Abstract
Presently, there is an increased demand for livestock products all over the world which has led to more devotion on improving livestock population. Although goats have been bred for a long time in Korea, but there is not much research conducted on traditional Korean black goat (Capra hircus coreanae) compared to other livestock populations. Mutton consumption has been dramatically changing from medicinal use to edible meat and this trend directs the black goat populations declining and also mutton import quantities are increasing consistently. The present study introduced a new estrus synchronizing technique with subsequent artificial insemination (AI) for Korean black goats to enable crossbreeding with non-native breeds for the small or subsistent farmers. Our data highlighted that, the percentage of motile sperm from the electro-ejaculated samples declined significantly after freezing and melting. In addition, the sperm motility significantly declined with regard to sperm incubation period (0, 5, 60, and 120 min at 37°C) and was negatively correlated (64.2 ± 7.9%, 63.3 ± 5.8%, 49.9 ± 6.3%, and 35.9 ± 7.6%, respectively) in frozen-thawed sperm samples. Moreover, the E2 levels were unchanged even 24 h after controlled internal drug releas (CIDR) withdrawal. But, 48 h and 72 h after CIDR removal, E2 levels increased significantly. These data helps us to consider the two time points for AI; CIDR removal after 24 h, at which E2 decreases, and after 48 h, as the time at which progesterone increases. Additionally, the AI after 48 h of CIDR removal group exhibited significantly higher pregnancy and parturition rates (42.9%) compared to AI after 24 h after CIDR removal 28.6% group. In conclusion, these studies will propose an optimal estrus synchronisation process with subsequent timing of AI and also will promote the Korean black goat breeding industry.
Keywords: Artificial insemination, Estrus synchronization, Frozen-thawed semen, Korean black goat
INTRODUCTION
In order to develop a promising breeding line to mitigate the increased demand of livestock products assisted reproductive technologies (ARTs) like as, semen cryopreservation, estrus synchronization, and artificial insemination (AI) have increased dramatically in the last decades [1]. Semen cryopreservation or freezing is usually used to reserve the germline of certain biologically, scientifically or economically valuable males, including livestock or endangered animal populations. Semen cryopreservation methods are species specific and therefore, semen cryopreservation methods have been established for a limited number of species because significant reduction in sperm motility and viability post-thawing (in comparison to freshly collected samples). Conservation of the Korean black goat (Capra hircus coreanae) which is the only native species in the Korean animal breeding industry with predominantly (80%) black fur. The Korean black goat population contains roughly 348,776 individuals disseminated among approximately 11,860 farms, constituting the majority of small ruminants in Korea. The multiple farming methods like commercial and subsistent goat farms are often upheld on a small scale. Goat production, despite having a long history in Korean agriculture, has grown more slowly compared to other livestock species because of its poor segment in the food supply market. Recently, Korean black goat meat has gained popularity among the people and is on equivalence with beef, pork, and chicken.
To improve the efficiency of natural reproduction in goats, several assisted-ARTs have been developed, including semen cryopreservation, AI, and estrus synchronization. However, their efficiency is low because they are simply modifications of the processes used originally in bovines.
Goats exhibit seasonal reproductive cycles that can be controlled using different methods of estrus synchronization [2], which is a simple, cost-effective, and significant method for AI in goat breeding. In addition, it is a key factor in the management of reproduction [2,3]. As observed in other ruminants, the breed, feed management, and environment can influence goat estrus synchronization. To date, estrus synchronization protocols optimized for cattle breeding have been used to breed small ruminants [4,5].
Goat reproduction either commercially or in small scale circumstances depends mainly on estrus synchronization, ovulation, and subsequent AI to be performed within a fixed time [6]. Commonly, the basis for estrus synchronisation is to increase the population rate during estrus or anestrus periods [7]. The synchronisation technique proposed the opportunity to increase the reproductive efficiency of an animal, as it permits mating or AI at a predetermined time. Moreover, it decreases the time needed for the recognition of estrus.
Several techniques have been recognized for goat estrus synchronization [5,8,9]. Progestogen administration is usually used either alone or in combination with other hormones [10]. Synchronization methods based on progesterone (P4), prostaglandin F2α (PGF2α), and equine chorionic gonadotropin (eCG) have shown promising outcomes for detecting estrus [11]. First, gonadotropin-releasing hormone (GnRH) is injected for estrus synchronization, resulting in the release of follicular stimulating hormone (FSH) and a surge of luteinizing hormone (LH) from the anterior pituitary gland. The administration of PGF2α then induces regression of the corpus luteum or luteinized follicle. Then, a new dominant follicle is prepared for ovulation, which is triggered by the second GnRH administration [12,13]. Alternatively, estrus and ovulation can be synchronized by sponge-eCG in goats [5]. To improve the pregnancy rate of Korean black goats, several previous reports have been described the efficacy of controlled internal drug release (CIDR) treatments [14–16]. however, the effect of CIDR on estrus synchronization in Korean black goats has not been studied yet.
Although several studies have attempted to improve the breeding efficiency and reproductive techniques but only a few studies have been conducted in Korean black goats [10,17]. Therefore, the need for techniques that are suitable and appropriate for Korean black goats has emerged as a major research interest. Therefore, the present study was designed to standardize the optimal estrus synchronisation approaches with timing of AI through hormonal evaluation (P4 and estrogen [E2]) after estrus synchronization, and also semen quality evaluation post cryopreservation by using CIDR protocol to improve the chances of pregnancy in Korean black goat.
MATERIALS AND METHODS
Experimental design
Experiments were carried out during seasonal anestrus. Goats were kept under intensive care and management. The 21 female Korean black goats (C. hircus coreanae) with number of parity (2–3) and average body weight: 36 kg were used in this experiment. All part of the experiments was performed in accordance with the Guidelines for the Care and Use of Experimental Animals (National Institute of Animal Science, Wanju, Korea). All of the techniques and experimental procedures were conducted according to the Rural Development Administration, under the National Institute of Animal Science, Korea and received approval from the Ethical Committee on Animal Experiments (Approval Number: 2019-320). Concentrated fodder feed (maximum quantity: 1.8% body weight) was provided once daily to the experimental animals. Over the experimental period bulk forage and water were supplied ad libitum. Semen was collected via electro-ejaculation and preserved using a conventional and simple freezing method. Pre- and post-freezing semen was analyzed using computer-assisted sperm analysis (CASA). AI was performed after estrus synchronization through CIDR insertion protocol. Additionally, blood P4 and E2 levels were analyzed to determine the optimal AI time in estrus-synchronized Korean black goats.
Semen collection and cryopreservation
A Lane Ram Ejaculator (HeatWatch, RAU immobilizer, model number IM2000) with a rechargeable electric stimulator was used for semen collection. The probe was lubricated, inserted into the rectum, and semen was collected by applying 3–4 V for 4–5 s, 2–3 times. On average, ejaculation was achieved after three stimulations, and the semen samples were collected in 50-mL tubes (BD Falcon, Corning, NY, USA) placed at the end-point of the penis. The electro-ejaculated semen was transported to the laboratory within 15 min with semen washing media (SWM). The SWM was composed of NaCl 130 mM, KCl 5.1 mM, glucose 10.54 mM, KH2PO4 1.19 mM, Na2HPO4 9.86 mM, MgSO4·7H2O 1.47 mM, and CaCl2·2H2O 0.93 mM. The seminal plasma was removed by centrifugation at 500×g for 15 min and diluted in a Triladyl®-egg yolk buffer. Prior to cryopreservation, the samples were examined microscopically to determine the sperm volume, concentration, motility, viability, and morphology, and total sperm count by CASA (Medical Supply, Wonju, Korea) using the Food Standards Agency 2011 guidelines. The final diluted semen at the intended concentratio was transferred and sealed in a fresh 50-mL conical tube, stored initially at 25°C for 30 min, and then placed in a medium-sized bath container within an icebox (30 × 26 × 15 cm) containing ice slurry for 2 to 3 h set at 5°C. Using a low-temperature semen treatment device (FHK, Fujihira Industry, Tokyo, Japan), the cooled, diluted semen was moved to 0.5-mL straws. Then, the semen straws were treated with liquid nitrogen (LN2) in a foam box (26 × 45.7 × 22.3 cm), applying a simple freeze method. The semen straws were placed 5 cm above the LN2 surface to hold vapors for 10 min. The straws were then immersed into LN2 to cryopreserve the semen. After 3 days of freezing, the cryopreserved semen was thawed in a water bath at 37°C for 45 s for experimental use and AI. For the sperm motility analysis, frozen-thawed semen was incubated at 37°C for 0, 5, 60, or 120 min.
Estrus synchronization and artificial insemination
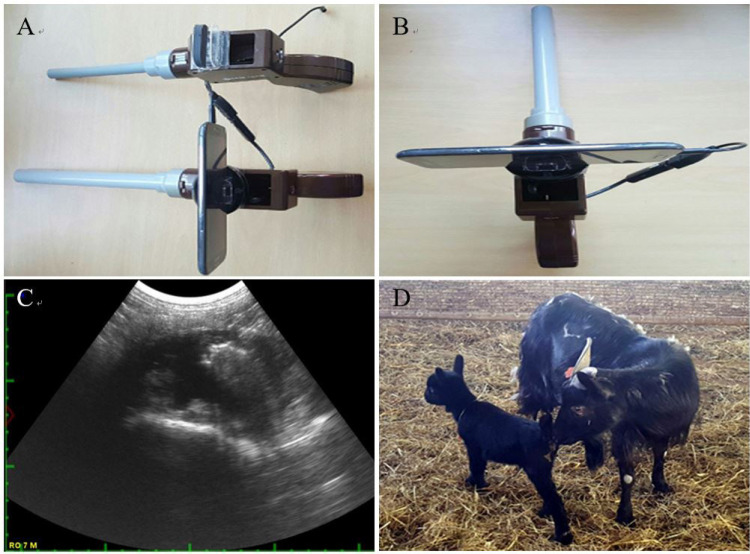
The control animals were treated to perform estrus synchronization as follows: sterile saline and 70% ethanol cotton swabs were used to clean the vulva and Eazi-Breed™-CIDR (sheep and goat; Zoetis Australia Pty., Rhodes, Australia) was inserted into the vagina for 9 days. On the seventh day post-insertion, 15 mg of PGF2α (Lutalyse, Zoetis, Belgium) was injected intramuscularly. Nine days after CIDR insertion, CIDR was removed and 200 IU pregnant mare serum gonadotropin (PMSG) was injected. The AI was performed 24 or 48 h after PMSG injection. AI was performed and visually confirmed by observing the cervix using equipment that we invented for small domestic animals in the laboratory (Figs. 1A and 1B).
Fig. 1. Artificial insemination (AI) using frozen-thawed semen of the Korean black goat (Capra hircus coreanae).
(A), (B) AI instrument for domestic animals. (C) Pregnancy diagnosis by abdominal ultrasonography. (D) A Korean black goat kid obtained by AI using frozen-thawed semen.
Evaluation of progesterone and estrogen
To evaluate the P4 and E2 level, blood samples were collected from the jugular vein at around 9 AM daily for 5 days. Each sample was refrigerated for 12 h and then centrifuged at 1,000×g for 10 min to separate the serum. The collected serum was stored at −70°C until analysis. The serum concentration of P4 and E2 were evaluated using the electric chemiluminescence immunoassay method.
Pregnancy diagnosis
To confirm pregnancy, ultrasound (Draminski 4Vet mini, Draminski S.A., Olsztyn, Poland) was used on days 40–60 afterAI. The conception, parturition, and twin fetus parturition rates were analyzed.
Statistical analysis
Statistical analyses were performed by using Duncan’s multiple range tests using SAS v.9.2. The results with a p-value ≤ 0.05 were considered significant. Inter-group assessments data from all replicates were analyzed using one-way analysis of variance. Depending on the variables, values were represented as the mean ± SE or as percentages.
RESULTS
Semen cryopreservation and thawing
Semen collection and sperm cryopreservation data were represented in the Table 1. The percentage of motile sperm was 91.4 ± 2.4% after dilution but significantly (p < 0.05) declined to 64.2 ± 7.9% in frozen-thawed semen samples. However, this did not affect the usage of the sample in AI. Semen motility parameters by using CASA, including total motility, velocity of curvilinear, plenty of lateral head displacement, speed of average pathway, beat-cross frequency, and straightness, were significantly (p < 0.05) lower in frozen-thawed semen related to fresh semen (Table 2).
Table 1. Characteristics of sperm from fresh and frozen-thawed semen (mean ± SE).
| Semen volume (mL) | Sperm concentration (× 108 mL−1) | Motile spermatozoa (%) | ||
|---|---|---|---|---|
| After collection | After dilution | After frozen-thawed | ||
| 1.4 ± 0.7 | 28.5 ± 11.6 | 94.7 ± 3.2a | 91.4 ± 2.4a | 64.2 ± 7.9b |
Experiments were completed with 10 biological replicates.
Values with different superscript letters were significantly different (p < 0.05).
Table 2. Computer-assisted semen analysis of fresh and frozen-thawed semen (mean ± SE).
| Semen status | MOT (%) | VCL (µm s−1) | ALH (µm s−1) | VAP (µm s−1) | BCF (Hz) | STR (%) |
|---|---|---|---|---|---|---|
| Fresh (After collection) | 94.7 ± 3.2a | 268.7 ± 20.8a | 6.5 ± 0.4a | 139.4 ± 8.2a | 11.7 ± 0.2a | 62.1 ± 4.2a |
| Frozen-thawed | 64.2 ± 7.9b | 178.9 ± 13.8b | 4.8 ± 0.3b | 77.8 ± 7.3b | 7.8 ± 0.9b | 51.1 ± 1.1b |
Experiments were repeated five times in each group.
Within columns, values with different superscript letters were significantly different (p < 0.05).
MOT, total motility; VCL, velocity of curvilinear; ALH, amplitude of lateral head displacement; VAP, velocity of average path; BCF, beat-cross frequency; STR, straightness.
Incubation of frozen-thawed semen
Frozen-thawed sperm samples were incubated for 0, 5, 60, and 120 min at 37°C (p < 0.05, Table 3). Our data demonstrated that, the incubation length negatively affected sperm motility (64.2 ± 7.9%, 63.3 ± 5.8%, 49.9 ± 6.3%, and 35.9 ± 7.6%, respectively).
Table 3. Effect of incubation time on motility of frozen-thawed semen (mean ± SE).
Experiments were repeated five times.
Within columns, values with different superscript letters were significantly different (p < 0.05).
Evaluation of progesterone and estrogen levels in the blood
Variations of P4 and E2 levels within the blood among estrus-synchronized Korean black goats were represented in Table 4. P4 levels were decreased to less than 1 ng mL−1 after 24 h of CIDR removal (9.53 ± 2.18 to 0.580 ± 0.09) and gradually decreased up to 96 h. The E2 levels were unchanging even 24 h after CIDR removal. However, 48 h and 72 h after CIDR removal, E2 levels increased. This data help us to get the two distinctive time points for AI; after 24 h of CIDR withdrawal, as the time at which E2 increases, and after 48 h, as the time at which P4 decreases.
Table 4. Changes in progesterone and estrogen levels.
| Parameter (ng mL−1) | After CIDR removal | ||||
|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | |
| P4 | 9.53 ± 2.18 | 0.580 ± 0.09 | 0.316 ± 0.08 | 0.259 ± 0.06 | 0.255 ± 0.07 |
| E2 | 18.9 ± 5.19 | 14.0 ± 2.15 | 24.2 ± 3.35 | 24.4 ± 6.93 | 13.3 ± 2.44 |
The experiments were completed within seven biological replicates.
Data were presented as mean ± SE.
P4, progesterone; E2, estrogen; CIDR, controlled internal drug releas.
Pregnancy and parturition rates after AI
The pregnancy and parturition rates after AI conferring to the method of estrus synchronization are shown in Table 5. Fig. 1C demonstrated the confirmation of pregnancy following 40–60 d after AI and pregnancy and fetus status were confirmed by abdominal ultrasonography. The comparison of pregnancy and parturition rates after AI at 24 h vs. 48 h were demonstrated in the Table 5. Our data highlighted that, 48 h after CIDR removal group exhibited significantly higher pregnancy and parturition rates. Fig. 1D shows a Korean black goat kid obtained by AI using frozen-thawed semen.
Table 5. Results of artificial insemination in Korean black goats using frozen-thawed semen.
| Recipient treatment | Pregnancy (%) | Parturition (%) |
|---|---|---|
| AI after 24 h of CIDR removal | 28.6 | 14.3 |
| AI after 48 h of CIDR removal | 42.9 | 42.9 |
The experiments were completed within seven biological replicates.
AI, artificial insemination; CIDR, controlled internal drug releas.
DISCUSSION
The estrus synchronization protocols before AI differs during the course of the breeding or non-breeding season. In the breeding season, natural ovulation has to be taken into line, while in the non-breeding season; induced ovulations must be organized accordingly. During the breeding season, the simplest method is to administer two concomitant injections of PGF. Regulatory actions at the time of estrus and ovulation need to be maintained with balance if satisfactory fertility is to be accomplished [18]. The stimulation of estrus synchronisation in small scale or subsistent level thus has a proposed and practical potential for future researchers and farmers. The present study demonstrated the effectiveness of sperm cryopreservation for AI was analyzed in Korean black goats.
In mammalian species, egg yolk and glycerol are conventionally used as cryoprotectants. Egg yolk prevents the dilution and cold shock of changing the sperm membrane [19]. Moreover, egg yolk might be a key factor in sperm cryopreservation, especially stabilization, owing to the inhibition of the acrosome reaction before freezing [20]. Lipase, an important inhibitory factor of sperm viability and cryopreservation, is secreted from the bulbourethral gland in goats but not in other livestock [6]. Several studies have investigated the cryopreservation of black goat semen and have shown the survival rate of sperm was ranges from 60% to 65% [21], in semen by AI. The present study also found 64.2 ± 7.9 % sperm motility in frozen-thawed semen (Table 1). However, in the consequences of CASA motility parameters (Table 2), were significantly lower in frozen-thawed semen compared to fresh semen. This result might be due to the damage by crystallization process during the freezing. The CASA motility parameters of frozen-thawed semen declined, but cryopreserved semen can be used for AI in Korean black goats. Still, further studies are needed to increase the survival rate and motility of sperm for the breeding industry.
Our studies on estrus synchronization has been imposing the optimum protocols to enable favorable synchrony and fertility in Korean black goats, there by generating and introducing simple, practical, and reliable methods for the commercial as well as subsistent farmers. Usually in the commercial goat industry, FSH+CIDR or PMSG+CIDR are most commonly used as effective protocols for estrus synchronisation or superovulation [21]. In ruminants, the estrus cycle can be synchronized via the insertion of a P4-impregnated intravaginal device, such as CIDR united with eCG [15]. however, this method cannot synchronize ovulation confidently. Therefore, the present study introduces an estrus synchronization technique ordinarily P4-based synchronization system [5]. After CIDR removal, it was found to be rapidly lowered to less than 1ng/mL of P4, and showed a tendency to stay low for about 4 days and gradually increase from the 5th day after CIDR removal. E2 levels rose sharply after the removal of CIDRs, showing numbers maintained between 24h and 48h. Previous studies highlighted some approaches for estrus synchronization and ovulation for small ruminants [22]. In particular, the synchronizing system using GnRH and PGF2α has been shown to synchronize the pre-ovulatory surge in gonadotropin, allowing ovulation to be relatively synchronous in cows [4,5]. In addition, the GnRH-PGF2α-based synchronization method is applicable for the AI of dairy goats and has been compared to CIDR-based treatments for dairy cows in a previous study; thus, the combination of P4 and a GnRH-PGF2α-based program may increase the pregnancy rate [23].
Our data accompany [24] who demonstrated intravaginal P4 treatment (CIDR) for 5-days in which PGF2α is administered on the day of sponge insertion, and removal of eCG (400 IU) by sponge. He mentioned that, the LH level becomes peak at 40 h after treatment withdrawal. Subsequently AI was performed at around 54 h and consequences good fertility [24]. Other researchers mentioned alternative protocols for inducing synchronization and fixed-time AI and was directed to reduce the use of hormones, especially those that leave residues, while maintaining the degree of synchronization and ovulation [24]. Several other studies have investigated estrus synchronization protocol in ruminants, including cows, dairy cows, sheep, and goats. In the present study, blood hormone levels remained similar across treatments: the P4 level remained less than 1 ng mL−1 for 4 d and the E2 level increased for a 2-d period. The effectiveness of fixed-time AI should be determined by confirming the optimum interval between the end of synchronization treatment and AI. The best way of establishing the optimum time for AI could be accomplished by confirming the preovulatory LH surge after synchronization. The farmers have to keep in mind that the time between the onset of estrus and the presence of the preovulatory LH surge besides comparatively 22 h between the ovulation.
Moreover, hormonal variation data could be useful key to predict ovarian follicle status. Our results showed that the parturition rate was optimal whenAI was performed 48 h after CIDR removal and PMSG injection. A kid from an estrus-synchronized Korean black goat was successfully produced by AI using frozen-thawed semen. Our data indicate that estrus synchronization methods should be used separately from AI. If these methods are used prior to AI, other programs with modified AI timings based on hormone levels will be necessary. However, previous studies have revealed that, after PGF2α treatment, ovarian follicle becomes a dominant mature follicle and after ovulation, the P4 level decreases significantly owing to corpus luteum regression. Follicular growth then continues, and a pre-ovulatory follicle grows. GnRH injection reinitiates the pituitary release of LH and pre-ovulatory follicle ovulation. Therefore, the period of 16–20 h after GnRH injection might be suitable for AI [25–27]. Previous studies with ovariectomized ewes were inserted with CIDR devices, plasma P4 get peaked within 2 h of insertion (5.5 ng/mL), while a rapid curvilinear decline happens thereafter [28]. But, subsequent studies by Wheaton and coworkers [29], found peak plasma P4 values of 2.1 ng/mL within 24 h and was comparatively stable levels between d 1 and 13 (1.9 ng/mL). They hypothesize the standard protocols for the usages of CIDR devices are typically identical to protocols for intravaginal sponges. Furthermore, [30], advising to reduce the dose of P4 had little effect on fertility.
Finally, provision of estrus synchronisation with subsequent AI (by using fresh or frozen semen) has been increasing and perhaps would be the most powerful tool for the reproductive biotechnologist and also will provide the Korean black goat industry with enlightening reproductive efficiency and genetic up-gradation. One of the most exciting developments would be possible in the reproduction of Korean native black goats by combining estrus synchronization with AI.
CONCLUSION
In conclusion, successful estrus synchronisation with AI by using Korean native black goat’s semen was confirmed by sperm motility and pregnancy rate. We hope that these studies contribute to the Korean black goat’s breeding industry and also suggest indications regarding estrus synchronisation with subsequent AI and also overcome the constraints that lead to infertility. Further studies are going on regarding establishment of a trustworthy estrus synchronization protocol for the AI of Korean native black goats.
Acknowledgements
Not applicable.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This research was supported by the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01431502)” and Fellowship Program of National Institute of Animal Science (Rural Development Administration, Korea).
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Kim KW, Lee J.
Data curation: Kim KW, Lee J.
Formal analysis: Kim KW, Lee J.
Methodology: Kim KW, Lee ED, Kim SW.
Validation: Kim KW, Kim KJ.
Investigation: Kim KW, Lee J.
Writing - original draft: Kim KW.
Writing - review & editing: Lee SS, Lee SH.
Ethics approval and consent to participate
All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the National Institute of Animal Science, Korea (No. 2019-329). This article does not require IRB/IACUC approval because there are no human and animal participants.
REFERENCES
- 1.Chowdhury MMR, Xu L, Kong R, Park BY, Mesalam A, Joo MD, et al. In vitro production of sex preselected cattle embryos using a monoclonal antibody raised against bull sperm epitopes. Anim Reprod Sci. 2019;205:156–64. doi: 10.1016/j.anireprosci.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Zhang J, Wei H, Sun X, Mu B, Yu M. Efficiency of methods applied for goat estrous synchronization in subtropical monsoonal climate zone of Southwest China. Trop Anim Health Prod. 2010;42:1257–62. doi: 10.1007/s11250-010-9558-6. [DOI] [PubMed] [Google Scholar]
- 3.Baldassarre H, Karatzas CN. Advanced assisted reproduction technologies (ART) in goats. Anim Reprod Sci. 2004;82-3:255–66. doi: 10.1016/j.anireprosci.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 1995;44:915–23. doi: 10.1016/0093-691X(95)00279-H. [DOI] [PubMed] [Google Scholar]
- 5.Holtz W, Sohnrey B, Gerland M, Driancourt MA. Ovsynch synchronization and fixed-time insemination in goats. Theriogenology. 2008;69:785–92. doi: 10.1016/j.theriogenology.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Leboeuf B, Restall B, Salamon S. Production and storage of goat semen for artificial insemination. Anim Reprod Sci. 2000;62:113–41. doi: 10.1016/S0378-4320(00)00156-1. [DOI] [PubMed] [Google Scholar]
- 7.Bogdan L, Groza I, Ciupe S, Neagu R, Tutulan C, Petrean A, et al. Research concerning estrus induction and synchronization in anestrous (out of season) goats. Vet Med. 2008;65:91–5. [Google Scholar]
- 8.Martínez MF, Kastelic JP, Colazo MG, Mapletoft RJ. Effects of estradiol on gonadotrophin release, estrus and ovulation in CIDR-treated beef cattle. Domest Anim Endocrinol. 2007;33:77–90. doi: 10.1016/j.domaniend.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Manjarin R, Dominguez JC, Castro MJ, Vallado B, Rodriguez S, Sprecher DJ, et al. Effect of prior FSH treatment on the estrus and ovulation responses to eCG in prepubertal gilts. Anim Reprod Sci. 2009;110:123–7. doi: 10.1016/j.anireprosci.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Kusina NT, Tarwirei F, Hamudikuwanda H, Agumba G, Mukwena J. A comparison of the effects of progesterone sponges and ear implants, PGF2alpha, and their combination on efficacy of estrus synchronization and fertility of mashona goat does. Theriogenology. 2000;53:1567–80. doi: 10.1016/S0093-691X(00)00298-3. [DOI] [PubMed] [Google Scholar]
- 11.Leboeuf B, Forgerit Y, Bernelas D, Pougnard JL, Senty E, Driancourt MA. Efficacy of two types of vaginal sponges to control onset of oestrus, time of preovulatory LH peak and kidding rate in goats inseminated with variable numbers of spermatozoa. Theriogenology. 2003;60:1371–8. doi: 10.1016/S0093-691X(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 12.Twagiramungu H, Guilbault LA, Dufour JJ. Synchronization of ovarian follicular waves with a gonadotropin-releasing hormone agonist to increase the precision of estrus in cattle: a review. J Anim Sci. 1995;73:3141–51. doi: 10.2527/1995.73103141x. [DOI] [PubMed] [Google Scholar]
- 13.Nebel RL, Jobst SM. Evaluation of systematic breeding programs for lactating dairy cows: a review. J Dairy Sci. 1998;81:1169–74. doi: 10.3168/jds.S0022-0302(98)75679-6. [DOI] [PubMed] [Google Scholar]
- 14.Miura H, Kotani S, Kohiruimaki M, Ohtsuka H, Kikuchi M, Ohnami Y. Relationships between the conception rate of estrus synchronization using estradiol benzoate and CIDR (progesterone) and other parameters in Holstein lactating dairy cows. J Reprod Dev. 2008;54:214–6. doi: 10.1262/jrd.18084. [DOI] [PubMed] [Google Scholar]
- 15.Long ST, Nakao T, Wakatake S, Okakoi M. Effect of CIDR 12 to 19 days after AI on detection of returning estrus and conception rate in dairy cows. J Reprod Dev. 2010;56:251–5. doi: 10.1262/jrd.09-134T. [DOI] [PubMed] [Google Scholar]
- 16.Azevedo C, Maia I, Canada N, Simões J. Comparison of fertility, regular returns-to-estrus, and calving interval between Ovsynch and CO-synch + CIDR protocols in dairy cows. Theriogenology. 2014;82:910–4. doi: 10.1016/j.theriogenology.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Stelletta C, Tekin K, Tirpan MB, Alemdar H, Cil B. Oztutar Stelletta F, et al. Vulvar thermal pattern following synchronization of estrus is linked to fertility after timed artificial insemination in goat. Theriogenology. 2017;103:137–42. doi: 10.1016/j.theriogenology.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Rahman ANMA, Abdullah RB, Wan-Khadijah WE. Estrus synchronisation and superovulation in goats: a review. J Biol Sci. 2008;8:1129–37. doi: 10.3923/jbs.2008.1129.1137. [DOI] [Google Scholar]
- 19.Quinn P, Stanger JD. Effect of purification of bovine serum albumin on the interaction of human semen with mouse ova in vitro. Biol Reprod. 1980;22:134–40. doi: 10.1095/biolreprod22.2.134. [DOI] [PubMed] [Google Scholar]
- 20.Witte TS, Schäfer-Somi S, Kuchar A, Möstl E, Iben C, Aurich C. Effect of hen’s egg yolk on capacitation and acrosome reaction of diluted canine spermatozoa. Anim Reprod Sci. 2009;110:293–305. doi: 10.1016/j.anireprosci.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Luo J, Wang W, Sun S. Research advances in reproduction for dairy goats. Asian-Australas J Anim Sci. 2019;32:1284–95. doi: 10.5713/ajas.19.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Titi HH, Kridli RT, Alnimer MA. Estrus synchronization in sheep and goats using combinations of GnRH, progestagen and prostaglandin F2α. Reprod Domest Anim. 2010;45:594–9. doi: 10.1111/j.1439-0531.2008.01309.x. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson JS, Pursley JR, Garverick HA, Fricke PM, Kesler DJ, Ottobre JS, et al. Treatment of cycling and noncycling lactating dairy cows with progesterone during Ovsynch. J Dairy Sci. 2006;89:2567–78. doi: 10.3168/jds.S0022-0302(06)72333-5. [DOI] [PubMed] [Google Scholar]
- 24.Menchaca A, Rubianes E. Pregnancy rate obtained with short-term protocol for timed artificial insemination in goats. Reprod Domest Anim. 2007;42:590–3. doi: 10.1111/j.1439-0531.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 25.Pursley JR, Wiltbank MC, Stevenson JS, Ottobre JS, Garverick HA, Anderson LL. Pregnancy rates per artificial insemination for cows and heifers inseminated at a synchronized ovulation or synchronized estrus. J Dairy Sci. 1997;80:295–300. doi: 10.3168/jds.S0022-0302(97)75937-X. [DOI] [PubMed] [Google Scholar]
- 26.Geary TW, Whittier JC, Downing ER, LeFever DG, Silcox RW, Holland MD, et al. Pregnancy rates of postpartum beef cows that were synchronized using Syncro-Mate-B or the Ovsynch protocol. J Anim Sci. 1998;76:1523–7. doi: 10.2527/1998.7661523x. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson JS, Kobayashi Y, Thompson KE. Reproductive performance of dairy cows in various programmed breeding systems including Ovsynch and combinations of gonadotropin-releasing hormone and prostaglandin F2α. J Dairy Sci. 1999;82:506–15. doi: 10.3168/jds.S0022-0302(99)75261-6. [DOI] [PubMed] [Google Scholar]
- 28.Ainsworth L, Downey BR. A controlled internal drug-release dispenser containing progesterone for control of the estrous cycle of ewes. Theriogenology. 1986;26:847–56. doi: 10.1016/0093-691X(86)90014-2. [DOI] [Google Scholar]
- 29.Wheaton JE, Carlson KM, Windels HF, Johnston LJ. CIDR: a new progesterone-releasing intravaginal device for induction of estrus and cycle control in sheep and goats. Anim Reprod Sci. 1993;33:127–41. doi: 10.1016/0378-4320(93)90111-4. [DOI] [Google Scholar]
- 30.Vilariño M, Rubianes E, Menchaca A. Re-use of intravaginal progesterone devices associated with the short-term protocol for timed artificial insemination in goats. Theriogenology. 2011;75:1195–200. doi: 10.1016/j.theriogenology.2010.11.030. [DOI] [PubMed] [Google Scholar]