Abstract
The blood-brain barrier (BBB), which protects the CNS from pathogens, is composed of specialized brain microvascular endothelial cells (BMECs) joined by tight junctions and ensheathed by pericytes and astrocyte endfeet. The stability of the BBB structure and function is of great significance for the maintenance of brain homeostasis. When a neurotropic virus invades the CNS via a hematogenous or non-hematogenous route, it may cause structural and functional disorders of the BBB, and also activate the BBB anti-inflammatory or pro-inflammatory innate immune response. This article focuses on the structural and functional changes that occur in the three main components of the BBB (endothelial cells, astrocytes, and pericytes) in response to infection with neurotropic viruses transmitted by hematogenous routes, and also briefly describes the supportive effect of three cells on the BBB under normal physiological conditions. For example, all three types of cells express several PRRs, which can quickly sense the virus and make corresponding immune responses. The pro-inflammatory immune response will exacerbate the destruction of the BBB, while the anti-inflammatory immune response, based on type I IFN, consolidates the stability of the BBB. Exploring the details of the interaction between the host and the pathogen at the BBB during neurotropic virus infection will help to propose new treatments for viral encephalitis. Enhancing the defense function of the BBB, maintaining the integrity of the BBB, and suppressing the pro-inflammatory immune response of the BBB provide more ideas for limiting the neuroinvasion of neurotropic viruses. In the future, these new treatments are expected to cooperate with traditional antiviral methods to improve the therapeutic effect of viral encephalitis.
Keywords: Blood-brain barrier (BBB), innate immune response, IFN signaling, viral neuroinvasion, SARS-CoV-2
Introduction
The blood-brain barrier (BBB) is centrally positioned within the neurovascular unit (NVU) and is mainly formed by a monolayer of tightly sealed endothelial cells along the vascular tree, expressing low paracellular and transcellular permeability. Under normal physiological conditions, the BBB prevents neurotoxic plasma components, blood cells, and pathogens from entering the brain. It also regulates the transport of molecules into and out of the nervous system, strictly controls the chemical composition of the neuronal microenvironment, and maintains the normal function of neurons. In disease states, BBB breakdown and dysfunction lead to leakages of harmful blood components into the CNS, cellular infiltration, and aberrant transport and clearance of molecules, which is associated with cerebral blood-flow reductions and dys-regulation, thus contributing to neurological deficits.1 The breakdown of the BBB can be seen in many diseases, such as several neurodegenerative diseases, including Alzheimer’s disease,2 brain tumors and brain metastases,3 and ischemic stroke.4 An imbalance of BBB function may be related to these diseases. In addition, a study pointed out that the breakdown of the BBB is an independent early biomarker of cognitive impairment, not related to Aβ and tau.5 More and more research in the future will help to reveal how BBB dysfunction is involved in the process of disease.
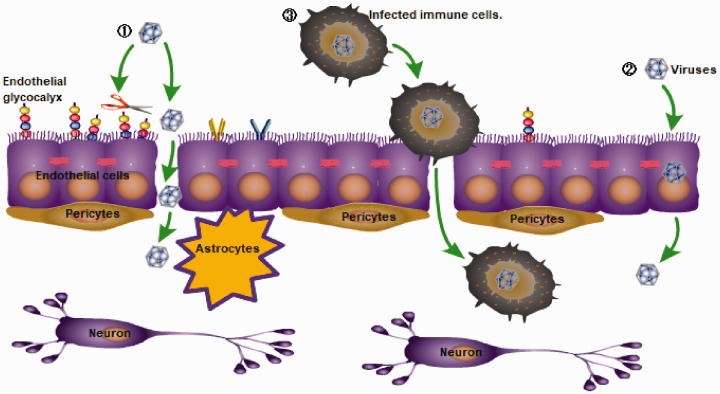
Not surprisingly, neurotropic virus infection also more or less affects the functional homeostasis of the BBB, changes the permeability of the BBB, and induces the BBB pro-inflammatory and anti-inflammatory innate immune responses. Many neurotropic viruses can cross the BBB via a hematogenous route and eventually invade the CNS, such as Japanese encephalitis virus (JEV), Zika virus (ZIKV), West Nile virus (WNV), tick-borne encephalitis virus of the Flaviviridae,6 Eastern, Western, Venezuelan equine encephalitis viruses of the Togaviridae,7 HIV of the Retroviridae, and others. Viruses can cross the BBB through three different mechanisms (Figure 1): the paracellular pathway (between cells),8 the transcellular pathway (through cells),9 or a ‘Trojan Horse’ mechanism through diapedesis of infected immune cells.10,11 For example, VEEV and WEEV can enter the CNS via hematogenous dissemination across an intact BBB by utilizing caveolin-mediated transcytosis.7 The non-hematogenous routes by which viruses enter the CNS are retrograde axonal transport12,13 and transynaptic trafficking.14 Considering viruses transmitted by hematogenous routes (such as flaviviruses, HIV, etc.) that rely on crossing the BBB to invade the CNS as an example, this article will mainly introduce the interaction between the host and the pathogen at the BBB to explore the relevant immune response.
Figure 1.
Three main ways by which viruses pass through the blood-brain barrier (BBB): (1) the paracellular pathway (between cells), (2) the transcellular pathway (through cells), or (3) a ‘Trojan Horse’ mechanism through diapedesis of infected immune cells.
How do endothelial cells of the BBB respond to viral infections?
BBB endothelial cells under normal physiological conditions
As is known, the BBB occurs at the level of post-capillary venules and capillaries. The BBB endothelial cells have unique biological characteristics as the first physical and immune barriers against pathogen invasion. First, CNS endothelial cells have specialized tight junctions (TJs) to prevent free paracellular passage through the vessel wall. Second, they express designated transporters to regulate the dynamic influx and efflux of specific substrates. Third, they display extremely low rates of transcellular vesicle trafficking, termed transcytosis, to limit transcellular transport through the vessel wall. Last, CNS endothelial cells have low expression levels of leukocyte adhesion molecules to limit the entry of immune cells into the brain.15,16 In addition, Rho GTPase (including RhoA, Rac1, etc.) of the BBB endothelial cells also plays an important role in maintaining the steady state of the BBB. Rho GTPase signaling pathway changes the steady state of endothelial cell-cell junction by controlling the assembly and disassembly of endothelial cytoskeletal protein, which, in turn, affects BBB permeability. For example, pro-inflammatory cytokines or growth factors can activate the RhoA/ROCK/pMLC signaling pathway, which promotes the formation of stress fibers and disrupts junctions, causing an increase in paracellular permeability. In general, hyperactivation of RhoA and its downstream effectors is associated with endothelial barrier disruption. Rac1, however, promotes endothelial barrier properties and is related to the down-regulation of RhoA.17,18 When a virus invades the CNS through the BBB, endothelial cells are the front-line cells that sense the virus and can quickly form immune responses and interact with other cells to intervene in the neuroinvasion of the virus.
When a neurotropic virus reaches the BBB following microcirculation
Damage effects of viral proteins on endothelial cells
Some neurotropic viruses directly or indirectly destroy the integrity of the BBB endothelial barrier through viral proteins. For example, nonstructural protein 1 (NS1) of WNV and JEV in Flaviviridae can up-regulate the expression of cathepsin L and endoglycosidase heparanase in endothelial cells, leading to the degradation of glycocalyx-like layer (EGL) components (Figure 1), which ultimately leads to the high permeability of the BBB.19 Inhibitors of heparanase and cathepsin L can prevent the in vivo EGL destruction and vascular leakage caused by NS1.20 In addition, the HIV trans-activator of transcription (Tat) protein also changes the integrity of endothelial cells. On one hand, the activation of the RhoA signaling pathway destroys the TJ and simultaneously induces the nuclear localization of ZO-1. On the other hand, it activates matrix metalloproteinases (MMPs) and proteasome, which promotes the degradation of TJ proteins and ultimately leads to increased permeability of the endothelial barrier.21 Enterovirus A71 (EV-A71), as the pathogen of hand, foot, and mouth diseases, was recently thought to invade the CNS by crossing the BBB, inducing encephalitis and other CNS symptoms.22 In vivo and in vitro experiments found that the EV-A71 capsid protein VP can reduce the expression of claudin-5 and increase the expression of the virus receptor vimentin in endothelial cells, which destroys the stability of the BBB and facilitates the ability of the virus to enter the Abs parenchyma.23 Viral protein-neutralizing antibodies and blockers have the opportunity to reduce the BBB penetration of neurotropic viruses.
Interactions between the BBB endothelial cell-specific receptors and viruses
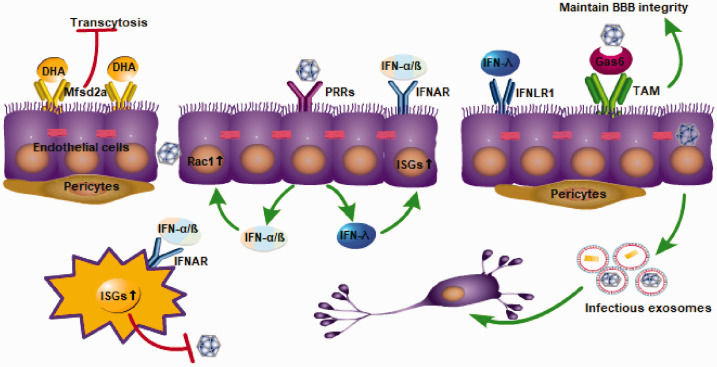
The BBB endothelial cells express a variety of receptors, such as the classic PRR receptor and the specifically expressed major facilitator super family domain containing 2a (Mfsd2a) receptor and TAM receptor, which play an important role in the maintenance of the BBB homeostasis, virus recognition, and the initiation of immune responses (Figure 2). Here, we mainly discuss the Mfsd2a receptor and TAM receptor; the PRR receptor and its signaling pathway will be discussed later.
Figure 2.
Receptors expressed by endothelial cells, such as PRRs, TAM, and major facilitator super family domain containing 2a (Mfsd2a), have the function of stabilizing the BBB. Endothelial cells express a variety of PRRs, and after recognizing PAMPs of viruses, they can activate the IFN signaling pathway and induce the production of type I IFN (IFN-1) and type III IFN in endothelial cells. The combination of IFN-1s and their receptor, IFNAR, can activate the JAK-STAT signaling cascade, and promote the expression of a series of antiviral gene IFN-stimulated genes. In addition, IFN-1 can activate Rac1 in endothelial cells to stabilize the integrity of the BBB.
The Mfsd2a is selectively expressed in CNS endothelial cells and plays a role in inhibiting transcytosis, thereby maintaining the integrity of the BBB. Mfsd2a had a 78.8-fold higher expression in the cortical endothelium compared to the lung endothelium at embryonic stages (E15.5). Mfsd2a-deficient mice have demonstrated increased transcytosis from embryonic stages to adulthood.24 In addition, pericytes also regulate the expression of Mfsd2a in endothelial cells through an unknown method. The expression of Mfsd2a is down-regulated and vesicular trafficking is increased in pericyte-deficient mice. The reduction in Mfsd2a gene expression is directly related to the degree of pericyte coverage.24 In addition to maintaining the stability of the BBB, Mfsd2a is also the major transporter for docosahexaenoic acid (DHA) uptake into the brain (Figure 2). Mfsd2a transports DHA in the form of lysophosphatidylcholine in a sodium-dependent manner. Mfsd2a-deficient mice show markedly reduced levels of DHA in the brain, accompanied by neuronal cell loss in the hippocampus and cerebellum, as well as cognitive deficits and severe anxiety and microcephaly.25 Unexpectedly, the role of Mfsd2a in maintaining BBB stability is not independent of DHA transport but rather interrelated. That is, lipids transported by Mfsd2a establish a unique lipid environment that inhibits caveolae vesicle formation in CNS endothelial cells to suppress transcytosis and ensure BBB integrity.26 The ZIKV envelope (E) protein specifically interacts with Mfsd2a and promotes Mfsd2a polyubiquitination for proteasome-dependent degradation. ZIKV inhibited brain Mfsd2a protein levels in mice without influencing Mfsd2a mRNA levels. In addition, the down-regulation of the Mfsd2a protein is fairly sensitive to ZIKV E but not to the WNV envelopes. Providing DHA to ZIKV-infected newborn mice at early stages (at postnatal day 0 and day 3) increased the brain Mfsd2a level and inhibited ZIKV RNA replication.27 In summary, TAM and Mfsd2a receptors provide us with new ideas for the regulation of BBB stability and the inhibition of viral neuroinvasion.
The TAM receptors Tyro3, Axl, and Mertk are receptor tyrosine kinases that, after binding to their ligand Gas6 and protein S, can recognize phospholipid molecules on enveloped viruses. There are two main types of TAM, Axl and Mertk, which are expressed on endothelial cells and are essential for maintaining the integrity of the BBB. Compared to wild type (WT) cells, Axl–/–MerTK–/– BMECs have lower transendothelial electrical resistance and higher WNV transit and invasion, which suggests a higher BBB permeability in the absence of TAM. TAM receptor signaling maintains the integrity of the endothelial barrier and limits WNV transport.28 To date, there are few studies about the role and specific mechanisms of TAM receptors in viral infection, and more evidence is still required to explore this.
Endothelial cells initiate type I and type III IFN signaling pathways through PRRs
Endothelial cells express a variety of PRRs, and after recognizing PAMPs of viruses, they can activate the type I IFN signaling pathway and induce the production of type I IFN (IFN-1) in endothelial cells.29 The combination of IFN-1 and the heterodimeric receptor IFNAR produces a cellular response that initiates a signaling cascade, which promotes heterodimers STAT1/2 nuclear translocation and transcriptional activation of IFN-stimulated genes (ISGs; Figure 2).30 The rapid expression of hundreds of ISGs is critical for controlling viral infections because these proteins block the entry, translation, transcription, assembly, and efflux of the virus.31 For example, WNV-sensing PRRs of endothelial cells such as retinoic acid-inducible gene I (RIG-I), MDA-5, and TLR7, after sensing WNV, activate the IFN-1 signaling pathway to produce IFN-1. In addition to inducing a large number of antiviral ISGs, IFN-1 also activates Rac1 and inhibits RhoA to maintain the integrity of endothelial cell TJs. In addition, the enhancement of BBB function by IFN-1 is also reflected in the counteraction of the effect of barrier-disrupting factors (such as TNF-α and IL-1β).29
Recently, the role of type III IFN (IFN-λ) in stabilizing the BBB has also received more attention.32 IFN-λ is also induced by PRRs after recognition of PAMPs, and has almost the same signal pathway as IFN-1. The combination of IFN-λ and IFN-λ receptor (IFNLR1) also activates the downstream signaling cascade and promotes the expression of a series of antiviral genes, such as ISGs.33 Recently, using an in vitro three-dimensional BBB model, it was found that after PRRs were stimulated by synthetic viral RNA mimetic poly(I:C), human BMECs (hBMECs) enhanced the expression of IFN-λ1, IFN-λ2, and IFN-β, with the level of IFN-λ higher than that of IFN-β.34 It can be seen that endothelial cells have the ability to produce IFN-1 and IFN-λ after sensing the virus. However, due to the small breadth and magnitude of ISGs induced by IFN-λ, sometimes the expression of ISGs induced by IFN-λ is not enough to exert an antiviral effect (such as anti-WNV infection).35 More importantly, the IFN-λ signaling pathway can regulate endothelial cell TJ integrity in a STAT1-independent manner, thereby reducing the permeability of the BBB and limiting virus neuroinvasion. Compared to WT mice, IFNLR1-deficient mice displayed higher BBB permeability and higher virus titers in the brain after WNV infection.35 IFN-λ plays an important role in the stability of the BBB, and its specific mechanism still needs further exploration.
When a neurotropic virus enters endothelial cells
In vitro experiments have confirmed that some flaviviruses can infect and replicate in BMECs in vertebrates.36,37 However, the infection and replication of flavivirus in BMECs of human patients still lack sufficient direct evidence and require histological confirmation. ZIKV persistently infects and continuously replicates in primary hBMECs in vitro, without obvious cytopathology or increased endothelial cell permeability. hBMECs, as a reservoir of persistent ZIKV replication, can release ZIKV basolaterally and potentially provide ZIKV the ability to enter neuronal compartments.37,38 In addition, under some flavivirus infections, the infected endothelium has a tendency to release infectious exosomes, which was recently discovered in LGTV and has not been found in other flaviviruses. At 24-to-48 hours after LGTV infection, brain endothelial cell–derived exosomes contain higher loads of LGTV-positive and -negative-sense RNA strands and viral proteins (Figure 2). These released exosomes are infectious transporters that could lead to the dissemination of flaviviruses to neuronal cells at earlier time points.39 In addition to certain flaviviruses, brain endothelial cells have been found to release virus-related exosomes during HIV infection, which plays a role in HIV brain transmission and HIV-1-related Aβ pathology.40,41 The discovery of infectious exosomes provides new opportunities to prevent the virus from invading the brain. In addition, certain flaviviruses, such as JEV/ZIKV, have the ability to change the expression of adhesion molecules and chemotactic molecules in cerebral vascular endothelial cells, which facilitates the infiltration of leukocytes into the CNS.38,42 Blocking the release of infectious exosomes and inhibiting the abnormal immune response of endothelial cells have a great potential to reduce the neural damage caused by viruses.
Pericytes of the BBB
Pericytes under normal physiological conditions
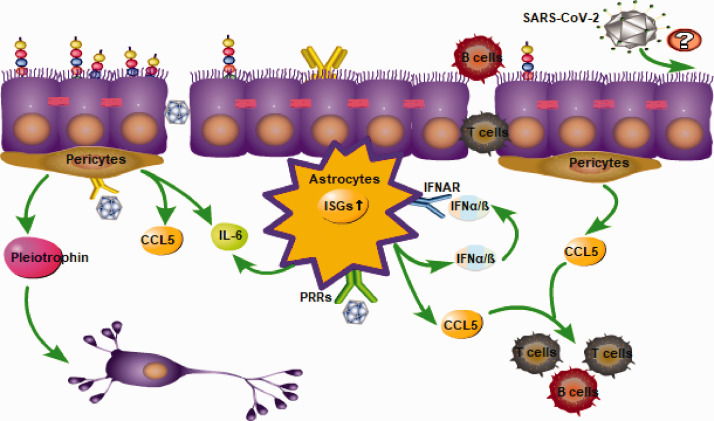
Pericytes play a role in maintaining the stability of the BBB, and pericyte-derived pleiotrophin, a neurotrophic growth factor, plays a role in maintaining the survival of neurons (Figure 3).43 The function of pericytes at the BBB is mainly reflected in the regulation of endothelial cell transcytosis and the induction of astrocyte endfeet polarization surrounding the CNS blood vessels. In pericyte-deficient mutant mice, increased endothelial transcytosis and abnormal polarization of the astrocyte endfeet can be seen.44 In an adult pericyte-deficient murine model, the loss of pericytes, on the one hand, reduces the cerebral microcirculation and ultimately leads to chronic perfusion stress and hypoxia. On the other hand, the breakdown of the BBB leads to accumulation of serum proteins and several vasculotoxic and/or neurotoxic macromolecules. The combined effect of these two processes eventually leads to secondary neuronal injury and neurodegeneration.45 It is worth mentioning that occludin is also expressed in pericytes, but occludin in pericytes is not a TJ protein, although it plays a role in regulating cell metabolism. At present, there are few studies on the role of occludin in pericytes, which are known to function like NADH oxidase,46 in addition to regulating glucose uptake and ATP production.47
Figure 3.
Pericytes and astrocytes induce pro-inflammatory and anti-inflammatory innate immune responses. The released chemotactic molecules promote the infiltration of leukocytes. Neuroinvasion characteristics of severe acute respiratory syndrome coronavirus 2.
Changes in the structure and function of pericytes during virus infection
Pericytes were susceptible to JEV infection in vitro, but were without signs of remarkable cytotoxicity.48 JEV-infected pericytes up-regulated the TLR7/MyD88 signaling axis, leading to a profound production of IL-6 and CCL5 (Figure 3).49 IL-6 released by JEV-infected pericytes induced the expression of the ubiquitin-protein ligase E3 component n-recognin-1 (Ubr 1) in brain endothelial cells, which led to proteasomal degradation of ZO-1, thereby causing disruption of endothelial barrier integrity.48 The released CCL5, as a chemokine, has the ability to drive the migration of monocytes and T cells to the BBB.50,51 A large number of peripheral immune cells infiltrate the CNS by crossing the BBB during viral infection, and while controlling the viral load, immunopathology occurs.52 Interestingly, the latest research on a murine model of ZIKV infection suggests that the ZIKV may invade the CNS by crossing the blood-CSF barrier rather than the BBB by infecting pericytes in the choroid plexus.53 This puts forward new opportunities for us to study the mechanism of flavivirus invasion of the CNS.
In addition to flaviviruses, recent results from in vitro, in vivo, and human samples of HIV-associated neurocognitive disorder (HAND) patients clearly indicate that HIV can infect pericytes. HIV-infected pericytes can form a latent infection state and become a potential HIV reservoir.54 Moreover, an analysis of cortical brain tissue samples from simian immunodeficiency virus (SIV)-infected macaques and HIV-1 encephalitis (HIVE) patients found that during HIV/SIV infection, the morphology of viral protein-positive pericytes changed, and these pericytes were visible as hypertrophied pericytes. In addition, the loss of pericytes has been found in the lesions of SIVE/HIVE. Hypertrophic pericytes may be involved in the initiation of the BBB destabilization, and the loss of pericytes further disrupts the stability of the BBB.55 In vitro experiments have also found that during HIV infection, occludin exerts its role as NADH oxidase and activates class III histone deacetylase sirtuin, which has the function of partially inhibiting HIV replication.46 During viral infection, it is important to regulate the function of pericytes properly and suppress their abnormal immune activation.
Astrocytes of the BBB
Astrocytes under normal physiological conditions
Astrocyte-ensheathing capillaries constitute the most abluminal layer of the NVU. These cells contact the outer basement membrane of the brain vasculature via polarized endfeet that express the water channel aquaporin 4.16 Significantly, astrocytes are important in the development and maintenance of the BBB characteristics in endothelial cells through the release of growth factors, such as vascular endothelial growth factor (VEGF), glial cell line-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), and ANG-1.56 Moreover, recent studies have found that astrocyte-derived glutathione plays a strategic role in endothelial stability by suppressing endothelial cell TJ phosphorylation and delocalization.57
Performance of astrocytes during virus infection
Astrocytes express a variety of PRRs, such as TLR3, 7, and 9, and RIG-I-like receptors (RLRs), which can induce the production of IFN-1 after identifying viral PAMPs (Figure 3).58 As mentioned before, the combination of IFN-1s and their receptor, IFNAR, can activate the JAK-STAT signaling cascade, and promote the expression of a series of antiviral gene ISGs. In addition, IFNs can regulate the Rho GTPase of endothelial cells, and activate Rac1 to stabilize the integrity of the BBB. Because of the regional heterogeneity of astrocytes,59 cerebellar astrocytes have a higher basal and IFN-induced expression of PRRs and ISGs compared to cerebral cortex astrocytes. During WNV infection, astrocyte IFNAR signaling-deficient mice have increased BBB permeability in the hindbrain, suggesting that astrocyte type I IFNAR signaling plays a more important role in regulating cerebellar BBB permeability.60
In addition to the production of anti-inflammatory cytokines based on IFNs, astrocytes also exhibit some pro-inflammatory effects during virus neuroinvasion. JEV-infected astrocytes produce a number of barrier-damaging molecules, including VEGF, IL-6, and MMP-2/MMP-9. These bioactive molecules activate ubiquitin proteasome, leading to the degradation of ZO-1 and claudin-5, which causes the destruction of the endothelial barrier.61 In addition, in vitro studies have found that the use of dexamethasone after JEV infection significantly reduces the level of pro-inflammatory mediators and restores the integrity of the BBB.62 However, whether dexamethasone can play the same role in the human body is unknown. Moreover, in HIV-infected states, increased IFN-γ from the CNS reduces the expression of heme oxygenase-1 in astrocytes via immunoproteasome degradation, which leads to increased levels of neurotoxin glutamate and likely contributes to neurocognitive impairment in HAND.63,64 In short, astrocytes play a double-edged role during viral infection. According to the characteristics of different virus infections, reasonable inhibition of the pro-inflammatory effect of astrocytes is of great significance to maintain the stability of the BBB.
Future perspectives
There are so many viruses that can infect the CNS.65 The first part of this article mainly discussed viruses that spread via hematogenous routes to invade the CNS by crossing the BBB, focusing on the interaction between the pathogen and the three structural cells of the BBB. In fact, there are some viruses that do not rely on the BBB to infect the CNS, such as HSV-1, which depends on retrograde axonal transport. The inflammatory response induced by HSV-1 after entering the brain parenchyma will, in turn, act on the BBB and even destroy the stability of the BBB.13,66 Recently, much attention has been paid to autoimmune encephalitis induced by herpes simplex encephalitis. Surprisingly, 9/11 (82%) patients with autoimmune encephalitis after herpes simplex encephalitis had areas of contrast enhancement (similar to those found during viral encephalitis), suggesting BBB destruction.67 However, there is little imaging data about autoimmune encephalitis after herpes simplex encephalitis, and no definitive conclusion can be drawn as yet. There is no definitive evidence as yet whether the destruction of the BBB induced by HSV-1 is related to the occurrence of autoimmune encephalitis. However, recently, a study has established a murine model of NMDAR autoimmune encephalitis after HSV-1 infection.68 The establishment of this murine model will provide a useful weapon with which to study the relationship between HSV-1 infection, BBB destruction, and the occurrence of autoimmune encephalitis.
The novel coronavirus, severe acute respiratory virus coronavirus 2 (SARS-CoV-2), which is currently responsible for a worldwide pandemic, has also shown some neuroinvasive properties.69 Hematogenous and neuronal retrograde routes have been proposed as the two main pathways by which neurotropic respiratory viruses enter the CNS.70 At present, the specific neuroinvasion mechanism of SARS-CoV-2 is still unclear. However, it is already known that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) and type II transmembrane serine protease as receptors by which to enter cells.71 Recently, the expression of ACE2 was detected in the capillaries of brain tissue specimens and primary human brain microvascular endothelial cells cultured in vitro. Further in vitro analysis found that SARS-CoV-2 spike proteins can induce a pro-inflammatory response of brain endothelial cells, which may lead to changes in the functioning of the BBB.72 Moreover, virus-like particles were readily detectable by electron microscopy in the frontal lobe endothelium.73 This evidence suggests that SARS-CoV-2 may use brain endothelial cells to cross the BBB and ultimately invade the CNS, but to confirm this conclusion, more evidence is still needed. Compared to healthy adults, SARS-CoV-2-infected patients have higher levels of IFN-γ, TNF-α, and VEGF, among others, and TNF-α levels in intensive care unit (ICU) patients are higher than those in non-ICU patients.74 The above cytokines have a certain barrier destruction effect, but it is unclear whether the systemic inflammation induced by SARS-CoV-2 changes the permeability of the BBB and accelerates the neuropenetrance of SARS-CoV-2. Research on SARS-CoV-2 around the world is ongoing and will provide more direct evidence about the neuroinvasion mechanisms of SARS-CoV-2.
Conclusions
This article mainly discusses neurotropic viruses transmitted through hematogenous routes (especially flaviviruses and HIV) as examples of the interaction between hosts and pathogens at the BBB. Because of the differences in the immune responses induced by different viral infections, more precise targeted treatment measures have been proposed. For instance, blocking the damage caused by viral proteins, enhancing the IFN-1 and IFN-λ responses of endothelial cells, stabilizing the integrity of endothelial TJs, and inhibiting the production of endothelial infectious exosomes have all shown some promise. With regard to pericytes and astrocytes, their abnormal immune activation can be suppressed to prevent further damage to the BBB. We have discussed more possibilities for immunomodulatory treatment based on traditional methods. In addition, there are many areas worthy of further exploration, including whether the occurrence of autoimmune encephalitis after HSV-1 infection is related to HSV-1-induced BBB dysfunction, and whether SARS-CoV-2 can invade the CNS by crossing the BBB.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zhuangzhuang Chen https://orcid.org/0000-0001-6704-2991
References
- 1.Sweeney MD, Zhao Z, Montagne A, et al. Blood-brain barrier: from physiology to disease and back. Physiol Rev 2019; 99: 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018; 14: 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer 2020; 20: 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang X, Andjelkovic AV, Zhu L, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 2018; 163–164: 144–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019; 25: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustafá YM, Meuren LM, Coelho SVA, et al. Pathways exploited by flaviviruses to counteract the blood-brain barrier and invade the central nervous system. Front Microbiol 2019; 10: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salimi H, Cain MD, Jiang X, et al. Encephalitic alphaviruses exploit caveola-mediated transcytosis at the blood-brain barrier for central nervous system entry. mBio 2020; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Obaidi MMJ, Bahadoran A, Wang SM, et al. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol 2018; 62: 16–27. [DOI] [PubMed] [Google Scholar]
- 9.Papa MP, Meuren LM, Coelho SVA, et al. Zika virus infects, activates, and crosses brain microvascular endothelial cells, without barrier disruption. Front Microbiol 2017; 8: 2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michlmayr D, Andrade P, Gonzalez K, et al. CD14+CD16+ monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat Microbiol 2017; 2: 1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meltzer MS, Skillman DR, Gomatos PJ, et al. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol 1990; 8: 169–194. [DOI] [PubMed] [Google Scholar]
- 12.Aravamudhan P, Raghunathan K, Konopka-Anstadt J, et al. Reovirus uses macropinocytosis-mediated entry and fast axonal transport to infect neurons. PLoS Pathog 2020; 16: e1008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Qiu K, He Q, et al. Mechanisms of blood-brain barrier disruption in herpes simplex encephalitis. J Neuroimmune Pharmacol 2019; 14: 157–172. [DOI] [PubMed] [Google Scholar]
- 14.Maximova OA, Bernbaum JG, Pletnev AG. West Nile virus spreads transsynaptically within the pathways of motor control: anatomical and ultrastructural mapping of neuronal virus infection in the primate central nervous system. PLoS Negl Trop Dis 2016; 10:e0004980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegenthaler JA, Sohet F, Daneman R. ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Curr Opin Neurobiol 2013; 23: 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langen UH, Ayloo S, Gu C. Development and cell biology of the blood-brain barrier. Annu Rev Cell Dev Biol 2019; 35: 591–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos CJ, Antonetti DA. The role of small GTPases and EPAC-Rap signaling in the regulation of the blood-brain and blood-retinal barriers. Tissue Barriers 2017; 5: e1339768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans 2005; 33: 891–895. [DOI] [PubMed] [Google Scholar]
- 19.Puerta-Guardo H, Glasner DR, Espinosa DA, et al. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep 2019; 26: 1598–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasner DR, Ratnasiri K, Puerta-Guardo H, et al. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog 2017; 13: e1006673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong Y, Zhang B, Eum SY, et al. HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J Neurosci 2012; 32: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng M, Guo S, Fan S, et al. The preferential infection of astrocytes by enterovirus 71 plays a key role in the viral neurogenic pathogenesis. Front Cell Infect Microbiol 2016; 6: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Sun J, Wang N, et al. Enterovirus A71 capsid protein VP1 increases blood-brain barrier permeability and virus receptor vimentin on the brain endothelial cells. J Neurovirol 2020; 26: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014; 509: 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen LN, Ma D, Shui G, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014; 509: 503–506. [DOI] [PubMed] [Google Scholar]
- 26.Andreone BJ, Chow BW, Tata A, et al. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 2017; 94: 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Chi X, Cheng M, et al. Zika virus degrades the ω-3 fatty acid transporter Mfsd2a in brain microvascular endothelial cells and impairs lipid homeostasis. Sci Adv 2019; 5: eaax7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miner JJ, Daniels BP, Shrestha B, et al. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med 2015; 21: 1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels BP, Holman DW, Cruz-Orengo L, et al. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. mBio 2014; 5: e01476-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005; 5: 375–386. [DOI] [PubMed] [Google Scholar]
- 31.Lazear HM, Diamond MS. New insights into innate immune restriction of West Nile virus infection. Curr Opin Virol 2015; 11: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells AI, Coyne CB. Type III interferons in antiviral defenses at barrier surfaces. Trends Immunol 2018; 39: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity 2019; 50: 907–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bramley JC, Drummond CG, Lennemann NJ, et al. A three-dimensional cell culture system to model RNA virus infections at the blood-brain barrier. mSphere 2017; 2: e00206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazear HM, Daniels BP, Pinto AK, et al. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med 2015; 7: 284ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Chen Y, Wang X, Zhao P, et al. Ezrin is essential for the entry of Japanese encephalitis virus into the human brain microvascular endothelial cells. Emerg Microbes Infect 2020; 9: 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu CF, Chu LW, Liao IC, et al. The mechanism of the Zika virus crossing the placental barrier and the blood-brain barrier. Front Microbiol 2020; 11: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mladinich MC, Schwedes J, Mackow ER. Zika virus persistently infects and is basolaterally released from primary human brain microvascular endothelial cells. MBio 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Woodson M, Neupane B, et al. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog 2018; 14: e1006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madison MN, Okeoma CM. Exosomes: implications in HIV-1 pathogenesis. Viruses 2015; 7: 4093–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.András IE, Leda A, Contreras MG, et al. Extracellular vesicles of the blood-brain barrier: role in the HIV-1 associated amyloid beta pathology. Mol Cell Neurosci 2017; 79: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai CY, Ou YC, Chang CY, et al. Endothelial Japanese encephalitis virus infection enhances migration and adhesion of leukocytes to brain microvascular endothelia via MEK-dependent expression of ICAM1 and the CINC and RANTES chemokines. J Neurochem 2012; 123: 250–261. [DOI] [PubMed] [Google Scholar]
- 43.Nikolakopoulou AM, Montagne A, Kisler K, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci 2019; 22: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 45.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro V, Bertrand L, Luethen M, et al. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J 2016; 30: 1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castro V, Skowronska M, Lombardi J, et al. Occludin regulates glucose uptake and ATP production in pericytes by influencing AMP-activated protein kinase activity. J Cereb Blood Flow Metab 2018; 38: 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CJ, Ou YC, Li JR, et al. Infection of pericytes in vitro by Japanese encephalitis virus disrupts the integrity of the endothelial barrier. J Virol 2014; 88: 1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang CY, Li JR, Ou YC, et al. Interplay of inflammatory gene expression in pericytes following Japanese encephalitis virus infection. Brain Behav Immun 2017; 66: 230–243. [DOI] [PubMed] [Google Scholar]
- 50.Ubogu EE, Callahan MK, Tucky BH, et al. Determinants of CCL5-driven mononuclear cell migration across the blood-brain barrier. Implications for therapeutically modulating neuroinflammation. J Neuroimmunol 2006; 179: 132–144. [DOI] [PubMed] [Google Scholar]
- 51.Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci 2006; 27: 48–55. [DOI] [PubMed] [Google Scholar]
- 52.Jurado KA, Yockey LJ, Wong PW, et al. Antiviral CD8 T cells induce Zika virus associated paralysis in mice. Nat Microbiol 2018; 3: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J, Alejandro B, Hetman M, et al. Zika virus infects pericytes in the choroid plexus and enters the central nervous system through the blood–cerebrospinal fluid barrier. PLoS Pathog 2020; 16: e1008204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertrand L, Cho HJ, Toborek M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 2019; 142: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bohannon DG, Ko A, Filipowicz AR, et al. Dysregulation of sonic hedgehog pathway and pericytes in the brain after lentiviral infection. J Neuroinflammation 2019; 16: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia 2013; 61: 1939–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang SF, Othman A, Koshkin A, et al. Astrocyte glutathione maintains endothelial barrier stability. Redox Biol 2020; 34: 101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soung A, Klein RS. Viral encephalitis and neurologic diseases: focus on astrocytes. Trends Mol Med 2018; 24: 950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 2010; 20: 588–594. [DOI] [PubMed] [Google Scholar]
- 60.Daniels BP, Jujjavarapu H, Durrant DM, et al. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J Clin Invest 2017; 127: 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang CY, Li JR, Chen WY, et al. Disruption of in vitro endothelial barrier integrity by Japanese encephalitis virus-infected astrocytes. Glia 2015; 63: 1915–1932. [DOI] [PubMed] [Google Scholar]
- 62.Patabendige A, Michael BD, Craig AG, et al. Brain microvascular endothelial-astrocyte cell responses following Japanese encephalitis virus infection in an in vitro human blood-brain barrier model. Mol Cell Neurosci 2018; 89: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gill AJ, Kovacsics CE, Cross SA, et al. Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest 2014; 124: 4459–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kovacsics CE, Gill AJ, Ambegaokar SS, et al. Degradation of heme oxygenase-1 by the immunoproteasome in astrocytes: a potential interferon-γ-dependent mechanism contributing to HIV neuropathogenesis. Glia 2017; 65: 1264–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe 2013; 13: 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He Q, Liu H, Huang C, et al. Herpes simplex virus 1-induced blood-brain barrier damage involves apoptosis associated with GM130-mediated golgi stress. Front Mol Neurosci 2020; 13: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018; 17: 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linnoila J, Pulli B, Armangué T, et al. Mouse model of anti-NMDA receptor post-herpes simplex encephalitis. Neurol Neuroimmunol Neuroinflamm 2019; 6: e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020; 92: 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in vitro models of the human blood-brain barrier. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol 2020; 92: 699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]