Abstract
The geographical origin of watermelon (Citrullus lanatus) remains debated. While a first hypothesis suggests the center of origin to be West Africa, where the endemic sister species C. mucosospermus thrives, a second hypothesis suggests northeastern Africa where the white‐fleshed Sudanese Kordophan melon is cultivated. In this study, we infer biogeographical and haplotype genealogy for C. lanatus, C. mucosospermus, C. amarus, and C. colocynthis using noncoding cpDNA sequences (trnT‐trnL and ndhF‐rpl32 regions) from a global collection of 135 accessions. In total, we identified 38 haplotypes in C. lanatus, C. mucosospermus, C. amarus, and C. colocynthis; of these, 21 were found in Africa and 17 appear endemic to the continent. The least diverse species was C. mucosospermus (5 haplotypes) and the most diverse was C. colocynthis (16 haplotypes). Some haplotypes of C. mucosospermus were nearly exclusive to West Africa, and C. lanatus and C. mucosospermus shared haplotypes that were distinct from those of both C. amarus and C. colocynthis. The results support previous findings that revealed C. mucosospermus to be the closest relative to C. lanatus (including subsp. cordophanus). West Africa, as a center of endemism of C. mucosospermus, is an area of interest in the search of the origin of C. lanatus. This calls for further historical and phylogeographical investigations and wider collection of samples in West and northeastern Africa.
Keywords: biogeography, center of origin, Citrullus spp., colonization routes, cpDNA, Watermelon, West Africa
While the origin of watermelon is still debated, two endemic close relative are separately found in northeastern Africa and West Africa. Our paper presents the current chloroplast haplotypes distribution to infer the possible colonization route of watermelon using two cultivated and two wild relatives gathered from four continents.

1. INTRODUCTION
Watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai) is a cultivated species of high economic importance, accounting for nearly 103.9 million metric tons of global fruit production in 2018 from 3.2 million ha (FAOSTAT, 2017). Over the last two decades, questions regarding the origin and taxonomy of Citrullus spp. have fuelled numerous studies to clarify phylogenetic relationships and nomenclature, identify wild relatives, and determine both centers of origin and divergence times (Chomicki & Renner, 2015; Chomicki et al., 2020; Dane et al., 2004, 2007; Dane & Liu, 2007; Dje et al., 2010; Hammer & Gladis, 2014; Jarret et al., 1997; Jarret & Newman, 2000; Levi et al., 2001, 2004, 2013; Levi & Thomas, 2005; Mujaju et al., 2013; Nesom, 2011; Renner et al., 2019; Solmaz & Sari, 2009; Solmaz et al., 2010). Despite these efforts, uncertainty vis‐à‐vis these questions remains as no wild relatives were found neither in West nor in northern East Africa; and comparatively few studies have focused on the distribution of the genetic variation within Citrullus or the likely colonization routes of various species within the genus.
The challenge of tracing the historical colonization routes of watermelon was for many years confounded by significant taxonomic confusion among species, subspecies, and varieties, all of which exhibit high morphological diversity. Citrullus Schrad. ex Eckl & Zeyh. is one of 95 genera of Cucurbitaceae (Jeffrey, 2005; Kocyan et al., 2007; Schaefer & Renner, 2011a, 2011b). To date, there seems to be a consensus regarding its complex taxonomy. According to recent research, including phylogenetic analyses and nomenclatural reviews (Chomicki et al., 2020; Renner et al., 2014) as well as a phenetic comparison within the genus (Achigan‐Dako et al., 2015), Citrullus encompasses the following seven species: (a) the widely cultivated C. lanatus, a juicy fruit found in tropical and subtropical climates including var. cordophanus (Ter‐Avan.) Fursa; (b) the tsamma melon C. amarus Schrad syn. C. caffer Schrad. or C. lanatus var. citroides (Bailey) Mansf., which grows in southern Africa (Whitaker & Bemis, 1976); (c) the egusi melon C. mucosospermus Fursa, previously referred to as a subtaxon of C. lanatus by many authors but which was raised to specific rank many decades ago (Fursa, 1972, 1981, 1983); (d) the bitter apple C. colocynthis (L.) Schrad., a perennial species growing in sandy areas throughout northern Africa and Near‐East; (e) C. ecirrhosus Cogn., another perennial wild species (De‐Winter, 1990); (f) C. rehmii, a wild annual species, with small fruits used for feeding desert animals; and (g) C. naudinianus (Sond.) Hook.f. from the Namib‐Kalahari region, previously placed in the genus Acanthosicyos Welw. ex Hook. f. and sister group to all other species. Citrullus ecirrhosus, C. rehmii and C. naudinianus, currently, are considered endemic and restricted to the desert region of Namibia with very little intraspecific variation (Dane & Lang, 2004). This understanding may however change with more extensive sampling.
Given recent clarification of Citrullus taxonomy, it is appropriate to revisit the question of genealogy. In a recent phylogenetic study, Chomicki and Renner (2015) indicated West Africa as the possible center of origin of C. lanatus, a claim at odds with earlier assertions. Indeed, whereas some experts believe watermelon originated from southern Africa, based on the distribution of wild relatives in the Namibian desert (Bates & Robinson, 1995), others point to northern or northeastern Africa, especially the Nile river area in Sudan, as the likely center of origin based on archaeological data (Paris, 2015; Renner et al., 2019; Wasylikowa & Van Der Veen, 2004). According to these latter studies, very few archaeological records of watermelon are known from southern Africa, and all date to a relatively recent period between the 8th and 13th centuries A.D. Furthermore, a cultigen is known to have been cultivated in the Nile Valley when farming was not yet practiced in southwest Africa (Zohary & Hopf, 2000). In contrast, archaeological records from West Africa are scanty, except for the presence of one endemic cultivated species (C. mucosospermus) previously deemed to be a subspecies or variety of C. lanatus (Achigan‐Dako et al., 2015; Hammer & Gladis, 2014; Nesom, 2011; Renner et al., 2014).
The fundamental questions remain: how did watermelon spread throughout the world if it has originated from West or northeastern Africa? How did the extant cultigens distribute throughout the world and how do they relate to wild types such as C. colocynthis or C. amarus? To contribute to our understanding of these questions, this paper presents a chloroplast phylogeography of Citrullus lanatus and three related species, one cultivated (C. mucosospermus) and two wild (C. amarus and C. colocynthis), using a large sample size collected from four continents. The objective is to characterize the geographical distribution of Citrullus haplotypes and shed specific light of the chloroplast sequence evolution of C. lanatus, hypothesizing that such information will help clarify our understanding of the history of this globally significant agricultural species.
2. MATERIALS AND METHODS
2.1. Taxon sampling and total genomic DNA isolation
To investigate the geographical distribution of watermelon haplotypes, we included in the study the four most economically important Citrullus species: (a) C. lanatus, widely cultivated throughout the world (78 accessions from four continents out of which only 14 were from West Africa); (b) C. mucosospermus, restricted to West Africa and the closest sister species of cultivated watermelon (13 accessions); (c) C. amarus, a wild species from southern Africa that has spread to Europe and the closest relative to C. ecirrhosus (22 accessions); and (d) C. colocynthis, a wild species found in northern Africa and East Asia (22 accessions). In total, 135 accessions were assessed, including 53 from Africa, 41 from Asia, 25 from Europe and 16 from North America (Table 1). Voucher specimens of all accessions were deposited in the herbarium of The Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) (Achigan‐Dako et al., 2015).
TABLE 1.
List of Citrullus accessions, their geographical origin, and accession numbers
| No | Taxon | Haplotype number | Accession number | Origin | Source of collection | NBCI number for ndhF‐rpl32 | NBCI number for trnT‐L |
|---|---|---|---|---|---|---|---|
| 1 | Citrullus lanatus var. lanatus | 9 | PI 494527 | Nigeria | USDA | KX773568 | KX773717 |
| 2 | Citrullus mucosospermus | 1 | PI 559993 | Nigeria | USDA | KX773569 | KX773718 |
| 3 | Citrullus mucosospermus | 26 | PI 559994 | Nigeria | USDA | KX773570 | KX773719 |
| 4 | Citrullus mucosospermus | 9 | PI 560000 | Nigeria | USDA | KX773571 | KX773720 |
| 5 | Citrullus lanatus var. lanatus | 17 | PI 560002 | Nigeria | USDA | KX773572 | KX773721 |
| 6 | Citrullus mucosospermus | 1 | PI 560008 | Nigeria | USDA | KX773573 | KX773722 |
| 7 | Citrullus mucosospermus | 1 | PI 560010 | Nigeria | USDA | KX773574 | KX773723 |
| 8 | Citrullus mucosospermus | 1 | PI 560013 | Nigeria | USDA | KX773575 | KX773724 |
| 9 | Citrullus mucosospermus | 1 | PI 560018 | Nigeria | USDA | KX773576 | KX773725 |
| 10 | Citrullus lanatus var. lanatus | 1 | PI 560024 | Nigeria | USDA | KX773577 | KX773726 |
| 11 | Citrullus mucosospermus | 1 | 849 BSN 001 | Benin | Prospection | KX773578 | KX773727 |
| 12 | Citrullus mucosospermus | 1 | 975 MAT 007 | Benin | Prospection | KX773579 | KX773728 |
| 13 | Citrullus mucosospermus | 1 | 977 MAT 008 | Benin | Prospection | KX773580 | KX773729 |
| 14 | Citrullus mucosospermus | 1 | 1068 SN 045 | Benin | Prospection | KX773581 | KX773730 |
| 15 | Citrullus lanatus var. lanatus | 19 | GRIF 12336 | China | USDA | KX773582 | KX773731 |
| 16 | Citrullus lanatus var. lanatus | 1 | GRIF 14199 | India | USDA | KX773583 | KX773732 |
| 17 | Citrullus lanatus var. lanatus | 1 | GRIF 17300 | China | USDA | KX773584 | KX773733 |
| 18 | Citrullus lanatus var. lanatus | 2 | GRIF 17310 | China | USDA | KX773585 | KX773734 |
| 19 | Citrullus lanatus var. lanatus | 1 | GRIF 17330 | China | USDA | KX773586 | KX773735 |
| 20 | Citrullus mucosospermus | 6 | PI 186975 | Ghana | USDA | KX773587 | KX773736 |
| 21 | Citrullus lanatus var. lanatus | 1 | PI 192937 | China | USDA | KX773588 | KX773737 |
| 22 | Citrullus mucosospermus | 1 | PI 249010 | Nigeria | USDA | KX773589 | KX773738 |
| 23 | Citrullus lanatus | 1 | PI 271778 | South Africa | USDA | KX773590 | KX773739 |
| 24 | Citrullus lanatus var. lanatus | 10 | GRIF 55960 | India | USDA | KX773591 | KX773740 |
| 25 | Citrullus lanatus var. lanatus | 1 | GRIF 55990 | India | USDA | KX773592 | KX773741 |
| 26 | Citrullus amarus | 3 | PI 596662 | South Africa | USDA | KX773593 | KX773742 |
| 27 | Citrullus amarus | 4 | GRIF 15896 | Russia | USDA | KX773595 | KX773744 |
| 28 | Citrullus amarus | 4 | GRIF 15897 | Russia | USDA | KX773596 | KX773745 |
| 29 | Citrullus amarus | 6 | PI 179881 | India | USDA | KX773597 | KX773746 |
| 30 | Citrullus amarus | 4 | PI 189225 | Democratic Republic of Congo | USDA | KX773598 | KX773747 |
| 31 | Citrullus amarus | 3 | PI 299378 | South Africa | USDA | KX773599 | KX773748 |
| 32 | Citrullus amarus | 4 | PI 299379 | South Africa | USDA | KX773600 | KX773749 |
| 33 | Citrullus amarus | 3 | PI 244018 | South Africa | USDA | KX773601 | KX773750 |
| 34 | Citrullus amarus | 3 | PI 244019 | South Africa | USDA | KX773602 | KX773751 |
| 35 | Citrullus amarus | 4 | PI 255137 | South Africa | USDA | KX773603 | KX773752 |
| 36 | Citrullus amarus | 4 | PI 270563 | South Africa | USDA | KX773604 | KX773753 |
| 37 | Citrullus amarus | 6 | PI 271779 | South Africa | USDA | KX773605 | KX773754 |
| 38 | Citrullus amarus | 32 | PI 525,083 | Egypt | USDA | KX773606 | KX773755 |
| 39 | Citrullus amarus | 8 | PI 596659 | South Africa | USDA | KX773607 | KX773756 |
| 40 | Citrullus amarus | 8 | PI 596669 | South Africa | USDA | KX773608 | KX773757 |
| 41 | Citrullus amarus | 14 | PI 596671 | South Africa | USDA | KX773609 | KX773758 |
| 42 | Citrullus amarus | 3 | PI 596676 | South Africa | USDA | KX773610 | KX773759 |
| 43 | Citrullus amarus | 15 | CIT 101 | Ukraine | IPK | KX773611 | KX773760 |
| 44 | Citrullus amarus | 4 | CIT 139 | Russia | IPK | KX773612 | KX773761 |
| 45 | Citrullus amarus | 3 | CIT 152 | Zimbabwe | IPK | KX773613 | KX773762 |
| 46 | Citrullus amarus | 3 | CIT 310 | South Africa | IPK | KX773614 | KX773763 |
| 47 | Citrullus amarus | 2 | CIT 313 | Yemen | IPK | KX773615 | KX773764 |
| 48 | Citrullus lanatus subsp. vulgaris | 2 | CIT 207 | France | IPK | KX773616 | KX773765 |
| 49 | Citrullus lanatus subsp. vulgaris | 1 | CIT 31 | Ukraine | IPK | KX773617 | KX773766 |
| 50 | Citrullus lanatus subsp. vulgaris | 1 | CIT 44 | Yugoslavia | IPK | KX773618 | KX773767 |
| 51 | Citrullus lanatus subsp. vulgaris | 18 | CIT 60 | Croatia | IPK | KX773619 | KX773768 |
| 52 | Citrullus lanatus subsp. vulgaris | 1 | CIT 67 | Italy | IPK | KX773620 | KX773769 |
| 53 | Citrullus lanatus subsp. vulgaris | 1 | CIT 69 | Italy | IPK | KX773621 | KX773770 |
| 54 | Citrullus lanatus subsp. vulgaris | 1 | CIT 86 | Greece | IPK | KX773623 | KX773772 |
| 55 | Citrullus lanatus subsp. vulgaris | 1 | CIT 97 | Hungary | IPK | KX773624 | KX773773 |
| 56 | Citrullus lanatus subsp. vulgaris | 1 | CIT 99 | China | IPK | KX773625 | KX773774 |
| 57 | Citrullus lanatus subsp. vulgaris | 1 | CIT 102 | USA | IPK | KX773626 | KX773775 |
| 68 | Citrullus lanatus subsp. vulgaris | 1 | CIT 103 | Russia | IPK | KX773627 | KX773776 |
| 59 | Citrullus lanatus subsp. vulgaris | 1 | CIT 105 | Ukraine | IPK | KX773628 | KX773777 |
| 60 | Citrullus lanatus subsp. Vulgaris | 1 | CIT 107 | Russia | IPK | KX773629 | KX773778 |
| 61 | Citrullus lanatus subsp. Vulgaris | 1 | CIT 109 | Russia | IPK | KX773630 | KX773779 |
| 62 | Citrullus lanatus subsp. vulgaris | 1 | CIT 112 | Ukraine | IPK | KX773631 | KX773780 |
| 63 | Citrullus lanatus subsp. vulgaris | 2 | CIT 126 | Armenia | IPK | KX773634 | KX773783 |
| 64 | Citrullus lanatus subsp. vulgaris | 1 | CIT 128 | Mongolia | IPK | KX773635 | KX773784 |
| 65 | Citrullus lanatus subsp. vulgaris | 18 | CIT 130 | Yugoslavia | IPK | KX773636 | KX773785 |
| 66 | Citrullus lanatus subsp. vulgaris | 1 | CIT 135 | Bulgaria | IPK | KX773637 | KX773786 |
| 67 | Citrullus lanatus subsp. vulgaris | 1 | CIT 142 | Bulgaria | IPK | KX773638 | KX773787 |
| 68 | Citrullus lanatus subsp. vulgaris | 1 | CIT 143 | Bulgaria | IPK | KX773639 | KX773788 |
| 69 | Citrullus lanatus subsp. vulgaris | 1 | CIT 156 | Georgia | IPK | KX773641 | KX773790 |
| 70 | Citrullus lanatus subsp. vulgaris | 1 | CIT 158 | Georgia | IPK | KX773642 | KX773791 |
| 71 | Citrullus lanatus subsp. vulgaris | 1 | CIT 160 | Georgia | IPK | KX773643 | KX773792 |
| 72 | Citrullus lanatus subsp. vulgaris | 1 | CIT 164 | Russia | IPK | KX773644 | KX773793 |
| 73 | Citrullus lanatus subsp. vulgaris | 2 | CIT 167 | North Korea | IPK | KX773645 | KX773794 |
| 74 | Citrullus lanatus subsp. vulgaris | 1 | CIT 235 | USA | IPK | KX773646 | KX773795 |
| 75 | Citrullus lanatus subsp. vulgaris | 2 | CIT 237 | Japan | IPK | KX773647 | KX773796 |
| 76 | Citrullus lanatus subsp. vulgaris | 1 | CIT 239 | USA | IPK | KX773648 | KX773797 |
| 77 | Citrullus lanatus subsp. vulgaris | 1 | CIT 242 | USA | IPK | KX773649 | KX773798 |
| 78 | Citrullus lanatus subsp. vulgaris | 11 | CIT 244 | USA | IPK | KX773650 | KX773799 |
| 79 | Citrullus lanatus | 11 | CIT 259 | USA | IPK | KX773651 | KX773800 |
| 80 | Citrullus lanatus subsp. vulgaris | 22 | CIT 253 | Japan | IPK | KX773652 | KX773801 |
| 81 | Citrullus lanatus subsp. vulgaris | 1 | CIT 303 | Turkey | IPK | KX773653 | KX773802 |
| 82 | Citrullus lanatus subsp. vulgaris | 1 | CIT 306 | Portugal | IPK | KX773654 | KX773803 |
| 83 | Citrullus lanatus subsp. vulgaris | 1 | 06 NIA 224 | Mali | Prospection | KX773656 | KX773805 |
| 84 | Citrullus lanatus subsp. vulgaris | 2 | 06 NIA 567 | Benin | Prospection | KX773657 | KX773806 |
| 85 | Citrullus lanatus subsp. vulgaris | 2 | 07 NIA 995 | Ghana | Prospection | KX773658 | KX773807 |
| 86 | Citrullus lanatus subsp. vulgaris | 1 | 846 BAX1 | Mali | Prospection | KX773659 | KX773808 |
| 87 | Citrullus lanatus subsp. vulgaris | 1 | 1005 SE 032 | Mali | Prospection | KX773660 | KX773809 |
| 88 | Citrullus lanatus subsp. vulgaris | 1 | CIT 168 | North Korea | IPK | KX773661 | KX773810 |
| 89 | Citrullus lanatus | 24 | CIT 175 | Italy | IPK | KX773662 | KX773811 |
| 90 | Citrullus lanatus | 2 | CIT 182 | Mongolia | IPK | KX773663 | KX773812 |
| 91 | Citrullus lanatus | 1 | CIT 193 | Ukraine | IPK | KX773665 | KX773814 |
| 92 | Citrullus lanatus | 1 | CIT 195 | Georgia | IPK | KX773666 | KX773815 |
| 93 | Citrullus lanatus | 1 | CIT 200 | Tajikistan | IPK | KX773668 | KX773817 |
| 94 | Citrullus lanatus | 1 | CIT 203 | Tunisia | IPK | KX773669 | KX773818 |
| 95 | Citrullus lanatus | 2 | CIT 206 | China | IPK | KX773670 | KX773819 |
| 96 | Citrullus lanatus | 1 | CIT 226 | USA | IPK | KX773671 | KX773820 |
| 97 | Citrullus lanatus | 1 | CIT 230 | Israel | IPK | KX773672 | KX773821 |
| 98 | Citrullus lanatus | 1 | CIT 234 | USA | IPK | KX773673 | KX773822 |
| 99 | Citrullus lanatus | 1 | CIT 260 | USA | IPK | KX773674 | KX773823 |
| 100 | Citrullus lanatus | 2 | CIT 264 | USA | IPK | KX773675 | KX773824 |
| 101 | Citrullus lanatus | 21 | CIT 270 | USA | IPK | KX773676 | KX773825 |
| 102 | Citrullus lanatus | 1 | CIT 271 | Canada | IPK | KX773677 | KX773826 |
| 103 | Citrullus lanatus | 1 | CIT 273 | USA | IPK | KX773678 | KX773827 |
| 104 | Citrullus lanatus | 1 | CIT 278 | USA | IPK | KX773679 | KX773828 |
| 105 | Citrulus lanatus subsp. lanatus | 16 | CIT 309 | South Africa | IPK | KX773680 | KX773829 |
| 106 | Citrullus colocynthis | 36 | CIT 150 | Canary Island | IPK | KX773687 | KX773836 |
| 107 | Citrullus colocynthis | 28 | CIT 154 | Turkmenistan | IPK | KX773688 | KX773837 |
| 108 | Citrullus colocynthis | 33 | CIT 166 | Cape Verde | IPK | KX773689 | KX773838 |
| 109 | Citrullus colocynthis | 35 | CIT 190 | Morocco | IPK | KX773690 | KX773839 |
| 110 | Citrullus colocynthis | 12 | CIT 192 | India | IPK | KX773691 | KX773840 |
| 111 | Citrullus colocynthis | 12 | CIT 199 | Egypt | IPK | KX773692 | KX773841 |
| 112 | Citrullus colocynthis | 38 | CIT 281 | Cyprus | IPK | KX773693 | KX773842 |
| 113 | Citrullus colocynthis | 13 | CIT 307 | Namibia | IPK | KX773694 | KX773843 |
| 114 | Citrullus colocynthis | 30 | PI 195927 | Ethiopia | USDA | KX773695 | KX773844 |
| 115 | Citrullus colocynthis | 7 | PI 220778 | Afghanistan | USDA | KX773696 | KX773845 |
| 116 | Citrullus colocynthis | 7 | PI 346082 | Afghanistan | USDA | KX773697 | KX773846 |
| 117 | Citrullus colocynthis | 5 | PI 386014 | Iran | USDA | KX773698 | KX773847 |
| 118 | Citrullus colocynthis | 5 | PI 386015 | Iran | USDA | KX773699 | KX773848 |
| 119 | Citrullus colocynthis | 5 | PI 386016 | Iran | USDA | KX773700 | KX773849 |
| 120 | Citrullus colocynthis | 5 | PI 386018 | Iran | USDA | KX773701 | KX773850 |
| 121 | Citrullus colocynthis | 7 | PI 386021 | Iran | USDA | KX773702 | KX773851 |
| 122 | Citrullus colocynthis | 27 | PI 386024 | Iran | USDA | KX773703 | KX773852 |
| 123 | Citrullus colocynthis | 29 | PI 386026 | Iran | USDA | KX773704 | KX773853 |
| 124 | Citrullus colocynthis | 37 | PI 432337 | Cyprus | USDA | KX773705 | KX773854 |
| 125 | Citrullus colocynthis | 34 | PI 525082 | Egypt | USDA | KX773706 | KX773855 |
| 126 | Citrullus colocynthis | 31 | PI 537277 | Pakistan | USDA | KX773707 | KX773856 |
| 127 | Citrullus lanatus subsp. vulgaris | 2 | 824 AE 60 | Burkina Faso | Prospection | KX773708 | KX773857 |
| 128 | Citrullus lanatus subsp. vulgaris | 23 | 825 AE 60 | Burkina Faso | Prospection | KX773709 | KX773858 |
| 129 | Citrullus lanatus subsp. vulgaris | 2 | 831 AE 031 | Burkina Faso | Prospection | KX773710 | KX773859 |
| 130 | Citrullus colocynthis | 25 | 962 KU 026 | Burkina Faso | Prospection | KX773711 | KX773860 |
| 131 | Citrullus lantus cv. neri | 1 | 06 NIA 095 | Ghana | Prospection | KX773712 | KX773861 |
| 132 | Citrullus lantus cv. neri | 20 | 06 NIA 103 | Ghana | Prospection | KX773713 | KX773862 |
| 133 | Citrullus lantus cv. neri | 1 | 06 NIA 111 | Ghana | Prospection | KX773714 | KX773863 |
| 134 | Citrullus lanatus vulgaris sugar baby | 2 | GRIF 15895 | Canada | USDA | KX773715 | KX773864 |
| 135 | Citrullus lanatus vulgaris sugar baby | 2 | GRIF 15898 | USA | USDA | KX773716 | KX773865 |
As indicated in Table 1, a total of 53 accessions were received from the USDA National Plant Germplasm System, 66 were received from IPK Gatersleben, and 16 were collected throughout West Africa as part of this study. Seeds of all accessions were germinated in a greenhouse at IPK‐Gatersleben, and approximately 100 mg of leaf tissue was collected from one seedling per accession and dried with silica gel. Total genomic DNA was extracted from the dried leaf tissues using the QIAGEN DNAeasy Plant Kit, and one washing step was added according to the manufacturer's instructions to increase the quality of the DNA. Concentrations were estimated on 1% agarose gels stained with ethidium bromide. Samples exhibiting suboptimal PCR amplification were purified via the QIAquick PCR Purification Kit (QIAGEN) and resuspended in 50 ml 1× TE buffer.
2.2. Choice of chloroplast regions
Based on the work of Shaw et al. (2007), the following nine noncoding chloroplast regions were chosen for initial screening of one accession each of C. lanatus, C. mucosospermus, C. amarus and C. colocynthis: rpl32‐trnL, trnQ‐5’rps16, 3’trnV‐ndhC, ndhF‐rpl32, psbD‐trnT, psbJ‐petA, 3’rps16‐5’trnK, atpI‐atpH and trnT‐trnL. For most of these regions, total levels of variation were low and exclusively interspecific. However, for ndhF‐rpl32 and trnT‐trnL, polymorphisms were observed both within and among species; thus, these two regions were selected for more in‐depth investigation. These two regions of the chloroplast genome were amplified using the following primer pairs: (a) ndhF (5′‐GAAAGGTATKATCAAYGMATATT‐3′) and rpl32‐R (5′‐CCAATATCCCTTYYTTTTCCAA‐3′); and (b) trnL(UAG) (5′‐CTGCTTCCTAAGAGCAGCCT‐3′) and trnT(GGU) (5′‐CCCTTTTAACTCAGTGGTAG‐3′).
2.3. Amplification and sequencing
PCR amplifications were performed using a Gene Amp 9700 PCR System (PE Biosystems) thermal cycler. For the trnT‐trnL region, we used a reaction volume of 50 µl consisting of 26.6 µl H2O, 5 µl of supply buffer (10×), an additional 2.5 µl of 15 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate, 10 µl Q‐solution (Qiagen), 1.5 U Taq DNA polymerase (QIAGEN), 50 pmol of each primer, and approximately 20 ng of genomic DNA. Cycling conditions for trnT‐trnL region: 95°C for 3 min; 10 cycles of 30 s at 95°C, 35 s at 56°C, and 90 s at 68°C; 35 cycles of 30 s at 95°C, 35 s at 53°C, and 90 s at 68°C; and a final extension of 10 min at 68°C. For the ndhF‐rpl32 region, PCR amplification was carried out using the Phusion Hot Start Kit (Thermo Scientific) in a reaction volume of 30 µl consisting of 17.7 µl H2O, 6 µl of supply buffer (10×), an additional 1.5 µl of 15 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate, 50 pmol of each primer, and approximately 20 ng of genomic DNA. Cycling conditions for ndhF‐rpl32 region: 98°C for 3 min; 35 cycles of 30 s at 98°C, 35 s at 58°C, and 80 s at 72°C; and a final extension of 15 min at 72°C. All PCR products were purified using the QIAquick PCR Purification Kit (QIAGEN), following manufacturer's instructions, and resuspended in 28 µl warmed 1× TE buffer. Sequencing was performed on either a MegaBACE 1000 (Amersham Biosciences) or an ABI 3730 XL (Applied Biosciences) capillary sequencer.
2.4. Sequence analysis and haplotype coding
For each chloroplast region, the forward and reverse sequences were manually edited and combined into a single sequence using Geneious 5.5.6 (Kearse et al., 2012). These merged sequences were submitted to NCBI GenBank to make them publicly available. Following merging, three alignments were generated: (a) species‐pairwise alignments of C. lanatus accessions with those of C. mucosospermus, C. amarus, and C. colocynthis for the chloroplast region trnT‐L; (b) the same species‐pairwise alignments for the region ndhF‐rpl32; and (c) a combined alignment of all species, containing both trnT‐L and ndhF‐rpl32 regions, yielding a matrix of 1,611 aligned nucleotides. In the combined alignment, for the purpose of constructing coherent and parsimonious haplotypes, repeats and indels were re‐coded as single bp polymorphisms. In the trnT‐L region: (a) a microsatellite ACATA at position 366 was coded as A (repeat presence) or a single gap "‐" (absence); (b) a TATT indel at position 405 was coded as a T (presence) or a single gap (absence); and (c) another TTTATA microsatellite at position 423 was coded as T (presence) or a single gap (absence). In the ndhF‐rpl32 region: (a) a poly AT, usually six to eight units (position 1149), was just replaced by a single gap for 6*(AT), A for 7*(AT), and T for 8*(AT); and (b) a TGATT microsatellite at position 1198 was coded as a T (presence) or a single gap (absence).
2.5. Data analysis
2.5.1. Analysis of genetic diversity
Statistical parameters including sequence diversity, nucleotide diversity (Nei, 1987; Nei & Tajima, 1983), A + T content, and substitution, inversion, and transversion rates (Baier, 2011; Chiu et al., 2013; Librado & Rozas, 2009; Rozas & Rozas, 1997) were computed using DnaSP software version 5.10.01 (Chiu et al., 2013; Librado & Rozas, 2009). Pairwise intra‐ and interspecific sequence divergences for each chloroplast region were computed as the mean number of nucleotide differences per site, following the formula:
where Tv is the number of transversions, Ts is the number of transitions, ID is the number of insertions/deletions, and L is the total length of the sequence (Dane et al., 2007; O’donnell, 1992). We used the PERMUT software package (Pons & Petit, 1996) to calculate the mean within‐population gene diversity (Ching‐Yi et al., 2012) and the total gene diversity (hT) (Chiu et al., 2013; Guicking et al., 2011; Martin et al., 2003; Sun et al., 2019; Zhao et al., 2019). Other intrapopulation metrics such as the number of haplotypes per population, the number of singleton haplotypes (haplotype that occurs only once in the study), the number of effective haplotypes, and the overall haplotype diversity were also estimated (Baier, 2011).
2.5.2. Population differentiation and genetic structure
To infer genetic differentiation parameters, haplotypes grouped by continent or subregion were considered to comprise distinct geographic populations. We assessed the genetic differentiation among geographic populations by computing the gene differentiation statistic developed by Nei and Chesser (1983), an allele frequency‐based approach that relies on estimates of genetic differentiation among geographic subpopulations. We further used Hudson et al. (1992)’s statistical test, a simple nonparametric method based on Monte Carlos permutations. That method, compared to the traditional chi‐square analysis of genetic differentiation estimates, helped understand whether the geographical populations are genetically different from one another. In addition, genetic differentiation among populations was estimated by computing a distance matrix based on the number of mutational steps between haplotypes (Nst) and by using haplotype frequencies (Gst). Phylogeographical structure was tested based on the difference between GST and NST using PERMUT 2.0 (Chiu et al., 2013; Pons & Petit, 1996) with 1,000 permutations. In contrast to Gst, Nst considers sequence differences between the haplotypes. Thus, Nst > Gst indicates that closely related haplotypes are observed more often in a given geographical area than would be expected by chance (Burban et al., 1999; Chávez‐Pesqueira & Núñez‐Farfán, 2016; Chiu et al., 2013; Grivet, 2002; Guicking et al., 2011; Pons & Petit, 1996; Sun et al., 2019). Following Templeton (1996), we tested the null hypothesis of homogeneity of nucleotide mutations using Fisher's exact test to investigate haplotypic differentiation within the overall population. We also performed Fu's Fs (Fu, 1997) to analyze the expansion level of the population under the hypothesis of selective neutrality and population equilibrium. Tajima's D test also was implemented for comparison with the Fu's Fs.
2.5.3. Statistical parsimony network
Parsimony networks were constructed to infer phylogeographical relationships among haplotypes using TCS v1.21 (Clément et al., 2000). TCS estimates genealogical relationships of sequences and collapses identical sequences into haplotypes (HT). To account for the different mutation rates underlying base substitutions, indels, and microsatellites, we followed the two‐step strategy described by Bänfer et al. (2006) and performed by Guicking et al. (2011). The network was re‐drawn from the TCS output using Adobe Illustrator.
3. RESULTS
3.1. Nucleotide variations, intra‐ and interspecific diversity
The length of the amplified trnT‐trnL region within C. lanatus ranged from 951 to 954 bp. No parsimony‐informative site was found within C. lanatus, but 3 indels were found at positions 242, 295, and 296. The amplified ndhF‐rpl32 region ranged from 650 to 652 bp in the species, also with no parsimony‐informative site, though 5 indels were found at positions 970, 1,028, 1,143, 1,178, and 1,198 (Table S1). The combined length of the two cpDNA regions was found equal to 1,601–1,605 bp and included 1 SNP (position 1,399) and 1 microsatellite (position 366); but no polymorphisms were parsimony‐informative. In total, the sampled accessions of this species comprise 12 distinct haplotypes, among which 10 were singletons, with an overall haplotype diversity of 0.5656 (Table 2).
TABLE 2.
Genetic statistics based on the trnT‐L, ndhF‐rpl32, and their combination in Citrullus spp
| CpDNA regions | Taxonomic groups | Number of accessions | Total Length (bp) | Parsimony‐informative sites | Number of haplotypes | Haplotypes diversity | Nucleotide diversity (Pi) | Average number of nucleotide difference (k) | Indel events | A + T (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| trnT‐L | Citrullus lanatus | 78 | 951–954 | 0 | 4 | 0.44 | 0 | 0 | 3 | 76.1 |
| C. mucosospermus | 16 | 950–953 | 0 | 3 | 0.34 | 0 | 0 | 2 | 75.8 | |
| C. amarus | 22 | 950–953 | 0 | 5 | 0.52 | 1 × 10–4 | 0.09 | 4 | 75.9 | |
| C. colocynthis | 22 | 948–954 | 6 | 12 | 0.92 | 28 × 10–4 | 2.65 | 5 | 76.0 | |
| ndhF‐rpl32 | C. lanatus | 78 | 650–652 | 0 | 8 | 0.24 | 0.4 × 10–4 | 0.027 | 5 | 76.3 |
| C. mucosospermus | 16 | 651–652 | 0 | 3 | 0.25 | 1.9 × 10–4 | 0.125 | 0 | 76.8 | |
| C. amarus | 22 | 651–653 | 2 | 6 | 0.71 | 10.5 × 10–4 | 0.68 | 1 | 76.8 | |
| C. colocynthis | 22 | 650–653 | 1 | 11 | 0.80 | 7 × 10–4 | 0.45 | 6 | 76.3 | |
| trnT‐L&ndhF‐rpl32 | C. lanatus | 78 | 1601–1605 | 0 | 12 | 0.56 | 0.2 × 10–4 | 0.025 | 8 | 76.2 |
| C. mucosospermus | 16 | 1601–1604 | 0 | 5 | 0.53 | 0.8 × 10–4 | 0.125 | 2 | 76.2 | |
| C. amarus | 22 | 1602–1604 | 2 | 8 | 0.81 | 4 0.8 × 10–4 | 0.78 | 6 | 76.2 | |
| C. colocynthis | 22 | 1599–1605 | 7 | 16 | 0.96 | 19.5 × 10–4 | 3.10 | 12 | 76.1 |
Parsimony‐informative sites: Polymorphic sites with a minimum of two alleles that are each present at least twice in the population.
Noninformative sites: Polymorphic sites that are unique in the population (singleton sites).
Haplotype diversity: The probability that two given sequences from two different haplotypes belong to two different regions or populations.
Nucleotide diversity: The average number of nucleotide substitutions per site between two sequences (Lynch and Crease 1990).
Average number of nucleotide differences: The average number of nucleotide differences (either Indels or SNPs) within a given population.
Indel events: The number of insertions/deletions in the genomic region.
A + T (%): A + T content in the genomic region.
Sequence lengths within C. mucosospermus were similar, with the combined length of the two regions spanning by 1,601–1,604 bp. One SNP (nonparsimony informative) was identified in the ndhF‐rpl32 region (position 1,397), as well as two indels in trnT‐trnL region (positions 242 and 296). Of the five haplotypes found among the sampled accessions of this species, three were singletons; and overall haplotype diversity is 0.5333.
The combined sequence length in C. amarus ranged between 1,602–1,604 bp (950–953 bp in trnT‐trnL and 651–653 bp in ndhF‐rpl32) and contained ten polymorphic sites. Of those, 4 indels were observed in trnT‐L (positions 295, 296, 297, 405) and 1 in ndhF‐rpl32 (positions 1,198). Four SNPs were found at positions 918, 1,149, 1,397, and 1,526; and there is a microsatellite at position 1,149. C. amarus was characterized by eight haplotypes, among which six were private; and overall haplotype diversity is 0.81.
Citrullus colocynthis was characterized by a combined sequence length of 1,599–1,605 bp (948–954 bp for trnT‐trnL and 650–653 bp for ndhF‐rpl32) that features 10 SNPs (positions 406, 455, 487, 882, 918, 949, 1,111, 1,286, 1,397, and 1,526) and 3 microsatellites (positions 366, 423, 1,149). In addition, there were 11 indels (positions 199, 242, 295, 296, 297, 972, 1,179, 1,180, 1,200, 1,262, and 1,530), 7 of which were parsimony informative (6 within trnT‐trnL and 1 within ndhF‐rpl32). The collection of this species contains 16 haplotypes, all private, and has an overall haplotype diversity of 0.96.
Based on the 29 polymorphic sites detected within the two cpDNA regions, 38 haplotypes were detected among the sampled accessions (Table 3). The most ancient haplotype (H1), according to TCS analysis, is exclusive to the cultivated species C. lanatus and C. mucosospermus. Of the 26 singleton haplotypes detected, 13 (50%) were found within C. colocynthis, indicating recent haplotype divergence in that species (Figure 1).
TABLE 3.
Haplotype codes for the combined trnT‐L and ndhF‐rpl32 chloroplast regions for the global collections of the four Citrullus species in this study

Red colour letters highlight sequence variations
FIGURE 1.
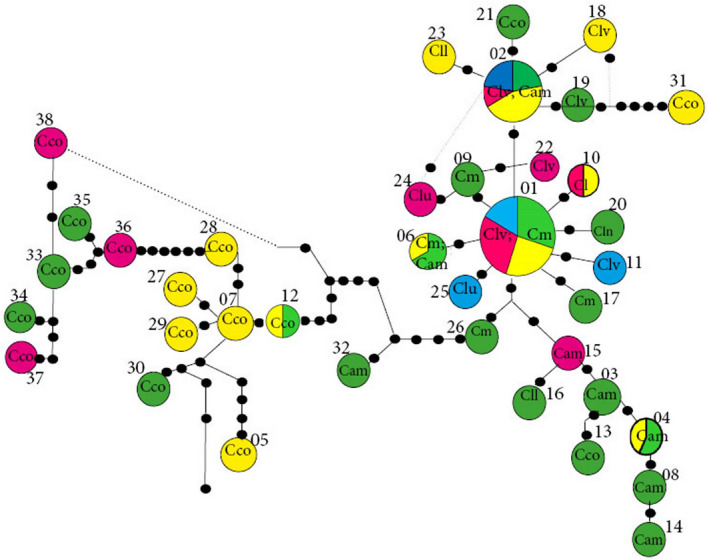
TCS network of 38 Citrullus spp. haplotypes. Circle size is proportional to haplotype frequency. Taxon names are abbreviated with two or three letters. Clv: C. lanatus subsp. vulgaris; Cll: C. lanatus subsp. lanatus; Cm: C. mucosospermus; Cam: C. amarus; and Cco: C. colocynthis. The numbers are arbitrary haplotype ID numbers (see Table S1), and the colors indicate geographical distribution: Africa (green), Asia (yellow); Europe (red), and North America (blue)
3.2. Geographical distribution, genetic differentiation of haplotypes, and population expansion
The pattern of polymorphism suggested non neutral selection as revealed by both Fu's Fs statistic and Tajima's D (Fs = −3.624, p = 0.016; D: −0.59858; not statistical significant, p > 0.10). Moreover, Ficher's exact test used to investigate haplotypic differentiation within the overall population suggested the rejection of the null hypothesis of homogeneity of nucleotide substitutions (LD = 0.1958, p < 0.001) following the neutral theory of molecular evolution.
Within‐continent gene diversity (Hs) varied from 0.57 (in Europe) to 0.85 (in Africa), with the majority of haplotypes being specific to certain regions. For instance, of the 21 haplotypes found in Africa, 16 were specific to the continent; of the 14 haplotypes found in Asia, eight were specific; of the nine found in Europe, six were specific; and of the four recovered from America, two were specific to that region (see Figures 2, 3, 4, 5).
FIGURE 2.

Distribution and frequencies of Citrullus spp. haplotypes in Africa
FIGURE 3.

Distribution and frequencies of Citrullus spp. haplotypes in Asia
FIGURE 4.

Distribution and frequencies of Citrullus spp. haplotypes in Europe
FIGURE 5.

Distribution and frequencies of Citrullus spp. haplotypes in North America
Haplotypes of C. mucosospermus were almost uniquely restricted to West Africa, and C. amarus haplotypes appeared specific to southern Africa. Haplotypes of C. colocynthis shared by Namibia, Ethiopia, and northern Africa were also found widespread throughout Asia. Across that continent, some haplotypes of C. colocynthis were specific to different countries (Figure 1). Six C. colocynthis haplotypes were specific to Asia, and six were specific to Africa. For this species, Iran contributed the highest number of haplotypes in Asia (Figure 1), as Egypt did in Africa (Figure 1).
Within C. lanatus, although all regions shared most haplotypes, Africa exhibited the highest number of singletons. The ancient haplotype H1 was found not only among West African countries but also in Europe (Georgia, former Yugoslavia, Italy, and Ukraine), Asia (Russia, Japan, China, India), and North America (United States and Canada). North Africa (Egypt) and southern Asia (India) shared C. colocynthis haplotype H12; and haplotype H4, specific to C. amarus, was shared by African countries (e.g., South Africa and the Democratic Republic of Congo) and Russia (Figure 1). Haplotype H2 was found throughout West Africa (Benin, Burkina‐Faso, and Ghana) as well as in Asia (China, Japan, Yemen, North‐Korean Republic, Mongolia, and Armenia), France, and North America (United States and Canada). Haplotype H2 is shared by C. lanatus and C. amarus; and haplotype H6 is shared by C. mucosospermus and C. amarus species (see Figures 2, 3, 4, 5).
Analysis of interspecific genetic differentiation revealed a high level of total genetic differentiation among continents (Tables 4 and 5). Coefficients of pairwise genetic differentiation values were highest between Africa and Europe, on the one hand, and Asia and Europe, on the other; Gst was lower between Africa and Asia (0.006). The coefficient of population differentiation Gst was 0.196, and the pairwise difference between haplotypes Nst = 0.374.
TABLE 4.
Diversity and differentiation statistics for the four Citrullus spp. in this study, based on combined cpDNA haplotypes, according to Pons and Petit (1996) and adapted from Guicking et al. (2011)
| Genetic parameters | Value | Standard error |
|---|---|---|
| Expected mean within‐population gene diversity (hS) | 0.737 | 0.0671 |
| Expected total gene diversity (hT) | 0.917 | 0.0320 |
| Expected coefficient of genetic differentiation (Gst) | 0.196 | 0.0812 |
| Observed mean within‐population gene diversity (Vs) | 0.668 | 0.1878 |
| Observed total gene diversity, accounting for similarities among haplotypes (VT) | 1.067 | 0.1609 |
| Observed coefficient of genetic differentiation (Nst) | 0.374 | 0.1274 |
hS: The average permuted value of gene diversity within the four geographical regions (Africa, America, Asia, and Europe).
hT: The permuted value of gene diversity across all four geographical regions.
GSt: The permuted value of genetic differentiation among the four geographical regions.
VS: The average observed value of gene diversity within the four geographical regions.
VT: The observed value of gene diversity across all four geographical regions.
NSt: The observed value of genetic differentiation among the four geographical regions.
TABLE 5.
Pairwise genetic differentiation between continents (a), between African regions (b) and between Asian regions (c)
| Region 1 | Region 2 | Hs | Ks | Kxy | Gst | Chi‐square |
|---|---|---|---|---|---|---|
| a: Pairwise genetic differentiation between continents (Hudson, 1992) | ||||||
| Africa | Asia | 0.85 | 0.85 | 4.78 | 0.006 |
χ2 = 135.067 p‐value = 0.05 |
| Africa | Europe | 0.76 | 0.76 | 3.84 | 0.035 | |
| Africa | America | 0.81 | 0.81 | 2.92 | 0.023 | |
| Asia | Europe | 0.73 | 0.73 | 4.41 | 0.038 | |
| Asia | America | 0.77 | 0.77 | 3.43 | 0.014 | |
| Europe | America | 0.57 | 0.57 | 2.12 | 0.0079 | |
| b: Pairwise genetic differentiation between African regions (Hudson, 1992) | ||||||
| West Africa | South Africa | 0.73 | 1.92 | 3.79 | 0.12 |
χ2 = 84.02 p‐value = 0.0001 |
| West Africa | South Africa | 0.72 | 3.14 | 9.02 | 0.043 | |
| South Africa | North Africa | 0.85 | 3.88 | 9.34 | 0.05 | |
| c: Pairwise genetic differentiation between Asian regions (Hudson, 1992) | ||||||
| East Asia | West Asia | 0.77 | 3.50 | 6.30 | 0.04 |
χ2 = 65.75 p‐value = 0.0047 |
| East Asia | South Asia | 0.76 | 2.65 | 4.73 | 0.06 | |
| East Asia | North Asia | 0.64 | 1.30 | 2.37 | 0.09 | |
| West Asia | South Asia | 0.89 | 6.20 | 6.20 | 0.014 | |
| West Asia | North Asia | 0.78 | 4.97 | 6.64 | 0.08 | |
| South Asia | North Asia | 0.77 | 4.19 | 5.11 | 0.07 | |
Hs: The mean within‐continent gene diversity.
Ks: A weighted average of the number of differences between sequences from continents i and j.
Kxy: The average number of differences between two samples, regardless of their provenance.
GST: The coefficient of genetic differentiation between continents.
4. DISCUSSION
4.1. Genetic diversity and sequence variation
Within the genus Citrullus genetic diversity analyses have been conducted since the second half of the 20th century (Hashizume et al. 1996) revealing various trends. Previous knowledge revealed lower genetic diversity in Citrullus for breeding purpose (Levi et al., 2001, 2004). Recent studies shed light on obvious genetic diversity within the genus. For instance, a study using High Frequency Oligonucleotide Target Active Genes (HFO‐TAGs) revealed high genetic diversity among Citrullus spp. and highlighted the potential importance of PI accessions as sources of valuable traits like disease resistance (Levi et al., 2013).
Our findings revealed low cpDNA variability among C. lanatus and C. mucosospermus. This was also observed by Dane and Lang (2004) and Dane et al. (2004) who found low nucleotide variability based on a low number of parsimony‐informative sites within each of the studied species. Most haplotypes were found within noncultivated (C. colocynthis) rather than cultivated (C. lanatus and C. mucosospermus) species. Taxa were clearly separated from one another with divergence based mainly on indels and transition events (Dane et al., 2004). However, there was sufficient resolution of the trnT‐L and ndhF‐rpl32 noncoding regions to reveal intraspecific variability.
Chloroplast sequence analysis revealed that the ndhF‐rpl32 region exhibits comparatively higher variability within the two cultivated species than the trnT‐L region. Dane and Lang (2004) analyzed four chloroplast regions (nhdF, ycf6‐psbM, ycf9‐trnG and atpA‐trnR) and found no variability within cultivated accessions, grouped either by morphological traits or geographical origin. In this study, we used a large number of C. lanatus accessions from a wide geographical range and observed low haplotype diversity within that species, as also revealed by Guo et al. (2013). While many factors can influence sequence diversity, selection is a major contributor via the imposition of bottlenecks that can substantially reduce diversity (Dane & Lang, 2004; Levi et al., 2013). The lack of haplotype divergence within C. lanatus and C. mucosospermus is likely the result of selection or other bottlenecks in the domestication histories of watermelon and egusi melon. Certainly, selection for sweet red‐fleshed cultivars with high lycopene content or selection of seed type as source of protein/oil for consumption might contribute to current genetic structure in those cultivated species (Achigan‐Dako et al., 2015; Renner et al., 2019).
Citrullus colocynthis exhibited a relatively high number of parsimony‐informative characters. Dane et al. (2004) revealed that haplotypes detected within C. colocynthis were associated with geographical origin and that was also confirmed by Levi et al. (2017). The haplotype diversity within C. colocynthis suggests cryptic evolution and calls for a comprehensive morphological comparison of Asian and African colocynths. Such an investigation is exemplified by the recent studies on Cucumis melo that revealed modern melon cultivars go back to two lineages and was domesticated at least twice: in Asia and in Africa (Endl et al., 2018).
4.2. Citrullus haplotype evolution
Thirty‐eight haplotypes were detected among the cultivated and wild Citrullus accessions used in this study. Dane et al. (2004) found seven haplotypes within the genus, using 55 accessions of C. lanatus, 15 accessions of C. colocynthis, and a total of seven cpDNA regions. With two cpDNA regions and 135 accessions carefully selected to represent a wide geographical region, we detected an even higher haplotype diversity among Citrullus spp. This situation can be expected to continue to evolve as more watermelon accessions from Sudan or northeast Africa are sequenced, particularly, the Sudanese sweet white‐fleshed melon. Unfortunately, sampling of C. lanatus from the Darfur region of Sudan has been scarce (Renner et al., 2019).
On average, we observed 9.5 haplotypes per species, varying from 5 to 16. In comparison with other species, Guicking et al. (2011) found 9.8 haplotypes per species in Macaranga and Jakob and Blattner (2006) found 2.83 haplotypes per species in Hordeum. In Citrullus spp., nucleotide substitutions appear to have evolved at different rates, an observation supported by the Fisher's test for homogeneity of nucleotide substitution. Fu's test Fs also rejected the null hypothesis of neutrality of evolution of nucleotide substitution, further supporting the hypothesis that the polymorphism pattern observed is nonrandom. Population expansions tend to produce significantly negative values of D, while population bottlenecks tend to produce significantly positive values of D. In our case the departure from neutrality might indicate that there is a high demographic expansion and a pattern of isolation by distance would be occurred between the continents (Jiang et al., 2016).
4.3. Genetic differentiation and geographical structure
The coefficient of population differentiation (Gst), that uses allelic frequencies and does not take into account the distances among haplotypes, and the coefficient of differentiation (Nst) based on the pairwise difference between alleles were found respectively, equal to 0.196 and 0.374; but the difference was not significant (p > 0.05). In Citrullus spp. Mujaju et al. (2011) found Gst = 0.56 and Nst = 0.49 for sweet watermelon and Gst = 0.71, Nst = 0.81 for cow watermelon. The fact that the differentiation parameter based on the pairwise difference between alleles is greater than the one calculated without permutation (i.e., Nst > Gst) indicates that the collection is characterized by clear geographic structure (Dane et al., 2007; Grivet, 2002; Guicking et al., 2011). Also, the significant value of the total gene diversity across all four geographical regions (hT = 0.917, standard error = 0.0320) is indicating a strong structure in the population (Pons & Petit, 1996; Sun et al., 2019; Zhao et al., 2019).
Levi et al. (2017) observed that accessions of C. colocynthis were subdivided into five groups in general agreement with their centers of diversification and origin. Our findings indicated that regional genetic differentiation statistics support Levi et al. (2017)’s conclusions, with subsamples from different regions exhibiting genetic differentiation associated with their likely centers of diversification. Also, haplotypes of C. amarus were mostly grouped in Southern Africa, which is assumed to be the origin of that species (Chomicki & Renner, 2015; Dane & Liu, 2007).
Citrullus chloroplast sequences analysis with TCS 1.21 resulted in a network where haplotypes widely sampled throughout West Africa were placed at the root. While coalescence theory predicts that older alleles will prevail in a population due to a higher number of descending lineages and associated wider geographic distributions (Crandall & Templeton, 1993), such an observation may depend on sample sizes and evolutionary/domestication histories and also the lack of subsp. cordophanus (from northeast Africa) in the germplasm studied. In this study, H1 is the most frequently sampled haplotype and has the most connections with other haplotypes; thus, H1 may be considered the most ancient haplotype. This ancient haplotype was sampled most frequently in West Africa (i.e., Nigeria and Benin) and was highly shared by accessions of both C. lanatus and C. mucosospermus. These results support the findings of Chomicki and Renner (2015) and Renner et al. (2019) who used eleven gene regions to infer phylogeny of Citrullus species, and also a 3,500‐year‐old leaf sample from the Egyptian tomb to infer close relationship between C. lanatus and C. mucosospermus. Our findings, based upon a large set of egusi melon and watermelon accessions from four continents, provide further evidence of that close relationship between these two species. However, they are indeed two different species, as previous crosses between them (e.g., Charleston Gray x PI 560006) resulted in high levels of sterility (Gusmini et al., 2004). The very limited haplotype diversity among the two species suggests an old split with chlorotype fixation (Dane & Liu, 2007) and ancient types of C. mucosospermus originating from West Africa (Renner et al., 2014). However, to the best of our knowledge, no wild populations have been confirmed in West Africa. Spontaneous plants may have been found earlier, but those individuals certainly escaped from cultivation. A region‐wide collecting mission by the first author yielded no wild population of C. mucosospermus in West Africa (Achigan‐Dako et al., 2015) though, the presence in West Africa of the “neri” type [figure 9f in Achigan‐Dako et al. (2015) and figure 1 in Minsart et al. (2011)], another cultivated egusi melon that exhibits smaller seeds with yellow soft coat, should be highlighted as a contributor to the genepool of Citrullus is the region. While this neri type (C. lanatus) is morphologically distinct from C. mucosospermus, it has been rarely studied.
Archaeological evidence indicates the northeast of Africa as a center of origin and domestication (Chomicki et al., 2020). Authors reported wild dessert watermelon in that region (Paris, 2015) or the genetic affinity with the C. lanatus var. cordophanus (a sweet white‐fleshed cultivar) (Renner et al., 2019). However, within the genus Citrullus mucosospermus remains the closest relative species to C. lanatus. The presence of an ancient haplotype in West Africa on the one hand and the close relationship between C. lanatus and subsp. cordophanus of Darfur in northeastern Africa as revealed by Renner et al. (2019) on the second hand, calls for further molecular and archaeological investigations to generate sufficient knowledge on newly published results, including those reported here. New molecular investigations should include more materials from Sudan and neighboring countries where wild populations of watermelon have been found (Paris, 2015). Moreover, our data showed that one of the Egyptian accessions (PI 525083), indicated to be C. amarus and observed by Levi et al. (2013) to cluster with dessert watermelon, exhibits a unique haplotype (H32). That accession is several mutations away from C. colocynthis and closer to watermelon and egusi melon haplotype. Previous findings of Levi et al. (2017) showed that PI 525083 rather clustered with C. lanatus var. lanatus. In addition, the hypothesis that watermelon is from northeastern Africa does not explain how an endemic species such as C. mucosospermus shares the same haplotype with dessert watermelon, while other accessions from the region (e.g., PI 525083) shows unique haplotype. If C. lanatus did indeed spread to the world from West or northeastern Africa, how and when was it domesticated in those regions as New Kingdom Egyptians were cultivating sweet red‐fleshed watermelon more than 3,500 years ago? From which species was C. mucosospermus domesticated? Through what mechanisms was C. lanatus spread to Asia and when? More germplasm collections from all continents are necessary to fully understand the phylogeographical relationships among Citrullus species. In Africa, the focus should be on both west and northeastern regions to resolve the domestication history of modern cultivars.
5. CONCLUSION
The genus Citrullus includes seven species that may originate from different parts of the world, according to previous and current data. Our results reveal 38 distinct chloroplast haplotypes among Citrullus spp. and the distribution of those haplotypes across the world. The close relationship of egusi melon and Kordofan melon to watermelon raised new questions regarding the colonization routes of major crops and the current status of extant genetic diversity of wild relatives in places of origin.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Enoch G. Achigan‐Dako: Conceptualization (equal); Data curation (equal), Formal analysis (equal); Funding acquisition, Investigation (equal); Methodology (equal); Project administration (equal); Supervision (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review and editing (equal). Hervé Degbey: Data curation (equal), Formal analysis (equal); Investigation (equal); Methodology (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review and editing (equal). Iago Hale: Methodology (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review and editing (equal). Frank Blattner: Conceptualization (equal); Investigation (equal); Methodology (equal); Project administration (equal); Supervision (equal); Visualization (equal); Writing‐review and editing (equal).
Supporting information
Table S1
ACKNOWLEDGEMENTS
This study was financially supported by the Vavilov‐Frankel Fellowship to the first author under grant CONT/08/136/RF and the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK). We acknowledge contribution and technical support from Christina Koch, Petra Oswald, Birgit Wohlbier, R.S. Vodouhe, and Adam Ahanchede. We thank Augustin Ganse for assistant in map making and Hanno Schaefer for useful comments on the manuscript.
Achigan‐Dako EG, Degbey H, Hale I, Blattner FR. Georeferenced phylogenetic analysis of a global collection of wild and cultivated Citrullus species. Ecol Evol. 2021;11:1918–1936. 10.1002/ece3.7189
DATA AVAILABILITY STATEMENT
DNA sequences: NCBI GenBank accession numbers are provided in Table S1. Dryad: https://doi.org/10.5061/dryad.31zcrjdjw
REFERENCES
- Achigan‐Dako, E. G. , Avohou, E. S. , Linsoussi, C. , Ahanchede, A. , Vodouhe, R. S. , & Blattner, F. R. (2015). Phenetic characterization of Citrullus spp. (Cucurbitaceae) and differentiation of egusi‐type (C. mucosospermus). Genetic Resources and Crop Evolution, 62(8), 1159–1179. 10.1007/s10722-015-0220-z [DOI] [Google Scholar]
- Baier, C. (2011). Comparative phylogeographic and population genetic analyses of three tropical pioneer trees, Macaranga winkleri, M. winkleriella and M. tanarius (Euphorbiaceae). Martin‐Luther‐Universität Halle‐Wittenberg. [Google Scholar]
- Bänfer, G. , Moog, U. , Fiala, B. , Mohamed, M. , Weising, K. , & Blattner, F. R. (2006). A chloroplast genealogy of myrmecophytic Macaranga species (Euphorbiaceae) in Southeast Asia reveals hybridization, vicariance and long‐distance dispersals. Molecular Ecology, 15, 4409–4424. 10.1111/j.1365-294X.2006.03064.x [DOI] [PubMed] [Google Scholar]
- Bates, D. M. , & Robinson, R. (1995). Cucumber, melon and watermelons: Cucumis and Citrullus (Cucurbitaceae) In Smart J., & Simmonds N. M. (Eds), Evolution of crop plants, 2nd ed. (pp. 89–96). Longman. [Google Scholar]
- Burban, C. , Petit, R. J. , Carcreff, E. , & Jactel, H. (1999). Range wide variation of the maritime pine bast scale Matsucoccus feytaudi Duc. (Homoptera: Matsucoccidae) in relation to the genetic structure of its host. Molecular Ecology, 8, 1593–1602. 10.1046/j.1365-294x.1999.00739.x [DOI] [PubMed] [Google Scholar]
- Chávez‐Pesqueira, M. , & Núñez‐Farfán, J. (2016). Genetic diversity and structure of wild populations of Carica papaya in Northern Mesoamerica inferred by nuclear microsatellites and chloroplast markers. Annals of Botany, 118(7), 1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching‐Yi, L. , Hsin‐Mei, K. U. , Chiang, Y.‐H. , Ho, H.‐Y. , Tsong‐Ann, Y. U. , & Jan, F.‐J. (2012). Development of transgenic watermelon resistant to Cucumber mosaic virus and Watermelon mosaic virus by using a single chimeric transgene construct. Transgenic Research, 21(5), 983–993. 10.1007/s11248-011-9585-8 [DOI] [PubMed] [Google Scholar]
- Chiu, Y.‐W. , Bor, H. , Tan, M.‐S. , Lin, H.‐D. , & Jean, C.‐T. (2013). Phylogeography and genetic differentiation among populations of the moon turban snail Lunella granulata Gmelin, 1791 (Gastropoda: Turbinidae). International Journal of Molecular Sciences, 14(5), 9062–9079. 10.3390/ijms14059062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomicki, G. , & Renner, S. S. (2015). Watermelon origin solved with molecular phylogenetics including Linnaean material: Another example of museomics. New Phytologist, 205, 526–532. 10.1111/nph.1363 [DOI] [PubMed] [Google Scholar]
- Chomicki, G. , Schaefer, H. , & Renner, S. S. (2020). Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytologist, 226(5), 1240–1255. 10.1111/nph.16015 [DOI] [PubMed] [Google Scholar]
- Clément, M. , Posadu, D. , & Crandall, K. (2000). a computer program to estimate gene genealogies. Molecular Ecology, 9, 1657–1660. [DOI] [PubMed] [Google Scholar]
- Crandall, K. A. , & Templeton, A. R. (1993). Empirical tests of some predictions from coalescent theory with aplications to intraspecific phylogeny reconstruction. Genetics, 143, 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane, F. , & Lang, P. (2004). Sequence variation at cpDNA regions of watermelon and related wild species: Implications for the evolution of Citrullus haplotypes. American Journal of Botany, 91(11), 1922–1929. 10.3732/ajb.91.11.1922 [DOI] [PubMed] [Google Scholar]
- Dane, F. , Lang, P. , & Bakhtiyarova, R. (2004). Comparative analysis of chloroplast DNA variability in wild and cultivated Citrullus species. Theoretical and Applied Genetics, 108(5), 958–966. 10.1007/s00122-003-1512-9 [DOI] [PubMed] [Google Scholar]
- Dane, F. , & Liu, J. (2007). Diversity and origin of cultivated and citron type watermelon (Citrullus lanatus). Genetic Resources and Crop Evolution, 54(6), 1255–1265. 10.1007/s10722-006-9107-3 [DOI] [Google Scholar]
- Dane, F. , Liu, J. , & Zhang, C. (2007). Phylogeography of the bitter apple, Citrullus colocynthis . Genetic Resources and Crop Evolution, 54, 327–336. 10.1007/s10722-005-4897-2 [DOI] [Google Scholar]
- De‐Winter, B. (1990). A new species of Citrullus (Benincaseae) from the Namib desert. Namibia. Bothalia, 3(20), 209–211. [Google Scholar]
- Dje, Y. , Tahi, C. G. , Bi, A. I. , Baudoin, J.‐P. , & Bertin, P. (2010). Use of ISSR markers to assess genetic diversity of African edible seeded Citrullus lanatus landraces. Scientia Horticulturae, 124(2), 159–164. [Google Scholar]
- Endl, J. , Achigan‐Dako, E. G. , Pandey, A. K. , Monforte, A. J. , Pico, B. , & Schaefer, H. (2018). Repeated domestication of melon (Cucumis melo) in Africa and Asia and a new close relative from India. American Journal of Botany, 105(10), 1662–1671. [DOI] [PubMed] [Google Scholar]
- FAOSTAT (2017). Watermelon production in 2014. from Crops/Regions (World list)/Production quantity (from pick lists).
- Fu, Y. X. (1997). Statistical neutrality of mutations against population growth, hitchhiking and background selection. Genetics, 147, 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fursa, T. B. (1972). K sistematike roda Citrullus Schrad. [On the taxonomy of genus Citrullus Schrad.]. Botanicheskii Zhurnal, 57, 31–41. [Google Scholar]
- Fursa, T. B. (1981). Intraspecific classification of water‐melon under cultivation. Die Kulturpflanze, 29(1), 297–300. [Google Scholar]
- Fursa, T. B. (1983). Novyi vid arbuza Citrullus mucosospermus (Fursa) Fursa. (A new species of watermelon Citrullus mucosospermus (Fursa) Fursa.). Trudy Po Prikladnoi Botanike Genetike I Selektsii 1(81), 108–112. [Google Scholar]
- Grivet, D. (2002). Phylogéographie et évolution moléculaire comparée d’arbres forestiers à l’aide des marqueurs chloroplastiques. (PhD), Université Henri Poincaré, Nancy‐I [Google Scholar]
- Guicking, D. , Fiala, B. , Blattner, F. R. , Slik, F. , Mohamed, M. , & Weising, K. (2011). Comparative chloroplast DNA phylogeography of two tropical pioneer tree, Macaranga gigantea and Macaranga pearsonii (Euphorbiaceae). Tree Genetics & Genomes, 7, 573–585. [Google Scholar]
- Guo, S. , Zhang, J. , Sun, H. , Salse, J. , Lucas, W. J. , Zhang, H. , … Xu, Y. (2013). The draft genome of Watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nature Genetics, 45, 51–58. [DOI] [PubMed] [Google Scholar]
- Gusmini, G. , Whener, C. T. , & Jarret, L. R. (2004). Inheritance of egusi seed type in watermelon. Journal of Heredity, 95, 268–270. [DOI] [PubMed] [Google Scholar]
- Hammer, K. , & Gladis, T. (2014). Notes on infraspecific nomenclature and classifications of cultivated plants in Compositae, Cruciferae, Graminae (with a remark on Triticum dicoccon Schrank) and Leguminosae. Genetic Resources and Crop Evolution, 61(8), 1455–1467. [Google Scholar]
- Hashizume, T. , Ikuhiro, S. , Yoshiaki, H. , Mamiko, Y. , Takanori, S. , & Tsuyoshi, I. H. (1996). Construction of a linkage map for watermelon (Citrullus lanatus (Thunb.) Matsum & Nakai) using random amplified polymorphic DNA (RAPD). Euphytica, 90(3), 265–273. [Google Scholar]
- Hudson, R. R. , Boos, D. D. , & Kaplan, N. L. (1992). A statistical test for detecting geographic subdivision. Molecular Biology and Evolution, 9(1), 138–151. [DOI] [PubMed] [Google Scholar]
- Jakob, S. S. , & Blattner, F. R. (2006). A chloroplast genealogy of Hordeum (Poaceae): Long‐term persisting haplotypes, incomplete lineage sorting, regional extinction, and the consequence for phylogenetic inference. Molecular Biology and Evolution, 23(8), 1602–1612. [DOI] [PubMed] [Google Scholar]
- Jarret, R. L. , Merrick, L. C. , Holms, T. , Evans, J. , & Aradhya, M. K. (1997). Simple sequence repeats in watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai). Genome, 40, 433–441. [DOI] [PubMed] [Google Scholar]
- Jarret, R. L. , & Newman, M. (2000). Phylogenetic relationships among species of Citrullus and the placement of C. rehmii De Winter as determined by internal transcribed spacer (ITS) sequence heterogeneity. Genetic Resources and Crop Evolution, 47(2), 215–222. [Google Scholar]
- Jeffrey, C. (2005). A new system of Cucurbitaceae. Bot Zhur, 90, 332–335. [Google Scholar]
- Jiang, N. , Man, L. , Zhang, W. , Dong, H.‐X. , Wang, H.‐Y. , Li, M.‐R. , Shi, F.‐X. , & Sun, M.‐Z. (2016). Chloroplast view of the population genetics and phylogeography of a widely distributed shrub species, Rhododendron dauricum (Ericaceae). Systematic Botany, 41(3), 626–633. [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. , Buxton, S. , Cooper, A. , Markowitz, S. , Duran, C. , Thierer, T. , Ashton, B. , Mentjies, P. , & Drummond, A. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocyan, A. , Zhang, L.‐B. , Schaefer, H. , & Renner, S. S. (2007). A multi‐locus chloroplast phylogeny for the Cucurbitaceae and its implications for character evolution and classification. Molecular Phylogenetics and Evolution, 44(2), 553–577. 10.1016/j.ympev.2006.12.022 [DOI] [PubMed] [Google Scholar]
- Levi, A. , Simmons, A. M. , Massey, L. , Coffey, J. , Wechter, W. P. , Jarret, R. L. , Tadmor, Y. , Nimmakayala, P. , & Reddy, U. K. (2017). Genetic diversity in the desert watermelon Citrullus colocynthis and its relationship with Citrullus species as determined by high‐frequency oligonucleotides‐targeting active gene markers. Journal of the American Society for Horticultural Science, 142(1), 47–56. 10.21273/JASHS03834-16 [DOI] [Google Scholar]
- Levi, A. , Thies, J. A. , Wechter, P. W. , Harrison, H. F. , Simmons, A. M. , Reddy, U. K. , Nimmakayala, P. , & Fei, Z. (2013). High frequency oligonucleotides: Targeting active gene (HFO‐TAG) markers revealed wide genetic diversity among Citrullus Spp. accession useful for enhancing diseases or pest resistance in watermelon cultivars. Genetic Resources and Crop Evolution, 14(60), 427–440. [Google Scholar]
- Levi, A. , & Thomas, C. E. (2005). Polymorphisms among chloroplast and mitochondrial genomes of Citrullus species and subspecies. Genetic Resources and Crop Evolution, 52(5), 609–617. 10.1007/s10722-004-2600-7 [DOI] [Google Scholar]
- Levi, A. , Thomas, C. E. , Keinath, A. P. , & Wehner, T. C. (2001). Genetic diversity among watermelon (Citrullus lanatus and Citrullus colocynthis) accessions. Genetic Resources and Crop Evolution, 48(6), 559–566. [Google Scholar]
- Levi, A. , Thomas, C. E. , Newman, M. , Reddy, O. , Zhang, X. , & Xu, Y. (2004). ISSR and AFLP markers differ among American watermelon cultivars with limited genetic diversity. Journal of the American Society for Horticultural Science, 129(4), 553–558. 10.21273/JASHS.129.4.0553 [DOI] [Google Scholar]
- Librado, P. , & Rozas, J. (2009). DnaSP v5: A software for comprehensive of analysis of DNA polymorphism data. Bioinformatics, 25, 1451–1452. [DOI] [PubMed] [Google Scholar]
- Lynch, M. , & Crease, T. J. (1990). The analysis of population survey data on DNA sequence variation. Molecular Biology and Evolution, 7(4), 377–94. 10.1093/oxfordjournals.molbev.a040607. [DOI] [PubMed] [Google Scholar]
- Martin, J.‐F. , Novoa, C. , Lanc‐manel, S. B. , & Taberlet, P. (2003). Les populations de perdrix grise des Pyrénées (Perdix perdix hispaniensis) ont‐elles subi une introgression génétique à partir d'individus d'élevage ? Analyse du polymorphisme de l'ADN mitochondrial. Les Actes Du BRG, 4, 115–126. [Google Scholar]
- Minsart, L.‐A. , Zoro bi, I. A. , Djè, Y. , Baudoin, J.‐P. , Jacquemart, A.‐L. , & Bertin, P. (2011). Set up of simple sequence repeat markers and first investigation of the genetic diversity of West‐African watermelon (Citrullus lanatus ssp. vulgaris oleaginous type). Genetic Resources and Crop Evolution, 58(6), 805–814. 10.1007/s10722-010-9617-x [DOI] [Google Scholar]
- Mujaju, C. , Sehic, J. , & Nybom, H. (2013). Assessement of EST‐SSR markers for Evaluating genetic diversity in watermelon accessions from Zimbabwe. American Journal of Plant Sciences, 4, 1448–1456. [Google Scholar]
- Mujaju, C. , Zborowska, A. , Werlemark, G. , Garkava‐Gustavssson, L. , Andersen, S. B. , & Nybom, H. (2011). Genetic diversity among and within watermelon (Citrullus lanatus) landraces in Southern Africa. Journal of Horticculture Sciences and Biotechnology, 86, 353–358. [Google Scholar]
- Nei, M. (1987). Molecular evolutionary genetics. New York: Columbia University Press. [Google Scholar]
- Nei, M. , & Chesser, R. K. (1983). Estimation of fixation indices and gene diversities. Annals of Human Genetics, 47, 253–259. 10.1111/j.1469-1809.1983.tb00993.x [DOI] [PubMed] [Google Scholar]
- Nei, M. , & Tajima, F. (1983). Maximum likelihood estimation of the number of nucleotide substitutions from restriction site data. Genetics, 105, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesom, G. L. (2011). Toward consistency of taxonomic rank in wild/domesticated Cucurbitaceae. Phytoneuron, 13, 1–33. [Google Scholar]
- O’Donnell, K. (1992). Ribosomal DNA internal transcribed spacers are highly divergent in the phytopathogenic ascomycete Fusarium sambucinum (Gibberella pulicaris). Current Genetics, 22, 213–220. 10.1007/BF00351728 [DOI] [PubMed] [Google Scholar]
- Paris, H. S. (2015). Origin and emergence of the sweet dessert watermelon, Citrullus lanatus . Annals of Botany, 116, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons, O. , & Petit, R. J. (1996). Measuring and testing genetic differentiation with ordered versus unordered allells. Genetics, 144, 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner, S. S. , Chomicki, G. , & Greuter, W. (2014). Proposal to conserve the name Momordica lanata (Citrullus lanatus) (watermelon, Cucurbitaceae), with a conserved type, against Citrullus battich . Taxon, 63(4), 941–942. [Google Scholar]
- Renner, S. S. , Pérez‐Escobar, O. A. , Silber, M. V. , Nesbitt, M. , Preick, M. , Hofreiter, M. , & Chomicki, G. (2019). A 3500‐year‐old leaf from a pharaonic tomb reveals that new kingdom egyptians were cultivating domesticated watermelon. bioRxiv, 642785. [Google Scholar]
- Rozas, J. , & Rozas, R. (1997). DnaSP version 3: An integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics, 2(15), 174–175. [DOI] [PubMed] [Google Scholar]
- Schaefer, H. , & Renner, S. S. (2011a). Cucurbitaceae In Kubitzki K. (Ed.), The families and genera of vascular plants, vol. 10, Sapindales, Cucurbitales, Myrtaceae (pp. 112–174). : Springer. [Google Scholar]
- Schaefer, H. , & Renner, S. S. (2011b). Phylogenetic relationships in the order Cucurbitales and a new classification of the gourd family (Cucurbitaceae). Taxon, 60(1), 122–138. 10.1002/tax.601011 [DOI] [Google Scholar]
- Shaw, J. , Lickey, E. B. , Schilling, E. E. , & Small, R. L. (2007). Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in Angiosperm: The tortoise and hare III. American Journal of Botany, 94(3), 275–288. [DOI] [PubMed] [Google Scholar]
- Solmaz, I. , & Sari, N. (2009). Characterization of watermelon (Citrullus lanatus) accessions collected from Turkey for morphological traits. Genetic Resources and Crop Evolution, 56(2), 173–188. 10.1007/s10722-008-9353-7 [DOI] [Google Scholar]
- Solmaz, I. , Sari, N. , Aka‐Kacar, Y. , & Yalcin‐Mendi, N. Y. (2010). The genetic characterization of Turkish watermelon (Citrullus lanatus) accessions using RAPD markers. Genetic Resources and Crop Evolution, 57(5), 763–771. 10.1007/s10722-009-9515-2 [DOI] [Google Scholar]
- Sun, R. , Lin, F. , Huang, P. , Ye, X. , Lai, J. , & Zheng, Y. (2019). Phylogeographical structure of Liquidambar formosana Hance revealed by chloroplast phylogeography and species distribution models. Forests, 10(10), 858 10.3390/f10100858 [DOI] [Google Scholar]
- Templeton, A. R. (1996). Contingency tests of neutrality using intra/interspecific gene trees: The rejection of neutrality for the evolution of the mitochondrial cytochrome oxidase II gene in hominoid primates. Genetics, 144, 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylikowa, K. , & Van der Veen, M. (2004). An archaeobotanical contribution to the history of watermelon, Citrullus lanatus (Thunb.) Matsum. & Nakai (syn. C. vulgaris Schrad.). Vegetation History & Archaeobotany, 13(4), 213–217. [Google Scholar]
- Whitaker, T. W. , & Bemis, B. W. (1976). Cucurbits Simmonds N. W (e.d). Evolution of Crop Plants, 6, 64–69. [Google Scholar]
- Zhao, Y. , Pan, B. , & Zhang, M. (2019). Phylogeography and conservation genetics of the endangered Tugarinovia mongolica (Asteraceae) from Inner Mongolia, Northwest China. PLoS One, 14(2), e0211696 10.1371/journal.pone.0211696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary, D. , & Hopf, M. (2000). Domestication of plants in the old World. : Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
DNA sequences: NCBI GenBank accession numbers are provided in Table S1. Dryad: https://doi.org/10.5061/dryad.31zcrjdjw


