Abstract
Arctic animals inhabit some of the coldest environments on the planet and have evolved physiological mechanisms for minimizing heat loss under extreme cold. However, the Arctic is warming faster than the global average and how well Arctic animals tolerate even moderately high air temperatures (T a) is unknown.
Using flow‐through respirometry, we investigated the heat tolerance and evaporative cooling capacity of snow buntings (Plectrophenax nivalis; ≈31 g, N = 42), a cold specialist, Arctic songbird. We exposed buntings to increasing T a and measured body temperature (T b), resting metabolic rate (RMR), rates of evaporative water loss (EWL), and evaporative cooling efficiency (the ratio of evaporative heat loss to metabolic heat production).
Buntings had an average (±SD) T b of 41.3 ± 0.2°C at thermoneutral T a and increased T b to a maximum of 43.5 ± 0.3°C. Buntings started panting at T a of 33.2 ± 1.7°C, with rapid increases in EWL starting at T a = 34.6°C, meaning they experienced heat stress when air temperatures were well below their body temperature. Maximum rates of EWL were only 2.9× baseline rates at thermoneutral T a, a markedly lower increase than seen in more heat‐tolerant arid‐zone species (e.g., ≥4.7× baseline rates). Heat‐stressed buntings also had low evaporative cooling efficiencies, with 95% of individuals unable to evaporatively dissipate an amount of heat equivalent to their own metabolic heat production.
Our results suggest that buntings’ well‐developed cold tolerance may come at the cost of reduced heat tolerance. As the Arctic warms, and this and other species experience increased periods of heat stress, a limited capacity for evaporative cooling may force birds to increasingly rely on behavioral thermoregulation, such as minimizing activity, at the expense of diminished performance or reproductive investment.
Keywords: Arctic climate change, evaporative cooling efficiency, evaporative water loss, heat dissipation, snow bunting, thermal physiology, thermoregulatory polygon
We tested the hypothesis that the evolved physiological mechanisms promoting cold tolerance in snow buntings (Plectrophenax nivalis) would result in a limited capacity to tolerate moderate heat. We show that buntings experiencing increasing air temperature begin to evaporate water at temperatures much lower than what is typically seen in more heat‐tolerant songbirds. Most importantly, buntings were extremely inefficient at evaporative cooling, with 95% of heat‐stressed buntings unable to dissipate an amount of heat equivalent to their own metabolic heat production

1. INTRODUCTION
The Arctic is warming faster than the global average (Overland et al., 2019), impacting both the flora and fauna (CAFF, 2013). Among Arctic birds, climate change has already impacted populations through habitat loss (Gilg et al., 2016), phenological shifts (Moe et al., 2009), and increased predation risk (Smith et al., 2010). However, the direct costs of increasing ambient temperature on the thermoregulatory demands of Arctic birds have garnered far less attention (Gaston et al., 2002). This is particularly concerning given that Arctic species are highly adapted to cold environments and the physiological mechanisms enhancing cold tolerance may increase thermal sensitivity to, and reduce thermoregulatory capacity at, warmer temperatures (Angilletta et al., 2010; Boyles et al., 2011). For example, thick‐billed murres (Uria lomvia) can die during incubation when exposed to full sun and daily maximum air temperature of only 16°C (Gaston & Elliott, 2013; Gaston et al., 2002). The paucity of information on Arctic birds’ capacity to physiologically tolerate warmer temperatures is a major impediment to predicting community responses to climate change, especially given their potentially limited ability to cope with heat.
Quantifying avian physiological capacity to tolerate warmer temperatures is fundamental for predicting the impact of climate change on avian biodiversity across biomes (Albright et al., 2017; McKechnie & Wolf, 2010). For example, avian sensitivity to heat has helped link population declines in Mojave Desert birds to climate change‐driven increases in evaporative cooling demands (Riddell et al., 2019). In Australia, intense heat waves have exceeded species' physiological heat tolerance limits, resulting in mass die‐off events (McKechnie et al., 2012). While heat waves produce the most dramatic effects, recent evidence suggests that the fitness costs of climate change will likely occur via sublethal effects from chronic exposure to warmer temperatures (Conradie et al., 2019; Gardner et al., 2015). Specifically, rising temperatures may force birds to increase thermoregulatory behaviors (e.g., shade seeking) at the expense of other essential activities (e.g., foraging; Oswald et al., 2019; Silva et al., 2015). These trade‐offs could significantly affect body condition and fitness of adults and/or nestlings (Cunningham et al., 2013; du Plessis et al., 2012; Van de Ven et al., 2019). Currently, investigations of behavioral trade‐offs and consequent fitness costs from chronic exposure to sublethal temperatures come mostly from arid bird communities, despite recent evidence suggesting that temperate species may also face thermal constraints to increasingly warm temperatures (e.g., Milne et al., 2015; Nilsson & Nord, 2018; Nord & Nilsson, 2019; Oswald et al., 2018; Tapper et al., 2020). However, we also require data on the heat tolerance capacity of Arctic birds as this is necessary to inform predictions of when and how increasing temperatures could impose thermal constraints that would force behavioral trade‐offs and, ultimately, impact fitness.
To address this issue, we investigated the heat tolerance and evaporative cooling capacity of a free‐living population of snow buntings (Plectrophenax nivalis) in the Canadian High Arctic. Buntings are a cold specialized, circumpolar migrant passerine that spends most of its life in cold environments. Indeed, buntings overwinter in snowy climates before migrating north through equally harsh conditions during the spring, only to arrive on their Arctic breeding grounds when Ta remains below freezing (Meltofte, 1983; Snell et al., 2018). Consequently, buntings have evolved physiological traits to withstand extreme cold (Scholander et al., 1950) and can tolerate experimental temperatures as low as −90°C (Le Pogam et al., 2020). Only later in the season, near the breeding period, do buntings regularly experience maximum temperatures above freezing (Meltofte, 1983). Importantly, in many northern locations temperatures are increasing during the breeding months (Zhang et al., 2019), and this period coincides with the energetically demanding behavior of feeding nestlings. Hence, snow buntings offer an excellent opportunity to examine whether cold‐adapted birds have a limited capacity to physiologically tolerate increasing exposure to moderate heat.
We examined heat tolerance in buntings by measuring responses in body temperature (T b), resting metabolic rate (RMR), and rates of evaporative water loss (EWL) of individuals exposed to increasing air temperature (T a). For each physiological trait, we determined the onset of heat stress by identifying T a inflection points, which represent the T a where the trait starts to change abruptly due to increasing heat. We predicted that relative to previously studied, noncold specialist songbirds, buntings would display inflection points at lower T a, resulting in an inability to tolerate maximum T a typically observed in more heat‐tolerant species.
2. MATERIALS AND METHODS
2.1. Study species and site
We studied snow buntings between May and July 2018 (n = 22 birds) and 2019 (n = 20) at Alert, Nunavut, Canada (82°30′05″N, 62°20′20″W). We used walk‐in traps baited with mixed seeds, or potter traps paired with a decoy bird and playback of a bunting call, to capture birds. Additionally, we captured nestling‐provisioning adults at nest entrances after they entered a nest, in which case only one adult was captured at a time, allowing the other parent to continue provisioning. Once captured, we transferred buntings to our field laboratory where they were held in indoor cages (76 cm W × 46 cm D × 45 cm H) for an average of 1.9 ± 2.2 days before respirometry measurements. Birds were maintained on a diet of mixed seeds supplemented with mealworms. All birds were weighed at capture and before respirometry measurements. Average (±SD) body mass (M b) at capture was 33.7 ± 2.5 g and before respirometry measurements was 31.0 ± 2.1 g.
2.2. Temperature and gas exchange measurements
We recorded Tb using two methods. In 2018, we measured core T b using a type‐T thermocouple inserted ≈1 cm into the cloaca. Prior to insertion, the thermocouple tip was lubricated with Vaseline. The thermocouple wire was secured in place on the underside of the tail with masking tape. This technique has been extensively used (see Milne et al., 2015; Prinzinger et al., 1991) and birds calmed down within minutes after insertion. The thermocouple was connected to a Sable Systems thermocouple meter (model TC‐2000, Las Vegas, NV, USA) that measured T b every second. In 2019, we measured subcutaneous T b using a temperature‐sensitive passive integrated transponder (PIT) tag (Biomark) implanted subcutaneously into the right flank under the wing (Nord et al., 2016). As with the thermocouple, birds calmed down within minutes after implantation. We recorded T b every 20 s using a portable transceiver system (model HPR Plus, Biomark) connected to an external racket antenna placed beside the metabolic chamber. After the 2019 field season, we compared a subset of 30 PIT tags in a circulating water bath against a type‐T thermocouple and thermocouple meter (model TC‐2000, Sable Systems). Thermocouple and PIT tag readings were recorded at water temperatures between 40 and 46°C at 2°C increments. On average, PIT tag readings deviated from the thermocouple by 0.2 ± 0.1°C.
To determine RMR and rates of EWL, we measured oxygen consumption (ml/min) and water vapor pressure (WVP; kPa), respectively, using flow‐through respirometry. We placed buntings individually inside a 2.6‐L plastic metabolic chamber fitted with a mesh base with spaces large enough for urine and feces to fall through and into a reservoir of mineral oil. The oil prevented evaporation from excrement affecting WVP measurements. We placed the metabolic chamber inside a temperature‐controlled cabinet fitted with a Peltier heating unit (model T35 DC‐S, Mobicool International). We monitored and regulated the T a inside the cabinet using an Omega benchtop controller (model CSi32T). We measured Ta inside the metabolic chamber with a type T thermocouple secured underneath the lid and connected to the thermocouple meter.
We pushed atmospheric air through the metabolic chamber with an aquarium air pump (model AAPA15L, Active AQUA). Atmospheric air first passed through columns of silica gel, soda lime, and drierite connected in series to scrub the airstream of water vapor and CO2. Once scrubbed, the airstream was split into a baseline channel, which went directly to the analyzers and another channel, which flowed toward the metabolic chamber. We controlled the flow rate of air entering the metabolic chamber with an Omega mass flow controller (model FMA5418A), calibrated against a soap bubble meter (Bubble‐O‐Meter). We maintained flow rates at 2,000 ml/min during the 2018 field season and at 2,500 ml/min in the 2019 field season. These flow rates produced chamber dew points ranging from −20.0 to 9.5°C (maximum absolute humidity = 8.2 g/m3 at T a = 42.2°C) and the system reached 95% of its final value after either 3.1 or 3.9 min, based on equation 8.1 of Lighton (2019).
We subsampled the incurrent baseline and excurrent chamber airstreams by manually switching between them using a MUX Flow‐Multiplexer (Sable Systems). Subsampled air first passed through a relative humidity and dew point analyzer (model RH‐300; Sable Systems) for the measurement of WVP. The airstream was then scrubbed of water vapor and CO2 before entering a Foxbox field gas analysis system (Sable Systems) for the measurement of oxygen consumption. We digitized voltage outputs from all the analyzers using a Sable Systems Universal Interface (model UI‐2) and logged analyzer outputs at a sampling rate of 1 s with Expedata software (Sable Systems).
2.3. Experimental protocol
We performed respirometry measurements between 10:00 and 00:00 hr depending on the time of capture and the need to process birds as quickly as possible. Once placed inside the metabolic chamber, we gave buntings a 30‐min habituation period to acclimate to the chamber before being exposed to a ramped T a profile. In 2018, we started birds at T a ≈ 25°C with an increase to 30°C and then at 2°C increments. In 2019, we started measurements at T a ≈ 30°C with subsequent increases at 2°C increments. We began measurements at 30°C in 2019 as our primary goal during this second field season was to increase sample sizes at higher T a, and this T a typically did not invoke heat stress in 2018. Once chamber T a stabilized, we recorded data on buntings for 10–20 min before increasing T a. A 10‐min baseline was recorded at the beginning and end of each run to control for analyzer drift. We continuously monitored the behavior of each focal bird using a SmoTecQ dome infrared camera (model DF‐3500‐AHD 1080P) and video capture software (ArcSoft ShowBiz, v. 3.5.15.68). We ended runs if buntings displayed continuous escape behavior (e.g., pecking at the walls of the chamber or jumping), or a T b ≥ 45°C. After each run, we immediately measured the bird's mass, provided them with fresh water, and returned them to their cage for release.
2.4. Data analyses
We first corrected the oxygen consumption and WVP traces for drift and time lag using the appropriate operations in Expedata. At each T a, we measured resting values of oxygen consumption, WVP, and T b using the mean of the most stable 5‐min period from the oxygen consumption trace. We did not include any data from birds that did not remain calm for at least 5 min at a given T a. We calculated rates of oxygen consumption using equation 10.1 of Lighton (2019). To transform oxygen consumption into RMR (Watts [W]), we used equation 9.13 of Lighton (2019) to derive energy equivalents (J/mlO2) assuming a respiratory quotient (RQ) of 0.71. However, in some cases (12%), birds were not fasted for more than 62 min (mean retention time for a 31 g bird; Karasov, 1990), and for these birds, we assumed an RQ of 0.80 (Lighton, 2019). We calculated rates of EWL (mg/min) by converting WVP into water vapor density and then multiplying by the incurrent flow rate. We converted rates of EWL into evaporative heat loss (EHL; W) assuming 2.406 J/mgH2O. We determined how efficient buntings were at dissipating body heat by calculating their evaporative cooling efficiency, which represents the ratio between EHL and metabolic heat production (EHL/MHP). Higher EHL/MHP values indicate greater evaporative cooling efficiency (Lasiewski et al., 1966).
We performed all statistical analyses in R 4.0.0 (R Core Team, 2020), and all values reported are means ± standard deviation (SD), unless noted otherwise. During our initial analyses, we found that T b varied considerably at a given T a depending on measuring technique (Supporting information). However, because recent heat tolerance investigations measured core T b (e.g., McKechnie et al., 2017; Smith et al., 2017; Whitfield et al., 2015), and thus to facilitate comparisons, we decided to only report our core T b values measured in the cloaca.
We first located an inflection point for each response variable, namely T b, RMR, EWL, and EHL/MHP, by fitting a piecewise linear regression model to the data with all birds combined using the SiZer package (Sonderegger, 2020). For each response variable, we subsequently fitted a linear mixed‐effect model to the data above the inflection point using the lme4 package (Bates et al., 2015). Each mixed‐effect model included T a and M b as continuous predictors. We included bird identity as a random intercept in all our models to account for repeated measurements within the same bird. We built a global model with all predictors and their two‐way interaction (i.e., T a:M b). We performed model selection on the global models using the “dredge” function in the MuMIn package (Bartoń, 2020). Models with an Akaike information criterion adjusted for small sample sizes (AICc) less than 8 (i.e., ∆AICc < 8) were considered to fit the data equally well (Burnham et al., 2011). Additionally, we used the model weights for each model to assess their relative strength of support, with models having a weight > 0.90 considered to exhibit overwhelming support as the best approximating model relative to all the other candidate models (Grueber et al., 2011). We further explored each top model and report the parameter estimates and accompanying standard errors (β ± SE), 95% confidence intervals (95% CI), and t‐values for each fixed effect in the model.
We assessed the overall fit of the global and top candidate models by visually inspecting the residuals for normality and homogeneity. Additionally, we tested for outliers in all the models by calculating a Cook's distance value for every bird using the influence.ME package (Nieuwenhuis et al., 2012). We considered birds with a Cook's distance value > 1 as highly influential on the parameter estimates (Logan, 2010). One model had Cook's distance values > 1, and instead of removing these values from the data set, we fitted a robust mixed‐effect model to the data using the robustlmm package (Koller, 2016). All figures were made using ggplot2 (Wickham, 2016), and the 95% CI around the regression predictions was calculated in ggeffects (Lüdecke, 2018).
3. RESULTS
3.1. Body temperature
Snow bunting body temperature showed an inflection point at T a = 32.6°C (95% CI = 31.0–34.4°C; Figure 1). Above the inflection point, the top candidate model fitted to the data included only T a and was much better supported than models including M b or the T a:M b interaction (Table 1). Body temperature had a positive linear relationship with Ta above the inflection point (n = 21, Figure 1; Table 2). Body temperature increased from 41.3 ± 0.2°C (n = 6) at T a ≈ 26°C to 43.5 ± 0.3°C (n = 5) at T a ≈ 39.0°C.
FIGURE 1.
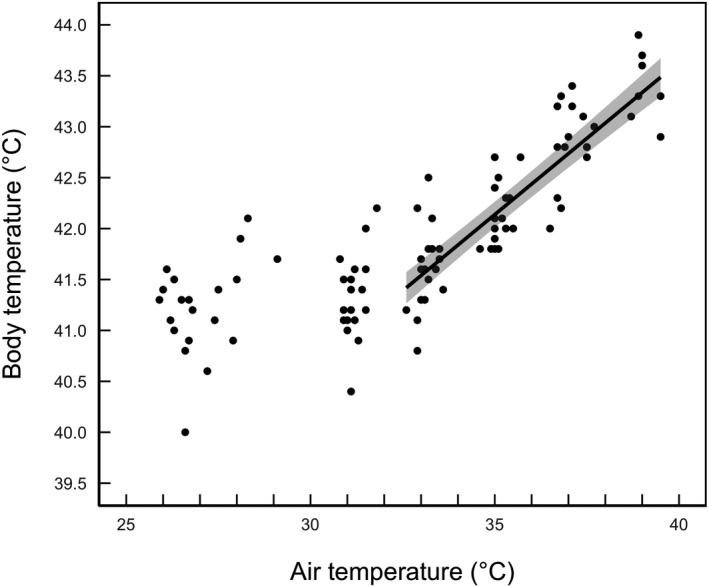
The relationship between core body temperature (T b) measured in the cloaca and air temperature (T a). The regression line represents the slope from a linear mixed‐effects model of T b regressed against T a above the inflection point (32.6°C). The shaded area represents the 95% confidence intervals around the predicted values
TABLE 1.
Top candidate models after model selection with an Akaike information criterion adjusted for small sample size less than 8 (i.e., ∆AICc < 8)
| Variable | Top models a | logLik | AICc | ∆AICc | Model weight |
|---|---|---|---|---|---|
| T b | T a | −17.759 | 44.29 | 0.000 | 0.940 |
| T a + M b | −19.322 | 49.82 | 5.532 | 0.059 | |
| RMR | T a | 82.842 | −157.39 | 0.000 | 0.965 |
| T a + M b | 80.484 | −150.53 | 6.865 | 0.031 | |
| EWL | T a | 59.491 | −110.33 | 0.000 | 0.991 |
| EHL/MHP | T a | 13.147 | −17.04 | 0.000 | 0.641 |
| T a + M b | 13.910 | −15.88 | 1.161 | 0.359 |
Models reflect data above the inflection points. Model selection was performed on four separate global models, each with a different response variable, namely body temperature (T b), resting metabolic rate (RMR), evaporative water loss (EWL), and the ratio of evaporative heat loss to metabolic heat production (i.e., evaporative cooling efficiency; EHL/MHP). Model fixed effects were air temperature (T a) and body mass (M b). Models with a weight > 0.90 were considered to have overwhelming support.
Global model included T a + M b + T a:M b.
TABLE 2.
Parameter estimates (β ± standard error) from the top linear mixed‐effects models (see Table 1) explaining variation in body temperature (T b), resting metabolic rate (RMR), rates of evaporative water loss rate (EWL), and the ratio of evaporative heat loss to metabolic heat production (i.e., evaporative cooling efficiency; EHL/MHP)
| Variable | T a inflection | β ± SE | 95% CI | t‐Value |
|---|---|---|---|---|
| T b (°C) | 32.6°C | – | – | – |
| Intercept | – | 31.66 ± 0.60 | 30.47 to 32.84 | 52.49 |
| T a | – | 0.299 ± 0.017 | 0.266 to 0.333 | 17.62 |
| RMR (Watts) | 29.8°C | – | – | – |
| Intercept | – | 0.208 ± 0.103 | 0.007 to 0.410 | 2.03 |
| T a | – | 0.014 ± 0.003 | 0.009 to 0.020 | 5.10 |
| EWL (g/hr) | 34.6°C | – | – | – |
| Intercept | – | −2.00 ± 0.15 | −2.30 to −1.70 | −13.22 |
| T a | – | 0.068 ± 0.004 | 0.060 to 0.076 | 16.66 |
| EHL/MHP | 36.7°C | – | – | – |
| Intercept | – | −2.05 ± 0.32 | −2.68 to −1.43 | −6.43 |
| T a | – | 0.068 ± 0.008 | 0.052 to 0.084 | 8.24 |
Parameter estimates are derived from models fitted to the data above the calculated air temperature inflection points (T a inflection). The 95% confidence intervals (95% CI) and t‐values from the models are included.
3.2. Resting metabolic rate
Resting metabolic rate had an inflection point at T a = 29.8°C (95% CI = 27.9–42.2; Figure 2). Above the inflection point, the top candidate model included only T a and had overwhelming support compared with the other candidate models (Table 1). Above the inflection point, RMR increased gradually with T a (Table 2, Figure 2). Over the range of temperatures measured, RMR displayed a 1.4‐fold increase, from 0.588 ± 0.105 W at T a ≈ 26 to 0.804 ± 0.517 W at T a ≈ 43°C.
FIGURE 2.
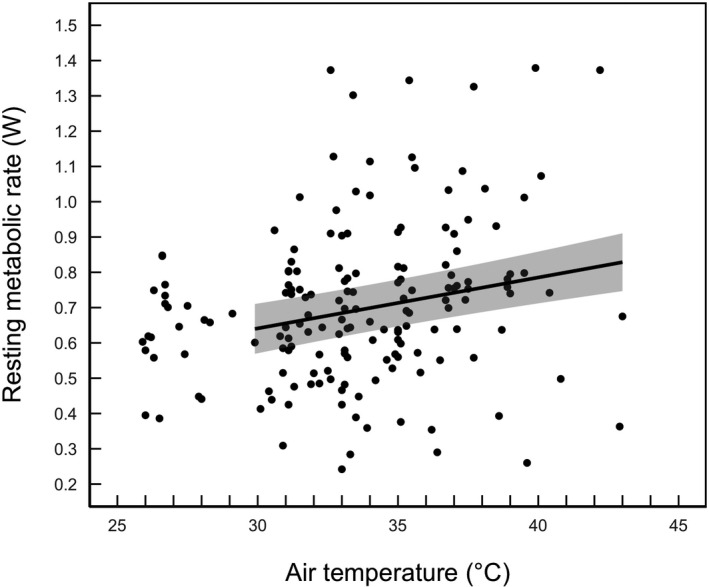
The relationship between resting metabolic rate (RMR) and air temperature (T a) in snow buntings. The regression line represents the slope from a linear mixed‐effects model of RMR against T a fitted to data above the inflection point (29.8°C). The shaded area represents the 95% confidence intervals around the predicted values
3.3. Evaporative water loss
On average, buntings began panting at T a = 33.2 ± 1.7°C in 2018 and 33.6 ± 1.8 in 2019. The average Tb at the start of panting was 42.0 ± 0.8°C. The onset of panting coincided with the EWL inflection point at T a = 34.6°C (95% CI = 31.1–36.2; Figure 3). Above the inflection point, the top candidate model only included T a and had overwhelming support relative to the other candidate models (Table 1). Above the inflection point, EWL displayed a positive linear relationship with T a (Table 2 and Figure 3). Buntings increased their rate of EWL 2.9‐fold relative to baseline rates at T a ≈ 26°C, reaching a maximum average rate of EWL = 0.913 ± 0.206 g/hr at T a ≈ 43°C.
FIGURE 3.
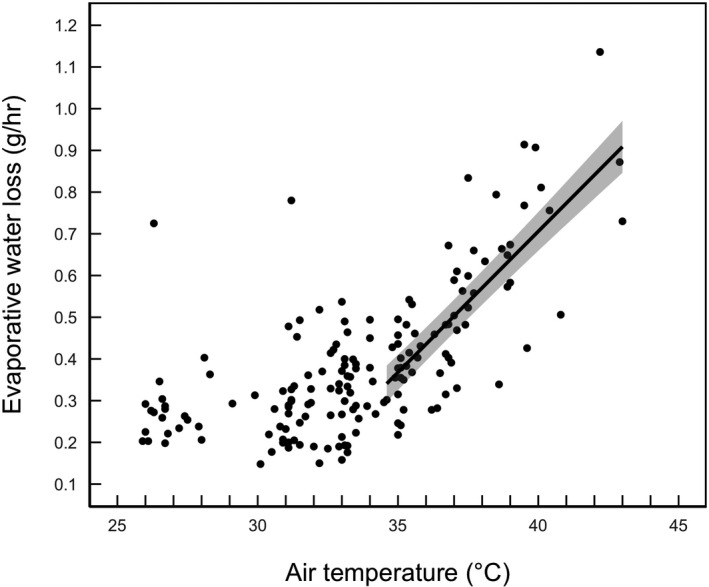
The relationship between rates of evaporative water loss (EWL) and air temperature (T a) in snow buntings. The regression line represents the slope from a linear mixed‐effects model of EWL against T a fitted to the data above the inflection point (34.6°C). The shaded area represents the 95% confidence intervals around the predicted values
3.4. Evaporative cooling efficiency
Buntings exhibited an EHL/MHP inflection point at T a = 36.7 (95% CI = 31.0–42.3°C; Figure 4). Above the inflection point, the top model explaining variation in EHL/MHP only included T a (Table 1). However, there was some support for the second model, which included M b and T a (Table 1). There was a positive linear relationship between EHL/MHP and T a above the inflection point (Table 2, Figure 4). Only two birds exceeded an EHL/MHP of 1.0 (Figure 4), indicating that most buntings were always producing more heat metabolically than they were losing evaporatively. Moreover, only 5 birds (i.e., 12%) had EHL/MHP values exceeding 0.70 (Figure 4), highlighting that buntings were extremely inefficient at dissipating heat evaporatively. The magnitude of increase in EHL/MHP was 2.8‐fold, increasing from an average of 0.348 ± 0.144 at T a ≈ 26.0°C up to an average of 0.960 ± 0.565 at T a ≈ 43.0°C.
FIGURE 4.
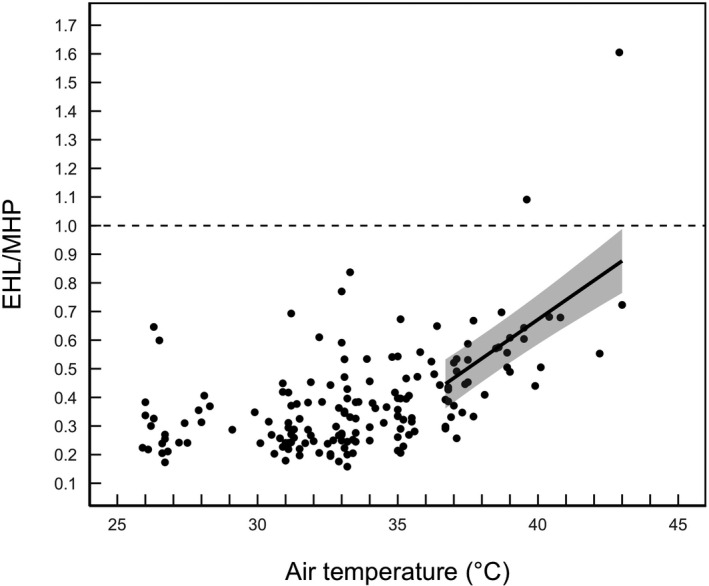
The relationship between the ratio of evaporative heat loss (EHL) to metabolic heat production (MHP) and air temperature (T a) in snow buntings. The black regression line represents the slope from a linear mixed‐effects model of EHL/MHP against T a above the inflection point (36.7°C). The shaded area represents the 95% confidence intervals around the predicted values. The horizontal dashed line represents the ratio when birds are able to evaporatively dissipate 100% of their MHP
4. DISCUSSION
Our goal was to examine the heat tolerance and evaporative cooling capacity of an Arctic songbird. As predicted, snow buntings increased their resting metabolic rate and rate of evaporative water loss at air temperatures well below their body temperature, indicating an early onset of heat stress. Moreover, buntings tolerated consistently lower ambient temperatures than previously studied heat‐tolerant songbirds. Thus, the physiological mechanisms permitting buntings’ extreme cold tolerance seem to adversely affect their heat tolerance. Indeed, heat stressed buntings exhibited low evaporative cooling efficiencies, with most individuals unable to evaporatively dissipate more than 70% of their metabolic heat production. Thus, we predict buntings will become increasingly challenged to physiologically dissipate body heat as the Arctic warms. Indeed, preliminary data collected within buntings’ Arctic breeding range show that maximum environmental operative temperatures (Bakken, 1976) can exceed 30°C (R. S. O’Connor, O. P. Love, K. H. Elliott, & F. Vézina unpublished data). Below, we compare our findings with recent heat tolerance studies on songbirds and conclude by discussing the ecological implications of our findings.
4.1. Body temperature
When exposed to increasing heat loads, birds often allow T b to increase with T a (i.e., facultative hyperthermia; Gerson et al., 2019; Tieleman & Williams, 1999). Buntings displayed increases in T b starting at T a of 32.6°C, which is within the range reported for 26 desert and nondesert species (Tieleman & Williams, 1999). Similarly, the rate at which bunting T b changed with T a is comparable to that found in other passerines (Czenze et al., 2020; McKechnie et al., 2017; Weathers, 1981). Thus, body temperature patterns of buntings under heat stress appear broadly similar to those of other avian species.
The maximum T a at which birds can defend a sublethal T b frequently correlates with the climate of origin, with species from warmer, more arid environments generally tolerating hotter temperatures (e.g., Hudson & Kimzey, 1966; McKechnie & Wolf, 2019; Noakes et al., 2016; Noakes et al., 2016; Tieleman et al., 2002). Our data support this trend, as the maximum T a at which buntings regulated T b was lower than for 24 arid‐zone passerines (Figure 5). This suggests that the physiological mechanisms enhancing heat tolerance are less pronounced in buntings and they may need to adjust their behavior at much lower environmental temperatures to avoid overheating.
FIGURE 5.
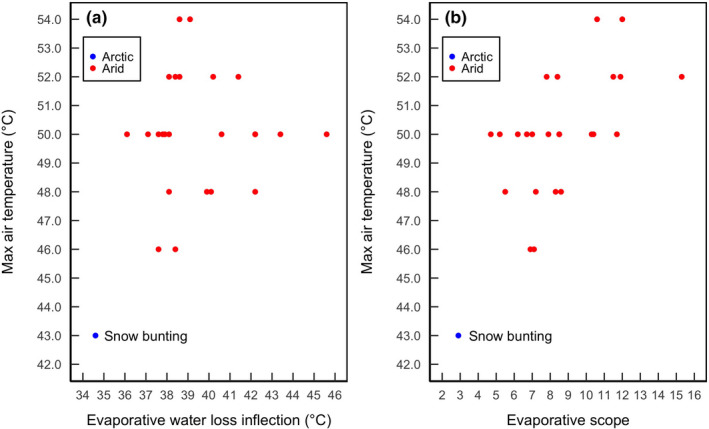
Maximum air temperature tolerated as a function of (a) evaporative water loss inflection point and (b) average evaporative scope among 24 arid‐zone passerines and Arctic snow buntings. Evaporative scope represents the ratio between the maximum rate of EWL and the minimum rate of EWL (sensu Czenze et al., 2020). Arid species data are from McKechnie et al. (2017), Smith et al. (2017), and Czenze et al. (2020)
4.2. Resting metabolic rate
Resting energy expenditure tends to vary inversely with habitat temperature, wherein birds from warmer environments have lower basal rates of heat production than species from colder environments (Jetz et al., 2008; Tieleman & Williams, 2000; Weathers, 1979; White et al., 2007). We found that at T a ≈ 26°C (i.e., within buntings’ thermoneutral zone; Scholander et al., 1950), mean RMR was 31% higher than the average minimum RMR reported in an arid population of similarly sized red‐eyed bulbuls (Pycnonotus nigricans, 30.1 g; Czenze et al., 2020). Furthermore, the basal metabolic rate (BMR) for 138 buntings (mean M b ≈ 33.1 g) measured at Alert was 0.563 W (unpublished data from authors), a value 154% of the prediction for a passerine of its size (Londoño et al., 2015). Hence, our findings are consistent with the trend that species from colder environments have higher rates of resting energy expenditure. Although a higher metabolic rate is likely advantageous for life in the cold, it presumably becomes a hindrance in warmer conditions because the higher metabolic heat production will lead to a greater total heat load that must be dissipated (Bartholomew et al., 1962).
Buntings displayed an upper critical temperature (T uc) in RMR at T a of 29.8°C, which is lower than the T uc values reported for some arid and mesic passerines, ranging from 33.9 to 44.9°C (McKechnie et al., 2017; Smith et al., 2017; Tieleman et al., 2002; Weathers, 1981). This is consistent with our prediction that buntings would exhibit signs of heat stress at lower T a relative to noncold specialist songbirds. Lower T uc values are also apparent in species from cooler habitats when directly compared to closely related species from warmer regions (e.g., Hayworth & Weathers, 1984; Tieleman et al., 2002; Weathers & van Riper, 1982). A lower T uc should limit a species heat tolerance because the early contribution of metabolic heat above basal levels will add to the total heat load that must be dissipated at a given T a.
The slope of RMR above the T uc represents the cost of thermoregulation (Weathers, 1981). Shallower slopes (i.e., less heat produced per unit of increase in T a) are expected in more heat‐tolerant species (Cooper & Gessaman, 2004) because the minimization of metabolic heat production above basal levels will lower an individual's total heat load (Bartholomew et al., 1962). Our results do not support this expectation. For example, the slope of RMR against T a for buntings (0.014 W/°C) is 43% shallower than the slope for the similarly sized, more heat‐tolerant red‐eyed bulbul (Czenze et al., 2020). Furthermore, buntings’ fractional increase in metabolic rate (i.e., maximum RMR/minimum RMR) of 1.4 is identical to the average fractional increase reported for six Sonoran Desert songbirds (Smith et al., 2017). Hence, although buntings appear to have high resting energy expenditures and a low T uc, both of which should adversely affect their heat tolerance by contributing to a greater total heat load at high T a, the incurred metabolic cost from panting does not seem to exceed that of more heat‐tolerant species who also use panting.
4.3. Evaporative water loss
Buntings started panting at a low mean T a of 33.2°C, with a subsequent increase in EWL at T a of 34.6°C. Milne et al. (2015) reported a panting T a value of 33.6°C in a population of cape rockjumpers (Chaetops frenatus) inhabiting the cool, high‐altitude regions of South Africa. Together, these findings suggest that species originating from cooler regions may experience heat stress at lower T a. These patterns starkly contrast those of more heat‐tolerant passerines. For example, among 17 arid‐zone passerines, the lowest average T a at the onset of panting was 38.0°C (Czenze et al., 2020). Moreover, the EWL inflection point and evaporative scope (i.e., max EWL/min EWL; sensu Czenze et al., 2020) were consistently lower for buntings than for 24 arid‐zone passerines (Figure 5). Recently, Czenze et al. (2020) observed that heat tolerance limits among arid‐zone passerines correlated with higher evaporative scopes. Our data conform to this pattern, as buntings displayed a low evaporative scope and a correspondingly low maximum T a (Figure 5). Buntings’ low evaporative scope presumably contributed, in part, to their limited heat tolerance capacity by constraining the amount of heat they could dissipate evaporatively.
4.4. Evaporative cooling efficiency
In contrast to more heat‐tolerant passerines (McKechnie et al., 2017; Whitfield et al., 2015), buntings exhibited generally low evaporative cooling efficiencies, with only two individuals evaporatively dissipating more heat than produced metabolically (cooling efficiencies of 1.09 and 1.61). Moreover, 88% of buntings could not dissipate more than 70% of their own metabolic heat through evaporation, further exemplifying how inefficient buntings are at dissipating body heat. Interestingly, Oswald et al. (2018) measured cape rockjumpers up to T a of 42°C and found that no birds exceeded an EHL/MHP value of 1, further suggesting that species that regularly inhabit cooler climates are potentially more vulnerable to moderate heat. The inability to efficiently dissipate their own metabolic heat production must severely limit buntings’ capacity to tolerate moderately high temperatures.
4.5. Conclusions and ecological implications
To our knowledge, this is the first study investigating how an Arctic songbird responds physiologically to warmer temperatures. We found that buntings had a limited capacity to tolerate increasing temperatures, manifested through several interacting physiological traits: (a) high rates of resting energy expenditure (e.g., basal heat production), (b) early onset of increases in resting metabolic rate and evaporative water loss under warming conditions, and (c) a limited evaporative scope. These factors culminated in buntings having generally low evaporative cooling efficiencies. Indeed, most buntings were incapable of evaporatively dissipating an amount of heat equivalent to their own metabolic heat production. These findings suggest that the physiological mechanisms permitting extreme cold tolerance in buntings, and possibly Arctic birds generally, inhibit their capacity to tolerate even moderately warm conditions. By the late 21st century, annual mean temperature across Canada could increase by more than 6°C, with the greatest warming occurring in northern regions (Zhang et al., 2019). Under this scenario, buntings will increasingly encounter environmental temperatures exceeding their physiological thresholds for heat stress. Given buntings’ extreme inefficiency for evaporative cooling, we predict they will increasingly rely on behavioral strategies for thermoregulation, which can interfere with provisioning rates and foraging efficiency (Cunningham et al., 2013; du Plessis et al., 2012). Ultimately, we expect behavioral trade‐offs to significantly impact performance during the summer breeding season, creating another example of sublethal effects of warming reducing avian fitness (Conradie et al., 2019). Hence, we argue that Arctic birds will not be exempt from thermal constraints due to increasing temperatures.
A major hurdle for leveraging thermal physiology data to predict climate change responses is extrapolating laboratory data to field scenarios (Bakken, 1976). One critical issue is that laboratory data, like ours, are collected on resting birds, whereas free‐living individuals are active and have higher sustained metabolic rates. This means that active birds will have a greater total heat load at any given temperature and should thus experience heat stress at lower environmental temperatures than predicted for resting birds. Recently, Rezende and Bacigalupe (2015) proposed a novel approach (i.e., thermoregulatory polygon) for combining the metabolic contribution of an active animal with standard respirometry variables to predict the range of conditions under which passive thermoregulation is possible. Using this framework, we estimate that buntings operating at 4 times BMR could maintain a constant Tb up to environmental temperatures of 22°C, above which they would either have to begin evaporative cooling or reduce activity to avoid hyperthermia. Hence, an active bunting would have to increase its rate of EWL at an environmental temperature of 11.2°C below the EWL inflection point reported here for resting birds. Importantly, buntings already experience environmental temperatures exceeding 22°C across their breeding range (unpublished data from authors). Thus, although we expect bunting populations to increasingly experience thermal constraints in the future, it is possible that sublethal effects of Arctic warming occurring via thermal trade‐offs (e.g., increasing thermoregulatory behaviors at the expense of nestling provisioning and development; Cunningham et al., 2013) are already occurring in these cold specialists, and possibly in cold adapted Arctic species generally.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Ryan S. O'Connor: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Writing‐original draft (equal). Audrey Le Pogam: Investigation (equal); Writing‐review & editing (equal). Kevin G. Young: Investigation (equal); Writing‐review & editing (equal). Francis Robitaille: Investigation (equal); Writing‐review & editing (equal). Emily S. Choy: Writing‐review & editing (equal). Oliver P. Love: Conceptualization (equal); Writing‐review & editing (equal). Kyle H. Elliott: Conceptualization (equal); Writing‐review & editing (equal). Anna L. Hargreaves: Conceptualization (equal); Writing‐review & editing (equal). Dominique Berteaux: Writing‐review & editing (equal). Andrew Tam: Writing‐review & editing (equal). François Vézina: Conceptualization (equal); Writing‐original draft (equal).
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank Justine Drolet and Gabrielle Roy for providing assistance in the field. This study is part of the Arctic Scope project and was supported by a FRQNT‐Équipe grant to F.V., K.H.E., A.L.H., and O.P.L. Fieldwork at Alert was also supported by Discovery grants and Northern Research Supplements from the Natural Sciences and Engineering Research Council (NSERC) of Canada to F.V. and O.P.L and by logistical and funding support by the Department of National Defence for Canada to F.V. and D.B. A travel award to R.S.O was generously provided by the Society for Experimental Biology and the Company of Biologists. All bird handling was approved by the animal care committee of the Université du Québec à Rimouski (CPA‐71‐17‐194) and was conducted under scientific (NUN‐SCI‐15‐05) and banding (10889) permits from Environment and Climate Change Canada.
O'Connor RS, Le Pogam A, Young KG, et al. Limited heat tolerance in an Arctic passerine: Thermoregulatory implications for cold‐specialized birds in a rapidly warming world. Ecol Evol. 2021;11:1609–1619. 10.1002/ece3.7141
DATA AVAILABILITY STATEMENT
Respirometry data analyzed for this study are available from the Dryad Digital Repository (https://doi.org/10.5061/dryad.4mw6m908g).
REFERENCES
- Albright, T. P. , Mutiibwa, D. , Gerson, A. R. , Smith, E. K. , Talbot, W. A. , O'Neill, J. J. , McKechnie, A. E. , & Wolf, B. O. (2017). Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proceedings of the National Academy of Sciences of the United States of America, 114, 2283–2288. 10.1073/pnas.1613625114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta, M. J., Jr. , Cooper, B. S. , Schuler, M. S. , & Boyles, J. G. (2010). The evolution of thermal physiology in endotherms. Frontiers in Bioscience, E2, 861–881. [DOI] [PubMed] [Google Scholar]
- Bakken, G. S. (1976). A heat transfer analysis of animals: Unifying concepts and the application of metabolism chamber data to field ecology. Journal of Theoretical Biology, 60, 337–384. 10.1016/0022-5193(76)90063-1 [DOI] [PubMed] [Google Scholar]
- Bartholomew, G. A. , Hudson, J. W. , & Howell, T. R. (1962). Body temperature, oxygen consumption, evaporative water loss, and heart rate in the poor‐will. Condor, 64, 117–125. 10.2307/1365480 [DOI] [Google Scholar]
- Bartoń, K. (2020). MuMIn: multi‐model inference. R package version 1.43.17. https://CRAN.R‐project.org/package=MuMIn [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Boyles, J. G. , Seebacher, F. , Smit, B. , & McKechnie, A. E. (2011). Adaptive thermoregulation in endotherms may alter responses to climate change. Integrative and Comparative Biology, 51, 676–690. 10.1093/icb/icr053 [DOI] [PubMed] [Google Scholar]
- Burnham, K. , Anderson, D. R. , & Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behavioral Ecology and Sociobiology, 65, 23–35. 10.1007/s00265-010-1029-6 [DOI] [Google Scholar]
- CAFF (2013). Arctic Biodiversity Assessment. Status and trends in Arctic biodiversity. Conservation of Arctic Flora and Fauna. [Google Scholar]
- Conradie, S. R. , Woodborne, S. M. , Cunningham, S. J. , & McKechnie, A. E. (2019). Chronic, sublethal effects of high temperatures will cause severe declines in southern African arid‐zone birds during the 21st century. Proceedings of the National Academy of Sciences of the United States of America, 116, 14065–14070. 10.1073/pnas.1821312116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, S. J. , & Gessaman, J. A. (2004). Thermoregulation and habitat preference in mountain chickadees and juniper titmice. Condor, 106, 852–861. 10.1093/condor/106.4.852 [DOI] [Google Scholar]
- Cunningham, S. J. , Martin, R. O. , Hojem, C. L. , & Hockey, P. A. R. (2013). Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly‐warming arid savanna: A study of common fiscals. PLoS One, 8(9), e74613 10.1371/journal.pone.0074613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czenze, Z. J. , Kemp, R. , van Jaarsveld, B. , Freeman, M. T. , Smit, B. , Wolf, B. O. , & McKechnie, A. E. (2020). Regularly drinking desert birds have greater evaporative cooling capacity and higher heat tolerance limits than non‐drinking species. Functional Ecology, 00, 1–12. 10.1111/1365-2435.13573 [DOI] [Google Scholar]
- du Plessis, K. L. , Martin, R. O. , Hockey, P. A. R. , Cunningham, S. J. , & Ridley, A. R. (2012). The costs of keeping cool in a warming world: Implications of high temperatures for foraging, thermoregulation and body condition of an arid‐zone bird. Global Change Biology, 18, 3063–3070. 10.1111/j.1365-2486.2012.02778.x [DOI] [PubMed] [Google Scholar]
- Gardner, J. L. , Amano, T. , Sutherland, W. J. , Clayton, M. , & Peters, A. (2015). Individual and demographic consequences of reduced body condition following repeated exposure to high temperatures. Ecology, 97, 786–795. 10.1890/15-0642.1 [DOI] [PubMed] [Google Scholar]
- Gaston, A. J. , & Elliott, K. H. (2013). Effects of climate‐induced changes in parasitism, predation and predator‐predator interactions on reproduction and survival of an Arctic marine bird. Arctic, 66, 43–51. 10.14430/arctic4265 [DOI] [Google Scholar]
- Gaston, A. J. , Hipfner, J. M. , & Campbell, D. (2002). Heat and mosquitoes cause breeding failures and adult mortality in an Arctic‐nesting seabird. Ibis, 144, 185–191. 10.1046/j.1474-919X.2002.00038.x [DOI] [Google Scholar]
- Gerson, A. R. , McKechnie, A. E. , Smit, B. , Whitfield, M. C. , Smith, E. K. , Talbot, W. A. , McWhorter, T. J. , & Wolf, B. O. (2019). The functional significance of facultative hyperthermia varies with body size and phylogeny in birds. Functional Ecology, 33, 597–607. 10.1111/1365-2435.13274 [DOI] [Google Scholar]
- Gilg, O. , Istomina, L. , Heygster, G. , Strøm, H. , Gavrilo, M. V. , Mallory, M. L. , Gilchrist, G. , Aebischer, A. , Sabard, B. , Huntemann, M. , Mosbech, A. , & Yannic, G. (2016). Living on the edge of a shrinking habitat: The ivory gull, Pagophila eburnea, an endangered sea‐ice specialist. Biology Letters, 12, 20160277 10.1098/rsbl.2016.0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber, C. E. , Nakagawa, S. , Laws, R. J. , & Jamieson, I. G. (2011). Multimodel inference in ecology and evolution: Challenges and solutions. Journal of Evolutionary Biology, 24, 699–711. 10.1111/j.1420-9101.2010.02210.x [DOI] [PubMed] [Google Scholar]
- Hayworth, A. M. , & Weathers, W. W. (1984). Temperature regulation and climatic adaptation in black‐billed and yellow‐billed magpies. Condor, 86, 19–26. 10.2307/1367336 [DOI] [Google Scholar]
- Hudson, J. W. , & Kimzey, S. L. (1966). Temperature regulation and metabolic rhythms in populations of the house sparrow, Passer domesticus . Comparative Biochemistry and Physiology, 17, 203–217. 10.1016/0010-406X(66)90021-1 [DOI] [PubMed] [Google Scholar]
- Jetz, W. , Freckleton, R. P. , & McKechnie, A. E. (2008). Environment, migratory tendency, phylogeny and basal metabolic rate in birds. PLoS One, 3(9), e3261 10.1371/journal.pone.0003261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov, W. H. (1990). Digestion in birds: Chemical and physiological determinants and implications In Morrison M. L., Ralph C. J., Verner J., & Jehl J. R. (Eds.), Studies in avian biology (vol. 13, pp. 391–415). Cooper Ornithological Society. [Google Scholar]
- Koller, M. (2016). robustlmm: An R package for robust estimation of linear mixed‐effects models. Journal of Statistical Software, 75, 1–24. 10.18637/jss.v075.i06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiewski, R. C. , Acosta, A. L. , & Bernstein, M. H. (1966). Evaporative water loss in birds – I. Characteristics of the open flow method of determination, and their relation to estimates of thermoregulatory ability. Comparative Biochemistry and Physiology, 19, 445–457. [Google Scholar]
- Le Pogam, A. , Love, O. P. , Régimbald, L. , Dubois, K. , Hallot, F. , Milbergue, M. , Petit, M. , O’Connor, R. S. , & Vézina, F. (2020). Wintering snow buntings elevate cold hardiness to extreme levels but show no changes in maintenance costs. Physiological and Biochemical Zoology, 93, 417–433. 10.1086/711370 [DOI] [PubMed] [Google Scholar]
- Lighton, J. R. B. (2019). Measuring metabolic rates: A manual for scientists (2nd ed.). Oxford University Press. [Google Scholar]
- Logan, M. (2010). Biostatistical design and analysis using R: A practical guide. Wiley‐Blackwell. [Google Scholar]
- Londoño, G. A. , Chappell, M. A. , del Rosario Castañeda, M. , Jankowski, J. E. , & Robinson, S. K. (2015). Basal metabolism in tropical birds: Latitude, altitude, and the ‘pace of life’. Functional Ecology, 29, 338–346. 10.1111/1365-2435.12348 [DOI] [Google Scholar]
- Lüdecke, D. (2018). ggeffects: Tidy data frames of marginal effects from regression models. Journal of Open Source Software, 3(26), 772 10.21105/joss.00772 [DOI] [Google Scholar]
- McKechnie, A. E. , Gerson, A. R. , McWhorter, T. J. , Smith, E. K. , Talbot, W. A. , & Wolf, B. O. (2017). Avian thermoregulation in the heat: Evaporative cooling in five Australian passerines reveals within‐order biogeographic variation in heat tolerance. Journal of Experimental Biology, 220, 2436–2444. 10.1242/jeb.155507 [DOI] [PubMed] [Google Scholar]
- McKechnie, A. E. , Hockey, P. A. R. , & Wolf, B. O. (2012). Feeling the heat: Australian landbirds and climate change. Emu, 112, 1–7. 10.1071/MUv112n2_ED [DOI] [Google Scholar]
- McKechnie, A. E. , & Wolf, B. O. (2010). Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biology Letters, 6, 253–256. 10.1098/rsbl.2009.0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie, A. E. , & Wolf, B. O. (2019). The physiology of heat tolerance in small endotherms. Physiology, 34, 302–313. 10.1152/physiol.00011.2019 [DOI] [PubMed] [Google Scholar]
- Meltofte, H. (1983). Arrival and pre‐nesting period of the snow bunting Plectrophenax nivalis in east Greenland. Polar Research, 1, 185–198. 10.1111/j.1751-8369.1983.tb00702.x [DOI] [Google Scholar]
- Milne, R. , Cunningham, S. J. , Lee, A. T. K. , & Smit, B. (2015). The role of thermal physiology in recent declines of birds in a biodiversity hotspot. Conservation Physiology, 3(1), cov048 10.1093/conphys/cov048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe, B. , Stempniewicz, L. , Jakubas, D. , Angelier, F. , Chastel, O. , Dinessen, F. , Gabrielsen, G. W. , Hanssen, F. , Karnovsky, N. J. , Rønning, B. , Welcker, J. , Wojczulanis‐Jakubas, K. , & Bech, C. (2009). Climate change and phenological responses of two seabird species breeding in the high‐Arctic. Marine Ecology Progress Series, 393, 235–246. 10.3354/meps08222 [DOI] [Google Scholar]
- Nieuwenhuis, R. , te Grotenhuis, M. , & Pelzer, B. (2012). influence.ME: Tools for detecting influential data in mixed effects models. R Journal, 4, 38–47. [Google Scholar]
- Nilsson, J. , & Nord, A. (2018). Testing the heat dissipation limit theory in a breeding passerine. Proceedings of the Royal Society B: Biological Sciences, 285, 20180652 10.1098/rspb.2018.0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes, M. J. , Wolf, B. O. , & McKechnie, A. E. (2016). Seasonal and geographical variation in heat tolerance and evaporative cooling capacity in a passerine bird. Journal of Experimental Biology, 219, 859–869. 10.1242/jeb.132001 [DOI] [PubMed] [Google Scholar]
- Nord, A. , Lehmann, M. , MacLeod, R. , McCafferty, D. J. , Nager, R. G. , Nilsson, J. , & Helm, B. (2016). Evaluation of two methods for minimally invasive peripheral body temperature measurement in birds. Journal of Avian Biology, 47, 417–427. 10.1111/jav.00845 [DOI] [Google Scholar]
- Nord, A. , & Nilsson, J. (2019). Heat dissipation rate constrains reproductive investment in a wild bird. Functional Ecology, 33, 250–259. 10.1111/1365-2435.13243 [DOI] [Google Scholar]
- Oswald, K. N. , Lee, A. T. K. , & Smit, B. (2018). Seasonal physiological responses to heat in an alpine range‐restricted bird: The Cape Rockjumber (Chaetops frenatus). Journal of Ornithology, 159, 1063–1072. 10.1007/s10336-018-1582-8 [DOI] [Google Scholar]
- Oswald, K. N. , Smit, B. , Lee, A. T. K. , & Cunningham, S. J. (2019). Behaviour of an alpine range‐restricted species is described by interactions between microsite use and temperature. Animal Behavior, 157, 177–187. 10.1016/j.anbehav.2019.09.006 [DOI] [Google Scholar]
- Overland, J. E. , Hanna, E. , Hanssen‐Bauer, I. , Kim, S.‐J. , Walsh, J. E. , Wang, M. , Bhatt, U. S. , Thoman, R. L. , & Ballinger, T. J. (2019). Surface air temperature InRichter‐Menge J., Druckenmiller M. L., & Jeffries M. (Eds.), Arctic Report Card 2019. http://www.artic.noaa.gov/Repor‐Card [Google Scholar]
- Prinzinger, R. , Preßmar, A. , & Schleucher, E. (1991). Body temperature in birds. Comparative Biochemistry and Physiology Part A: Physiology, 99, 499–506. 10.1016/0300-9629(91)90122-S [DOI] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing; https://www.R‐project.org/ [Google Scholar]
- Rezende, E. L. , & Bacigalupe, L. D. (2015). Thermoregulation in endotherms: Physiological principles and ecological consequences. Journal of Comparative Physiology B, 185, 709–727. 10.1007/s00360-015-0909-5 [DOI] [PubMed] [Google Scholar]
- Riddell, E. A. , Iknayan, K. J. , Wolf, B. O. , Sinervo, B. , & Beissinger, S. R. (2019). Cooling requirements fueled the collapse of a desert bird community from climate change. Proceedings of the National Academy of Sciences of the United States of America, 116, 21609–21615. 10.1073/pnas.1908791116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander, P. F. , Hock, R. , Walters, V. , Johnson, F. , & Irving, L. (1950). Heat regulation in some Arctic and tropical mammals and birds. Biological Bulletin, 99, 237–258. 10.2307/1538741 [DOI] [PubMed] [Google Scholar]
- Silva, J. P. , Catry, I. , Palmeirim, J. M. , & Moreira, F. (2015). Freezing heat: Thermally imposed constraints on the daily activity patterns of a free‐ranging grassland bird. Ecosphere, 6(7), 119 10.1890/ES14-00454.1 [DOI] [Google Scholar]
- Smith, E. K. , O'Neill, J. J. , Gerson, A. R. , McKechnie, A. E. , & Wolf, B. O. (2017). Avian thermoregulation in the heat: Resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert songbirds. Journal of Experimental Biology, 220, 3290–3300. 10.1242/jeb.161141 [DOI] [PubMed] [Google Scholar]
- Smith, P. A. , Elliott, K. H. , Gaston, A. J. , & Gilchrist, H. G. (2010). Has early ice clearance increased predation on breeding birds by polar bears? Polar Biology, 33, 1149–1153. 10.1007/s00300-010-0791-2 [DOI] [Google Scholar]
- Snell, K. R. S. , Stokke, B. G. , Moksnes, A. , Thorup, K. , & Fossøy, F. (2018). From Svalbard to Siberia: Passerines breeding in the high Arctic also endure the extreme cold of the western Steppe. PLoS One, 13(9), e0202114 10.1371/journal.pone.0202114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger, D. (2020). SiZer: significant zero crossings. R package version 0.1‐7. https://CRAN.R‐project.org/package=SiZer [Google Scholar]
- Tapper, S. , Nocera, J. J. , & Burness, G. (2020). Heat dissipation capacity influences reproductive performance in an aerial insectivore. Journal of Experimental Biology, 223, jeb222232 10.1242/jeb.222232 [DOI] [PubMed] [Google Scholar]
- Tieleman, B. I. , & Williams, J. B. (1999). The role of hyperthermia in the water economy of desert birds. Physiological and Biochemical Zoology, 72, 87–100. 10.1086/316640 [DOI] [PubMed] [Google Scholar]
- Tieleman, B. I. , & Williams, J. B. (2000). The adjustment of avian metabolic rates and water fluxes to desert environments. Physiological and Biochemical Zoology, 73, 461–479. 10.1086/317740 [DOI] [PubMed] [Google Scholar]
- Tieleman, B. I. , Williams, J. B. , & Buschur, M. E. (2002). Physiological adjustments to arid and mesic environments in larks (Alaudidae). Physiological and Biochemical Zoology, 75, 305–313. 10.1086/341998 [DOI] [PubMed] [Google Scholar]
- Van de Ven, T. M. F. N. , McKechnie, A. E. , & Cunningham, S. J. (2019). The costs of keeping cool: Behavioural trade‐offs between foraging and thermoregulation are associated with significant mass losses in an arid‐zone bird. Oecologia, 191, 205–215. 10.1007/s00442-019-04486-x [DOI] [PubMed] [Google Scholar]
- Weathers, W. W. (1979). Climatic adaptation in avian standard metabolic rate. Oecologia, 42, 81–89. [DOI] [PubMed] [Google Scholar]
- Weathers, W. W. (1981). Physiological thermoregulation in heat‐stressed birds: Consequences of body size. Physiological Zoology, 54, 345–361. 10.1086/physzool.54.3.30159949 [DOI] [Google Scholar]
- Weathers, W. W. , & van Riper III, C. (1982). Temperature regulation in two endangered Hawaiian honeycreepers: The palila (Psittirostra bailleui) and the laysan finch (Psittirostra cantans). The Auk, 99, 667–674. [Google Scholar]
- White, C. R. , Blackburn, T. M. , Martin, G. R. , & Butler, P. J. (2007). Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proceedings of the Royal Society B: Biological Sciences, 274, 287–293. 10.1098/rspb.2006.3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield, M. C. , Smit, B. , McKechnie, A. E. , & Wolf, B. O. (2015). Avian thermoregulation in the heat: Scaling of heat tolerance and evaporative cooling capacity in three southern African arid‐zone passerines. Journal of Experimental Biology, 218, 1705–1714. 10.1242/jeb.121749 [DOI] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant graphics for data analysis. Springer. [Google Scholar]
- Zhang, X. , Flato, G. , Kirchmeier‐Young, M. , Vincent, L. , Wan, H. , Wang, X. , Rong, R. , Fyfe, J. , Li, G. , & Kharin, V. V. (2019). Changes in temperature and precipitation across Canada; Chapter 4 In Bush E., & Lemmen D. S. (Eds.), Canada's changing climate report (pp. 112–193). Government of Canada. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Respirometry data analyzed for this study are available from the Dryad Digital Repository (https://doi.org/10.5061/dryad.4mw6m908g).


