Abstract
A stochastic epidemic model with infectivity rate in incubation period and homestead–isolation on the susceptible is developed with the aim of revealing the effect of stochastic white noise on the long time behavior. A good understanding of extinction and strong persistence in the mean of the disease is obtained. Also, we derive sufficient criteria for the existence of a unique ergodic stationary distribution of the model. Our theoretical results show that the suitably large noise can make the disease extinct while the relatively small noise is advantageous for persistence of the disease and stationary distribution.
Keywords: Stochastic epidemic model, Homestead–isolation, Infectivity in incubation period, Survival, Stationary distribution
Introduction
Over the last several decades, infectious disease models have gained increasing recognition as a powerful tool to reveal the mechanism spread of diseases. Based on systems design with deterministic and stochastic models, there are a lot of literatures to investigate the transmission rates of diseases, see [1–27] and the references therein. One of classic epidemic models is the SEIR model, which subdivides a homogeneous host population into categories containing susceptible, exposed, infectious and recovered individuals, with their population sizes denoted by S, E, I and R, respectively. Anderson and May [2] first used a system of ordinary differential equations (ODEs) to describe a classical SEIR epidemic model in 1991. Later, ODEs, IDEs, PDEs and SDEs are heavily employed to investigate SEIR epidemic models and many good results are obtained, for details to see [3, 22–30]. In particular, Zhao et al. [30] established and studied an SEIR model with non-communicability in incubation period. But, for some diseases, such as COVID-19, the incubation period is infectious [31], and the COVID-19 outbreak control in China shows that both physical protection [32–34] and social isolation [35–37] play important roles in controlling the epidemic in the present absence of vaccines for the virus. With the idea of infectivity in incubation period, Jiao et al. [38] proposed a deterministic SEIR epidemic model with homestead–isolation on the susceptible
| 1.1 |
where , and the biological meanings of the parameters of model (1.1) are shown in Table 1 below.
Table 1.
The biological significance of each parameter for model (1.1)
| Notation | Biological meaning |
|---|---|
| The enrolling rate | |
| The infective rate from S to E | |
| The homestead–isolation rate of the susceptible | |
| The infective rate of the exposed in incubation period | |
| The natural death rate | |
| The transition rate from E to I | |
| The hospitalized rate of I for the disease | |
| The transition rate from I to R | |
| The recovery rate of I |
Since the variable R in the fourth equation is not involved in the first three equations of model (1.1), a reasonable idea is to consider the following model
| 1.2 |
It follows from [38] that the basic reproduction number is , and the threshold dynamics results of model (1.2) are summarized as follows:
The positive equilibrium (denoted by ) is globally asymptotically stable if ;
The disease-free equilibrium (denoted by ) is globally asymptotically stable provided that .
Admittedly, deterministic model has significant advantages in simplifying the complex system and facilitating the theoretical analysis, whereas there exist some limitations in describing the population dynamics by this modeling method, especially when the external interference is relatively large and the population number decreases dramatically due to large-scale natural disasters including hurricanes, tsunamis, volcanoes, earthquakes such that the law of large numbers becomes no longer available and the dynamical behaviors have changed even more radically compared with the corresponding deterministic models [39–41]. For example, Arnold et al. [39] found that the real environmental white noise could make the stable Lotka-Volterra system no longer stable, even a stationary solution no longer exists. Zhou et al. [40] revealed that random effects may lead the disease to extinction in scenarios where the deterministic model exhibits persistence. Since the fluctuations in the randomly varying environment, such as humidity, temperature, food supply, season, etc., constantly affect the biological population densities [42], which embodies the objectivity and universality of the stochasticity. Accordingly, some researchers have argued that stochastic differential equations (SDEs) should be used to model epidemic systems because they are inevitably affected by the environmental noises, thus their stochastic dynamics have been intensively studied, for example, persistence and extinction [12, 16–20, 23], asymptotic stability [24, 25], positive recurrent [20, 23], stationary distribution [6, 16–19, 43, 44], periodic solution [43, 44], optimal vaccination strategy [14] and so on.
In the last few years, numerous good works [23, 45–48] on stochastic SEIR epidemic model have sprung up that focused on the effects of stochastic perturbation on their transmission dynamics. In particular, Yang and Mao [23] observed that the dynamical behaviors of the perturbed SEIR models be considerably different from that of the deterministic counterpart, namely, large perturbations can accelerate the extinction of epidemics regardless of the magnitude of the basic reproduction number . Applying Lyapunov method Zhang and Wang [45] achieved the conditions for the stochastic stability, persistence and extinction of an SEIR model driven simultaneously by white and Lévy noises (termed jumps) which can capture the wide spread of infectious diseases caused by medical negligence. More recently, Boukanjime et al. [46] utilized a stochastic SEIR model to describe the COVID-19 transmission dynamics affected by mixture of white and telegraph noises and investigated the extinction and persistence in the mean of the COVID-19 epidemic in Indian states in terms of the stochastic threshold .
It should also be mentioned that several possible approaches incorporating stochastic effects into the epidemic models have been proposed and extensively studied. Thereinto, one well-known approach to construct the discrete-time or continuous-time Markov chain models [49–51], which are conducive to estimating parameters and exploring the implications of the models by using statistical methods and simulation (e.g., seeing [50, 51]). Artalejo and his coauthors [50] formulated a stochastic SEIR model described by a continuous-time Markov chain and developed efficient computational procedures for the distribution of the duration of an outbreak. Another frequently used approach is to incorporate the noises (such as the white, color, Lévy, telegraph noises or the coupling noises between them) into certain deterministic epidemic models [20, 43–46]. This approach is mainly involved in the following several different forms of perturbations: the noises may perturb model parameters [23, 52–56], or be proportional to the distance between the state variables and the endemic equilibria of the deterministic models [24, 57, 58], or proportional to the state variables measuring population densities [19, 25, 43, 47, 59], with the advantage of good understanding the stochastic dynamics in long time of these models from the theoretical analysis perspective. For instance, Liu et al. [25] analyzed asymptotic behaviors of the equilibria of a stochastic delayed nonlinear SEIR epidemic model via Lyapunov functions.
This contribution is interested in probing into stochastic dynamics of model (1.2) incorporating random perturbations by thorough mathematical analysis. Therefore, following [25, 47, 59], we suppose that random perturbations are proportional to the variables S, E and I in model (1.2) under the influence of white noises and get a stochastic model
| 1.3 |
where are the white noises which are regarded as the derivative of mutually independent standard Brownian motions . are the intensities of the noises and are defined on the complete probability space . Also, we assign .
Our main purpose of this work is to investigate the effects of environmental noises on the stochastic dynamics of model (1.3) and reveal whether the noises can inhibit the disease or not by comparing with the global dynamical results of model (1.2) established in [38]. The rest organization is as follows. Section 2 focuses on the extinction and strong persistence in the mean of the disease. Section 3 discusses the existence of a unique ergodic stationary distribution. Numerical simulations are conducted to substantiate our analytical results in Sect. 4. A brief biological discussion is provided in the final section.
Survival results of the disease
The section focuses on the survival results of the disease, and we first give the following two lemmas (Lemmas 2.1 and 2.2).
Lemma 2.1
For initial value , there is a unique solution (S(t), E(t), I(t)) of model (1.3) on and the solution remains in with probability one.
Assign , where , the rest proof is similar to Theorem 2.1 in [25], and we omit it here.
The following Lemma 2.2 is useful for the proofs of Theorems 2.1–2.2.
Lemma 2.2
Let (S(t), E(t), I(t)) be the solution of (1.3), then , , , , , , , a.s. And , , , a.s.
Proof
It follows from (1.3) that
| 2.1 |
and we obtain, by solving the above equation (2.1), that
| 2.2 |
Let
Clearly, M(t) is a continuous local martingale with .
Define
where
It follows from (2.2) that for all . Obviously, H(t) and Y(t) are continuous adapted increasing processes on with . Applying Theorem 3.9 in [60], we obtain . Hence
| 2.3 |
Thus, one easily derives
Assign
It follows from the quadratic variations that
By the large number theorem for martingale (see [60]) and (2.3), one has
Similarly, we have
The proof of the desired conclusions is finished.
Next, we consider the extinction of the disease.
Theorem 2.1
If , then the disease I(t) will be extinct.
Proof
Integrating both sides of (2.1) over the interval [0, t] and dividing by t lead to
from which we conclude that
We can know from Lemma 2.2 that
| 2.4 |
Let . Applying Itô’s formula yields
| 2.5 |
Integrating both sides of (2.5) over the interval [0, t], dividing by t, and combining with (2.4) and Lemma 2.2, we have
which implies that
Thus, the disease I(t) will be extinct.
Assumption A
.
Finally, we give the persistence of the disease.
Theorem 2.2
If Assumption A holds, then the disease I(t) will be strong persistent in the mean, and
Proof
Integrating on both sides of the last equation of (1.3) over the interval [0, t] and dividing by t lead to
We computer that
| 2.6 |
where
It follows from Lemma 2.2 that we have
| 2.7 |
Assign
where
Applying Itô’s formula to U, one obtains
where
Thus
| 2.8 |
Integrating (2.8) over the interval [0, t] and then dividing by t on both sides, we have
| 2.9 |
Taking the superior limit on both sides of (2.9) and combining with (2.6), (2.7) and Lemma 2.2, we have
The proof is complete.
Stationary distribution
In this section, we further study the stationary distribution for model (1.3) by using the theory of Hasminskii [61]. Let X(t) be a regular time-homogeneous Markov process in described by
and the corresponding diffusion matrix is defined as follows
Lemma 3.1
[61] The Markov process X(t) has a unique ergodic stationary distribution if there exists a bounded domain with regular bounded and
-
(i):
A positive number M satisfied that .
-
(ii):There exists V ( V is a nonnegative -function) such that LV is negative for any . If is a function integrable with respect to the measure , then
for all .
Theorem 3.1
If Assumption A holds, model (1.3) owns a unique ergodic stationary distribution .
Proof
We only need to verify the assumptions in Lemma 3.1. We first consider the diffusion matrix of model (1.3)
Assign , then
which implies that (i) in Lemma 3.1 is satisfied.
Now, we continue to verify (ii). Consider a -function with
where , are positive constants to be chosen later, is a constant satisfying and the constant is sufficient large such that , where ,
In addition, is continuous and tends to when (S, E, I) is close to the boundary of . Therefore, it has lower bounded and reaches the lower bound at this point in the interior of . Let us consider a nonnegative -function with
where and is a sufficiently large integer, , and
Applying Itô’s formula, one has
| 3.1 |
Similarly,
| 3.2 |
and
| 3.3 |
where
In addition, we can obtain
| 3.4 |
Combining with (3.1)–(3.4), we have
| 3.5 |
where
Assign a bounded closed set as follows
where is a suitably small constant satisfying the following inequalities
| 3.6 |
| 3.7 |
| 3.8 |
| 3.9 |
| 3.10 |
| 3.11 |
where
and we will determine the two positive constants , later. For convenience, we first divide into six domains with the forms
Clearly, . By verifying the above six cases, we will prove that on .
Case 1 When , then (3.5) and (3.6) imply that .
Case 2 When , it follows from (3.5) and (3.7) that
Case 3 When , we obtain from (3.5) and (3.8) that .
Case 4 When , (3.5) and (3.9) imply that .
Case 5 When , it follows from (3.5) and (3.10) that , where
Case 6 When , one obtains from (3.5) and (3.11) that , where .
From the above discussion, we obtain that on , which implies that (ii) in Lemma 3.1 also holds. This completes the proof.
Numerical simulations
In this section, our analytical results will be verified by numerical simulations. Let us choose initial value , and the parameter values are kept the same as in [38], see Table 2.
Table 2.
Parameter values used in model (1.2)
| Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Value | 10 | 0.2 | 0.3 | 0.1 | 0.3 | 0.2 | 0.2 |
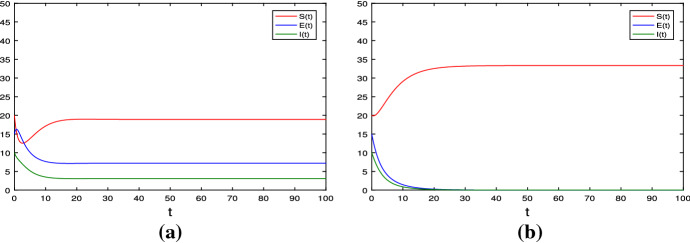
Jiao et al. [38] discussed the effect of (see Fig. 1a and b), that is, the disease I(t) of deterministic model (1.2) will die out if is large, and however the disease I(t) will be persistent if is relative small. This fact shows that the strategy of the homestead–isolation on the susceptible is very important in the epidemics of infectious diseases.
Fig. 1.
a , , then the positive equilibrium of deterministic model (1.2) is globally asymptotically stable. b , , then its disease-free equilibrium is globally asymptotically stable
We choose , , and the same as Fig. 1b, then . It follows from Theorem 2.1 that the disease goes to extinction (see Fig. 2). Compared with Fig. 1b, we can see that the noise will accelerate the extinction of the disease although the disease tends to be extinct in deterministic model (1.2).
Fig. 2.
a The disease of stochastic model (1.3) is extinct. b Frequency histogram at time 100
To illustrate the threshold of disease and the effect of the environment white noises. To compare with Fig. 1a, we continue to select the same as Fig. 1a, and smaller noises with , , . For the stochastic model (1.3), a calculation shows that , then the conditions of Theorems 2.2 and 3.1 are satisfied. As observed in Fig. 3 the disease of model (1.3) goes to strong persistence in the mean, and moreover the conditions support model (1.3) owns an ergodic stationary distribution, see Fig. 4. Recalling Fig. 1a, we know that the disease will persist if , and Figs. 3 and 4 shows that although it is affected by environmental noise, the disease will persist if the noise intensity is relatively low.
Fig. 3.
a The disease of stochastic model (1.3) will be strongly persistent in the mean. b Frequency histogram at time 100
Fig. 4.
a Solution of stochastic model (1.3). b Blue line frequency histogram of stochastic model (1.3) at time 100; Red line the probability density function (PDF) of its corresponding stationary distribution simulated by 3000 sample trajectories. (Color figure online)
Discussions
In this paper, based on a deterministic SEIR epidemic model with infectivity in incubation period and homestead–isolation on the susceptible proposed by Jiao et al. [38], we incorporate white noise into the above model and establish a stochastic version. We first discuss the extinction and strong persistence in the mean of the disease, and later the stationary distribution is investigated by using Hasminskiis method and Lyapunov function. Let us recall Theorems 2.1, 2.2 and 3.1, we reveal the biological conclusions as follows:
Theorem 2.1 shows that the disease will be extinct when . This fact implies that if the noise intensities are suitably large and the cooperation from the homestead–isolation rate is enough while the infective rate of the exposed in incubation period is relatively small, then the disease I(t) is extinct. That is, increasing the noise intensity and homestead–isolation rate are advantageous for the extinction of the disease while increasing the infective rate of the exposed in incubation period is harmful for it.
It follows from (see Theorem 2.2) that if the noise intensities are small and the cooperation from the homestead–isolation rate is inadequate, then the disease I(t) will be strong persistent in the mean.
When , Theorem 3.1 shows that model (1.3) has a unique stationary distribution which is ergodic. This result indicates that decreasing the noise intensities and the homestead–isolation rate may result in a high prevalence level of the disease, and hence the governments should strictly implement the isolation system to make every effort to curb propagation of disease.
In the end, it should be pointed out that, in order to compare with the global dynamical results of model (1.2) established in [38], this work is only concerned with the stochastic dynamics of the variables S, E, I in model (1.3) under the three noises , , however it also would deserve to introduce a noise into the variable R in the fourth equation of model (1.1) and investigate the four-dimension stochastic model. In addition, we would like to develop more complicated models by incorporating other forms of stochastic perturbations (e.g., perturbed parameters [23, 52–56]), Lévy jump [14, 45] or regime switching [62], and we leave these interesting and challenging issues for future consideration.
Acknowledgements
The work is supported by the NNSF of China (Nos. 11871201, 11961023), and the NSF of Hubei Province, China (No. 2019CFB241).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dongchen Shangguan, Email: shangguanmath@163.com.
Zhijun Liu, Email: zjliu@hbmzu.edu.cn, Email: zhijun_liu47@hotmail.com.
Lianwen Wang, Email: wanglianwen1987@hotmail.com.
Ronghua Tan, Email: ronghua_tan@hotmail.com.
References
- 1.Kermack WO, Mckendrick AG. Combined effects of prevention and quarantine on a breakout in SIR model. Proc. R. Soc. Edin. A. 1927;115:700–721. [Google Scholar]
- 2.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. New York: Oxford University Press; 1991. [Google Scholar]
- 3.Cooke K, Van Den Driessche P. Analysis of an SEIRS epidemic model with two delays. J. Math. Biol. 1996;35:240–260. doi: 10.1007/s002850050051. [DOI] [PubMed] [Google Scholar]
- 4.Ruan SG, Wang WD. Dynamical behavior of an epidemic model with a nonlinear incidence rate. J. Differ. Equ. 2003;188:135–163. [Google Scholar]
- 5.Wang WD. Epidemic models with nonlinear infection forces. Math. Biosci. Eng. 2006;3:267–279. doi: 10.3934/mbe.2006.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Cai YL, Kang Y, Banerjee M, Wang WM. A stochastic SIRS epidemic model with infectious force under intervention strategies. J. Differ. Equ. 2015;259:7463–7502. [Google Scholar]
- 7.Wang JL, Yang J, Kuniya T. Dynamics of a PDE viral infection model incorporating cell-to-cell transmission. J. Math. Anal. Appl. 2016;444:1542–1564. [Google Scholar]
- 8.Wang LW, Liu ZJ, Zhang XA. Global dynamics for an age-structured epidemic model with media impact and incomplete vaccination. Nonlinear Anal. Real World Appl. 2016;32:136–158. [Google Scholar]
- 9.Li SP, Jin Z. Impacts of cluster on network topology structure and epidemic spreading. Discrete Contin. Dyn. Syst. Ser. B. 2017;22:3749–3770. [Google Scholar]
- 10.Liu ZJ, Hu J, Wang LW. Modelling and analysis of global resurgence of mumps: a multi-group epidemic model with asymptomatic infection, general vaccinated and exposed distributions. Nonlinear Anal. Real World Appl. 2017;37:137–161. [Google Scholar]
- 11.Li JQ, Wang XQ, Lin XL. Impact of behavioral change on the epidemic characteristics of an epidemic model without vital dynamics. Math. Biosci. Eng. 2018;15:1425–1434. doi: 10.3934/mbe.2018065. [DOI] [PubMed] [Google Scholar]
- 12.Xu CY, Li XY. The threshold of a stochastic delayed SIRS epidemic model with temporary immunity and vaccination. Chaos Solitons Fractals. 2018;111:227–234. [Google Scholar]
- 13.Liu ZZ, Shen ZW, Wang H, Jin Z. Analysis of a local diffusive SIR model with seasonality and nonlocal incidence of infection. SIAM J. Appl. Math. 2019;79:2218–2241. [Google Scholar]
- 14.Mu XJ, Zhang QM, Rong LB. Optimal vaccination strategy for an SIRS model with imprecise parameters and Lévy noise. J. Frankl. Inst. 2019;356:11385–11413. [Google Scholar]
- 15.Liu LL, Xu R, Jin Z. Global dynamics of a spatial heterogeneous viral infection model with intracellular delay and nonlocal diffusion. Appl. Math. Model. 2020;82:150–167. [Google Scholar]
- 16.Gray A, Greenhalgh D, Hu L, Mao X, Pan J. A stochastic differnetial equation SIS epidemic model. SIAM J. Appl. Math. 2011;71:876–902. [Google Scholar]
- 17.Liu Q, Jiang DQ, Shi NZ, Hayat T, Ahmad B. Stationary distribution and extinction of a stochastic SEIR epidemic model with standard incidence. Phys. A. 2017;476:58–69. [Google Scholar]
- 18.Feng T, Qiu ZP, Meng XZ. Dynamics of a stochastic hepatitis C virus system with host immunity. Discrete Contin. Dyn. Syst. Ser. B. 2019;24:6367–6385. [Google Scholar]
- 19.Wei, F.Y., Chen, L.H.: Extinction and stationary distribution of an epidemic model with partial vaccination and nonlinear incidence rate. Phys. A 545, 122852, 10pp (2020)
- 20.Cao, Z.W., Shi, Y., Wen, X.D., Liu, L.Y., Hu, J.W.: Analysis of a hybrid switching SVIR epidemic model with vaccination and Lévy noise. Phys. A 537, 122749, 17pp (2020)
- 21.Liu XN, Wang Y, Zhao XQ. Dynamics of a climate-based periodic Chikungunya model with incubation period. Appl. Math. Model. 2020;80:151–168. [Google Scholar]
- 22.Zhao Z, Chen LS, Song XY. Impulsive vaccination of SEIR epidemic model with time delay and nonlinear incidence rate. Math. Comput. Simul. 2008;79:500–510. [Google Scholar]
- 23.Yang QS, Mao XR. Extinction and recurrence of multi-group SEIR epidemic models with stochastic perturbations. Nonlinear Anal. Real World Appl. 2013;14:1434–1456. [Google Scholar]
- 24.Liu M, Bai CZ, Wang K. Asymptotic stability of a two-group stochastic SEIR model with infinite delays. Commun. Nonlinear Sci. Numer. Simul. 2014;19:3444–3453. [Google Scholar]
- 25.Liu Q, Jiang DQ, Shi NZ, Hayat T, Ahmad A. Asymptotic behavior of a stochastic delayed SEIR epidemic model with nonlinear incidence. Phys. A. 2016;462:870–882. [Google Scholar]
- 26.Tian BC, Yuan R. Traveling waves for a diffusive SEIR epidemic model with non-local reaction. Appl. Math. Model. 2017;50:432–449. [Google Scholar]
- 27.Han SY, Lei CX. Global stability of equilibria of a diffusive SEIR epidemic model with nonlinear incidence. Appl. Math. Lett. 2019;98:114–120. [Google Scholar]
- 28.Abta A, Kaddar A, Alaoui HT. Global stability for delay SIR and SEIR epidemic models with saturated incidence rates. Electron. J. Differ. Equ. 2012;386:956–965. [Google Scholar]
- 29.Lv GC, Lu ZY. Global asymptotic stability for the SEIRS models with varying total population size. Math. Biosci. 2018;296:17–25. doi: 10.1016/j.mbs.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Zhao DL, Sun JB, Tan YJ, Wu JH, Dou YJ. An extended SEIR model considering homepage effect for the information propagation of online social networks. Phys. A. 2018;512:1019–1031. [Google Scholar]
- 31.National Health Commission of the People’s Republic of China. (2020). http://www.nhc.gov.cn/. Accessed 26 Jan 2020 [DOI] [PMC free article] [PubMed]
- 32.Cheng VCC, Wong SC, Chuang VWM, So SYC, Chen JHK, Sridhar S, To KKW, Chan JFW, Hung IFN, Ho PL, Yuen KY. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020;81:107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of sars-cov-2 and covid-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng S, Shen C, Xia N, Song W, Fan M, CowlingCowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020;8:434–436. doi: 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier BF, Brockmann D. Effective containment explains subexponential growth in recent confirmed COVID-19 cases in china. Science. 2020;368:742–746. doi: 10.1126/science.abb4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J. Travel Med. 2020;27:1–4. doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 38.Jiao, J.J., Liu, Z.Z., Cai, S.H.: Dynamics of an SEIR model with infectivity in incubation period and homestead-isolation on the susceptible. Appl. Math. Lett. 107, 106442, 7pp (2020) [DOI] [PMC free article] [PubMed]
- 39.Arnold L, Horsthemke W, Stucki JW. The influence of external real and white noise on the Lotka–Volterra model. Biom. J. 1979;21:451–471. [Google Scholar]
- 40.Zhou YL, Zhang WG, Yuan SL. Survival and stationary distribution of a SIR epidemic model with stochastic perturbations. Appl. Math. Comput. 2014;244:118–131. [Google Scholar]
- 41.Zhang, X.B., Wang, X.D., Huo, H. F.: Extinction and stationary distribution of a stochastic SIRS epidemic model with standard incidence rate and partial immunity. Phys. A 531, 121548, 14pp (2019)
- 42.Lewontin RC, Cohen D. On population growth in a randomly varying environment. Proc. Nat. Acad. Sci. 1969;62:1056–1060. doi: 10.1073/pnas.62.4.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang XH, Jiang DQ, Hayat T, Alsaedi A. Periodic solution and stationary distribution of stochastic S-DI-A epidemic models. Appl. Anal. 2018;97:179–193. [Google Scholar]
- 44.Qi HK, Leng XN, Meng XZ, Zhang TH. Periodic solution and ergodic stationary distribution of SEIS dynamical systems with active and latent patients. Qual. Theory Dyn. Syst. 2019;18:347–369. [Google Scholar]
- 45.Zhang XH, Wang K. Stochastic SEIR model with jumps. Appl. Math. Comput. 2014;239:133–143. [Google Scholar]
- 46.Boukanjime B, Caraballo T, El Fatini M, El Khalifi M. Dynamics of a stochastic coronavirus (COVID-19) epidemic model with Markovian switching. Chaos Solitons Fractals. 2020 doi: 10.1016/j.chaos.2020.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, F., Zhang, S.Q., Meng, X.Z.: Dynamics analysis and numerical simulations of a delayed stochastic epidemic model subject to a general response function. Comput. Appl. Math. 38, 95, 30pp (2019)
- 48.Xu R, Guo R. Pontryagin’s maximum principle for optimal control of stochastic SEIR models. Complexity. 2020;2020:1–5. [Google Scholar]
- 49.Allen LJS, Burgin AM. Comparison of deterministic and stochastic SIS and SIR models in discrete time. Math. Biosci. 2000;163:1–33. doi: 10.1016/s0025-5564(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 50.Artalejo JR, Economou A, Lopez-Herrero MJ. The stochastic SEIR model before extinction: computational approaches. Appl. Math. Comput. 2015;265:1026–1043. [Google Scholar]
- 51.Engbert R, Rabe MM, Kliegl R, Reich S. Sequential data assimilation of the stochastic SEIR epidemic model for regional COVID-19 dynamics. Bull. Math. Biol. 2021 doi: 10.1101/2020.04.13.20063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao XR, Marion G, Renshaw E. Environmental Brownian noise suppresses explosions in population dynamics. Stoch. Process. Appl. 2002;97:95–110. [Google Scholar]
- 53.Dalal N, Greenhalgh D, Mao XR. A stochastic model of AIDS and condom use. J. Math. Anal. Appl. 2007;325:36–53. [Google Scholar]
- 54.Zhao YN, Jiang DQ. The threshold of a stochastic SIRS epidemic model with saturated incidence. Appl. Math. Lett. 2014;34:90–93. [Google Scholar]
- 55.Cai S, Cai Y, Mao X. A stochastic differential equation SIS epidemic model with two correlated Brownian motions. Nonlinear Dyn. 2019;97:2175–2187. [Google Scholar]
- 56.Mummert A, Otunuga OM. Parameter identification for a stochastic SEIRS epidemic model: case study influenza. J. Math. Biol. 2019;79:705–729. doi: 10.1007/s00285-019-01374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beretta E, Kolmanovskii V, Shaikhet L. Stability of epidemic model with time delays influenced by stochastic perturbations. Math. Comput. Simul. 1998;45:269–277. [Google Scholar]
- 58.Hussain, G., Khan, A., Zahri, M., Zaman, G.: Stochastic permanence of an epidemic model with a saturated incidence rate. Chaos Solitons Fractals 139, 110005, 7pp (2020)
- 59.Jiang DQ, Yu JJ, Ji CY, Shi NZ. Asymptotic behavior of global positive solution to a stochastic SIR model. Math. Comput. Modell. 2011;54:221–232. [Google Scholar]
- 60.Mao XR. Stochastic Differential Equations and Applications. Chichester: Horwood Publishing; 2007. [Google Scholar]
- 61.Hasminskii RZ. Stochastic Stability of Differential Equations. The Netherlands: Sijthoff Noordhoff, Alphen aan den Rijn; 1980. [Google Scholar]
- 62.Liu Q, Jiang DQ, Shi NZ. Threshold behavior in a stochastic SIQR epidemic model with stansard incidence and regime switching. Appl. Math. Comput. 2018;316:310–325. [Google Scholar]