Abstract
Background:
Anti-citrullinated protein antibodies (ACPAs) and rheumatoid factor (RF) are key factors in the American College of Rheumatology/European League Against Rheumatism rheumatoid arthritis (RA) classification criteria markers. However, about 30% of patients diagnosed with RA are seronegative, rationalizing the need for new serologic markers for RA. Antibodies against carbamylated proteins (anti-CarP) and against peptidyl-arginine deiminase type 4 (anti-PAD4) have been postulated to be useful RA markers. The purpose of this study is to evaluate the value of anti-CarP and anti-PAD4 in a well-characterized population of RA patients and healthy controls (HCs).
Methods:
A total of 122 RA patients and 30 HCs were enrolled in the study. Serum levels of ACPA, anti-PAD4, anti-CarP and RF were determined by enzyme-linked immunosorbent immunoassays (ELISAs). Synthetic carbamylated peptides were used in the ELISA assay to determine the protein targets of the anti-CarP antibodies.
Results:
Rates of ACPA, RF, anti-PAD4 and anti-CarP positivity were 85.2%, 67.2%, 55.7% and 46.7% in RA, and 0%, 0%, 6.7% and 6.7% in HC respectively. In the RA population, 25.4% of patients had all four types of antibodies positive, while 6.6% had no antibodies. There was a significant correlation between anti-PAD4 and ACPAs (rs = 0.39), RF and ACPAs, (rs = 0.3) and RF and anti-CarP, (rs = 0.3). There was no correlation between ACPAs and anti-CarP. Anti-CarP positivity was noted in 49 (47.1%) and 45 (54.9%) of ACPAs and RF positive patients respectively. In addition, five anti-CarP+ patients did not have ACPA nor RF.
Conclusion:
Anti-CarP but not anti-PAD4 may be a useful biomarker in identifying ACPA/RF negative RA patients. This antibody may identify an additional RA population who may benefit from early implementation of aggressive therapy.
Keywords: anti-CarP, anti-PAD4, DNA methylation, Rheumatoid arthritis, RA markers
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease of unknown origin which affects about 0.5–1% of the northern hemisphere population in rural and urban areas.1 Currently, serological markers including rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs) are widely used to assist in the diagnosis of RA.2 Despite the addition of ACPA as a serologic marker in 2010, about 30% of RA patients remain seronegative, and new serologic markers are being investigated. Anti-carbamylated proteins antibodies (anti-CarP) and antibodies against peptidyl-arginine deiminase type four (anti-PAD4) have been proposed as novel markers of RA.3,4
The first widely used marker of RA was RF.5 RF is an IgM class antibody against the Fc portion of IgG. Its sensitivity in advanced stages of disease can be as high as 79% and overall specificity does not exceed 84%.6,7 The origin of RF has not been precisely explained. It was recently hypothesized by Tan and Smolen that RFs recognize the Fc domains of IgG inside immunological complexes (ICs). The targets are usually post-translationally modified proteins and the antibodies against them are mostly ACPAs or anti-CarP. The authors suggest that the complexes of RF/IC initiate complement binding and activation, causing the initiation of inflammation of the synovium.8 The RF positivity at diagnosis in RA patients is associated with higher baseline disease activity. The structural damage of joints is strongly associated with the presence of RF and ACPA.9 The presence of RF, ACPAs, anti-CarP and anti-PAD4 antedate RA diagnosis on average 2–3, 6, 5–7 and 4.5 years respectively.8,10–12
ACPAs recognize citrulline-containing proteins. IgG class ACPAs are considered the most specific marker for RA. ACPAs are formed against a wide range of auto-antigens via epitope spreading.13,14 ACPAs bind with citrullinated vimentin, fibrinogen, fibronectin, alfa-enolase, type I and type II collagen and histones, among others.15 At least 53 citrullinated proteins have been found in synovial fluid or sera, and the epitope spreading process results in a wide diversity of specific ACPAs.13,16 ACPA diversity and concentrations increase over years until symptoms of early arthritis have developed.17 ACPAs are measured using aCCP (anti-cyclic citrullinated peptide antibody) tests. The latest third generation of this test has a sensitivity and specificity of 70% and 96% respectively. ACPA positivity is a predictor of RA development in early undifferentiated arthritis, and also correlates with more severe RA disease.18
Several additional antibodies have been proposed to assist in RA diagnosis. One such antibody is anti-CarP. Similar to ACPAs, the target of these antibodies is a post-translationally modified protein which in this case involves carbamylation and synthesis of homocitrulline. Anti-CarP antibodies are also referred to as anti-homocitrullinated protein antibodies. Homocitrulline is a neo amino-acid structurally similar to citrulline with an additional methylene group. ACPAs usually do not recognize homocitrulline, but some cross-reactivity may exist.13,19 While citrullination is an enzymatic reaction, carbamylation is caused by a chemical reaction involving the action of isocyanic acid on the primary amine group of lysine.20 In prior studies, the sensitivity and specificity of anti-CarP in RA ranged from 36.2% to 47.7% and from 92.9% to 97.0% respectively.21 An association was observed between anti-CarP positivity and radiographic progression in both ACPA positive and negative RA patients.22,23 The presence of anti-CarP in a cohort of arthralgia patients predicted RA development independently of ACPA.24 In the recent study, Chila-Moreno et al. suggest a significant role for anti-CarP specifically directed against carbamylated peptides of fibrinogen (anti-Ca-Fib2, anti-Ca-Fib3) as early biomarkers of RA.25
Anti-PAD4 antibodies have also been proposed to be useful RA markers. Antigen formation occurs as a result of PAD4 post-translational modifications.26 It is not currently known whether this process plays a regulatory function or is a pathological phenomenon that accelerates autoimmune reactions.27 Apart from synovial tissue and plasma, PAD4 is expressed also in monocytes and granulocytes.28 Of the five isoforms of the PAD enzyme, PAD4 seems to be the most important in RA pathogenesis.29 The enzyme plays an important role in ACPA formation but also may undergo auto-citrullination. PAD4 citrullination causes remodeling of its tertiary structure and decrease of its activity.11,27 PAD also undergoes other post-translational modifications causing anti-PAD4 antibodies production. In the presence of anti-PAD4, the PAD4 enzyme is much more sensitive to calcium ions. Its maximum activity is enhanced in normal calcium concentrations instead physiologically required higher calcium ions concentration. Anti-PAD4 positivity correlates with radiological progression of RA, and can be detected 4–5 years before RA clinical symptoms appear.30 Interestingly, successful RA treatment can lead to loss of anti-PAD4 positivity, which is rare with ACPA and RF.11 The sensitivity of anti-PAD4 is low and ranges from 30% to 40%, while specificity exceeds 95%. These antibodies are associated with erosive RA and with a more severe RA course.26 The presence of a specific subgroup of anti-PAD4 positive patients who also had antibodies that cross-reacted to PAD3 has been shown to increase susceptibility for interstitial lung disease development, especially in patients with a history of tobacco use.30,31 Additionally it has been proposed that some PAD4 antibodies are formed against Porphyromonas gingivalis-originated PAD. There are contrary reports concerning its role in the pathophysiology of RA and periodontal disease (PD). Its existence may conserve the immune response in the context of existing cross-reactivity with citrullinated proteins or play a protective role in PD development and RA.32,33
There are still challenges in proper classification and diagnosis of early arthritis patients. The confirmation of seropositive RA results in early implementation of aggressive treatment and better RA outcomes. There are contrary opinions concerning the utility of new serologic markers in RA, and anti-CarP is particularly controversial. Some authors suggest the possibility of its use, while others conclude that it is of moderate benefit, or of little use in RA diagnosis. The aim of our study is to evaluate the potential usefulness of anti-CarP and anti-PAD4 in a well-characterized population of RA patients, and to correlate the presence of these new antibodies with RF and ACPA positivity.
Materials and methods
A total of 122 RA patients and 30 healthy controls (HCs) were enrolled in the study. RA patients were consecutively admitted to the Department of Rheumatology and Connective Tissue Diseases, Medical University of Lublin, Poland during March 2016–April 2017 and can be considered representative of a larger RA population. RA diagnosis was established using the American College of Rheumatology (ACR)/European League Against Rheumatism 2010 classification criteria, or the 1987 ACR criteria depending on the time of diagnosis. Exclusion criteria included infection or severe illness during hospitalization. The HCs were a group of patients with no joint complaints or who carried a diagnosis of osteoarthritis, with no evidence of inflammatory rheumatic diseases. Written informed consent was obtained from every participant before entering the study. The Ethics Committee of the Medical University in Lublin approved the study (protocol number KE-0254/7/2016). Serum samples were collected and stored at −80°C until analysis. The characteristics of the patients and controls are described in Table 1.
Table 1.
Characteristics of the groups.
| Characteristics | RA n = 122 |
HC n = 30 |
|---|---|---|
| Age (years) | 52.1 ± 12.34 | 52.75 ± 8.23 |
| Females | 102 (84.3) | 21 (75) |
| Disease duration (years) | 11.82 ± 9.17 | N/A |
| ESR (mm/h) | 31.73 ± 24.75 | 13.84 ± 8.9 |
| CRP (mg/dl) | 14.08 ± 25.72 | 1.37 ± 1.71 |
| VAS PGA | 33.79 ± 26.68 | N/A |
| VAS PhGA | 28.9 ± 24.35 | NA/A |
ESR, erythrocyte sedimentation rate; HC, healthy control; RA, rheumatoid arthritis; VAS PGA, visual analog scale patient global assessment; VAS PhGA, visual analog scale physician global assessment.
ACPAs (Anti CCP assay, DiaMetra, Italy), anti-PAD4 antibodies (PAD4 Autoantibody assay, CAYMAN Chemical, USA), anti-CarP antibodies (Carp-Ab assay, Novateinbio, USA) and RF (EIA RF IgG, TestLine Clinical Diagnostics, Czech Republic) were determined in serum by enzyme-linked immunosorbent immunoassay (ELISA) and absorbance reader (Tecan infinite M200 Pro reader and Magellan software, version 7.1). All procedures were performed according to the manufacturer’s recommendation. The cutoff reference range for ACPAs was 30 U/ml and for RF was 22 U/ml. Anti-PAD4 (U/ml) and anti-CarP (ng/ml) reference values were estimated based on results in a control group as below 95th percentile. There is no cross reactivity between ACPA and anti-PAD4 or anti-CarP.10 The Novateinbio assay used in our study uses carbamylated peptides from fetal calf serum (FCS).
Statistical analysis
Quantitative values are presented as mean ± SD or median (interquartile range). The relationship between two continuous variables was analyzed by Spearman’s correlation coefficient. Qualitative parameters are provided as numbers with percentage and were evaluated using contingency tables with a χ2 test with Yates’s correction. A p-value < 0.05 was considered statistically significant. Analysis was performed with STATISTICA Version 13 (StatSoft Inc., USA).
Results
The prevalence of ACPAs, RF, anti-PAD4 and anti-CarP in our study was 85.2%, 67.2%, 55.7% and 46.7% in RA and 0%, 0%, 6.7% and 6.7% in HCs respectively. We also compared combinations of positive results in the RA cohort. In the 122 RA patients, we found 114 patients positive for any marker from the panel. We found 114 positive results for ACPAs or anti-CarP or anti-PAD4, and 112 positive results for ACPAs or anti-CarP or RF. The numbers and percentages of various combinations of RA markers are presented in Table 2.
Table 2.
The prevalence of positivity of various antibodies in ACPA-, RF-, anti-CarP- and anti-PAD4-positive and -negative patients. Results shown in columns in n = numbers of patients and percentage of total RA patients.
| RA patients, N = 122 | Anti-PAD4 (+) | Anti-PAD4 (–) | Anti-CarP (+) | Anti-CarP (–) | ACPA (+) | ACPA (–) | RF (+) | RF (–) |
|---|---|---|---|---|---|---|---|---|
| n = 68 (55.7%) | n = 54 (44.3%) | n = 57 (46.7%) | n = 65 (53.3%) | n = 104 (85.2%) | n = 18 (14.8%) | n = 82 (67.2%) | n = 40 (32.8%) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| ACPA (+) | 65 (95.6) | 39 (72.2) | 49 (86) | 55 (84.6) | N/A | N/A | 78 (95.1) | 26 (65) |
| RF (+) | 57 (83.8) | 25 (46.3) | 45 (79) | 37 (56.9) | 78 (75) | 4 (22.2) | N/A | N/A |
| Anti-CarP (+) | 35 (51.5) | 22 (40.7) | N/A | N/A | 49 (47.1) | 8 (44.4) | 45 (54.9) | 12 (30) |
| Anti-PAD4 (+) | N/A | N/A | 35 (61.4) | 33 (50.8) | 65 (62.5) | 3 (16.7) | 57 (69.5) | 11 (27.5) |
| ACPA (+) and RF (+) | 55 (80.9) | 23 (42.6) | 42 (73.7) | 36 (55.4) | N/A | N/A | N/A | N/A |
| ACPA (+) and anti-CarP (+) | 34 (50) | 15 (27.8) | N/A | N/A | N/A | N/A | 42 (51.2) | 7 (17.5) |
| RF (+) and anti-CarP (+) | 32 (47.1) | 13 (24.1) | N/A | N/A | 42 (40.4) | 3 (16.7) | N/A | N/A |
| RF (+) and anti-PAD4 (+) | N/A | N/A | 32 (56.1) | 25 (38.5) | 55 (52.9) | 2 (11.1) | N/A | N/A |
| Anti-PAD4 (+) and ACPA (+) | N/A | N/A | 34 (59.7) | 31 (47.7) | N/A | N/A | 55 (67.1) | 10 (25) |
| Anti-PAD4 (+) and anti-CarP (+) | N/A | N/A | N/A | N/A | 34 (32.7) | 1 (5.6) | 32 (39) | 3 (7.5) |
| ACPA (+) and RF (+) and anti-CarP (+) | 31 (44.6) | 11 (20.4) | N/A | N/A | N/A | N/A | N/A | N/A |
| ACPA (+) and RF (+) and anti-PAD4 (+) | N/A | N/A | 31 (54.4) | 24 (36.9) | N/A | N/A | N/A | N/A |
| Anti-CarP (+) and RF (+) and anti-PAD4 (+) | N/A | N/A | N/A | N/A | 31 (29.8) | 1 (5.6) | N/A | N/A |
| ACPA (+) and anti-CarP (+) and anti-PAD4 (+) | N/A | N/A | N/A | N/A | N/A | N/A | 31 (37.8) | 3 (7.5) |
ACPA, anti-citrullinated protein antibody; anti-CarP, anticarbamylated proteins antibodies; anti-PAD4, antibodies against peptidyl-arginine deiminase type 4; RA, rheumatoid arthritis; RF, rheumatoid factor.
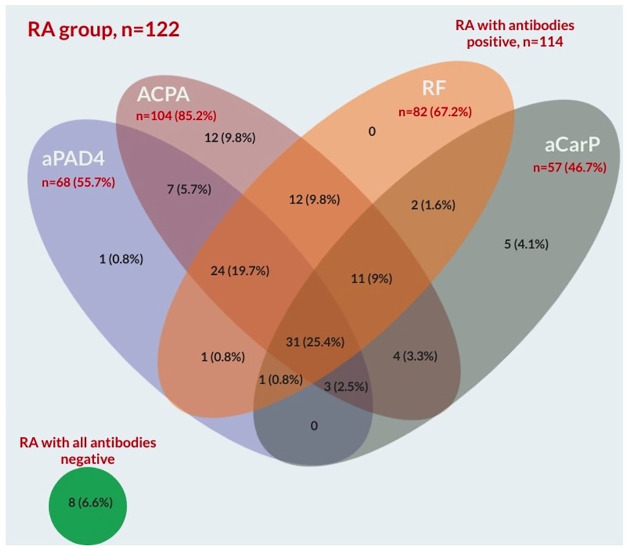
Positivity for all four examined markers was found in 31 of 122 RA patients (25.4%). We surprisingly found in our study that RF was not as useful a marker of RA as anti-CarP and a larger benefit was achieved by including anti-CarP. The group examined in our study included patients with an established RA diagnosis, and average disease duration was over 11 years, so we cannot comment on the distribution of the results in early RA. The Venn diagram in Figure 1 summarizes these results.
Figure 1.
Venn diagram with number and percentage of positive results from the whole 122 RA patients group.
ACPA, anti-citrullinated protein antibody; aCarP, anticarbamylated proteins antibodies; aPAD4, antibodies against peptidyl-arginine deiminase type 4; RA, rheumatoid arthritis; RF, rheumatoid factor.
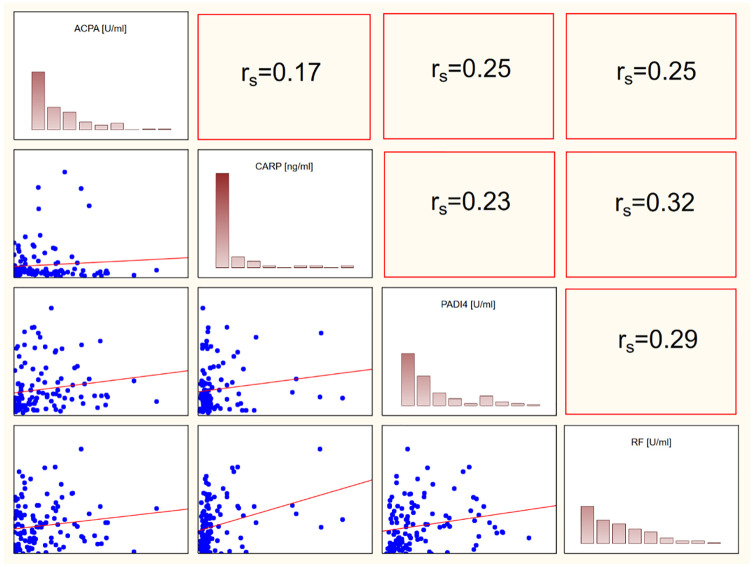
We also found a significant correlation between anti-PAD4 and ACPAs (rs = 0.39), RF and ACPAs (rs = 0.3) and RF and anti-CarP (rs = 0.32). Interestingly, we did not find a correlation between ACPAs and anti-CarP. This may support the concept that anti-CarP is an independent RA marker and confirm that these two markers are of different origin. Graphical representation of correlations is shown in Figure 2.
Figure 2.
Spearman correlations between ACPAs, anti-CarP, anti-PAD4 and RF. Statistically significant correlations were found between ACPAs and PAD4, ACPA and RF, RF and anti-PAD4. There was no significant correlation between ACPAs and anti-CarP.
ACPA, anti-citrullinated protein antibody; CARP, anticarbamylated proteins antibodies; PADI4, antibodies against peptidyl-arginine deiminase type 4; RF, rheumatoid factor.
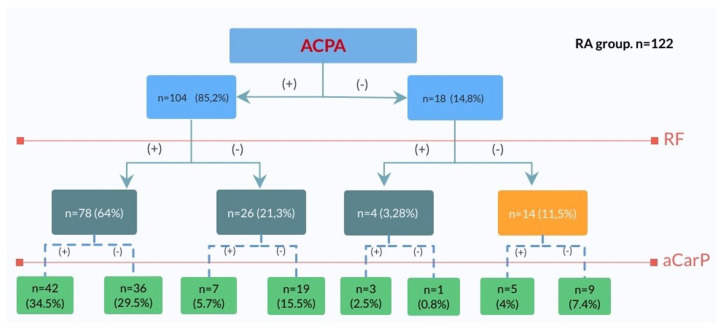
Among ACPA and RF positive patients in our study, we found a significant number of anti-CarP+ results. Forty-nine (47.1%) and 45 (54.9%) were positive for these antibodies. We found five anti-CarP+ patients who were not ACPA+ nor RR+. It is of interest that there are 1 and 5 positive results outside the ACPA/anti-CarP and ACPA/RF groups respectively. This places anti-CarP in a position to be a very valuable RA marker. The decision tree shown in Figure 3 demonstrates the anti-CarP serology in various groups. In the whole RA group (n = 122) we found eight patients (6.6%) were negative for all four antibodies. We found mono-positivity for ACPAs, anti-CarP, anti-PAD4 and RF in 9.8%, 4.1%, 0.8% and 0% respectively. Interestingly we found eight anti-CarP positive results in the ACPA negative group (44%). In the group of RF negative patients (n = 40), there were 12 anti-CarP+, 26 ACPA+ and 11 anti-PAD4+ results. In the group of 82 RF+ patients, there were 37 anti-CarP– and 57 anti-CarP+. In the group of 57 anti-CarP+ patients there were 12 RF–.
Figure 3.
The decision tree. The group of 122 RA patients divided into subgroups according to APCA, RF and anti-CarP positivity presented as number of patients and percentage. ACPA, anti-citrullinated protein antibody; aCarP, anticarbamylated proteins antibodies; RA, rheumatoid arthritis; RF, rheumatoid factor.
Discussion
Anti-CarP
In summary we found in our study a significant proportion of anti-CarP positive results. Surprisingly, the number of anti-CarP mono-positive patients was even bigger than RF mono-positive results. Our results confirm the potential clinical impact of adding anti-CarP to identify seropositive RA patients.34–36 This marker is associated with joint damage, disease severity, DAS28 activity scores37–39 and mortality.40,41 According to a meta-analysis by Li et al., its pooled sensitivity and specificity were 41% [95% confidence interval (CI), 38–45%) and 96% (95% CI, 95–97%) respectively.21 The prevalence of anti-CarP in our study was 46.7%. Its positivity among ACPA-positive and ACPA-negative patients was 47.1% and 44.4% respectively. Rigby et al., in a French cohort of RA patients, found 41.8% anti-CarP positive results among ACPA-positive patients. In the same study, the authors found 23.6% anti-CarP positive results in ACPA-negative and RF-negative patients.42
Among 14 ACPA- and RF-negative patients we found five anti-CarP positive ones, which represents 4% of the whole RA group. Shi et al. previously showed that 16% and 30% of ACPA-negative patients had anti-CarP IgG and IgA and that anti-CarP were positive in 73% and 51% in the ACPA-positive group.10 This confirms that anti-CarP may be independent from other markers and thus may identify additional patients as seropositive.
The natural proteins recognized by anti-CarP were unknown until recently. Recently published results of Verheul et al., using proteome analyses and mass-spectrometry, identified the carbamylated proteins in the synovium recognized by anti-CarP antibodies. The authors discovered carbamylated collagen alpha, fibronectin, vimentin, fibrillin-1 among others.43 Most anti-CarP assays detect in vitro carbamylated fibrinogen or peptides originating from FCS.10 The Novateinbio anti-CarP assay used in our study uses carbamylated peptides from FCS. The differences of anti-CarP prevalence between our study and that in previous research are not very large and may result from difference in the population groups. For example, the fact that the average RA duration was over 10 years in the population we studied might have affected the results. The prevalence of ACPAs in our study was 85%, which is consistent with general data indicating 80–90% ACPA positivity in patients with established RA when conventional assays are used.44 It is of interest that widely used aCCP tests do not recognize all specific ACPAs. Wagner et al. discovered that up to 10% more ACPA-positive patients are identified with custom-designed tests used to detect specific ACPAs from a wide range of anti-citrullinated antibodies generated during the epitope spreading process.45 Therefore, there are two ways to improve the detection of seropositive ACPA results in a RA. The first would involve the preparation of new substrates for ACPA. The second would be to prepare an assay which recognizes more antigens.
The high specificity of anti-CarP and its positivity in RF-negative and ACPA-negative patients may benefit RA diagnosis. It is clear that the additional value of any new marker added to the dyad of RF and ACPA will never be huge. There are contrary opinions concerning using anti-CarP for RA diagnosis. Some authors suggest the possibility of its use, while others conclude that it is of moderate benefit and may not be cost-effective.21,46 We suggest a continuing discussion on the use of anti-CarP in the RA classification criteria or its use as an auxiliary and supportive marker in the face of negative results of ACPA and RF. In connection with the above, there will be a chance to diagnose an additional group of high-risk patients, who will benefit from early implementation of aggressive therapy.
Shi et al. initially showed and then Truchetet et al. confirmed that anti-CarP positivity may identify patients with a higher risk of joint destruction in early RA.23,47 Similarly Verheutel et al. and lately Regueiro et al. proposed using three markers (RF, ACPA, anti-CarP) to properly classify early RA patients.48,49 Therefore, anti-CarP positivity may identify ACPA–/RF– patients, who are in danger of developing rapid radiographic progression and more severe disease.34 Moreover, anti-CarP positivity (determined using ELISA kits against FCS – Anti-FCS, fibrinogen – Anti-Fib, and chimeric fibrine/filagrine homocitrullinated peptide – Anti-CFFHP) was found to be connected with the higher frequency of interstitial lung disease (ILD) in RA patients. Castellanos-Moreira et al. showed more frequent anti-CarP positivity in ILD-RA patients – Anti-FCS 70% versus 43%; Anti-Fib 73% versus 51%; Anti-CFFHP 38% versus 19%.50
In our analysis, of 122 RA patients there were 12 ACPA+ only patients (9.8%), five (4.1%) anti-CarP+ only and 0 (0%) RF+ only. Our results are in line with the results of other authors. In our opinion the consistency of these results is sufficient to consider anti-CarP as a supplementary test in RA diagnosis. Taking into account that the mechanism of carbamylation is completely different and independent from citrullination and the cross-reactivity between ACPA and anti-CarP is limited, anti-CarP might be a unique marker which can recognize an additional group of seropositive patients with an increased risk of severe RA and ILD development.
Anti-PAD4
Among the European population, auto-antibodies to the PAD4 enzyme were proclaimed to be 42% sensitive and 99% specific for RA in a cross-sectional study, and were associated with a more aggressive RA phenotype, characterized by faster appearance of erosions and more intensive joint damage.1,23,30 In a recently published study of 1473 patients (528 with RA), anti-PAD4 positivity reached 35% in the RA cohort. This antibody was found in both ACPA-positive and -negative patients. In the ACPA-negative population, the odds ratio for RA was 5.9.3 In our study, the prevalence of anti-PAD4 was 55.7% in the whole RA group and we found 62.5% anti-PAD4-positive results among ACPA-positive RA patients and only 16.7% (n = 3) in ACPA-negative ones. The low additive value of anti-PAD4 as a supportive RA marker is demonstrated by that fact that anti-PAD was the only antibody present in one RA patient (0.8%). These results question the suitability of this marker for the diagnosis of RA. The positivity of anti-PAD4 mainly occurred in ACPA+ patients. The presence of anti-PAD4 antibodies facilitates PAD enzyme activity in the normal calcium range, resulting in the protein’s over-citrullination and ACPA over-production. This explains, in our view, their concurrent expression in RA patients.
Our study had some limitations. We performed our study on a group of patients with well-established RA. To better describe the role of anti-CarP in the diagnosis of RA at the early arthritis stage, further investigations will be needed. Our study population was quite small and larger confirmatory studies are necessary. The anti-CarP ELISA kit was a non-diagnostic test, and we created an arbitrary cutoff point for the pathological values. Thus, the results may differ from kits from other manufacturers.
Conclusion
The position of ACPA and RF in diagnosing RA is incontestable, but adding anti-CarP, the new player to the team, may enhance the chance to better fit the initial treatment to the individual patient. Apart from the diagnostic value of anti-CarP in RA it may also be possible to incorporate anti-CarP to the panel of markers for establishing diagnosis of pre-RA.
Acknowledgments
We warmly thank Anna Selwa, MD for assistance in collecting patients’ clinical data, and Zofia Kielbik for help in blood sample collection. The results were in part delivered in the form of a poster presentation and abstract at the EULAR 2019, Madrid, 12–15 June 2019.
Footnotes
Author contributions: BK conceived and planned the study, wrote the manuscript, analyzed and interpreted the data, and contributed to blood sample collection. MC performed all the experiments, analyzed the data, performed statistical analysis. MD collected blood samples. AKR oversight for the research activity and external mentor to the core team and final manuscript review. MM contributed to the study design and manuscript review.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: The study was approved by the Bioethics Board at the Medical University in Lublin, protocol number KE-0254/7/2016.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent: Obtained.
ORCID iD: Bogdan Kolarz  https://orcid.org/0000-0001-8496-6846
https://orcid.org/0000-0001-8496-6846
Contributor Information
Bogdan Kolarz, Faculty of Medicine, University of Rzeszow, Kopisto 2A, Rzeszow, 35-959, Poland.
Marek Ciesla, Faculty of Medicine, University of Rzeszow, Rzeszow, Poland.
Ann K. Rosenthal, Division of Rheumatology, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA
Magdalena Dryglewska, Department of Rheumatology and Connective Tissue Disease, Medical University of Lublin, Lublin, Lubelskie, Poland.
Maria Majdan, Department of Rheumatology and Connective Tissue Disease, Medical University of Lublin, Lublin, Lubelskie, Poland.
References
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016; 388: 2023–2038. [DOI] [PubMed] [Google Scholar]
- 2. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 3. Martinez-Prat L, Lucia D, Ibarra C, et al. Antibodies targeting Protein-Arginine Deiminase 4 (PAD4) demonstrate diagnostic value in rheumatoid arthritis. Ann Rheum Dis 2019; 78: 434–436. [DOI] [PubMed] [Google Scholar]
- 4. Shi J, van de Stadt LA, Levarht E, et al. Anti carbamylated protein antibodies (anti-CarP) are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum 2012; 21: 37830. [DOI] [PubMed] [Google Scholar]
- 5. Aho K, Heliövaara M, Maatela J, et al. Rheumatoid factors antedating clinical rheumatoid arthritis. J Rheumatol 1991; 18: 1282–1284. [PubMed] [Google Scholar]
- 6. Chatfield SM, Wicks IP, Sturgess AD, et al. Anti-citrullinated peptide antibody: death of the rheumatoid factor? Med J Aust 2009; 190: 693–695. [DOI] [PubMed] [Google Scholar]
- 7. Niewold TB, Harrison MJ, Paget SA. Anti-CCP antibody testing as a diagnostic and prognostic tool in rheumatoid arthritis. QJM 2007; 100: 193–201. [DOI] [PubMed] [Google Scholar]
- 8. Tan EM, Smolen JS. Historical observations contributing insights on etiopathogenesis of rheumatoid arthritis and role of rheumatoid factor. J Exp Med 2016; 213: 1937–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aletaha D, Alasti F, Smolen JS. Rheumatoid factor, not antibodies against citrullinated proteins, is associated with baseline disease activity in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015; 17: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi J, Stadt LA, Levarht EWN, et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis 2014; 73: 780–783. [DOI] [PubMed] [Google Scholar]
- 11. Kolfenbach JR, Deane KD, Derber LA, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum 2010; 62: 2633–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004; 50: 380–386. [DOI] [PubMed] [Google Scholar]
- 13. Trouw L, Huizinga TW, Toes RE. Autoimmunity in rheumatoid arthritis: different antigens—common principles. Ann Rheum Dis 2013; 72: ii132–ii136. [DOI] [PubMed] [Google Scholar]
- 14. Reed E, Jiang X, Kharlamova N, et al. Antibodies to carbamylated α-enolase epitopes in rheumatoid arthritis also bind citrullinated epitopes and are largely indistinct from anti-citrullinated protein antibodies. Arthritis Res Ther 2016; 18: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013; 5: 178ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Beers JJBC, Schwarte CM, Stammen-Vogelzangs J, et al. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and β-actin. Arthritis Rheum 2013; 65: 69–80. [DOI] [PubMed] [Google Scholar]
- 17. Hafkenscheid L, de Moel E, Smolik I, et al. N-linked glycans in the variable domain of ACPA-IgG predict the development of rheumatoid arthritis. Arthritis Rheumatol 2019; 71: 1626–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stadt LA, Horst AR, Koning MHMT, et al. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann Rheum Dis 2011; 70: 128–133. [DOI] [PubMed] [Google Scholar]
- 19. Shi J, Willemze A, Janssen GMC, et al. Recognition of citrullinated and carbamylated proteins by human antibodies: specificity, cross-reactivity and the “AMC-Senshu” method. Ann Rheum Dis 2013; 72: 148–150. [DOI] [PubMed] [Google Scholar]
- 20. Jaisson S, Gillery P. Evaluation of nonenzymatic posttranslational modification–derived products as biomarkers of molecular aging of proteins. Clin Chem 2010; 56: 1401–1412. [DOI] [PubMed] [Google Scholar]
- 21. Li L, Deng C, Chen S, et al. Meta-analysis: diagnostic accuracy of anti-carbamylated protein antibody for rheumatoid arthritis. PLoS One 2016; 11: e0159000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ajeganova S, van Steenbergen H, Verheul M, et al. The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: a study exploring replication and the added value to ACPA and rheumatoid factor. Ann Rheum Dis 2017; 76: 112–118. [DOI] [PubMed] [Google Scholar]
- 23. Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A 2011; 108: 17372–17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi J, van de Stadt LA, Levarht EN, et al. Brief report: anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum 2013; 65: 911–915. [DOI] [PubMed] [Google Scholar]
- 25. Chila-Moreno L, Rodríguez L-S, Bautista-Molano W, et al. Anti-carbamylated protein and peptide antibodies as potential inflammatory joint biomarkers in the relatives of rheumatoid arthritis patients. Int J Rheum Dis; 23: 1698–1706. [DOI] [PubMed] [Google Scholar]
- 26. Halvorsen EH, Haavardsholm EA, Pollmann S, et al. Serum IgG antibodies to peptidylarginine deiminase 4 predict radiographic progression in patients with rheumatoid arthritis treated with tumour necrosis factor-α blocking agents. Ann Rheum Dis 2009; 68: 249–252. [DOI] [PubMed] [Google Scholar]
- 27. Andrade F, Darrah E, Gucek M, et al. Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation. Arthritis Rheum 2010; 62: 1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foulquier C, Sebbag M, Clavel C, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum 2007; 56: 3541–3553. [DOI] [PubMed] [Google Scholar]
- 29. Auger I, Charpin C, Balandraud N, et al. Autoantibodies to PAD4 and BRAF in rheumatoid arthritis. Autoimmun Rev 2012; 11: 801–803. [DOI] [PubMed] [Google Scholar]
- 30. Darrah E, Giles JT, Ols ML, et al. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci Transl Med 2013; 5: 186ra165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giles JT, Darrah E, Danoff S, et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS One 2014; 9: e98794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Konig MF, Paracha AS, Moni M, et al. Defining the role of Porphyromonas Gingivalis Peptidylarginine Deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Ann Rheum Dis 2015; 74: 2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quirke A-M, Lugli EB, Wegner N, et al. Heightened immune response to autocitrullinated porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis 2014; 73: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trouw LA, Mahler M. Closing the serological gap: promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun Rev 2012; 12: 318–322. [DOI] [PubMed] [Google Scholar]
- 35. van der Helm-van Mil AHM, Zink A. What is rheumatoid arthritis? Considering consequences of changed classification criteria. Ann Rheum Dis 2017; 76: 315–317. [DOI] [PubMed] [Google Scholar]
- 36. van Nies JAB, Krabben A, Schoones JW, et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014; 73: 861–870. [DOI] [PubMed] [Google Scholar]
- 37. Brink M, Verheul MK, Rönnelid J, et al. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res Ther 2015; 17: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montes A, Regueiro C, Perez-Pampin E, et al. Anti-carbamylated protein antibodies as a reproducible independent type of rheumatoid arthritis autoantibodies. PLoS One 2016; 11: e0161141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Humphreys JH, Verheul MK, Barton A, et al. Anticarbamylated protein antibodies are associated with long-term disability and increased disease activity in patients with early inflammatory arthritis: results from the Norfolk arthritis register. Ann Rheum Dis 2016; 75: 1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vidal-Bralo L, Perez-Pampin E, Regueiro C, et al. Anti-carbamylated protein autoantibodies associated with mortality in Spanish rheumatoid arthritis patients. PLoS One 2017; 12: e0180144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ajeganova S, Humphreys JH, Verheul MK, et al. Anticitrullinated protein antibodies and rheumatoid factor are associated with increased mortality but with different causes of death in patients with rheumatoid arthritis: a longitudinal study in three European cohorts. Ann Rheum Dis 2016; 75: 1924–1932. [DOI] [PubMed] [Google Scholar]
- 42. Rigby WF, Skopelja-Gardner S, Jones JD. Anti-citrullinated protein antibody, anti-carbamylated protein antibody, and rheumatoid arthritis: azurophilic granules sing the blues. Arthritis Rheumatol 2017; 69: 2251–2255. [DOI] [PubMed] [Google Scholar]
- 43. Verheul MK, Janssen GMC, de Ru A, et al. Mass-spectrometric identification of carbamylated proteins present in the joints of rheumatoid arthritis patients and controls. Clin Exp Rheumatol. Epub ahead of print 1 September 2020. [PubMed] [Google Scholar]
- 44. Demoruelle MK, Parish MC, Derber LA, et al. Performance of anti–cyclic citrullinated peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheumatol 2013; 65: 2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wagner CA, Sokolove J, Lahey LJ, et al. Identification of anticitrullinated protein antibody reactivities in a subset of anti-CCP-negative rheumatoid arthritis: association with cigarette smoking and HLA-DRB1 ‘shared epitope’ alleles. Ann Rheum Dis 2015; 74: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Regueiro C, Nuño L, Ortiz AM, et al. Value of measuring anti-carbamylated protein antibodies for classification on early arthritis patients. Sci Rep 2017; 7: 12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Truchetet ME, Dublanc S, Barnetche T, et al. Association of the presence of anti–carbamylated protein antibodies in early arthritis with a poorer clinical and radiologic outcome: data from the French ESPOIR cohort. Arthritis Rheumatol 2017; 69: 2292–2302. [DOI] [PubMed] [Google Scholar]
- 48. Regueiro C, Rodríguez-Martínez L, Nuño L, et al. Improved RA classification among early arthritis patients with the concordant presence of three RA autoantibodies: analysis in two early arthritis clinics. Arthritis Res Ther 2019; 21: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Verheul MK, Böhringer S, van Delft MAM, et al. Triple positivity for anti–citrullinated protein autoantibodies, rheumatoid factor, and anti–carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: implications for very early identification of at-risk individuals. Arthritis Rheumatol 2018; 70: 1721–1731. [DOI] [PubMed] [Google Scholar]
- 50. Castellanos-Moreira R, Rodríguez-García SC, Gomara MJ, et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease. Ann Rheum Dis 2020; 79: 587–594. [DOI] [PubMed] [Google Scholar]