Abstract
The aim of the present study was to examine the protein expression levels of E-, N- and P-cadherin, which are involved in the proliferation of neoplastic cells, in cancer tissue from patients with endometrial cancer. Furthermore, the present study aimed to investigate the effect of these proteins on clinicopathological parameters. Immunohistochemistry was performed to detect the protein expression levels of the aforementioned cadherins in 38 primary endometrial tumors, 20 metastatic tumors (nine metastases to the lymph nodes and 11 distant metastases) and five cases of atypical hyperplasia as the control group. It was found that the E-, N- and P-cadherin proteins in hyperplastic endometrial lesions with atypia were weakly expressed in the cytoplasm, while the expression levels of E-, N- and P-cadherin proteins, in endometrial cancer tissue, were located in the membrane and/or in the cytoplasm, and was found to be unevenly distributed. Furthermore, increased expressed level of the three cadherin proteins was observed at the tumor front, as opposed to in the main mass, of endometrial cancer tumor. It was demonstrated that membrane expression levels of the 3 cadherin proteins were lower in metastatic cancer cells compared with that in the primary tumor cells. In addition, a significantly higher cytoplasmic expression level of E-cadherin and increased membranous and cytoplasmic expression of P-cadherin, were associated with high-grade tumor budding. Furthermore, a higher percentage of P-cadherin membrane expression level was associated with poorly differentiated cancer cell types. The present results suggested that the increased membrane expression level of E-cadherin was associated with the presence of local lymph node involvement.
Keywords: E-cadherin, endometrial cancer, N-cadherin, P-cadherin, tumor budding
Introduction
Endometrial cancer is the most common type of neoplasm affecting one of the female reproductive organs, and primarily occurs in postmenopausal women. It has been revealed that there is an increase in the incidence rate of endometrial cancer with age, particularly in developing countries. In 2006 to 2007, rates varied 10-fold across countries, with the highest rates in North America (United States), Eastern and Northern Europe (Slovakia), and the lowest rates were in middle-income countries (South Africa and India) (1). In 2012, endometrial cancer was the 6th most frequently diagnosed carcinoma, and the 14th most common cause of cancer-associated deaths in women worldwide (2). Furthermore, risk factors, such as early menarche, late menopause, infertility, taking menopausal hormones and obesity play a major role in endometrial cancer etiology (3).
Endometrial cancer has been associated with invasiveness and metastasis formation, which are multistage processes in which malignant cells detach from the primary tumor mass, travel via the lymph and blood to target tissues, where tumor cells can adhere to and penetrate the vascular endothelium; thus, forming new blood vessels in the developing neoplastic foci. Each of these stages requires specific interactions between cancer cells and the surrounding intercellular substance, and also with endothelial cells of blood and lymphatic vessels (4). These interactions are mediated by specific adhesion molecules, such as cadherins, which constitute a group of 100 classical and non-classical cadherins. E- and N-cadherin are well-known classical cadherins and can mediate homotypic intercellular interactions by interacting in a Ca2+-dependent manner with the same cadherin types on adjacent cells (5). E-cadherin is typically located on epithelial cell membranes, and promotes cell adhesion and integrity; thus, mediates differentiation of healthy epithelial tissue architecture (6). Furthermore, N-cadherin is characteristic of mesenchymal cells, particularly in cells with greater motility and reduced polarization. It has been shown that N-cadherins are representative markers of epithelial-mesenchymal transformation (EMT) (6,7). P-cadherin is present at adherens junctions, and is found, similar to E-cadherin, in several mature tissue types, including the epidermis, breast, prostate and mesothelium (8–10). Cadherin switching, which is important for the formation of the cell phenotype, occurs during normal processes of cell development (11). Furthermore, switching of E-cadherin to N-cadherin has been observed in carcinomas of the prostate (12), breast (13) and melanoma (14), whereas changes to P-cadherin were found in pancreatic (15) or gastric cancer types (16).
Therefore, the aim of the present study was to examine the protein expression levels of E-, N- and P-cadherin in cancer tissue from patients with endometrial cancer. In addition, the present study investigated the association of these proteins with clinicopathological parameters.
Materials and methods
Patients and tissue samples
The present study enrolled 38 patients (mean age 68.6 years; range 44–84 years old) (Table I) with endometrial cancer, who underwent hysterectomy at the Medical Center ‘Żelazna’ in Warsaw (Poland) between January 2008 and December 2015. The inclusion criteria for the study group was endometrial cancer in any of The International Federation of Gynecology and Obstetrics (FIGO) stages (17) with full histopathological documentation. The exclusion criteria were any cases with unspecified status of lymph node or distant organ involvement and refusal to participate. The postoperative tissue was fixed in 10% neutral, buffered formalin for 24 h at room temperature and embedded in paraffin. Sections (4-µm) were cut from the paraffin blocks and stained with hematoxylin for 5 min and eosin for 1 min at room temperature. Slides were assessed under a light microscope Olympus BX40 under magnifications ×200 and ×400. Routine histopathological analysis was performed to determine tumor histological type, malignancy grade (G), staging according to FIGO, the presence of tumor budding, local lymph node involvement and the presence of distant metastases (ovary, vagina and colon) (17) (Table I). Five sections with atypical endometrial proliferation from resected material of the study group were used as controls. A total of 12 patients (out of 38) had metastases to the lymph nodes and 8 to distant organs (2 of them had metastases to two or more organs at the same time). Tumor budding was counted in 5 high-power fields (magnification, ×40), and were classified as low-grade (<5 buds) or high-grade (≥5 buds; Table I). The study was approved by the local Bioethics Committee (Medical University of Bialystok; approval no. R-I-002/68/2016) and written informed consent, regarding the use of the tissue, was provided by each patient in the study.
Table I.
Clinicopathological characteristics of the patients with endometrial cancer.
| Clinicopathological parameters | Number (%) |
|---|---|
| Age, years | |
| <65 | 19 (50) |
| ≥65 | 19 (50) |
| Tumor type | |
| Endometrioid | 31 (81.6) |
| Serous | 7 (18.4) |
| Tumor grade, differentiated | |
| Well | 4 (10.5) |
| Medium | 25 (65.8) |
| Poorly | 9 (23.7) |
| Tumor stage | |
| I | 15 (39.5) |
| II | 3 (7.9) |
| III | 15 (39.5) |
| IV | 5 (13.1) |
| Tumor budding, grade | |
| Low | 27 (71) |
| High | 11 (29) |
| pN | |
| Absent | 26 (68.4) |
| Present | 12 (31.6) |
| pM | |
| Absent | 30 (78.9) |
| Present | 8 (21.1) |
Immunohistochemistry
Immunohistochemical analyses were performed using 38 primary endometrial tumors tissues and 20 metastatic tissues: 9 metastases to the lymph nodes (3 were removed due to micrometastases) and 11 distant metastases (7 ovaries, 2 vaginas and 2 colons). For comparison, five cases of atypical endometrial proliferation were also stained. Tissue blocks were cut on a microtome into 4-µm thick sections on silanized glass slides. The sections were deparaffinized in xylene and hydrated in a series of alcohols of decreasing concentration (two treatments with 99.9, then 96 and 70% ethanol) at room temperature. Then, sections were heated in a microwave oven for 15 min in citrate buffer (pH, 6.0) for antigen retrieval. For blocking endogenous peroxidase activity 3% hydrogen peroxide solution was used for 10 min at room temperature. For blocking non-specific antibody binding horse serum was used (anti-mouse/rabbit serum produced in horse; Vector Laboratories, Inc.) for 10 min at room temperature. Next, the sections were incubated with murine monoclonal anti-E-cadherin (cat. no. 36B5; 1:100; Leica Microsystems GmbH), anti-N-cadherin (cat. no. IAR06; 1:100; Leica Microsystems GmbH) and anti-P-cadherin (cat. no. HPA001767; 1:100; Sigma-Aldrich; Merck KGaA) antibodies for 60 min at room temperature. Next, the one-step system ImmPRESS™ Universal Antibody Polymer Reagent (30 min at room temperature; catalog no. : MP-7500; Vector Laboratories, Inc.) and chromogen ImmPACT DAB (5 min at room temperature; catalog no. : SK-4105, Vector Laboratories, Inc.) were used. Cellular nuclei were stained with hematoxylin for 5 min at room temperature. Positive and negative controls were performed according to the manufacturer's protocol (Leica Microsystems, Inc.; Sigma-Aldrich; Merck KGaA).
The results were determined by two independent pathologists (Department of General Pathomorphology, Medical University of Bialystok) under a light microscope. Protein expression was observed at random using 10 fields of view (FOV) and a high-power lens (magnification, 10×40), with each FOV counting ≥100 cells. The expression level was observed as cytoplasmic, membranous or mixed, and was classified as positive in the cytoplasm (only cytoplasmic reaction was visible) or positive in the membrane (only membranous or mixed reaction). The cytoplasm or membrane staining was calculated based on the percentage of immunoreactive cells and a range 1–100% indicated positive expression. Data obtained from the immunohistochemical analysis are presented as the mean percentage of the expression level.
Statistical analysis
Statistical analyses were conducted using the 38 primary tumors and 20 metastatic tumors of endometrial cancer. The Statistica 11 (v4.0; StatSoft; TIBCO Software, Inc.) and GraphPad Prism (v5.04; GraphPad Software, Inc.) programs were used for statistical analysis. The protein expression levels were compared between two groups using the Mann-Whitney U test. E-, N-, P-cadherin protein expression levels did not follow a normal distribution and therefore non-parametric statistical analyses were performed. Spearman's rank correlation analysis was used to assess the correlation between membrane and cytoplasmic E-, N-, P-cadherin expression level in primary and metastatic tumor of endometrial cancer. The association between membrane and cytoplasmic E-, N-, P-cadherin protein expression level between the primary tumor and the clinicopathological features of patients with endometrial cancer was analyzed using the Mann-Whitney test for two groups, while the Kruskal-Wallis test was used for three or more groups plus Dunn's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
E-, N- and P-cadherin protein expression levels in endometrial hyperplasia with atypia
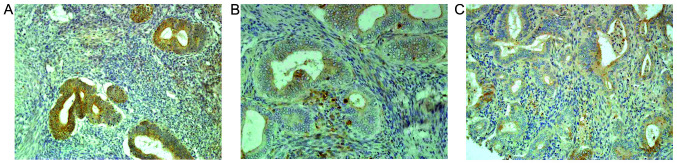
In hyperplastic endometrial lesions, the protein expression levels of E-, N- and P-cadherin were similar, and weak cytoplasmic staining was observed. Furthermore, the expression level was equally distributed throughout the hyperplastic epithelium. The membrane staining of E-cadherin was present in 3/5 cases (Fig. 1A), whereas that of P-cadherin was only observed in a few cells and was <10% (Fig. 1C). In addition, the protein expression level of N-cadherin was found to be negative (Fig. 1B).
Figure 1.
Comparison of E-, N-, P-cadherin expression levels in endometrial hyperplasia with atypia. (A) E-cadherin (magnification, ×100), (B) N-cadherin (magnification, ×200) and (C) P-cadherin (magnification, ×200) expression levels in endometrial hyperplasia with atypia. E-, N- and P-cadherin expression levels were similar and weak cytoplasmic staining was observed. Expression was equally distributed throughout the hyperplastic epithelium. The membrane staining of E-cadherin was present in 3/5 cases, whereas that of P-cadherin was only observed in few cells and was <10%. The expression of N-cadherin was absent.
E-, N- and P-cadherin protein expression levels in the primary tumor and metastatic tissues in endometrial cancer
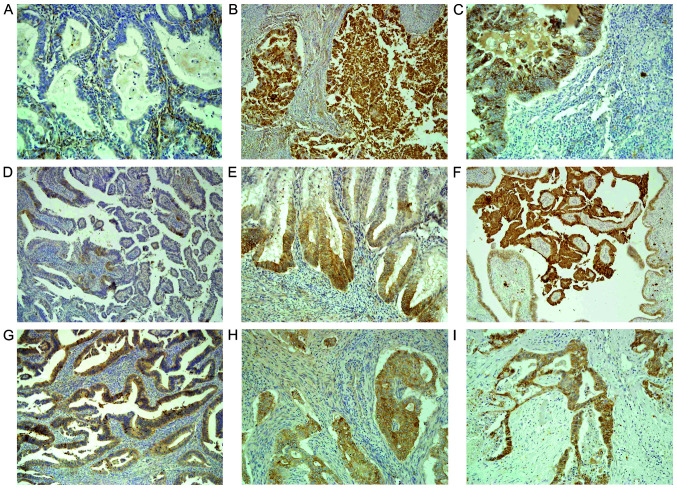
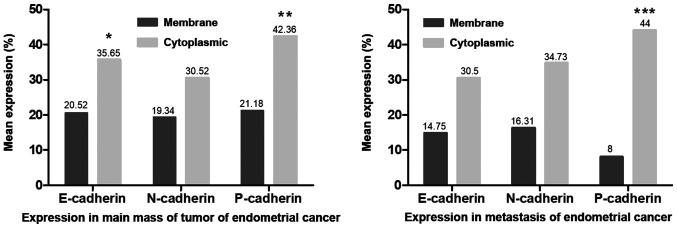
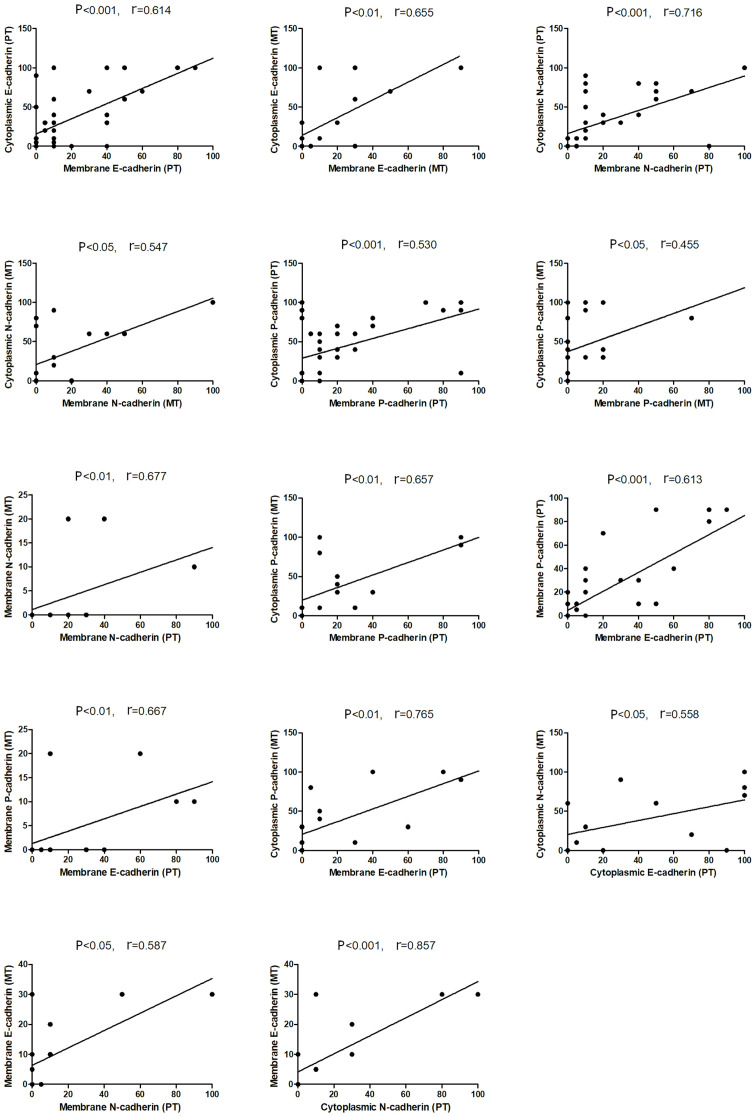
The present results indicated that the three cadherin proteins were expressed either in the membrane and/or in the cytoplasm and were unevenly distributed in endometrial cancer tissue (Fig. 2A, B, D, E, G and H). Furthermore, the staining of the three cadherin proteins was stronger at the tumor front compared with that in the main tumor mass (N-cadherin staining; Fig. 2D and E). The cytoplasmic expression level was significantly higher compared with that in the membrane, with respect to E-cadherin (P<0.05) and P-cadherin (P<0.01) in the primary tumor tissues, while P-cadherin expression level was also significantly higher in the metastatic tissue (P<0.001) (Fig. 3). Furthermore, it was found that the membrane expression levels of the 3 cadherin proteins were lower in metastatic cancer cells compared with that in the primary tumor cells (Fig. 3). Moreover, a decrease in the membranous protein expression level of P-cadherin was found in the metastatic tumor (mean expression, 8%) compared with that in the primary endometrial cancer tissue (mean expression, 21.18%), although these findings were not statistically significant (Fig. 3). As shown in Fig. 4, the protein expression levels of cytoplasmic E-, N- and P-cadherin were also correlated with membrane expression level the in primary and metastatic tumors. Furthermore, positive correlations were found between membrane N-cadherin expression level in the primary tumor and with the membrane expression level in the metastatic tumor, also between membrane P-cadherin expression level in the primary tumor and with the membrane expression level in the metastatic tumor, and between membrane P-cadherin expression level in the primary tumor and with cytoplasmic expression level in the metastatic tumor. In addition, correlations were also identified between membrane E-cadherin and membrane P-cadherin expression level in the primary tumor; membrane P-cadherin in metastatic tumor and membrane E-cadherin in primary tumor; cytoplasmic P-cadherin in metastatic tumor and membrane E-cadherin in primary tumor, cytoplasmic N-cadherin in metastatic tumor and cytoplasmic E-cadherin in primary tumor; membrane E-cadherin in metastatic tumor and membrane N-cadherin in primary tumor; and also membrane E-cadherin in metastatic cancer and cytoplasmic N-cadherin in primary tumor (Fig. 4).
Figure 2.
E-, N-, P-cadherin expression levels in the primary and metastasis tissues from endometrial cancer. The 3 aforementioned cadherin proteins were expressed in the membranes and/or in the cytoplasm and were unevenly distributed in the endometrial cancer tissue. The staining of the three cadherin proteins were stronger at the tumor front compared with that in the main tumor mass. Cytoplasmic expression level was higher compared with that in the membrane. In the main mass of the primary tumor there was (A) negative and (B) strong cytoplasmic E-cadherin staining in cancer cells (magnification, ×200 and ×100, respectively). (C) Positive E-cadherin expression level in the metastatic cancer cells in the lymph node. Magnification, ×200. (D) Cytoplasmic N-cadherin expression level was found in a few cells in the main mass of the tumor. Magnification, ×100. (E) Stronger membranous and cytoplasmic N-cadherin expression level at the tumor front. Magnification, ×200. (F) N-cadherin expression level in the metastasis tissue of the ovary. Magnification, ×100. (G) Weak (magnification, ×200) and (H) medium cytoplasmic P-cadherin expression level in main mass of the primary tumor (magnification, ×200). (I) P-cadherin expression level in the metastasis to the vagina. Magnification, ×100.
Figure 3.
Comparison of E-, N-, P-cadherin expression levels in main mass of the tumor and in the metastasis tissue of endometrial cancer. Comparison of protein expression levels in groups was performed using a Student's t-test. *P<0.05 membrane vs. cytoplasmic E-cadherin expression level in the primary tumor. **P<0.01 membrane vs. cytoplasmic P-cadherin expression level in the primary tumor. ***P<0.001 membrane vs. cytoplasmic P-cadherin level expression in the metastasis tissue. Non-statistically results are omitted.
Figure 4.
Correlation analysis between E-, N-, P-cadherin expression levels in the PT and MT of endometrial cancer. Only statistically significant correlations are presented. Spearman's rank correlation analysis was used. PT, primary tumor; MT, metastatic tumor.
Association between E-, P- and N-cadherin protein expression levels in primary endometrial cancer and clinicopathological parameters
The present results suggested that there were no significant associations between the age of the patients, histological type of the tumor, FIGO grade and the presence of distant metastases and the E-, N- and P-cadherin protein expression levels (Table II). Furthermore, a significant association was found between the membrane expression level of P-cadherin and endometrial carcinoma grade; a higher percentage of P-cadherin membrane expression level was associated with histologically poorly differentiated cancer types (P=0.023). In addition, a significant association was found between high-grade tumor budding and higher cytoplasmic expression level of E-cadherin (P=0.042), and higher membrane and cytoplasmic expression levels of P-cadherin (P=0.012 and P=0.002, respectively). The present results indicated that a higher membrane expression of E-cadherin was also associated with high-grade tumor budding; however, the result was not statistically significant. Furthermore, increases in the membrane expression level of E-cadherin was associated with the presence of local lymph node involvement (P=0.044; Table II).
Table II.
Association between E-, P- and N-cadherin protein expression level in the primary tumor tissue and clinicopathological parameters.
| E-cadherin | N-cadherin | P-cadherin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Membrane | Cytoplasmic | Membrane | Cytoplasmic | Membrane | Cytoplasmic | ||||||||
| Clinicopathological parameters | Number | Mean percentage | P-value | Mean percentage | P-value | Mean percentage | P-value | Mean percentage | P-value | Mean percentage | P-value | Mean percentage | P-value |
| Age, years | 0.752 | 0.315 | 0.904 | 0.247 | 0.940 | 0.410 | |||||||
| <65 | 19 | 19.5 | 40.3 | 19.7 | 36.3 | 17.1 | 47.4 | ||||||
| ≥65 | 19 | 21.6 | 31.3 | 18.9 | 24.7 | 25.3 | 37.4 | ||||||
| Tumor type | 0.522 | 0.746 | 0.449 | 0.329 | 0.061 | 0.436 | |||||||
| Endometrioid | 31 | 21.9 | 35.9 | 19.2 | 28.7 | 24.7 | 44.2 | ||||||
| Serous | 7 | 14.3 | 34.3 | 20 | 38.6 | 5.7 | 34.3 | ||||||
| Tumor grade, differentiated | 0.521 | 0.383 | 0.277 | 0.331 | 0.023a | 0.595 | |||||||
| Well | 4 | 15 | 26.7 | 40 | 40 | 8.3 | 36.7 | ||||||
| Medium | 25 | 18.9 | 32.5 | 15.7 | 29.1 | 15 | 35.9 | ||||||
| Poorly | 9 | 33.7 | 61.2 | 30 | 32.5 | 41.2 | 50 | ||||||
| Tumor stage | 0.924 | 0.938 | 0.063 | 0.373 | 0.620 | 0.236 | |||||||
| I | 15 | 17 | 30.3 | 20.7 | 31.3 | 13.7 | 33.3 | ||||||
| II | 3 | 6.7 | 18.3 | 23.3 | 40 | 13.3 | 23.3 | ||||||
| III | 15 | 25 | 41 | 23 | 34.7 | 28 | 48 | ||||||
| IV | 5 | 26 | 46 | 2 | 10 | 28 | 64 | ||||||
| Tumor budding, grade | 0.153 | 0.042a | 0.288 | 0.432 | 0.012a | 0.002a | |||||||
| Low | 27 | 15.5 | 26.8 | 15 | 27 | 14.2 | 31.5 | ||||||
| High | 11 | 32.7 | 57.3 | 30 | 39.1 | 38.2 | 69.1 | ||||||
| pN | 0.044a | 0.556 | 0.323 | 0.786 | 0.122 | 0.303 | |||||||
| Absent | 26 | 14.8 | 34.4 | 22.1 | 28.5 | 15.6 | 38.5 | ||||||
| Present | 12 | 32.9 | 38.3 | 13.3 | 35 | 33.3 | 50.8 | ||||||
| pM | 0.506 | 0.293 | 0.171 | 0.135 | 0.239 | 0.104 | |||||||
| Absent | 30 | 18 | 31.7 | 19.8 | 34 | 17.8 | 37.3 | ||||||
| Present | 8 | 30 | 50.6 | 17.5 | 17.5 | 33.7 | 61.2 | ||||||
P<0.05. A Mann-Whitney test was used to compare 2 groups, while the Kruskal-Wallis test and Dunn's post hoc test was used for >3 groups.
Discussion
E-cadherin has been widely investigated and is therefore the best described cadherin. Previous studies have shown that in the healthy endometrium, the expression level of E-cadherin was moderate to strong in 60–90% of cases and did not differ between the proliferative and secretion phases (18–20). Furthermore, E-cadherin was stained in the membrane, and was located on the borders between polarized cells (18–20). Nguyen et al (21) revealed that N- and P-cadherin were found in the endometrium. In addition, N-cadherin was primarily visualized in the apical surface and at the lateral junctions of the plasma membrane of the epithelial cells in the basalis, while it was strongest in the basalis glands adjacent to the myometrium and found to a lesser extent in the functional glands. However, P-cadherin was shown to be located in the basal surface of epithelial glands in both the functionalis and basalis (21).
The present results suggested that E-, N- and P-cadherins in hyperplastic endometrial lesions with atypia were equally weakly expressed in the cytoplasm. Furthermore, the membrane staining was observed in a few cells, with <10%. The protein expression levels of E-, N- and P-cadherins in endometrial cancer were located in the membrane and/or in the cytoplasm, and were unevenly distributed in the neoplastic tissue. Furthermore, stronger staining of the three cadherins was identified at the tumor front compared with that in the main mass of endometrial cancer tissue. However, no significant differences were found in E-cadherin protein expression level between primary and metastasis tumors. The present results were consistent with those from previous studies, which have shown similar cadherin expression levels. Ahmed and Muhammad (18) revealed membranous protein expression level of E-cadherin in non-neoplastic endometrial lesions, along with proliferative, secretory and hyperplastic endometrial changes, while neoplastic endometrial lesions showed mixed membranous-cytoplasmic staining. In addition, Carico et al (19) showed that E-cadherin protein expression level in normal endometrial growth was reduced, but was not homogenous. However, in atypical endometrium and in endometrial cancer cells, the membranous and cytoplasmic protein expression level of E-cadherin was weaker compared with normal endometrial cells. Furthermore, various regions of neoplastic tissue (for example tumor front, main mass of tumor and free-floating tumor cells in the ascitic fluid) showed differentiated expression level of E-cadherin, which reflected the heterogeneity of the neoplastic epithelium (21–24). Therefore, the loss of E-cadherin interaction with the cadherin-catenin complex could be attenuated at an early stage of the hyperplastic process and could be involved in endometrial cancer progression.
Only one study compared expression of N-cadherin in normal and neoplastic cells of uterus. Xie et al (20) observed moderate and strong N-cadherin protein expression level in endometrial cancer, compared with low or moderate expression level in normal endometrial epithelium. Comparison of N-cadherin expression with E-cadherin expression in endometrial cancer did not reveal any statistical significance (20). The present study found differences in the protein expression level of cytoplasmic N-cadherin in metastatic and cytoplasmic E-cadherin in primary tumors; membrane E-cadherin in metastatic and membrane N-cadherin in primary tumors and also membrane E-cadherin in metastatic cancer and cytoplasmic N-cadherin in primary tumors.
It has been demonstrated that P-cadherin protein expression level is increasing in endometrial cancer cells in comparison with normal cells. Moreno-Bueno et al (24) identified positive P-cadherin expression in <10% cases of atypical endometrial hyperplasia. Furthermore, P-cadherin staining was higher in endometrioid cancer types and in non-endometrioid neoplasms, accounting for ~46% of cases. However, positive expression level of P-cadherin was considered when ≥10% of cells had immunohistochemical staining, due to low expression of this protein. The present results suggested that the membranous protein expression level of P-cadherin was similar to that of E- and N-cadherin, whereas its cytoplasmic expression was significantly higher. Furthermore, a decrease in the membranous protein expression level of P-cadherin was observed in the metastatic tumor compared with that in the primary endometrial cancer tissue, although these findings were not statistically significant.
However, changes in the expression levels of 2 of the cadherin proteins were associated with clinicopathological factors in endometrial carcinoma. The present results indicated that higher cytoplasmic protein expression level of E-cadherin and increased membranous and cytoplasmic expression level of P-cadherin, was associated with high-grade tumor budding. Furthermore, Koyuncuoglu et al (25) conducted a similar analysis of the association between E-cadherin and tumor budding, revealing that its positive expression was higher in low-grade tumor budding, even though the results were not significant. While loss of membranous E-cadherin expression level was frequently observed, histological analysis of the immunohistochemical reaction identified higher expression at the tumor front in the present study research. In other carcinomas, such as colorectal cancer, a decrease in membranous E-cadherin has been shown at the tumor front, which allows for the loss of stability of intercellular junctions and enables cells to detach from the main tumor mass. Thus, in colorectal cancer epithelial-mesenchymal transformation occurs during tumor budding (26,27). The present results indicated the opposite E-cadherin protein expression level in endometrial cancer, which suggested that EMT does not occur in tumor budding of endometrial cancer. However, this requires verification in further studies, also detailed analysis of E-cadherin expression at the front and in the main mass of endometrial cancer.
In addition, it was found that the increase in the membranous protein expression level of E-cadherin in endometrial cancer was associated with local lymph node involvement. However, there was no statistically significant association between E-cadherin expression level and local lymph node metastases. Previous studies have shown associations between E-cadherin and other prognostic factors of endometrial cancer; however, the results have been inconsistent. Ahmed and Muhammad (18) revealed an association between lower E-cadherin expression level and infiltration of lymphatic and blood vessels in endometrial cancer cells. In addition, Koyuncuoglu et al (25) identified an association between low E-cadherin protein expression level and FIGO stage III+IV, compared with that in stage I+II. However, a meta-analysis investigating the reduced expression level of E-cadherin in endometrial cancer revealed a statistically significant association with total postoperative survival time (28). Thus, female patients with endometrial cancer and a reduced E-cadherin expression level may have poorer prognosis, compared with that in patients with endometrial cancer and normal or higher E-cadherin expression levels.
Furthermore, the present results suggested that the higher percentage of P-cadherin membrane protein expression level was associated with histologically poorly differentiated cancer types, which was in line with Piura et al (29). However, Stefansson et al (30) revealed an association between higher P-cadherin expression level and high FIGO grade, increasing FIGO stage, vascular invasion and depth of myometrial invasion.
The present study found no association between N-cadherin protein expression level and clinicopathological parameters. However, Singh et al (31) showed that higher protein expression level of N-cadherin was more frequent in non-endometrial cancer types. Furthermore, Xie et al (20) showed that positive N-cadherin protein expression level was associated with infiltration depth, higher FIGO stage and lower histological differentiation of the tumor.
The present study has a limitation. For the control group, endometrial hyperplasia with atypia was chosen from the vicinity of the tumor obtained during standard surgical procedures. A total of 5 sections of atypical endometrial proliferation were analyzed qualitatively to visualize differences with the cancer cells. For significant differences a larger sample size should be considered to ensure a representative distribution.
In conclusion, the present results indicated the involvement of the cadherin family adhesion proteins in the development of endometrial cancer. Furthermore, loss of E-cadherin membrane protein expression level and the appearance of membrane-cytoplasmic expression levels were identified. Contrary to E-cadherin, an increase in membrane and cytoplasmic staining of N- and P-cadherin proteins in endometrial cancer was found. Therefore, differences in the protein expression levels of the cell adhesion molecules may be involved in differentiation of the histological type of the tumor and the formation of tumor budding. Thus, the present results may provide potential prognosis targets, particularly with respect to changes in P-cadherin expression level in cancer cells, which may be associated with tumor budding and aggressiveness.
Acknowledgements
Not applicable.
Funding Statement
The study was supported by the Medical University of Bialystok, Poland (grant no. N/ST/ZB/16/007/3314).
Funding
The study was supported by the Medical University of Bialystok, Poland (grant no. N/ST/ZB/16/007/3314).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author's contributions
ŁL performed the experiment and analyzed data, and was a major contributor in writing the manuscript. AP performed the experiment, analyzed data and prepared figures. KGU designed the experiment, analyzed and interpreted the data. AP and KGU confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the local Bioethics Committee (Medical University of Bialystok; approval no. R-I-002/68/2016) and written informed consent, regarding the use of tissue, was provided by each patient in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. J Natl Cancer Inst. 2018;110:354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, editors. IARC CancerBase; Lyon: 2013. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 v1.0. No. 11. [Google Scholar]
- 3.Prat J, Franceschi S, Denny L, Lazcano Ponce E, Stewart BW, Wild CP. Endometrial cancer. In: Steward BW, Wild CP, editors. World Cancer Report 2014. International Agency for Research on Cancer; Lyon: 2014. pp. 465–481. [Google Scholar]
- 4.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Halbleib JM, Nelson WJ. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 6.van Roy F. Beyond E-cadherin: Roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014;14:121–134. doi: 10.1038/nrc3647. [DOI] [PubMed] [Google Scholar]
- 7.Pal M, Bhattacharya S, Kalyan G, Hazra S. Cadherin profiling for therapeutic interventions in epithelial mesenchymal transition (EMT) and tumorigenesis. Exp Cell Res. 2018;368:137–146. doi: 10.1016/j.yexcr.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Son H, Moon A. Epithelial-mesenchymal transition and cell invasion. Toxicol Res. 2010;26:245–252. doi: 10.5487/TR.2010.26.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda H, Tsukita S, Takeichi M. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev Biol. 1998;203:435–450. doi: 10.1006/dbio.1998.9047. [DOI] [PubMed] [Google Scholar]
- 10.Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Höfler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 12.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 13.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- 15.Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005;65:3092–3099. doi: 10.1158/0008.5472.CAN-04-3646. [DOI] [PubMed] [Google Scholar]
- 16.Shimoyama Y, Hirohashi S. Expression of E- and P-cadherin in gastric carcinomas. Cancer Res. 1991;51:2185–2192. [PubMed] [Google Scholar]
- 17.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed AR, Muhammad EM. E-cadherin and CD10 expression in atypical hyperplastic and malignant endometrial lesions. J Egypt Natl Canc Inst. 2014;26:211–217. doi: 10.1016/j.jnci.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Carico E, Atlante M, Giarnieri E, Raffa S, Bucci B, Giovagnoli MR, Vecchione A. E-cadherin and alpha-catenin expression in normal, hyperplastic and neoplastic endometrium. Anticancer Res. 2010;30:4993–4997. [PubMed] [Google Scholar]
- 20.Xie X, Zheng X, Wang J, Chen L. Clinical significance of Twist, E-cadherin, and N-cadherin protein expression in endometrioid adenocarcinoma. J Cancer Res Ther. 2017;13:817–822. doi: 10.4103/jcrt.JCRT_405_17. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen HP, Xiao L, Deane JA, Tan KS, Cousins FL, Masuda H, Sprung CN, Rosamilia A, Gargett CE. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum Reprod. 2017;32:2254–2268. doi: 10.1093/humrep/dex289. [DOI] [PubMed] [Google Scholar]
- 22.Veatch AL, Carson LF, Ramakrishnan S. Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int J Cancer. 1994;58:393–399. doi: 10.1002/ijc.2910580315. [DOI] [PubMed] [Google Scholar]
- 23.Risinger JI, Berchuck A, Kohler MF, Boyd J. Mutations of the E-cadherin gene in human gynecologic cancers. Nat Genet. 1994;7:98–102. doi: 10.1038/ng0594-98. [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Bueno G, Hardisson D, Sarrió D, Sánchez C, Cassia R, Prat J, Herman JG, Esteller M, Matías-Guiu X, Palacios J. Abnormalities of E- and P-cadherin and catenin (beta-, gamma-catenin, and p120ctn) expression in endometrial cancer and endometrial atypical hyperplasia. J Pathol. 2003;199:471–478. doi: 10.1002/path.1310. [DOI] [PubMed] [Google Scholar]
- 25.Koyuncuoglu M, Okyay E, Saatli B, Olgan S, Akin M, Saygili U. Tumor budding and E-cadherin expression in endometrial carcinoma: Are they prognostic factors in endometrial cancer? Gynecol Oncol. 2012;125:208–213. doi: 10.1016/j.ygyno.2011.12.433. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Choi SY, Kim WJ, Ji M, Lee TG, Son BR, Yoon SM, Sung R, Lee EJ, Youn SJ, Park SM. Combined aberrant expression of E-cadherin and S100A4, but not β-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn Pathol. 2013;8:99. doi: 10.1186/1746-1596-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karamitopoulou E, Zlobec I, Panayiotides I, Patsouris ES, Peros G, Rallis G, Lapas C, Karakitsos P, Terracciano LM, Lugli A. Systematic analysis of proteins from different signaling pathways in the tumor center and the invasive front of colorectal cancer. Hum Pathol. 2011;42:1888–1896. doi: 10.1016/j.humpath.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Zheng X, Du XL, Jiang T. Prognostic significance of reduced immunohistochemical expression of E-cadherin in endometrial cancer-results of a meta-analysis. Int J Clin Exp Med. 2015;8:18689–18696. [PMC free article] [PubMed] [Google Scholar]
- 29.Piura B, Rabinovich A, Aizenberg N, Wolfson M. Cadherins in malignancies of the female genital tract. Harefuah. 2005;144:261–265. 303, 302. (In Hebrew) [PubMed] [Google Scholar]
- 30.Stefansson IM, Salvesen HB, Akslen LA. Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol. 2004;22:1242–1252. doi: 10.1200/JCO.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Singh M, Darcy KM, Brady WE, Clubwala R, Weber Z, Rittenbach JV, Akalin A, Whitney CW, Zaino R, Ramirez NC, Leslie KK. Cadherins, catenins and cell cycle regulators: Impact on survival in a gynecologic oncology group phase II endometrial cancer trial. Gynecol Oncol. 2011;123:320–328. doi: 10.1016/j.ygyno.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.