Abstract
The nociceptive flexion reflex (NFR) is used in neurophysiological research as an objective measure of nociception. NFR thresholds are reduced in numerous chronic pain pathologies, which are indicative of common central hyperexcitability within conditions. However, variation exists in both the NFR assessment and determinants of NFR threshold among research groups. Our purpose was to provide a review of the recent literature to (a) confirm the NFR threshold’s efficacy in identifying those with chronic pain compared to controls and (b) provide a narrative synthesis on the current methodology used to assess the NFR in clinical populations. We conducted a review of multiple databases (MEDLINE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Google Scholar and Cochrane Library), including articles that reported controlled clinical studies of humans, in English, comparing NFR thresholds within chronic pain conditions to matched control subjects, published since the last NFR review in 2010. Our search resulted in nine studies included in our narrative synthesis and eight studies included in a meta-analysis. There was a significant pooled standardized mean difference in NFR threshold between chronic pain conditions and controls (−0.94, 95% confidence interval (CI) −1.33 to −0.55, p < 0.0001), with substantial heterogeneity of pooled estimates (I2 = 87%, τ2 = 0.41, Q = 76.13, the degrees of freedom (df) = 11, p < 0.0001). Significant variations in participant positioning, stimulation parameters and determinants of the NFR threshold were evident among included studies. We provided a narrative synthesis on the methodologies of included studies, as a recommendation for future studies in the assessment of the NFR in chronic pain.
Keywords: Nociceptive flexion reflex, NFR, chronic pain, electromyography, neurophysiology
Introduction
Chronic pain affects nearly one in five North Americans.1,2 At an estimated cost of $43–60 billion per year in healthcare costs and lost productivity in Canada,3,4 and with an estimated cost of $560 billion in the United States,2 the economic burden of chronic pain is greater than cancer, heart disease and HIV combined.5 Along with its economic impact, the assessment and the subsequent treatment of chronic pain are hindered due to its complexity as a multidimensional clinical entity.6 Up until recently, chronic pain has been evaluated using primarily subjective methods.6
The nociceptive flexion reflex (NFR), also known as the RIII reflex, has been proposed as an objective and reproducible neurophysiological tool for the evaluation of nociception.7 The NFR is a polysynaptic reflex that facilitates the withdrawal of an affected body region in response to noxious stimulation.8 The NFR is usually elicited by stimulation of the sural nerve at the retromalleolar space, and the impulse is recorded over the ipsilateral short head of the biceps muscle via standard surface electromyography (EMG).9 The theoretical advantage of the NFR is its objectivity in the assessment of nociception via involuntary reflex,7 compared to numerous other quantitative sensory tests that rely heavily on participant’s verbal/written perception to various stimuli.10 The initial descriptions of the NFR methodology for clinical practice were provided by Willer7 and Sandrini et al.11 They describe delivering electrical stimuli to the sural nerve in trains of 5–10 rectangular wave pulses of 1.0 ms duration at a frequency of 200–300 Hz. The trains should be delivered randomly to avoid habituation.12 The intensity of stimulation eliciting a response at a rate of 60–90% in a series of approximately 20 stimuli is considered the NFR threshold.7,11,13,14 This loosely describes a technical administration and associated primary outcome measure of the NFR technique. Unfortunately, this description does not provide adequate information to allow a researcher who is unfamiliar with the technique to elicit the NFR in the assessment of individuals affected by chronic pain.
Since its original investigation by Kugelberg et al.8 and subsequent correlation to pain perception by Willer,7 the NFR technique has been a consistent tool in the study of nociception and chronic pain.15 However, with the increasing popularity of the NFR technique over the last 40 years and further evolution of its outcome measures,16–18 there lacks a standardized protocol of noxious stimulation and associated NFR assessment. This is especially true in the examination of individuals with altered nociception, such as those living with chronic pain. With the added intrasubject variation within chronic pain conditions, the variability in NFR techniques among researchers presents a further challenge for multi-centred randomized controlled trials, as well as for meta-analyses of NFR outcomes. A standardized protocol may improve the diagnostic efficacy of the NFR technique, both during assessments of those living with chronic pain and in future meta-analyses of NFR outcomes.
One previous study systematically reviewed the NFR in 2010, and reported that reduced NFR thresholds were commonly observed in chronic pain pathologies compared to healthy matched controls.15 This review also noted variation in methodological techniques used to assess the NFR threshold, but made few recommendations on a standardized methodology moving forward.15 As such, there likely remain methodological discrepancies in the use of the NFR for the assessment of chronic pain pathologies over the last 10 years.
We set out to address this knowledge gap through a review of the recently available studies that have employed the NFR in the assessment of humans who are affected by chronic pain. We provide a synopsis of the methodological procedures used in the acquisition of the NFR within humans affected by chronic pain.
Methods
We searched the following databases using methodology outlined by the Cochrane collaboration: MEDLINE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Google Scholar and Cochrane Library (Cochrane Reviews and Cochrane Central Register of Controlled Trials). We used the following search terms: nocicept*⁄flex*⁄adj7 reflex, nociceptive withdrawal reflex, flexor withdrawal reflex, flexor reflex, withdrawal reflex, RIII reflex, spinal-mediated reflex, central hyperexcitability, central sensitization, musculoskeletal system and musculoskeletal diseases and pain. The only limits applied to our search strategy were ‘human’ and ‘English’. We included articles published after 2010, as a previous review by Lim et al.15 examined the efficacy of the NFR threshold to dissociate between chronic pain pathology and healthy controls. We included articles in our study provided they compared NFR thresholds within clinically diagnosed chronic musculoskeletal pain conditions to matched healthy controls. Review articles, commentaries and case reports were excluded.
All the articles produced by the search were reviewed by two authors (H.E. and K.M.) and disagreements during article screening were resolved by a third reviewer (D.A.K.). Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram that was created from the results of the search and subsequent inclusion/exclusion based on the criteria outlined above.
Figure 1.
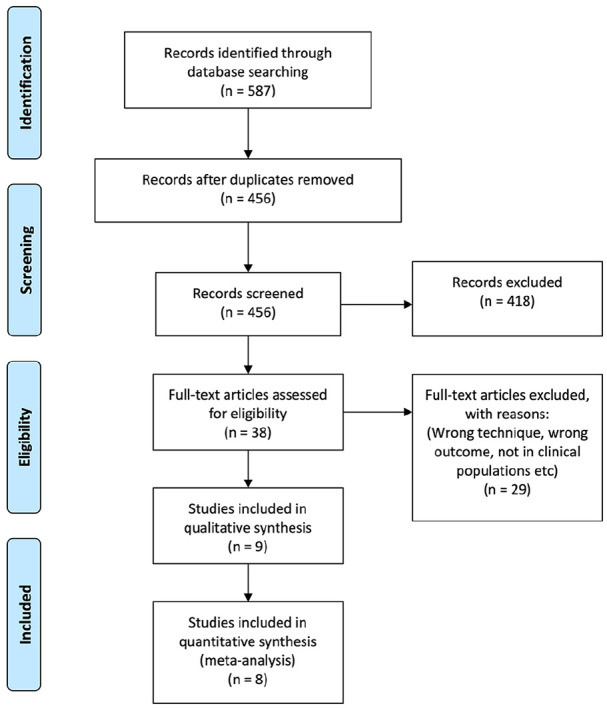
PRISMA diagram.
Data were extracted from each of the articles by one of the authors (H.E.) and confirmed by another author (K.M.). The following data were extracted from each study: study characteristics such as sample size, type of chronic pain condition, NFR thresholds (reported as mean and standard deviation) for both clinical and control populations, as well as testing procedures, including participant positioning, noxious stimulation and EMG recording parameters. We evaluated the efficacy of the NFR threshold to identify those living with chronic pain, while participant positioning, noxious stimulation and EMG recording parameters contributed to a narrative synthesis of current methodological practices for NFR assessments.
We assessed the included articles for risk of bias using appropriate quality appraisal tools based on study design. Specifically, the quality of randomized controlled trials was appraised using the Jadad et al.19 scoring, while the internal validity and risk of bias was assessed using the updated Risk of Bias 2 (RoB 2) tool,20 in accordance with the Cochrane handbook for systematic reviews of interventions.21 Non-randomized studies were assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool.22 Two independent reviewers (L.D.L. and F.C.K.D.) appraised all eligible studies and provided scores. Disagreements were mediated by a third reviewer (D.A.K.).
Our primary purpose was to provide a narrative synthesis on the methods used to assess the NFR in clinical populations. We included studies published since the last systematic review and meta-analysis on the NFR threshold in 2011.15 We conducted a meta-analysis of our included studies to confirm the continued efficacy of the NFR threshold to accurately identify individuals living with chronic pain compared to matched controls since this previous review.15 For studies with multiple clinical pathologies, each pathology was included separately.
All statistical analyses were completed in R (Version 3.5.3, macOS Mojave). Mean, standard deviation and sample size were extracted for the NFR thresholds from control and clinical populations for the included studies. We calculated standardized mean differences between control and clinical pathologies23 along with 95% confidence intervals (CIs) and performed tests of heterogeneity. Data were pooled using a random effects model to account for inter-study variability. In line with the previous review by Lim et al.,15 differences were plotted such that negative differences represent a reduction in NFR threshold in chronic pain conditions compared to control. We plotted standardized differences versus standard errors and assessed symmetry of the funnel plot visually.
Results
Included studies and risk of bias
We obtained a total of 587 articles that satisfied our search strategy, and after removing duplicates, we were left with a total of 456 articles. Two authors applied the inclusion/exclusion criteria to all the articles using the information found within the title and abstract. After applying the inclusion/exclusion criteria, 38 articles were included for full-text screening. Additional 29 articles were excluded because of the following reasons: wrong study population, wrong outcome measures and wrong study design (Figure 1). This left a total of nine articles from which we extracted information regarding NFR stimulation and recording procedures, along with NFR thresholds in clinical populations and healthy matched controls.24–32 Of these studies, only one was excluded from meta-analysis, due to a lack of reported comparison of NFR thresholds between clinical condition and matched control.25 Their findings remain in the narrative synthesis of our proposed methodology for the NFR threshold assessment.
The cases examined by the authors of the included studies ranged from chronic widespread pain such as fibromyalgia to other chronic regional musculoskeletal conditions such as whiplash, along with neck and lumbar chronic pain. All included studies were non-randomized cohort designed studies. Risk of bias assessments, via the ROBINS-I tool for non-randomized studies, determined all included articles to have low to moderate risk of bias.
Meta-analysis
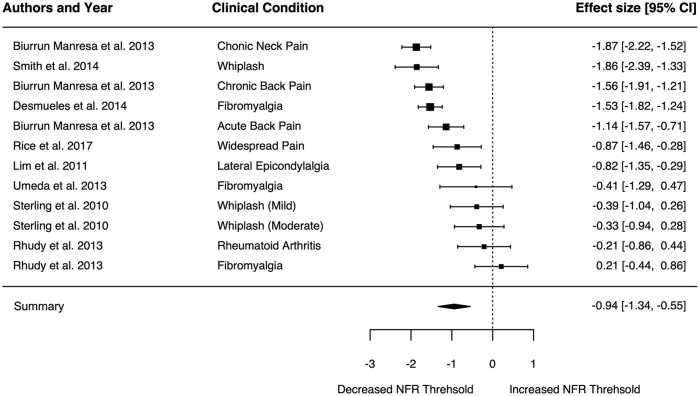
There was a significant pooled standardized mean difference in NFR threshold between chronic pain conditions and controls (−0.94, 95% CI −1.33 to −0.55, p < 0.0001; Figure 2). There was a high level of heterogeneity displayed in the pooled estimates (I2 = 87%, τ2 = 0.41, Q = 76.13, the degrees of freedom (df) = 11, p < 0.0001). The funnel plot displayed evidence of asymmetry in included studies (Figure 3).
Figure 2.
Pooled estimates of nociceptive flexion reflex (NFR) threshold standardized mean difference between clinical populations and healthy matched controls. Negative effect represents reduced NFR threshold in the clinical population compared to controls.
Figure 3.
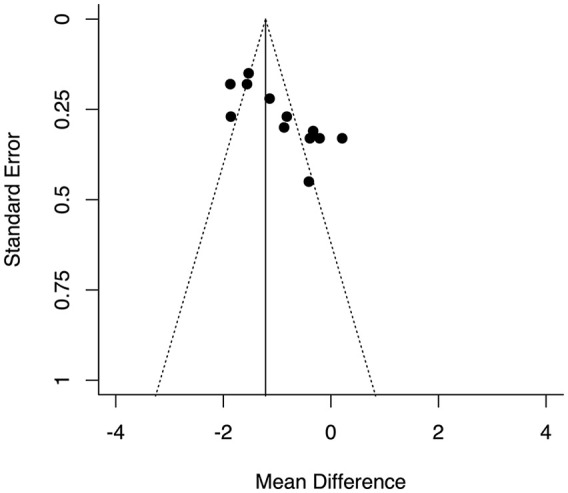
Funnel plot of standardized mean difference against standard error.
Participant positioning
Details on participants’ positioning during NFR assessments were reported for seven of the nine included studies (Table 1).26–28,30–32 Three studies evaluated the NFR with participants in prone lying, with their right knee flexed at 30°.26,30,31 Two studies had participants in sitting/reclined sitting, with the knee flexed at 60°32 and 160° (20),28 respectively. One study had participants in lying posture with the knee flexed at 10°,27 while one study had participants in standing posture, with their tested leg unsupported.29
Table 1.
Summary of participant positioning, stimulation and recording site locations for each study reviewed.
| Desmeules et al. 2014 | Krafft et al. 2017 | Lim et al. 2012 | Biurrun Manresa et al., 2013 | Rhudy et al. 2013 | Rice et al., 2017 | Smith et al., 2014 | Sterling et al., 2010 | Umeda et al., 2013 | |
|---|---|---|---|---|---|---|---|---|---|
| Participant position | Not reported | Not reported | Prone | Lying | Reclined sitting | Standing on the nondominant leg | Prone | Prone | Upright sitting |
| Knee angle | Not reported | Not reported | Right knee with 30 degrees of flexion | 10 degrees flexion. | The knee at 160 degrees | Not applicable | Knee flexed 30 degree | Knee flexed 30 degree | Hip flexed at 90 degrees, Knee flexion was 60 degrees. |
| Site of Electrical Stimulation | Sural N., in the retromalleolar track | Sural N., in the retromalleolar track | Sural nerve just inferior to the lateral malleolus. |
Lateral malleolus | Retromalleolar pathway of the sural nerve | Plantar aspect of the foot of the dominant leg | Distal to the left lateral malleolus | Inferior to the lateral malleolus right ankle |
Retromalleolar pathway of the sural nerve |
| Site of recording | Ipsilateral biceps femoris muscle |
Ipsilateral biceps femoris muscle) | Right biceps femoris muscle | Belly of the biceps femoris and the rectus femoris muscles |
Biceps femoris muscle |
Biceps femoris muscle | Biceps femoris muscle | Biceps femoris muscle | Right biceps femoris muscle |
Noxious stimuli parameters
The noxious stimulation parameters to elicit the NFR were extracted from each study (Table 2). The pulse duration was 0.5 ms in one study,24 whereas it was 1 ms in the other eight studies.25–32 The number of pulses in each stimulant was five pulses in six studies,25,27,29–32 ten pulses in one study,26 one pulse in one study24 and not reported in one study.28 Inter-pulse duration was 2 ms in one study,26 3 ms in two studies,29,32 4 ms in one study25 and 5 ms in two studies.30,31 Two studies did not report inter-pulse duration,27,28 and in one case, it was not applicable as the stimulus was just one pulse.24 Inter-stimulant intervals were 6–10 ms in one study,24 8–12 ms in four studies,25,27–29 4–8 ms in two studies,26,31 5–10 ms in one study and not reported in one study.30 The total duration of each stimulus was 0.5 ms in one study,24 21 ms in one study,25 17 ms in one study29 and 25 ms in two studies,30,31 while the remaining four studies did not report.26–28,32 Maximum stimulus intensity was 100 mA in one study,24 50 mA in one study28 and 40 mA in one study,32 while all other studies did not report.25–27,29–31
Table 2.
Summary of the details of the stimulation procedure used within each study.
| Desmeules et al., 2014 | Krafft et al., 2017 | Lim et al., 2012 | Biurrun Manresa et al., 2013 | Rhudy et al., 2013 | Rice et al., 2017 | Smith et al., 2014 | Sterling et al., 2010 | Umeda et al., 2013 | |
|---|---|---|---|---|---|---|---|---|---|
| Chronic Pain Pathology | Fibromyalgia | Back Pain | Lateral Epicondylalgia | Neck and low back pain | Fibromyalgia Rheumatoid Arthritis |
Fibromyalgia | Whiplash | Whiplash | Fibromyalgia |
| Sample Size | Fibromyalgia, N=137 Control, N=99 |
Back Pain, N=33 Control, N=15 |
Elbow Pain, N=38 Control, N=38 |
Neck Pain, N=40 Back Pain, N=24 Chronic Pain, N=40 Control, N=300 |
Fibromyalgia, N=17 Rheumatoid, N=17, Control, N=19 |
Fibromyalgia, N=26 Control, N=26 |
Whiplash, N=23 Control, N=30 |
Whiplash (mild), N=20 Whiplash (moderate), N=17 Control, N=22 |
Fibromyalgia, N=8 Control, N=13 |
| Pulse duration (ms) | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Number of pulse(s) | Single pulse | Train of 5 pulses | Train of 10 pulses | Train of 5 pulses | Not reported | Train of 5 pulses | Train of 5 pulses | Train of 5 pulses | Train of 5 pulses |
| Inter-pulse duration (ms) | Not reported | 4 | 2 | Delivered at 200 Hz | Not reported | 3 | 5 | 5 | 3 |
| Interstimulus interval(s) | Randomly between 6-10 | Randomly between 8-12 | Randomly between 4-8 | Randomly between 8-12 | Randomly between 8-12 | Randomly between 8-12 | Random | Randomly between 4-8 | Randomly between 5-10 |
| Total stimulus duration (ms) | 0.5 | 21 | Not reported | Not reported | Not reported | 17 | 25 | 25 | Not reported |
| Max Stimulus intensity (mA) | 100 | Not reported | Not reported | Not reported | 50 | Not reported | Not reported | Not reported | 40 |
| Increments | Not reported | 0.5 mA steps | 2 mA increments | 0.5 mA steps | 3 ascending /descending steps, 2mA then 1mA | Staircase up and down 4-2-1mA | 2 mA increments | 2 mA increments | Staircase up and down 4-2-1mA |
NFR threshold assessments
Studies differed in their assessment of NFR threshold in both stimulus parameters and methods to calculate NFR threshold (Tables 2 and 3). For stimulus parameters, three studies used a single series of 2 mA increments of increasing stimulus intensity26,30,31 and two studies used 0.5 mA increments of increasing stimulus intensity.25,27 Three studies used staircase methods of increasing and decreasing stimuli; one of these involved increases in 2 mA increments followed by 1 mA increases and decreases, respectively,28 while two studies used 4 mA increases, then 2 mA decreases, followed by an additional two series of 1 mA increases and decreases (4-2-1 method).29,32 One study did not report their method of increasing/decreasing stimulus intensity.24
Table 3.
Summary of the details of the recording procedure used for each study.
| Desmeules et al. 2014 | Krafft et al. 2017 | Lim et al. 2012 | Biurrun Manresa et al., 2013 | Rhudy et al. 2013 | Rice et al., 2017 | Smith et al., 2014 | Sterling et al., 2010 | Umeda et al., 2013 | |
|---|---|---|---|---|---|---|---|---|---|
| Recording before stim | Not reported | 90-30 ms | Not reported | Not reported | 60 ms | 70-5 ms | 60 ms | 60 ms | 60 ms |
| Latency after stim for NFR | 90-200 ms | 90- 120 ms | 90-150 ms | 60–180 ms | 90–150 ms | 85-150 ms | 90-150 ms | 90-150 ms | 90–150 ms |
| EMG amplification | Not reported | up to 10,000 times | 2,000 | 50,000 times | 10,000 | 1000 | Not reported | Not reported | 1,000 |
| Determinant of NFR-Threshold | The intensity of the current inducing 50% of positive responses to a series of 30 to 40 stimulations, |
The stimulus intensity that first evoked a reflex response exceeding a baseline-corrected area of 100 lV•ms]. The mean of three RIII thresholds was calculated. |
Interval peak z score of greater than 10.32 | A response with an amplitude exceeding 20 uV for at least 10 ms | 1 standard deviation more than the base line before stimulation | The intensity that elicit response (z score) in 3 out of 4 stimuli of last two ascending / descending stimuli. | Interval peak z score of greater than 10.32 | Interval peak z score of greater than 10.32 | 1 standard deviation more than the base line before stimulation The average during the two ascending sequences |
For the calculation of NFR threshold (Table 3), three studies used the lowest stimulus intensity that elicited a z-score greater than 10.23 during a single series of stimulations.26,30,31 Two studies classified NFR threshold as the lowest stimulus intensity to elicit amplitude increase of 20 µV amplitude for over 10 ms27 or an area of 100 μV ms with respect to baseline.25 Two studies used a 4-2-1 staircase method of stimulus delivery, with NFR threshold classified as the stimulus intensity to generate a z-score greater than 10.23 in at least three of the four 1 mA ascending/descending series of stimuli,29 or the average stimulus intensity of the last two series of stimuli that elicited a response greater than 1 standard deviation of baseline,32 respectively. One study classified NFR threshold as the stimulus intensity to induce 50% of responses to a series of stimuli.24
Discussion
Meta-analysis
The meta-analysis on the NFR threshold compared between chronic pain pathologies and healthy matched controls provides an update on that previously reported by Lim et al.15 They reported reduced NFR thresholds across chronic pain pathologies compared to healthy matched controls, suggesting the NFR threshold as a measure of central excitability. In line with this finding, we also observed a significant pooled standardized mean difference in the NFR threshold, with reduced NFR thresholds observed in clinical pathologies compared to control. Interestingly, the studies included within our analysis resulted in a greater effect (−0.94, 95% CI −1.33 to −0.55) compared to that reported by Lim et al.15 (−0.68, 95% CI −0.92 to −0.44). Contrary to the previous study by Lim et al.,15 we observed a high level of heterogeneity for our reported pooled effect. This was likely due to the smaller sample of studies included in our analysis, as well as a trend for larger studies to demonstrate larger effects.33 Specifically, the larger sample sizes by the works of Biurrun Manresa et al.27 and Smith et al.30 also demonstrated the largest effects, compared to the smaller sample sizes by the works of Umeda et al.,32 Sterling31 and Rhudy et al.28 Despite noted heterogeneity among included studies, our pooled effect replicated that which was previously reported,15 and suggests that reduced NFR thresholds continue to be associated with those living with chronic pain.
Participant positioning
The seminal work by Kugelberg et al.8 demonstrated evidence of stimulus location and postural dependent responses of the NFR, which have been further explored in the upper limb.34 As such, participant positioning and the site of noxious stimulus undoubtedly influence the NFR. Among the studies included in our review, there was significant variation in their reported participant positioning, which likely contributed to the heterogeneity of the pooled effect of the NFR threshold. A lying posture with the knee flexed and supported was the most reported among our included studies.26,27,30,31 However, two studies adopted a sitting posture with the knee flexed,28,32 two studies did not report their participant position24,25 and another had participants in standing posture.29 While the maintenance of a consistent posture within studies is critical for consistent NFR assessments, the known influence of postural variations on NFR responses34 is a major limitation in the comparison of NFR thresholds across studies. The adoption of a consistent posture during NFR assessments across research groups would improve the comparability of future study findings.
Noxious stimuli parameters
There were subtle variations in the stimulus parameters used to assess the NFR among included studies. The majority of studies used five 1 ms pulses to deliver noxious stimuli.25,27,29–32 The time between progressive stimuli was randomized in all studies, with the majority of studies reporting an interval ranging from 4 to 12 seconds.24–29,31,32 While inter-pulse intervals and the overall length of each stimuli varied between studies, these subtle differences likely did not have a substantial influence on NFR assessments, as all stimulation parameters would be perceived as a single stimulus to participants. In addition, these respective parameters within each study were kept consistent in comparisons between chronic pain conditions and healthy matched controls. Conversely, the environment in which stimuli were delivered was seldom reported among included studies. Only one study specified the use of a standardized testing space.26 Possible examiner effects during NFR assessments were also seldom addressed, with two studies indicating the same examiner completing all NFR assessments30,34 – one study indicating blinded examiners31 and one study making use of a separated testing room with microphones to mitigate any examiner influence.28 Given well-documented influences of examiner’s sex on pain-related outcomes,35,36 as well as the influence of examiner’s position and posture on pain ratings,37 this is an area in need of improved reporting as well as further exploration within NFR assessments.
NFR threshold assessments
The assessment of NFR thresholds – the primary outcome measure used to compare chronic pain to healthy matched controls – varied among included studies. Two main components of NFR threshold assessment involve the method of stimulus delivery and the criteria for determining both NFR responses and NFR thresholds. While variation was evident in the methodology of stimulus delivery, patterns emerged for either the use of a single series of ascending stimuli25–27,30,31 or a staircase method of increasing and decreasing stimuli.28,29,32 The use of a single series of ascending stimuli compared to a staircase method was previously validated,38 suggesting that the use of prolonged staircase methods may expose participants to additional noxious stimuli with little advantage in ascertaining reliable NFR thresholds. However, more recent studies have suggested the use of more complex algorithms for stimulus delivery during NFR assessments.12,39 In addition, a recent simulation study recommended dynamic staircases of stimulus delivery, which adapt in step sized based on NFR threshold stability, in comparison to the more traditional staircase methods reported from studies included in this review.40 Further research is needed to explore the use of these more novel NFR threshold algorithms in the assessment of clinical pathologies compared to healthy matched controls.
The included studies varied in their determination of the NFR threshold; however, clear patterns emerged with respect to NFR threshold calculation along with respective stimulus delivery. The three studies that used 2 mA increases in stimulus intensity also all shared the same method of NFR threshold determination, via the stimulus intensity to produce an interval peak z-score greater than 10.32.26,30,31 The two studies that used 0.5 mA increases in stimulus intensity used similar amplitude-based metrics, either 20 µV amplitude for over 10 ms27 or an area of 100 IV ms with respect to baseline.25 The two studies that used the 4-2-1 staircase method of stimulus delivery varied in their determination of NFR threshold; Rice et al.29 used the stimulus intensity to generate a z-score greater than 10.23 in at least three of the four 1 mA ascending/descending series of stimuli, while Umeda et al.32 took the average stimulus intensity of the last two series of stimuli that elicited a response greater than 1 standard deviation of baseline. When considering both stimulus delivery and NFR threshold criteria, the only consistent method between more than two studies was the use of a single series of increasing 2 mA increments, with the NFR threshold defined as the stimulus intensity that elicited a z-score greater than 10.32.26,30,31
Proposed methodology for NFR assessment
After assessing the literature as described above, we propose the following methodology for the elicitation of the NFR within chronic pain populations.
Two surface EMG electrodes can be used to deliver the (noxious) electrical stimulus and should have a 2 cm inter-electrode distance along the infra-lateral aspect of the lateral malleolus. A single electrical stimulation consisting of five 1 ms square wave pulses should be delivered at 200 Hz.25,27,29–32 The recording electrodes can be placed on a muscle that is supplied by nerves that are within the segment of innervation that corresponds to the location where the stimulation electrodes were placed. For example, the most common muscle used for recording is the biceps femoris. The skin over each muscle belly should be cleaned with alcohol prior to electrode placement. The identification of motor points should be used to maintain consistent electrode placement between sessions.41 The positioning of the participant should be consistent, in either reclined lying or lying with the leg supported. For optimal results, we recommend approximately 60° of flexion at the hip and knee of the leg that is being evaluated.
The detection threshold can be defined as the lowest detectable stimulus intensity after three successive trials.42 Following the establishment of the detection threshold, progressively increasing stimulations should be delivered with an 8 to 12-second inter-stimulus delay at increasing intensities of 2 mA.26,30,31 These stimuli will familiarize participants to noxious stimulation intensities required to elicit NFR responses, to both limit anxiety to oncoming noxious stimuli and reduce background EMG activity during subsequent assessments.25,27,29,32 During this process, the participants are asked to report their subjective pain rating on a visual analogue scale (VAS) from 0 to 10 following each stimulus. The stimulus intensity should be progressively increased in 2 mA increments from the detection threshold until the participant can no longer tolerate further increases in stimulus intensity. We suggest that the intensity of stimulations not exceed a VAS score of 7/10 to ensure participant safety.31
Following familiarization, two options are available for determining the NFR threshold, either relying on increasing stimuli at 2 mA intensities until NFR responses are present26,30,31 or using a staircase approach with multiple increasing and decreasing series of stimuli in 1 mA increments.29,32 Our review of the literature supported both approaches. A previous study validated the use of a single series of stimuli compared to a repeated staircase method.38 However, a repeated staircase method provides the advantage of multiple series of stimuli to evaluate and re-evaluate NFR threshold, contributing to improved consistency of the outcome. While this choice remains challenging for researchers, we strongly suggest the use of a series of familiarization stimuli (which may be the first series in a staircase method) prior to either a single series or repeated staircase of stimuli.
To analyse the raw EMG signals, they should be band-pass filtered at 50–500 Hz using a second-order Butterworth filter prior to full-wave rectification.34,43,44 The EMG signals are typically amplified 500–1000 times and sampled at a rate of 2000 Hz. NFR responses should be visually identified based on rectified EMG activity in the 90–150 ms post-stimulus window26,28,30–32 that exceeded baseline mean EMG, sampled from 60 ms pre-stimulus.30–32 This response window is preferred to avoid contamination from tactile-mediated responses, typically with latencies of 40–70 ms in the lower limb7 and 35–55 ms in the upper limb,45 as well as to movement response that occurs 250 ms onwards. To expedite the process of NFR response identification, a z-score approach can be used, which is based on methods described by Rhudy and France18 and used by numerous studies within this review.26,29–31
The determination of the primary outcome measure of the NFR, the NFR threshold, remains variable both in the assessment of chronic pain conditions presented within this review and elsewhere.9,15,24–32,46 The use of differing classifications of NFR responses, as well as the use of single versus multiple series of stimuli, was a noted source of variability among the included studies of this review.24–32 Ultimately, the standardization of both the definition of the NFR threshold and its acquisition in chronic pain populations should be a long-term goal within the field. Based on the findings of this review, we suggest a familiarization series of stimuli, followed by either a single series of 2 mA increasing increments only26,30,31 or in combination with an additional two series of increasing and decreasing stimuli in 1 mA increments.29,32 NFR responses should be classified as present, if EMG activity in the 90–150 ms response window25,26,28–32 is greater than 1 standard deviation above baseline28,32 or reaches a z-score value greater than 10.32.26,29–31 The lowest stimulus intensity during each series of stimulations should be recorded, and the average of these values (if appropriate) would comprise the NFR threshold.
Additional factors that influence NFR
There are several factors that can influence the NFR. Sex effects of NFR thresholds have been reported in a variety of studies, with inconclusive findings.47,48 Previous studies have reported lower NFR thresholds in males,47 while other studies have reported reduced thresholds in females.48–50 The most recent study – with sample sizes of over 152 males and 148 females, respectively – concluded no significant effect of sex on NFR threshold.51
It has also been shown that the time of day can affect the NFR threshold. Sandrini et al.52 showed that the NFR threshold progressively increases with the phases of sleep reaching its peak during the rapid eye movement (REM) phase. In addition, it was during REM sleep that the maximum increase in the amplitude and duration of the NFR was determined.52 Edwards et al.53 showed that the NFR threshold is also affected by the cardiac cycle, being attenuated during systole. While studies often report similar time of day of assessments in a repeated measure designed experiments, a few studies have accommodated the influence of cardiac cycle on the NFR since the discovered relationship by Edwards et al.53 None of the articles presented within the scope of our review monitored heart rate or accounted for the cardiac cycle in their NFR assessments of individuals living with chronic pain. This is an area of research that may require future investigation in the determination of optimal assessment protocols for the NFR for individuals living with chronic pain.
There are numerous technical aspects to obtaining the NFR since it can vary based upon the site of stimulation.34 The maximum amplitude is obtained when the stimulation site and muscle from which it is recorded correspond in regard to their innervation.54 The NFR threshold has been shown to be higher outside the zone of innervation of the receptive field.55
Known issues in NFR assessments
Efforts should be made to maintain a consistent position between participants during NFR acquisition. Changes in participant position within or between trials will influence the NFR, as the NFR is known to adapt to both stimulus location and posture.34 A baseline level of muscle contraction increases the difficulty in ascertaining NFR responses. Participants should be at rest, with little to no background EMG activity during NFR assessments. Consistent participant positioning along with a familiarization session to limit anxiety to oncoming noxious stimuli can improve background EMG activity during NFR assessments. The use of steadily increasing stimulus intensity may bias participants to overestimate their pain ratings during NFR assessments. While some studies have proposed alternative delivery methods in experimental pain models and young healthy populations,39,56 further studies exploring the use of randomized stimuli delivery algorithms are needed in NFR assessments for those living with chronic pain.
Participants’ perceptual response to noxious stimulations is also a limiting factor in NFR assessments. Previous studies have reported difficulty in ascertaining NFR thresholds due to stimulus intensities required to elicit responses being too painful for some participants.57–60 There is some evidence among the studies within this review26,29,31 that this may be disproportionate in those affected by chronic pain compared to matched healthy controls. Some recent studies have proposed altering stimulus location or methods of NFR threshold acquisition to combat these issues;39,57 however, there remains a need for further investigation of these methods within clinical populations.
Conclusion
Following a review of the literature, we have synthesized the current state of NFR acquisition for those living with chronic pain. While the NFR technique continues to show promise as an objective assessment tool of nociception, the between-study variability in NFR acquisition methodology remains a cause for concern within the field. We have provided a proposed methodology for NFR acquisition within individuals living with chronic pain as a starting point to shift the research field towards the adoption of a standardized methodology in both research and clinical practice. Future studies should continue to develop novel approaches to limit participant expectations to oncoming noxious stimuli and contribute to the development of a standardized method to objectively assess nociception for individuals living with chronic pain.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: D.A.K. is the guarantor of this article.
References
- 1. Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain Res Manag 2011; 16(6): 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuehn B. Chronic pain prevalence. JAMA 2018; 320(16): 1632. [DOI] [PubMed] [Google Scholar]
- 3. Lynch ME. The need for a Canadian pain strategy. Pain Res Manag 2011; 16(2): 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choiniere M, Dion D, Peng P, et al. The Canadian STOP-PAIN project – part 1: who are the patients on the waitlists of multidisciplinary pain treatment facilities? Can J Anaesth 2010; 57(6): 539–548. [DOI] [PubMed] [Google Scholar]
- 5. Phillips CJ. The cost and burden of chronic pain. Rev Pain 2009; 3(1): 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Younger J, McCue R, Mackey S. Pain outcomes: a brief review of instruments and techniques. Curr Pain Headache Rep 2009; 13(1): 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain 1977; 3(1): 69–80. [DOI] [PubMed] [Google Scholar]
- 8. Kugelberg E, Eklund K, Grimby L. An electromyographic study of the nociceptive reflexes of the lower limb: mechanism of the plantar responses. Brain 1960; 83(3): 394–410. [DOI] [PubMed] [Google Scholar]
- 9. Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans – review article. Pain 2002; 96(1–2): 3–8. [DOI] [PubMed] [Google Scholar]
- 10. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006; 123(3): 231–243. [DOI] [PubMed] [Google Scholar]
- 11. Sandrini G, Alfonsi E, Bono G, et al. Circadian variations of human flexion reflex. Pain 1986; 25(3): 403–410. [DOI] [PubMed] [Google Scholar]
- 12. von Dincklage F, Hackbarth M, Schneider M, et al. Introduction of a continual RIII reflex threshold tracking algorithm. Brain Res 2009; 1260: 24–29. [DOI] [PubMed] [Google Scholar]
- 13. Sandrini G, Degli Uberti EC, Salvadori S, et al. Dermorphin inhibits spinal nociceptive flexion reflex in humans. Brain Res 1986; 371(2): 364–367. [DOI] [PubMed] [Google Scholar]
- 14. Sandrini G, Alfonsi E, De Rysky C, et al. Evidence for serotonin-S2 receptor involvement in analgesia in humans. Eur J Pharmacol 1986; 130(3): 311–314. [DOI] [PubMed] [Google Scholar]
- 15. Lim ECW, Sterling M, Stone A, et al. Central hyperexcitability as measured with nociceptive flexor reflex threshold in chronic musculoskeletal pain: a systematic review. Pain 2011; 152(8): 1811–1820. [DOI] [PubMed] [Google Scholar]
- 16. Andersen OK, Sonnenborg FA, Arendt-Nielsen L. Reflex receptive fields for human withdrawal reflexes elicited by non-painful and painful electrical stimulation of the foot sole. Clin Neurophysiol 2001; 112(4): 641–649. [DOI] [PubMed] [Google Scholar]
- 17. Arendt-Nielsen L, Brennum J, Sindrup S, et al. Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. Eur J Appl Physiol Occup Physiol 1994; 68(3): 266–273. [DOI] [PubMed] [Google Scholar]
- 18. Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria. Pain 2007; 128(3): 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 20. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 21. Higgins J, Thomas J, Chandler J, et al. (eds). Cochrane handbook for systematic reviews of interventions version 6.0 (Updated July 2019). Cochrane, 2019. www.training.cochrane.org/handbook
- 22. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hedges LV, Pustejovsky JE, Shadish WR. A standardized mean difference effect size for single case designs: an effect size for single case designs. Res Syn Meth 2012; 3(3): 224–239. [DOI] [PubMed] [Google Scholar]
- 24. Desmeules J, Chabert J, Rebsamen M, et al. Central pain sensitization, COMT Val158Met polymorphism, and emotional factors in fibromyalgia. J Pain 2014; 15(2): 129–135. [DOI] [PubMed] [Google Scholar]
- 25. Krafft S, Göhmann HD, Sommer J, et al. Learned control over spinal nociception in patients with chronic back pain. Eur J Pain 2017; 21(9): 1538–1549. [DOI] [PubMed] [Google Scholar]
- 26. Lim ECW, Sterling M, Pedler A, et al. Evidence of spinal cord hyperexcitability as measured with nociceptive flexion reflex (NFR) threshold in chronic lateral epicondylalgia with or without a positive neurodynamic test. J Pain 2012; 13(7): 676–684. [DOI] [PubMed] [Google Scholar]
- 27. Biurrun Manresa JA, Neziri AY, Curatolo M, et al. Reflex receptive fields are enlarged in patients with musculoskeletal low back and neck pain. Pain 2013; 154(8): 1318–1324. [DOI] [PubMed] [Google Scholar]
- 28. Rhudy JL, DelVentura JL, Terry EL, et al. Emotional modulation of pain and spinal nociception in fibromyalgia. Pain 2013; 154(7): 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rice DA, Parker RS, Lewis GN, et al. Pain catastrophizing is not associated with spinal nociceptive processing in people with chronic widespread pain. Clin J Pain 2017; 33(9): 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith AD, Jull G, Schneider G, et al. Cervical radiofrequency neurotomy reduces central hyperexcitability and improves neck movement in individuals with chronic whiplash. Pain Med 2014; 15(1): 128–141. [DOI] [PubMed] [Google Scholar]
- 31. Sterling M. Differential development of sensory hypersensitivity and a measure of spinal cord hyperexcitability following whiplash injury. Pain 2010; 150(3): 501–506. [DOI] [PubMed] [Google Scholar]
- 32. Umeda M, Corbin LW, Maluf KS. Preliminary investigation of absent nociceptive flexion reflex responses among more symptomatic women with fibromyalgia syndrome. Rheumatol Int 2013; 33(9): 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 34. Peterson CL, Riley ZA, Krepkovich ET, et al. Withdrawal reflexes in the upper limb adapt to arm posture and stimulus location. Muscle Nerv 2014; 49(5): 716–723. [DOI] [PubMed] [Google Scholar]
- 35. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013; 111(1): 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hurley RW, Adams MCB. Sex, gender, and pain: an overview of a complex field. Anesth Analg 2008; 107(1): 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bohns VK, Wiltermuth SS. It hurts when I do this (or you do that): posture and pain tolerance. J Exp Soc Psychol 2012; 48(1): 341–345. [Google Scholar]
- 38. Rhudy JL, France CR. Reliability and validity of a brief method to assess nociceptive flexion reflex (NFR) threshold. J Pain 2011; 12(7): 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jensen MB, Frahm KS, Biurrun Manresa J, et al. Novel cross correlation technique allows crosstalk resistant reflex detection from surface EMG. In: Proceedings of the annual international conference of the IEEE engineering in medicine and biology society (EMBS), San Diego, CA, 28 August–1 September 2012, pp. 3531–3534. New York: IEEE. [DOI] [PubMed] [Google Scholar]
- 40. Jurth C, Dörig TM, Lichtner G, et al. Development, validation and utility of a simulation model of the nociceptive flexion reflex threshold. Clin Neurophysiol 2018; 129(3): 572–583. [DOI] [PubMed] [Google Scholar]
- 41. De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech 1997; 13(2): 135–163. [Google Scholar]
- 42. Andersen OK, Gracely RH, Arendt-Nielsen L. Facilitation of the human nociceptive reflex by stimulation of A beta-fibres in a secondary hyperalgesic area sustained by nociceptive input from the primary hyperalgesic area. Acta Physiol Scand 1995; 155(1): 87–97. [DOI] [PubMed] [Google Scholar]
- 43. Serrao M, Pierelli F, Don R, et al. Kinematic and electromyographic study of the nociceptive withdrawal reflex in the upper limbs during rest and movement. J Neurosci 2006; 26(13): 3505–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serrao M, Ranavolo A, Andersen OK, et al. Reorganization of multi-muscle and joint withdrawal reflex during arm movements in post-stroke hemiparetic patients. Clin Neurophysiol 2012; 123(3): 527–540. [DOI] [PubMed] [Google Scholar]
- 45. Cambier J, Dehen H, Bathien N. Upper limb cutaneous polysynaptic reflexes. J Neurol Sci 1974; 22(1): 39–49. [DOI] [PubMed] [Google Scholar]
- 46. Sandrini G, Serrao M, Rossi P, et al. The lower limb flexion reflex in humans. Prog Neurobiol 2005; 77(6): 353–395. [DOI] [PubMed] [Google Scholar]
- 47. Page GD, France CR. Objective evidence of decreased pain perception in normotensives at risk for hypertension. Pain 1997; 73(2): 173–180. [DOI] [PubMed] [Google Scholar]
- 48. France CR, Suchowiecki S. A comparison of diffuse noxious inhibitory controls in men and women. Pain 1999; 81(1–2): 77–84. [DOI] [PubMed] [Google Scholar]
- 49. Mylius V, Kunz M, Schepelmann K, et al. Sex differences in nociceptive withdrawal reflex and pain perception. Somatosens Mot Res 2005; 22(3): 207–211. [DOI] [PubMed] [Google Scholar]
- 50. Serrao M, Rossi P, Sandrini G, et al. Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain 2004; 112(3): 353–360. [DOI] [PubMed] [Google Scholar]
- 51. Neziri AY, Andersen OK, Petersen-Felix S, et al. The nociceptive withdrawal reflex: normative values of thresholds and reflex receptive fields. Eur J Pain 2010; 14(2): 134–141. [DOI] [PubMed] [Google Scholar]
- 52. Sandrini G, Milanov I, Rossi B, et al. Effects of sleep on spinal nociceptive reflexes in humans. Sleep 2001; 24(1): 13–17. [DOI] [PubMed] [Google Scholar]
- 53. Edwards L, Ring C, McIntyre D, et al. Modulation of the human nociceptive flexion reflex across the cardiac cycle. Psychophysiology 2001; 38(4): 712–718. [PubMed] [Google Scholar]
- 54. Syrovegin AV, Kukushkin ML, Gnezdilov AV, et al. EMG responses in humans during painful heterosegmentary stimulation. Bull Exp Biol Med 2000; 130(5): 1069–1073. [PubMed] [Google Scholar]
- 55. Schouenborg J, Kalliomaki J. Functional organization of the nociceptive withdrawal reflexes. I. Activation of hindlimb muscles in the rat. Exp Brain Res 1990; 83(1): 67–78. [DOI] [PubMed] [Google Scholar]
- 56. Gronroos M, Pertovaara A. Capsaicin-induced central facilitation of a nociceptive flexion reflex in humans. Neurosci Lett 1993; 159(1–2): 215–218. [DOI] [PubMed] [Google Scholar]
- 57. Jensen MB, Biurrun Manresa J, Andersen OK. Reliable estimation of nociceptive withdrawal reflex thresholds. J Neurosci Methods 2015; 253: 110–115. [DOI] [PubMed] [Google Scholar]
- 58. Banic B, Petersen-Felix S, Andersen OK, et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 2004; 107(1–2): 7–15. [DOI] [PubMed] [Google Scholar]
- 59. Biurrun Manresa J, a Fritsche R, Vuilleumier PH, et al. Is the conditioned pain modulation paradigm reliable? A test-retest assessment using the nociceptive withdrawal reflex. PLoS ONE 2014; 9(6): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sterling M, Hodkinson E, Pettiford C, et al. Psychologic factors are related to some sensory pain thresholds but not nociceptive flexion reflex threshold in chronic whiplash. Clin J Pain 2008; 24(2): 124–130. [DOI] [PubMed] [Google Scholar]