Abstract
Pancreatic adenocarcinoma is one of the deadliest types of cancer worldwide, with a 5-year survival rate of 8% despite recent treatment advancements. The present systematic review aimed to investigate the role of hyperthermic intraperitoneal chemotherapy (HIPEC) following surgical resection for pancreatic adenocarcinoma, with or without peritoneal carcinomatosis. A systematic search of the MEDLINE and SCOPUS electronic databases was performed according to PRISMA guidelines. All possible relevant articles published between January 1980 and May 2019 were retrieved using multiple search terms associated with HIPEC and pancreatic adenocarcinoma. The initial search resulted in 1,244 reports, which condensed to 41 reports following screening of titles and abstracts, and subsequently to four reports following full-text thorough examination. The four reports included involved a prospective cohort study of HIPEC use in resectable pancreatic adenocarcinoma, and three retrospective studies of HIPEC use following cytoreductive surgery for peritoneal carcinomatosis due to pancreatic adenocarcinoma, resulting in a total of 47 patients. The overall survival ranged between 2 and 62 months, and the hospital mortality rate was 8.5%. Morbidity (34%) was mainly attributed to anastomotic leak or respiratory failure. Due to the small sample size and low quality of evidence of the included studies, no valid conclusions could be drawn. Therefore, further studies are required to justify the use of HIPEC as an adjuvant therapy in resectable pancreatic adenocarcinoma, while cytoreductive surgery and HIPEC in peritoneal carcinomatosis of pancreatic origin seems not only not useful but also unsafe at this level of evidence.
Keywords: hyperthermic intraperitoneal chemotherapy, cytoreductive surgery, pancreatic cancer, peritoneal carcinomatosis, pancreatic adenocarcinoma
Introduction
Despite its relatively low incidence, pancreatic adenocarcinoma is the fourth deadliest cancer in the West and eighth worldwide, with a 5-year survival rate of only 8% following initial diagnosis (1–7). Complete surgical resection at an early stage is the only treatment option with a curative potential (8,9); however, lack of early symptomatology makes this effort feasible for <20% of newly diagnosed patients (10–13). The remaining cases are considered to have unresectable locally advanced, borderline resectable, or metastatic disease (14). In this setting, the introduction of neoadjuvant chemotherapy or radiochemotherapy seems to offer potential advantages, such as the increase of the R0 resection rates in borderline resectable tumors or the conversion of locally advanced tumors to resectable ones (14). Examples of neoadjuvant chemotherapy that have been successfully used in the setting of pancreatic cancer includes FOLFIRINOX regimen (leucovorin, fluorouracil, irinotecan, oxaliplatin) or gemcitabine-based chemotherapy or capecitabine-based chemotherapy (14). The dissemination pattern of pancreatic adenocarcinoma is through its microenvironment, which plays a crucial role to local invasion of anatomical structures, lymphatics and blood vessels, leading to early metastases (15).
Pancreatic adenocarcinoma can also lead to peritoneal carcinomatosis (PC) (4,16,17). Peritoneal metastases are the second most common following liver metastases and are found in half of the patients at the time of death due to pancreatic adenocarcinoma (18,19). Additionally, 20–30% of pancreatic cancer patients with no metastases have malignant cells in the peritoneal cavity (18). Also, following curative resection, one-third of the patients develop peritoneal metastases and 75% of them have local recurrence (19,20). In this respect, a number of highly selected patients with locoregional pancreatic adenocarcinoma with or without peritoneal metastases, and without evidence of systemic disease, has been treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) (21). CRS/HIPEC has been introduced in the past three decades and has led to favorable 5-year survival rates in several PC pathologies (22–24). Pseudomyxoma peritonei was the first indication for CRS/HIPEC (25). In addition, CRS/HIPEC has been demonstrated to offer improved outcomes in PC of colorectal origin for selected patients (26–28). There are similar results from retrospective studies involving pathologies of ovarian and gastric origin that have led to clinical trial assessments (23,29,30). However, whether this treatment approach offers any benefit in locoregional (with or without peritoneal metastases) pancreatic adenocarcinoma without evidence of systemic disease remains unclear.
The present systematic review aimed to investigate whether HIPEC can be used to effectively treat pancreatic adenocarcinoma, with or without peritoneal carcinomatosis.
Materials and methods
Search strategy
A systematic search was performed on the medical literature in MEDLINE and SCOPUS databases, between January 1980 and May 2019, guided by the PRISMA protocol (31,32). All retrieved articles were initially screened for relevant titles and abstracts, and full-text inspection followed. Medical Subject Heading (MeSH) terms and text words were used based on the following search strategy: Group A terms: ‘crs’ OR ‘cytoreduction’ OR ‘cytoreductive surgery’ OR ‘debulking’ OR ‘hipec’ OR ‘hyperthermic intraperitoneal chemotherapy’. Group B terms: ‘pancreas’ OR ‘pancreatic’. Group A and group B terms were combined and no limits were applied.
Inclusion and exclusion criteria
The following criteria were applied to the articles retrieved from the search: i) Cohort studies on CRS/HIPEC or resection plus HIPEC treatment for pancreatic adenocarcinoma, with or without peritoneal carcinomatosis, were included; ii) case reports were excluded; iii) histologies other than pancreatic adenocarcinoma were excluded; iv) articles that lacked outcome data were excluded; v) non-human studies were excluded; and vi) review articles, meta-analyses, and book chapters were excluded; however, their reference lists were used to retrieve any relevant studies of any publishing date.
Data collection and extraction
A total of three reviewers (AL, GZV, and KG) independently screened titles and abstracts of the retrieved studies. Articles classified as relevant were full-text reviewed in order to identify studies to be included in the present systematic review. Disagreements were resolved by a third reviewer (EA).
All existing details were included and the following data were extracted: i) Demographics of population, including age and sex; ii) disease-specific and clinical characteristics of population, including type and location of tumor, TNM staging, performance status and cytoreduction level, and iii) HIPEC characteristics, such as type of chemotherapeutic agent(s), type and quantity of the dialysate, temperature and duration of HIPEC. Details on methodology, inclusion and exclusion criteria, number of patients excluded or lost to follow-up, and intervention and declaration of competing interests were also collected. A total of three reviewers (EA, GZV and KG) independently extracted data from the full version of the articles included in the present systematic review. Disagreements were resolved by a fourth reviewer (AL).
The primary outcome was overall survival (OS). All types of baseline and postintervention outcomes were recorded, including mean, median and 5-year survival. Secondary outcomes were mortality and morbidity, including any local or systematic complication that were attributed to HIPEC.
Assessment of risk of bias and quality of the included studies
Due to the low prevalence of pancreatic adenocarcinoma and its short course, cohort studies without control groups and case series were the only evidence available. These sources are often considered among the lowest levels of evidence, thus, all observational studies were rated as fair or low quality. The summary of findings grades the quality of evidence as very low. A total of two different reviewers (AL and EA) performed the assessment of risk of bias, using a table formatted based on different tools (Table I), as there are currently no widely accepted tools to assess case series studies (33).
Table I.
Quality assessment of the included studies.
| Authors, year | Article type | Question/hypothesis clearly defined | Control group | Prospective study | Intervention adequately described | FU | Outcome adequately ascertained | Each patient reported in detail | Declaration of no conflict of interest provided | Quality of evidence | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tentes et al, 2016 | Prospective cohort | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Fair | (34) |
| Tentes et al, 2018 | Retrospective cohort | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Fair | (35) |
| Farma et al, 2005 | Retrospective cohort | Yes | No | No | Yes | Yes | Yes | No | No | Low | (36) |
| Fujimura et al, 1999 | Retrospective cohort | Yes | No | No | Yes | Yes | Yes | Yes | No | Fair | (37) |
FU, follow-up.
Statistical analysis
Statistical analysis was performed on the data from the included studies. Wherever feasible, outcomes regarding median survival were synthesized by pooling data for patients that underwent HIPEC. Due to the high heterogeneity of the intervention, low quality of evidence and small sample size, a meta-analysis was not performed.
Results
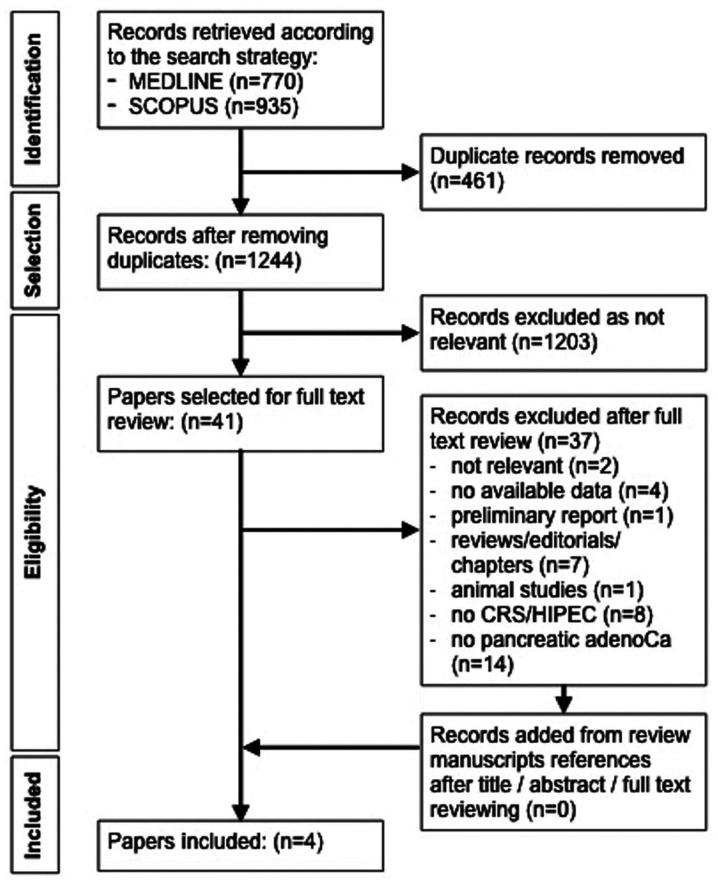
The search strategy yielded 1,244 articles following removal of duplicates. Of these articles, 1,203 were rejected according to the predefined criteria through title and abstract screening, and 41 articles remained for further assessment. Following full-text review, 37 out of 41 articles were excluded as not relevant (n=2), no outcome data (n=4), preliminary report (n=1), reviews/editorials/book chapters (n=7), animal study (n=1), no CRS/HIPEC nor resection plus HIPEC treatment (n=8) and no pancreatic adenocarcinoma (n=14). The remaining four articles were included in the present study. The flow diagram of the selection process is presented in Fig. 1.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flow diagram for the selection of reports included in the present study. CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; adenoCa, adenocarcinoma.
Of the four final reports included in this systematic review, one was a prospective cohort study of pancreatic adenocarcinoma cases treated with resection plus HIPEC (34), one was a retrospective cohort study of PC of pancreatic adenocarcinoma origin treated with CRS/HIPEC (35), and two were retrospective cohort studies regarding PC cases that were treated with CRS/HIPEC and included some pancreatic adenocarcinoma cases (36,37). The work of Fujimura et al (37), was a retrospective cohort regarding PC cases that were treated with CRS/HIPEC and included one pancreatic adenocarcinoma case. This study was not considered to be a case report; thus, this work did not meet exclusion criteria. The quality assessment of the included studies is presented in Table I.
The four studies included 47 patients with pancreatic adenocarcinoma treated with a combination of primary tumor resection and/or cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. The main data of the included studies are presented in Table II. Of the 47 patients, 33 received HIPEC as an adjuvant for resectable pancreatic adenocarcinoma with no peritoneal disease (TNM I: 4, TMN II: 9, TNM III: 20), while the remaining 14 patients had PC of pancreatic origin and were treated with CRS/HIPEC. A total of nine cases had metachronous peritoneal metastases and five cases had synchronous ones. None of the studies included any type of comparison or control groups. The data regarding sex and age were missing in one study (36). The location of the original pancreatic tumor was available in 42 out of 47 cases (28 head, 2 body, 11 tail and 1 mixed). Regarding the HIPEC chemotherapeutic agent used, choices varied between mitomycin C, cisplatin, etoposide, gemcitabine and combinations of these. Details regarding the characteristics of HIPEC, including open/closed technique, dosage of drug, dialysate, temperature and duration are presented in Table III.
Table II.
Main data of the included studies.
| Authors, year | No. of cases | Sex | PC | Tumor original location, n | Histology | PCI | CC | OS | Morbidity, % | Mortality, % | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tentes et al, 2016 | 33 | 14 M, 19 F | No | Head, 26; body, 2; tail, 4; mixed, 1 | adenoCa | n/a | n/a | 5-year: 24%. | 24.2 | 6.1 | (34) |
| Tentes et al, 2018a | 6 | 3 M, 3 F | Yes - 2 synchronous, 4 metachronous | Tail, 6 | adenoCa | 3–23 | CC-0 n=6; CC-1 n=1; CC-3 n=1 | 4–36 mo | 66.67 | 33.3 | (35) |
| Farma et al, 2005 | 7 | N/A | Yes - 3 synchronous, 4 metachronous | Head, 2; tail, 1; N/A, 4 | adenoCa | N/A | CC-0 n=6; CC-2 n=1 | 2–62 mo | 57 | 0 | (36) |
| Fujimura et al, 1999 | 1 | M | Yes - metachronous | N/A | adenoCa | N/A | N/A | 18 mo | 0 | 0 | (37) |
This study included 6 patients who underwent 8 procedures in total. M, male; F, female; PC, peritoneal carcinomatosis; adenoCa, adenocarcinoma; OS, overall survival; PCI, peritoneal carcinomatosis index; CC, completeness of cytoreduction; mo, months; n/a, not applicable; N/A, not available.
Table III.
Data regarding the intervention characteristics (open/closed technique, drug type and dosage, dialysate type and volume, temperature and hyperthermic intraperitoneal chemotherapy duration) of the four studies.
| First author, year | Technique | Drug | Drug dose | Solution | Solution volume | Temperature | Duration | Refs. |
|---|---|---|---|---|---|---|---|---|
| Tentes et al, 2016 | O | Gemcitabine | 1000 mg/m2 | N/A | 3,000 ml | 42.5–43°C | 60 min | (34) |
| Tentes et al, 2018 | O | Gemcitabine or | 1,000 mg/m2 or | N/A | N/A | N/A | N/A | (35) |
| Cisplatin + MMC | 50 mg/m2 + 15 mg/m2 | |||||||
| Farma et al, 2005 | C | Cisplatin | 425–676 mg/m2 | N/A | 3,000-7,000 ml | 41.4°C | 90 min | (36) |
| Fujimura et al, 1999 | O | Cisplatin + MMC + Etoposide | 300 mg + 60 mg + 100 mg | Saline | N/A | 42-42.5°C | 60 min | (37) |
N/A, not available; O, open technique; C, closed technique; RL, Ringer's lactate; MMC, mitomycin C.
The OS of pancreas-originated PC cases (n=14) treated with CRS/HIPEC was reported individually for each patient, with a median survival of 12 months (range 2–62 months). The group of 33 patients with pancreatic adenocarcinoma and no PC had an overall mean and median survival of 33±6 and 13 months, respectively; the 5-year survival was 24%, with a median follow-up of 11 months. The recurrence rate was 60.6% (20/33 patients), whereby three patients had local-regional failure and the remaining 17 had liver metastases. Detailed information on the overall morbidity (34%) and mortality (8.5%) rates are presented in Tables II and IV.
Table IV.
Postoperative complications and times each was encountered in the 47 patients studied.
| Complication | Times encountered, n | Percentage among the 47 patients |
|---|---|---|
| Anastomotic leak | 6 | 12.8 |
| Respiratory failure | 5 | 10.6 |
| Sepsis | 4 | 8.5 |
| Surgical wound infection | 2 | 4.3 |
| Neutropenia | 2 | 4.3 |
| Postoperative bleeding | 1 | 2.1 |
| Liver failure | 1 | 2.1 |
| Renal failure | 1 | 2.1 |
| Delayed gastric emptying | 1 | 2.1 |
| Small bowel obstruction | 1 | 2.1 |
| Enterocutaneous fistula | 1 | 2.1 |
Discussion
Standard treatment for pancreatic adenocarcinoma includes surgical resection, along with neoadjuvant and/or adjuvant chemotherapy or chemotherapy alone, depending on whether the tumor or the disease is amenable to resection following initial diagnosis (3). Despite advancements in operative, anesthetic and chemotherapeutic fields, there has been little improvement in the patients' prognosis(1,2,5). Peritoneum is the second most common site of metastases among pancreatic adenocarcinoma patients and is found to have metastatic disease in >9% of the cases following initial diagnosis (19,38). A percentage of 42.5% of these cases are found to have peritoneum as the only site of metastases (38). Additionally, 9–12% of pancreatic adenocarcinoma patients are found to have peritoneal metastases as the only metastatic site during staging laparoscopy (39–41), Also 20–30% of pancreatic adenocarcinoma patients, with no metastases, have positive for malignant cells peritoneal washing cytology (18). Finally, one-third of the patients develop peritoneal metastases and 75% of them have local recurrence, following initial curative resection (19,20). In this respect, HIPEC, a method to deliver the chemotherapeutic agents intraperitoneally, may offer an alternative to improve survival in select patients with pancreatic adenocarcinoma (34).
The perioperative intraperitoneal administration of chemotherapy seems to provide high concentrations of the drug by targeting the site of disease, whilst detouring systemic side effects (42). Administering heated chemotherapy is thought to facilitate the process of cytotoxicity both by a direct effect on tumor cells and by potentiating the effect of the chemotherapeutic drug (42).
CRS/HIPEC has been used to treat PC for the past 35 years. Tumors that used to be considered unresectable may receive CRS/HIPEC with a clear survival benefit, depending mainly on the histology of the primary disease, the abdominal burden of the disease, expressed as peritoneal carcinomatosis index (PCI) score (43,44), and the completeness of cytoreduction (CC) expressed as CC-score (45), among other factors (22–24,27). One of the less investigated histologies is that of pancreatic adenocarcinoma. The purpose of this study was to determine whether HIPEC has a positive effect in the treatment of pancreatic adenocarcinoma. The results presented here demonstrate that no valid conclusions can be made, both for the concept of treating peritoneal carcinomatosis of pancreatic adenocarcinoma origin with CRS/HIPEC, and for the concept of adjuvant (prophylactic) HIPEC for non-peritoneal carcinomatosis resectable pancreatic adenocarcinoma. This is a hugely controversial subject in terms of effectiveness and safety, especially regarding the approach of CRS/HIPEC for PC of pancreatic origin.
Notably, there is a series of patients with prophylactic use of HIPEC after R0 resection of pancreatic adenocarcinoma without peritoneal disease (34). This approach is innovative in pancreatic cancer treatment. The survival results are among the highest in the pancreatic cancer literature. However, these results should be perceived with the greatest possible caution in terms of stage relative survival, reproducibility, morbidity, and mortality since the methodology of the study (no control group) is not able to strongly support the data.
A limitation of the present systematic review is the small sample size published on HIPEC and pancreatic adenocarcinoma. The exhaustive, systematic search of the databases only yielded 47 patients. This may be partially explained by the aggressive, rapid progression of the disease and short course from initial diagnosis to death, and the lack of symptoms at an early stage (3,4). In addition, the quality of studies was fair to low with different research questions and designs. Only two reports focused primarily on HIPEC or CRS/HIPEC in patients with pancreatic adenocarcinoma (34,35), while the other two studies included patients with different types of cancer who were treated with CRS/HIPEC (36,37), with characteristics of population and intervention partly mentioned. Despite the small sample size, analysis was performed on the selected studies in the hope of providing novel insight on HIPEC and pancreatic adenocarcinoma.
There were discrepancies associated with the in-hospital mortality of the four included studies (Table II). Notably, two different settings of HIPEC are used, one as adjuvant following curative resection in non-PC patients (34), and one as CRS/HIPEC for PC cases (35–37). From the data available, Tentes et al (34) reported, in 2016, that non-PC patients (TNM I: 4, TMN II: 9, TNM III: 20) had a mortality rate of 6.1%. These patients underwent curative resection of the primary tumor plus HIPEC. The 33.3% mortality rate reported in 2018, by Tentes et al (35), was associated with 2 synchronous and 4 metachronous PC cases, with a PCI between 3 and 23, who underwent CRS/HIPEC. Furthermore, the 0% mortality rate reported by Farma et al (36) and Fujimura et al (37) was associated with 3 synchronous and 5 metachronous PC cases, without data regarding PCI. Similar CC score rates were reported by Tentes et al (35) and Farma et al (36); however, there was no such information available by Fujimura et al (37). Thus, the differences in the reported mortality rates should be taken into consideration in relation to the burden of the intra-abdominal disease and the corresponding extent of the resections required. Notably, the above-mentioned mortality rates, which are very high either in the setting of non-PC patients (6,1%) or in the setting of patients with pancreatic origin PC (33.3%), are quite discouraging regarding CRS/HIPEC or HIPEC application in pancreatic adenocarcinoma patients. As of that, extreme caution should be spent in selecting patients for CRS-HIPEC with peritoneal metastases outside a proper designed study protocol.
HIPEC involves extensively alternating variables, and thus any attempt to study its unique entity, particularly in meta-analyses, should be performed with caution or avoided. The chemotherapy regimen constitutes of 1–3 drugs from a choice of at least four different agents, the temperature ranges from 41–43°C, the duration from 60–90 min and the volume of solution from 3,000-7,000 ml. Notably, comparisons between the outcomes in subgroups of different characteristics of the interventions have not yet been investigated. Thus, well-designed randomized clinical trials should focus on answering questions regarding the burden of disease that is amenable to HIPEC treatment, including which drug is the best, what is the optimal dosage and drug solution, and what is the optimal temperature. According to clinical trials registry (ClinicalTrials.gov), there are two registered clinical trials on pancreatic cancer and HIPEC. The first clinical trial (NCT02850874) designed to study HIPEC as neoadjuvant treatment in pancreatic adenocarcinoma was withdrawn due to no recruitment (46). The second clinical trial (NCT03251365) designed to study CRS/HIPEC for locally/regionally resectable pancreatic adenocarcinoma is still recruiting (47).
As more surgical oncology fellows are trained in these techniques and more centers offer this approach, it is unlikely that this specialized technique will be abandoned. HIPEC is one of several modalities used for intraperitoneal or bi-directional chemotherapy administration, the combination of which may be worthy for further investigation on the treatment of pancreatic adenocarcinoma (48). However, there are several randomized trials regarding HIPEC in other than pancreatic adenocarcinoma pathologies that should be mentioned; the negative results in the colorectal cancer prophylaxis setting (49) and colorectal PC setting (50,51) have been greatly challenged, mainly due to the drugs used for HIPEC (52–55); the positive (23) and negative (56) results regarding HIPEC in advanced ovarian cancer have been greatly challenged regarding their methodology and interpretation (57,58); the positive results in gastric cancer prophylactic HIPEC is also a relatively recent concept (29).
In conclusion, the body of evidence presented in this review is extremely limited and of low quality to effectively conclude the use of HIPEC as prophylaxis on resectable pancreatic adenocarcinoma; thus, further evidence is needed within a proper designed study protocol. However, the use of cytoreductive surgery plus HIPEC should be considered un-safe in patients with peritoneal carcinomatosis of pancreatic origin.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CRS
cytoreductive surgery
- CC
completeness of cytoreduction
- HIPEC
hyperthermic intraperitoneal chemotherapy
- MeSH
medical subject heading
- OS
overall survival
- PC
peritoneal carcinomatosis
- PCI
peritoneal carcinomatosis index
- PRISMA
preferred reporting items for systematic reviews and meta-analyses
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AL, GCZ and KGT contributed to the conception and design of the present review. AL, EA, GZV and KG contributed to the acquisition of data. AL and EA assessed the authenticity of all the raw data AL, EA, GZV, KG and CGZ contributed to the analysis and interpretation of the data. EA, KG and CGZ equally contributed to drafting the initial manuscript. AL, GCZ, GZV and KGT critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14:1320–1326. doi: 10.1245/s10434-006-9249-8. [DOI] [PubMed] [Google Scholar]
- 2.Beger HG, Rau B, Gansauge F, Leder G, Schwarz M, Poch B. Pancreatic Cancer - Low Survival Rates. Dtsch Arztebl Int. 2008;105:255–262. doi: 10.3238/arztebl.2008.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, et al. ESMO Guidelines Committee Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 4.Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: Basic and clinical aspects. Gastroenterology. 2005;128:1606–1625. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Sirri E, Castro FA, Kieschke J, Jansen L, Emrich K, Gondos A, Holleczek B, Katalinic A, Urbschat I, Vohmann C, et al. Recent Trends in Survival of Patients With Pancreatic Cancer in Germany and the United States. Pancreas. 2016;45:908–914. doi: 10.1097/MPA.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 6.Minicozzi P, Cassetti T, Vener C, Sant M. Analysis of incidence, mortality and survival for pancreatic and biliary tract cancers across Europe, with assessment of influence of revised European age standardisation on estimates. Cancer Epidemiol. 2018;55:52–60. doi: 10.1016/j.canep.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 8.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, Strasberg S, Hanna S, Taylor B, Langer B, et al. Prognostic factors in resected pancreatic adenocarcinoma: Analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R, Shapiro M, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2541–2556. doi: 10.1200/JCO.2016.67.5553. [DOI] [PubMed] [Google Scholar]
- 10.Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: An epidemiological study. Br J Surg. 1995;82:111–115. doi: 10.1002/bjs.1800820137. [DOI] [PubMed] [Google Scholar]
- 11.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. 2014;20:11142–11159. doi: 10.3748/wjg.v20.i32.11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller PC, Frey MC, Ruzza CM, Nickel F, Jost C, Gwerder C, Hackert T, Z'graggen K, Kessler U. Neoadjuvant Chemotherapy in Pancreatic Cancer: An Appraisal of the Current High-Level Evidence. Pharmacology. 2020:1–11. doi: 10.1159/000510343. doi: 10.1159/000510343. [DOI] [PubMed] [Google Scholar]
- 15.Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. doi: 10.1186/s12943-018-0858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spratt JS, Edwards M, Kubota T, Lindberg R, Tseng MT. Peritoneal carcinomatosis: Anatomy, physiology, diagnosis, management. Curr Probl Cancer. 1986;10:553–584. doi: 10.1016/S0147-0272(86)80009-5. [DOI] [Google Scholar]
- 17.Sugarbaker PH, editor. Principles of Management. Cancer Treatment and Research. Vol. 82. Springer; Boston, MA: 1996. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology; pp. 79–100. [DOI] [PubMed] [Google Scholar]
- 18.del Castillo CF, Warshaw L. Peritoneal metastases in pancreatic carcinoma. Hepatogastroenterology. 1993;40:430–432. [PubMed] [Google Scholar]
- 19.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 20.Hishinuma S, Ogata Y, Matsui J, Ozawa I. Results of surgery and adjuvant radiotherapy for pancreatic cancer. J Hepatobiliary Pancreat Surg. 1998;5:143–150. doi: 10.1007/s005340050025. [DOI] [PubMed] [Google Scholar]
- 21.Sugarbaker P. Cytoreductive Surgery and Perioperative Chemotherapy for Peritoneal Surface Malignancy. Ciné-Med, Inc.; Woodbury, CT, USA: 2012. An overview of peritonectomy, visceral resections, and perioperative chemotherapy for peritoneal surface malignancy; pp. 1–30. [Google Scholar]
- 22.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–2456. doi: 10.1200/jco.2012.30.4_suppl.532. [DOI] [PubMed] [Google Scholar]
- 23.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 24.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P, Mohamed F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J Clin Oncol. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 25.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 26.Elias D, Blot F, El Otmany A, Antoun S, Lasser P, Boige V, Rougier P, Ducreux M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. doi: 10.1002/1097-0142(20010701)92:1<71::AID-CNCR1293>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 28.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 29.Beeharry MK, Zhu Z-L, Liu W-T, Yao X–X, Yan M, Zhu Z-G. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: Personal experience from a randomized case control study. BMC Cancer. 2019;19:932–932. doi: 10.1186/s12885-019-6411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X-J, Huang C-Q, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JP, Altman DG, Sterne JA. Assessing risk of bias in included studies. Higgins JP, Green S, The Cochrane Collaboration, corp-author, editors. https://handbook-5-1.cochrane.org. [Oct 17;2019 ];Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. doi: 10.1002/9781119536604. [DOI]
- 34.Tentes A-A, Stamou K, Pallas N, Karamveri C, Kyziridis D, Hristakis C. The effect of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) as an adjuvant in patients with resectable pancreatic cancer. Int J Hyperthermia. 2016;32:895–899. doi: 10.1080/02656736.2016.1227094. [DOI] [PubMed] [Google Scholar]
- 35.Tentes A-A, Pallas N, Karamveri C, Kyziridis D, Hristakis C. Cytoreduction and HIPEC for peritoneal carcinomatosis of pancreatic cancer. J BUON. 2018;23:482–487. [PubMed] [Google Scholar]
- 36.Farma JM, Pingpank JF, Libutti SK, Bartlett DL, Ohl S, Beresneva T, Alexander HR. Limited survival in patients with carcinomatosis from foregut malignancies after cytoreduction and continuous hyperthermic peritoneal perfusion. J Gastrointest Surg. 2005;9:1346–1353. doi: 10.1016/j.gassur.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Fujimura T, Yonemura Y, Fujita H, Michiwa Y, Kawamura T, Nojima N, Sato T, Fushida S, Nishimura G, Miwa K, et al. Chemohyperthermic peritoneal perfusion for peritoneal dissemination in various intra-abdominal malignancies. Int Surg. 1999;84:60–66. [PubMed] [Google Scholar]
- 38.Thomassen I, Lemmens VE, Nienhuijs SW, Luyer MD, Klaver YL, de Hingh IH. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: A population-based study. Pancreas. 2013;42:72–75. doi: 10.1097/MPA.0b013e31825abf8c. [DOI] [PubMed] [Google Scholar]
- 39.Conlon KC, Dougherty E, Klimstra DS, Coit DG, Turnbull AD, Brennan MF. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223:134–140. doi: 10.1097/00000658-199602000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merchant NB, Conlon KC. Laparoscopic evaluation in pancreatic cancer. Semin Surg Oncol. 1998;15:155–165. doi: 10.1002/(SICI)1098-2388(199810/11)15:3<155::AID-SSU4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB., Jr MDCT in Pancreatic adenocarcinoma: Prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182:419–425. doi: 10.2214/ajr.182.2.1820419. [DOI] [PubMed] [Google Scholar]
- 42.González-Moreno S, González-Bayón LA, Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol. 2010;2:68–75. doi: 10.4251/wjgo.v2.i2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005;2:3. doi: 10.1186/1477-7800-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portilla AG, Sugarbaker PH, Chang D. Second-look surgery after cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: Analysis of prognostic features. World J Surg. 1999;23:23–29. doi: 10.1007/s002689900560. [DOI] [PubMed] [Google Scholar]
- 45.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–731. doi: 10.1007/s10434-999-0727-7. [DOI] [PubMed] [Google Scholar]
- 46.HIPEC as Neoadjuvant Treatment for Resectable Pancreatic Adenocarcinoma. Clinicaltrials.gov. [Jul 28;2019 ]; NCT02850874.
- 47.Intrabdominal Hyperthermic Chemotherapy and Pancreatic Cancer. Clinicaltrials.gov. [Jul 28;2019 ]; NCT03251365.
- 48.Péron J, Giai J, Maucort-Boulch D, Buyse M. The Benefit-Risk Balance of Nab-Paclitaxel in Metastatic Pancreatic Adenocarcinoma. Pancreas. 2019;48:275–280. doi: 10.1097/MPA.0000000000001234. [DOI] [PubMed] [Google Scholar]
- 49.Klaver CEL, Wisselink DD, Punt CJA, Snaebjornsson P, Crezee J, Aalbers AGJ, Brandt A, Bremers AJA, Burger JWA, Fabry HFJ, et al. COLOPEC collaborators group Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4:761–770. doi: 10.1016/S2468-1253(19)30239-0. [DOI] [PubMed] [Google Scholar]
- 50.Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol. 2018;36(Suppl. 18):LBA3503–LBA3503. doi: 10.1200/JCO.2018.36.18_suppl.LBA3503. [DOI] [Google Scholar]
- 51.Goéré D, Glehen O, Quenet F, Guilloit JM, Bereder JM, Lorimier G, Thibaudeau E, Ghouti L, Pinto A, Tuech JJ, et al. BIG-RENAPE group Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020;21:1147–1154. doi: 10.1016/S1470-2045(20)30322-3. [DOI] [PubMed] [Google Scholar]
- 52.Koh CE, Ansari N, Morris D, Moran B, Australian and New Zealand Peritoneal Malignancy Collaborative (ANZ PMC) Beware mis-representation of PRODIGE 7: Danger of throwing out the cytoreductive surgery baby with the hyperthermic intraperitoneal chemotherapy bathwater. ANZ J Surg. 2019;89:992–994. doi: 10.1111/ans.15424. [DOI] [PubMed] [Google Scholar]
- 53.Nagourney RA, Evans S, Tran PH, Nagourney AJ, Sugarbaker PH. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur J Surg Oncol. 2020 doi: 10.1016/j.ejso.2020.09.017. S0748-7983(20)30789-7. doi: 10.1016/j.ejso.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Lei ZY, Guan TP, Luo JL, Tang HS, Cui SZ. Rationality of performing hyperthermic intraperitoneal chemotherapy 5–8 weeks after primary tumor resection for patients with locally advanced colorectal cancer-based on COLOPEC. Zhonghua Wei Chang Wai Ke Za Zhi. 2019;22:1115–1117. doi: 10.3760/cma.j.issn.1671-0274.2019.12.004. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 55.Segura-Sampedro JJ, Morales-Soriano R. Prophylactic HIPEC with oxaliplatin might be of benefit in T4 and perforated colon cancer: Another possible interpretation of the COLOPEC results. Rev Esp Enferm Dig. 2020;112:666. doi: 10.17235/reed.2020.6755/2019. [DOI] [PubMed] [Google Scholar]
- 56.Lim MC, Chang S-J, Yoo HJ, Nam B-H, Bristow R, Park S-Y. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J Clin Oncol. 2017;35(Suppl 15):5520–5520. doi: 10.1200/JCO.2017.35.15_suppl.5520. [DOI] [Google Scholar]
- 57.de Bree E, Michelakis D. An overview and update of hyperthermic intraperitoneal chemotherapy in ovarian cancer. Expert Opin Pharmacother. 2020;21:1479–1492. doi: 10.1080/14656566.2020.1766024. [DOI] [PubMed] [Google Scholar]
- 58.Fotopoulou C, Sehouli J, Mahner S, Harter P, Van Nieuwenhuysen E, Gonzalez-Martin A, Vergote I, Chiva L, Du Bois A. HIPEC: HOPE or HYPE in the fight against advanced ovarian cancer? Ann Oncol. 2018;29:1610–1613. doi: 10.1093/annonc/mdy198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.