Abstract
Amplifex Diene reagent was employed to derivatize estradiol (E2) to enhance the analyte signal at low picogram concentrations. This derivatization enabled measurement of E2 (and other estrogens) in ESI+ mode, earlier retention times for analytes than other methods, avoidance of MS harmful ammonium fluoride in mobile phases, and an LLOQ below 1 pg/mL. The sample preparation workflow involved liquid–liquid extraction followed by Amplifex Diene derivatization for 10 min at ambient temperature. Samples were chromatographed using a standard C18 column and analyzed using a SCIEX 6500+ mass spectrometer. The assay calibrators were prepared in-house, traceable to certified reference materials, and ranged from 1.29 to 624 pg/mL. A method comparison to samples from the CDC HoSt program yielded a correlation coefficient of 0.9858 and bias of −1.37%. The LLOQ using certified reference material was 0.66 pg/mL. The intra-run precision was <9.00% for low- and high-level samples, whereas the inter-run precision was 15.2 and 5.43% for low- and high-level samples, respectively. No interference from other clinically relevant steroids was found. Amplifex Diene derivatized E2 and estrone (E1) was found to be stable for over 6 months, both refrigerated and frozen.
Introduction
Clinical applications of estrogen determination challenge the analytical sensitivity of the instrumentation. As clinical significance exists in the low picogram range, Amplifex Diene (SCIEX, Framingham, MA), a reagent commonly used for 1,25-dihydroxy vitamin D analysis, was employed to combat the sensitivity challenges observed when measuring samples from patients with low estradiol levels. Due to its ability to react with phenolic groups, the Amplifex Diene reagent is able to derivatize estrogens and other chemically similar structures. Amplifex Diene provides a permanent positive charge via a quaternary nitrogen (Figure 1). Unlike other derivatization reagents, derivatization with Amplifex Diene occurs at room temperature and neutral pH, and only requires 10 min. Some notable advantages of using Amplifex Diene to measure estrogens include the ability to operate the mass spectrometer in positive mode, drastically shorter retention times compared to underivatized methods because of increased hydrophilicity and the use of mobile phases that are not harmful to the mass spectrometer (e.g., formic acid, water, acetonitrile).
Figure 1.
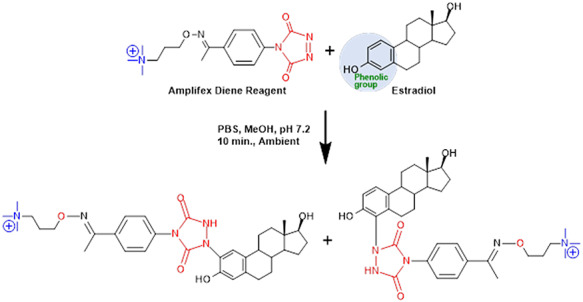
Amplifex Diene derivatization reaction with estradiol. The Amplifex Diene reagent reacts with the phenolic group of estradiol, and two potential derivatives are formed with permanent positive charges. The same reaction occurs with estrone which also contains the same phenolic group as estradiol.
Estradiol is produced in the ovaries and is the most potent estrogen. As a group, estrogens are lipid hormones responsible for developing and maintaining the female reproductive organs, the menstrual cycle and pregnancy, while also impacting female secondary sex characteristics. In the bloodstream, estradiol is generally bound to protein (1).
Premenopausal females typically have concentrations of estradiol ranging from 40 to 500 pg/mL (Supplementary Figure S1). Measuring samples from this population is not nearly as challenging as measuring estradiol concentrations in postmenopausal women, prepubertal children and males. Clinical conditions, such as precocious puberty, delayed puberty, osteoporosis, gynecomastia and ambiguous genitalia in newborns, all require sensitive instrumentation to accurately quantitate the low level of estradiol present in samples from patients with these conditions (2).
Benign prostatic hyperplasia (BPH) is a condition that occurs in aging males and generally manifests while androgen levels are in decline (3). Studies have demonstrated that estrogens may play a role in the pathophysiology of BPH (3,4). The present study measured the estradiol levels in 12 male subjects with BPH using the method presented here (Supplementary Figure S5).
Amplifex Diene can also be used to measure estrone alongside estradiol, creating a panel. Estrone contributes in both reproductive and postmenopausal women, but also in men (5). Estrone is structurally similar to estradiol. Both structures have a phenol available for Amplifex Diene interactions. The major structural difference is that estrone has one hydroxyl group and estradiol has two.
The challenges associated with measuring estradiol at low picogram concentrations have been well documented. A series of method validation studies, including linearity, precision, accuracy, specificity and limit of detection, were performed to demonstrate the robustness of the estradiol assay. We have included some data using estrone, however, the general focus was to demonstrate the performance of Amplifex Diene for estradiol quantification. We have leveraged existing chemistries to address the problems of clinical laboratories and researchers assaying estrogens and demonstrated the broad utility of the Amplifex Diene reagent. Here, we utilized Amplifex Diene to enhance the detection limits and increase the overall efficiency of clinical estrogen testing using LC–MS/MS.
Materials and Methods
Stock Materials
The 17β-estradiol, estrone and their 13C3 internal standards were purchased from Cerilliant (Round Rock, TX), whereas powder versions of 17β-estradiol and estrone were purchased from United States Pharmacopeia (USP, North Bethesda, MD) (Supplementary Table S1).
Sample Preparation
The following were added to a 2.0 mL plastic microcentrifuge tube (Eppendorf, Hamburg, Germany): 100 μL of 200 nM D-norgestrel (Millipore-Sigma, Burlington, MA) and 500 μL of sample. After a brief vortex, the tubes were incubated for 30 min at 25°C while rotating at 800 RPM. Liquid–liquid extraction was performed by adding 1000 μL of diisopropyl ether (Sigma-Aldrich, St. Louis, MO) to the mixture and a 10 μL aliquot of 13C3 internal standards (25 pg/μL). A quick vortex was performed before the contents in the tube were inverted for 30 min at 25°C. The microcentrifuge tubes were then centrifuged (Eppendorf Centrifuge 5430, Hamburg, Germany) for 5 min at 15,000 RCF. A total of 800 μL of supernatant was pipetted and placed in a glass tube. The organic layer in the glass tubes was dried down using a speed vacuum (Savant, Thermo Fisher Scientific, Waltham, MA). To ensure that all contents along the walls of the tubes were available for derivatization, 200 μL of methanol was added to each glass tube, and then the tubes were vortexed for 10 s, followed by drying.
Derivatization
Amplifex Diene was the derivatization agent used for the analysis. This was prepared with the provided diluent to yield a working concentration of 5 mg/mL. To a tube containing the dried sample, we added 25 μL of methanol, 15 μL of phosphate buffered saline (PBS, pH 7.2) and 20 μL of Amplifex Diene reagent. The tube was then vortexed for 10 s. After 10 min at 25°C, the samples were dried again for 10 min using the speed vacuum. The samples were then reconstituted with 60 μL of 50/50 methanol/water with 0.1% formic acid and vortexed for 10 s. A total of 60 μL was then transferred to a 1.5 mL plastic centrifuge vial and centrifuged for 5 min at 15,000 RCF. After centrifugation, 55 μL of supernatant was transferred to 300 μL plastic vials (Thermo Fisher Scientific, Waltham, MA) and capped with plastic caps (Agilent Technologies, Santa Clara, CA).
Instrumentation
Liquid chromatography was performed on an Agilent Technologies (Santa Clara, CA) Infinity 1290 series HPLC system. The mass spectrometry analysis was performed on a SCIEX (Framingham, MA) 6500+ QTRAP mass spectrometer. The mass spectrometer analysis method utilized a positive MRM scan (Supplementary Tables S2 and S3). A SCIEX 5500 QTRAP mass spectrometer and SCIEX 4500 mass spectrometer were also used for certain aspects of the study.
Liquid Chromatography Conditions
Mobile phase A consisted of water and 0.1% formic acid, and mobile phase B consisted of acetonitrile and 0.1% formic acid. The analytical column was an Imtakt (Portland, OR) Cadenza CD-C18 HT (100 × 2.0 mm, 3 μm). Refer to Supplementary Table S4 for the chromatographic flow program.
System Suitability Test
Prior to each analysis, system suitability test (SST) samples were analyzed to verify instrument performance. The SST solution was composed of each analyte and internal standard and was analyzed before a double blank sample containing no analyte and no internal standard. If the SST and double blank showed acceptable performance, the batch was analyzed. Acceptability was based on expected retention time, expected intensity and overall peak shape.
High concentration stock SST samples were created by pipetting 3 μL of 17β-estradiol, 17β-estradiol C13 and estrone into a glass tube and then dried. The dried sample was then derivatized with 25 μL of methanol, 15 μL of PBS and 20 μL of 5.0 mg/mL Amplifex diene. The contents of the glass tube were incubated for 10 min at 25°C and were then dried again. The contents were reconstituted with 60 μL of 50/50 methanol/water and 0.1% formic acid. A 1000-fold dilution was performed to create a working-level solution of 250 pg/mL for E1 and E2.
The SST bulk was stored at −40°C and transferred to a vial for injection onboard the autosampler at 10°C. Stability of the SST was monitored over a 6-month period using the 5500 QTRAP mass spectrometer as the detector. Over the 6-month period, four aliquots from the bulk material were transferred to HPLC vials and stored on-board the autosampler at 10°C. Data were collected over a 13-day period, 14-day period, 44-day period and 94-day period. Data for the entire 183-day study were also analyzed.
Certified Reference Material
Certified reference material for estradiol (E2) was purchased from the European Commission, Joint Research Centre (JRC). Samples BCR-576, BCR-577 and BCR-578 contained 31.05, 188.0 and 365.0 pg/mL of E2, respectively.
UTAK Calibrators and Controls
A set of calibrators and controls were custom ordered through UTAK (Valencia, CA). The UTAK calibrators and controls were of human serum origin and used as a secondary means of calibration and control. Prior to formulation, a human serum sample from UTAK was analyzed in our laboratory to verify that the starting material was free of analytes. The concentrations of the six individual calibrators were 5, 15, 25, 100, 300 and 600 pg/mL. The quality control concentrations were 10, 50 and 250 pg/mL.
Preparation of SCIEX Calibrators and Controls
A series of calibrators and controls were prepared in 5% BSA and subsequently assigned values by comparing them with the JRC reference materials. Values for both estrogens ranged from 2.34 to 600 pg/mL. The USP estrone and estradiol material was dissolved in N-methyl-2-pyrrolidone (NMP) to make a 700 μg/mL stock solution. This was further diluted to an intermediate stock solution with a concentration of 24 ng/mL in NMP. A syringe pump was then used to slowly deliver 2.5 mL of the 24 ng/mL intermediate stock to 47.5 mL of 5% BSA while mixing. This produced a high-level working formulation of the estrogens of 1,200 pg/mL, which was subsequently serially diluted to form working standards with concentrations that were assigned values by comparing them with the JRC reference material.
Samples for Studies
The samples used for the accuracy study were acquired from the Centers for Disease Control and Prevention (CDC, Atlanta, GA) hormone standardization program. Specifically, the accuracy study samples were phase 1 samples from the hormone standardization program for estradiol. Serum samples from child-bearing-aged females, postmenopausal females, males with BPH and hemolyzed and icteric samples for matrix effect studies were purchased from BioIVT (Westbury, NY) and analyzed by the present method.
Comparison of Instruments
A three-way comparison between the SCIEX 4500, 5500 and 6500+ series instruments was performed to demonstrate the sensitivity associated with each platform. SST samples were assayed in duplicate on each platform and the average values plotted.
Accuracy
A total of 41 reference samples were used to test the accuracy of the assay: 38 of the hormone standardization phase 1 samples from the CDC and 3 JRC reference serum samples assigned values using the SCIEX calibration curve. The CDC reference values were established by ID-LC-MS–MS (6). One replicate was processed per sample due to limited sample volume.
Precision
Small-scale precision studies were performed to evaluate the assay’s intra- and inter-run precision using low and high levels of spiked samples in 5% BSA. The target of the low sample’s concentration reflected a common clinical decision point. The intra-run precision was determined by assaying the low and high sample five times within a batch. The inter-run precision was determined by assaying the low and high samples five times over the course of 5 days. Samples were injected in an alternating pattern of low and high.
Linearity
The linearity study utilized four samples containing estradiol spiked into 5% BSA that were assigned values by the SCIEX calibrators that were originally assigned values from the JRC samples. The linearity samples had concentrations of 4.89, 13.99, 60.25 and 326.54 pg/mL and were run in triplicate to evaluate the linearity (particularly the low-end, clinical decision region) of the assay. Samples in this concentration range represent some of the most clinically relevant concentrations.
Lower Limit of Quantitation
The lower limit of quantitation (LLOQ) was determined by diluting reference material BCR-576 with 5% BSA to obtain five low-level samples: 6.21, 3.11, 1.55, 0.776 and 0.388 pg/mL. This was achieved by diluting BCR-576 by a factor of five, and then serially diluting by a factor of two. The subsequent low-level samples were then assayed five times on a SCIEX 6500+ QTRAP. The lowest mean value generated which achieved both a % CV and % bias value within ±20% was designated the LLOQ. In addition, the low-level samples were assayed on a SCIEX 5500 QTRAP and a SCIEX 4500 triple quadrupole for a proof of concept version of the LLOQ study.
Matrix Effect
Estradiol in hemolyzed and icteric human serum was measured to obtain a baseline value prior to evaluating the matrix effect. SCIEX control samples (5% BSA) were also measured to obtain baseline values. This serum was then combined in a 1:1 ratio with low- and high-level controls in 5% BSA. Expected estradiol results were calculated by adding the baseline low- and high-level control sample values with the baseline hemolyzed and icteric serum values. Constituents were mixed, processed, measured and then the expected results compared against the measured results. The percentage matrix effect at low- and high-levels was then calculated.
Mixing Study (Recovery)
Low- and high-level control samples in 5% BSA were mixed with other samples containing low and high doses of estradiol. The samples were mixed in a 1:1 ratio, resulting in four different combinations: low QC + low dose, low QC + high dose, high QC + low dose and high QC + high dose. Expected values were calculated by adding the individually measured QC and dose sample values together. The expected values were compared to the measured values and percent recovery calculated. Samples were processed in duplicate.
Interference
The assay was evaluated for interference from five steroids of clinical relevance: 17α-hydroxyprogesterone, androstenedione, cortisol, progesterone and testosterone (Cerilliant). Low and high levels of estrogen quality controls in 5% BSA were mixed 1:1 with 50 and 500 pg/mL interference samples, which were also prepared in 5% BSA. This provided four different interference scenarios: low-level sample + low-level interference, low-level sample + high-level interference, high-level sample + low-level interference and high-level sample + high-level interference.
Results
System Suitability Test
The performance of the SST samples was acceptable in terms of peak shape and separation from geometric isomers (Supplementary Figure S2). The 6500+ instrument yielded the greatest peak intensity for both estradiol and estrone, followed by the 5500 and 4500 instruments (Figures 2 and 3). The Amplifex Diene-derivatized SST was relatively stable over the 6-month period (Figures 4 and 5). The coefficient of variation for the 13-day period was 28% for estradiol and 16% for estrone, for the 14-day period 29% for estradiol and 20% for estrone, for the 44-day period 21% for estradiol and 23% for estrone and the 94-day period 28% for estradiol and 32% for estrone.
Figure 2.
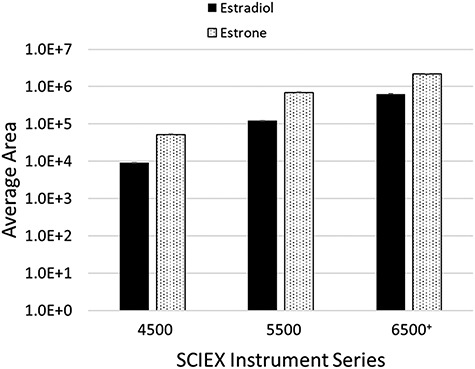
Average peak area obtained from SST samples run in duplicate across three different instrument platforms. Error bars represent 1 SEM.
Figure 3.
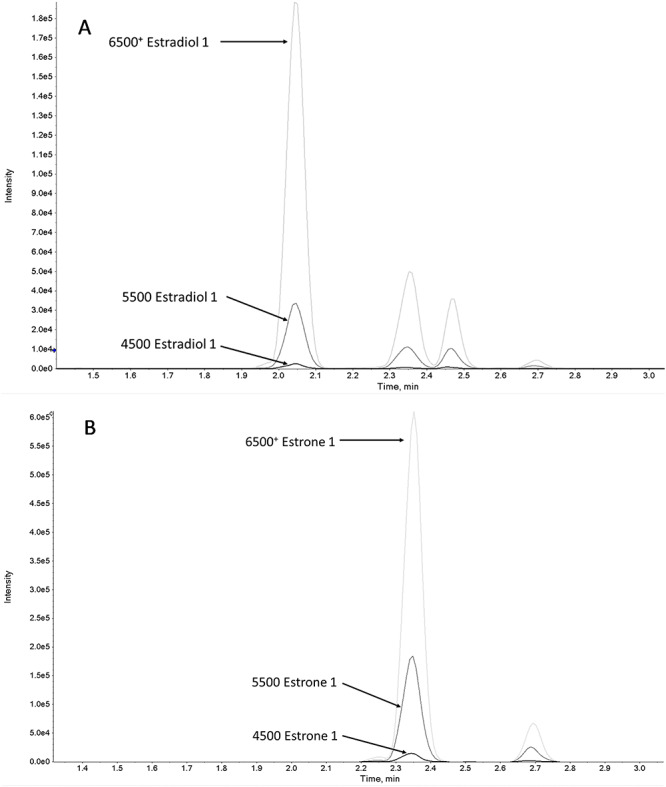
Overlays of estradiol (A) and estrone (B) from three different instrument platforms.
Figure 4.
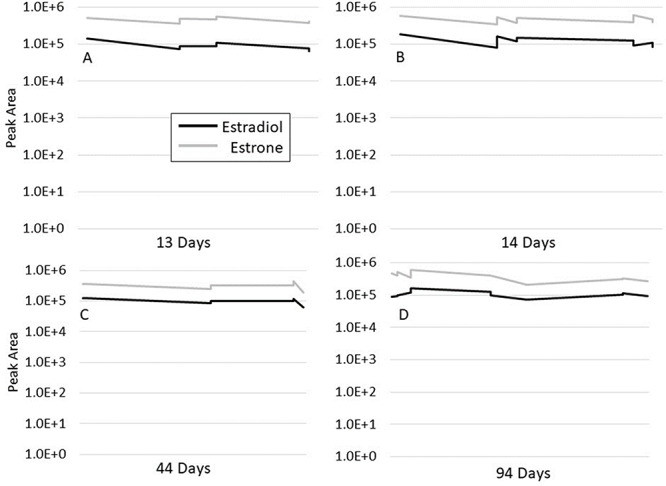
Amplifex Diene-derivatized SST peak area measured over 13 days (A), 14 days (B), 44 days (C) and 94 days (D). Long-term storage before being transferred to the autosampler was −40°C. The sampling temperature and autosampler temperature was 10°C.
Figure 5.
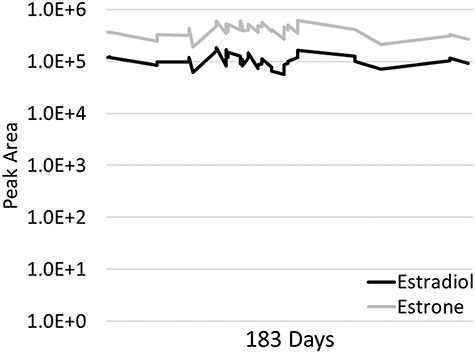
Amplifex Diene-derivatized SST peak area measured over 183 days. Storage of bulk material occurred at −40°C. The sampling temperature and autosampler temperature was 10°C.
Calibration
The JRC reference samples produced a linear calibration curve from 1.55 to 365 pg/mL. The r value was 0.9999 with an equation of y = 2.47x + 0.468. The UTAK curve was linear from 5 to 600 pg/mL and had an r value of 0.9969 and an equation of y = 1.06x + 0.035. The SCIEX value-assigned calibrators produced a linear calibration curve from 1.29 to 624 pg/mL, with an equation of y = 1.972x + 0.165 and an r value of 0.9997 (Supplementary Figure S3). The SCIEX curve was used for the studies performed in this paper except for the BPH sample study, in which the UTAK curve was used due to the presence of estrone calibrators.
Accuracy (Method Comparison)
The equation between the CDC and SCIEX methods was y = 0.9837x – 0.0623 with an r value of 0.9858 (Figure 6). The overall bias of the SCIEX assay compared to the CDC assay was determined to be −1.37% (Figure 7).
Figure 6.
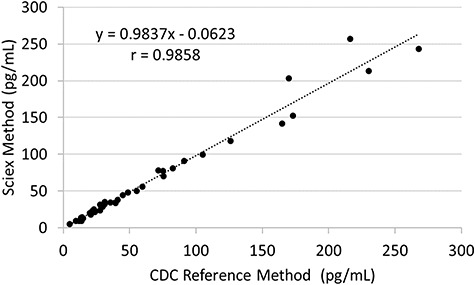
Estradiol method comparison between the CDC reference method and the SCIEX method.
Figure 7.
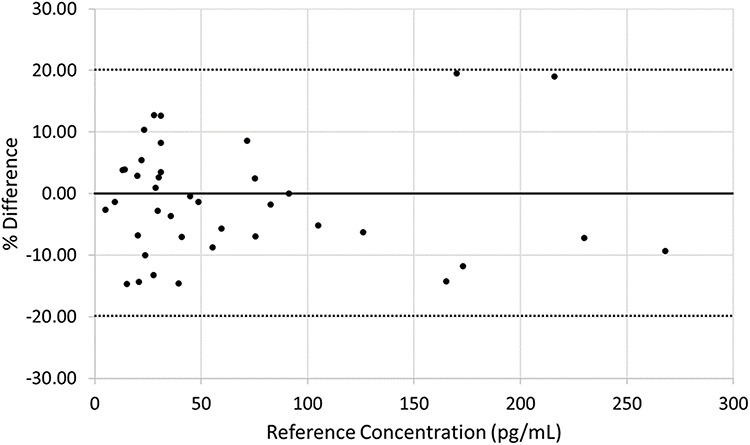
Bias plot for the estradiol accuracy study. The bold, solid line represents a percent difference of 0. The bold, dotted lines represent ±20% difference.
Precision
The intra-run precision yielded a mean value of 13.1 pg/mL with a %CV of 8.83 for the low-level sample and a mean value of 255 pg/mL with a %CV of 8.43 for the high-level sample (Supplementary Table S5).
The inter-run precision yielded a mean value of 12.6 pg/mL with a %CV of 15.2 for the low-level sample and a mean value of 781.8 pg/mL with a %CV of 5.43 for the high-level sample (Supplementary Table S6).
Linearity
The samples for linearity (which represented clinically relevant samples) produced a linear equation with a slope of 1, a y-intercept of 0.0017 and an r value of 0.9994 (Supplementary Figure S4). Values ranged from 4.89 to 327 pg/mL for the linearity study.
Interference
Assay performance was generally unaffected by low and high levels of 17α-hydroxyprogesterone, androstenedione, cortisol, progesterone and testosterone in low and high control samples. The low and high control samples were all recovered within 15% of their expected value when mixed with low and high concentrations of potentially interfering substances, with the exception of one high control sample that recovered −15.3% of the expected value when mixed with a high concentration of testosterone (Supplementary Table S7). There were no inherent estrogens found in the individual interference samples before mixing with the low- and high-level controls, except for a small amount of estradiol in the low- and high-level testosterone interference samples. This value was subtracted from the measured value of the test sample (control sample + interference sample).
Matrix Effect
A matrix effect of both hemolyzed and icteric serum was observed for samples toward the low end of the quantitation range. In hemolyzed samples spiked with a low-level calibrator, the matrix effect caused −23.5% recovery, whereas in icteric samples spiked with a low-level calibrator, the matrix effect caused −68.6% recovery. The matrix effect was not so prominent, however, in samples spiked with the high-level calibrator. In hemolyzed and icteric serum spiked with a high-level calibrator, the measured matrix effect was 2.19 and 3.31%, respectively (Figure 8).
Figure 8.
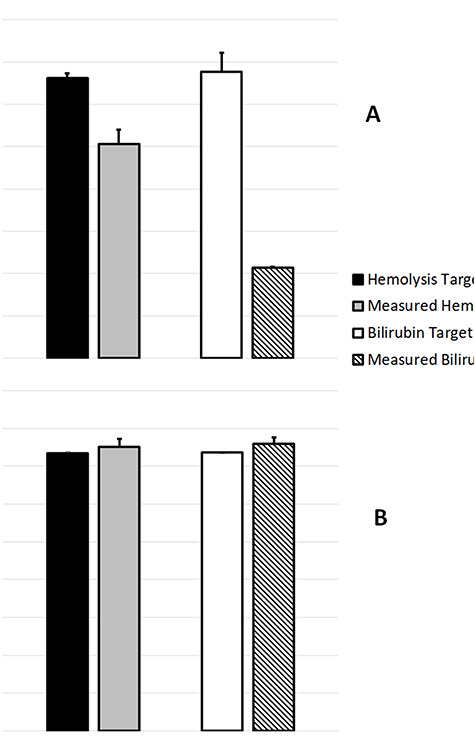
Target values versus measured values of estradiol in hemolyzed and icteric serum spiked with a (A) low-level and (B) high-level control serum.
Mixing Study (Recovery)
Estradiol was recovered at an acceptable level across all four different possible outcomes. Generally, as the estradiol concentration increased, the percent recovery also increased (Figure 9, Supplementary Table S8).
Figure 9.
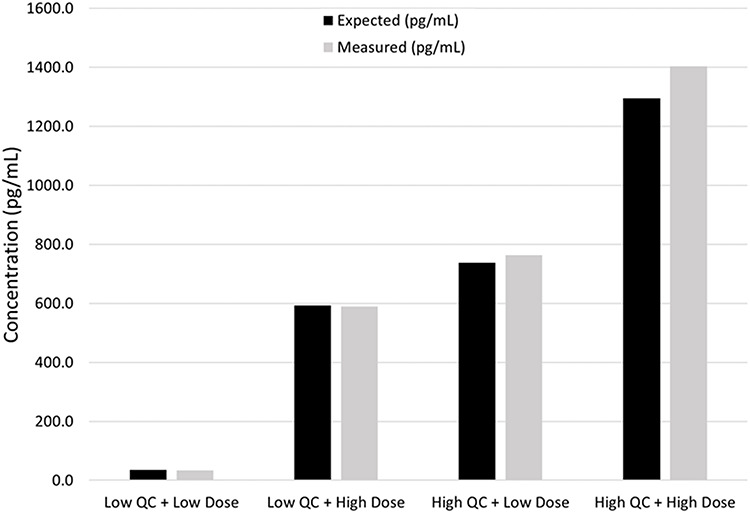
Comparison between expected and measured estradiol values for the mixing study.
LLOQ
The LLOQ on the SCIEX 6500+ QTRAP was determined to be 0.663 pg/mL. At this point, the coefficient of variation was 18.8% and the bias was −14.5% (Figure 10).
Figure 10.
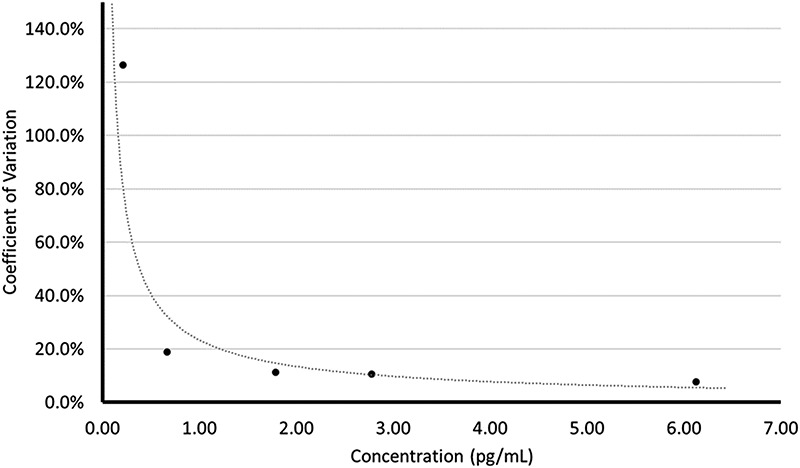
Lower limit of quantitation plot for estradiol using the SCIEX 6500+ QTRAP.
The low-level E2 samples run on a SCIEX 5500 QTRAP and a 4500 triple quadrupole yielded less sensitive results, as expected. The 6.21 pg/mL sample assayed on the SCIEX 5500 QTRAP was the only sample to yield a sufficient signal-to-noise ratio (≥20) for quantitation (Supplementary Table S9). All other samples assayed on either instrument did not chromatograph well and did not generate adequate signal.
Males with BPH
A variety of reproducible results were obtained with 12 serum samples from males with BPH. Quantitative results for estradiol and estrone were obtained using the UTAK calibration curve. A total of eight samples had estradiol concentrations <16 pg/mL, whereas only four male samples had estradiol concentrations ranging from 24.4 to 41.3 pg/mL. Estrone concentrations were slightly higher, ranging from 21.2 to 54.2 pg/mL, and generally, but not always, increased with increasing estradiol concentration (Supplementary Figure S5).
Discussion
Amplifex Diene reagent derivatization provides three advantages over other techniques: the mass spectrometer can be operated in positive mode, the retention time of the analyte is significantly reduced after derivatization and Q3-specific fragments are formed. A great amount of low-level estradiol research has utilized mass spectrometers operating in negative ion mode with ammonium fluoride and/or ammonium hydroxide as an additive in the mobile phase (7–9). Although these conditions allow for estradiol analysis without derivatization, the effects of ammonium fluoride (10) can be detrimental to critical equipment, and the highly basic mobile phase reduces the longevity of HPLC columns. The common dansyl-derivatized estradiol produces Q3 ions, which are dansyl group-specific and not analyte-specific (11). Guo et al. (12) reported an LLOQ of 1.0 pg/mL while using ESI-negative mode without derivatization and without harmful mobile phases, but the injection volume required to achieve this level of sensitivity was 1,200 μL.
As low-level estradiol testing is essential for monitoring and/or diagnosing certain medical conditions, low picogram quantitation is required. The SCIEX 6500+ QTRAP was capable of achieving accurate quantitation at 0.663 pg/mL. The SCIEX 5500 QTRAP was also able to achieve accurate quantitation at low pg/mL concentrations, but was ~10-fold less sensitive than the SCIEX 6500+ QTRAP. In studies using the UTAK calibrators (5% BSA), the SCIEX 5500 QTRAP provided sufficient signal for a 5 pg/mL calibrator. When assaying human serum reference material, the SCIEX 5500 QTRAP produced sufficient signal for the 6.21 pg/mL sample but struggled to produce a sufficient signal for the 3.11 pg/mL sample. The SCIEX 5500 QTRAP remains a good, economical option if quantitation of around 6 pg/mL is acceptable.
The sample preparation method was based on a method developed by NIST. Specifically, the use of D-norgestrel was incorporated to facilitate the release of estradiol from its binding protein (1). This competitive action increased the available free estradiol for measurement. Other methods to dissociate estradiol from the binding protein, such as using zinc sulfate and acetonitrile, did not increase the amount of free estradiol as much as 200 nM D-norgestrel did.
No significant interference was discovered for five common steroids of clinical interest. This was expected because of the derivatization chemistry that occurs with the Amplifex Diene regent. None of the five clinically important steroids tested here have a phenol or a diene in their structure. Furthermore, the retention times for estradiol and estrone were not interfered with and only a minor recovery difference was observed. The vitamin D vitamers are common, clinically relevant substances that do possess dienes in their chemical structure and will derivatize with Amplifex Diene. These vitamers, however, had a longer retention time than estradiol and estrone and did not interfere with estrogen testing (Supplementary Figure S6).
A limitation we discovered was that hemolysis and bilirubin caused notable matrix effects when measuring low-level concentrations of estradiol. At high levels, this matrix effect is overcome and not a threat to the quantitation. This is consistent with the study performed by Chen et al. (13), who found that samples containing low levels of estradiol are more susceptible to matrix effects than samples containing high levels of estradiol.
With regards to estrone, it has been shown to positively correlate with bone mineral density. Postmenopausal females generally lose bone mineral density and, therefore, may suffer from lower estrone levels (14). Although not as clinically relevant as estradiol, our method was able to detect low-level estrone values in postmenopausal females compared to child-bearing-aged females (Supplementary Figure S7). As with estradiol, the Amplifex Diene reagent provided Q3-specific fragments for derivatized estrone.
Conclusions
In conclusion, the Amplifex Diene regent provided a novel method for low-level estrogen testing. The reagent provided Q3-specific fragments, allowed for measurement in positive ion mode, displayed a retention time around 2 min, and did not react with other steroids. The accuracy study using CDC HoSt program estradiol samples showed that the present method is also highly accurate (bias = −1.37%). The sensitivities achieved using Amplifex Diene for estradiol quantitation on a SCIEX 6500+ QTRAP meet the demands of clinical laboratories, and equally as important, when using calibrators that are traceable to certified reference material, the results achieved here are accurate.
Supplementary Material
Acknowledgments
Jason Cournoyer.
Michal Weinstock.
Alicia Prater.
Contributor Information
Aaron Stella, SCIEX, 500 Old Connecticut Pathway, Framingham, MA 01701, USA.
Subhakar Dey, SCIEX, 500 Old Connecticut Pathway, Framingham, MA 01701, USA.
References
- 1. Tai, S.S.-C., Welch, M.J.; Development and evaluation of a reference measurement procedure for the determination of Estradiol-17B in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry; Analytical Chemistry, (2005); 6359–6363. [DOI] [PubMed] [Google Scholar]
- 2. Yi, X., Leung, E.K., Bridgman, R., Koo, S., Yeo, K.-T.J.; High Sensitivity micro LC-MS/MS assay for serum estradiol without derivatization; Journal of Applied Laboratory Medicine, (2016); 14–24. [DOI] [PubMed] [Google Scholar]
- 3. Ho, C.K., Nanda, J., Chapman, K.E., Habib, F.K.; Oestrogen and benign prostatic hyperplasia: Effects on stromal cell proliferation and local formation from androgen; Journal of Endocrinology, (2008); 483–491. [DOI] [PubMed] [Google Scholar]
- 4. Matsuda, T., Abe, H., Suda, K.; Relation between benign prostatic hyperplasia and obesity and Estrogen. Japanese; Japanese Journal of Clinical Pathology, (2004); 52(4): 291–294. [PubMed] [Google Scholar]
- 5. Watson, C.S., Jeng, Y.-J., Kochukov, M.Y.; Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation; The FASEB Journal, (2008); 3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thienpont, L.P.; Estradiol-17B quantified in serum by isotope dilution-gas chromatography-mass spectrometry: Reversed-phase C18 high-performance liquid chromatography compared with immune-affinity chromatography for sample Pretreatment; Clinical Chemistry, (1988); 2066–2069. [PubMed] [Google Scholar]
- 7. Fiers, T., Casetta, B., Bernaert, B., Vandersypt, E., Debock, M., Kaufman, J.-M.; Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization; Journal of Chromatography B, (2012); 893-4: 57–62. [DOI] [PubMed] [Google Scholar]
- 8. Pauwels, S., Antonio, L., Jans, I., Lintermans, A., Claessens, F., Decallonne, B. et al. ; Sensitive routine liquid chromatography-tandem mass spectrometry method for serum estradiol and estrone without derivatization; Analytical and Bioanalytical Chemistry, (2013); 405(26): 8569–8577. [DOI] [PubMed] [Google Scholar]
- 9. Wooding, K.M., Hankin, J.A., Johnson, C.A., Chosich, J.D., Baek, S.W., Bradford, A.P. et al. ; Measurement of estradiol, estrone, and testosterone in postmenopausal human serum by isotope dilution liquid chromatography tandem mass spectrometry without derivatization; Steroids, (2015); 89–94. doi: 10.1016/j.steroids.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pesek, J.J., Matyska, M.T.; Ammonium fluoride as a mobile phase additive in aqueous normal phase chromatography; Journal of Chromatography A, (2015); 1401: 69–74. [DOI] [PubMed] [Google Scholar]
- 11. Keski-Rahkonen, P., Desai, R., Jimenez, M., Harwood, D.T., Handelsman, D.J.; Measurement of estradiol in human serum by LC-MS/MS using a novel estrogen-specific derivatization reagent; Analytical Chemistry, (2015). doi: 10.1021/acs.analchem.5b01042. [DOI] [PubMed] [Google Scholar]
- 12. Guo, T., Gu, J., Soldin, O.P., Singh, R.J., Soldin, S.J.; Rapid measurement of estrogens and their metabolites in human serum by liquid chromatography-tandem mass spectrometry without derivatization; Clinical Biochemistry, (2008); 41(9): 736–741. doi: 10.1016/j.clinbiochem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen, Y., Kinney, L., Soldin, S.J.; Performance evaluation of Siemens ADVIA centaur enhanced estradiol assay and a split sample comparison with liquid chromatography-tandem mass spectrometry; Clinical Biochemistry, (2012); 45: 811–815. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki, N., Yano, T., Nakazawa, N., Yoshikawa, H., Taketani, Y.; A possible role of estrone produced in adipose tissues in modulating postmenopausal bone density; Maturitas, (1995); 9–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


