Abstract
Recent data argue for a pro-inflammatory role of CAMP (cathelicidin antimicrobial peptide) in adipocytes and adipose tissue (AT) and for regulatory circuits involving TLRs. In order to investigate regulatory effects of TLR2 and TLR4, 3T3-L1 adipocytes were stimulated with TLR2 agonistic lipopeptide MALP-2 and with TLR4 agonist LPS in presence or absence of signal transduction inhibitors. CAMP gene expression was analysed by quantitative real-time PCR in adipocytes and in murine AT compartments and cellular subfractions. CAMP expression was higher in gonadal than in subcutaneous AT and there was a gender-specific effect with higher levels in males. Adipocytes had higher CAMP expression than the stroma-vascular cell (SVC) fraction. MALP-2 up-regulated CAMP expression significantly, mediated by STAT3 and PI3K and potentially (non-significant trend) by NF-κB and MAPK, but not by raf-activated MEK-1/-2. Moreover, LPS proved to act as a potent inducer of CAMP via NF-κB, PI3K and STAT3, whereas specific inhibition of MAPK and MEK-1/-2 had no effect. In conclusion, activation of TLR2 and TLR4 by classical ligands up-regulates adipocyte CAMP expression involving classical signal transduction elements. These might represent future drug targets for pharmacological modulation of CAMP expression in adipocytes, especially in the context of metabolic and infectious diseases.
Keywords: Adipocyte, adipose tissue, cathelicidin antimicrobial peptide, LPS, MALP-2, Toll-like receptor
Introduction
It is a common clinical observation that inflammatory diseases are associated with metabolic consequences such as hyperglycaemia, insulin resistance, and increased fatty acids.1 On the other hand, metabolic diseases are characterized by inflammatory implications. Most important, there is a chronic and low grade of inflammation (metaflammation) in obesity and type 2 diabetes, both systemically and locally in visceral adipose tissue (adipoflammation).2–5 Thus, high emphasis has been increasingly put on the pro-inflammatory role of the adipose tissue as part of the innate immune system.4,6,7 The innate immune system recognizes bacterial, viral, fungal, and PAMPs as well as host-derived damage-associated molecular patterns (DAMPs) by specific PRRs.8 Several excellent reviews discuss the general role of five families of PRRs such as TLRs, C-type lectin-like receptors (CLRs), retinoic-acid-inducible gene I (RIG-I)-like receptors (RLRs), absent-in-melanoma (AIM)-like receptors, and NLRs.8,9 In an adipocyte-specific context, the expression, regulation, and functionality of nearly all members of the TLR family have been demonstrated in several adipocytic cells.4,7,10,11 Most importantly, the TLR system in adipocytes has only recently been linked to antimicrobial peptides expressed and secreted by these cells.7,12–15
Antimicrobial peptides (AMPs) represent secreted molecules that are able to kill bacteria via attacking the integrity of their membrane.12 AMPs can additionally act as pleiotropic and immune-modulating molecules in the context of cellular differentiation, migration and proliferation, wound healing, or angiogenesis. CAMP (cathelicidin antimicrobial peptide, also named LL-37 or CAP18) represents the only known human member of the cathelicidin family and is expressed in a variety of cells and organs, also in adipocytes and in adipose tissue.7,12
Zhang et al. reported on a pathophysiological link between CAMP and adipose tissue defense against Staphylococcus aureus.13 The authors demonstrated that expansion of adipose tissue occurs after infection of subdermal adipose tissue by S. aureus;13 the disease course was more severe in mice with an impaired adipogenesis.13 Moreover, inhibition of adipogenesis decreased CAMP expression and CAMP knockout mice were less effective in fighting against S. aureus growth.13 A review article by Alcorn and Kolls commented on these results by using the term “killer fat”.14
Gram-positive bacteria such S. aureus and their specific lipopeptides are sensed and recognized by TLR2 that signals either as heterodimer TLR1/2 or TLR2/6. Interestingly, the TLR-2-CAMP-pathway is expressed and highly inducible in adipocytes.7 In detail, TLR2 and CAMP are significantly up-regulated during differentiation of murine 3T3-L1 pre-adipocytes into mature adipocytes.7 Moreover, the lipopeptide MALP-2, a well described agonistic ligand to TLR2, is highly effective to induce expression of TLR2 and CAMP when applying doses between 50 and 100 ng/ml.7,15 Besides Gram-positive bacteria, Gram-negative bacteria are also able to modulate adipocyte pathways by releasing LPS. LPS is a strong inducer and activator of TLR4 in monocytes and it up-regulates CAMP expression in neutrophils.16 However, numerous publications report on a wide variety of pro-inflammatory and metabolic effects of TLR4 activation by LPS also in adipocytes.13,17–22
Based on these considerations, we aimed to investigate:
the basal expression of CAMP mRNA in adipose tissue compartments and cellular subfractions of mice
the effect of TLR2 activation (by MALP-2) and TLR4 activation (by LPS) on CAMP expression in mature 3T3-L1 adipocytes
several signal transduction components (NF-κB, MAPK, raf-activated MEK–1/–2, PI3K, STAT3) that could potentially be involved in TLR-mediated CAMP expression.
Materials and methods
Adipocyte cell culture and stimulation experiments
3T3-L1 Pre-adipocytes are fibroblast-like cells that represent unipotent cells derived from mesenchymal pluripotent stem cells. These unipotent cells can be differentiated into mature and lipid-storing adipocytes by using a well-established hormonal differentiation protocol over 7–9 d as summarized below. Originally, the 3T3-L1 cell line was derived from disaggregated mouse embryos. The cell line is provided by ATCC (American Type Culture Collection; Manassas, VA, USA).
3T3-L1 pre-adipocytes were cultured and differentiated into mature adipocytes as described previously.6,23 Briefly, cells were cultured at 37°C and 5% CO2 in DMEM (Biochrom AG, Berlin, Germany) supplemented with 10% newborn calf serum (Sigma-Aldrich, Deisenhofen, Germany) and 1% penicillin/streptomycin (Aidenbach, Germany), and were differentiated into adipocytes in DMEM/F12/glutamate medium (Lonza, Basel, Switzerland) supplemented with 20 µM 3-isobutyl-methyl-xanthine (Serva, Heidelberg, Germany), 1 µM corticosterone, 100 nM insulin, 200 µM ascorbate, 2 µg/ml transferrin, 5% FCS (Sigma-Aldrich, Deisenhofen, Germany), 1 µM biotin, 17 µM panthothenate, 1% penicillin/streptomycin (all from Sigma Aldrich, Deisenhofen, Germany) and 300 µg/ml Pedersen-fetuin (MP Biomedicals, Illkirch, France).24,25 A differentiation protocol reported in the literature was used with slight modifications, with light-microscopy control of adipocyte phenotype.23,26–29 Viability, integrity and differentiation of cells was regularly controlled by using light microscopy at d 0, 3, 5, 7 and 9 of differentiation and by assessing cytotoxicity via the lactate dehydrogenase (LDH) assay. LDH concentration was measured in the supernatants of all stimulation experiments (Cytotoxicity Detection Kit, Roche, Mannheim, Germany) to exclude any unexpected cytotoxic effects. The fully differentiated cellular phenotype is regularly reached between d 7 and d 9 of differentiation. The fully differentiated phenotype can be defined when cells at confluence have a round shape (instead of a fibroblastic spindle-shaped form) with an extensive, intracellular accumulation of multi-locular lipid droplets that are reactive to Oil-red O staining. Mature adipocytes were incubated under serum-free conditions prior to stimulation experiments. The cells were stimulated with LPS (10 ng/ml; from Sigma-Aldrich) and MALP-2 (50 ng/ml; from Axxora, Loerrach, Germany). LPS was isolated from Escherichia coli strain O55:B5, purified via gel-filtration chromatography (protein impurities < 3% warranted by the supplier), and provided as a lyophilised powder. The LPS concentrations used were as low as possible to cause pro-inflammatory effects in adipocytes (only 10 ng/ml).
Co-stimulation experiments were performed with the NF-κB inhibitor BAY-11 (5 µM), the STAT3 inhibitor S3I-201 (50 µM), the selective MAPK inhibitor SB239063 (5 µM), the MEK-1/-2 inhibitor U0126 (5 µM) and the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (5 µM) (all purchased from Merck and Sigma-Aldrich), applying doses similar to those reported in the literature.30–34
Isolation of mRNA and quantitative real-time PCR analysis of murine CAMP mRNA expression in adipose tissues of mice and in murine 3T3-L1 adipocytes
Gonadal and subcutaneous adipose tissue compartments were resected from wild type C57BL/6N mice (sacrificed for organ samples conformable to §4 Abs. 3 Tierschutzgesetz). A specific announcement was made at the local ethical committee (Regierungspraesidium Giessen: internal registration number: 544_M) that was approved subsequently. Small portions of fresh gonadal and subcutaneous adipose tissue were digested with 0.225 U/ml of collagenase NB 6 (#17458, SERVA Electrophoresis; Heidelberg, Germany). Adipocytes were then separated from SVC via centrifugation. Total mRNA was isolated from frozen murine gonadal and subcutaneous adipose, from isolated mature adipocytes, and from the SVC fraction as described previously.6 Briefly, tissues were homogenized in TRIzol®-Reagent (Life Technologies GmbH, Darmstadt, Germany) in combination with gentleMACS dissociator and M-tubes (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) for dissociation and RNA was isolated applying RNeasy® Mini Kit (Qiagen, Hilden, Germany) including DNase digestion (RNase-Free DNase Set, Qiagen).
For gene expression analysis, reverse transcription of RNA (QuantiTect Reverse Transcription Kit from Qiagen) was performed to generate corresponding cDNA for RT-PCR (iTaq Universal SYBR Green Supermix, CFX Connect RT-PCR system; Bio-Rad, Munich, Germany). Expression levels of the target gene Cathelicidin were normalized to the gene expression of GAPDH as a housekeeping gene as done by other experienced groups.35 The following primer pairs were used:
Murine cathelicidin: 5′-CCCAAGTCTGTGAGGTTCCG-3′/5′-GTGCACCAGGCTCGTTACA -3′
Murine GAPDH: 5′-TGTCCGTCGTGGATCTGAC-3′/5′-AGGGAGATGCTCAGTGT TGG-3′.
All oligonucleotides used were purchased from Metabion (Martinsried, Germany). The PCR mix contained the following components: 4 µl of cDNA (in H2O), 1 µl of specific primer-pair (5 µM in H2O), 5 µl of reaction mix (iTaq Universal SYBR Green Supermix, Bio-Rad).
Statistical analysis
For explorative data analysis, a statistical software package (SPSS 26.0) was used. Non-parametric numerical parameters were analyzed by the Mann–Whitney U test (for two unrelated samples), the Kruskal–Wallis test (more than two unrelated samples), the Wilcoxon test (for two related samples) or the Friedman test (more than two related samples). A P value below 0.05 (two tailed) was considered as statistically significant. In the figures, box plots are shown indicating median, upper/lower quartiles, interquartile range and minimum/maximum values and outliers.
Results
Adipose tissue expression of CAMP mRNA in wild type C57BL/6N mice
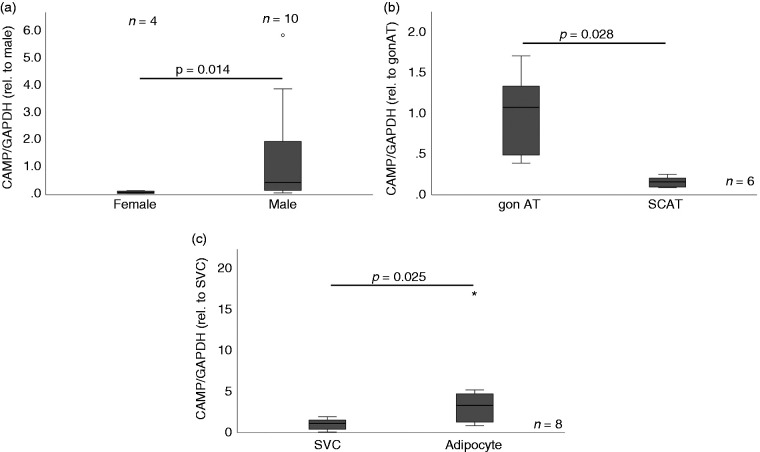
As shown in Figure 1a, a gender-specific effect with a higher expression level of CAMP in adipose tissue existed in male mice when compared with female mice (P = 0.014). This gender-specific effect was evident only in gonadal adipose tissue (which resembles visceral adipose tissue in mice) but not in subcutaneous adipose tissue (data not shown). Moreover, when gonadal and subcutaneous adipose tissue gene expression levels were compared directly in male mice, gonadal adipose tissue CAMP expression was significantly (P = 0.028) higher than subcutaneous adipose tissue CAMP expression (Figure 1b). Finally, CAMP expression was investigated in cellular subfractions prepared from gonadal murine adipose tissue (Figure 1c). Interestingly, CAMP expression was significantly higher (P = 0.025) in mature adipocytes when compared with the stroma-vascular cell fraction. These results matched our data obtained from differentiating 3T3-L1 pre-adipocytes.7 In this murine cell line, CAMP expression increased continuously with highest levels in fully differentiated adipocytes at d 9 of hormonally induced cellular differentiation.
Figure 1.
Quantitative CAMP mRNA expression in adipose tissue compartments and cellular fractions of mice. CAMP expression was investigated by quantitative real-time PCR in tissues and cellular fractions of wild type C57BL/6N mice. AT, adipose tissue; CAMP, cathelicidin anti-microbial peptide; gon., gonadal; SVC, stroma-vascular cell fraction; * outlier. (a) CAMP expression is higher in gonadal adipose tissue of male mice compared with female mice (P = 0.014; Mann–Whitney U test). (b) CAMP expression is significantly higher in gonadal adipose tissue compared with subcutaneous adipose tissue in male mice (P = 0.028; Wilcoxon test). (c) CAMP expression is higher in mature adipocytes compared with the stroma-vascular cell fraction (P = 0.025; Wilcoxon test) prepared from gonadal adipose tissue.
Signalling pathways involving NF-κB, MAPK and STAT3 mediate MALP-2-induced CAMP expression in adipocytes
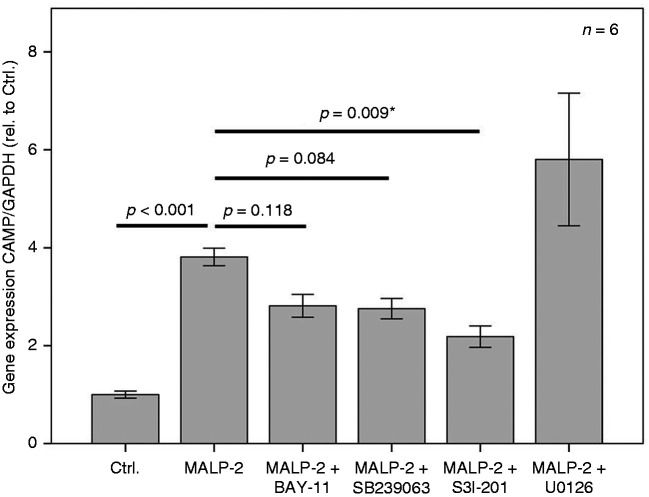
The lipopeptide MALP-2 represents a potent ligand for TLR2. We demonstrated previously that CAMP expression and TLR2 expression were up-regulated in parallel by MALP-2 not only in fibroblasts but also in mature 3T3-L1 adipocytes.7 Therefore, we aimed to test classical pro-inflammatory signalling pathways in adipocytes for their involvement in MALP-2-induced CAMP expression. As shown in Figures 2 and 3, MALP-2 significantly (P < 0.001 and P = 0.005, respectively) up-regulated CAMP expression. MALP-2-induced CAMP expression was potentially antagonized (Figure 2) by the NF-κB-Inhibitor BAY-11 (not significant; P = 0.118) and by the selective MAPK-inhibitor SB239063 (not significant; P = 0.084). Importantly, the STAT3-inhibitor S3I-201 could significantly (P = 0.009) antagonize MALP-2-induced CAMP expression. The raf-activated MEK-1/-2 inhibitor U0126 completely failed to modulate MALP-2-induced CAMP expression (Figure 2).
Figure 2.
MALP-2-induced expression of CAMP in 3T3-L1 adipocytes is antagonized by the STAT3-inhibitor S3I-201 and potentially antagonized by the NF-κB-Inhibitor BAY-11 and by the selective MAPK-inhibitor SB239063 but not by the MEK-1/-2 inhibitor U0126. MALP-2 (TLR-2/-6 activating lipopeptide); BAY-11 (irreversible inhibitor of IKKα and phosphorylation inhibitor of IκBα); SB239063 (selective MAPK-inhibitor); S3I-201 (STAT3-inhibitor; signal transducer and activator of transcription-3); U0126 (inhibitor of Raf-activated MEK-1/-2). The overnight incubation time was 18 h. The Kruskal–Wallis test was applied for calculating P values and statistical significance (*). Ctrl.: control.
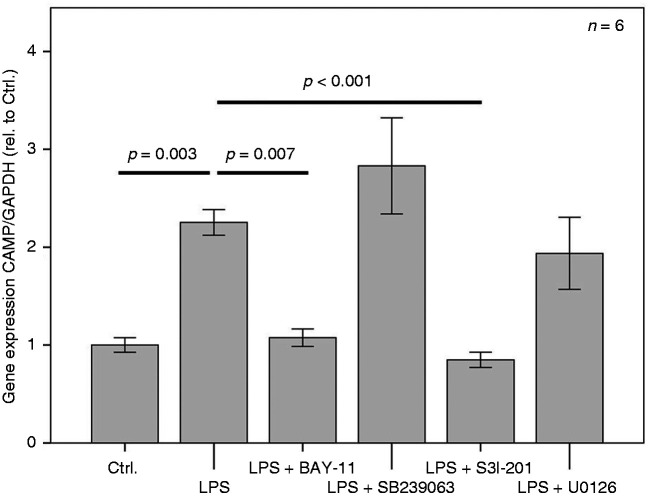
Figure 3.
LPS-induced expression of CAMP in 3T3-L1 adipocytes is abrogated by the NF-κB-Inhibitor BAY-11 and by the STAT3-inhibitor S3I-201 but not by the selective MAPK-inhibitor SB239063 or by the MEK-1/-2 inhibitor U0126. BAY-11 (irreversible inhibitor of IKKα and phosphorylation inhibitor of IκBα); SB239063 (selective MAPK-inhibitor); S3I-201 (STAT3-inhibitor; signal transducer and activator of transcription-3); U0126 (inhibitor of Raf-activated MEK-1/-2). The overnight incubation time was 18 h. The Kruskal–Wallis test was applied for calculating statistical significance. Ctrl.: control.
Signalling pathways involving NF-κB and STAT3 but not MAPK or MEK-1/-2 mediate LPS-induced CAMP expression in adipocytes
Since adipocytes are well known to express TLR4 and to respond to pro-inflammatory activation by LPS, we aimed to test whether LPS up-regulates CAMP expression. As shown in Figures 3 and 4, LPS up-regulated CAMP expression significantly (P = 0.003 and P = 0.002, respectively. To investigate putative signal transduction pathways involved in LPS-induced CAMP expression in adipocytes (Figure 3), the NF-κB-inhibitor BAY-11, the STAT3-inhibitor S3I-201, the selective MAPK-inhibitor SB239063, and the MEK-1/-2 inhibitor U0126 were applied. LPS-induced expression of CAMP was abrogated completely by the NF-κB-inhibitor BAY-11 and by the STAT3-inhibitor S3I-201, whereas the selective MAPK-inhibitor SB239063 or the MEK-1/-2 inhibitor U0126 had no effect.
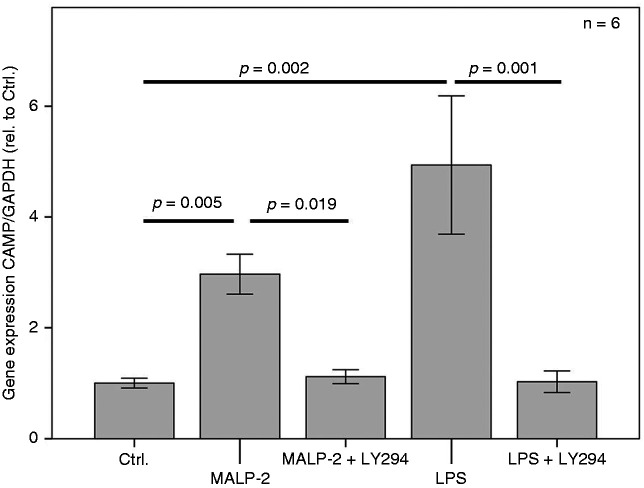
Figure 4.
MALP-2 and LPS-induced expression of CAMP in 3T3-L1 adipocytes is abrogated by the PI3K potent inhibitor LY294002. The overnight incubation time was 18 h. The Kruskal–Wallis test was applied for calculating statistical significance. Ctrl.: control.
Impact of PI3K on MALP-2- and LPS-induced CAMP expression
The induction of CAMP expression in 3T3-L1 adipocytes by the TLR2 ligand MALP-2 and by the TLR4 agonist LPS was completely abrogated by the potent and permeable PI3K inhibitor LY294002 (Figure 4).
Discussion
Functional TLRs such as TLR2 and TLR4 are expressed in adipocytes,4,5,17,36,37 and ligand-specific activation is able to modulate cytokine, adipokine and chemokine expression13,17–20 in adipocytes and in adipose tissue. We demonstrated previously that stimulation of pre-adipocytes and mature adipocytes with the TLR2 agonistic lipopeptide MALP-2 (50–100 ng/ml) up-regulated TLR2 and CAMP expression in parallel.7 Moreover, it was documented that CAMP expression increased during differentiation of murine 3T3-L1 adipocytes with highest levels in mature and fully differentiated adipocytes.7 Based on these data, it was our aim to verify these observations and to identify potential signal transduction elements involved in MALP-2-induced effects on CAMP expression.
In the present study, five intracellular signalling molecules/pathways were studied since these might play a role in adipocytes in addition to their established role in immune cells or mast cells. Moreover, there was some evidence from the literature and from prior publications of our group that the regulation of immuno-modulating adipokines such as progranulin, CTRP-3, resistin, adiponectin, and leptin comprises several of these pathways.6,7,10,17,18,38–40 However, exact data on the involvement of these pathways in the adipocytic regulation of CAMP expression are lacking. The present data show that MALP-2 was able to up-regulate CAMP expression significantly. This effect was mediated by STAT3 and PI3K and potentially (non-significant trend) by NF-κB and MAPK, but not by raf-activated MEK-1/-2. These regulatory mechanisms can be hypothesized to be involved in processes with a pro-inflammatory activation of adipocytes such as host defense in general and infection by Gram-positive bacteria and S. aureus in particular.7 Moreover, our data strongly support the data published by Zhang et al. and Schmid et al.7,13 Future mechanistic studies in vitro and in vivo are necessary to describe the molecular mechanisms of S. aureus/lipopeptide sensing by TLR2 in adipocytes and the subsequent up-regulation of CAMP as a defense mechanism against Gram-positive bacteria.
LPS represents a potent and well-known TLR4 ligand in monocytes21,41,42 and in adipocytes21,36,43–45. Interestingly, LPS was shown to induce CAMP mRNA expression in murine mast cells via NF-κB,38 whereas the involvement of the MAPK pathway remained obscure. Data on the effects of LPS on CAMP expression in adipocytes are not available. Thus, we aimed to test whether LPS also up-regulates CAMP expression in adipocytes in a similar way. To the best of our knowledge, the present study showed for the first time that CAMP was induced by LPS in adipocytes in vitro. Moreover, classical signal transduction elements such as NF-κB, PI3K and STAT3 were involved during this process, whereas specific inhibition of MAPK and MEK-1/-2 did not play a role. These mechanisms might be involved in host defense of adipose tissue against Gram-negative bacteria such as E. coli that are sensed by LPS. Whereas LPS induces CAMP mRNA expression in murine mast cells selectively via NF-κB but not via by MAPK/MEK pathways,38 LPS activated CAMP expression in adipocytes not only via NF-κB but also via PI3K and STAT3.
Very recently, we could demonstrate the quantitative measurement of CAMP peptide concentrations by ELISA in human obesity and during weight loss.46 However, it is a limitation of the present study that CAMP peptide levels in cell supernatants could not be presented quantitatively. Several ELISA kits detecting the murine peptide (3T3-L1 cells are of murine origin) were used. In principle, these kits failed to give reliable and quantitative results when using the supernatants of murine and differentiated 3T3-L1 adipocytes. This was due mainly to the very low rate of CAMP peptide secretion into the supernatants. Thus, ELISA-based results have not yet been published in the literature. Future work is under way to develop more sensitive ELISAs that are applicable for the murine system and for adipocytes.
Conclusions
Taken together, the activation of TLR2 and TLR4 in adipocytes up-regulated CAMP expression involving classical signal transduction elements that could be a drug target for pharmacological modulation of CAMP expression in the context of metabolic and infectious diseases. Future work is necessary to investigate more downstream elements of signal transduction as well as mechanisms of transactivation of the CAMP gene in adipocytes. CAMP might play a role in adipose tissue defense against Gram-positive and Gram-negative bacteria, since adipocytes express the functional sensing machinery (TLR2 and TLR4) with subsequent up-regulation of the highly effective anti-microbial peptide CAMP.
Acknowledgements
The laboratory work of K. Ebeling and L. Knüpfer is highly appreciated.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andreas Schmid https://orcid.org/0000-0003-4054-1971
References
- 1.von Stebut E, Boehncke WH, Ghoreschi K, et al. IL-17A in psoriasis and beyond: cardiovascular and metabolic implications. Front Immunol 2020; 10: 3096. [DOI] [PMC free article] [PubMed]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860–867. [DOI] [PubMed]
- 3.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017; 542:177–185. [DOI] [PubMed] [Google Scholar]
- 4.Schäffler A, Schölmerich J, Salzberger B. Adipose tissue as an immunological organ: Toll-like receptors, C1q/TNFs and CTRPs. Trends Immunol 2007; 28: 393–399. [DOI] [PubMed] [Google Scholar]
- 5.Schäffler A, Schölmerich J. Innate immunity and adipose tissue biology. Trends Immunol 2010; 31: 228–235. [DOI] [PubMed] [Google Scholar]
- 6.Schmid A, Hochberg A, Kreiß AF, et al. Role of progranulin in adipose tissue innate immunity. Cytokine 2020; 125: 154796. [DOI] [PubMed] [Google Scholar]
- 7.Schmid A, Karrasch T, Thomalla M, et al. Innate immunity of adipose tissue in rodent models of local and systemic Staphylococcus aureus infection. Mediators Inflamm 2017; 2017: 5315602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agier J, Pastwińska J, Brzezińska-Błaszczyk E. An overview of mast cell pattern recognition receptors. Inflammation Res 2018; 67: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agier J, Brzezińska-Błaszczyk E, Zelechowska P, et al. Cathelicidin LL-37 affects surface and intracellular toll-like receptor expression in tissue mast cells. J Immunol Res 2018; 2018: 7357162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid A, Kopp A, Hanses F, et al. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/–2 phosphorylation in mice in vivo. Biochem Biophys Res Commun 2014; 452: 8–13. [DOI] [PubMed] [Google Scholar]
- 11.Karrasch T, Schmid A, Kopp A, et al. Impact of toll-like-receptor-9 (TLR9) deficiency on visceral adipose tissue adipokine expression during chronic DSS-induced colitis in mice. Exp Clin Endocrinol Diabetes 2015; 123: 80–87. [DOI] [PubMed] [Google Scholar]
- 12.Lee EY, Lee MW, Wong GCL. Modulation of toll-like receptor signaling by antimicrobial peptides. Semin Cell Dev Biol 2019; 88: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang LJ, Guerrero-Juarez CF, Hata T, et al. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015; 347: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcorn JF, Kolls JK. Killer fat. Science 2015; 347: 26–27. [DOI] [PubMed] [Google Scholar]
- 15.Liggins MC, Li F, Zhang L, et al. Retinoids enhance the expression of cathelicidin antimicrobial peptide during reactive dermal adipogenesis. J Immunol 2019; 203: 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T-T, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004; 173: 2909–2912. [DOI] [PubMed] [Google Scholar]
- 17.Kopp A, Buechler C, Neumeier M, et al. Innate immunity and adipocyte function: ligand-specific activation of multiple toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity 2009; 17: 648–656. [DOI] [PubMed] [Google Scholar]
- 18.Kopp A, Buechler C, Bala M, et al. Toll-like receptor ligands cause pro-inflammatory and prodiabetic activation of adipocytes via phosphorylation of extracellular signal-regulated kinase and c-Jun N-terminal kinase but not interferon regulatory factor-3. Endocrinology 2010; 151: 1097–1108. [DOI] [PubMed] [Google Scholar]
- 19.Ajuwon KM, Banz W, Winters TA. Stimulation with peptidoglycan induces interleukin 6 and TLR2 expression and a concomitant downregulation of expression of adiponectin receptors 1 and 2 in 3T3-L1 adipocytes. J Inflamm 2009; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietsch J, Batra A, Stroh T, et al. Toll-like receptor expression and response to specific stimulation in adipocytes and preadipocytes: on the role of fat in inflammation. Ann N Y Acad Sci 2006; 1072: 407–409. [DOI] [PubMed]
- 21.Yamashita A, Soga Y, Iwamoto Y, et al. Macrophage-adipocyte interaction: marked interleukin-6 production by lipopolysaccharide. Obesity 2007; 15: 2549–2552. [DOI] [PubMed]
- 22.Lira FS, Rosa JC, Pimentel GD, et al. Both adiponectin and interleukin-10 inhibit LPS-induced activation of the NF-κB pathway in 3T3-L1 adipocytes. Cytokine 2012; 57: 98–106. [DOI] [PubMed]
- 23.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell 1975; 5: 19–27. [DOI] [PubMed]
- 24.Zaitsu H, Serrero G. Pedersen fetuin contains three adipogenic factors with distinct biochemical characteristics. J Cell Physiol 1990; 144: 485–491. [DOI] [PubMed]
- 25.Bachmeier M, Loffler G. Adipogenic activities in commercial preparations of fetuin. Horm Metab Res 1994; 26: 92–96. [DOI] [PubMed]
- 26.Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol 1979; 101: 169–171. [DOI] [PubMed]
- 27.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell 1975; 5: 19–27. [DOI] [PubMed]
- 28.MacDougald OA. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem 1995; 64: 345–373. [DOI] [PubMed]
- 29.Cornelius P. Regulation of adipocyte development. Annu Rev Nutr 1994; 14: 99–129. [DOI] [PubMed]
- 30.Takahashi K, Yamaguchi S, Shimoyama T, et al. JNK- and IκB-dependent pathways regulate MCP-1 but not adiponectin release from artificially hypertrophied 3T3-L1 adipocytes preloaded with palmitate in vitro. Am J Physiol Endocrinol Metab 2008; 294: E898–E909. [DOI] [PubMed]
- 31.Rios FJ, Neves KB, Cat AND, et al. Cholesteryl ester-transfer protein inhibitors stimulate aldosterone biosynthesis in adipocytes through nox-dependent processes. J Pharmacol Exp Ther 2015: 353: 27–34. [DOI] [PubMed]
- 32.Chen C, Jiang J, Lü JM, et al. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol 2020; 299: H193–H201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi W, Clark JM, Timme-Laragy AR, et al. Perfluorobutanesulfonic acid (PFBS) potentiates adipogenesis of 3T3-L1 adipocytes. Food Chem Toxicol 2018; 120: 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Oliveira M, Rodrigues BM, Olimpio RMC, et al. Adiponectin and serine/threonine kinase Akt modulation by triiodothyronine and/or LY294002 in 3T3-L1 adipocytes. Lipids 2019; 54: 133–140. [DOI] [PubMed] [Google Scholar]
- 35.Singh P, Zhang Y, Sharma P, et al. Statins decrease leptin expression in human white adipocytes. Physiol Rep 2018; 6: e13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bès-Houtmann S, Roche R, Hoareau L, et al. Presence of functional TLR2 and TLR4 on human adipocytes. Histochem Cell Biol 2007; 127: 131–137. [DOI] [PubMed] [Google Scholar]
- 37.Song MJ, Kim KH, Yoon JM, et al. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 2006: 346: 739–745. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Domenico J, Jia Y, et al. NF-κB-dependent induction of cathelicidin-related antimicrobial peptide in murine mast cells by lipopolysaccharide. Int Arch Allergy Immunol 2009; 150: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schäffler A, Müller-Ladner U, Schölmerich J, et al. Role of adipose tissue as an inflammatory organ in human diseases. Endocrine Rev 2006; 27: 449–467. [DOI] [PubMed] [Google Scholar]
- 40.Kopp A, Bala M, Buechler C, et al. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 2010; 151: 5267–5278. [DOI] [PubMed] [Google Scholar]
- 41.Rossol M, Heine H, Meusch U, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol 2011; 31: 379–446. [DOI] [PubMed] [Google Scholar]
- 42.Tsan M-F, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol 2004; 76: 514–519. [DOI] [PubMed] [Google Scholar]
- 43.Poulain-Godefroy O, Froguel P. Preadipocyte response and impairment of differentiation in an inflammatory environment. Biochem Biophys Res Commun 2007; 356: 662–667. [DOI] [PubMed] [Google Scholar]
- 44.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitseva OI, Tanriverdi K, Tchkonia TT, et al. Inducible toll-like receptor and NF-κB regulatory pathway expression in human adipose tissue. Obesity 2008; 16: 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hochberg A, Patz M, Karrasch T, et al. Serum levels and adipose tissue gene expression of cathelicidin antimicrobial peptide (CAMP) in obesity and during weight loss. Horm Metab Res. 2021 Jan 12. doi: 10.1055/a-1323-3050. Online ahead of print. [DOI] [PMC free article] [PubMed]