Abstract
We currently find ourselves in the midst of a global coronavirus disease 2019 (COVID-19) pandemic, caused by the highly infectious novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Here, we discuss aspects of SARS-CoV-2 biology and pathology and how these might interact with the circadian clock of the host. We further focus on the severe manifestation of the illness, leading to hospitalization in an intensive care unit. The most common severe complications of COVID-19 relate to clock-regulated human physiology. We speculate on how the pandemic might be used to gain insights on the circadian clock but, more importantly, on how knowledge of the circadian clock might be used to mitigate the disease expression and the clinical course of COVID-19.
Keywords: circadian clock, critical care, COVID-19, SARS-CoV-2, nutrition, zeitgeber, rhythm
Introduction
Coronavirus disease 2019 (COVID-19) is a new pandemic respiratory illness with a highly variable clinical presentation, ranging from asymptomatic infection to profound respiratory and multiorgan failure (Wang et al., 2020b; Zhou et al., 2020a). With over 100 million people infected and 2 million fatalities, COVID-19 ranks as one of the most lethal infectious diseases in the year 2020 (World Health Organization, 2020). Amid enormous worldwide effort to stem this disease much about the pathophysiology of COVID-19 and its causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; Zhu et al., 2020), remains to be clarified. Here and in the accompanying series of articles (Herz et al., 2021; Kronfeld-Schor et al., 2021; Sengupta et al., 2021), we discuss potential contributions of the field of chronobiology to the prevention, risk mitigation, prognosis, and rehabilitation of critically ill COVID-19 patients. This work is based on discussions at the European Biological Rhythms Society–convened workshop on Chronobiology and COVID-19 (Chronobiology of COVID-19 CARE Conference). Convened in July 2020, this virtual workshop brought together 250 researchers and clinicians worldwide. They engaged in a wide-ranging dialogue to consider how chronobiology and COVID-19 pathology might relate, what kinds of model systems are needed, and how clinical data might best be harnessed to explore such connections. Here, we provide a brief overview of COVID-19 with an emphasis on elements potentially sensitive to circadian regulation based on the existing pre-COVID literature. We conclude with a review of how critical illness and the intensive care setting influence circadian output, which is relevant to patients with severe COVID-19.
A Brief Overview of COVID-19
The causative agent of COVID-19, SARS-CoV-2, is a novel beta-coronavirus that bears genetic and clinical resemblance to two prior endemic coronaviruses of global concern: SARS, severe acute respiratory syndrome, which appeared in 2002 and MERS, Middle East respiratory syndrome, which appeared in 2012 (Drosten et al., 2003; de Groot et al., 2013; Haagmans et al., 2014; Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020; Kim et al., 2020; Zhu et al., 2020). While all three coronaviruses produce a similar picture of hypoxemic respiratory failure in humans with a 20%-70% fatality rate once mechanical ventilation becomes necessary (Yam et al., 2003; Alsolamy and Arabi, 2015; Auld et al., 2020; Richardson et al., 2020), only SARS-CoV-2 achieved pandemic spread. This may be related to a greater ability of SARS-CoV-2 to productively replicate in the nasopharynx without inciting symptoms of systemic illness that would otherwise cause infected individuals to self-isolate (Wölfel et al., 2020). Epidemiologic information about the scope of asymptomatic spread remains incomplete due to the lack of comprehensive virus testing in most countries and a variable rate of false-negative tests. Among symptomatic patients, roughly 81% experience mild disease, 14% experience moderate symptoms requiring hospitalization, and 5% require hospitalization plus advanced respiratory support in intensive care units (ICUs; Huang et al., 2020; Richardson et al., 2020; Yang et al., 2020). While prior chronic lung disease represents a potent risk factor for severe COVID-19, most COVID-19 risk factors are extrapulmonary in nature and include older age, obesity, hypertension, diabetes, and cardiovascular disease (Wang et al., 2020a; Zheng et al., 2020).
SARS-CoV-2 infection begins in the upper respiratory tract with differentiated airway epithelial cells being the initial sites of infection, particularly multiciliated cells and secretory (club) cells (Hui et al., 2020). Infection proceeds distally down the conducting airways to the alveolar space leading to the infection of type I and type II pneumocytes as well as alveolar macrophages (Grant et al., 2020; Hou et al., 2020; Hui et al., 2020). While SARS-CoV-2 is cytopathic in immortalized cells, it is the host inflammatory response that is thought to be primarily responsible for acute lung injury and breakdown of alveolar architecture known as the acute respiratory distress syndrome (ARDS; Thompson et al., 2017; Bryce et al., 2020; Schaller et al., 2020). The advent of ARDS frequently requires the initiation of mechanical ventilation to maintain life, but the elevated airway pressure this intervention imposes is itself injurious and represents a second inflammatory “hit” (Slutsky and Ranieri, 2013). If the host immune system succeeds in controlling the viral infection early, ARDS lung pathology can fully resolve (Doglioni et al., 2020). However, in some individuals, ARDS lung pathology proceeds to a fibroproliferative stage rendering lungs mechanically stiff, permanently impairing ventilation, and unsustainably increasing the work of breathing (Thompson et al., 2017). Autopsy studies show that respiratory failure is usually the proximate cause of death due SARS-CoV-2, although in critically ill patients, multisystem organ dysfunction frequently occurs as well (Bryce et al., 2020; Schaller et al., 2020; Wang et al., 2020b).
Two forms of systemic pathology bear special mention in severe COVID-19. The first is macrophage hyperactivation, leading to the elaboration of high levels of circulating pro-inflammatory cytokines such as interleukin 6 (IL-6; Moore and June, 2020). The term “cytokine storm” has been applied to COVID-19; however, it is unclear whether this condition is unique to or even representative of most patients with this disease (Lukan, 2020; Mudd et al., 2020). Emerging evidence suggests that mechanically ventilated COVID-19 patients may have suppressed type I interferon responses, suggesting a more complex picture than simple hypercytokinemia (Giamarellos-Bourboulis et al., 2020; Hadjadj et al., 2020; Mudd et al., 2020). The second form of systemic pathology is hypercoagulation, resulting in microthrombus formation and, somewhat counterintuitively, bleeding (Al-Samkari et al., 2020; Bryce et al., 2020). While sharing features with the more commonly known disseminated intravascular coagulation (DIC), COVID-induced hypercoagulability exhibits unique aspects including preserved levels of circulating fibrinogen and massively increased levels of von Willebrand factor from activated or damaged endothelial cells (Ward et al., 2020; Zhang et al., 2020). The clinical ramifications are that COVID-19 patients have a dramatically increased risk of stroke, myocardial infarction, pulmonary and deep venous thromboembolism, and major hemorrhage (Helms et al., 2020b; Castro and Frishman, 2021). The mechanism underlying thrombosis in COVID-19 remains to be clarified, although defects in platelet function were recently described in patients (Iba et al., 2020; Zaid et al., 2020).
The prognosis of COVID-19 largely depends on the severity of respiratory failure. Patients with mild to moderate disease appear to clinically recover within 2 weeks, although viral RNA can be detected in many patients for longer periods (Sun et al., 2020; Wölfel et al., 2020). At present, patients who progress to mechanical ventilation spend a median of 10-17 days intubated (Almeshari et al., 2020). The long-term recovery of severely ill COVID-19 patients has yet to be charted, although reports of protracted debility in the weeks following hospital discharge are common (Lambert and Corps, 2020; Halpin et al., 2021). In general, survival from critical illnesses is followed by protracted functional and cognitive impairment that persist even a year after hospital discharge (Schelling et al., 1998; Bein et al., 2018).
Brief Overview of SARS-CoV-2
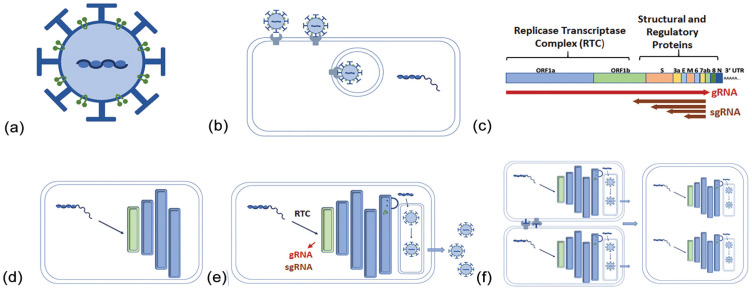
SARS-CoV-2 (Figure 1) is an enveloped single-stranded RNA virus that, typical of coronaviruses, is distinguished morphologically by its spike (S) protein which forms crown-like projections around the viral particles on electron micrographs (Masters and Perlman, 2013; Brahim Belhaouari et al., 2020). There are three additional structural proteins in the virion: the matrix (M) and envelope (E) proteins which reside in the membrane, and the nucleocapsid (N) protein that spools and protects the genomic RNA (Masters and Perlman, 2013; Kim et al., 2020). The S protein catalyzes viral entry and is thought to be the main determinant of tissue and species tropism (Dinnon et al., 2020; Ou et al., 2020). It is a type I transmembrane protein with a prominent extracellular region composed of two segments: an N-terminal (S1) segment that engages cell-surface angiotensin-converting enzyme 2 (ACE2), and a C-terminal S2 segment that promotes trimerization and contains a membrane fusion peptide (Cai et al., 2020; Ou et al., 2020). The bioactivity of the fusion peptide is promoted by cleavage of the S protein, which is catalyzed by extracellular proteases like TMPRSS2 or by lysosomal cathepsins (Hoffmann et al., 2020; Ou et al., 2020).
Figure 1.
Life cycle of the SARS-CoV-2 virus. (a) Spike ( ), envelope, and matrix (
), envelope, and matrix ( ) proteins are expressed on the surface of this single-stranded RNA virus. Nucleocapsid protein binds and protects the genomic RNA (gRNA) until (b) the virus enters the cells via spike protein interaction with cellular ACE2. (c) The gRNA of the positive-stranded RNA is transcribed and translated as a single ORF, yielding the RTC. Structural proteins are transcribed subsequently from the (subgenomic, sg) 5’ end of the gRNA. (d) The RTC integrates with the endoplasmic reticulum to form a double-membrane vesicle (DMV). (e) This structure produces virus which is released. (f) Local viral load leads to the formation of multinucleated giant cells through the binding of spike and ACE2 proteins on the surface of local cells. Abbreviations: SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; ORF = open reading frame; RTC = replicase-transcriptase complex; ACE2 = angiotensin-converting enzyme 2.
) proteins are expressed on the surface of this single-stranded RNA virus. Nucleocapsid protein binds and protects the genomic RNA (gRNA) until (b) the virus enters the cells via spike protein interaction with cellular ACE2. (c) The gRNA of the positive-stranded RNA is transcribed and translated as a single ORF, yielding the RTC. Structural proteins are transcribed subsequently from the (subgenomic, sg) 5’ end of the gRNA. (d) The RTC integrates with the endoplasmic reticulum to form a double-membrane vesicle (DMV). (e) This structure produces virus which is released. (f) Local viral load leads to the formation of multinucleated giant cells through the binding of spike and ACE2 proteins on the surface of local cells. Abbreviations: SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; ORF = open reading frame; RTC = replicase-transcriptase complex; ACE2 = angiotensin-converting enzyme 2.
The genome of SARS-CoV-2 consists of a single positive-strand RNA roughly 30 kb in length that is dominated by a single open reading frame (Orf1a/1b) taking up two thirds of its length (Kim et al., 2020). Orf1a/1b is translated off the genomic RNA as a single polypeptide that is autoproteolyzed into multiple subunits forming the “Replicase-Transcriptase Complex” (RTC; Kim et al., 2020). The RTC is believed to be responsible for all subsequent steps of viral replication (Masters and Perlman, 2013). The structural proteins that comprise the viral capsid are expressed later in infection as a series of subgenomic RNAs (sgRNAs) that have a common 3’ end and sport an identical leader sequence derived from the 5’ end of the viral genome (Masters and Perlman, 2013; Kim et al., 2020). The leader sequence allows for capping and translation of all viral transcripts (Masters and Perlman, 2013; Kim et al., 2020).
The next step in the SARS-CoV-2 replication involves the establishment of a specialized organelle in continuity with the endoplasmic reticulum (ER) called a double-membrane vesicle (DMV) structure (Klein et al., 2020). The DMV is thought to coordinate viral replication and to sequester viral factors away from innate immune sensors in the cytoplasm (Klein et al., 2020). Of note, DMV membranes bear ultrastructural resemblance to autophagosome membranes, and autophagy markers are upregulated in SARS-CoV-2–infected cells (Stukalov et al., 2020).
Viral RNA synthesis by coronaviruses generally involves the production of a complementary negative-strand RNA template starting from the 3’ end of the viral genome (Masters and Perlman, 2013; Kim et al., 2020). Full-length templates are used for synthesis of progeny + RNA viral genomes, while the shorter sgRNA templates drive the expression of the four viral structural proteins plus accessory proteins that may play a role in virus-host interactions (Masters and Perlman, 2013; Liu et al., 2014). Rather than engaging in RNA splicing, coronaviruses have a unique method of ensuring inclusion of the genomic 5’ leader sequence in each sgRNA, which is essential for translation. Directly upstream of the body of each structural gene is a conserved “Transcription Regulation Sequence” (TRS-B), which is identical to a motif in the 5’ leader sequence (TRS-L). As the RTC engages the negative-strand RNA synthesis, the polymerase can cross over from the TRS-B motif, located upstream of the coding sequence in each nascent sgRNA template, to the TRS-L sequence, thereby appending the 5’ end of the genome to each RNA. As a result, coronaviruses rely heavily on genetic recombination to complete its life cycle and have a proclivity for rapid viral evolution (Banner and Lai, 1991). Reflecting this, the dominant SARS-CoV-2 genotype appears to have changed with enhanced infectivity as the virus spread from China to Europe and beyond (Korber et al., 2020). Even in the same infected individual, SAR-CoV-2 genomes can vary based on the anatomic location of sampling within the respiratory system (Wölfel et al., 2020).
The final step in the SARS-CoV-2 life cycle is the assembly and release of virions. For coronaviruses, structural proteins of the viral envelope (the S, M, and E proteins) are cotranslationally inserted into the ER membrane and traffic to the ER-Golgi intermediate compartment (ERGIC; Masters and Perlman, 2013; Klein et al., 2020). There, nucleocapsids consisting of progeny genomes bound by N protein interact with the envelope proteins and bud into the ERGIC lumen. Virions are believed to reach the extracellular space by bulk transport, although transport through lysosomal compartments was recently suggested as an alternative (Ghosh et al., 2020). A fraction of S protein reaches the plasma membrane, causing cell fusion with neighboring infected cells and the formation of multinucleated giant cells that are characteristic of COVID-19 lung pathology (Masters and Perlman, 2013; Bryce et al., 2020).
To summarize, SARS-CoV-2 engages infected hosts at multiple levels during severe infection, inserting itself into the host’s physiological processes, immune responses, and fundamental cellular machineries. All these layers of biological organization are influenced by circadian rhythms, whose basic structure in mammals is summarized in the next section.
Relevant Features of Circadian Clock Organization
Circadian clocks impose a 24-h temporal structure on more processes than we are able to count (Zhang et al., 2014). In 1972, Arnold Eskin conceptualized circadian clocks as possessing an input pathway, an oscillator and output pathway (Eskin, 1979). We now know that this is an oversimplification of the system (Roenneberg and Merrow, 1998; Duguay and Cermakian, 2009; Ince et al., 2019; Reinke and Asher, 2019), but it remains a useful tool for conceptualization of circadian organization. The input pathway processes zeitgeber signals. Zeitgebers are the regular, predictable signals emanating from the environment that organisms and cells use to synchronize to time of day. For human behavior, light is the main zeitgeber, acting via specialized retinal cells that directly innervate the suprachiasmatic nuclei (SCN) of the hypothalamus (the pacemaker for human behavior). A well-known confounder of human synchronization is self-regulated and socially regulated exposure to light such that the natural photic environment is not faithfully represented to the brain. We do this by turning off the lights and closing our eyes when we sleep as well as by living indoors and therefore changing the amplitude of the light/dark cycle or, in extreme examples, by changing the timing of our light environment due to shift work. The consequences of “living at the wrong time” (shift work) are dire. Long-term shift workers have increased risks of metabolic syndrome, coronary heart disease, and certain types of cancers (Schernhammer et al., 2001, 2003, 2006; Vetter et al., 2016). The phenomenon called social jetlag refers to the generally smaller shift in timing due to the mismatch of social (e.g., school or work) and biological clocks (Roenneberg et al., 2012). Social jetlag is also characterized by behavioral and physiological deficits. It is remarkable that even relatively short bouts of desynchrony can result in metabolic disturbance. After an experimental protocol called forced desynchrony, whereby human subjects are forced into a situation where they cannot synchronize to the short or long zeitgeber cycle, individuals continue to show circa 24-h daily oscillations in physiology but prediabetic features become evident (Scheer et al., 2009). A common consequence of controlled phase shifts, as occurs in transmeridian travel or shift work, is decreased amplitude of the circadian clock (Dijk et al., 2012).
In addition to synchronization of the organism as a whole, each cell within us possesses a circadian clock. Indeed, the organismal clock is built on individual cellular circadian clocks. They respond to a cocktail of endogenous zeitgebers for their synchronization. Already in 2000, Basolobre et al. (2000b) showed that cyclic adenosine monophosphate (cAMP) analogs, dexamethasone, protein kinase C, and Ca2+ could synchronize and phase shift clock gene oscillations in Rat-1 fibroblasts. That this is relevant for human entrainment and clock-regulated gene expression was shown with administration of glucocorticoids once a day, in the afternoon, resulting in reentrainment of clock gene expression in peripheral blood mononuclear cells (PBMCs; Cuesta et al., 2015). Importantly, the timing of nutrient uptake has remarkable effects on the timing of clock gene expression in the liver. Experiments showed that the timing or phase of rhythmic gene expression in peripheral organs can resemble that of the SCN (entrained using light) or that of the liver (entrained using food) depending on the tissue and the relative strengths of the zeitgebers (Stokkan et al., 2001). Cellular clocks may be amplitude - attenuated by irregular zeitgeber timing (Cuesta et al., 2015), as has been observed at the organismal level, leading to a dysregulated circadian system. Obviously, entrainment or synchronization is a dominant feature of the circadian clock.
Concerning the oscillator mechanism, genetic data show transcription factor networks toggling between activation and repression to execute daily timing at the molecular level (Aryal et al., 2017). As with all other known transcription factors, the clock operates in large multimeric protein complexes. This formula creates many opportunities for fine tuning of daily timing. Changes in the timing of transcription, translation, and posttranslational modifications of any of the clock regulators, and changes in the temporal structure of zeitgebers could result in fundamental changes in characteristics of the oscillator and thus its synchronization properties.
The output pathways are like the hands of a clock, influencing clock-controlled regulation. Rhythmic gene expression—beyond the transcription factors thought to be at the center of the process—is generally tissue specific (Zhang et al., 2014). Historically, there have been reports of some tissues lacking clock-regulated gene expression, namely testis and thymus (Alvarez and Sehgal, 2005). More recently, the baboon transcriptome showed rhythms in these tissues (Mure et al., 2018). It may be that all tissues show daily temporal structures at the molecular level. By extrapolation, we suggest that all tissues will exhibit functional, tissue-specific circadian clock regulation.
Relevance of the Circadian Clock to SARS-CoV-2 Biology and Pathology
Is there any reason to connect rhythms to the disease process—from infection to pathology—associated with critically ill COVID-19 patients? One could look at this from a level of the biology of the virus as it leads to (different degrees of) pathology to how the circadian clock may contribute to containment or exacerbation of symptoms to considerations in the clinical critical care environment. Any of these aspects may lead to novel opportunities for interventions.
Concerning viral biology, are ACE2, TMPRSS2, and FURIN clock regulated within cells? We interrogated the database that describes gene expression in 64 tissues over 24 h in young, healthy, male baboons (Table 1; Mure et al., 2018). These genes are broadly expressed, with BACE2 (the baboon ACE2 ortholog) and FURIN giving a signal in all assayed tissues. TMPRSS2 is similarly broadly expressed except in the hippocampus, the putamen, and the retina. Rhythmicity of RNA expression corresponding to these genes, however, occurs seldomly. The gene for ACE2 is rhythmic only in abdominal muscle. TMPRSS2 RNA is rhythmic only in omental fat tissue. FURIN-encoding RNA was rhythmic in nine tissues (bladder, cornea, heart, gastrocnemius muscle, testicles, thyroid, and three areas of the brain). ACE2 gene expression measured in homogenized mouse or human lung samples did not exhibit rhythmic expression (Zhang et al., 2014; Ruben et al., 2018). However, the lung has a high degree of cellular diversity, consisting of more than 40 cell types (Franks et al., 2008; Montoro et al., 2018; Reyfman et al., 2019). It remains possible that ACE2 can be rhythmically expressed in specific cells types, in patients with inhaled exposures to irritants, or in the setting of chronic lung diseases.
Table 1.
Daily rhythms in expression of severe acute respiratory syndrome coronavirus 2–relevant genes in tissues isolated from baboons (Mure et al., 2018).
| Gene | Tissue |
|---|---|
| BACE2 (BABOON ACE2) | Abdominal muscle |
| TMPRSS2 | Omental fat |
| FURIN | Bladder |
| Cornea | |
| Heart | |
| Medial globus pallidus | |
| Gastrocnemius muscle | |
| Prefrontal cortex | |
| Testicles | |
| Thyroid | |
| Ventromedial hypothalamus |
Abbreviation: ACE2 = angiotensin-converting enzyme 2.
ACE2, angiotensin-converting enzyme 2, catalyzes the hydrolysis of angiotensin 2 into angiotensin 1-7, which has important anti-inflammatory properties (Kuba et al., 2013). As a result, the relationship between ACE2 levels and COVID-19 clinical severity is more complex than it appears at first glance. For example, ACE2 and angiotensin 1-7 levels tend to be reduced in older individuals, a group that it is nevertheless at high risk for clinically severe COVID (AlGhatrif et al., 2020; Miller et al., 2020). In this case, whatever benefit might be gleaned by lower ACE2 levels on viral entry is apparently outweighed by the magnification of host inflammatory responses that result from the reduced amounts of angiotensin 1-7 in older patients. While rhythmicity of ACE2 levels in the lung has not yet been shown, ACE2 shows a diurnal variation in the blood (Veglio et al., 1987). RAS has also been shown to influence the circadian clock by modulating the expression of core clock genes such as PER2 and BMAL1 (Nonaka et al., 2001).
Biological functions involved in viral processing within cells (lysosomes, endocytic trafficking, and various extracellular proteases) commonly exhibit circadian oscillations in activity (Mazzoccoli et al., 2015; Cuddapah et al., 2019; Chang et al., 2020). In general, a single rhythmic rate-limiting step in viral cell biology is sufficient to impose rhythmicity on the entire process. Therefore, there are a number of possibilities that could lead to rhythms in in vivo viral processing.
Concerning how the circadian clock may contribute to exacerbation of pathology in critically ill patients with COVID-19, several symptoms are particularly relevant. These patients may experience respiratory failure, microcoagulation events, and cytokine storm. The molecular mechanisms involved in each of these are regulated by the circadian clock. For instance, human respiratory function shows clock regulation in mechanics (airway resistance and flow characteristics), ventilatory response to hypercapnia, and airway responses to inhaled allergens (Shimoda and Semenza, 2011). Concerning hypoxemic respiratory failure that can occur in COVID-19, it is important to note that circadian clocks are sensitive to cellular oxygenation status via the action of hypoxia inducible factor 1 alpha (HIF1a; Adamovich et al., 2017; Peek et al., 2017). As such, COVID-19 respiratory failure and its treatment likely alter clock-regulated gene expression in the lung, similar to what has been described in other acute lung injuries (Jafri et al., 1992; Richardson Jones et al., 1996; Bremner et al., 2000; Numminen et al., 2000). Regarding the coagulation cascade, both clotting function and clotting factors show complex time-of-day differences. Several studies have demonstrated a diurnal rhythmicity in human platelet function (platelet counts and activation), endothelial function, and several coagulation and fibrinolytic parameters (activated factor VII, factor IX, beta-thromboglobulin, platelet factor 4, and fibrinogen; Rosing et al., 1970; Tofler et al., 1987; Akiyama et al., 1990; Willich et al., 1992, 1993; Etsuda et al., 1999; Ündar et al., 1999; Guagnano et al., 2000; Ringqvist et al., 2000; Gaenzer et al., 2001; Osmancik et al., 2004; Otto et al., 2004; Rudnicka et al., 2007). The circadian clock has been shown to modulate both intrinsic and extrinsic coagulation pathways, and the phase relations of the rhythms of different coagulation parameters may contribute to the known circadian variations in the frequency of arterial (e.g., myocardial infarction, sudden cardiac death, cerebral infarction; Willich et al., 1993; Montagnana et al., 2009) and venous (e.g., deep vein thrombosis and pulmonary embolism) thromboembolic events as well as hemorrhagic phenomena (intracerebral hemorrhage, rupture of aortic aneurysms; Maemura et al., 2006; Maas et al., 2020c).
With regard to the expression of cytokines, both the transcription of cytokine-encoding genes as well as stimulation of cytokine release show strong time-of-day regulation. Clock proteins are known to be activators of some cytokines and suppressors of others (Timmons et al., 2020). Clock regulation occurs in macrophages and monocytes (Figure 2) as well as for T cells, thus impacting the immune responses of these cells to pathogens (Scheiermann et al., 2018). It has been suggested that mechanically ventilated COVID-19 patients may have suppressed type I interferon responses (Hadjadj et al., 2020). It is possible that a misalignment here could influence the probability of the occurrence of a cytokine storm. This aspect is discussed in the companion paper on the clock and the immune system with respect to COVID-19.
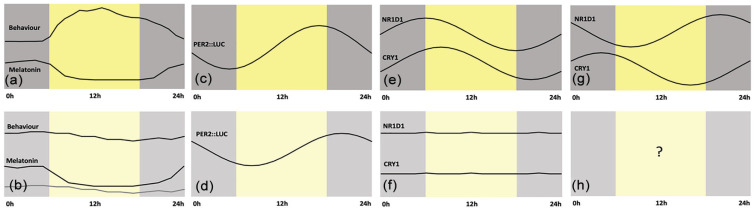
Figure 2.
Circadian rhythms in critical illness. (a) Daily behavior and melatonin profiles in healthy individuals. (b) In intensive care unit patients with neurological and systemic critical illness, behavioral rhythms are absent and melatonin rhythms show a variety of changes depending on severity (as characterized by the Glasgow Coma Scale) and medication (the use of pressors or not). Data from Maas et al. (2020c). (c) At the level of the lung, experiments in mice show expression of the PER2::LUC transgene (d) is delayed by approximately 4.5 h in hypoxic conditions. Data from Manella et al. (2020). (e) Peripheral blood mononuclear cells show rhythms of NR1D1 and CRY1 gene expression (f) which are absent in cells from critically ill patients. Data from Maas et al. (2020a). (g) These same genes, NR1D1 and CRY1, show an 8-h difference in phase of expression in monocytes obtained from healthy human subjects. Data from Wittenbrink et al. (2018). (h) We do not know what the status of molecular rhythms is in these cells in critically ill patients. Time is expressed as external time (ExT), a convention that runs for 24 h starting at midnight (Daan et al., 2002).
COVID-19 and Chronopharmacology
The development of therapies to mitigate COVID-19 is an intense area of focus. Current therapies available to critically ill patients include anti-inflammatory drugs like glucocorticoids and tocilizumab, direct antivirals like remdesivir, and SARS-CoV-2–neutralizing antibodies conferred by convalescent plasma. Whether time of day plays a role in the efficacy of these drugs in COVID-19 patients is unclear. However, the pharmacokinetics and pharmacodynamics of many drugs are known to change over the 24 h. Accordingly, so do the efficacy and tolerance to chemotherapy (Dallmann et al., 2016), and antibody response to vaccination (Long et al., 2016). A recent study has shown that approximately 50% of all currently used drugs target the product of a gene under circadian control (Anafi et al., 2017). Finally, the outcomes of certain types of surgery (e.g., aortic valve replacement; Montaigne et al., 2018) also vary across time of day. Time of day differences may reflect the time of maximum efficacy and success, or alternatively, the time of minimum adverse effects (Ballesta et al., 2017).
Recently, a prospective meta-analysis from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group pooled data from seven trials totaling 1703 patients and showed that administration of systemic corticosteroids is associated with a lower 28-day mortality in critically ill COVID-19 patients (Horby et al., 2020; Sterne et al., 2020). Commonly known for their potent anti-inflammatory properties, glucocorticoids also act to synchronize the cellular circadian clock in most cells. Interestingly, dexamethasone does not act on the SCN clock, only on clocks in nonpacemaker cells (Balsalobre et al., 2000a). Theoretically, glucocorticoid administration has the potential to synchronize peripheral clocks in patients, although there are no studies demonstrating glucocorticoids can do this in the setting of critical illness. Whether the salutary effects of glucocorticoids on COVID-19 can be ascribed to circadian regulation is unknown, but it represents a topic for future investigation. Another strong synchronizer of circadian rhythms, melatonin, came to the attention of COVID-19 researchers (Cardinali et al., 2020; Zhou et al., 2020b). In a large registry of patients undergoing testing for suspected COVID-19, routine use of melatonin was statistically associated with a negative COVID-19 quantitative polymerase chain reaction (qPCR) result, suggesting melatonin might suppress SARS-CoV-2 replication (Zhou et al., 2020b). Melatonin is currently under investigation as a potential SARS-CoV-2 antiviral therapy (Rodríguez-Rubio et al., 2020).
Implications of the Critical Care Environment and the Circadian Clock on COVID-19
The potential effect of the ICU environment on a patient’s circadian clock has been acknowledged for years. This may be especially relevant in critically ill COVID-19 patients who require a prolonged ICU stay with limited exposure to normal environmental zeitgebers. ICUs are well known to feature low, noncycling or weakly cycling light environments and excessive noise due to equipment and alarms, conditions which disturb normal sleep (Boyko et al., 2017). Furthermore, many patients are sedated due to various medical problems, interrupting behavioral control of light-dark cycles. In addition, feeding occurs intravenously and continuously, rather than by more physiological boluses. Various measures have confirmed that many ICU patients show either suppressed or inappropriately synchronized circadian rhythms. For example, Gazendem et al. (2013) showed that the phase of the rhythm in core body temperature was displaced by more hours in sicker patients, defined by a higher APACHE III score. A recent paper confirmed this general finding but used heart rate variability as a measure. Knauert et al. (2015) found that most patients (>80%) showed daily rhythms but that, already in the first 2 days in the ICU, the phase of the rhythms was misaligned relative to a control population. Based on these observations, we anticipate that many critically ill COVID-19 patients may have circadian rhythms, but the entrained phase (synchrony relative to the natural day) will not be normal and it will be difficult to predict with current information.
Certain severe illnesses may lead to disrupted circadian clock regulation independent of the environment. Patients with neurologic or systemic critical illness have been shown to rapidly enter a state of profound behavioral quiescence with the onset of illness, during which rest-activity rhythm showed severe temporal disorganization and dissociation from melatonin rhythms (Maas et al., 2020c). Dampening of melatonin amplitude has been associated with worsening encephalopathy, although improvement in encephalopathy was not associated with corresponding change in melatonin amplitude (Maas et al., 2020b). The altered oxygenation status in the lung can shift the phase of clock gene expression in the lung (Figure 2c and 2d; (Manella et al., 2020)). Furthermore, circadian gene expression becomes dampened in the ICU (Figure 2e and 2e; (Maas et al., 2020a)). A key question remains with respect to the state of the clock specifically in cells involved in acute inflammation via production of cytokines, potentially contributing to cytokine storm. Some of the highest amplitude circadian rhythms described to date occur in peritoneal macrophages (Keller et al., 2009). The monocyte subset of PBMCs show distinct phase relationships between clock gene expression relative to the whole blood cell population (Figure 2g vs 2e; (Wittenbrink et al., 2018)). It is not known how these cells function in critically ill patients or those in the ICU (Figure 2h).
Interestingly, strong physiologic stressors can induce a state of sleep-like behavioral quiescence in animal models, which occurs in tandem with protective mechanisms to restore cellular homeostasis and recover from cellular stress (Hill et al., 2014; Iannacone and Raizen, 2016; Nath et al., 2016; Trojanowski and Raizen, 2016). During critical illness, normal sleep architectures on traditional electroencephalography (EEG) frequently disappear and are replaced with various abnormal patterns that are neither sleep nor wake in a healthy state. It is therefore difficult to apply traditional EEG scoring methods for sleep and infer underlying sleep-associated neurophysiologic processes to critically ill individuals (Watson, 2007; Watson et al., 2013; Schabus et al., 2018; Wislowska et al., 2018).
Nearly 30% of patients admitted to an ICU develop delirium, which increases the mortality risk (Salluh et al., 2015). Patients with COVID-19 are at increased risk of delirium due to multiple factors, including (1) direct central nervous system (CNS) invasion, (2) induction of CNS inflammatory mediators, (3) secondary effect of noncerebral organ system failures, (4) effect of sedative strategies, (5) prolonged mechanical ventilation, (6) immobilization, and (7) isolation without family (Kotfis et al., 2020). Accordingly, a high rate of delirium (up to 84%) has been reported in critically ill patients with COVID-19 infection (Helms et al., 2020a). Basic research suggests a link between the circadian clock and delirium. In animal models, constant light exposure, inflammation, and midazolam exposure can induce delirium-like phenotype (i.e., impaired executive and memory function) and reduced expression of PER2 in the SCN. This delirium-like phenotype was abolished by the PER2 enhancer nobiletin (Gile et al., 2018).
Although it is clear that the circadian clock is challenged in the ICU, the origin of circadian manifestations in critically ill patients cannot be definitively attributed to the ICU environments. For example, severe illnesses may have direct effects on the circadian clock, regardless of the ICU environments. Furthermore, we have been operating with a bias that an intact circadian system is always optimal. This is partly due to observations of disruptions of metabolism with short-term clock disruption and increased chronic pathologies with long-term disruption (Schernhammer et al., 2001; Scheer et al., 2009). Stress-induced disruption of circadian rhythms or behavioral quiescence may rather represent a distinct adaptive mechanism. If this is correct, then restoration of daily rhythms in physiologic variables may be harmful. In other words, the “optimal state of biological rhythms” during critical illness may be different from that in health.
Incorporation of the Circadian Clock Into Critical Care Paradigms for Health and Knowledge
Discovering the State of the Circadian Clock in the ICU
Chronobiologists are often biased to think that the presence of a circadian rhythm is the preferred state. Here, we have identified an ambiguous situation with a testable hypothesis. Our alternative hypothesis is that supporting the circadian clock with zeitgebers will lead to entrainment and daily rhythms, and this in turn will lead to a better outcome. The null hypothesis would be that the presence of rhythms is an exacerbation and that the suppression of rhythms sometimes seen in serious illness is adaptive. These hypotheses could be tested descriptively via collection of continuous data from ICU stations and analysis for rhythmicity and appropriate entrained phase as correlated with outcome. The data routinely collected in the ICU—e.g., temperature, oxygen saturation, and heart rate—show circadian rhythms in normal individuals. It represents a huge amount of information if it can be harnessed and analyzed. The hypotheses could also be tested experimentally, for example, by examining whether the circadian phase, amplitude, or robustness in temperature rhythms is associated with patient outcomes from severe COVID-19.
Given that the mediators of the most common complications in COVID-19 (hypercoagulation/bleeding, cytokine storm) are extensively regulated by the circadian clock, it may be expected that they occur at discrete times of day. Understanding this would allow for preventive treatments. Without deeper knowledge of the endogenous circadian clock characteristics specifically in the ICU, correct determination of clock regulation of these complications is not possible. What is needed is a comprehensive atlas of potential circadian outputs in ICU patients that include minimally invasive measurements such as blood pressure, heart rate, body temperature, as well as serial peripheral blood sampling over at least two cycles. From this dataset, ICU-appropriate biomarkers of the circadian clock can be derived to determine the circadian profile of the patient (rhythmic/nonrhythmic, amplitude, phase) and to assess the internal time of greatest risk for complications and that of optimal medication dosing schedules.
Delivering a Zeitgeber Cycle in the ICU
It can be challenging to administer a high-amplitude zeitgeber, whether light/dark or administration of corticosteroids with a <24-h half-life, to sedated or septic patients. Glucocorticoid administration should be weighed against its potentially life-threatening side effects including immunosuppression, and creating a dark environment for the patient during the night may interfere with patient care. On the other hand, modifying timing of nutritional support is feasible in the ICU for many patients and could potentially entrain peripheral clocks. We know of no efforts to administer food in the ICU intermittently, mimicking normal eating behaviors, in sedated patients. How is feeding accomplished in the ICU and what does nutrition therapy look like? Clinical trials and guidelines on nutrition therapy during and after critical illness have largely focused on optimal timing (early vs late initiation), route of delivery (gastric vs jejunal vs parenteral), and caloric/protein target. Enteral nutrition (EN) is the preferred route of artificial nutrition therapy in critically ill patients. Initiation of nutrition support therapy in the form of early EN within 24-48 h is recommended in the critically ill patient who is unable to maintain volitional intake, unless there are reasons to delay EN such as enteral obstruction, active gastrointestinal bleeding, and compromised splanchnic circulation (Taylor et al., 2016; Singer et al., 2019).
Several methods of EN administration exist, including continuous, cyclic, intermittent, and bolus techniques. Continuous feeding involves hourly administration of EN over 24 h assisted by a feeding pump; cyclic feeding involves administration of EN over a time period of <24 h generally assisted by a feeding pump; intermittent feeding involves administration of EN over 20-60 min every 4-6 h via pump assist or gravity assist; and bolus feeding involves administration of EN over a 4- to 10-min period using a syringe or gravity drip.
In practice, pump-assisted continuous feeding is generally acceptable for critically ill patients to reduce EN-related complications such as aspiration, feeding intolerance/high gastric residual, underfeeding, and diarrhea. However, a limited number of studies have been conducted to support this practice (Hiebert et al., 1981; Kocan and Hickisch, 1986; Ciocon et al., 1992; MacLeod et al., 2007). Small randomized controlled studies comparing bolus to continuous feeding in ventilated critically ill adults have shown greater volume with fewer interruptions in continuous feeding but no significant difference between feeding techniques with regard to patient outcome (Hiebert et al., 1981; Kocan and Hickisch, 1986; Ciocon et al., 1992; Bonten et al., 1996; Steevens et al., 2002; MacLeod et al., 2007). Based on existing evidence, the Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) suggest switching from bolus to intermittent EN in cases of feeding intolerance, whereas European Society for Clinical Nutrition and Metabolism (ESPEN) recommends continuous rather than bolus EN (Grade B—strong consensus; Taylor et al., 2016).
Time-restricted feeding (TRF) is an approach to resetting biological rhythmicity via metabolic entrainment of peripheral tissues. It may be time to explore the possibility of pursuing circadian realignment by optimizing the timing of feeding in relation to day-night cycle for critically ill patients receiving EN (Sunderram et al., 2014). Currently, no study has been performed to investigate the effects of timing of feeding in either bolus or continuous feeding in the ICUs. Future investigation will need to be equipped with a thoughtful selection of potential biomarkers of rhythmicity in peripheral tissues that can be collected in the clinical setting (e.g., microbiome, metabolomics/transcriptomics) and of clinically meaningful outcome variables.
Summary
Taken together there are numerous aspects of the COVID-19 pandemic that may have a relationship to circadian and other biological rhythms, ranging from behavior of asymptotic carriers, organ physiology in the sick, the viral life cycle within infected cells, and the host immune response. Although biologically plausible, there has been limited attention to the potential protective effects of healthy sleep and circadian rhythms. Interestingly, the consideration of the available data on the ICU environment and critically ill patients points out the lack of data demonstrating if and how sleep and a synchronized clock contribute to healing. There are numerous examples of circadian disruption leading to illness but few examples of synchronization leading to healing. With judicious collection of time-stamped data, the current pandemic can be used to better understand how the circadian clock is involved in critical illness. We also propose that knowledge of the state of the circadian clock may be used to mitigate all stages of COVID-19.
Acknowledgments
We are grateful for the participation of so many colleagues in the European Biological Rhythms Society (EBRS) Workshop “Chonobiology of COVID-19.” The open discussion helped us to solidify our ideas and concepts for how chronobiology could intersect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) biology and COVID-19 severe illness. The work of M.M. is supported by the Volkswagen Foundation (Life? Funding Program: “The Fourth Dimension”) and by the Friedrich Bauer Stiftung and the Verein zur Förderung von Wissenschaft und Forschung of the LMU Munich. The work of A.A. is supported by the Munich Excellence Training Initiative for Physician Scientists (Metiphys) program of the LMU Munich. The work of T.S. is supported by the Förderprogramm für Forschung und Lehre (FöFoLe) of the LMU Munich.
Footnotes
Conflict of Interest Statement: The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Tanja Schwarzmeier  https://orcid.org/0000-0003-0807-5093
https://orcid.org/0000-0003-0807-5093
Sara Montagnese  https://orcid.org/0000-0003-2800-9923
https://orcid.org/0000-0003-2800-9923
Martha Merrow  https://orcid.org/0000-0002-8688-2360
https://orcid.org/0000-0002-8688-2360
References
- Adamovich Y, Ladeuix B, Golik M, Koeners MP, Asher G. (2017) Rhythmic oxygen levels reset circadian clocks through HIF1α. Cell Metab 25:93-101. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Kazama M, Tahara C, Shimazu C, Otake J, Kamei K, Nakatake T, Sakurai N, Yasumuro Y, Suzuki S, et al. (1990) Reference values of hemostasis related factors of healthy Japanese adults I: circadian fluctuation. Thromb Res 60:281-289. [DOI] [PubMed] [Google Scholar]
- AlGhatrif M, Cingolani O, Lakatta EG. (2020) The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol 5:747-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeshari MA, Alobaidi NY, Al Asmri M, Alhuthail E, Alshehri Z, Alenezi F, Sapey E, Parekh D. (2020) Mechanical ventilation utilization in COVID-19: a systematic review and meta-analysis. medRxiv. doi: 10.1101/2020.06.04.20122069. [DOI] [Google Scholar]
- Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, et al. (2020) COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 136:489-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsolamy S, Arabi YM. (2015) Infection with Middle East respiratory syndrome coronavirus. Can J Respir Ther 51:102. [PMC free article] [PubMed] [Google Scholar]
- Alvarez JD, Sehgal A. (2005) The thymus is similar to the testis in its pattern of circadian clock gene expression. J Biol Rhythms 20:111-121. [DOI] [PubMed] [Google Scholar]
- Anafi RC, Francey LJ, Hogenesch JB, Kim J. (2017) CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci U S A 114:5312-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal RP, Kwak PB, Tamayo AG, Gebert M, Chiu PL, Walz T, Weitz CJ. (2017) Macromolecular assemblies of the mammalian circadian clock. Mol Cell 67:770-782.e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld S, Caridi-Scheible M, Blum JM, Robichaux CJ, Kraft CS, Jacob JT, Jabaley CS, Carpenter D, Kaplow R, Hernandez AC, et al. (2020) ICU and ventilator mortality among critically ill adults with COVID-19. medRxiv. doi: 10.1101/2020.04.23.20076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta A, Innominato PF, Dallmann R, Rand DA, Lévi FA. (2017) Systems Chronotherapeutics. Pharmacological reviews 69:161-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. (2000. a) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289:2344-2347. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Marcacci L, Schibler U. (2000. b) Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol 10:1291-1294. [DOI] [PubMed] [Google Scholar]
- Banner LR, Lai MM. (1991) Random nature of coronavirus RNA recombination in the absence of selection pressure. Virology 185:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bein T, Weber-Carstens S, Apfelbacher C. (2018) Long-term outcome after the acute respiratory distress syndrome: different from general critical illness? Curr Opin Crit Care 24:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonten MJ, Gaillard CA, van der Hulst R, de Leeuw PW, van der Geest S, Stobberingh EE, Soeters PB. (1996) Intermittent enteral feeding: the influence on respiratory and digestive tract colonization in mechanically ventilated intensive-care-unit patients. Am J Respir Crit Care Med 154:394-399. [DOI] [PubMed] [Google Scholar]
- Boyko Y, Jennum P, Nikolic M, Holst R, Oerding H, Toft P. (2017) Sleep in intensive care unit: the role of environment. J Crit Care 37:99-105. [DOI] [PubMed] [Google Scholar]
- Brahim Belhaouari D, Fontanini A, Baudoin JP, Haddad G, Le Bideau M, Bou Khalil JY, Raoult D, La Scola B. (2020) The strengths of scanning electron microscopy in deciphering SARS-CoV-2 infectious cycle. Front Microbiol 11:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner WF, Sothern RB, Kanabrocki EL, Ryan M, McCormick JB, Dawson S, Connors ES, Rothschild R, Third JLHC, Vahed S, Nemchausky BM, et al. (2000) Relation between circadian patterns in levels of circulating lipoprotein(a), fibrinogen, platelets, and related lipid variables in men. Am Heart J 139:164-173. [DOI] [PubMed] [Google Scholar]
- Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, Al Rasheed M, et al. (2020) Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. doi: 10.1101/2020.05.18.20099960. [DOI] [Google Scholar]
- Cai Y, Zhang J, Xiao T, Peng H, Sterling SM, Walsh RM, Rawson S, Rits-Volloch S, Chen B. (2020) Distinct conformational states of SARS-CoV-2 spike protein. Science 369:1586-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali DP, Brown GM, Pandi-Perumal SR. (2020) Can melatonin be a potential “silver bullet” in treating COVID-19 patients? Diseases 8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro RA, Frishman WH. (2021) Thrombotic complications of COVID-19 infection: a review. Cardiol Rev 29:43-47. [DOI] [PubMed] [Google Scholar]
- Chang J, Garva R, Pickard A, Yeung CC, Mallikarjun V, Swift J, Holmes DF, Calverley B, Lu Y, Adamson A, et al. (2020) Circadian control of the secretory pathway maintains collagen homeostasis. Nat Cell Biol 22:74-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocon JO, Galindo-Ciocon DJ, Tiessen C, Galindo D. (1992) Continuous compared with intermittent tube feeding in the elderly. J Parenter Enteral Nutr 16:525-528. [DOI] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020) The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah VA, Zhang SL, Sehgal A. (2019) Regulation of the blood-brain barrier by circadian rhythms and sleep. Trends Neurosci 42:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M, Cermakian N, Boivin DB. (2015) Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J 29:1360-1370. [DOI] [PubMed] [Google Scholar]
- Daan S, Merrow M, Roenneberg T. (2002) External time–internal time. J Biol Rhythms 17:107-109. [DOI] [PubMed] [Google Scholar]
- Dallmann R, Okyar A, Lévi F. (2016) Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends in molecular medicine 22:430-445. [DOI] [PubMed] [Google Scholar]
- de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish ZA, et al. (2013) Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 87:7790-7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, Czeisler CA. (2012) Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS ONE 7:e30037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnon KH, III, Leist SR, Schäfer A, Edwards CE, Martinez DR, Montgomery SA, West A, Yount BL, Jr, Hou YJ, Adams LE, et al. (2020) A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586:560-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doglioni C, Ravaglia C, Rossi G, Dubini A, Pedica F, Piciucchi S, Vizzuso A, Pecciarini L, Stella F, Maitan S, et al. (2020) Acute lung injury evolution in Covid-19. medRxiv. doi: 10.1101/2020.08.09.20170910. [DOI] [Google Scholar]
- Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, et al. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- Duguay D, Cermakian N. (2009) The crosstalk between physiology and circadian clock proteins. Chronobiol Int 26:1479-1513. [DOI] [PubMed] [Google Scholar]
- Eskin A. (1979) Identification and physiology of circadian pacemakers. Introduction. Fed Proc 38:2570-2572. [PubMed] [Google Scholar]
- Etsuda H, Takase B, Uehata A, Kusano H, Hamabe A, Kuhara R, Akima T, Matsushima Y, Arakawa K, Satomura K, et al. (1999) Morning attenuation of endothelium-dependent, flow-mediated dilation in healthy young men: possible connection to morning peak of cardiac events? Clin Cardiol 22:417-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks TJ, Colby TV, Travis WD, Tuder RM, Reynolds HY, Brody AR, Cardoso WV, Crystal RG, Drake CJ, Engelhardt J, et al. (2008) Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc Am Thorac Soc 5:763-766. [DOI] [PubMed] [Google Scholar]
- Gaenzer H, Neumayr G, Marschang P, Sturm W, Kirchmair R, Patsch JR. (2001) Flow-mediated vasodilation of the femoral and brachial artery induced by exercise in healthy nonsmoking and smoking men. J Am Coll Cardiol 38:1313-1319. [DOI] [PubMed] [Google Scholar]
- Gazendam JAC, Van Dongen HPA, Grant DA, Freedman NS, Zwaveling JH, Schwab RJ. (2013) Altered circadian rhythmicity in patients in the ICU. Chest 144:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Dellibovi-Ragheb T, Pak E, Qiu Q, Fisher M, Takvorian P, Bleck C, Hsu V, Fehr A, Perlman S, et al. (2020) β-coronaviruses use lysosomal organelles for cellular egress. bioRxiv. doi: 10.1101/2020.07.25.192310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, et al. (2020) Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27:992-1000.e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gile J, Scott B, Eckle T. (2018) The period 2 enhancer nobiletin as novel therapy in murine models of circadian disruption resembling delirium. Crit Care Med 46:e600-e608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RA, Morales-Nebreda L, Markov NS, Swaminathan S, Guzman ER, Abbott DA, Donnelly HK, Donayre A, Goldberg IA, Klug ZM, et al. (2020) Alveolitis in severe SARS-CoV-2 pneumonia is driven by self-sustaining circuits between infected alveolar macrophages and T cells. bioRxiv. doi: 10.1101/2020.08.05.238188. [DOI] [Google Scholar]
- Guagnano MT, Davì G, Sensi S. (2000) Morning sudden cardiac death. Int J Immunopathol Pharmacol 13:55-60. [DOI] [PubMed] [Google Scholar]
- Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, et al. (2014) Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis 14:140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, et al. (2020) Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369:718-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, et al. (2021) Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 93:1013-1022. [DOI] [PubMed] [Google Scholar]
- Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, Studer A, Radosavljevic M, Kummerlen C, Monnier A, et al. (2020. a) Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care 24:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, et al. (2020. b) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 46:1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz RSH, Merrow M, Noya SB. (2021) The circadian clock, the brain, and COVID-19: the cases of olfaction and the timing of sleep. J Biol Rhythms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert JM, Brown A, Anderson RG, Halfacre S, Rodeheaver GT, Edlich RF. (1981) Comparison of continuous vs intermittent tube feedings in adult burn patients. J Parenter Enteral Nutr 5:73-75. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Mansfield R, Lopez JM, Raizen DM, Van Buskirk C. (2014) Cellular stress induces a protective sleep-like state in C. elegans. Curr Biol 24:2399-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271-280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. (2020) Dexamethasone in hospitalized patients with covid—19—preliminary report. N Engl J Med. Epub ahead of print July. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, III, Kato T, Lee RE, Yount BL, Mascenik TM, et al. (2020) SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182:429-446.e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KPY, Cheung MC, Perera R, Ng KC, Bui CHT, Ho JCW, Ng MMT, Kuok DIT, Shih KC, Tsao SW, et al. (2020) Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med 8:687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannacone MJ, Raizen DM. (2016) Sleep: how many switches does it take to turn off the lights? Curr Biol 26:R847-R849. [DOI] [PubMed] [Google Scholar]
- Iba T, Connors JM, Levy JH. (2020) The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res 69:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince LM, Zhang Z, Beesley S, Vonslow RM, Saer BR, Matthews LC, Begley N, Gibbs JE, Ray DW, Loudon ASI. (2019) Circadian variation in pulmonary inflammatory responses is independent of rhythmic glucocorticoid signaling in airway epithelial cells. FASEB J 33:126-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri SM, VanRollins M, Ozawa T, Mammen EF, Goldberg AD, Goldstein S. (1992) Circadian variation in platelet function in healthy volunteers. Am J Cardiol 69:951-954. [DOI] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. (2009) A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A 106:21407-21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. (2020) The architecture of SARS-CoV-2 transcriptome. Cell 181:914-921.e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Cortese M, Winter SL, Wachsmuth-Melm M, Neufeldt CJ, Cerikan B, Stanifer ML, Boulant S, Bartenschlager R, Chlanda P. (2020) SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat Commun 11:5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauert MP, Haspel JA, Pisani MA. (2015) Sleep loss and circadian rhythm disruption in the intensive care unit. Clin Chest Med 36:419-429. [DOI] [PubMed] [Google Scholar]
- Kocan MJ, Hickisch SM. (1986) A comparison of continuous and intermittent enteral nutrition in NICU patients. J Neurosci Nurs 18:333-337. [DOI] [PubMed] [Google Scholar]
- Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, Hengartner N, Giorgi EE, Bhattacharya T, Foley B, et al. (2020) Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 182:812-827.e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. (2020) COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care 24:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfeld-Schor N, Stevenson TJ, Nickbakhsh S, Schernhammer E, Dopico XC, Dayan T, Martinez M, Helm B. (2021) Drivers of infectious disease seasonality: potential implications for COVID-19. J Biol Rhythms 36:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Penninger JM. (2013) Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J 77:301-308. [DOI] [PubMed] [Google Scholar]
- Lambert NJ, Corps S. (2020) COVID-19 “long hauler” symptoms survey report. Indiana University School of Medicine. https://dig.abclocal.go.com/wls/documents/2020/072720-wls-covid-symptom-study-doc.pdf.
- Liu DX, Fung TS, Chong KK, Shukla A, Hilgenfeld R. (2014) Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res 109:97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC. (2016) Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 34:2679-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukan N. (2020) “Cytokine storm,” not only in COVID-19 patients. Mini-review. Immunol Lett 228:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas MB, Iwanaszko M, Lizza BD, Reid KJ, Braun RI, Zee PC. (2020. a) Circadian gene expression rhythms during critical illness. Crit Care Med 48:e1294-e1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas MB, Lizza BD, Abbott SM, Liotta EM, Gendy M, Eed J, Naidech AM, Reid KJ, Zee PC. (2020. b) Factors disrupting melatonin secretion rhythms during critical illness. Crit Care Med 48:854-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas MB, Lizza BD, Kim M, Abbott SM, Gendy M, Reid KJ, Zee PC. (2020. c) Stress-induced behavioral quiescence and abnormal rest-activity rhythms during critical illness. Crit Care Med 48:862-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod JBA, Lefton J, Houghton D, Roland C, Doherty J, Cohn SM, Barquist ES. (2007) Prospective randomized control trial of intermittent versus continuous gastric feeds for critically Ill trauma patients. J Trauma 63:57-61. [DOI] [PubMed] [Google Scholar]
- Maemura K, Layne MD, Watanabe M, Perrell MA, Nagai R, Lee M-E. (2006) Molecular mechanisms of morning onset of myocardial infarction. Ann N Y Acad Sci 947:398-402. [DOI] [PubMed] [Google Scholar]
- Manella G, Aviram R, Bolshette N, Muvkadi S, Golik M, Smith DF, Asher G. (2020) Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc Natl Acad Sci U S A 117:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters PS, Perlman S. (2013) Coronaviridae. In: Knipe DM, Howley PM, editors. Fields virology. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health. p. 826-847. [Google Scholar]
- Mazzoccoli G, Mazza T, Vinciguerra M, Castellana S, Scarpa M. (2015) The biological clock and the molecular basis of lysosomal storage diseases. JIMD Rep 18:93-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Bingaman SS, Mehay D, Medina D, Arnold AC. (2020) Angiotensin-(1-7) improves integrated cardiometabolic function in aged mice. Int J Mol Sci 21:5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnana M, Salvagno GL, Lippi G. (2009) Circadian variation within hemostasis: an underrecognized link between biology and disease? Semin Thromb Hemost 35:23-33. [DOI] [PubMed] [Google Scholar]
- Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme JL, Lefebvre P, Staels B. (2018) Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erb-antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 391:59-69. [DOI] [PubMed] [Google Scholar]
- Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, et al. (2018) A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JB, June CH. (2020) Cytokine release syndrome in severe COVID-19. Science 368:473-474. [DOI] [PubMed] [Google Scholar]
- Mudd PA, Crawford JC, Turner JS, Souquette A, Reynolds D, Bender D, Bosanquet JP, Anand NJ, Striker DA, Martin RS, et al. (2020) Targeted immunosuppression distinguishes COVID-19 from influenza in moderate and severe disease. medRxiv. doi: 10.1101/2020.05.28.20115667. [DOI] [Google Scholar]
- Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, et al. (2018) Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359:eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath RD, Chow ES, Wang H, Schwarz EM, Sternberg PW. (2016) C. elegans stress-induced sleep emerges from the collective action of multiple neuropeptides. Curr Biol 26:2446-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. (2001) Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation 104:1746-1748. [DOI] [PubMed] [Google Scholar]
- Numminen H, Syrjälä M, Benthin G, Kaste M, Hillbom M. (2000) The effect of acute ingestion of a large dose of alcohol on the hemostatic system and its circadian variation. Stroke 31:1269-1273. [DOI] [PubMed] [Google Scholar]
- Osmancik P, Kvasnicka J, Widimsky P, Tarnok A. (2004) Diurnal variation of soluble E- and P-selectin, and intercellular adhesion molecule-1 in patients with and without coronary artery disease. Cardiology 102:194-199. [DOI] [PubMed] [Google Scholar]
- Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. (2004) Early morning attenuation of endothelial function in healthy humans. Circulation 109:2507-2510. [DOI] [PubMed] [Google Scholar]
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, et al. (2020) Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, Bass J. (2017) Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab 25:86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke H, Asher G. (2019) Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol 20:227-241. [DOI] [PubMed] [Google Scholar]
- Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, Fernandez R, Akbarpour M, Chen CI, Ren Z, et al. (2019) Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 199:1517-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, et al. (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323:2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson Jones A, Twedt D, Swaim W, Gottfried E. (1996) Diurnal change of blood count analytes in normal subjects. Am J Clin Pathol 106:723-727. [DOI] [PubMed] [Google Scholar]
- Ringqvist Å, Caidahl K, Petersson AS, Wennmalm Å. (2000) Diurnal variation of flow-mediated vasodilation in healthy premenopausal women. Am J Physiol Heart Circ Physiol 279:H2720-H2725. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rubio M, Figueira JC, Acuña-Castroviejo D, Borobia AM, Escames G, de la Oliva P. (2020) A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial. Trials 21:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. (2012) Social jetlag and obesity. Curr Biol 22:939-943. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. (1998) Molecular circadian oscillators: an alternative hypothesis. J Biol Rhythms 13:167-179. [DOI] [PubMed] [Google Scholar]
- Rosing DR, Brakman P, Redwood DR, Goldstein RE, Beiser GD, Astrup T, Epstein SE. (1970) Blood fibrinolytic activity in man. Circul Res 27:171-184. [DOI] [PubMed] [Google Scholar]
- Ruben MD, Wu G, Smith DF, Schmidt RE, Francey LJ, Lee YY, Anafi RC, Hogenesch JB. (2018) A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Science Transl Med 10:eaat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicka AR, Rumley A, Lowe GDO, Strachan DP. (2007) Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation 115:996-1003. [DOI] [PubMed] [Google Scholar]
- Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, Serafim RB, Stevens RD. (2015) Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ 350:h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Wislowska M, Angerer M, Blume C. (2018) Sleep and circadian rhythms in severely brain-injured patients: a comment. Clin Neurophysiol 129:1780-1784. [DOI] [PubMed] [Google Scholar]
- Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, Claus R. (2020) Postmortem examination of patients with COVID-19. JAMA 323:2518-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106:4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Gibbs J, Ince L, Loudon A. (2018) Clocking in to immunity. Nat Rev Immunol 18:423-437. [DOI] [PubMed] [Google Scholar]
- Schelling G, Stoll C, Haller M, Briegel J, Manert W, Hummel T, Lenhart A, Heyduck M, Polasek J, Meier M, et al. (1998) Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med 26:651-659. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. (2006) Night work and risk of breast cancer. Epidemiology 17:108-111. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. (2001) Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 93:1563-1568. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. (2003) Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Ins 95:825-828. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Ince L, Sartor F, Borrmann H, Zhuang X, Naik A, Curtis A, McKeating J. (2021) Clocks, viruses and immunity: lessons for the COVID-19 pandemic. J Biol Rhythms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda LA, Semenza GL. (2011) HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med 183:152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, et al. (2019) ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 38:48-79. [DOI] [PubMed] [Google Scholar]
- Slutsky AS, Ranieri VM. (2013) Ventilator-induced lung injury. N Engl J Med 369:2126-2136. [DOI] [PubMed] [Google Scholar]
- Steevens EC, Lipscomb AF, Poole GV, Sacks GS. (2002) Comparison of continuous vs intermittent nasogastric enteral feeding in trauma patients: perceptions and practice. Nutr Clin Pract 17:118-122. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, et al. (2020) Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324:1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. (2001) Entrainment of the circadian clock in the liver by feeding. Science 291:490-493. [DOI] [PubMed] [Google Scholar]
- Stukalov A, Girault V, Grass V, Bergant V, Karayel O, Urban C, Haas DA, Huang Y, Oubraham L, Wang A, et al. (2020) Multi-level proteomics reveals host-perturbation strategies of SARS-CoV-2 and SARS-CoV. bioRxiv. doi: 10.1101/2020.06.17.156455. [DOI] [PubMed] [Google Scholar]
- Sun J, Xiao J, Sun R, Tang X, Liang C, Lin H, Zeng L, Hu J, Yuan R, Zhou P, et al. (2020) Prolonged persistence of SARS-CoV-2 RNA in body fluids. Emerg Infect Dis 26:1834-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderram J, Sofou S, Kamisoglu K, Karantza V, Androulakis IP. (2014) Time-restricted feeding and the realignment of biological rhythms: translational opportunities and challenges. J Trans Med 12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, et al. (2016) Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). Crit Care Med 44:390-438. [DOI] [PubMed] [Google Scholar]
- Thompson BT, Chambers RC, Liu KD. (2017) Acute respiratory distress syndrome. N Engl J Med 377:562-572. [DOI] [PubMed] [Google Scholar]
- Timmons GA, O’Siorain JR, Kennedy OD, Curtis AM, Early JO. (2020) Innate rhythms: clocks at the center of monocyte and macrophage function. Front Immunol 11:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE. (1987) Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med 316:1514-1518. [DOI] [PubMed] [Google Scholar]
- Trojanowski NF, Raizen DM. (2016) Call it worm sleep. Trends Neurosci 39:54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ündar B, Akkoç N, Alakavuklar MN, Ĉehreli C, Ündar L. (1999) Flow cytometric analysis of circadian changes in platelet activation using anti-GMP-140 monoclonal antibody. Chronobiol Int 16:335-342. [DOI] [PubMed] [Google Scholar]
- Veglio F, Pietrandrea R, Ossola M, Vignani A, Angeli A. (1987) Circadian rhythm of the angiotensin converting enzyme (ACE) activity in serum of healthy adult subjects. Chronobiologia 14:21-25. [PubMed] [Google Scholar]
- Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, Rosner B, Stampfer MJ, Schernhammer ES. (2016) Association between rotating night shift work and risk of coronary heart disease among women. JAMA 315:1726-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li R, Lu Z, Huang Y. (2020. a) Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging 12:6049-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. (2020. b) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SE, Curley GF, Lavin M, Fogarty H, Karampini E, McEvoy NL, Clarke J, Boylan M, Alalqam R, Worrall AP, et al. (2020) Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br J Haematol. Epub ahead of print December. doi: 10.1111/bjh.17273. [DOI] [PubMed] [Google Scholar]
- Watson PL. (2007) Measuring sleep in critically ill patients: beware the pitfalls. Crit Care 11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PL, Pandharipande P, Gehlbach BK, Thompson JL, Shintani AK, Dittus BS, Bernard GR, Malow BA, Ely EW. (2013) Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med 41:1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. (1992) Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol 70:65-68. [DOI] [PubMed] [Google Scholar]
- Willich SN, Maclure M, Mittleman M, Arntz HR, Muller JE. (1993) Sudden cardiac death. Support for a role of triggering in causation. Circulation 87:1442-1450. [DOI] [PubMed] [Google Scholar]
- Wislowska M, Blume C, Angerer M, Wielek T, Schabus M. (2018) Approaches to sleep in severely brain damaged patients: further comments and replies to Kotchoubey & Pavlov. Clin Neurophysiol 129:2680-2681. [DOI] [PubMed] [Google Scholar]
- Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A, et al. (2018) High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest 128:3826-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581:465-469. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020) Coronavirus disease (COVID-19) weekly epidemiological update. Geneva (Switzerland): World Health Organization. [Google Scholar]
- Yam LY, Chen RC, Zhong NS. (2003) SARS: ventilatory and intensive care. Respirology 8:S31-S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, Khalki L, Limami Y, Zaid N, Sadki K, Ben El, Haj R, et al. (2020) Platelets can contain SARS-CoV-2 RNA and are hyperactivated in COVID-19. medRxiv. doi: 10.1101/2020.06.23.20137596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111:16219-16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang X, Jiao H, Liu X. (2020) Coagulopathy in patients with COVID-19: a systematic review and meta-analysis. Aging 12:24535-24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, et al. (2020) Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 81:e16-e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. (2020. a) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hou Y, Shen J, Kallianpur A, Zein J, Culver DA, Farha S, Comhair S, Fiocchi C, Gack MU, et al. (2020. b) A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. ChemRxiv. doi: 10.26434/chemrxiv.12579137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]