Abstract
Study Design:
Retrospective review of a multicenter prospective registry.
Objectives:
Our goal was to develop a method to risk-stratify adult spinal deformity (ASD) patients on the basis of their accumulated health deficits. We developed a novel comorbidity score (CS) specific to patients with ASD based on their preoperative health state and investigated whether it was associated with major complications, length of hospital stay (LOS), and self-reported outcomes after ASD surgery.
Methods:
We identified 273 operatively treated ASD patients with 2-year follow-up. We assessed associations between major complications and age, comorbidities, Charlson Comorbidity Index score, and Oswestry Disability Index score. Significant factors were used to construct the ASD-CS. Associations of ASD-CS with major complications, LOS, and patient-reported outcomes were analyzed.
Results:
Major complications increased significantly with ASD-CS (P < .01). Compared with patients with ASD-CS of 0, the odds of major complications were 2.8-fold higher (P = .068) in patients with ASD-CS of 1 through 3; 4.5-fold higher (P < .01) in patients with ASD-CS of 4 through 6; and 7.5-fold higher (P < .01) in patients with ASD-CS of 7 or 8. Patients with ASD-CS of 7 or 8 had the longest mean LOS (10.7 days) and worst mean Scoliosis Research Society–22r total score at baseline; however, they experienced the greatest mean improvement (0.98 points) over 2 years.
Conclusions:
The ASD-CS is significantly associated with major complications, LOS, and patient-reported outcomes in operatively treated ASD patients.
Keywords: adult, comorbidity, frail elderly, intraoperative complications, spinal curvatures
Introduction
Surgical treatment of adult spinal deformity (ASD) is associated with substantial medical and surgical complications.1-8 Studies have reported major complication rates from 20% to 40%.1-10 Reported rates of revision surgery in ASD patients are as high as 21%.10 In patients undergoing 3-column osteotomies, reported rates of new neurologic deficits are as high as 10%,11 and rates of major surgical complications are as high as 25%.12 In patients aged 60 years or older, reported rates of major complications after 3-column osteotomy for ASD treatment are as high as 18%.13
Frailty, defined as a state of decreased homeostatic reserve,14 is an independent risk factor for postoperative complications in the elderly in various surgical settings.15,16 In a study examining outcomes of geriatric trauma patients, frailty was an independent risk factor for in-hospital complications and adverse discharge disposition and was superior to patient age as a predictor of complications.17 In another study, the authors examined outcomes of elderly patients who underwent elective surgery and found that a frailty index independently predicted postoperative complications, length of hospital stay (LOS), and discharge to a skilled nursing or assisted-living facility.18
Various frailty indices have been proposed, including those based on a standard comprehensive geriatric clinical examination,19 those based on routine laboratory tests,20-22 and those defining frailty as an accumulation of systemic health disorders.23-25 However, to our knowledge, the associations of frailty with complications and patient-reported outcomes have not been investigated in surgical ASD patients. Our goal was to develop a method to risk-stratify ASD patients on the basis of preoperative frailty. We constructed a novel comorbidity score (CS) for the ASD population, as a surrogate for frailty, to risk-stratify patients according to their preoperative health state. We assessed whether the ASD-CS can predict common quality and value metrics: major complications, LOS, and self-reported outcomes after surgery for spinal deformity correction.
Methods
A multicenter prospective registry of operatively treated patients with ASD was examined. Institutional review board approval was obtained at each participating institution.
Patient Population
We identified 564 patients aged 18 years or older who underwent surgery for ASD between October 2008 and August 2013 and agreed to be enrolled in the study. Of these patients, 405 (72%) were treated operatively and were eligible for 2-year follow-up; 273 of these patients (67%) had complete 2-year radiographic and clinical follow-up. Patient characteristics, surgical variables (Table 1), comorbidities (Table 2), and radiographic characteristics (Table 3) were noted.
Table 1.
Characteristics of 273 Patients Surgically Treated for Adult Spinal Deformity, 2008-2013.
| Variable | Mean ± SD | % |
|---|---|---|
| Age (y) | 56 ± 15 | |
| Female sex | 84 | |
| Body mass index (kg/m2) | 27 ± 6.1 | |
| History of spine surgery | 45 | |
| History of spinal arthrodesisa | 78 | |
| History of smoking | 9 | |
| Number of levels fused | 11 ± 4.5 | |
| Operative time (min) | 395 ± 134 | |
| Operative blood loss (L) | 1.8 ± 1.6 | |
| Length of hospital stay (d) | 8.0 ± 4.5 |
a In patients who had previous spine surgery.
Table 2.
Comorbidities Present in Preoperative History of 273 Patients Surgically Treated for Adult Spinal Deformity, 2008-2013.
| Patient History | No. (%) of Patients |
|---|---|
| Hypertension | 96 (35) |
| Depression | 68 (25) |
| Bladder incontinence | 52 (19) |
| Stomach ulcer | 41 (15) |
| Osteoporosis | 30 (11) |
| Bowel incontinence | 27 (10) |
| Cancer | 22 (8) |
| Heart disease | 22 (8) |
| Pseudarthrosis | 19 (7) |
| Deep wound infection | 16 (6) |
| Diabetes mellitus | 16 (6) |
| Lung disease | 14 (5) |
| Neurologic weakness | 14 (5) |
| DVT or pulmonary embolism | 11 (4) |
| Chronic kidney disease | 8 (3) |
| Peripheral vascular disease | 3 (1) |
| Liver disease | 1 (0.4) |
Abbreviation: DVT, deep vein thrombosis.
Table 3.
Radiographic Characteristics of 273 Patients Surgically Treated for Adult Spinal Deformity, 2008-2013.
| Deformity Characteristic | Preoperative (Mean ± SD) | 2-Year Follow-up (Mean ± SD) |
|---|---|---|
| C7 sagittal vertical axis (cm) | 6.1 ± 7.6 | 3.2 ± 5.7 |
| Lumbar lordosis (deg) | 37 ± 22 | 50 ± 15 |
| Major curve | ||
| Lumbar (deg) | 17 ± 33 | 9 ± 19 |
| Thoracic (deg) | 24 ± 35 | 14 ± 24 |
| Pelvic incidence (deg) | 55 ± 13 | 55 ± 13 |
| Pelvic tilt (deg) | 23 ± 11 | 21 ± 10 |
| Sacral slope (deg) | 32 ± 12 | 34 ± 11 |
| Thoracic kyphosis (deg) | 34 ± 19 | 48 ± 18 |
Intraoperative, perioperative, and postoperative major complications were noted during 2 years of follow-up. Major complications were categorized as medical or surgical. Medical complications of interest were new-onset acute respiratory distress syndrome, cardiac arrhythmia, congestive heart failure, cardiac arrest, myocardial infarction, deep vein thrombosis, pulmonary embolism, stroke, acute renal failure, reintubation, pneumonia, and delirium. Surgical complications of interest were deep wound infection, motor deficit, new myelopathy, nerve root injury, new radiculopathy, and failure of fixation (ie, screw dislodgement, vertebral fracture, interbody dislodgement, rod breakage, screw breakage), and proximal junctional kyphosis, distal junctional kyphosis, and pseudarthrosis. Preoperative and 2-year follow-up Scoliosis Research Society–22r (SRS-22r) scores were examined for each patient.
Surgical Procedures
All patients in this study underwent multilevel instrumented fusion of the thoracolumbar spine via an open posterior approach with pedicle screw fixation. Though instrumentation techniques varied by center and surgeon, most surgeons used a similar approach, placing all screws freehand on the basis of preoperative computed tomography measurements. For thoracic screws, this involves clearing the posterior elements out to the tips of the transverse processes and cannulating the pedicles using a starting point just lateral to the superior facet. Guide holes are formed using a high-speed drill, and the screw path is then formed using a curved gearshift, taking care to remain lateral until a depth of 1.5 to 2.0 cm is achieved. The probe is then rotated to aim medially until the full screw tract has been mapped. After confirming that all 4 walls are intact using a ball-tip probe, the screw tracts are under-tapped, and screws are placed. A similar technique was used for the placement of lumbar screws; however, the starting trajectory is usually more lateral and the screw tract angulation is more medial compared with screws placed in the mid-thoracic spine. Sacropelvic instrumentation was used because of the tendency for the lumbosacral junction to function as a stress riser in long constructs ending at the L5 level.26 Our preference is to use the S2-alar-iliac (S2AI) technique as opposed to the older iliac bolt technique because the S2AI technique is associated with lower rates of screw prominence and associated pain27 while offering similar or superior biomechanical properties. Intraoperative radiographs were acquired at most centers after placement of screws to confirm positioning.
For patients with concurrent central stenosis, laminectomy or sublaminar decompression was also performed to alleviate symptoms of neural element compression. In all cases, the primary goals of surgery were to relieve symptoms and correct the underlying spinal deformity. Where the patient’s deformity was identified as fixed on preoperative computed tomography images and radiographs, facet-based or 3-column osteotomies were performed; interbody fusion with lordotic Harms cages was also performed when additional segmental lordosis was required. Briefly, 3-column osteotomies involve performing a Gill laminectomy over the target level with partial laminectomies over the adjacent segment. A temporary rod is placed on one side, and the pedicle and a wedge of the posterior vertebral body are resected unilaterally using an osteotome, ultrasonic cutter, or high-speed drill. The same process is repeated contralaterally, after which the osteotomy is closed with continuous neurophysiological monitoring to look for new-onset neurological compromise. Because of the multi-institutional nature of this cohort, there was no single protocol regarding which level should be fused or when osteotomies or interbody fusion were to be used.
Statistical Analysis
Univariate logistic regression was used to analyze the association between development of major complications and the following variables: patient age; sex; body mass index; and history of smoking, pseudarthrosis, deep spinal infection, bowel or bladder incontinence, drug or alcohol abuse, kidney disease, liver disease, lung disease, depression, diabetes, osteoporosis, cancer, heart disease, and hypertension. Univariate regression models were constructed to assess the association between the development of major complications and the American Society of Anesthesiologists (ASA) functional classification, the Charlson Comorbidity Index (CCI), and the Oswestry Disability Index (ODI).
Variables that were significantly associated with the development of major complications were used in a multivariate model to construct the ASD-CS. Differences in rates of major complications and ASD-CS and ASA scores were assessed using χ2 tests and logistic regression. Variation in LOS by ASD-CS was assessed using the analysis of variance test. The association between changes in SRS-22r scores and the ASD-CS was assessed using linear regression. For validation of the ASD-CS, a bootstrap random-sampling algorithm was used, and 10 000 runs were executed.
Stata, version 12, software (StataCorp LP, College Station, Texas) was used for all analyses. Significance was set at P < .05.
Results
Results of univariate analyses showed the following comorbidities to be significantly associated with development of major complications: history of osteoporosis (odds ratio [OR] = 1.69; P = .049), history of hypertension (OR = 1.45; P = .049), older age (OR = 1.02; P = .004), higher CCI score (OR = 1.14; P = .01), and higher ODI score (OR = 1.02; P = .01).
Adult Spinal Deformity Comorbidity Score Components
The ASD-CS (Table 4) assigns 1 point each for history of osteoporosis and history of hypertension. For age at surgery, CCI score, and ODI score, the 25th and 75th percentile distribution cutoffs were determined for the population. The 25th and 75th percentiles, respectively, were as follows: for age at surgery, 51 years and 66 years; for CCI score, 0 and 2; and for ODI score, 29 and 58. Zero points were assigned if the score was less than the 25th percentile for a given category; 1 point was assigned if the score was between the 25th and 74th percentiles, and 2 points were assigned if the score was greater than or equal to the 75th percentile.
Table 4.
Adult Spinal Deformity Comorbidity Score Components.
| Component | Points |
|---|---|
| Age at surgerya | 0 if <25th percentile; 1 if 25th to <75th percentile; 2 if ≥75th percentile |
| Patient history | |
| Osteoporosis | 1 if present, 0 if absent |
| Hypertension | 1 if present, 0 if absent |
| Charlson Comorbidity Index scoreb | 0 if <25th percentile; 1 if 25th to <75th percentile; 2 if ≥75th percentile |
| Oswestry Disability Index scorec | 0 if <25th percentile; 1 if 25th to <75th percentile; 2 if ≥75th percentile |
a In the current study, 25th percentile was 51 years; 75th percentile was 66 years.
b In the current study, 25th percentile was 0; 75th percentile was 2.
c In the current study, 25th percentile was 29; 75th percentile was 58.
The total possible score for the ASD-CS is 8. Patients were classified into 4 comorbidity categories according to ASD-CS score: “no comorbidities” (ASD-CS: 0, 12% of patients), “mild comorbidities” (ASD-CS: 1-3, 39% of patients), “moderate comorbidities” (ASD-CS: 4-6, 45% of patients), or “severe comorbidities” (ASD-CS: 7 or 8, 4% of patients).
Preoperative Characteristics
There were no significant associations between ASD-CS category and the number of levels fused (P = .08), history of spinal arthrodesis (P = .10), or use of 3-column osteotomies (P = .09). There was a significant association between ASD-CS category and patient age (P < .001) but no association between ASD-CS category and patient race (P = .26).
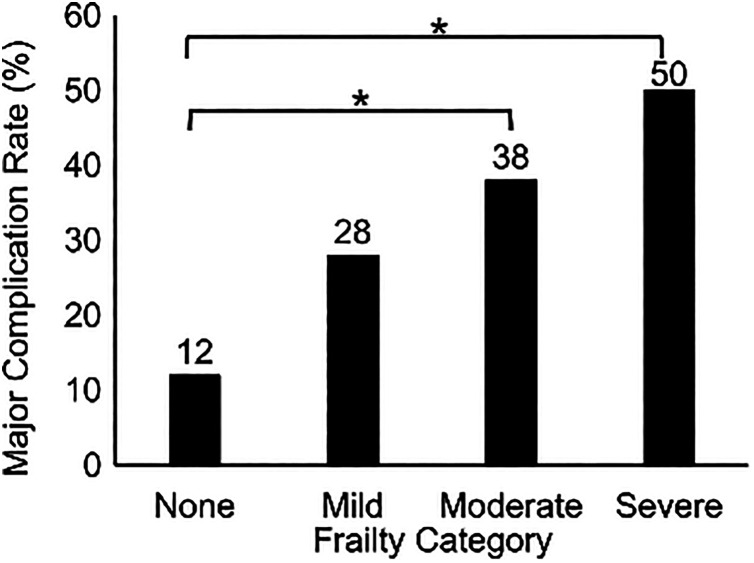
Major Complications
The rate of major complications in all patients was 31%. There were significant differences in major complication rates by ASD-CS and ASD-CS category (both P < .001; Figure 1). Compared with patients with no comorbidities, those in greater ASD-CS categories had higher odds of experiencing major complications (mild comorbidities, OR = 2.8, 95% confidence interval [CI] = 0.93-8.8, P = .068; moderate comorbidities, OR = 4.5, 95% CI = 1.5-14, P < .01; severe comorbidities, OR = 7.5, 95% CI = 1.6-35, P < .01).
Figure 1.
Major complication rate after spinal deformity surgery by adult spinal deformity comorbidity score category in 273 patients. Asterisks denote significant differences between groups at P < .05.
There were significant differences in major medical complications (P = .002) and major surgical complications (P = .03) by ASD-CS category. Compared with patients without comorbidities, patients with moderate and severe comorbidities combined had ORs of 6.5 (95% CI = 1.8-50) for developing major medical complications (P = .04) and 3.2 (95% CI = 1.3-7.9) for developing major surgical complications (P = .01).
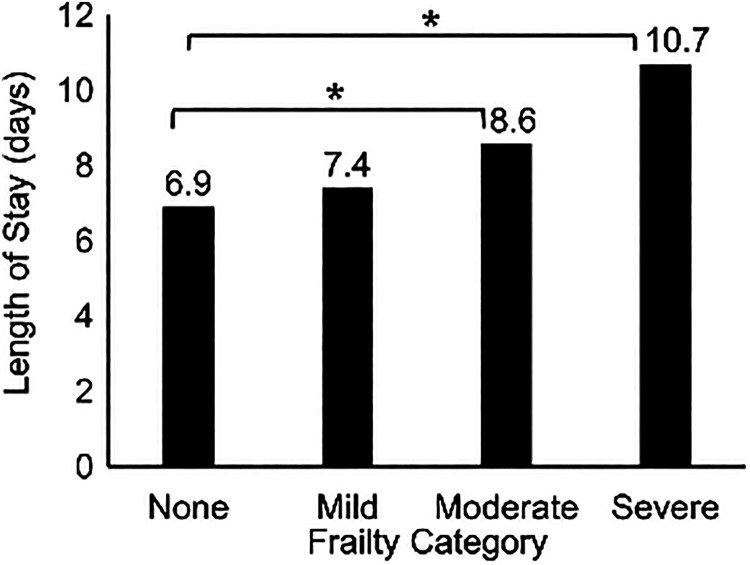
Length of Hospital Stay
Mean LOS for all patients was 8.0 ± 4.5 days. There were significant differences in mean LOS by ASD-CS and ASD-CS category (both P < .001; Figure 2). Compared with patients without comorbidities, those with comorbidities had longer mean LOS (mild comorbidities, mean 0.5 days longer [95% CI = –1.3 to 2.2, P = .59]; moderate comorbidities, mean 1.7 days longer [95% CI = 0.01-3.4, P = .49]; severe comorbidities, mean 3.8 days longer [95% CI = 0.8-6.7, P = .01]).
Figure 2.
Length of hospital stay after spinal deformity surgery stay by adult spinal deformity comorbidity score category for 273 patients. Asterisks denote significant differences between groups at P < .05.
Patient-Reported Outcomes
Changes in SRS-22r total scores between baseline and 2-year follow-up were significantly different by ASD-CS (P = .04) and ASD-CS category (P = .02; Table 5). Patients without comorbidities had the highest mean SRS-22r total scores at baseline and 2-year follow-up. Patients with severe comorbidities had the lowest scores at baseline and 2-year follow-up. By 2-year follow-up, patients without comorbidities had a mean 0.65-point improvement in SRS-22r total score, whereas those with moderate and severe comorbidities combined had a mean 0.98-point improvement (P = .02).
Table 5.
Variation in Scoliosis Research Society–22r (SRS-22r) Scores (Mean ± SD) by Adult Spinal Deformity Comorbidity Score Category.
| SRS-22r Domain by Time Point | Comorbidity Score Category | |||
|---|---|---|---|---|
| None | Mild | Moderate | Severe | |
| Activity | ||||
| Baseline | 4.0 ± 0.7 | 3.1 ± 0.9 | 2.6 ± 0.8 | 2.2 ± 0.5 |
| 2-year | 4.3 ± 0.8 | 3.5 ± 1.0 | 3.3 ± 1.0 | 3.0 ± 0.9 |
| Appearance | ||||
| Baseline | 3.1 ± 0.8 | 2.5 ± 0.7 | 2.2 ± 0.7 | 2.1 ± 0.7 |
| 2-year | 4.2 ± 0.7 | 3.7 ± 0.9 | 3.4 ± 0.9 | 3.3 ± 1.1 |
| Pain | ||||
| Baseline | 3.4 ± 0.7 | 2.6 ± 0.8 | 2.1 ± 0.8 | 2.1 ± 0.6 |
| 2-year | 4.0 ± 0.9 | 3.3 ± 1.1 | 3.3 ± 1.2 | 3.2 ± 1.2 |
| Mental health | ||||
| Baseline | 3.8 ± 0.8 | 3.5 ± 1.0 | 3.2 ± 0.9 | 3.2 ± 1.1 |
| 2-year | 4 ± 0.9 | 3.9 ± 0.9 | 3.7 ± 0.9 | 3.9 ± 0.9 |
| Satisfaction | ||||
| Baseline | 3.1 ± 1.0 | 2.8 ± 1.0 | 2.6 ± 1.0 | 3.1 ± 1.6 |
| 2-year | 4.5 ± 0.6 | 4.1 ± 1.0 | 4.2 ± 0.9 | 4.1 ± 1.2 |
| Total | ||||
| Baseline | 3.5 ± 0.5 | 2.9 ± 0.6 | 2.6 ± 0.6 | 2.5 ± 0.6 |
| 2-year | 4.2 ± 0.7 | 3.6 ± 0.8 | 3.5 ± 0.8 | 3.4 ± 0.8 |
Comparison With American Society of Anesthesiologists Functional Classification
Mean ASA score for the cohort was 2.3 ± 0.68. ASA distribution was as follows: 11% of patients were ASA I, 50% were ASA II, 38% were ASA III, and 1% were ASA IV. On χ2 analysis, there were no significant differences in major complication rates by ASA functional classification (P = .424). However, there were significant differences in major complication rates by ASD-CS (P = .001) and ASD-CS category (P = .01).
Validation
To validate the model for major complications as a function of ASD-CS, we applied a random sampling bootstrapping algorithm. After 10 000 runs, logistic regression analysis showed a significant difference in major complication rate by ASD-CS (P = .002).
Discussion
Despite the ability of frailty and comorbidities to affect risk profile in ASD surgery,9,10,13 there is no universally accepted, spine-specific metric for preoperative assessment of surgical risk based on comorbidities. We investigated whether such a score could predict major complications and patient-reported outcomes after ASD surgery, finding that our devised comorbidity score—a quantitative surrogate for frailty—had robust associations with both endpoints.
Reported rates of complications after ASD surgery are 20% to 40%.1-10 High scores on general measures of patient morbidity (eg, ASA score) correlate with high complication rates, as demonstrated by Schoenfeld et al28 who found that 30-day morbidity and mortality rates were positively associated with preoperative ASA scores in a multicenter cohort of nearly 6000 patients. Pateder et al29 reported similar findings in their single-center study of 407 patients treated for ASD. However, ASA score fails to account for several variables that are routinely considered during evaluation for ASD surgery, including patient age, preoperative disability, and comorbidities correlated with poorer outcomes in the spine surgery population, such as osteoporosis.
Collectively, these comorbidities may contribute to a state of decreased homeostatic reserve or frailty,14 which independently predicts higher morbidity and mortality in multiple surgical specialties.15,17,18,30 In some cases, frailty is a better predictor of complications than patient age16 or ASA score.31,32 To our knowledge, only 2 attempts have been made to formulate ASD-specific frailty indices. The first—the Seattle Spine Score33—shares many factors with the present score, notably hypertension and age. But unlike our index, the Seattle Spine Score omits comprehensive assessments of baseline disability and medical comorbidity, as are provided by the CCI and ODI scores in our index, respectively. In contrast, a second index—the Adult Spinal Deformity Frailty Index (ASD-FI)34—is similar to our own in that it considers multiple comorbidities, age, and patient-reported disability at baseline. Unlike the present score, the ASD-FI treats all metrics as equivalent and uses only select items from the validated patient questionnaires; we do not believe all components contribute equally to frailty. Additionally, by using the global patient-reported outcome score versus select items, our scoring metric is more user friendly and can predict long-term quality-of-life outcomes, as illustrated by the present results.
In addition to its simplicity, the ASD-CS benefits from the fact that all of its components have been previously correlated with postoperative outcomes. Hypertension,35 older age, and higher comorbidity burden have all been linked to higher complication rates after ASD surgery.9,36,37 Similarly, the ODI, a measure of spine-specific disability that is used extensively in ASD research,10,38-40 has been applied successfully to multiple spinal conditions41 and correlates with the 36-Item Short Form Health Survey score,42 a widely used marker of overall health.
The fact that all included components have been validated likely explains why LOS and major complication rates increased semilinearly across the four ASD-CS categories. This association of higher ASD-CS with higher complication rates persisted for both medical and surgical complications. Interestingly, 2-year follow-up rates did not differ by ASD-CS category, suggesting that these categories reflect true differences within the population.
Although patients in the higher frailty ASD-CS categories had worse absolute SRS-22r scores than less frail patients at all endpoints, they had significantly greater improvement in their SRS-22r scores, consistent with findings of the Spinal Deformity Study Group.43 This suggests a high risk–high reward scenario for patients with moderate or severe comorbidities, whereby they have the greatest risk of complications but can also have the greatest improvements in health-related quality-of-life outcomes.
Limitations
This study has several limitations. First, it lacks external validation because we had insufficient patients to both derive and validate an accurate model. To address this shortcoming, we attempted to internally validate our model using a bootstrapping algorithm that randomly samples the data and recalculates the regression analyses over the specified number of iterations. When using this technique, differences remained in the rates of major complications by ASD-CS over 10 000 random sampling iterations. Though this suggests that our results are valid, bootstrapping can lead to bias, and external validation of the ASD-CS model is required to show generalizability. Second, although the ASD-CS accurately predicted the endpoints of interest, the weighting of the included components was performed empirically, consistent with the technique used in prior studies.15-18,30-32 It is possible that altering component weighting may further improve the predictive power of the ASD-CS. Third, patients were risk-stratified into broad categories. Our goal was not to validate the categorization of the ASD-CS but to show that an ASD-CS index can be established for the ASD population, and that as the comorbidity score increases, LOS and the risk of complications increase. Fourth, we did not account for surgical invasiveness. A recent study demonstrated that a surgical invasiveness index in the ASD population independently predicted estimated blood loss and operative time.44 Patients with more extensive comorbidities may have had greater baseline deformity and underwent more invasive procedures. This seems unlikely, yet it cannot be ruled out given that we did not control for surgical invasiveness. Finally, the rates of various comorbidities were lower in our population relative to the general population. This likely represents a selection bias against patients with extensive comorbidities, and so it is possible that our results may not be generalizable to all spine patients. However, given that the reluctance of ASD surgeons to operate on highly frail patients is apt to persist, we believe that our tool is likely to remain applicable for the ASD population. Therefore, our data may be useful in benchmarking complication rates, patient counseling, treatment decision making, and efforts to improve quality of care for patients with ASD.
Conclusions
Patient age, comorbidities, and preoperative disability are significantly associated with development of major complications in patients who undergo surgery for ASD. We developed an ASD-specific comorbidity score that was found to be associated with development of major complications, LOS, and patient-reported outcomes. Patients in the moderate and severe ASD-CS categories had lower absolute SRS-22r scores at baseline and final follow-up compared with patients without comorbidities; however, they had significantly greater improvement in SRS-22r scores from baseline to 2-year follow-up compared with patients without comorbidities.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The International Spine Study Group receives funding from DePuy Synthes for administrative support and data collection. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Daniel Sciubba  https://orcid.org/0000-0001-7604-434X
https://orcid.org/0000-0001-7604-434X
Amit Jain  https://orcid.org/0000-0002-9983-3365
https://orcid.org/0000-0002-9983-3365
Khaled M. Kebaish  https://orcid.org/0000-0002-2186-2166
https://orcid.org/0000-0002-2186-2166
Han J. Kim  https://orcid.org/0000-0003-2170-3592
https://orcid.org/0000-0003-2170-3592
References
- 1. Bridwell KH, Lewis SJ, Edwards C, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine (Phila Pa 1976). 2003;28:2093–2101. [DOI] [PubMed] [Google Scholar]
- 2. Buchowski JM, Bridwell KH, Lenke LG, et al. Neurologic complications of lumbar pedicle subtraction osteotomy: a 10-year assessment. Spine (Phila Pa 1976). 2007;32:2245–2252. [DOI] [PubMed] [Google Scholar]
- 3. Cho KJ, Suk SI, Park SR, et al. Complications in posterior fusion and instrumentation for degenerative lumbar scoliosis. Spine (Phila Pa 1976). 2007;32:2232–2237. [DOI] [PubMed] [Google Scholar]
- 4. Kim YJ, Bridwell KH, Lenke LG, Rinella AS, Edwards C., 2nd Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine (Phila Pa 1976). 2005;30:468–474. [DOI] [PubMed] [Google Scholar]
- 5. Lapp MA, Bridwell KH, Lenke LG, et al. Long-term complications in adult spinal deformity patients having combined surgery: a comparison of primary to revision patients. Spine (Phila Pa 1976). 2001;26:973–983. [DOI] [PubMed] [Google Scholar]
- 6. Pichelmann MA, Lenke LG, Bridwell KH, Good CR, O’Leary PT, Sides BA. Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine (Phila Pa 1976). 2010;35:219–226. [DOI] [PubMed] [Google Scholar]
- 7. Schwab FJ, Hawkinson N, Lafage V, et al. Risk factors for major peri-operative complications in adult spinal deformity surgery: a multi-center review of 953 consecutive patients. Eur Spine J. 2012;21:2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weistroffer JK, Perra JH, Lonstein JE, et al. Complications in long fusions to the sacrum for adult scoliosis: minimum five-year analysis of fifty patients. Spine (Phila Pa 1976). 2008;33:1478–1483. [DOI] [PubMed] [Google Scholar]
- 9. Daubs MD, Lenke LG, Cheh G, Stobbs G, Bridwell KH. Adult spinal deformity surgery: complications and outcomes in patients over age 60. Spine (Phila Pa 1976). 2007;32:2238–2244. [DOI] [PubMed] [Google Scholar]
- 10. Hassanzadeh H, Jain A, El Dafrawy MH, et al. Clinical results and functional outcomes of primary and revision spinal deformity surgery in adults. J Bone Joint Surg Am. 2013;95:1413–1419. [DOI] [PubMed] [Google Scholar]
- 11. Kelly MP, Lenke LG, Shaffrey CI, et al. Evaluation of complications and neurological deficits with three-column spine reconstructions for complex spinal deformity: a retrospective Scoli-RISK-1 study. Neurosurg Focus. 2014;36:E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Auerbach JD, Lenke LG, Bridwell KH, et al. Major complications and comparison between 3-column osteotomy techniques in 105 consecutive spinal deformity procedures. Spine (Phila Pa 1976). 2012;37:1198–1210. [DOI] [PubMed] [Google Scholar]
- 13. Hassanzadeh H, Jain A, El Dafrawy MH, et al. Three-column osteotomies in the treatment of spinal deformity in adult patients 60 years old and older: outcome and complications. Spine (Phila Pa 1976). 2013;38:726–731. [DOI] [PubMed] [Google Scholar]
- 14. Amrock LG, Deiner S. The implication of frailty on preoperative risk assessment. Curr Opin Anaesthesiol. 2014;27:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48:78–83. [DOI] [PubMed] [Google Scholar]
- 16. Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joseph B, Pandit V, Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149:766–772. [DOI] [PubMed] [Google Scholar]
- 18. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. [DOI] [PubMed] [Google Scholar]
- 19. Jones D, Song X, Mitnitski A, Rockwood K. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res. 2005;17:465–471. [DOI] [PubMed] [Google Scholar]
- 20. Mitnitski A, Collerton J, Martin-Ruiz C, et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rockwood K, McMillan M, Mitnitski A, Howlett SE. A frailty index based on common laboratory tests in comparison with a clinical frailty index for older adults in long-term care facilities. J Am Med Dir Assoc. 2015;16:842–847. [DOI] [PubMed] [Google Scholar]
- 22. Schoufour JD, Echteld MA, Boonstra A, Groothuismink ZM, Evenhuis HM. Biochemical measures and frailty in people with intellectual disabilities. Age Ageing. 2016;45:142–148. [DOI] [PubMed] [Google Scholar]
- 23. Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev. 2006;127:840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. [DOI] [PubMed] [Google Scholar]
- 25. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 26. Wood KB, Schendel MJ, Ogilvie JW, Braun J, Major MC, Malcom JR. Effect of sacral and iliac instrumentation on strains in the pelvis. A biomechanical study. Spine (Phila Pa 1976). 1996;21:1185–1191. [DOI] [PubMed] [Google Scholar]
- 27. De la Garza Ramos R, Nakhla J, Sciubba DM, Yassari R. Iliac screw versus S2 alar-iliac screw fixation in adults: a meta-analysis. J Neurosurg Spine. 2018;30:253–258. [DOI] [PubMed] [Google Scholar]
- 28. Schoenfeld AJ, Carey PA, Cleveland AW, 3rd, Bader JO, Bono CM. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J. 2013;13:1171–1179. [DOI] [PubMed] [Google Scholar]
- 29. Pateder DB, Gonzales RA, Kebaish KM, Cohen DB, Chang JY, Kostuik JP. Short-term mortality and its association with independent risk factors in adult spinal deformity surgery. Spine (Phila Pa 1976). 2008;33:1224–1228. [DOI] [PubMed] [Google Scholar]
- 30. Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95–E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams P, Ghanem T, Stachler R, Hall F, Velanovich V, Rubinfeld I. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg. 2013;139:783–789. [DOI] [PubMed] [Google Scholar]
- 32. Karam J, Tsiouris A, Shepard A, Velanovich V, Rubinfeld I. Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg. 2013;27:904–908. [DOI] [PubMed] [Google Scholar]
- 33. Buchlak QD, Yanamadala V, Leveque JC, Edwards A, Nold K, Sethi R. The Seattle Spine Score: predicting 30-day complication risk in adult spinal deformity surgery. J Clin Neurosci. 2017;43:247–255. [DOI] [PubMed] [Google Scholar]
- 34. Miller EK, Neuman BJ, Jain A, et al. An assessment of frailty as a tool for risk stratification in adult spinal deformity surgery. Neurosurg Focus. 2017;43:E3. [DOI] [PubMed] [Google Scholar]
- 35. Acosta FL, Jr, McClendon J, Jr, O’Shaughnessy BA, et al. Morbidity and mortality after spinal deformity surgery in patients 75 years and older: complications and predictive factors. J Neurosurg Spine. 2011;15:667–674. [DOI] [PubMed] [Google Scholar]
- 36. Bhagat S, Vozar V, Lutchman L, Crawford RJ, Rai AS. Morbidity and mortality in adult spinal deformity surgery: Norwich Spinal Unit experience. Eur Spine J. 2013;22(suppl 1):S42–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho SK, Bridwell KH, Lenke LG, et al. Major complications in revision adult deformity surgery: risk factors and clinical outcomes with 2- to 7-year follow-up. Spine (Phila Pa 1976). 2012;37:489–500. [DOI] [PubMed] [Google Scholar]
- 38. Daubs MD, Lenke LG, Bridwell KH, et al. Does correction of preoperative coronal imbalance make a difference in outcomes of adult patients with deformity? Spine (Phila Pa 1976). 2013;38:476–483. [DOI] [PubMed] [Google Scholar]
- 39. Gum JL, Bridwell KH, Lenke LG, et al. SRS22R appearance domain correlates most with patient satisfaction after adult deformity surgery to the sacrum at 5-year follow-up. Spine (Phila Pa 1976). 2015;40:1297–1302. [DOI] [PubMed] [Google Scholar]
- 40. Dorward IG, Lenke LG, Stoker GE, Cho W, Koester LA, Sides BA. Radiographical and clinical outcomes of posterior column osteotomies in spinal deformity correction. Spine (Phila Pa 1976). 2014;39:870–880. [DOI] [PubMed] [Google Scholar]
- 41. Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25:2940–2952. [DOI] [PubMed] [Google Scholar]
- 42. Grevitt M, Khazim R, Webb J, Mulholland R, Shepperd J. The short form-36 health survey questionnaire in spine surgery. J Bone Joint Surg Br. 1997;79:48–52. [DOI] [PubMed] [Google Scholar]
- 43. Smith JS, Shaffrey CI, Glassman SD, et al. Risk-benefit assessment of surgery for adult scoliosis: an analysis based on patient age. Spine (Phila Pa 1976). 2011;36:817–824. [DOI] [PubMed] [Google Scholar]
- 44. Neuman BJ, Ailon T, Scheer JK, et al. Development and validation of a novel adult spinal deformity Surgical Invasiveness Score: analysis of 464 patients. Neurosurgery. 2018;82:847–853. [DOI] [PubMed] [Google Scholar]