Abstract
Study Design:
In vitro biomechanical study.
Objective:
The transverse ligament is the strongest ligament of the craniocervical junction and plays a critical role in atlanto-axial stability. The goal of this cadaveric study, and the subsequent study (part II), was to reevaluate the force required for the transverse ligament and alar ligament to fail in a more physiological biomechanical model in elderly specimens.
Methods:
Twelve C1-2 specimens were harvested from fresh-frozen Caucasian cadavers with a mean age at death of 81 years (range 68-89 years). Only the transverse ligament was preserved, and the bony C1-2 complex was left intact. The dens was pulled away from the anterior arch of C1 using a strength test machine that applies controlled increasing force. After testing, the axis was split in half to check for hidden pathologies and osteoporosis. The differences in the failure force between sex and age groups (group 1: <80 years, group 2: >80 years) were compared.
Results:
The mean force required for the transverse ligament to fail was 236.2 ± 66 N (range 132-326 N). All but 2 specimens had significant osteoporotic loss of trabecular bone. No significant differences between sex and age groups were found.
Conclusions:
The transverse ligament’s failure in elderly specimens occurred at an average force of 236 N, which was lower than that reported in the previous literature. The ligament’s failure force in younger patients differs and may be similar to the findings published to date.
Keywords: transverse ligament, dens, elderly, biomechanical study, failure force, osteoporosis
Introduction
The craniocervical junction (CCJ) serves as the transition point from the skull base to spine and protects vital structures such as the spinal cord and vascular supply to the head and neck, while also allowing substantial rotational movement despite not having an intervertebral disc.1 The transverse ligament serves as the CCJ’s major stabilizing ligament. The ligament is the largest and strongest of those in the upper cervical spine and limits the anterior displacement of the atlas while ensuring proper positioning of the dens’ anterior surface on the posterior face of the atlas’s anterior arch.2 The transverse ligament’s biomechanics as Dvorak et al3 determined indicated a total load strength of 350 N before ligament rupture. Fielding et al4 investigated the transverse ligament’s importance further, and their study highlighted its protective nature in preventing the atlas from anterior subluxation.
In the elderly population, type II dens fractures are extremely prevalent and occur most often in low-impact trauma, such as simple falls.5 Generally, the extent of transverse ligament rupture determines conservative or surgical intervention in type II dens fracture. The transverse ligament mediates the dens’ stability, and when type II dens fractures occur that rupture or avulse the transverse ligament completely, surgical intervention is indicated.
There have been no recent remarks in the literature about the biomechanics of the transverse ligament since Dvorak et al,3 Fielding et al,4 and Panjabi et al’s2 landmark studies. Furthermore, no effort has been made to reassess or reevaluate these earlier findings, even though the reported failure forces vary widely from study to study.
Investigation of the CCJ’s biomechanics will allow clinicians and surgeons alike to understand these injuries’ clinicopathology better, resulting in enhanced patient outcomes. In this study, we reevaluate the transverse ligament biomechanics in elderly specimens in a more physiological biomechanical model that will provide clinicians a deep understanding of the transverse ligament.
Methods
Twelve C1-C2 specimens in total were harvested from 3 male and 9 female fresh-frozen Caucasian cadavers. Only cadavers of individuals who were more than 65 years old at death were included. The mean age at death was 81 years (range 68-89 years). All muscles were removed, as well as the dura mater, spinal cord, alar ligaments, tectorial membrane, superior and inferior longitudinal bands, atlanto-dental ligament, anterior longitudinal ligament, and C1-C2 joint capsules. Only the transverse ligament was preserved (Figure 1). The C1-C2 specimens were inspected carefully for abnormalities, fractures, malformations, tumors, and other pathologies. Radiographic imaging was not available.
Figure 1.

Harvested C1-2 specimen with posterior view of the transverse ligament.
The specimens then were secured in a strength testing machine (M2-200, Mark-10 Corporation, Copiague, NY, USA); the dens was surrounded by a bony clamp and pulled against the anterior arch, which was held with 2 bony clamps that apply a horizontally controlled increasing force (Figure 2). The anterior arch of C1 was displaced anteriorly (Farch), while axial tension was exerted upon the dens (Fdens) to displace it posteriorly (Figure 3). The peak force (N) was recorded at the transverse ligament’s point of failure. Failure was defined as the ligament’s complete rupture, which was categorized into left- and right-sided, and mid-ligament rupture. In addition, the specimens were divided into 2 age groups (group 1: <80 years and group 2: >80 years), based on the hypothesis that specimens over 80 years old would demonstrate a lower mean force at transverse ligament failure. After testing, the dens was split in half along its sagittal axis to identify any hidden pathologies or cysts and to check for osteoporosis, observed as distinct loss of trabecular bone.
Figure 2.
Lateral (A) and superior (B) view of the C1-2 specimen secured in the testing machine. One bony clamp is placed around the dens, 2 clamps fix the anterior arch of the atlas.
Figure 3.

Forces on atlantoaxial junction (lateral view) during axial separation.
The differences in the failure force between sex and age groups were compared and evaluated using a Student’s t test. A P value of <.05 was considered to indicate a significant relation. SPSS v. 22 (SPSS, Munich, Germany) was used for statistical analysis.
The Anatomical Quality Assurance checklist (AQUA) was used for this cadaveric study.6,7 Institutional review board approval is not required at our institution for cadaveric studies, and the work was performed in accordance with the 1964 Helsinki Declaration and its later amendments.
Results
Mean age at death was 81 years (range 68-89 years). The mean force at which the transverse ligament failed was 236.2 ± 64 N (range 132-326 N; Table 1). In males, the mean force was 265.7 ± 52 N with a range of 214 to 318 N, while the mean in females was 226.4 ± 67.3 N and ranged from 132 to 326 N; the 2 means did not differ significantly (P > .05). Analysis of the 2 age groups revealed a mean failure force of 268.8 ± 53.5 N in group 1 (<80 years) and of 212.3 ± 63.3 N in group 2 (>80 years); the 2 groups also did not differ significantly (P > .05).
Table 1.
Characteristics and Results of the Tested Specimens.
| Specimen | Sex | Age (Years) | Osteoporosis | Mean Failure Force (N) | Rupture Location |
|---|---|---|---|---|---|
| 1 | Female | 73 | Yes | 245 | Left side |
| 2 | Female | 83 | Yes | 192 | Left side |
| 3 | Male | 83 | Yes | 214 | Midline |
| 4 | Female | 89 | Yes | 132 | Midline |
| 5 | Female | 68 | Yes | 263 | Left side |
| 6 | Male | 84 | Yes | 265 | Left side |
| 7 | Female | 88 | Yes | 142 | Right side |
| 8 | Male | 72 | No | 318 | Right sidea |
| 9 | Female | 87 | Yes | 234 | Midline |
| 10 | Female | 76 | Yes | 326 | Right side |
| 11 | Female | 89 | Yes | 307 | Left side |
| 12 | Female | 75 | No | 197 | Right side |
aBony avulsion.
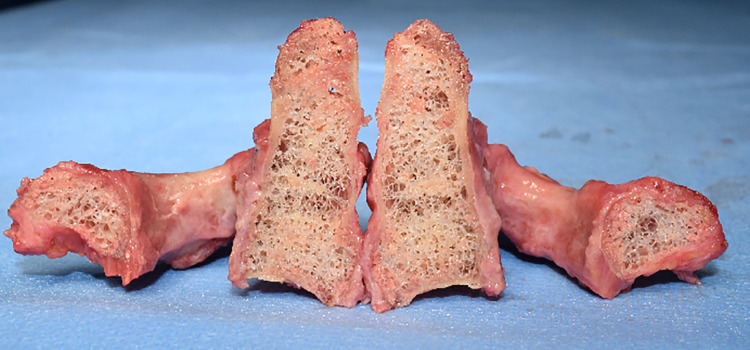
The transverse ligament’ failure occurred largely on the left side (n = 6), followed by mid-ligament rupture (n = 3), and right-sided failure (n = 3: Table 1). In one specimen, the ligament detached with a small piece of cortical bone on the right side, but remained grossly intact (Figure 4). Interestingly, this was the sole specimen that did not demonstrate severe osteoporosis (Figure 5), while the other 11 specimens did (Figure 6). No signs of congenital abnormalities, fractures, malformations, tumors, or other pathologies were seen in any specimen before or after testing.
Figure 4.

Bony avulsion on the right side occurred in one male specimen at a failure force of 326 N.
Figure 5.
A sagittal cut through the axis demonstrates an almost normal bone substance in a 75-year-old male specimen.
Figure 6.
Severe osteoporosis with loss of bone mass and trabecular interconnection is seen in an 89-year-old female specimen after the axis is cut in the sagittal plane.
Discussion
The incidence of type II dens fractures in the elderly in the United States has doubled over the past several decades.8 Fractures in this population are caused often by low-energy trauma, such as falls from sitting or standing height. Type II dens fractures are attributable in particular to osteoporosis and degenerative changes, both of which decrease the ability to absorb trauma.9 A ruptured transverse ligament in dens fractures can lead to atlanto-axial instability that requires surgical stabilization of the C1-C2 complex to provide sufficient stability during head and neck movement.10 There remains ongoing debate about the force required to rupture the transverse ligament, which recently has led to reasonable doubt regarding its strength.11-13
Sayama et al13 highlighted that the force generated to fracture the dens can target only the dens itself, and therefore, no force will act on the transverse ligament to cause its rupture. Conversely, the opposite is true. The force generated to rupture the transverse ligament rupture does not have the potential to fracture the dens as well. According to the authors, it is nearly impossible to cause the transverse ligament to fail and the base of the dens to fracture with the same force simultaneously. To date, there is limited knowledge about the transverse ligament’s biomechanics in an elderly population and its effects on spinal degeneration, which may compromise the ligament’s mechanical strength with age.14 Furthermore, no existing literature has examined the transverse ligament’s strength in a more physiological setting. Thus, the purpose of our biomechanical study was to evaluate its strength in elderly specimens in an accurate physiological model with the goal to provide a platform for future study of the C1-C2 junction and the biomechanics of its injury.
Transverse Ligament Biomechanics and Mechanism of Trauma
The transverse ligament is the strongest and thickest ligament of the CCJ, which runs around the dens’ posterior aspect and attaches to the atlas’s lateral tubercles.1 One of its key functions is to maintain the CCJ’s stability by locking the dens anteriorly, restricting posterior movement within the atlas, and inhibiting pathological flexion of the atlantoaxial region, while the atlantoaxial joint facets prevent extension.15 Rotation around the dens and avoidance of anteroposterior displacement are further primary functions of the transverse ligament. In Fielding et al’s4 biomechanical study, the transverse ligament was found to rupture under both rapid loading forces—such as high-speed-trauma in younger patients—and slow loading forces, which may explain the lower impact required in elderly patients. In high-speed trauma, a tremendous amount of force exerted upon the CCJ causes the head to flex with anterior translation of C1, while the C1-C2 joint extends and posterior translation of C2 follows.10 Experimental models have shown that the transverse ligament ruptures in the context of pure anteroposterior force,10,12 but there is no consensus in the current literature with regard to the loading path that causes dens fracture combined with transverse ligament rupture.16 The mechanism in elderly patients, who often sustain dens fractures as well as transverse ligament ruptures attributable to low-energy trauma, remains unclear and has not been evaluated.
The question of whether ligament failure or dens fracture occurring first is important; however, this phenomenon may not be testable with current models. The dens is an articulation site for several ligaments and soft tissues, not just its primary stabilizing ligaments. During high-impact traumas that lead to a dens fracture, a wide range of directional forces are imparted on this structure. Dens fracture seems to be an additive effect, and not simply due to one discrete ligamentous structure, therefore it is expected that dens fracture did not occur a single time in this study.
Previous Studies of the Transverse Ligament
CCJ instability can be misdiagnosed often and may result from the lack of consensus on the CCJ’s biomechanics attributable to the difficulties of applying cadaveric studies’ results to clinical implementation in vivo. In 1970, in one of the first articles of its kind, Spence et al17 studied the transverse ligament’s strength related to atlas burst fractures. The mean failure force was 58 kiloponds (kp), which is equivalent to 569 N, and ranged between 38 and 104 kp (≅373-1020 N).17 The age of the specimens was not reported. Similar to Spence et al,17 Dvorak et al3 developed a biomechanical model to test the transverse ligament’s tensile strength by cutting it with the bony ends attached. Seven specimens with a mean age of 76 years (range 45-90 years) were used, and the transverse ligament’s mean failure force was 354 N (range 170-700 N). One major limitation of this study is that significant bony portions of the C1-C2 complex were removed.
Fielding et al4 applied anterior translation of C1 over C2 and perpendicular force to the transverse ligament. The mean force in all specimens was 824 N (range 118-1765 N). The age of these specimen was not fully reported.
In general, these observations result in more confusion than clarity in improving our knowledge of the transverse ligament’s strength. Although the study focused on the transverse ligament, it remains unclear why the entire occipto-atlanto-dental complex was tested and all ligaments were left intact, which could be the reason that the transverse ligament’s mean strength was found to be much higher than reported in other literature.2,3,17 Fixing the axis and beginning the testing in flexion also could lead to methodological errors, which not only could influence the ligament’s failure force but also strays from the C1-C2 complex’s natural movement, and thus, would influence the failure force results and impart a rigidity to the C1-C2 complex or dens that is not found in vivo.
In 1993, Heller et al18 also described perpendicular load on the transverse ligament’s long axis that emulated anterior translation of C1 over C2.18 The mean failure force was 692 N (range 220-1590 N), and the specimens’ mean age was 56 years with a range of 18 to 84 years. Heller et al’s18 methodology, in which they altered the C1-C2 complex by removing the axis and replacing the dens with a steel rod, may not have had the greatest clinical effect. This would confound the findings substantially in the event that the dens fractured before the transverse ligament ruptured. Furthermore, microfractures, osteoporosis, and anatomical variations all may affect the dens’ structural integrity, and thus, must be taken into account.
Panjabi et al2 examined the transverse ligament’s strength when different extension rates were applied, slow (0.1 mm/s) and fast (920 mm/s). The mean force required to fail when evaluated at a fast extension rate was 367 N (range 289-486 N).
These studies found wide variability in the transverse ligament’s strength, which could be attributable not only to the nonphysiological methods the authors used but also to differences in the specimens’ age (Table 2). C1-2’s prior mobility and state of activity also could play an important role. Transverse ligament rupture results primarily from anterior translation of C1 over C2, and simulating a physiological C1-2 trauma mechanism in a cadaveric setting is nearly impossible, yet more rigorous models in the future may be capable of simulating this. Compared with the literature described previously and other literature available, our biomechanical method maintains the C1-C2 junction’s articulation without fixing any bony portions or replacing the dens, and thus, provides a closer approximation of the way C1-C2 trauma occurs under normal physiological circumstances. No external fixation was used, and no modification or removal of bone fragments was necessary in our study. To elucidate the ligament’s strength in elderly patients further, exclusively elderly specimens (>65 years) were used for the experiment. A mean failure force of 236 N was found in our study, which is lower than that of data published previously.
Table 2.
Main Literature Regarding Biomechanics of the Transverse Ligament.
| Authors | Mean Age (Years) | Mean Failure Force (N) | No. of Specimens | Limitations |
|---|---|---|---|---|
| Spence et al,17 1970 | N/A | 569 | 10 | Failed in 2 specimens |
| Fielding et al,4 1974 | N/A | 824 | 20 | All occipito-atlantal-axial ligaments were left intact |
| Dvorak et al,3 1988 | 76 | 354 | 7 | Bony structures were removed |
| Heller et al,18 1993 | 56 | 692 | 13 | Dens was replaced with a steel rod; 5 specimens were tested preliminary |
| Panjabi et al,2 1998 | 49 | 367 | 11 | Ligaments were tested twice, irreversible damage occurred in 4 specimens during experiment |
Role of Age and Osteoporosis
Age-related degeneration of the spinal column may contribute to global osteoligamentous weakening and ligaments’ ossification or calcification. This phenomenon involves the posterior longitudinal ligament, anterior longitudinal ligament, and ligamentum flavum most commonly.19,20 Currently, there is no literature available that documents the transverse ligament’s ossification or the degeneration of its insertion points at the tubercle of the atlas. The results of this study, as well as those of Heller et al,18 whose study included 5 elderly specimens, suggests that the transverse ligament may weaken with increasing age. Accordingly, in combination with osteoporosis, degeneration of atlanto-dental and atlanto-axial joints could provide a better understanding of the increase of type 2 dens fractures with transverse ligament injures in elderly patients.8,11,21
Osteoporosis in elderly patients is common and characterized by a deficiency in bone mass. The base of the dens itself is a region of least resistance attributable to a reduced volume of trabecular bone, trabecular interconnections, and a one-third smaller cortical thickness compared to the dens process.22 A 55% reduction of trabecular bone volume in the base of the dens compared to the body of the dens and C2 also has been described in histomorphometric analysis.22 Therefore, the structural weakness of the base of the dens combined with osteoporosis is a good explanation for the high rate of type II dens fractures in elderly patients, and helps explain why 95% of dens fractures occur at its base.22 Osteoporosis in this study was present in nearly every specimen, but the transverse ligament still ruptured before the dens fractured. Osteoporotic changes in bone also may result in progressive weakening of the bone-ligament connections that contribute to the lower failure forces in elderly specimens, especially those >80 years of age.
Limitations
One major limitation in our study is the relatively small number of cadaveric specimens tested. More specimens definitely would refine the transverse ligament’s threshold strength value. However, our sample size still is high compared with those found in the literature. Another drawback of this study may be the specimens’ age, whose mean age at time of death was 81 years (range 68-89 years), representing a geriatric population, which was this study’s main focus. The biomechanical properties of the transverse ligament in younger patients may differ; however, most dens fractures that involve the transverse ligament occur in elderly patients after minor trauma. Although we used fresh-frozen specimens, cadaveric and live tissue’s differences also must be considered. Osteoporosis was determined by visual inspection after splitting the C2 vertebral bodies in half and influenced by the individual investigator’s experience. Furthermore, bone density data was unavailable. Despite these limitations, we believe that this cadaveric study presents the most accurate data on the transverse ligament’s strength in a more physiological setting.
Conclusion
Our results suggest that the transverse ligament fails at an average of 236 N in elderly cadaveric specimens, which is less than the value reported in the previous literature. These results suggest that elderly patients are more likely to sustain transverse ligament rupture after low-impact trauma. However, even in osteoporotic specimens, the dens failed to fracture during the testing process. Further studies are needed to determine the transverse ligament’s ability to support similar forces when rotation and lateral bending are applied.
Acknowledgments
The authors thank the donors and their families for their gifts, which definitely have advanced our knowledge of the transverse ligament’s strength.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Basem Ishak, MD  https://orcid.org/0000-0002-6414-4295
https://orcid.org/0000-0002-6414-4295
Emre Yilmaz, MD  https://orcid.org/0000-0002-1492-1201
https://orcid.org/0000-0002-1492-1201
Alexander Von Glinski, MD  https://orcid.org/0000-0002-7141-138X
https://orcid.org/0000-0002-7141-138X
Shogo Kikuta, PhD  https://orcid.org/0000-0002-2236-2884
https://orcid.org/0000-0002-2236-2884
Joe Iwanaga, PhD  https://orcid.org/0000-0002-8502-7952
https://orcid.org/0000-0002-8502-7952
References
- 1. Tubbs RS, Hallock JD, Radcliff V, et al. Ligaments of the craniocervical junction. J Neurosurg Spine. 2011;14:697–709. [DOI] [PubMed] [Google Scholar]
- 2. Panjabi MM, Crisco JJ, 3rd, Lydon C, Dvorak J. The mechanical properties of human alar and transverse ligaments at slow and fast extension rates. Clin Biomech (Bristol, Avon). 1998;13:112–120. [DOI] [PubMed] [Google Scholar]
- 3. Dvorak J, Schneider E, Saldinger P, Rahn B. Biomechanics of the craniocervical region: the alar and transverse ligaments. J Orthop Res. 1988;6:452–461. [DOI] [PubMed] [Google Scholar]
- 4. Fielding JW, Cochran GV, Lawsing JF, 3rd, Hohl M. Tears of the transverse ligament of the atlas. A clinical and biomechanical study. J Bone Joint Surg Am. 1974;56:1683–1691. [PubMed] [Google Scholar]
- 5. Ishak B, Schneider T, Gimmy V, Unterberg AW, Kiening KL. Early complications, morbidity, and mortality in octogenarians and nonagenarians undergoing posterior intra-operative spinal navigation-based C1/2 fusion for type II odontoid process fractures. J Neurotrauma. 2017;34:3326–3335. [DOI] [PubMed] [Google Scholar]
- 6. Tomaszewski KA, Henry BM, Ramakrishnan PK, et al. Development of the Anatomical Quality Assurance (AQUA) checklist: guidelines for reporting original anatomical studies. Clin Anat. 2017;30:14–20. [DOI] [PubMed] [Google Scholar]
- 7. Henry BM, Tomaszewski KA, Ramakrishnan PK, et al. Development of the Anatomical Quality Assessment (AQUA) tool for the quality assessment of anatomical studies included in meta-analyses and systematic reviews. Clin Anat. 2017;30:6–13. [DOI] [PubMed] [Google Scholar]
- 8. Pearson AM, Martin BI, Lindsey M, Mirza SK. C2 vertebral fractures in the Medicare population: incidence, outcomes, and costs. J Bone Joint Surg Am. 2016;98:449–456. [DOI] [PubMed] [Google Scholar]
- 9. Malik SA, Murphy M, Connolly P, O’Byrne J. Evaluation of morbidity, mortality and outcome following cervical spine injuries in elderly patients. Eur Spine J. 2008;17:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greene KA, Dickman CA, Marciano FF, Drabier J, Drayer BP, Sonntag VK. Transverse atlantal ligament disruption associated with odontoid fractures. Spine (Phila Pa 1976). 1994;19:2307–2314. [DOI] [PubMed] [Google Scholar]
- 11. Iyer S, Hurlbert RJ, Albert TJ. Management of odontoid fractures in the elderly: a review of the literature and an evidence-based treatment algorithm. Neurosurgery. 2018;82:419–430. [DOI] [PubMed] [Google Scholar]
- 12. Debernardi A, D’Aliberti G, Talamonti G, Villa F, Piparo M, Cenzato M. Traumatic (type II) odontoid fracture with transverse atlantal ligament injury: a controversial event. World Neurosurg. 2013;79:779–783. [DOI] [PubMed] [Google Scholar]
- 13. Sayama CM, Fassett DR, Apfelbaum RI. The utility of MRI in the evaluation of odontoid fractures. J Spinal Disord Tech. 2008;21:524–526. [DOI] [PubMed] [Google Scholar]
- 14. Iida T, Abumi K, Kotani Y, Kaneda K. Effects of aging and spinal degeneration on mechanical properties of lumbar supraspinous and interspinous ligaments. Spine J. 2002;2:95–100. [DOI] [PubMed] [Google Scholar]
- 15. Dvorak J, Panjabi MM, Novotny JE, Antinnes JA. In vivo flexion/extension of the normal cervical spine. J Orthop Res. 1991;9:828–834. [DOI] [PubMed] [Google Scholar]
- 16. Puttlitz CM, Goel VK, Clark CR, Traynelis VC. Pathomechanisms of failures of the odontoid. Spine (Phila Pa 1976). 2000;25:2868–2876. [DOI] [PubMed] [Google Scholar]
- 17. Spence KF, Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52:543–549. [PubMed] [Google Scholar]
- 18. Heller JG, Viroslav S, Hudson T. Jefferson fractures: the role of magnification artifact in assessing transverse ligament integrity. J Spinal Disord. 1993;6:392–396. [PubMed] [Google Scholar]
- 19. Fujimori T, Watabe T, Iwamoto Y, Hamada S, Iwasaki M, Oda T. Prevalence, concomitance, and distribution of ossification of the spinal ligaments: results of whole spine CT scans in 1500 Japanese patients. Spine (Phila Pa 1976). 2016;41:1668–1676. [DOI] [PubMed] [Google Scholar]
- 20. Ohara Y. Ossification of the ligaments in the cervical spine, including ossification of the anterior longitudinal ligament, ossification of the posterior longitudinal ligament, and ossification of the ligamentum flavum. Neurosurg Clin N Am. 2018;29:63–68. [DOI] [PubMed] [Google Scholar]
- 21. Watanabe M, Sakai D, Yamamoto Y, Nagai T, Sato M, Mochida J. Analysis of predisposing factors in elderly people with type II odontoid fracture. Spine J. 2014;14:861–866. [DOI] [PubMed] [Google Scholar]
- 22. Amling M, Hahn M, Wening VJ, Grote HJ, Delling G. The microarchitecture of the axis as the predisposing factor for fracture of the base of the odontoid process. A histomorphometric analysis of twenty-two autopsy specimens. J Bone Joint Surg Am. 1994;76:1840–1846. [DOI] [PubMed] [Google Scholar]