Abstract
The pandemic of Serious Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) that produces corona virus disease (COVID-19) has challenged the entire mankind by rapidly spreading globally in 210 countries affecting over 25 million people and about 1 million deaths worldwide. It continues to spread, afflicting the health system globally. So far there is no remedy for the ailment and the available antiviral regimens have been unsatisfactory for the clinical outcomes and the mode of treatment has been mainly supportive for the prevention of COVID-19-induced morbidity and mortality. From the time immortal the traditional plant-based ethno-medicines have provided the leads for the treatment of infectious diseases. Phytopharmaceuticals have provided potential and less toxic antiviral drugs as compared to conventional modern therapeutics which are associated with severe toxicities. The ethnopharmacological knowledge about plants has provided food supplements and nutraceuticals as a promise for prevention and treatment of the current pandemic. In this review article, we have attempted to comprehend the information about the edible medicinal plant materials with potential antiviral activity specifically against RNA virus which additionally possess property to improve immunity along with external and internal respiration and exhibit anti-inflammatory properties for the prevention and treatment of the disease. This will open an arena for the development of novel nutraceutical herbal formulations as an alternative therapy that can be used for the prevention and treatment of COVID-19.
Keywords: Antiviral, Nutraceutical, Edible plants, Coronavirus, COVID-19
Introduction
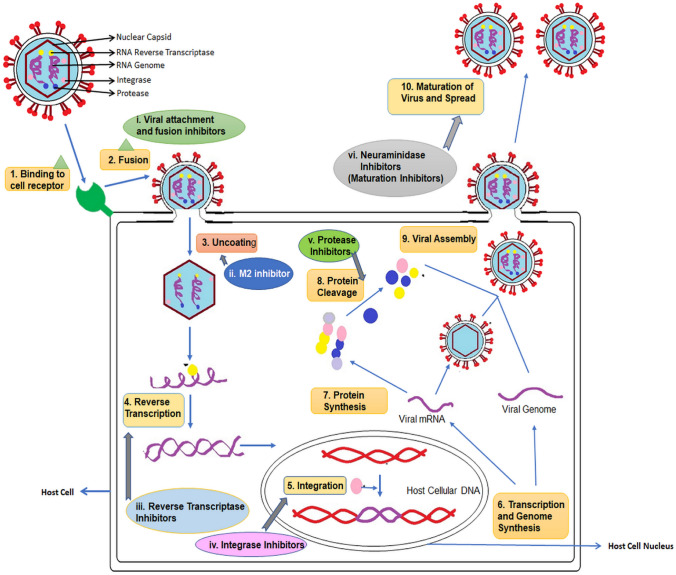
In Wuhan, China in December 2019, a newly emergent novel coronavirus SARS-CoV-2 was reported to cause severe acute respiratory tract infections, coronavirus disease 2019 (COVID-19) [1]. After the first case of corona reported from Wuhan, there has been an unprecedented outbreak of COVID-19. As of the last week of August 2020, over 25 million cases of this disease have been reported from 210 countries with 3.32% deaths [2, 3]. The United Nations called the pandemics of COVID-19 as the worst global humanitarian crisis since World War II. Countries all over the world are taking aggressive steps and adopting all possible preventive measures to combat the spread of this disease. The disease is associated with high mortality risk (2–8% in different countries), a very high transmission rate combined with the lack of WHO or FDA approved specific prophylactic vaccines or therapeutic protocols for the effective prevention, treatment or management of the disease. A typical viral disease mechanism involves the entry of virus into the host via specific receptor, followed by uncoating, transcription and genome synthesis finally forming viral assembly and releasing of multiple copies in the host. The antiviral drugs are designed to act on such varied targets (Fig. 1).
Fig. 1.
Typical viral disease mechanism and various targets for antiviral drugs
The current treatment appears to be mainly supportive in nature [4, 5]. From time immemorial, herbal medicine has provided remedies for majority of the diseases, for e.g., digitalis and reserpine for cardiac patients, artemisinin and quinine for malaria, vincristine and vinblastine for cancer. Some potential drug candidates including blockbuster antivirals like Remdesivir, Hydroxychloroquine, Lopinavir, Ritonavir, APN01 or Favipiravir are being tested for clinical trials across the globe. Still no therapy has been found to be effective or devoid of deleterious effects against COVID-19 as of now [6–8]. Keeping in view the shortcomings associated with available antiviral drugs therapies, i.e. viral resistance coupled with the problem of viral latency and conflicting efficacy in recurrent infection in immunocompromised patients, there is an increasing need for search of new compounds and therapy with antiviral activity that are highly efficacious and cost-effective for the management and control of viral infections. Moreover, the viral infections caused by Coronaviruses, Human immunodeficiency virus, Ebola virus, Nipah virus, Influenza virus, Enterovirus, Swine flu, Bird flu, Zika Virus, Hepatitis B and C, etc. have risen significantly and natural products play a vital role in the cure for some with no or less harmful effects [9, 10]. Novel bioactive phytomolecules bearing credible therapeutic potential against such viral diseases is the prime focus of the current medical research in order to gain an upper edge over such widespread infections and prevent the future ones [11, 12]. Identification of the antiviral mechanisms from these natural agents has shed light for further research targeting virus–host-specific interactions.
Indian subcontinent has been recognized as a treasure home for various plant species due to its varied agro-climatic zones and suitable topographical conditions and is placed among the list of top 12 mega diversity countries of the world. The Indian subcontinent is endowed with rich and diverse flora about which the ethnobotanical literature describes use as plant extracts, infusions and powders for diseases of infectious nature [13]. These medicinal herbs provide a wide approach in managing several diseases, including viral respiratory infections by modulating immune system and inflammatory responses. AYUSH system of medicine has provided a basic approach on prevention of infection through dietary modification, lifestyle management, remedies to boost immune system and preventive interventions based on the symptom [14]. Around 65–80% of the world population residing in developing countries utilizes traditional herbs in their primary health treatment. Additionally, the interest in the study of herbs have aroused due to their phytoactive/phytotherapeutic agents which can be utilized in the form of nutraceuticals, which possess drug-like actions, and in some cases can be traced directly through the existing links between a local and biomedical use [15]. The lack of preventive and curative treatment of COVID-19 till date compels the researchers to look onto therapeutic alternatives that can be added in our daily diets in order to both prevent and cure such life-threatening infections and provide long-term protection. For centuries, numerous plants have been used in daily diets which serve as folk remedies by supporting the body system in one way or the other [16].
This review aims to bring focus on the detailed information about herbal flora with antiviral activities that can be explored for development of novel nutraceutical herbal formulations. Advancement in separation technologies, adoption of modern drug discovery and the development of vector-based strategies for antiviral screening purposes offers a promise for edible medicinal plants usage in daily diet, and may serve as an alternative therapy for treatment of this pandemic and prevention of another one. The article also aimed to merge the ethnopharmacological knowledge with the modern technologies to devise drug targets for the SARS-COV-2 virus and identification of potential candidates from natural sources which may offer some preventive or even therapeutic value.
Pathogenesis of COVID-19 and strategy for using edible plants
For a better insight on how nutraceuticals or phytomolecules can effectively work against novel coronaviruses, it is imperative to understand the structural characteristics and culpable targets and receptors associated with it. Moreover, understanding the mechanism of action of conventional antivirals and liable targets for drug designing may be useful for development of therapy regimen for COVID-19 from natural sources. SARS-CoV-2 like other HCoVs is positive-sense single-stranded RNA viruses with two groups of protein forming its characteristic markers: structural protein, such as Spike (S), Nucleocapsid (N), Matrix (M), Envelope (E); and non-structural proteins such as nsp12-RNA-dependent RNA polymerase (RdRp), Nsp3- Papain-like proteinases, Nsp5-3C-like main protease and nsP13 SARS-CoV helicase [17, 18]. Primarily, nsP13 helicase, 3CL protease, nsp12-RNA-dependent RNA polymerase (RdRp) become primary target for drug development. Apart from these proteins, the viral spike glycoprotein (S) initial attachment and internalization within host cells ACE-2 receptor can also be targeted to prevent viral entry into newer host cells [19]. SARS-CoV-2 recognizes human angiotensin-converting-enzyme-2 (ACE-2), thereby proving its essentiality for host cell entry by invasion of alveolar epithelial cells, subsequent viral replication and primary host lung cells infection as ACE-2 is highly expressed in the heart, lungs, intestine, kidney and blood vessels [20]. The expression of ACE-2 is substantially increased in patients with diabetes and hypertension and the connecting link to this associated comorbidity has been the angiotensin-converting-enzyme-2 (ACE-2) receptor as it is the site of virus multiplication, and thus a strategy has been devised to develop antiviral newer drugs considering the ACE-2 as an attractive target [21]. Various plants produce phytomolecules that can be utilized in targeting these viral targets and so has been done previously in case of other viral diseases like SARS, HIV, HCV, etc. [22–24].
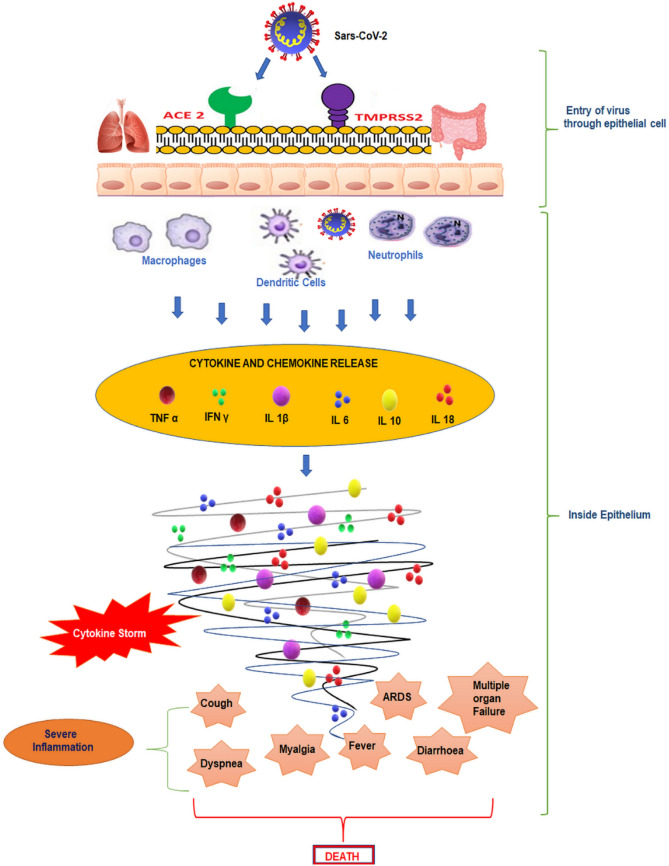
At the molecular level, the virus SARS-CoV-2 binds to the angiotensin-converting-enzyme-2 (ACE-2) present in the lungs of the human host. Binding of the virus to the host cells through its trimeric spike glycoprotein makes this protein a key target for potential therapies and diagnostics. It was reported that in SARS-CoV-2, the S2 subunit in each spike monomer contains a fusion peptide, a transmembrane domain, and cytoplasmic domain which is highly conserved and could be a possible target for antiviral (anti-S2) compounds [25]. There occurs multiplication of the viruses that induces cellular responses. There occurs infiltration of huge number of inflammatory cells which comprise innate immune cells and adaptive immune cells [26, 27]. Neutrophils are majorly the innate immune cells which produce injury to lungs [28]. On the other hand, the adaptive immune cells are majorly the T cells viz. cytotoxic CD8+ T cells which not just kill virus but again contribute injury to lungs [29]. This accelerates the progression of systemic inflammatory response, leading to extensive increase in various cytokines like TNFα, IL1, IL6, IL10, etc. This is termed as the cytokine surge. Due to increase in various cytokines, there occurs inflammation and apoptosis of Type-1 and Type-2 cells in the alveoli. This interrupts the functions of oxygen transport resulting in cell death in alveoli of the lungs and causing Acute Respiratory Distress or syndrome (ARDS) [30]. Figure 2 depicts the pathogenesis of disease leading to cytokine storm and multiple organ dysfunction and ultimately death.
Fig. 2.
Pathogenesis of COVID-19
Among the proposed mechanisms of pulmonary injury caused by SARS-CoV-2, there is a “cytokine storm” triggered by an imbalanced response by type-1 and type-2 T helper (Th) cells leading to an uncontrolled and generalized inflammatory response [31]. Increased pro-inflammatory cytokines (Interferon γ, interleukin (IL-) 1β, IL-6, IL-12) and chemokines (CXCL10 and CCL2) circulating in the body are associated with pulmonary inflammation and ARDS pathogenesis due to inflammatory injury to the alveolar-capillary membrane, resulting in increased lung permeability and the exudation of protein-rich pulmonary edema fluid into the airspaces culminating into respiratory insufficiency and the main causes of complications leading to multi-organ failure [32]. Antiviral plants with added anti-inflammatory properties protecting the lung against infections can be investigated, for that matter, to have a synergetic therapy.
Weak immune mechanisms coupled with cytokine surge is one of the major causes that finally leads to decreased cellular oxygenation at the level of alveoli, has been reported to be the main cause of death in COVD-19. Apart from this respiratory damage there occurs thrombotic events involving open reading frames (ORFs) especially the ORF8 proteins which upon binding with SARS-COV2 leading to dissociation of iron from the1-beta chain of hemoglobin getting attached to the surface glycoprotein porphyrin and thereby resulting in failure of internal respiration [33].
SARS-CoV2 has a longer incubation period of 2–14 days on an average inside the human body, probably due to their immune evasion properties, efficiently escaping host immune detection at the early stage of infection [34]. Herbal preparations that possess immunomodulatory activity may serve as prophylactic treatment, if added in daily diet, for prevention of infection acquisition during this spell of critical community level spread and help contain the disease in community as well as help faster healing post infection. Considering the above strategies for treatment, management and prevention of COVID- 19, a search for potential plants with above properties can help to devise natural plant-derived antiviral agents against the pandemic disease.
Edible plants exhibiting antiviral property against RNA viruses: initial signals for COVID-19
The secondary metabolites obtained from herbal drugs can also be utilized as nutraceuticals and can become a lead compound in the treatment therapy [35]. Studies have also shown promising results of nutraceuticals and phytomolecules in various pathological complications such as diabetes, atherosclerosis, cardiovascular diseases (CVDs), cancer and neurological disorders [14]. Since ages, herbs of Indian origin have been implemented in treatment and as preventive strategies for several diseases that include respiratory viral infections as the benefit of usage of these herbs against viral respiratory infections lies in immune stimulation and inflammation modulating effects. The AYUSH systems of medicine also promotes prevention of COVID-19 through lifestyle modification as well as dietary management and prophylactic interventions for improving the immunity [31]. All these have led to a revival of interest in herbal medicines, novel nutraceuticals and herbal formulations with antiviral potency based on any of the potential plants. Looking into the results of previously deciphered phytochemical-directed researches, a wide variety of phytomolecules present in Indian forest biodiversity can point towards their capability to be manipulated into devising antiviral drugs for SARS-CoV-2. Table 1 provides detailed information on edible plants used as food or nutraceutical showing antiviral activity against RNA viruses, their potential to be explored against COVID-19 on the basis of antiviral activity against various RNA viruses, their active phytoconstituents bearing potential anti-coronaviral activity and mechanism of action.
Table 1.
Edible plants present in Indian biodiversity being used as food or nutraceutical showing anti-retroviral activity
| S. No. | Plant species /family | Common name | Major chemical constituents | Used as | Virus type | Extract type/active compound | Mechanism of action |
|---|---|---|---|---|---|---|---|
| 1 | Abutilon indicum L. (Sweet)/Malvaceae | Indian lantern flower, Indian mallow, Kanghi | β-Sitosterol, asparagine [86] | Food [87] | Anti-mouse coronaviral activity (a surrogate of SARS-CoV) [66] | Aerial parts methanol extract [66] | Mechanism not clear |
| 2 | Acalypha indica L./Euphorbiaceae | Indian-nettle, Copperleaf, Kuppi, Kuppikhokhali | Acalyphin, kaempferol [88] | Food [89] | Vesicular stomatitis virus [90] | Ethanolic leaf extract [90] | Inhibitory activity by protein interaction [90] |
| 3 | Aegle marmelos (L.) Correa/Rutaceae | Bael | Marmin, marmesin [62] | Food [91] | Human coxsackieviruses B1-B6 infection [62] | Methanolic and aqueous methanolic (1:1) extract of Leaves, stem, stem bark, root, root bark/Marmelide [62] | Inhibits viral replication [62] |
| 4 | Agrimonia pilosa Ledeb./Rosaceae | Hairy agrimony | Catechin, hyperoside [50] | Food [92] | Influenza virus [50] | Whole plant ethanol extract/Flavonoids (catechin, hyperoside, quercetin, and rutin) [50] | Reacts with viral membrane, inhibits viral replication and viral mRNA synthesis [50] |
| 5 | Allium sativum L./Amaryllidaceae | Garlic | Allicin, Alliin [43] | Nutraceutical [93], Spice [94] |
SARS-CoV [37] Parainfluenza-3, Human rhinovirus, Vesicular stomatitis virus [43] |
Lectin ( ASA, ASA1) [37], fresh garlic clove extract/Ajoene, allicin, allyl methyl thiosulfinate, methyl allyl thiosulfinate [43] |
Interfere with the glycans on the spike protein during virus entry and virus release [37], Inhibits viral adsorption or penetration [43] |
| 6 | Aloe vera (L.) Burm. f./Asphodelaceae | Aloe vera, Gwarpatha, Ghritkumari | Polysaccharides, aloin [95] | Food [95] | Influenza A virus [96] | Aqueous leaf extract/polysaccharide [96] | Inhibits viral attachment to host cell [96] |
| 7 | Areca nut L./Arecaceae | Supari, Betelnut | Arecoline, guvacine [97] | Mouth fresher [98] | Human immunodeficiency virus type 1[2] | Aqueous and methanolic seed extract/arecatannins [2] | Inhibition of HIV type-1 protease enzyme [2] |
| 8 | Artemisia annua L. /Asteraceae | Sweet sagewort | Artemisinin [99] | Spice [100] | SARS-CoV [67] | Whole plant ethanol extract [66] | Mechanism not clear |
| 9 | Azadirachta indica A. Juss./Meliaceae | Neem, Indian-lilac | Azadirachtin [101] | Nutraceutical [101] | Group B Coxsackieviruses [72] | Methanolic leaf extract/Flavonoids, triterpenes [72] | Inhibits viral replication [72] |
| 10 | Camellia sinensis (L.) Kuntze/Theaceae | Black tea, Common tea, Green tea | Epigallocatechin gallate [102] | Beverage [103] | Bovine coronavirus [75], Influenza virus [104], HIV-1 [102] |
Epigallocatechin gallate [75], Aqueous leaf extract/Catechins [104], Hot aqueous leaf extract/Epigallocatechin gallate [102] |
Inhibitory effect by interacting with spike glycoprotein [75], Inhibits various virus lifecycle steps [104, 102] |
| 11 | Cassia occidentalis L./Fabaceae | Coffee senna | Rhein, emodin [105] | Food [106] | Human immunodeficiency virus [40] | Methanolic leaf extract [40] | Inhibiting HIV reverse transcriptase activity [40] |
| 12 | Cicer arietinum L./Fabaceae | Chick Pea, Bengal gram | Dietary minerals [107] | Food [107] | Parainfluensa-3 virus [48] | Methanolic extract of seed, fruit skin and aerial part/Phenolic compounds [48] | Inhibits parainfluensa-3 virus [48] |
| 13 | Commelina communis L./Commelinaceae | Asiatic dayflower | Homonojirimycin [108] | Food [109] | Influenza virus [108] | Ethanolic leaf and stem extract/Homonojirimycin [108] | Prevents inflammatory responses and strengthen host resistance against viral infection by activating secretion of IFN- and IL-10 [108] |
| 14 | Curcuma longa L. /Zingiberaceae | Haldi, turmeric | Curcumin [110] | Spice [111] | Respiratory syncytial virus [110] | Curcumin [110] | Inhibit viral replication [110] |
| 15 | Cynara Scolymus L./Asteraceae | Globe artichoke, Sharifa | Cynaropicrin [39] | Food, nutraceutical [112] | Hepatitis C virus [39] | Cynaropicrin [39] | Inhibits viral cell-entry [39] |
| 16 | Embelia ribes Burm. f./Primulaceae | Vidanga | Embelin [59] | Nutraceutical [113] | Influenza A virus (H1N1) [59] | Ethyl acetate fruit extract/Embelin [59] | Inhibits viral replication [59] |
| 17 | Eugenia jambolana Lam./Myrtaceae | Jamun, Jambul | Delphinidin, petunidin [114] | Food [114] | Influenza virus (H5N1) [115] | Methanolic, hydromethanolic and aqueous leaf extract; aqueous bark extract [115] | Interferes with viral envelop that are necessary for adsorption or entry into host cells [115] |
| 18 | Gingko biloba L./Ginkgoaceae | Maidenhair-tree, Ginkgo | Ginkgetin [46] | Nutraceutical [116] | Influenza virus [46] | Ginkgetin [46] | Inhibition of viral sialidase activity [46] |
| 19 | Glycyrrhiza glabra L./Fabaceae | Liquorice, Mulethi | Glycyrrhizin [42] | Nutraceutical [117], sweetener[118] | SARS- CoV [42] | Glycyrrhizin [42] | Inhibits viral adsorption, penetration and replication [42] |
| 20 | Gymnema sylvestre (Retz.) Schult./Apocynaceae | Gymnema, miracle-fruit, Gudmar | Gymnemic acid [119] | Nutraceutical [119] | Anti-mouse coronaviral activity (a surrogate of SARS-CoV) [66] | Aerial parts methanol extract [66] | Mechanism not clear |
| 21 | Hibiscus sabdariffa L./Malvaceae | Roselle, Indian-sorrel, Lal ambari | Hibiscus acid, citric acid [120] | Food [120] | H5N1 highly pathogenic avian influenza virus [121] | Aqueous tea extract [121] | Inhibited viral replication and viral antigens and genes expression [121] |
| 22 | Leucas aspera (Wild.) Link/Lamiaceae | Tumba, Chota halkusa | Asperphenamate, sitosterol [122] | Food [122] | Anti-mouse coronaviral activity (a surrogate of SARS-CoV) [66] | Aerial parts methanol extract [66] | Mechanism not clear |
| 23 | Mangifera indica L./Anacardiaceae | Mango | Mangiferin [123] | Food [124] | H2N2 influenza A virus, coxsackie B3 virus [52] | Hydroalcoholic stem bark extract/Penta-O-galloyl-glucose, tetra-O-galloyl-glucose [52] | Inhibits influenza neuraminidase and coxsackie virus 3C protease [52] |
| 24 | Momordica charantia L./Cucurbitaceae | Karela, Bitter gourd, Bitter melon | Momordicine, Charantin [125] | Food [125] | Human immunodeficiency virus [126] | MAP30 protein [126] | Inhibit various stages of viral life cycle [126] |
| 25 | Moringa oleifera Lam./Moringaceae | Drumstick tree | Quercetin, Linolenic acid [76] | Food [127] | Human immunodeficiency virus type-1 [128] | Methanolic, ethyl ether and aqueous extract of leaves/Saponins, tannins, flavonoids [128] | Inhibits viral replication [128] |
| 26 | Myrica esculenta Buch.-Ham. Ex D. Don/Myricaceae | Kaphal, Bayberry | Myricetin, gallic acid [54] [129] | Food [130] | SARS-CoV [54] | Myricetin [54] | Inhibits helicase protein [54] |
| 27 | Nigella sativa L./Ranunculaceae | Black Cumin, Kalonji | Thymoquinone, thymol [49] [131] | Spice [132] | H9N2 avian influenza virus [49] | Dried seeds/Thymoquinone [49] | Inhibit viral replication [49] |
| 28 | Ocimum sanctum L./Lamiaceae | Basil, Tulsi | Eugenol, linolenic acid [133] | Herbal tea [133] | Human immunodeficiency virus [57] | Aerial parts methanolic extract/Flavonoids [57] | Inhibit protease enzyme [57] |
| 29 | Olea europaea L./Oleaceae | Olive | Oleuropein [47] | Edible oil [134] | Viral hemorrhagic septicemia virus [47] | Ethanolic leaf extract/Oleuropein [47] | Direct inactivation, interacts with viral envelope [47] |
| 30 | Phaseolus vulgaris L./Fabaceae | Bean, Rajma | Phaseolin [135] | Food [135] | Human immunodeficiency virus type-1 [58] | Crude bean extract/Homodimeric lectin [58] | Inhibits HIV reverse transcriptase and alpha-glucosidase [58] |
| 31 | Phyllanthus emblica L./Phyllanthaceae | Amla, Indian Gooseberry | Phyllantidine, phyllantine [136] | Food [137] | Human immunodeficiency virus [138] | Methanolic fruit extract [138] | Inhibits HIV reverse transcriptase [138] |
| 32 | Punica granatum L./Lythraceae | Pomegranate | Polyphenols, ursolic acid [139] [140] | Food [139] | Influenza A virus [141] | Ethanolic peel extract [141] | Inhibits viral replication [141] |
| 33 | Solanum nigrum L./Solanaceae | Black Nightshade, Makoi | Solanine, solamargine [142] | Food [142] | Hepatitis C virus [65] | Chloroform and methanol seed extract [65] | Inhibits NS3 protease [65] |
| 34 | Syzygium cumini (L.) Skeels/Myrtaceae | Jaman, Jambolan | Ellagic acid, gallic acid [143] | Food [143] | Avian influenza virus (H5N1) [53] | Aqueous leaf extract, aqueous bark extract [53] | Interfere with viral envelop or mask viral structures which are necessary for adsorption or entry into host cells [53] |
| 35 | Terminalia chebula Retz./Combretaceae | Black myrobalan | Chebulagic acid, Chebulinic acid [144] | Food [145] | Respiratory syncytial virus [69] | Chebulagic acid [69] | Anti-inflammation, suppression of iNOS, COX-2 and PGE2 expressions and suppression of IKK-NF-KB and MAPK signaling pathway [69] |
| 36 | Trachyspermum ammi (L.) Sprague ex Turrill/Apiaceae | Ajwain, Bishop's weed | Thymol, carvacrol [146] | Spice [147] | Hepatitis C virus [64] | Methanolic fruit extract [64] | Inhibits viral protease enzymes [64] |
| 37 | Withania somnifera (L.) Dunal./ Solanaceae | Ashwagandha | Withaferin A [148] | Nutraceutical [149] | Influenza virus (H1N1) [148] | Withaferin A [148] | Inhibit neuraminidase, the key enzyme in the life cycle of influenza virus [148] |
| 38 | Zingiber officinale Roscoe./Zingiberaceae | Ginger | 6-Gingerol, 6-shogaol [150] | Nutraceutical [151], Spice [152] | Human respiratory syncytial virus [150] | Hot aqueous rhizome extract/Gingerols [150] | Inhibits viral attachment and penetration [150] |
Plants preventing entry of virus in the host
It has been reported that flavonoids can bind to the functional domains of the SARS-CoV-2S protein, a viral surface glycoprotein required for initial attachment and internalization within host cells. Emodin from plants of family polygonaceae can block the interaction with the SARS-coronavirus spike protein by inhibiting the 3a ion channel of SARS-CoV and HCoV-OC43 [36]. Lectins, the natural proteins, also target the sugar moieties of a SARS-CoV spike protein. In time-of-addition assay conducted to understand mechanism of antiviral action, glucose-, galactose-, N-acetyl glucosamine- and N-acetyl galactosamine binding lectins and most importantly mannose binding lectin indicated their interference with virus attachment to spike protein making them early entry inhibitors. Lectins also carry prophylactic potentials as it agglutinates viral particles by binding to it, thereby not allowing it to bind to human cell receptors and complete its pathogenic cycle [37]. As SARS-CoV-2 also uses host receptor ACE-2 for the cellular entrance similar to SARS-CoV [38], medicinal herbs with the capacity to target ACE-2 therefore holds a promising effect in the prevention and infection of SARS-CoV-2. Various edible medicinal plants, including Cynara scolymus [39], Cassia occidentalis [40] and Punica granatum [41], have shown ACE inhibitory effects, and the same can be explored for inhibition of ACE2 also.
Plants inhibiting viral replication
Studies on edible plants, such as Glycyrrhiza glabra [42], Allium sativum [43], showed the inhibition of viral replication of SARS-CoV that can be further utilized as leads against SARS-CoV-2, due to similar homology between SARS-CoV and SARS-CoV-2 [44]. Edible antiviral plants like Aloe vera [45], Gingko [46], Olea europaea [47], Cicer arietinum [48], Nigella sativa [49], Agrimonia Pilosa [50], Commelina communis [51], Mangifera indica [52], Syzygium cumini [53] that showed effects against influenza virus can be studied rigorously to investigate any relatable target between SARS-CoV-2 and influenza virus.
Myricetin and scutellarein can act as novel chemical inhibitors of the SARS coronavirus helicase, nsP13 [54]. Flavonoids isolated from medicinal plants have been reported to show antiviral activity. Quercetin, epigallocatechin gallate and gallocatechin gallate showed inhibitory activity against 3CLpro of SARS-CoV [55]. Plants showing inhibitory effects on HIV proteases, such as Eugenia jambolana, Areca nut [56], can be investigated for their effects on SARS-CoV-2. Similarly, plants like Ocimum sanctum [57], Phaseolus vulgaris [58], Phyllanthus emblica [59] having HIV reverse transcriptase activity can also be studied against SARS-CoV-2. Plants like Solanum nigrum [60] have been known to target the reverse transcriptase activity of HIV and can be studied for activity against SARS-CoV-2 as well; betulinic acid, savinin and some plant-based phenolic compounds are competitive inhibitors of SARS-CoV 3CL protease [61]. Azadirachta indica inhibits viral replication in Group B Coxsackieviruses virus and can be investigated for their possible effects against SARS-CoV-2 [62]. Another herb Aegle marmelos inhibited viral replication in human coxsackieviruses B1-B6 infection and can be used in the study against SARS-CoV-2 [62]. Another potential target that can be utilized for the inhibition of CoV replication is proteases [63]. Trachyspermum ammi [64] and Solanum nigrum [65] inhibited viral protease enzymes in hepatitis C virus (HCV) infection. Acalypha indica showed selective anti-VSV activity by protein interaction [64], and Ocimum sanctum also inhibited HIV protease enzyme [57]; therefore these plants can be studied against SARS-CoV-2 as they may target protease enzymes.
Plants inhibiting viral envelop formation
Sambucus ebulus has been known to inhibit the activity of enveloped viruses and can also be used to target this virus. Though the detailed mechanism remains unclear, Sambucus ebulus is indicated to inhibit the entry of enveloped viruses owing to the presence of lectins that block viral entry. Phenolic compounds like quertin 3–0-glucoside and isorhamnetin present in the plant have previously demonstrated the prophylactic potential against Ebola virus. The flavonoids, diosgenin and yomogenin of Sambucus species also showed viral entry inhibition against Hepatitis C viruses [61].
Antiviral plants with unknown mechanism
A study on Abutilon indicum, Gymnema sylvestre, Leucas aspera showed anti-mouse coronaviral activity which is a surrogate of human SARS virus but its mechanism of action is still unexplored and requires more research in this area [66]. Leucas aspera has been shown to have anti-MCV and anti-HSV activities, Abutilon indicum extract was found active against influenza virus and Sindbis virus which is a surrogate to Hepatitis B virus. Gymnemic acid from Gymnema sylvestre has virucidal activity against Asian influenza virus, whereas Artemisia annua showed inhibitory effects against SARS-CoV and likely against SARS-CoV-2 but their mechanism of action is still unknown [67].
Plants used in respiratory distress
As SARS-CoV-2 causes respiratory distress, plants used in human respiratory syncytial virus (HRSV) infection, such as Zingiber officinale [68], Olea europaea [47], Terminalia chebula [69], might act as a preventive treatment in COVID-19. Aqueous rhizome extract of Zingiber officinale contains allicin which acts against HRSV by reducing the plaque formation in respiratory mucosa induced by stimulation of the respiratory mucosal cells to secrete IFN-β. Olea europaea act via multiple antiviral mechanisms: interfering with critical amino acid production essential for viruses, preventing virus assembly at the cell membrane, penetrating infected cells and stopping the viral replication or else primarily by neutralizing the production of reverse transcriptase and protease [47, 68]. On the other hand, Chebulagic acid and chebulinic acid from Terminalia chebula have shown efficacy to inhibit virus attachment and penetration comparable to Acyclovir as well as implement neuraminidase-mediated viral release similar to the antiviral drug oseltamivir [69]. Curcumin (diferuloylmethane), which is found in the spice Curcuma longa, exhibits anti-inflammatory as well as immunomodulatory activity by inhibiting PHA-induced T-cell proliferation, interleukin-2 production, NO generation, and lipopolysaccharide-induced nuclear factor-kappa B (NF-kappa B), augments NK cell cytotoxicity as well as inhibits cell proliferation and cytokine production by inhibiting NF-kappa B target genes involved in the induction of these immune parameters [70].
Edible antiviral plants with additional activity against Covid-19
Medicinal plants such as Hibiscus sabdariffa [71], Ocimum sanctum [57], Azadirachta indica [72]. contain flavonoids which can be exploited for the development of the active compounds against COVID-19. Although numerous plants have been studied, a lot of scientific data are required to confirm their effects and hence further research needs to be maneuvered towards this direction. It should be noted that increased inflammatory responses occurs in the patients with COVID-19 which increases the death rate of the patients [73]; therefore anti-inflammatory herbal drugs like Withania somnifera [74], Zingiber officinale [68], Camellia sinensis [75], Nigella sativa [49], Moringa oleifera [76], Agrimonia Pilosa [50], Momordica charantia [77] can be investigated in supportive treatment against COVID-19 and can be incorporated in daily routine diet of patients, which could produce a reduction in the severity and mortality rate of the patients suffering from the disease. A study on herbal formulas also suggested that immunomodulators might show preventive effects against viral infections and likely COVID-19 [78, 79]. Edible herbs and nutraceuticals such as Allium sativum, Zingiber officinale, Glycyrrhiza glabra, Olea europaea, Cicer arietinum, Camellia sinensis can boost immune system, preventing the body from invading viruses [37]. As mentioned earlier, ACE2 is the entry point for the SARS-CoV-2. Considering this, search for the antiviral plants with added ACE2 inhibition property, anti-inflammatory and immunomodulatory activity should be the future line for research. Table 2 provides the details of the antiviral plants that show potential ACE inhibition, anti-inflammatory and/or immunomodulatory activity.
Table 2.
Plants having anti-inflammatory, immunomodulatory and/or ACE inhibitory activity
| S. No. | Plant species/family | Anti-inflammatory | Immunomodulatory | ACE inhibitor |
|---|---|---|---|---|
| 1. | Abutilon indicum L. (Sweet) | Yes [153] | Yes [154] | No |
| 2. | Acalypha indica L | Yes [88] | Yes [155] | No |
| 3. | Aegle marmelos (L.) Correa | Yes [156] | Yes [157] | No |
| 4 | Agrimonia pilosa Ledeb | Yes [158] | No | No |
| 5. | Allium sativum L | Yes [159] | Yes [160] | Yes [161] |
| 6. | Aloe vera (L.) Burm. f | Yes [162] | Yes[163] | No |
| 7. | Areca nut L | Yes [97] | No | Yes [56] |
| 8. | Artemisia annua L | Yes [99] | Yes [164] | No |
| 9. | Azadirachta indica A. Juss | Yes [165] | Yes [166] | Yes [167] |
| 10. | Camellia sinensis (L.) Kuntze | Yes [168] | Yes [169] | Yes [170] |
| 11. | Cassia occidentalis L | Yes [171] | Yes [105] | Yes [41] |
| 12. | Cicer arietinum L | Yes [172] | Yes [173] | Yes [174] |
| 13. | Commelina communis L | Yes [175] | No | No |
| 14. | Curcuma longa L | Yes [176] | Yes [177] | Yes [178] |
| 15. | Cynara Scolymus L | Yes [179] | No | Yes [41] |
| 16 | Embelia ribes Burm. f | Yes [180] | Yes [181] | Yes [41] |
| 17. | Eugenia jambolana Lam | Yes [182] | Yes [183] | Yes [184] |
| 18. | Gingko biloba L | Yes [185] | Yes [186] | Yes [187] |
| 19. | Glycyrrhiza glabra L | Yes [188] | Yes [189] | No |
| 20. | Gymnema sylvestre (Retz.) Schult | Yes [190] | Yes [191] | No |
| 21. | Hibiscus sabdariffa L | Yes [192] | Yes [193] | Yes [194] |
| 22. | Leucas aspera (Wild.) Link | Yes [195] | Yes [196] | No |
| 23. | Mangifera indica L | Yes [197] | Yes [198] | Yes [123] |
| 24. | Momordica charantia L | Yes [77] | Yes [199] | Yes [200] |
| 25. | Moringa oleifera Lam | Yes [76] | Yes [201] | Yes [202] |
| 26. | Myrica esculenta Buch.-Ham. Ex D. Don | Yes [203] | No | Yes [204] |
| 27. | Nigella sativa L | Yes [205] | Yes [206] | Yes [207] |
| 28. | Ocimum sanctum L | Yes [208] | Yes [209] | Yes [210] |
| 29. | Olea europaea L | Yes [211] | Yes [212] | Yes [213] |
| 30. | Phaseolus vulgaris L | Yes [214] | Yes [78] | Yes [215] |
| 31. | Phyllanthus emblica L | Yes [136] | Yes [216] | No |
| 32. | Punica granatum L | Yes [217] | Yes [218] | Yes [41] |
| 33. | Solanum nigrum L | Yes [219] | Yes [220] | No |
| 34. | Syzygium cumini (L.) Skeels | Yes [221] | Yes [183] | Yes [184] |
| 35. | Terminalia chebula Retz | Yes [144] | Yes [222] | Yes [223] |
| 36. | Trachyspermum ammi (L.) Sprague ex Turrill | Yes [146] | Yes [224] | No |
| 37. | Withania somnifera (L.) Dunal | Yes [225] | Yes [226] | Yes [227] |
| 38. | Zingiber officinale Roscoe | Yes [228] | Yes [229] | Yes [230] |
Clinical evidence of SARS treatment using herbals: paving the way for optimistic future
An epidemic of severe acute respiratory syndrome (SARS) that began in 2002 saw extensive usage and treatment with phytomolecules as auxiliary therapy to conventional medicine. Several anti-SARS formulae were recommended by the Ministry of Health of China to be used along conventional antiviral drugs. The very fact is that SARS-CoV-2 virus shares a striking similarity of 79.5% genetic homology to the SARS-CoV and MERS coronavirus as both are descendants of bat coronaviruses within the beta coronavirus genus [38]; this high genetic similarities between SARS-CoV-2 and SARS or MERS point towards the notion that similar-to-yester-years, administration of auxiliary therapy with plant-based product will prove effective and beneficial against novel coronaviral-generated pandemic too. As a matter of fact, phytomolecules and plant products as well as their analogues are already being employed as an early line of defense against SARS-CoV-2 also. In this section, we have described certain clinical studies for SARS-CoV and SARS-CoV-2 using herbal drugs.
Chen et al. (2007) studied and reported that treatment with herbal drugs which consisted of more than different herbal medicines mainly including Anemarrhena asphodeloides Bunge, Atractylodes macrocephala Koidz., Aspidium, Artemisia annua L., Bupleurum chinense DC., Paeonia mascula (L.) Mill., Coptis chinensis Franch., Coptis deltoidea, Coptis teeta Wall., Curcuma, Salvia miltiorrhiza Bunge, Fritillaria was more effective in clearing up the lung infiltrate as well as shortening the time to abatement of a fever in SARS-infected patients than conventional treatment alone. The study also suggests that adjunctive use of medicinal plants and phytomolecules could significantly bring the average daily use of corticosteroids for reducing inflammatory responses as well as in tackling the issue of low counts of CD4+ and CD8+. The patients who had received integrative medicine in the study showed to recover the lymphocyte cells with marked higher CD4+ counts at the end of study [80].
In another study by Hsu et al., four of the severely confirmed SARS patients received routine supplementary treatment with combination of herbs: Bupleurum chinense, Gardenia jasminoides, Siler divaricatum, Scutellaria baicalensis, Notopterygium incisium, Schizonepeta tenuifolia, Poria cocos, Paeonia lactiflora Pallas, Paeonia veitchii Lynch, Pinellia ternata, Platycodon grandiflorum and Ophiopogon japonicus showed less morbidity than the patient on western medicine and placebo [81]. While in a controlled clinical study by Hsu et al. (2006), the adjuvant treatment with plant-derived pharmaceuticals resulted in marked improvement of symptoms and shortened the disease course [82], in another study by Cinatl et al. (2003) Glycyrrhizin, an active constituent in liquorice root, showed potential inhibition of replication of clinical isolates of SARS virus [42]. Chloroquine phosphate extracted from the bark of cinchona trees and hydroxychloroquine are being currently used for treating COVID-19 patients [83]. Qingfei Paidu Decoction (QPD), a Chinese decoction of medicinal plants comprising phedra sinica, Glycyrrhiza glabra, Prunus armeniaca Linne var. ansu Maximowicz, Prunus mandshurica Koehne var. glabra Nakai, Cinnamomum cassia (L.) Presl, Alisma orientale (Sam.) Juzep, Polyporus, Aster tataricus L., Atractylodes macrocephala Koidz, Poria cocos (Schw.) Wolf., Bupleurum chinense DC., Bupleurum scorzonerifolium Willd., Scutellariae baicalensis Georgi, Pinellia ternate (Thunb.) Breit, Zingiber officinale Rosc, Tussilago farfara L., Belamcanda chinensis (L.) DC., Asarum spp, Dioscorea opposite Thunb, Citrus aurantium L., Citrus sinensis Osbeck, Citrus reticulata Blanco and Pogostemon cablin (Blanco) Benth., are being promoted in China and as per report of clinical studies there are already seem to be some promising results [84] and some of these plants belongs to Indian origin. In the trial study by Xu et al.(2020), 701 confirmed cases were treated by QPD; among them130 cases were cured as well as discharged, whereas clinical symptoms of 51 cases disappeared, symptoms of 268 cases showed improved and 212 of the cases had stable symptoms without aggravation [85].
Summary and conclusion
Despite the fact that a number of drug candidates are being tested for clinical trials for COVID-19 across the globe, no therapy has yet been found to be effective. Thus, there is need to look into any alternative solutions. Natural compounds have been used since decades in controlling infectious diseases. Based on previous experiences of coronavirus outbreaks (SARS-CoV in 2002 and MERS CoV in 2012), seasonal epidemics caused by various viruses showing effectiveness of natural products in the treatment of HIV, HCV and Influenza, herbal drugs and their phytoconstituents could be developed as a potential drug candidate against SARS-CoV-2. To be an effective therapy in treatment of COVID-19, the phytoconstituents need to have anti-inflammatory, antioxidants, antiviral activity and effects on cardiovascular targets in lieu of renin-angiotensin system being involved in COVID-19, with ACE-2 as the major target. Such herbal formulations can be used as complementary treatment for prevention of infection acquisition without causing any ill effects, provided the intake is on evidence-based protocols.
In the present review, a total of 38 plant species of Indian flora and biodiversity have been identified that are edible plants. They have potential antiviral activities on the basis of inhibition against various RNA viruses. Many of them contain phytoconstituents that possess the potential anti-coronaviral activity. The clinical spectrum of COVID-19 starts from simple cough, fever, chills, sore throat and headache. The edible nutraceuticals are being used in most countries with the history of complementary ancient medicine system and still being practiced to the extent of over 50%.
To conclude, there are several edible plants available as a source of natural antiviral agents and have a potential to develop as a nutraceutical for the COVID-19. More rigorous scientific research is needed to understand mechanistically their therapeutic value. The nutraceuticals thus developed may serve as adjuvant and complementary treatment to help the population in coping with such maliciously infectious pandemics and thereby protect the global population from current and future pandemics.
Compliance with ethical standards
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization,
- 2.Kusumoto IT, Nakabayashi T, Kida H, Miyashiro H, Hattori M, Namba T, Shimotohno K. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Phytother Res. 1995;9(3):180–184. doi: 10.1002/ptr.2650090305. [DOI] [Google Scholar]
- 3.University JH (2020) COVID-19 Dash board by the Center for Systems Science and Engineering (CSSE) at JHU. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed 13 May 2020
- 4.Dayer MR, Taleb-Gassabi S, Dayer MS. Lopinavir; a potent drug against coronavirus infection: insight from molecular docking study. Arch Clin Infect Dis. 2017 doi: 10.5812/archcid.13823. [DOI] [Google Scholar]
- 5.Omolo C, Soni N, Fasiku V, Mackraj I, Govender T. Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus. Eur J Pharmacol. 2020 doi: 10.1016/j.ejphar.2020.173348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 8.Jorge A, Ung C, Young LH, Melles RB, Choi HK. Hydroxychloroquine retinopathy—implications of research advances for rheumatology care. Nat Rev Rheumatol. 2018;14(12):693–703. doi: 10.1038/s41584-018-0111-8. [DOI] [PubMed] [Google Scholar]
- 9.Vijayan P, Raghu C, Ashok G, Dhanaraj S, Suresh B. Antiviral activity of medicinal plants of Nilgiris. Indian J Med Res. 2004;120:24–29. [PubMed] [Google Scholar]
- 10.Lin L-T, Hsu W-C, Lin C-C. Antiviral natural products and herbal medicines. J Tradit Complement Med. 2014;4(1):24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung T, Kim J, Kim M, Choi S, Kim S, Chung J, Lee I, Kim SH, Hahn K, Lee I. Investigation of Korean plant extracts for potential phytotherapeutic agents against B-virus hepatitis. Phytother Res. 1995;9(6):429–434. doi: 10.1002/ptr.2650090609. [DOI] [Google Scholar]
- 12.Akram M, Tahir IM, Shah SMA, Mahmood Z, Altaf A, Ahmad K, Munir N, Daniyal M, Nasir S, Mehboob H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: a systematic review. Phytother Res. 2018;32(5):811–822. doi: 10.1002/ptr.6024. [DOI] [PubMed] [Google Scholar]
- 13.Vijayalatha S. An Ornamental garden with medicinal plants an indirect approach for conservation of medicinal plants. J Indian J Arecanut Spices Med Plants. 2004;6(3):98–107. doi: 10.1002/mnfr.201601066. [DOI] [Google Scholar]
- 14.Zhao J. Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: a perspective on plant biotechnology application. Recent Pat Biotechnol. 2007;1(1):75–97. doi: 10.2174/187220807779813893. [DOI] [PubMed] [Google Scholar]
- 15.Patra JK, Das G, Kumar S, Thatoi H (2019) Ethnopharmacology and Biodiversity of Medicinal Plants. CRC Press, pp 470
- 16.Vanden Berghe D, Vlietinck A, Van Hoof L. Plant products as potential antiviral agents. Bull Inst Pasteur. 1986;84(2):101–147. doi: 10.3923/ajava.2011.1125.1152. [DOI] [Google Scholar]
- 17.Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnard DL, Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol. 2011;6(5):615–631. doi: 10.2217/fvl.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel Coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS Coronavirus. J Virol. 2020;94(7):e00127–e1120. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majeed J, Ajmera P, Goyal RK. Delineating clinical characteristics and comorbidities among 206 COVID-19deceasedpatients in India: Emerging significance of renin angiotensin system derangement. Diabetes Res Clin Pract. 2020 doi: 10.1016/j.diabres.2020.108349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scotti N, Buonaguro L, Tornesello ML, Cardi T, Buonaguro FM. Plant-based anti-HIV-1 strategies: vaccine molecules and antiviral approaches. Expert Rev Vaccines. 2010;9(8):925–936. doi: 10.1586/erv.10.79. [DOI] [PubMed] [Google Scholar]
- 23.Ho T-Y, Wu S-L, Chen J-C, Li C-C, Hsiang C-Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy BU, Mullick R, Kumar A, Sudha G, Srinivasan N, Das S. Small molecule inhibitors of HCV replication from pomegranate. Sci Rep. 2014;4:5411. doi: 10.1038/srep05411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020) Features, evaluation and treatment coronavirus (COVID-19). In: Statpearls [internet]. StatPearls Publishing, [PubMed]
- 26.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao S-Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomar B, Anders H-J, Desai J, Mulay SR. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9(6):1383. doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small BA, Dressel SA, Lawrence CW, Drake DR, III, Stoler MH, Enelow RI, Braciale TJ. CD8+ T cell–mediated injury in vivo progresses in the absence of effector T cells. J Exp Med. 2001;194(12):1835–1846. doi: 10.1084/jem.194.12.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Channappanavar R, Perlman S (2017) Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. In: Seminars in immunopathology vol 39. Springer Berlin Heidelberg., pp 529–539. doi:10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed]
- 31.Rizzo P, Dalla Sega FV, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol. 2020;115(3):31. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect. 2020;8(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 35.Heinrich M, Gibbons S. Ethnopharmacology in drug discovery: an analysis of its role and potential contribution. J Pharm Pharmacol. 2001;53(4):425–432. doi: 10.1211/0022357011775712. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz S, Wang K, Yu W, Sun B, Schwarz W. Emodin inhibits current through SARS-associated coronavirus 3a protein. Antiviral Res. 2011;90(1):64–69. doi: 10.1016/j.antiviral.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keyaerts E, Vijgen L, Pannecouque C, Van Damme E, Peumans W, Egberink H, Balzarini J, Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elsebai MF, Mocan A, Atanasov AG. Cynaropicrin: a comprehensive research review and therapeutic potential as an anti-hepatitis C virus agent. Front Pharmacol. 2016;7:472. doi: 10.3389/fphar.2016.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lunavath V, Estari (2012) Inhibition of Human Immunodeficiency Virus (HIV-1) Reverse Transcriptase by Casia occidentalis (L) Plant Extract. Int J Eng Res 3 (7)
- 41.Khan MY, Kumar V. Mechanism & inhibition kinetics of bioassay-guided fractions of Indian medicinal plants and foods as ACE inhibitors. J Tradit Complement Med. 2018;9(1):73–84. doi: 10.1016/j.jtcme.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. The Lancet. 2003;361(9374):2045–2046. doi: 10.1016/s0140-6736(03)13615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber ND, Andersen DO, North JA, Murray BK, Lawson LD, Hughes BG. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58(5):417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- 44.Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sydiskis R, Owen D, Lohr J, Rosler K, Blomster R. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother. 1991;35(12):2463–2466. doi: 10.1128/aac.35.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miki K, Nagai T, Suzuki K, Tsujimura R, Koyama K, Kinoshita K, Furuhata K, Yamada H, Takahashi K. Anti-influenza virus activity of biflavonoids. Bioorg Med Chem Lett. 2007;17(3):772–775. doi: 10.1016/j.bmcl.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 47.Micol V, Caturla N, Pérez-Fons L, Más V, Pérez L, Estepa A. The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV) Antiviral Res. 2005;66(2–3):129–136. doi: 10.1016/j.antiviral.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Kan A, Özçelİk B, Kartal M. In vitro antiviral activities under cytotoxic doses against herpes simples type-1 and parainfluensa-3 viruses of Cicer arietinum L. (Chickpea) Afr J Pharm Pharmacol. 2009;3(12):627–631. [Google Scholar]
- 49.Umar S, Munir MT, Subhan S, Azam T, Nisa Q (2016) Protective and antiviral activities of Nigella sativa against avian influenza (H9N2) in turkeys. J Saudi Soc Agric Sci
- 50.Shin WJ, Lee KH, Park MH, Seong BL. Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol Immunol. 2010;54(1):11–19. doi: 10.1111/j.1348-0421.2009.00173.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhang GB, Bing FH, Liu J, Li Z, Liao YF, Li J, Dong CY. Effect of total alkaloids from Commelina communis L on lung damage by influenza virus infection. Microbiol Immunol. 2010;54(12):754–757. doi: 10.1111/j.1348-0421.2010.00277.x. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Mageed WM, Bayoumi SAH, Chen C, Vavricka CJ, Li L, Malik A, Dai H, Song F, Wang L, Zhang J, Gao GF, Lv Y, Liu L, Liu X, Sayed HM, Zhang L. Benzophenone C-glucosides and gallotannins from mango tree stem bark with broad-spectrum anti-viral activity. Bioorg Med Chem. 2014;22(7):2236–2243. doi: 10.1016/j.bmc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Sood R, Swarup D, Bhatia S, Kulkarni D, Dey S, Saini M, Dubey S. Antiviral activity of crude extracts of Eugenia jambolana Lam. against highlypathogenic avian influenza (H5N1) virus. Indian J Exp Biol. 2012;50:179–186. [PubMed] [Google Scholar]
- 54.Yu M-S, Lee J, Lee JM, Kim Y, Chin Y-W, Jee J-G, Keum Y-S, Jeong Y-J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg Med Chem Lett. 2012;22(12):4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musarra-Pizzo M, Ginestra G, Smeriglio A, Pennisi R, Sciortino MT, Mandalari G. The antimicrobial and antiviral activity of polyphenols from Almond (Prunus dulcis L.) skin. Nutrients. 2019;11(10):2355. doi: 10.3390/nu11102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inokuchi J, Okabe H, Yamauchi T, Nagamatsu A, Nonaka G, Nishioka I. Antihypertensive substance in seeds of Areca catechu L. Life Sci. 1986;38(15):1375–1382. doi: 10.1016/0024-3205(86)90470-4. [DOI] [PubMed] [Google Scholar]
- 57.Rege A, Chowdhary AS. Evaluation of Ocimum sanctum and Tinospora cordifolia as probable HIV protease inhibitors. Int J of Pharm Sci Rev Res. 2014;25:315–318. [Google Scholar]
- 58.Ye XY, Ng TB, Tsang PW, Wang J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J Protein Chem. 2001;20(5):367–375. doi: 10.1023/A:1012276619686. [DOI] [PubMed] [Google Scholar]
- 59.Hossan MS, Fatima A, Rahmatullah M, Khoo T, Nissapatorn V, Galochkina A, Slita A, Shtro A, Nikolaeva Y, Zarubaev V, Wiart C. Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Arch Virol. 2018;163(8):2121–2131. doi: 10.1007/s00705-018-3842-6. [DOI] [PubMed] [Google Scholar]
- 60.Yu Y-B. The extracts of Solanum nigrum L for inhibitory effects on HIV-1 and its essential enzymes. Korean J Orient Med Prescr. 2004;10(1):119–126. [Google Scholar]
- 61.Chowdhury M, Shahid M, Kashem M (2020) Scope of natural plant extract to deactivate COVID-19. [Preprint]. 10.21203/rs.3.rs-19240/v1
- 62.Badam L, Bedekar SS, Sonawane KB, Joshi SP. In vitro antiviral activity of bael (Aegle marmelos Corr) upon human coxsackieviruses B1–B6. J Commun Dis. 2002;34(2):88–99. [PubMed] [Google Scholar]
- 63.Kilianski A, Mielech AM, Deng X, Baker SC. Assessing activity and inhibition of Middle East respiratory syndrome coronavirus papain-like and 3C-like proteases using luciferase-based biosensors. J Virol. 2013;87(21):11955–11962. doi: 10.1128/JVI.02105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, Shimotohno K. Inhibitory effects of sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother Res. 2000;14(7):510–516. doi: 10.1002/1099-1573(200011)14:7<510::aid-ptr646>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 65.Javed T, Ashfaq UA, Riaz S, Rehman S, Riazuddin S. In-vitro antiviral activity of Solanum nigrum against hepatitis C virus. Virol J. 2011;8:26. doi: 10.1186/1743-422X-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vimalanathan S, Ignacimuthu S, Hudson J. Medicinal plants of Tamil Nadu(Southern India) are a rich source of antiviral activities. Pharm Biol. 2009;47:422–429. doi: 10.1080/13880200902800196. [DOI] [Google Scholar]
- 67.Li SY, Chen C, Zhang HQ, Guo HY, Wang H, Wang L, Zhang X, Hua SN, Yu J, Xiao PG, Li RS, Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.San Chang J, Wang KC, Yeh CF, Shieh DE, Chiang LC. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. 2013;145(1):146–151. doi: 10.1016/j.jep.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 69.Xie F. Broad-spectrum antiviral effect of chebulagic acid and punicalagin on respiratory syncytial virus infection in a BALB/c model. Int J Clin Exp Pathol. 2016;9(2):611–619. doi: 10.1186/1471-2180-13-187. [DOI] [Google Scholar]
- 70.Yadav V, Mishra K, Singh D, Mehrotra S, Singh V. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol. 2005;27(3):485–497. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- 71.Im SA, Lee YR, Lee YH, Lee MK, Park YI, Lee S, Kim K, Lee CK. In vivo evidence of the immunomodulatory activity of orally administered Aloe vera gel. Arch Pharm Res. 2010;33(3):451–456. doi: 10.1007/s12272-010-0315-1. [DOI] [PubMed] [Google Scholar]
- 72.Badam L, Joshi S, Bedekar S. 'In vitro'antiviral activity of neem (Azadirachta indica. A. Juss) leaf extract against group B Coxsackieviruses. J Commun Dis. 1999;31(2):79–90. [PubMed] [Google Scholar]
- 73.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 74.Grover A, Agrawal V, Shandilya A, Bisaria VS, Sundar D Non-nucleosidic inhibition of Herpes simplex virus DNA polymerase: mechanistic insights into the anti-herpetic mode of action of herbal drug withaferin A. In: BMC bioinformatics, 2011. vol 13. BioMed Central, p S22. doi:10.1186/1471-2105-12-S13-S22 [DOI] [PMC free article] [PubMed]
- 75.Matsumoto M, Mukai T, Furukawa S, Ohori H. Inhibitory effects of epigallocatechin gallate on the propagation of bovine coronavirus in Madin-Darby bovine kidney cells. Anim Sci J. 2005;76(5):507–512. doi: 10.1111/j.1740-0929.2005.00297.x. [DOI] [Google Scholar]
- 76.Coppin JP, Xu Y, Chen H, Pan M-H, Ho C-T, Juliani R, Simon JE, Wu Q. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J Funct Foods. 2013;5(4):1892–1899. doi: 10.1016/j.jff.2013.09.010. [DOI] [Google Scholar]
- 77.Lii CK, Chen HW, Yun WT, Liu KL. Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviata ser) fruit extracts on inflammatory responses in RAW 2647 macrophages. J Ethnopharmacol. 2009;122(2):227–233. doi: 10.1016/j.jep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 78.Chan YS, Wong JH, Fang EF, Pan W, Ng TB. A hemagglutinin from northeast red beans with immunomodulatory activity and anti-proliferative and apoptosis-inducing activities toward tumor cells. Protein Pept Lett. 2013;20(10):1159–1169. doi: 10.2174/0929866511320100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding X, Zhu F, Gao S. Purification, antitumour and immunomodulatory activity of water-extractable and alkali-extractable polysaccharides from Solanum nigrum L. Food Chem. 2012;131(2):677–684. doi: 10.1016/j.foodchem.2011.09.060. [DOI] [Google Scholar]
- 80.Chen Y, Guo JJ, Healy DP, Zhan S. Effect of integrated traditional Chinese medicine and western medicine on the treatment of severe acute respiratory syndrome: a meta-analysis. Pharmacy Practice. 2007;5(1):1–9. doi: 10.4321/S1886-36552007000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsu C-H, Hwang K-C, Chao C-L, Chang SG, Ker C-C, Chien L-C, Ho M-S, Lin J-G, Chen Y-M, Chou P. The lesson of supplementary treatment with Chinese medicine on severe laboratory-confirmed SARS patients. Am J Chin Med. 2006;34(06):927–935. doi: 10.1142/S0192415X06004405. [DOI] [PubMed] [Google Scholar]
- 82.Hsu C-H, Hwang K-C, Chao C-L, Chang SG, Ho M-S, Chou P. Can herbal medicine assist against avian flu? Learning from the experience of using supplementary treatment with Chinese medicine on SARS or SARS-like infectious disease in 2003. J Altern Complement Med. 2006;12(6):505–506. doi: 10.1089/acm.2006.12.505. [DOI] [PubMed] [Google Scholar]
- 83.Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.New Coronavirus Pneumonia Dignosis and Treatment Program (2020) Office of the National Health and Health Commission Office of the State Administration of Traditional. http://www.nhc.gov.cn/xcs/zhengcwj/202002/8334a8326dd94d329df351d7da8aefc2.shtml. Accessed 3 May 2020
- 85.Xu X-W, Wu X-X, Jiang X-G, Xu K-J, Ying L-J, Ma C-L, Li S-B, Wang H-Y, Zhang S, Gao H-N. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saini A, Gahlawat DK, Chauhan C, Gulia SK, Ganie SA, Archita YSS. Ethnomedicinal uses and phytochemistry of Abutilon indicum (Linn.) Sweet: an overview. J Pharmacogn Phytochem. 2015;3(5):66–72. [Google Scholar]
- 87.Reddy KN, Pattanaik C, Reddy CS, Raju VS. Traditional knowledge on wild food plants in Andhra Pradesh. Indian J Tradit Know. 2007;6(1):223–229. [Google Scholar]
- 88.Muzammil S, Manikandan M, Jafar A, Sakthivel P, Geetha S, Malarkodi R. Anti-inflammatory studies on Acalypha indica L. leaves by membrane stabilization. Indian J Nat Prod Resour. 2014;5(2):195–197. [Google Scholar]
- 89.Amalraj A, Pius A. Bioavailability of calcium and its absorption inhibitors in raw and cooked green leafy vegetables commonly consumed in India: an in vitro study. Food Chem. 2015;170:430–436. doi: 10.1016/j.foodchem.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 90.Ali A, Mackeen M, Ei-Sharkawyl S, Hamidi J, Ismaili NOR, Ahmad H, Lajisi F. Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika J Trop Agric Sci. 1996;19:129–136. [Google Scholar]
- 91.Baliga MS, Mane PP, Joseph N, Jimmy R, Watson RR, Preedy VR (2013) Chapter 20 - Review on the Protective Effects of the Indigenous Indian Medicinal Plant, Bael (Aegle marmelos Correa), in Gastrointestinal Disorders. In: Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease. Academic Press, San Diego, pp 313–324. doi:https://doi.org/10.1016/B978-0-12-397154-8.00036-1
- 92.Kunkel G. Plants for human consumption. Koenigstein: Koeltz Scientific Books; 1984. [Google Scholar]
- 93.Touloupakis E, Ghanotakis D. Nutraceutical use of garlic sulfur-containing compounds. Adv Exp Med Biol. 2010;698:110–121. doi: 10.1007/978-1-4419-7347-4_9. [DOI] [PubMed] [Google Scholar]
- 94.Tiwari R. Garlic as food, spice and Medicine: as prospective. J Pharm Res. 2011;4:1857–1860. [Google Scholar]
- 95.Ahlawat KS, Khatkar BS. Processing, food applications and safety of aloe vera products: a review. J Food Sci Technol. 2011;48(5):525–533. doi: 10.1007/s13197-011-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Z, Yu C, Wang W, Yu G, Zhang T, Zhang L, Zhang J, Wei K Aloe Polysaccharides Inhibit Influenza A Virus Infection-A Promising Natural Anti-flu Drug. Front Microbiol 9 (2338). doi:10.3389/fmicb.2018.02338 [DOI] [PMC free article] [PubMed]
- 97.Khan S, Mehmood MH, Ali ANA, Ahmed FS, Dar A, Gilani A-H. Studies on anti-inflammatory and analgesic activities of betel nut in rodents. J Ethnopharmacol. 2011;135(3):654–661. doi: 10.1016/j.jep.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 98.Fazal F, Mane PP, Rai MP, Thilakchand KR, Bhat HP, Kamble PS, Palatty PL, Baliga MS. The phytochemistry, traditional uses and pharmacology of Piper betel linn (Betel Leaf): a pan-asiatic medicinal plant. Chin J Integr Med. 2014 doi: 10.1007/s11655-013-1334-1. [DOI] [PubMed] [Google Scholar]
- 99.Melillo de Magalhães P, Dupont I, Hendrickx A, Joly A, Raas T, Dessy S, Sergent T, Schneider Y. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012;134(2):864–871. doi: 10.1016/j.foodchem.2012.02.195. [DOI] [PubMed] [Google Scholar]
- 100.Agize M. Ethnobotany of spice and condiment plants and the associated indigenous knowledge on management, utilization and conservation of them in and around home gardens in Loma and Gena Bosa Districts ( Weredas ) of Dawuro Zone, Southern Ethiopia. Int J Agric Innov Res. 2016;4(3):426–442. [Google Scholar]
- 101.Keservani RK, Kesharwani RK, Sharma AK, Vyas N, Chadokar A. Nutritional supplements: an overview. J Curr Pharm Rev Res. 2010;1(1):59–75. [Google Scholar]
- 102.Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T. Inhibitory effects of (-)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1) Antiviral Res. 2002;53(1):19–34. doi: 10.1016/s0166-3542(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 103.Harbowy ME, Balentine DA, Davies AP, Cai Y. Tea Chemistry. Crit Rev Plant Sci. 1997;16(5):415–480. doi: 10.1080/07352689709701956. [DOI] [Google Scholar]
- 104.Lee HJ, Lee YN, Youn HN, Lee DH, Kwak JH, Seong BL, Lee JB, Park SY, Choi IS, Song CS. Anti-influenza virus activity of green tea by-products in vitro and efficacy against influenza virus infection in chickens. Poult Sci. 2012;91(1):66–73. doi: 10.3382/ps.2011-01645. [DOI] [PubMed] [Google Scholar]
- 105.Panigrahi G, Yadav A, Mandal P, Tripathi A. Immunomodulatory potential of Rhein, an anthraquinone moiety of Cassia occidentalis seeds. Toxicol Lett. 2016 doi: 10.1016/j.toxlet.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 106.Manikandaselvi S, Vadivel V, Brindha P. Studies on physicochemical and nutritional properties of aerial parts of Cassia occidentalis L. J Food Drug Anal. 2016;24(3):508–515. doi: 10.1016/j.jfda.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ibrikci H, Knewtson SJB, Grusak MA. Chickpea leaves as a vegetable green for humans: evaluation of mineral composition. J Sci Food Agric. 2003;83(9):945–950. doi: 10.1002/jsfa.1427. [DOI] [Google Scholar]
- 108.Zhang GB, Tian LQ, Li YM, Liao YF, Li J, Bing FH. Protective effect of homonojirimycin from Commelina communis (dayflower) on influenza virus infection in mice. Phytomedicine. 2013;20(11):964–958. doi: 10.1016/j.phymed.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 109.Britta MO, Ho Thi T, Hoang Nghia D, Nguyen Nhut Xuan D. Food, feed or medicine: the multiple functions of edible wild plants in Vietnam. Econ Bot. 2003;57(1):103–117. doi: 10.1663/0013-0001. [DOI] [Google Scholar]
- 110.Obata K, Kojima T, Masaki T, Okabayashi T, Yokota S, Hirakawa S, Nomura K, Takasawa A, Murata M, Tanaka S, Fuchimoto J, Fujii N, Tsutsumi H, Himi T, Sawada N. Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PLoS ONE. 2013;8(9):e70225. doi: 10.1371/journal.pone.0070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prasad S, Aggarwal BB. Turmeric, the golden spice: from traditional medicine to modern medicine. In: Benzie IFF, Wachtel-Galor S, editors. Herbal medicine: biomolecular and clinical aspects. 2. Boca Raton: CRC Press/Taylor & Francis; 2011. [PubMed] [Google Scholar]
- 112.Lattanzio V, Kroon PA, Linsalata V, Cardinali A. Globe artichoke: a functional food and source of nutraceutical ingredients. J Funct Foods. 2009;1(2):131–144. doi: 10.1016/j.jff.2009.01.002. [DOI] [Google Scholar]
- 113.Harish GU, Danapur V, Jain R, Patell VM. Endangered Medicinal Plant Embelia ribes Burm. f.: a review. Pharmacogn J. 2012;4(27):6–19. doi: 10.5530/pj.2012.27.2. [DOI] [Google Scholar]
- 114.Baliga MS, Bhat HP, Baliga BRV, Wilson R, Palatty PL. Phytochemistry, traditional uses and pharmacology of Eugenia jambolana Lam. (black plum): a review. Food Res Int. 2011;44(7):1776–1789. doi: 10.1016/j.foodres.2011.02.007. [DOI] [Google Scholar]
- 115.Sood R, Swarup D, Bhatia S, Kulkarni D, Dey S, Saini M, Dubey S. Antiviral activity of crude extracts of Eugenia jambolana Lam. against highlypathogenic avian influenza (H5N1) virus. Indian J Exp Biol. 2012;50(3):179–186. [PubMed] [Google Scholar]
- 116.Belwal T, Giri L, Bahukhandi A, Tariq M, Kewlani P, Bhatt ID, Rawal RS, Nabavi SM, Silva AS (2019) Chapter 319- Ginkgo biloba. In: Nonvitamin and Nonmineral Nutritional Supplements. Academic Press, pp 241–250. Doi:10.1016/B978-0-12-812491-8.00035-7
- 117.Thakur AK, Raj P. Pharmacological perspective of Glycyrrhiza Glabra Linn.: a mini-review. J Anal Pharm Res. 2017;5(5):156. doi: 10.15406/japlr.2017.05.00156. [DOI] [Google Scholar]
- 118.Hayashi H, Sudo H. Economic importance of licorice. Plant Biotechnol. 2009;26:101–104. doi: 10.5511/plantbiotechnology.26.101. [DOI] [Google Scholar]
- 119.Edell D, R.A H (2004) Herbal formulation of Gymnema sylvestre as a dietary aid. United States Patent
- 120.Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L.: a phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 121.Baatartsogt T, Bui VN, Trinh DQ, Yamaguchi E, Gronsang D, Thampaisarn R, Ogawa H, Imai K. High antiviral effects of hibiscus tea extract on the H5 subtypes of low and highly pathogenic avian influenza viruses. J Vet Med Sci. 2016;78(9):1405–1411. doi: 10.1292/jvms.16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rajyalakshmi P, Venkatalaxmi K, Venkatalakshmamma K, Jyothsna Y, Balachandramani Devi K, Suneetha V. Total carotenoid and beta-carotene contents of forest green leafy vegetables consumed by tribals of south India. Plant Foods Hum Nutr. 2001;56(3):225–238. doi: 10.1023/a:1011125232097. [DOI] [PubMed] [Google Scholar]
- 123.Costa H, Ronchi S, Brasil G, Nascimento A, Lima E, Scherer R, Romão W, Boëchat G, Lenz D, Fronza M, Bissoli N, Endringer D, Andrade T. Phytochemical and in vitro and in vivo biological investigation on the antihypertensive activity of mango leaves (Mangifera indica L.) Ther Adv Cardiovasc Dis. 2015;9(5):244–256. doi: 10.1177/1753944715572958. [DOI] [PubMed] [Google Scholar]
- 124.MacLeod AJ, de Troconis NG. Volatile flavour components of mango fruit. Phytochemistry. 1982;21(10):2523–2526. doi: 10.1016/0031-9422(82)85249-7. [DOI] [Google Scholar]
- 125.Morton JF. The balsam pear- an edible, medicinal and toxic plant. Econ Bot. 1967;21(1):57–68. doi: 10.1007/bf02897176. [DOI] [Google Scholar]
- 126.Lee-Huang S, Huang PL, Chen HC, Huang PL, Bourinbaiar A, Huang HI, Kung HF. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene. 1995;161(2):151–156. doi: 10.1016/0378-1119(95)00186-a. [DOI] [PubMed] [Google Scholar]
- 127.Sánchez-Machado D, Núñez-Gastélum JA, Reyes Moreno C, Ramirez-Wong B, López-Cervantes J. Nutritional quality of edible parts of Moringa oleifera. Food Anal Methods. 2010;3(3):175–180. doi: 10.1007/s12161-009-9106-z. [DOI] [Google Scholar]
- 128.Nworu EL, Esimone CO, Ezeifeka CS, Okoye GO. Extracts of Moringa oleifera Lam. showing inhibitory activity against early steps in the infectivity of HIV-1 lentiviral particles in a viral vector-based screening. Afr J Biotechnol. 2015;12:4866–4873. doi: 10.5897/AJB2013.12343. [DOI] [Google Scholar]
- 129.Rawat S, Jugran A, Giri L, Bhatt I, Rawal R. Assessment of antioxidant properties in fruits of Myrica esculenta: a popular wild edible species in indian Himalayan region. Evid Based Complement Alternat Med. 2011;2011:8. doi: 10.1093/ecam/neq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Makdoh K, Lynser MB, Pala KHM. Marketing of indigenous fruits: a source of income among Khasi Women of Meghalaya, North East India. J Agric Sci. 2014;5:1–9. doi: 10.1080/09766898.2014.11884707. [DOI] [Google Scholar]
- 131.Subratti A, Lalgee LJ, Jalsa NK. Efficient extraction of black cumin (Nigella sativa L.) seed oil containing thymol, using liquefied dimethyl ether (DME) J Food Process Preserv. 2019;43(4):13913. doi: 10.1111/jfpp.13913. [DOI] [Google Scholar]
- 132.Dubey PN, Singh B, Mishra B, Kant K, Solanki R. Nigella (Nigella sativa): a high value seed spice with immense medicinal potential. Indian J Agric Sci. 2016;86:967–979. [Google Scholar]
- 133.Cohen MM. Tulsi-Ocimum sanctum: A herb for all reasons. J Ayurveda Integr Med. 2014;5(4):251–259. doi: 10.4103/0975-9476.146554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.): a review. Int J Mol Sci. 2012;13(3):3291–3340. doi: 10.3390/ijms13033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Broughton WJ, Hernandez G, Blair M, Beebe S, Gepts P, Vanderleyden J. Beans (Phaseolus spp.)-model food legumes. Plant Soil. 2003;252:55–128. doi: 10.1023/A:1024146710611. [DOI] [Google Scholar]
- 136.Ihantola-Vormisto A, Summanen J, Kankaanranta H, Vuorela H, Asmawi M, Moilanen E. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1998;63(6):518–524. doi: 10.1055/s-2006-957754. [DOI] [PubMed] [Google Scholar]
- 137.Morton JF. The emblic (Phyllunthus emblica L.) Econ Bot. 1960;14:119–128. doi: 10.1007/BF02860014. [DOI] [Google Scholar]
- 138.Estari M, Venkanna L, Sripriya D, Lalitha R. Human Immunodeficiency Virus (HIV-1) reverse transcriptase inhibitory activity of Phyllanthus emblica plant extract. Biol Med. 2012;4(4):178–182. [Google Scholar]
- 139.Al-Maiman SA, Ahmad D. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 2002;76(4):437–441. doi: 10.1016/S0308-8146(01)00301-6. [DOI] [Google Scholar]
- 140.Li Z, Wang K, Zheng J, Cheung FSG, Chan T, Zhu L, Zhou F. Interactions of the active components of Punica granatum (pomegranate) with the essential renal and hepatic human Solute Carrier transporters. Pharm Biol. 2014;52(12):1510–1517. doi: 10.3109/13880209.2014.900809. [DOI] [PubMed] [Google Scholar]
- 141.Moradi MT, Karimi A, Shahrani M, Hashemi L, Ghaffari-Goosheh MS. Anti-Influenza virus activity and phenolic content of pomegranate (Punica granatum L.) peel extract and fractions. Avicenna J Med Biotechnol. 2019;11(4):285–291. [PMC free article] [PubMed] [Google Scholar]
- 142.Kuete V (2014) Chapter 22-physical, hematological, and histopathological signs of toxicity induced by African medicinal plants. In: Toxicological Survey of African Medicinal Plants. Elsevier, pp 635–657. https://doi.org/10.1016/B978-0-12-800018-2.00022-4
- 143.Tavares IMdC, Lago-Vanzela ES, Rebello LPG, Ramos AM, Gómez-Alonso S, García-Romero E, Da-Silva R, Hermosín-Gutiérrez I. Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels) Food Res Int. 2016;82:1–13. doi: 10.1016/j.foodres.2016.01.014. [DOI] [Google Scholar]
- 144.Yang MH, Ali Z, Khan IA, Khan SI. Anti-inflammatory activity of constituents isolated from Terminalia chebula. Nat Prod Commun. 2014;9(7):965–968. [PubMed] [Google Scholar]
- 145.Barthakur NN, Arnold NP. Nutritive value of the chebulic myrobalan (Terminalia chebula Retz) and its potential as a food source. Food Chem. 1991;40(2):213–219. doi: 10.1016/0308-8146(91)90105-W. [DOI] [Google Scholar]
- 146.Korani M, Jamshidi M. The effect of aqueous extract of Trachyspermum ammi seeds and ibuprofen on inflammatory gene expression in the cartilage tissue of rats with collagen-induced arthritis. J Inflamm Res. 2020;13:133–139. doi: 10.2147/jir.s236242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Srivastava KC. Extract of a spice-Omum (Trachyspermum ammi)-shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins Leukot Essent Fatty Acids. 1988;33(1):1–6. doi: 10.1016/0952-3278(88)90115-9. [DOI] [PubMed] [Google Scholar]
- 148.Cai Z, Zhang G, Tang B, Liu Y, Fu X, Zhang X. Promising anti-influenza properties of active constituent of Withania somnifera ayurvedic herb in targeting neuraminidase of H1N1 influenza: computational study. Cell Biochem Biophys. 2015;72(3):727–739. doi: 10.1007/s12013-015-0524-9. [DOI] [PubMed] [Google Scholar]
- 149.Palliyaguru DL, Singh SV, Kensler TW. Withania somnifera: From prevention to treatment of cancer. Mol Nutr Food Res. 2016;60(6):1342–1353. doi: 10.1002/mnfr.201500756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang JS, Wang KC, Yeh CF, Shieh DE, Chiang LC. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. 2013;145(1):146–151. doi: 10.1016/j.jep.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 151.Semwal RB, Semwal DK, Combrinck S, Viljoen AM. Gingerols and shogaols: important nutraceutical principles from ginger. Phytochemistry. 2015;117:554–568. doi: 10.1016/j.phytochem.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 152.Nair KPP (2013) Chapter 26- Ginger as a Spice and Flavorant. In: The Agronomy and Economy of Turmeric and Ginger. Elsevier, Oxford, pp 497–510. doi:10.1007/978-3-030-29189-1_26
- 153.Tripathi P, Chauhan NS, Patel JR. Anti-inflammatory activity of Abutilon indicum extract. Nat Prod Res. 2012;26(17):1659–1661. doi: 10.1080/14786419.2011.616508. [DOI] [PubMed] [Google Scholar]
- 154.Dashputre N, Naikwade N. Immunomodulatory Activity of Abutilon Indicum linn on Albino Mice. Int J Pharm Sci Res. 2010;1(3):178–184. [Google Scholar]
- 155.Bhuvaneswari K, Michael D. Immunomodulation by leaf extract of Acalypha indica Linn in Oreochromis mossambicus (Peters) Hydrobiologia. 2000;430(1):113–120. [Google Scholar]
- 156.Benni JM, Jayanthi MK, Suresha RN. Evaluation of the anti-inflammatory activity of Aegle marmelos (Bilwa) root. Indian J Pharmacol. 2011;43(4):393–397. doi: 10.4103/0253-7613.83108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Govinda HV, Asdaq SMB. Immunomodulatory potential of methanol extract of Aegle marmelos in animals. Indian J Pharm Sci. 2011;73(2):235–240. doi: 10.4103/0250-474x.91571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim JJ, Jiang J, Shim DW, Kwon SC, Kim TJ, Ye SK, Kim MK, Shin YK, Koppula S, Kang TB, Choi DK, Lee KH. Anti-inflammatory and anti-allergic effects of Agrimonia pilosa Ledeb extract on murine cell lines and OVA-induced airway inflammation. J Ethnopharmacol. 2012;140(2):213–221. doi: 10.1016/j.jep.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 159.Lee DY, Li H, Lim HJ, Lee HJ, Jeon R, Ryu J-H. Anti-inflammatory activity of sulfur-containing compounds from garlic. J Med Food. 2012;15(11):992–999. doi: 10.1089/jmf.2012.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fallah-Rostami F, Tabari MA, Esfandiari B, Aghajanzadeh H, Behzadi MY. Immunomodulatory activity of aged garlic extract against implanted fibrosarcoma tumor in mice. N Am J Med Sci. 2013;5(3):207–212. doi: 10.4103/1947-2714.109191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ried K, Fakler P. Potential of garlic (Allium sativum) in lowering high blood pressure: mechanisms of action and clinical relevance. Integr Blood Press Control. 2014;7:71–82. doi: 10.2147/ibpc.s51434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vázquez B, Avila G, Segura D, Escalante B. Antiinflammatory activity of extracts from Aloe vera gel. J Ethnopharmacol. 1996;55(1):69–75. doi: 10.1016/s0378-8741(96)01476-6. [DOI] [PubMed] [Google Scholar]
- 163.Farahnejad Z, Ghazanfari T, Yaraee R. Immunomodulatory effects of Aloe vera and its fractions on response of macrophages against Candida albicans. Immunopharmacol Immunotoxicol. 2011;33(4):676–681. doi: 10.3109/08923973.2011.560158. [DOI] [PubMed] [Google Scholar]
- 164.Yao W, Wang F, Wang H. Immunomodulation of artemisinin and its derivatives. Sci Bull. 2016 doi: 10.1007/s11434-016-1105-z. [DOI] [Google Scholar]
- 165.Chattopadhyay RR. Possible biochemical mode of anti-inflammatory action of Azadirachta indica A. Juss. in rats. Indian J Exp Biol. 1998;36(4):418–420. [PubMed] [Google Scholar]
- 166.Upadhyay SN, Dhawan S, Garg S, Talwar GP. Immunomodulatory effects of neem (Azadirachta indica) oil. Int J Immunopharmacol. 1992;14(7):1187–1193. doi: 10.1016/0192-0561(92)90054-o. [DOI] [PubMed] [Google Scholar]
- 167.Arise RO, Acho MA, Yekeen AA, Omokanye IA, Sunday-Nwaso EO, Akiode OS, Malomo SO. Kinetics of angiotensin-1 converting enzyme inhibition and antioxidative properties of Azadirachta indica seed protein hydrolysates. Heliyon. 2019;5(5):e01747. doi: 10.1016/j.heliyon.2019.e01747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen BT, Li WX, He RR, Li YF, Tsoi B, Zhai YJ, Kurihara H. Anti-inflammatory effects of a polyphenols-rich extract from tea (Camellia sinensis) flowers in acute and chronic mice models. Oxid Med Cell Longev. 2012;2012:537923. doi: 10.1155/2012/537923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rahayu RP, Prasetyo RA, Purwanto DA, Kresnoadi U, Iskandar RPD, Rubianto M. The immunomodulatory effect of green tea (Camellia sinensis) leaves extract on immunocompromised Wistar rats infected by Candida albicans. Vet World. 2018;11(6):765–770. doi: 10.14202/vetworld.2018.765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dong J, Xu X, Liang Y, Head R, Bennett L. Inhibition of angiotensin converting enzyme (ACE) activity by polyphenols from tea (Camellia sinensis) and links to processing method. Food Funct. 2011;2(6):310–319. doi: 10.1016/j.foodchem.2011.09.060. [DOI] [PubMed] [Google Scholar]
- 171.Gopakumar S, Latha P, Shine VJ, Anuja G, Suja S, ShyamaPeriyaRajasekharan SSPS. Anti-allergic, anti-inflammatory and anti-lipidperoxidant effects of Cassia occidentalis Linn. Indian J Exp Biol. 2010;48(5):494–498. [PubMed] [Google Scholar]
- 172.Doppalapudi SC, Sandya L, Reddy Y, Nagarjuna S, Shafeen S. Anti-inflammatory activity of Cicer arietinum seed extracts. Asian J Pharm Clin Res. 2012;5:64–68. [Google Scholar]
- 173.Sathyanarayana S, Kumar P, Prashanth H. Pectic polysaccharides have relatively potent immunomodulatory activity compared to their hydrolysates from chickpea (Cicer arietinum L.) husk. Indian J Nutr Diet. 2019;56(2):94. doi: 10.21048/ijnd.2019.56.2.22687. [DOI] [Google Scholar]
- 174.Bhagyawant SS, Narvekar DT, Gupta N, Bhadkaria A, Gautam AK, Srivastava N. Chickpea (Cicer arietinum L.) lectin exhibit Inhibition of ACE-I, α-amylase and α-glucosidase activity. Protein Pept Lett. 2019;26(7):494–501. doi: 10.2174/0929866526666190327130037. [DOI] [PubMed] [Google Scholar]
- 175.Mensah AY, Mireku EA, Damoah AO, Amponsah IK. Anti-inflammatory and antioxidant activities of Commelina diffusa (Commelinaceae) World J Pharm Sci. 2014;2(10):1159–1165. [Google Scholar]
- 176.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 177.Chandrasekaran CV, Sundarajan K, Edwin JR, Gururaja GM, Mundkinajeddu D, Agarwal A. Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. J Pharm Pharmacogn Res. 2013;5(2):71–79. doi: 10.4103/0974-8490.110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lekshmi PC, Arimboor R, Nisha VM, Menon AN, Raghu KG. In vitro antidiabetic and inhibitory potential of turmeric (Curcuma longa L) rhizome against cellular and LDL oxidation and angiotensin converting enzyme. Int J Food Sci Technol. 2014;51(12):3910–3917. doi: 10.1007/s13197-013-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ben Salem M, Affes H, Athmouni K, Ksouda K, Dhouibi R, Sahnoun Z, Hammami S, Zeghal KM. Chemicals compositions, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evid Based Complement Alternat Med. 2017;2017:4951937. doi: 10.1155/2017/4951937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chitra M, Sukumar E, Suja V, Devi CSS. Antitumor, anti-inflammatory and analgesic property of Embelin, a plant product. Chemotherapy. 1994;40(2):109–113. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- 181.Sethi J, Singh J. Role of medicinal plants as immunostimulants in health and disease. Ann Med Chem Res. 2015;1(2):1009. doi: 10.1016/j.fsi.2017.05.034. [DOI] [Google Scholar]
- 182.Kota P, Prasad PD, Rao AN, Reddy PD, Abhinay G. Anti-inflammatory activity of Eugenia Jambolana in Albino rats. Int J Pharma Bio Sci. 2010;1(4):435–438. [Google Scholar]
- 183.Mastan SK, Saraseeruha A, Gourishankar V, Chaitanya G, Raghunandan N, Reddy GA, Eswar Kumar KE. Immunomodulatory activity of methanolic extract of Syzygium cumini seeds. Pharmacology online. 2008;3:895–903. [Google Scholar]
- 184.Syama HP, Arya AD, Dhanya R, Nisha P, Sundaresan A, Jacob E, Jayamurthy P. Quantification of phenolics in Syzygium cumini seed and their modulatory role on tertiary butyl-hydrogen peroxide-induced oxidative stress in H9c2 cell lines and key enzymes in cardioprotection. J Food Sci Technol. 2017;54(7):2115–2125. doi: 10.1007/s13197-017-2651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kaur S, Sharma N, Nehru B. Anti-inflammatory effects of Ginkgo biloba extract against trimethyltin-induced hippocampal neuronal injury. Inflammopharmacology. 2018;26(1):87–104. doi: 10.1007/s10787-017-0396-2. [DOI] [PubMed] [Google Scholar]
- 186.Xu AH, Ren L, Zheng YY, Chen HS. Immunomodulatory effect of Ginkgo biloba exocarp polysaccharides on immunosuppressive mice induced by cyclophosphamide. Chin J Pharmacol Toxicol. 2008;22(1):69–72. [Google Scholar]
- 187.Ma FF, Wang H, Wei CK, Thakur K, Wei ZJ, Jiang L. Three novel ACE inhibitory peptides isolated from Ginkgo biloba seeds: purification purification, inhibitory kinetic and mechanism. Front Pharmacol. 2018;9:1579. doi: 10.3389/fphar.2018.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Frattaruolo L, Carullo G, Brindisi M, Mazzotta S, Bellissimo L, Rago V, Curcio R, Dolce V, Aiello F, Cappello AR. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants. PLoS ONE. 2019;8(6):186. doi: 10.3390/antiox8060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mitra Mazumder P, Pattnayak S, Parvani H, Sasmal D, Rathinavelusamy P. Evaluation of immunomodulatory activity of Glycyrhiza glabra L. roots in combination with zing. Asian Pac J Trop Biomed. 2012;2(1):S15–S20. doi: 10.1016/S2221-1691(12)60122-1. [DOI] [Google Scholar]
- 190.Malik J, Manvi FV, Alagawadi KR, Noolvi M. Evaluation of anti-inflammatory activity of Gymnema sylvestre leaves extract in rats. Int J Green Pharm. 2008 doi: 10.4103/0973-8258.41184. [DOI] [Google Scholar]
- 191.Singh VK, Dwivedi P, Chaudhary BR, Singh R. Immunomodulatory effect of gymnema sylvestre (RBr). leaf extract: an in vitro study in rat model. PLoS ONE. 2015;10(10):0139631. doi: 10.1371/journal.pone.0139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bayani GFE, Marpaung NLE, Simorangkir DAS, Sianipar IR, Ibrahim N, Kartinah NT, Mansur IG, Purba JS, Ilyas EII. Anti-inflammatory effects of Hibiscus Sabdariffa Linn. on the IL-1β/IL-1ra ratio in plasma and hippocampus of overtrained rats and correlation with spatial memory. Kobe J Med Sci. 2018;64(2):E73–E83. [PMC free article] [PubMed] [Google Scholar]
- 193.Fakeye TO, Pal A, Bawankule DU, Khanuja SP. Immunomodulatory effect of extracts of Hibiscus sabdariffa L. (Family Malvaceae) in a mouse model. Phytother Res. 2008;22(5):664–648. doi: 10.1002/ptr.2370. [DOI] [PubMed] [Google Scholar]
- 194.Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol. 2010;127(1):7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 195.Patil N, Kotian R, Reddy S, Nayak V, Bairy L, Parida A, Malalur C (2014) Evaluation of anti-inflammatory activity of alcoholic extract of leaves of Leucas Aspera in albino rats. Int J Pharm Pharm Sci 6(2)
- 196.Augustine B, Dash S, Lahkar M, Amara V, Samudrala P, Thomas J. Evaluation of immunomodulatory activity of ethyl acetate extract of Leucas aspera in Swiss albino mice. Int J Green Pharm. 2014;8(2):84. doi: 10.4103/0973-8258.129574. [DOI] [Google Scholar]
- 197.Knödler MC, Jr, Wenzig EM, Bauer R, Lacorn M, Beifuss U, Carle R, Schieber A. Anti-inflammatory 5-(11’Z-heptadecenyl)- and 5-(8’Z,11’Z-heptadecadienyl)-resorcinols from mango (Mangifera indica L.) peels. Phytochemistry. 2008;69(4):988–993. doi: 10.1016/j.phytochem.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 198.Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78(2):133–137. doi: 10.1016/S0378-8741(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 199.Juvekar A, Hule A, Sakat S, Chaughule V. In vitro and in vivo evaluation of immunomodulatory activity of methanol extract of Momordica charantia fruits. Drug Invent Today. 2009;1(2):89–94. [Google Scholar]
- 200.Priyanto AD, Doerksen RJ, Chang CI, Sung W-C, Widjanarko SB, Kusnadi J, Lin YC, Wang TC, Hsu JL. Screening, discovery, and characterization of angiotensin-I converting enzyme inhibitory peptides derived from proteolytic hydrolysate of bitter melon seed proteins. J Proteom. 2015;128:424–435. doi: 10.1016/j.jprot.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 201.Anudeep S, Prasanna VK, Adya SM, Radha C. Characterization of soluble dietary fiber from Moringa oleifera seeds and its immunomodulatory effects. Int J Biol Macromol. 2016;91:656–662. doi: 10.1016/j.ijbiomac.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 202.Abdulazeez A, Ajiboye O, Wudil A, Abubakar H. Partial purification and characterization of angiotensin converting enzyme inhibitory alkaloids and flavonoids from the leaves and seeds of Moringa oleifera. J adv biol biotechnol. 2016;5:1–11. doi: 10.9734/jabb/2016/21974. [DOI] [Google Scholar]
- 203.Patel KG, Rao NJ, Gajera VG, Bhatt PA, Patel KV, Gandhi TR. Anti-allergic activity of stem bark of Myrica esculenta Buch-Ham (Myricaceae) J Young Pharm. 2010;2(1):74–78. doi: 10.4103/0975-1483.62219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nguyen XN, Phan VK, Chau VM, Bui HT, Nguyen XC, Vu KT, le Hoang TA, Jo SH, Jang HD, Kwon YI, Kim YH. A new monoterpenoid glycoside from Myrica esculenta and the inhibition of Angiotensin I-converting enzyme. Chem Pharm Bull. 2010;58:1408–1410. doi: 10.1248/cpb.58.1408. [DOI] [PubMed] [Google Scholar]
- 205.Ikhsan M, Hiedayati N, Maeyama K, Nurwidya F. Nigella sativa as an anti-inflammatory agent in asthma. BMC Res Notes. 2018;11(1):744–744. doi: 10.1186/s13104-018-3858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Boskabady MH, Keyhanmanesh R, Khameneh S, Doostdar Y, Khakzad MR. Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. J Zhejiang Univ Sci B. 2011;12(3):201–209. doi: 10.1631/jzus.B1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sutopo CCY, Sutrisno A, Wang LF, Hsu JL. Identification of a potent Angiotensin-I converting enzyme inhibitory peptide from Black cumin seed hydrolysate using orthogonal bioassay-guided fractionations coupled with in silico screening. Process Biochem. 2020 doi: 10.1016/j.procbio.2020.02.010. [DOI] [Google Scholar]
- 208.Kalabharathi HL, Suresha RN, Pragathi B, Pushpa VH, Satish AM. Anti inflammatory activity of fresh tulsi leaves (Ocimum Sanctum) in albino rats. Int J Pharma Bio Sci. 2011;2(4):45–50. [Google Scholar]
- 209.Mediratta PK, Sharma KK, Singh S. Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol. 2002;80(1):15–20. doi: 10.1016/s0378-8741(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 210.Chaudhary S, Mukherjee P, Maity N, Nema N, Bhadra S, Saha BP. Ocimum sanctum L. a potential angiotensin converting enzyme (ACE) inhibitor useful in hypertension. Indian J Nat Prod Resour. 2014;5(1):83–87. [Google Scholar]
- 211.Song C, Hong YH, Park JG, Kim HG, Jeong D, Oh J, Sung G-H, Hossain MA, Taamalli A, Kim JH, Kim J-H, Cho JY. Suppression of Src and Syk in the NF-κB signaling pathway by Olea europaea methanol extract is leading to its anti-inflammatory effects. J Ethnopharmacol. 2019;235:38–46. doi: 10.1016/j.jep.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 212.Vezza T, Algieri F, Rodríguez-Nogales A, Garrido-Mesa J, Utrilla MP, Talhaoui N, Gómez-Caravaca AM, Segura-Carretero A, Rodríguez-Cabezas ME, Monteleone G, Gálvez J (2017) Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol Nutr Food Res 61(10) [DOI] [PubMed]
- 213.Hansen K, Adsersen A, Christensen SB, Jensen SR, Nyman U, Smitt UW. Isolation of an angiotensin converting enzyme (ACE) inhibitor from Olea europaea and Olea lancea. Phytomedicine. 1996;2(4):319–325. doi: 10.1016/S0944-7113(96)80076-6. [DOI] [PubMed] [Google Scholar]
- 214.Oomah BD, Corbé A, Balasubramanian P. Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J Agric Food Chem. 2010;58(14):8225–8230. doi: 10.1021/jf1011193. [DOI] [PubMed] [Google Scholar]
- 215.Tagliazucchi D, Martini S, Bellesia A, Conte A. Identification of ACE-inhibitory peptides from Phaseolus vulgaris after in vitro gastrointestinal digestion. Int J Food Sci Nutr. 2015;66(7):774–782. doi: 10.3109/09637486.2015.1088940. [DOI] [PubMed] [Google Scholar]
- 216.Suresh K, Vasudevan DM. Augmentation of murine natural killer cell and antibody dependent cellular cytotoxicity activities by Phyllanthus emblica, a new immunomodulator. J Ethnopharmacol. 1994;44:55–60. doi: 10.1016/0378-8741(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 217.Lee CJ, Chen LG, Liang WL, Wang CC. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010;118(2):315–322. doi: 10.1016/j.foodchem.2009.04.123. [DOI] [Google Scholar]
- 218.Gracious Ross R, Selvasubramanian S, Jayasundar S. Immunomodulatory activity of Punica granatum in rabbits: a preliminary study. J Ethnopharmacol. 2001;78(1):85–87. doi: 10.1016/s0378-8741(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 219.Wang Y, Xiang L, Yi X, He X. Potential anti-inflammatory steroidal saponins from the berries of Solanum nigrum L. (European Black Nightshade) J Agric Food Chem. 2017;65(21):4262–4272. doi: 10.1021/acs.jafc.7b00985. [DOI] [PubMed] [Google Scholar]
- 220.Li J, Li QW, Gao DW, Han ZS, Lu WZ. Antitumor and immunomodulating effects of polysaccharides isolated from Solanum nigrum Linne. Phytother Res. 2009;23(11):1524–1530. doi: 10.1002/ptr.2769. [DOI] [PubMed] [Google Scholar]
- 221.Chaudhuri AKN, Pal S, Gomes A, Bhattacharya S. Anti-inflammatory and related actions of Syzygium cuminii seed extract. Phytother Res. 1990;4(1):5–10. doi: 10.1002/ptr.2650040103. [DOI] [Google Scholar]
- 222.Shivaprasad HN, Kharya MD, Rana AC, Mohan S. Preliminary immunomodulatory activities of the aqueous extract of Terminalia chebula. Pharm Biol. 2006;44(1):32–34. doi: 10.1080/13880200500530542. [DOI] [Google Scholar]
- 223.Sornwatana T, Bangphoomi K, Roytrakul S, Wetprasit N, Choowongkomon K, Ratanapo S. Chebulin: Terminalia chebula Retz. fruit-derived peptide with angiotensin-I-converting enzyme inhibitory activity. Biotechnol Appl Biochem. 2015;62(6):746–753. doi: 10.1002/bab.1321. [DOI] [PubMed] [Google Scholar]
- 224.Shruthi RR, Venkatesh Y, Gudipati M. In vitro immunomodulatory potential of macromolecular components derived from the aqueous extract of ajowan [Trachyspermum ammi (L.) Sprague] Indian J Tradit Know. 2017;16(3):506–513. [Google Scholar]
- 225.Gupta A, Singh S. Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharm Biol. 2014;52(3):308–320. doi: 10.1186/s12906-016-1466-5. [DOI] [PubMed] [Google Scholar]
- 226.Davis L, Kuttan G. Immunomodulatory activity of Withania somnifera. J Ethnopharmacol. 2000;71(1–2):193–200. doi: 10.1016/s0378-8741(99)00206-8. [DOI] [PubMed] [Google Scholar]
- 227.Ravindran R, Sharma N, Roy S, Thakur A, Ganesh S, Kumar S, Devi J, Rajkumar J. Interaction studies of Withania somnifera's key metabolite withaferin a with different receptors associated with cardiovascular disease. Curr Comput Aided Drug Des. 2015;11(3):212–221. doi: 10.2174/1573409912666151106115848. [DOI] [PubMed] [Google Scholar]
- 228.Funk JL, Frye JB, Oyarzo JN, Chen J, Zhang H, Timmermann BN. Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. Pharmanutrition. 2016;4(3):123–131. doi: 10.1016/j.phanu.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Amri M, Touil-Boukoffa C. In vitro anti-hydatic and immunomodulatory effects of ginger and [6]-gingerol. Asian Pac J Trop Med. 2016;9(8):749–756. doi: 10.1016/j.apjtm.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 230.Akinyemi A, Ademiluyi A, Oboh G. Inhibition of angiotensin-1-converting enzyme activity by two varieties of ginger ( Zingiber officinale ) in rats fed a high cholesterol diet. J Med Food. 2014 doi: 10.1089/jmf.2012.0264. [DOI] [PubMed] [Google Scholar]