD of five separate measurements. Statistical significance is indicated as (**P < 0.001) and (***P < 0.0001). Values above the bars in (a), indicate the mean RWC
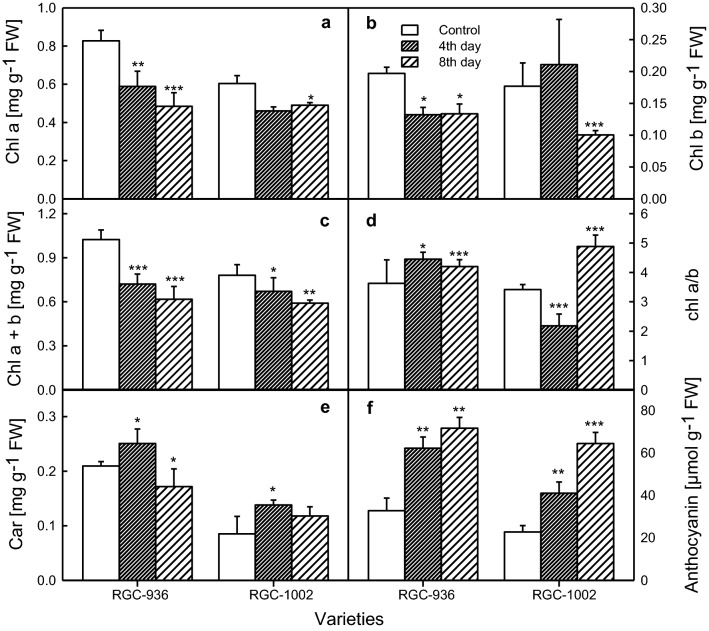
Fig. 2.
Changes in the pigment contents between the guar varieties in response to watered (Control), 4th day and 8th day of water stress. Chlorophyll a, Chl a (a), chlorophyll b, Chl b (b), total chlorophyll a + b, Chl a + b (c), ratio of Chl a/Chl b (d), carotenoid, Car, (e), and anthocyanin (f). Data represent the means ± SD from leaves of five independent plants. Statistical significance is indicated as (*P < 0.05), (**P < 0.001) and (***P < 0.0001)
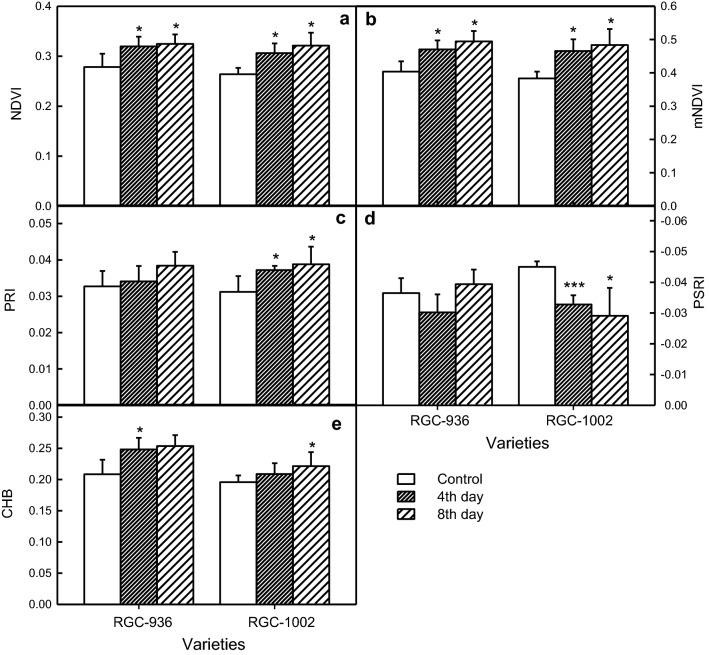
Fig. 4.
Variations in leaf reflectance indices in water-stressed and watered (control) plants, were calculated from the derived spectral reflectance curves. Normalized difference vegetative index, NDVI (a), modified normalized difference vegetative index, mNDVI (b), Photosynthetic reflectance index, PRI (c), plant senescence reflectance index, PSRI (d), and cold hard band, CHB (e). Data represent the means ± SD of five separate measurements. Statistical significance is indicated as (*P < 0.05), (**P < 0.001) and (***P < 0.0001)
Results
Both the guar varieties were monitored periodically for their physiological performance by studying the relative water content, gas exchange parameters, pigment quantity and reflectance studies under drought and watered conditions.
In watered plants, RWC was recorded to be approximately 94% in both the varieties and in water deficit conditions, relative water content decreased gradually and it reached up to 59.7% and 65.4% in RGC-936 and RGC-1002, respectively, on the 8th day of drought (RWC values are mentioned above the bar as a percentage in Fig. 1a).
Effect of water treatments on gas exchange
In water deficit conditions, the net photosynthetic rate decreased significantly by 5.3- and 2.3-fold on 8th day of desiccation in RGC-936 and RGC-1002, respectively (Fig. 1a). Along with PN, gs and E also decreased. Stomatal conductance decreased significantly by 14.9- and 2.2-fold while, E decreased significantly by 13.3- and 4.2-fold in RGC-936 and RGC-1002, respectively, on the last day of drought (Fig. 1b, c). Water use efficiency (WUE) which is an important determinant of plant drought resistance and was calculated as the ratio of PN and E increased significantly as desiccation stress increased (Fig. 1d).
Chl a content showed a significant decrease in both the varieties as water stress increased. RGC-936 showed a decrement of 41.45% and RGC-1002 with a decrement of 18.82% (Fig. 2a). Along with the decrease in chl (Fig. 2a–c), the chl a/b ratio showed significant changes under water stress (Fig. 2d). The carotenoid content decreased in RGC-936 with 18% and increased with 39% in RGC-1002 on the 8th day of desiccation, while it increased significantly on the 4th day of drought in both the varieties (Fig. 2e). As the days of desiccation proceeds, anthocyanin content increased by 118.5% and 183.3% in RGC-936 and RGC-1002, respectively (Fig. 2f).
Decrease in biomass accumulation under drought
At the end of drought treatments, both the varieties showed a decrement in dry biomass. The root, stem and leaves dry biomass was comparatively higher in RGC-1002 before and after subjecting to water treatments in comparison to RGC-936. On the 8th day of drought, the decrease in root biomass accumulation was by 10% and 12%, in stem biomass by 20% and 25% and in leaves biomass by 9% and 14%, in RGC-1002 and RGC-936, respectively, as compared to zero-day watered plants (Supplementary Fig. 1).
Leaf optical properties
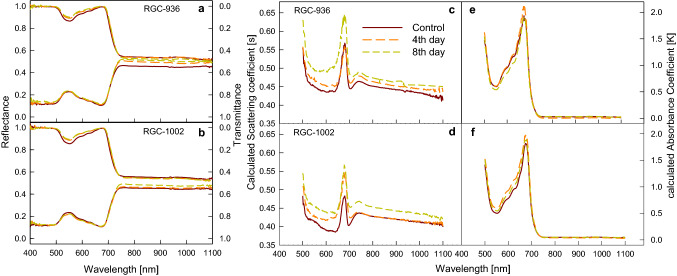
The guar leaf showed major changes in visible and near-infrared regions of the spectra on different days of desiccation in both the varieties. The reflectance and scattering coefficient spectra increased while transmittance spectra decreased in the visible range between 500 and 750 nm of spectra under water stress (Fig. 3a–d). The absorption coefficient becomes narrower and decreased in RGC-936 (Fig. 3e) and increased in RGC-1002 with increasing days of desiccation in the visible spectrum (Fig. 3f).
Fig. 3.
Variations in reflectance and transmittance measured using spectroradiometer, due to water stress of the adaxial surface in leaves of contrasting guar varieties, RGC-936 (a) and RGC-1002 (b). The Changes in Scattering coefficient, s (c, d), and variation in absorption coefficient, k (e, f), between the guar varieties under water stress were calculated by using the reflectance data between 500 and 750 nm. Each trace is the average of five independent leaves
Both the varieties studied showed an increase in NDVI and mNDVI under drought but RGC-1002 showed higher value of increment by 21.67% and 26.21%, respectively, in both the indices (Fig. 4a, b). The PRI showed a significant increase of 24.36% in RGC-1002 and 17.43% in RGC-936 under drought (Fig. 4c). PSRI showed a non-significant decrement in RGC-936 than RGC-1002, which RGC-1002 showed a significant increment by 35%, under drought condition due to chlorophyll degradation and drought-induced senescence (Fig. 4d). The Plant Senescence Reflectance Index (PSRI) is designed to maximize the sensitivity of the index to the ratio of bulk carotenoids to chlorophyll. CHB increased as days of drought progressed and recorded by 21.73% and 13.06% in RGC-936 and RGC-1002, respectively (Fig. 4e).
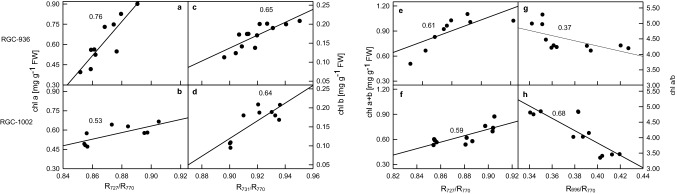
In our study, the physiological status of the plant was predicted by reflectance studies at 696, 727, 731 and 770 nm, which represent the pigmentation of the leaf. There was a strong correlation between chlorophyll content with reflectance at different wavelengths. Chl a showed positive correlation with reflectance measurement (R727/R770) in both the varieties with r2 of 0.76 and 0.53 in RGC-936 and RGC-1002, respectively (Fig. 5a, b). Similarly, chl b also showed a positive correlation with the reflectance measurement (R731/R770) in both the varieties with a coefficient of 0.65 and 0.64 in RGC-936 and RGC-1002, respectively (Fig. 5c, d). Correlation between the chlorophyll content (Chl a + b) and R727/R770 showed r2 of 0.61 and 0.59 (Fig. 5e, f) while, correlation coefficient between R696/R770 and chl a/b ratio was 0.37 and 0.68 in RGC-936 and RGC-1002, respectively (Fig. 5g, h).
Fig. 5.
Correlation between chlorophyll content and leaf reflectance. Correlation between Chl a and R727/R770 (a, b), Chl b and R731/R770 (c, d), Chl a + b and R727/R770 (e, f), and the ratio of Chl a and b with R696/R770 (g, h). Data represent the means ± SD of five separate measurements in leaves of guar varieties RGC-936 (a, c, e, g) and RGC-1002 (b, d, f, h) in watered, 4th and 8th days of water-stressed plants
The relationship between reflectance indices and photochemical reflectance index (PRI) along with the correlation coefficients (r2) of both the varieties was also calculated (Fig. 6). RGC-1002 showed higher value of correlation of 0.84 with R696/R770 (chl a/b), r2 of 0.78 with R727/R770 chl (a + b), coefficient of 0.81 with R731/R770 chl b and 0.78 with R727/R770 (chl a).
Fig. 6.
Correlation between photochemical reflectance index (PRI) and leaf reflectance in leaves of guar plants in watered, 4th and 8th days of drought. Correlation between PRI with R727/R770 (a, b), R731/R770 (c, d) and R696/R770 (e, f). Data represent the means ± SD of five separate measurements in guar variety RGC-936 (a, c, e), and RGC-1002 (b, d, f) plants in watered, 4th and 8th days of water stress conditions
Discussion
Correlation between water status with gas exchange, pigment content, and growth
Leaf spectral alterations, gas exchange parameters and pigment changes in guar leaves were studied under drought. In water stress condition, the reduction in photosynthesis is mainly due to decrement in RWC, stomatal conductance, and degradation of chlorophyll (Chl). This decrease in photosynthesis is maintained by photosynthetic pigments which eventually ensure the proper functioning of the two photosystems of photosynthetic cells (Singh et al. 2014; Ranjan et al. 2014). These pigments degrade during water stress but also help plants to withstand the consequences of drought (Singh et al. 2014).
From the studied varieties, the variety RGC-1002 showed minimum percentage decrease in RWC and anthocyanin; while, it also showed a minimum decrement in photosynthesis, stomatal conductance, transpiration, and chlorophyll pigments with the increase in severity of water stress. The decrease under drought in biomass accumulation of RGC-936 guar plants (Supplementary Fig. 1c) is coupled with the chlorophyll content (Fig. 2c), while comparatively higher increased chlorophyll content in RGC-1002 (Fig. 2c) is linked with higher biomass accumulation (Supplementary Fig. 1c) and drought tolerance. Our results support that accumulation of biomass is affected by chlorophyll concentration (Zhou et al. 2017; Liu et al. 2018).
An increase in carotenoid content during drought has been shown to scavenge ROS (reactive oxygen species) and also interfere in their generation (Edge et al. 1997). Similarly higher carotenoid content has been shown to better preserve the membrane integrity in tolerant cotton genotypes (Yildiz‐Aktas et al. 2009). These two roles of carotenoid support our observation (Fig. 2e) and also validate better tolerance of RGC-1002 towards drought.
Anthocyanin another class of plant pigments contributes to stress tolerance by protecting the photosynthetic apparatus from light stress by attenuation of high light energy (Ranjan et al. 2014; Moustaka et al. 2018). RGC-1002 showed significant increase in anthocyanin as compared to RGC-936 with increase in drought stress. This increment in anthocyanin in RGC-1002 may protect the leaves from high light stress. The enhancement in anthocyanin content under drought is also well studied in cotton leaves (Deeba et al. 2012; Singh et al. 2014).
Leaf spectral reflectance in water stress condition
Due to a change in water content of the leaves and pigmentation, there is an increase in leaf reflectance and a decrease in its light absorption (Fig. 3b, f). These reflectance properties are controlled by leaf chemical constituents such as chl a and b, carotenoids and anthocyanin due to their role in the light-harvesting step of photosynthesis (Ollinger 2011; Gitelson et al. 2019). Weber et al. (2012) found that the leaf reflectance was higher under water deficit conditions than well-watered conditions in the PAR region (400–700 nm). This increase in reflectance could be explained by the fact that as drought stress progresses there is a decrease in photosynthetic pigments or decrease in light absorption by chloroplasts in the PAR region (400–700 nm). Further analysis of the reflectance spectra was performed which included reflectance indices, scattering and absorbance coefficient (Figs. 3, 4). The increase in scattering coefficient in both the varieties (Fig. 3c, d), and the decrease in absorption coefficient in the variety RGC-936 (Fig. 3e), is due to the increase in the intercellular air spaces formed as a result of water loss from the cells and degradation of the chlorophyll (Merzlyak et al. 1999; Singh et al. 2014).
Vegetative indices in water stress conditions
The Red Edge Normalized Difference Vegetation Index (NDVI) gives an estimate of the photosynthetically absorbed radiation over the leaf surface and mNDVI is a modification of the Red Edge NDVI (Sims and Gamon 2002). Both NDVI and mNDVI increases as desiccation increased and the variety RGC-1002 showed a significantly higher increase in both indices, which demonstrate that there is maximum absorption of red light by the chlorophyll molecules in drought (Fig. 4a, b). NDVI is used for improved monitoring of vegetation and is sensitive to the fraction of absorbed photosynthetic active radiation. In dry season especially in arid and semiarid regions, there are spectral variations associated with geologic substrate materials, where lower NIR reflectance resulted in higher NDVI values (Heute et al. 1997). Studies have reported that PRI is strongly correlated with photosynthetic radiation-use efficiency in green leaves (Zhang et al. 2016). It is the measurement of photosynthetic efficiency of the leaves or the rate of carbon dioxide uptake by foliage per unit energy absorbed. PRI increases in both the varieties while it increases significantly in the variety RGC-1002 under drought (Fig. 4c). PRI also correlated strongly with the ratio of reflectance and pigments in leaves (Fig. 6). An increase in PSRI indicates increased carotenoid pigment and a decrease in chlorophyll content. The variety, RGC-1002 showed a significant increase in PSRI under drought condition due to less chlorophyll degradation and less decrease in carotenoid content and drought-induced senescence than in RGC-936. This relationship of PSRI with car/chl ratio has been, also studied in coleus, maple, and chestnut (Merzlyak et al. 1999).
Earlier, reflectance spectra have been used to predict the concentration of pigments in leaf tissues (Blackburn 1998; Sims and Gamon 2002; Mishra et al. 2017). The absorption spectra of different pigments are convoluted as they absorb light in the same regions. The ratio analyses of reflectance spectra were initially developed by Chappelle et al. (1992) in soybean leaves. The ratios of reflectance in selected narrow bands were found to accurately estimate or correlate well with the concentration of pigments (Peñuelas et al. 1995).
Correlation between chlorophyll content and leaf reflectance
A positive linear correlation between chl b and R731/R770 indicates that both chl b and R731/R770 decrease, and similarly chl a and chl a + b relate with R727/R770 under drought (Fig. 5) which indicates that both variables decrease with increase in days of drought. Under drought, chl b declines more remarkably than chl a, which increases chl a/b ratio (Fig. 2). This may be related to an increase in anthocyanin content (Fig. 2f) (Nicotra et al. 2003; Mishra et al. 2017). Chappelle et al. (1992) found a similar positive linear relation between ratio analyses of reflectance spectra and chlorophyll pigments. This linear relation is explained by the fact that the ratio of the reflectance spectrum responds to changes in chlorophyll concentrations. As drought progresses chlorophyll pigments decline and the internal structure of leaf disintegrates. Thus, leaf structural changes become a major controlling factor on the reflectance spectrum or red edge specifically (Blackburn 1998; Sims and Gamon 2002).
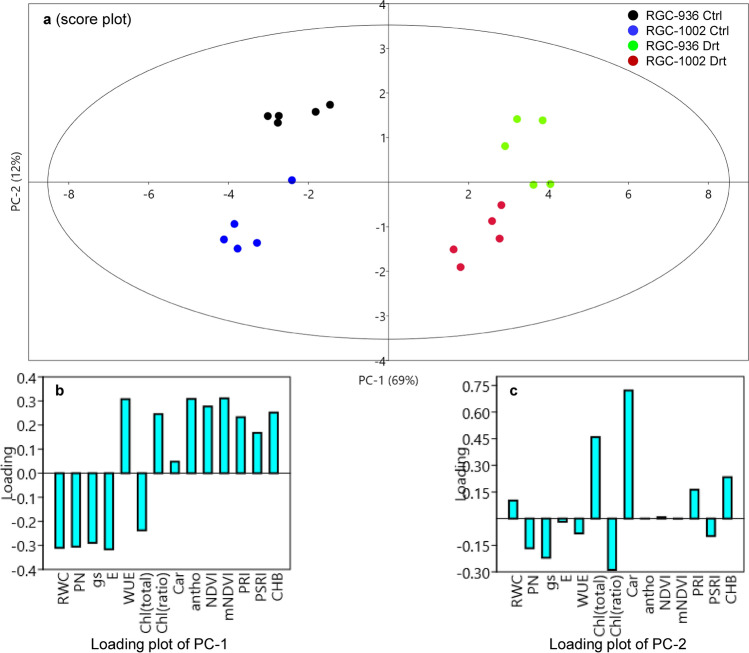
PCA analysis
The principal component analysis was carried out to analyze the effect of studied parameters between the guar varieties. It was observed that both the varieties fall into different quadrants (Fig. 7a). However, watered and water-stressed conditions of the varieties occupied two different groups which show that drought affects the parameters included. PCA results showed that the PCA axes (PC-1 and PC-2) define most of the variations (81%). The first PCA axis (PC1) defines 69% of the total variations and positively correlated with the responsive traits viz. WUE, Chl (ratio), Car, antho, NDVI, mNDVI, PRI, PSRI and CHB though, negatively correlated with RWC, PN, gs, E and Chl (total) (Fig. 7b). While PC-2 defines only 12% of the total variations and positively correlated with RWC, Chl (total), Car, PRI and CHB, and negatively correlated with PN, gs, E, WUE, Chl (ratio) and PSRI (Fig. 7c).
Fig. 7.
Principal component analysis, PCA score plot of anthocyanin, Antho; carotenoid, Car; Relative water content, RWC; Net Photosynthesis, PN; stomatal conductance, gs; transpiration rate, E; water use efficiency, WUE; total chlorophyll, Chl (a + b); chlorophyll (a/b), chl (a/b); normalized difference vegetation index, NDVI; modified red edge normalized difference vegetation index, mNDVI; plant senescence reflectance index, PSRI; photochemical reflectance index, PRI; cold hard band, CHB of both the guar varieties; RGC-936 and RGC-1002 in watered and 8th day of water stress condition (a) and loading plot of PC-1 (b) and loading plot of PC-2 (c)
The leaf spectral results of the present study indicate that the increase in reflectance and transmittance are responses of leaf optics to plant stress. These optical responses due to water stress are explained by the tendency of water-stressed leaves to lose chlorophyll and its absorption properties. Thus, reflectance and transmittance increase significantly and absorption may decrease in the far-red region (Carter and Knapp 2001).
Conclusion
Drought is multidimensional stress, affecting various physiological properties that subsequently influence leaf reflectance spectra. In the present study, the RGC-1002 guar variety exhibited greater photosynthesis, stomatal conductance rate, and a higher percentage of RWC in water stress as compared to RGC-936. It was also observed that chlorophyll pigment decrement is minimal and increment in the percentage of anthocyanin was significantly higher in RGC-1002 under water stress. The reflectance indices of Normalized difference vegetative index, NDVI, Photosynthetic reflectance index, PRI and, plant senescence reflectance index, PSRI, correlate well with the pigment concentration and help in explaining the physiological condition of varieties under water stress.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankful to The Director, CSIR-National Botanical Research Institute, Lucknow, for the facilities and for the financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, India, under the 12th Five Year Plan program (SIMPLE, BSC-0109). A Senior Research Fellowship provided to SN by University Grants Commission (UGC) is, gratefully acknowledged. This manuscript bears CSIR-NBRI Communication Number CSIRNBRI_MS/2020/12/11.
Abbreviations
- Antho
Anthocyanin
- CHB
Cold hard band
- gs
Stomatal conductance to H2O
- k
Absorbance coefficient represents the fraction of light absorbed
- mNDVI
Modified red edge normalized difference vegetation index
- NDVI
Normalized difference vegetation index
- PN
Net photosynthesis rate
- PRI
Photochemical reflectance index
- PSRI
Plant senescence reflectance index
- RWC
Relative water content
- s
Scattering coefficient associated with fraction of light scattered
- E
Transpiration
- VPD
Leaf-to-air vapor pressure deficit
- WUE
Water use efficiency
Author contributions
PU and SN performed the experiments, analyzed the data and drafted the manuscript; FK made a contribution to perform the experiments; LMT helped in critical revision and final approval of the manuscript; PAS formulated the original research plans, supervised the research and revised and finalized the manuscript. All authors have read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors wish to state that they do not have any conflict of interest.
References
- Aguirrezabal L, Bouchier-Combaud S, Radziejwoski A, Dauzat M, Cookson SJ, Granier C. Plasticity to soil water deficit in Arabidopsis thaliana: dissection of leaf development into underlying growth dynamic and cellular variables reveals invisible phenotypes. Plant Cell Environ. 2006;29:2216–2227. doi: 10.1111/j.1365-3040.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- Ahmed AKM, Jiang L, Wang F, Chen S, Zhou X, Pei X, Zhao X, Qu G. Variation analysis of growth traits of four poplar clones under different water and fertilizer management. J For Res. 2020;31:45–55. doi: 10.1007/s11676-019-00888-y. [DOI] [Google Scholar]
- Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- Beamish AL, Coops N, Chabrillat S, Heim B. A phenological approach to spectral differentiation of low-arctic tundra vegetation communities, north slope. Alaska Remote Sens. 2017;9:1200. doi: 10.3390/rs9111200. [DOI] [Google Scholar]
- Blackburn GA. Quantifying chlorophylls and carotenoids at leaf and canopy scales: an evaluation of some hyperspectral approaches. Remote Sens Environ. 1998;66:273–285. doi: 10.1016/S0034-4257(98)00059-5. [DOI] [Google Scholar]
- Carter GA, Knapp AK. Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll concentration. Am J Bot. 2001;88:677–684. doi: 10.2307/2657068. [DOI] [PubMed] [Google Scholar]
- Chappelle EW, Kim MS, McMurtrey JE., III Ratio analysis of reflectance spectra (RARS): an algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens Environ. 1992;39:239–247. doi: 10.1016/0034-4257(92)90089-3. [DOI] [Google Scholar]
- Cordón GB, Lagorio MG. Optical properties of the adaxial and abaxial faces of leaves. Chlorophyll fluorescence, absorption and scattering coefficients. Photoch Photobio Sci. 2007;6:873–882. doi: 10.1039/b617685b. [DOI] [PubMed] [Google Scholar]
- Croft H, Chen JM, Zhang Y. The applicability of empirical vegetation indices for determining leaf chlorophyll content over different leaf and canopy structures. Ecol Complex. 2014;17:119–130. doi: 10.1016/j.ecocom.2013.11.005. [DOI] [Google Scholar]
- Curran PJ, Dungan JL, Peterson DL. Estimating the foliar biochemical concentration of leaves with reflectance spectrometry: testing the Kokaly and Clark methodologies. Remote Sens Environ. 2001;76:349–359. doi: 10.1016/S0034-4257(01)00182-1. [DOI] [Google Scholar]
- Deeba F, Pandey AK, Ranjan S, Mishra A, Singh R, Sharma YK, Shirke PA, Pandey V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol Biochem. 2012;53:6–18. doi: 10.1016/j.plaphy.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Edge R, McGarvey DJ, Truscott TG. The carotenoids as anti-oxidants—a review. J Photoch Photobio B. 1997;41:189–200. doi: 10.1016/S1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Gamon JA, Peñuelas J, Field CB. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens Environ. 1992;41:35–44. doi: 10.1016/0034-4257(92)90059-S. [DOI] [Google Scholar]
- Garriga M, Retamales JB, Romero-Bravo S, Caligari PD, Lobos GA. Chlorophyll, anthocyanin, and gas exchange changes assessed by spectroradiometry in Fragaria chiloensis under salt stress. J Integr Plant Biol. 2014;56:505–515. doi: 10.1111/jipb.12193. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Ball MC. Protection and storage of chlorophyll in overwintering evergreens. Proc Nat Acad Sci. 2000;97:11098–11101. doi: 10.1073/pnas.150237697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelson A, Solovchenko A. Non-invasive quantification of foliar pigments: possibilities and limitations of reflectance-and absorbance-based approaches. J Photoch Photobio B. 2018;178:537–544. doi: 10.1016/j.jphotobiol.2017.11.023. [DOI] [PubMed] [Google Scholar]
- Gitelson A, Viña A, Solovchenko A, Arkebauer T, Inoue Y. Derivation of canopy light absorption coefficient from reflectance spectra. Remote Sens Environ. 2019;231:111276. doi: 10.1016/j.rse.2019.111276. [DOI] [Google Scholar]
- Heute AR, Liu HQ, Batchily K, Van Leeuwen W. A comparison of vegetation indices over a global set of TM images for EOS-MODIS. Remote Sens Environ. 1997;59:440–451. doi: 10.1016/S0034-4257(96)00112-5. [DOI] [Google Scholar]
- Inamullah IA. Adaptive responses of soybean and cotton to water stress II. Changes in CO2 assimilation rate, chlorophyll fluorescence and photochemical reflectance index in relation to leaf temperature. Plant Prod Sci. 2005;8:131–138. doi: 10.1626/pps.8.131. [DOI] [Google Scholar]
- Katsoulas N, Elvanidi A, Ferentinos KP, Kacira M, Bartzanas T, Kittas C. Crop reflectance monitoring as a tool for water stress detection in greenhouses: a review. Biosyst Eng. 2016;151:374–398. doi: 10.1016/j.biosystemseng.2016.10.003. [DOI] [Google Scholar]
- Liu X, Li L, Li M, Su L, Lian S, Zhang B, Li X, Ge K, Li L. AhGLK1 affects chlorophyll biosynthesis and photosynthesis in peanut leaves during recovery from drought. Sci Rep. 2018;8:2250. doi: 10.1038/s41598-018-20542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobos GA, Escobar-Opazo A, Estrada F, Romero-Bravo S, Garriga M, Del Pozo A, Poblete-Echeverría C, Gonzalez-Talice J, González-Martinez L, Caligari P. Spectral reflectance modeling by wavelength selection: studying the scope for blueberry physiological breeding under contrasting water supply and heat conditions. Remote Sens. 2019;11:329. doi: 10.3390/rs11030329. [DOI] [Google Scholar]
- Lovelock CE, Robinson SA. Surface reflectance properties of Antarctic moss and their relationship to plant species, pigment composition and photosynthetic function. Plant Cell Environ. 2002;25:1239–1250. doi: 10.1046/j.1365-3040.2002.00916.x. [DOI] [Google Scholar]
- Ma S, Zhou Y, Gowda PH, Dong J, Zhang G, Kakani VG, Wagle P, Chen L, Flynn KC, Jiang W. Application of the water-related spectral reflectance indices: a review. Ecol Indic. 2019;98:68–79. doi: 10.1016/j.ecolind.2018.10.049. [DOI] [Google Scholar]
- Meftahizadeh H, Ghorbanpour M, Asareh MH. Changes in phenological attributes, yield and phytochemical compositions of guar (Cyamopsis tetragonoloba L.) landaraces under various irrigation regimes and planting dates. Sci Hortic. 2019;256:108577. doi: 10.1016/j.scienta.2019.108577. [DOI] [Google Scholar]
- Merzlyak MN, Gitelson AA, Chivkunova OB, Rakitin VY. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol Plantarum. 1999;106:135–141. doi: 10.1034/j.1399-3054.1999.106119.x. [DOI] [Google Scholar]
- Middleton EM, Huemmrich KF, Landis DR, Black TA, Barr AG, McCaughey JH. Photosynthetic efficiency of northern forest ecosystems using a MODIS-derived Photochemical Reflectance Index (PRI) Remote Sens Environ. 2016;187:345–366. doi: 10.1016/j.rse.2016.10.021. [DOI] [Google Scholar]
- Mishra P, Asaari MSM, Herrero-Langreo A, Lohumi S, Diezma B, Scheunders P. Close range hyperspectral imaging of plants: a review. Biosyst Eng. 2017;164:49–67. doi: 10.1016/j.biosystemseng.2017.09.009. [DOI] [Google Scholar]
- Moustaka J, Panteris E, Adamakis IDS, Tanou G, Giannakoula A, Eleftheriou EP, Moustakas M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ROS formation. Environ Exp Bot. 2018;154:44–55. doi: 10.1016/j.envexpbot.2018.01.006. [DOI] [Google Scholar]
- Murray JR, Hackett WP. Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L. Plant Physiol. 1991;97:343–351. doi: 10.1104/pp.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra AB, Hofmann M, Siebke K, Ball MC. Spatial patterning of pigmentation in evergreen leaves in response to freezing stress. Plant Cell Environ. 2003;26:1893–1904. doi: 10.1046/j.1365-3040.2003.01106.x. [DOI] [Google Scholar]
- Ollinger SV. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011;189:375–394. doi: 10.1111/j.1469-8137.2010.03536.x. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Struik PC, Yin X, Yang J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J Exp Bot. 2017;68:5191–5205. doi: 10.1093/jxb/erx314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñuelas J, Inoue Y. Reflectance indices indicative of changes in water and pigment contents of peanut and wheat leaves. Photosynthetica. 1999;36:355–360. doi: 10.1023/A:1007033503276. [DOI] [Google Scholar]
- Peñuelas J, Baret F, Filella I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica. 1995;31:221–230. [Google Scholar]
- Ranjan S, Singh R, Singh M, Pathre UV, Shirke PA. Characterizing photoinhibition and photosynthesis in juvenile-red versus mature-green leaves of Jatropha curcas L. Plant Physiol Biochem. 2014;79:48–59. doi: 10.1016/j.plaphy.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Silva-Perez V, Molero G, Serbin SP, Condon AG, Reynolds MP, Furbank RT, Evans JR. Hyperspectral reflectance as a tool to measure biochemical and physiological traits in wheat. J Exp Bot. 2017;69:483–496. doi: 10.1093/jxb/erx421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DA, Gamon JA. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens Environ. 2002;81:337–354. doi: 10.1016/S0034-4257(02)00010-X. [DOI] [Google Scholar]
- Singh R, Naskar J, Pathre UV, Shirke PA. Reflectance and cyclic electron flow as an indicator of drought stress in cotton (Gossypium hirsutum) Photochem Photobiol. 2014;90:544–551. doi: 10.1111/php.12213. [DOI] [PubMed] [Google Scholar]
- Tan C, Samanta A, Jin X, Tong L, Ma C, Guo W, Knyazikhin Y, Myneni RB. Using hyperspectral vegetation indices to estimate the fraction of photosynthetically active radiation absorbed by corn canopies. Int J Remote Sens. 2013;34:8789–8802. doi: 10.1080/01431161.2013.853143. [DOI] [Google Scholar]
- Tapia G, Méndez J, Inostroza L. Different combinations of morpho-physiological traits are responsible for tolerance to drought in wild tomatoes Solanum chilense and Solanum peruvianum. Plant Biol. 2016;18:406–416. doi: 10.1111/plb.12409. [DOI] [PubMed] [Google Scholar]
- Thapa S, Adams CB, Trostle C. Root nodulation in guar: effects of soils, Rhizobium inoculants, and guar varieties in a controlled environment. Ind Crop Prod. 2018;120:198–202. doi: 10.1016/j.indcrop.2018.04.060. [DOI] [Google Scholar]
- Verma S, Gill KS, Pruthi V, Dhugga KS, Randhawa GS. A novel combination of plant growth regulators for in vitro regeneration of complete plantlets of guar [Cyamopsis tetragonoloba (L.) Taub.] Indian J Exp Biol. 2013;51:1120–1124. [PubMed] [Google Scholar]
- Weber VS, Araus JL, Cairns JE, Sanchez C, Melchinger AE, Orsini E. Prediction of grain yield using reflectance spectra of canopy and leaves in maize plants grown under different water regimes. Field Crop Res. 2012;128:82–90. doi: 10.1016/j.fcr.2011.12.016. [DOI] [Google Scholar]
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Wong CY, Gamon JA. Three causes of variation in the photochemical reflectance index (PRI) in evergreen conifers. New Phytol. 2015;206:187–195. doi: 10.1111/nph.13159. [DOI] [PubMed] [Google Scholar]
- Yildiz-Aktas L, Dagnon S, Gurel A, Gesheva E, Edreva A. Drought tolerance in cotton: involvement of non-enzymatic ROS-scavenging compounds. J Agron Crop Sci. 2009;195:247–253. doi: 10.1111/j.1439-037X.2009.00366.x. [DOI] [Google Scholar]
- Zhang C, Filella I, Garbulsky MF, Peñuelas J. Affecting factors and recent improvements of the photochemical reflectance index (PRI) for remotely sensing foliar, canopy and ecosystemic radiation-use efficiencies. Remote Sens. 2016;8:677. doi: 10.3390/rs8090677. [DOI] [Google Scholar]
- Zhang YJ, Hou MY, Xue HY, Liu LT, Sun HC, Li CD, Dong XJ. Photochemical reflectance index and solar-induced fluorescence for assessing cotton photosynthesis under water-deficit stress. Biol Plant. 2018;62:817–825. doi: 10.1007/s10535-018-0821-4. [DOI] [Google Scholar]
- Zhou R, Yu X, Ottosen CO, Rosenqvist E, Zhao L, Wang Y, Yu W, Zhao T, Wu Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017;17:24. doi: 10.1186/s12870-017-0974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.