Abstract
Antiretroviral therapy effectively controls human immunodeficiency virus (HIV) infection. However, a reservoir of latently infected cells persists under suppressive therapy, constituting a major barrier to an HIV cure. The block-and-lock approach to a functional cure aims at the transcriptional and epigenetic silencing of proviruses, blocking viral reactivation in the absence of therapy, preventing disease progression and transmission, despite the presence of detectable integrated proviruses. This approach has been put forward for exploration based on the activity of didehydro–cortistatin A, an inhibitor of the HIV transcriptional activator Tat. Here we review the mechanisms by which didehydro–cortistatin A inhibition of Tat’s feedback loop transcriptional amplification results in epigenetic silencing of the HIV promoter, and we discuss the benefits and limitations of the block-and-lock approach for an HIV cure.
Keywords: block-and-lock, functional cure, didehydro, cortistatin A, HIV transcription, epigenetic silencing
Antiretroviral (ARV) therapy (ART) effectively restricts human immunodeficiency virus (HIV) replication and dramatically improves survival rates in infected individuals. Unfortunately, fully replication-competent integrated proviruses persist, which can reinitiate full-blown viral replication on treatment interruption (TI). This latent reservoir of proviruses is established very early during infection and is characterized by very low to undetectable levels of viral expression [1]. Early ART initiation, during the acute phase of infection, reduces the size of the reservoir, but not sufficiently to prevent its establishment [2]. Several approaches have been explored to eliminate the viral reservoir or limit viral reactivation from latency [3].
The “block-and-lock” strategy has been proposed as an HIV functional cure [4–6]. The premise is with the addition of latency-promoting agents (LPAs), such as HIV Tat inhibitors or HIV-specific transcriptional inhibitors, to an ART regimen, the HIV promoter would become progressively epigenetically suppressed, resulting in durable HIV transcriptional inhibition in the absence of therapy (Figure 1). Ideal LPAs, in addition to favorable pharmacokinetics and minimal toxicity, should “block” transcription so that a durable epigenetic silencing, “lock,” may be established over time through the accumulation of repressive epigenetic marks at the HIV promoter. The premise is that under an HIV transcriptional lock, very little to no residual viral production would occur in the absence of ART, and the immune system would hold any minimally occurring virus in check, achieving a functional cure.
Figure 1.
Proposed combination of antiretroviral therapy (ART) with Tat inhibitors or other latency-promoting agents (LPAs) in the block-and-lock strategy. With ART, human immunodeficiency virus (HIV) transcription from the integrated provirus is unaffected, and episodes of detectable viremia or viral load (VL) “blips” are commonly observed (blue line). Treatment interruption (TI) leads to rapid viral rebound. The addition of the Tat inhibitor didehydro–cortistatin A (dCA) or another LPA to ART accelerates viral control via an additional block at HIV transcription, and long-term treatment further promotes heterochromatin formation and silences the provirus epigenetically, which might diminish viral rebound after TI (red dashed line).
Much of the human genome is epigenetically silenced as part of cellular differentiation [7]; thus, epigenetic silencing of the HIV genome is not far-fetched. For instance, it is commonly observed with human endogenous retroviruses [8]. The block-and-lock approach is also supported by the few but important functional cure examples, such as post-treatment controllers. These individuals generally initiated ART early during acute infection and, after a substantial period of adherence, interrupted ART and maintained viral suppression in the absence of therapy. Examples can be found in the VISCONTI cohort [9], the CHAMP study [10], a French teenager [11], and a child infected perinatally [12]. Whether these individuals can transmit the virus to others and whether long-term disease progression will be observed remains to be investigated. Post-treatment controllers generally have HIV-specific (although weak) immune responses, low levels of immune inflammation, and smaller HIV reservoirs [9–12]. Ideally, such therapeutic benefits may also be achieved through block-and-lock approaches.
HIV transcriptional expression requires binding of a plethora of host transcription factors (TFs), including NF-κB, specificity protein 1 (Sp1), TATA-binding protein (TBP), the general TFs (TFIIA, TFIIB, TFIIE, TFIIF, and TFIIH), and the Mediator complex to the HIV promoter to drive recruitment of RNA polymerase II (RNAPII), formation of the preinitiation complex, and RNAPII promoter escape (reviewed in [13]). However, RNAPII stalls after transcribing a short stretch of RNA from the transcription start site, which forms a dynamic secondary structure hairpin termed the transactivation response element RNA (TAR). RNAPII promoter proximal stalling is relieved through recruitment of the viral protein Tat. Through an interaction with TAR, Tat recruits the positive elongation factor, P-TEFb (CDK9/cyclin T1), to promote crucial phosphorylation events, which drive RNAPII transcriptional elongation. This results in the establishment of a positive feedback loop to exponentially amplify HIV transcription (Figure 2A).
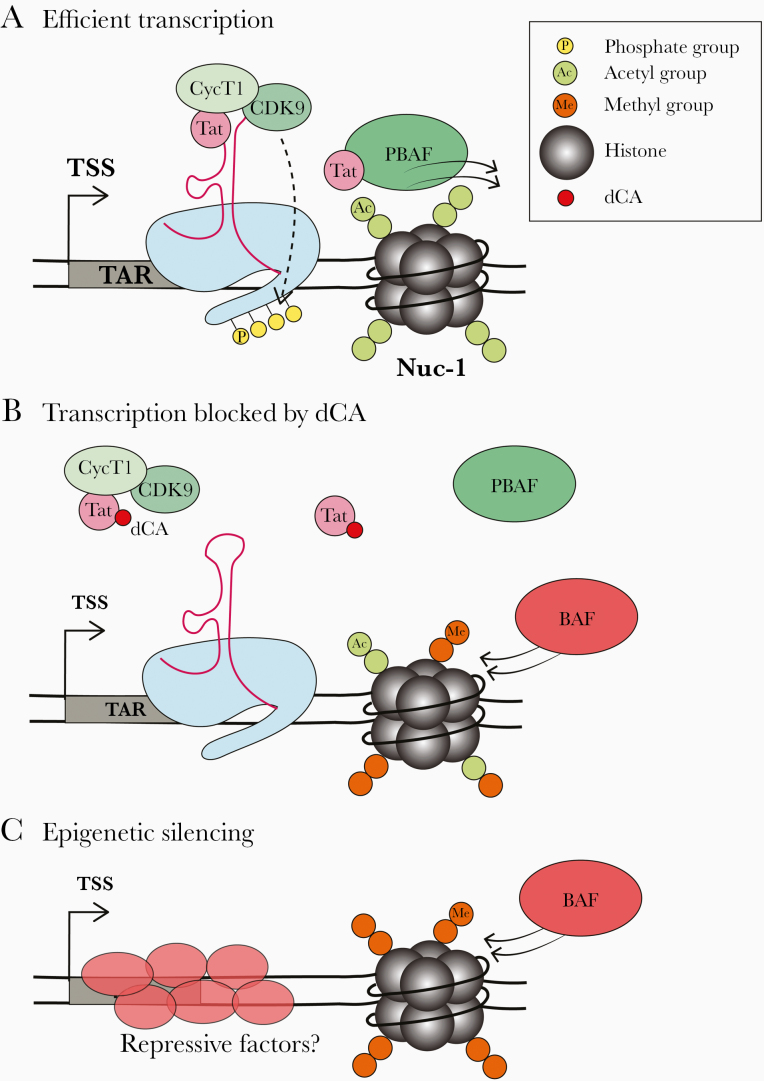
Figure 2.
Epigenetic silencing of the human immunodeficiency virus (HIV) promoter by the Tat inhibitor didehydro–cortistatin A (dCA). A, Tat drives efficient transcriptional elongation from the HIV promoter through specific recruitment of the positive elongation factor positive transcription elongation factor b (P-TEFb) to the HIV transactivation response element RNA (TAR) RNA hairpin structure driving further Tat expression and establishing a positive feedback loop. Tat also recruits the chromatin remodeling complex polybromo-associated factor (PBAF) to reposition Nuc-1 to promote elongation. B, dCA binds to the basic domain of Tat, blocking the Tat-TAR interaction and preventing recruitment of PBAF, breaking the Tat-TAR positive feedback loop. Epigenetic changes begin to appear at the HIV promoter, for example, decreased acetylation and increased methylation of Nuc-1 and increased recruitment of the repressive BRG1-associated facfor (BAF) chromatin remodeling complex, which positions Nuc-1 just downstream of the transcription start site (TSS) to physically occlude the transcription machinery. C, It is hoped that over time, long-term dCA treatment will lead to permanent epigenetic silencing of the promoter through methylation of Nuc-1, recruitment of BAF and recruitment of other repressive factors.
While limited Tat expression is usually correlated with latency [14], other mechanisms also participate in latency regulation, including the proviral integration site, levels of cellular transcription factors, epigenetic modifications, and chromatin remodeling [15]. Any of these mechanisms that influence HIV transcription could potentially be exploited for block-and-lock purposes (reviewed elsewhere [13, 16]). HIV inhibitors targeting host factors or signaling pathways required for viral expression, such as P-TEFb, heatshock protein 90 (HSP90), mammalian target of rapamycin (mTOR) complex, facilitates chromatin transactions (FACT), bromodomain containing protein 4 (BRD4), and xeroderma pigmentosum subtype B (XPB) have been reviewed recently by us and others [13, 17]. Importantly, though, direct inhibition of a viral protein is likely to provide a more specific and potent HIV suppression, by limiting off-target activity. Given the unique role of the Tat-TAR feedback circuitry in HIV transcription and absence of cellular homologues, blocking this interaction remains an outstanding approach. Through a series of in vitro and in vivo studies, our group discovered and characterized a potent Tat inhibitor, didehydro–cortistatin A (dCA), which activity proved the concept for the block-and-lock functional cure. Here we summarize current knowledge of the mechanisms by which dCA suppresses HIV transcription, ultimately resulting in long-lasting epigenetic silencing with inhibition of rebound on TI.
dCA BINDS TO THE BASIC DOMAIN OF TAT AND BLOCKS ITS INTERACTION WITH TAR
Our group’s previous studies suggested a role for cyclin-dependent kinase 11, CDK11 (PITSLRE, CDC2L), in the cleavage and polyadenylation of HIV messenger RNAs (mRNAs) [18, 19], suggesting that a CDK11 inhibitor might possess anti-HIV activity. Cortistatin A, a steroidal alkaloid isolated from the marine sponge Corticium simplex found in Southeast Asia, was reported as a high-affinity ligand of CDK11 [20], and a simpler-to-synthesize analog of cortistatin A, dCA, showed similar activity [21]. While dCA potently blocked HIV production, it was not correlated with CDK11 inhibition. In fact, dCA inhibits CDK19 and not CDK11 (PITSLRE, CDC2L); however, CDK19 was named CDK11 in the original human kinome nomenclature, leading to literature confusion [22].
Importantly though, from this mishap we discovered a potent activity of dCA as an inhibitor of Tat-mediated HIV-1 transcription [23]. dCA inhibits the Tat-TAR interaction by binding the basic domain of Tat, blocking Tat-dependent HIV transcriptional amplification in acutely and chronically infected cell line models (half-maximal effective concentration [EC50], 1 nM) [23]. The nanomolar affinity of dCA for Tat was shown by fluorescence isothermal titration calorimetry, and pull-down assays using biotinylated dCA confirmed interaction with Tat [24]. Molecular docking revealed dCA’s preferential binding to Tat’s basic patch [24], and structure-function relationship studies with dCA analog showed the importance of the isoquinoline group and intact cycloheptene ring for potent inhibitor activity [24]. Tat protein has a very loose structure, adopting specific conformations dependent on the binding partner [25]. Tat protein is stabilized by the interaction with dCA, locking it in a more stable conformer, with key residues in the basic domain forming a horse shoe-like patch that accommodates dCA. These results are supported by dCA dose-dependent protection of recombinant Tat from proteinase K digestion [24].
The dCA block of the Tat-TAR interaction was confirmed by electrophoretic mobility shift assays with radiolabeled TAR, and TAR DNA immunoprecipitation (ChIP) with transfected Tat [24]. Importantly, dCA blocks Tat-TAR interaction without altering basal HIV transcription. This was demonstrated using a long terminal repeat (LTR)–luciferase reporter system where the apical region of TAR was replaced with a 29-nucleotide stemloop II B (SIIB) subdomain of the REV-response element (RRE) [26]. In this system, a Tat-Rev fusion protein activates transcription when Rev binds the SIIB–RRE, bringing Tat in close proximity to P-TEFb. As expected, dCA did not interfere with transactivation in this system [26]. Furthermore, dCA did not interfere with the functions of HIV Rev protein or the cellular protein HEXIM-1, which have basic domains similar to those of Tat, confirming dCA specificity for Tat [24].
We also demonstrated that dCA’s inhibitory effect on HIV is independent of its role on the other known ligands, CDK19 and its paralog CDK8, based on the following: (1) short hairpin RNA (shRNA) knockdown of CDK8/CKD19 did not change HIV-1 transcription [24, 27]; (2) the cycloheptene ring and nitrogen positioning in the isoquinoline of dCA are important for Tat binding but not for CDK8 inhibition [24]; (3) high-affinity cortistatin A analog lacking the above features are potent CDK8 inhibitors [28], while inactive against HIV transcription [24]; and (4) analog of dCA without kinase activity remain very potent Tat inhibitors [24]. In sum, dCA specifically binds to the basic domain of Tat, interfering with the Tat-TAR interaction, a mechanism independent of its anti-CDK19/CDK8 activity.
dCA AND THE BLOCK-AND-LOCK CONCEPT
Based on the activity of dCA on HIV transcription, we proposed that Tat inhibitors might prove useful in block-and-lock functional cure approaches. We hypothesized that adding a Tat inhibitor, such as dCA, to an ART regimen could promote sustained epigenetic silencing of the HIV promoter, possibly allowing for ART interruption without viral rebound [23]. Thus, the long-term effects of dCA treatment were assessed in a series of cell lines, including a chronically infected HeLa-CD4 model, the promyelocytic OM-10.1 model, and J-Lat T-lymphocytic lines [29]. dCA treatment drastically reduced residual HIV transcription, inhibited RNAPII recruitment to the HIV promoter, blocked HIV reactivation on exposure to various latency reactivation reagents (LRAs), and delayed or prevented viral rebound on TI [29]. The specificity of dCA for the Tat-TAR interaction was also confirmed by little to no activity in cell lines with Tat/TAR-deficient viruses, namely, the T-lymphocytic ACH-2 cell line and the promonocyte U1 cell line [29, 30].
To assess the effects of dCA in a more relevant HIV latency model, we developed a primary cell model using CD4+ T cells isolated from aviremic, ART-suppressed individuals [31]. Using this model, primary CD4+ T cells were expanded and treated with ART or dCA plus ART. Viral mRNA levels in cells treated with dCA plus ART dropped below the limit of detection at day 21, about 14 days earlier than the ART-only control, suggesting that dCA treatment accelerates HIV suppression [6]. When all samples were exposed to the PKC activator, prostratin, dCA pre-treatment drastically inhibited (99%) viral reactivation [6]. Furthermore, dCA pre-treatment prevented viral rebound for up to 25 days on TI in cells from all subjects, while rebound was observed in all ART-only treated cells. Thus, long-term dCA treatment results in long-lasting latency refractory to viral reactivation.
Low-level virus production is often detected in many ART-suppressed individuals [32, 33], which is associated with immune activation and chronic inflammation [34]. There is also growing evidence of biologically relevant gene expression from the pool of defective proviruses that results in viral protein production, novel HIV RNAs, and chimeric HIV proteins that can drive immune responses [35–37]. Unlike conventional ART, blocking HIV transcription with dCA may have the added benefit of reducing chronic immune activation.
dCA ACTIVITY IN THE HUMANIZED MICE MODEL OF HIV INFECTION AND LATENCY
dCA is stable both in vitro and in vivo, has a drug-like structure, is easily formulated owing to its high aqueous solubility, acts additively with other ARVs, and crosses the blood-brain barrier [23, 38]. Daily intraperitoneal injection of mice with dCA has been shown to reach therapeutically relevant levels in plasma and viral sanctuary sites, such as the brain, without adverse effects [38]. Thus, we investigated the effects of dCA in the bone marrow-liver-thymus (BLT) mouse model of HIV latency [6]. First, we assessed dCA activity on plasma viral loads (VLs), human CD4+ T cell counts and cell-associated viral RNA in various lymphoid tissues. Briefly, BLT mice were infected with HIV-1JRCSF, and, once systemic infection was confirmed (at 2 weeks), ART was initiated and maintained for 5 weeks until plasma VLs were undetectable in all animals. Mice were then treated with vehicle or dCA for 2 weeks. dCA administration significantly reduced mean cell-associated viral RNA in lymphoid tissues (approximately 3.8-fold, including lymph node, spleen, peripheral blood mononuclear cells, and bone marrow), with an approximately 7-fold reduction in the brain [6].
Next, we determined the effects of dCA treatment on viral rebound after TI. BLT mice were again infected with HIV-1JRCSF, and, once systemic infection was confirmed (2 weeks), ART was initiated. After 3 weeks of ART, mice were treated with vehicle plus ART or dCA plus ART for a period of 4 weeks, after which treatment was interrupted and plasma VL was measured over time. dCA treatment significantly delayed viral rebound (by 12 days on average) and drastically reduced the viral rebound set point [6]. These exciting results highlighted the potential of dCA to promote long-term suppression of HIV in vivo, and we expect that even more impressive results could be observed with longer pretreatment periods before TI. These studies are currently ongoing.
dCA PROMOTES HETEROCHROMATINIZATION OF THE HIV PROMOTER
To understand the mechanism by which dCA blocks latent provirus reactivation, we characterized the epigenetic profile of the HIV promoter in several cell models, namely, chronically infected HeLa-CD4, OM-10.1, U1, and ACH-2. As expected, long-term dCA treatment correlated with reduced HIV transcription and RNAPII recruitment, even under strong reactivation conditions [30]. Micrococcal nuclease nucleosomal mapping revealed that dCA treatment significantly stabilized Nuc-1/DNA interaction, as evidenced by increased protection from micrococcal nuclease digestion. Increased nucleosomal occupancy was confirmed by ChIP, where dCA treated cells showed increased occupancy of deacetylated histone 3 at Nuc-1 (associated with latency) and decreased histone acetylation levels (associated with active transcription) [30]. Furthermore, in unstimulated cells we observed increased recruitment of the inhibitory SWI/SNF chromatin remodeling complex BRG-1 associated factor (BAF) and decreased occupancy of polybromo-associated factor (PBAF) (associated with active transcription) [30, 39]. Importantly, the specificity of dCA was demonstrated by the total absence of epigenetic changes at the HIV promoter in cell lines containing Tat-TAR incompetent proviruses.
In sum, dCA accelerates the establishment of proviral latency by facilitating and accelerating the corresponding epigenetic modifications (Figure 2B). These silencing epigenetic modifications reduce RNAPII recruitment and thus transcription initiation and viral mRNA synthesis, ultimately reducing viral protein production, including Tat [23, 30]. Reducing Tat levels to below a certain threshold drives the virus into transcriptional silencing, while a certain level of Tat production above that threshold is required for reactivation [40, 41]. Tat is thus a switch between latency and reactivation by establishing a positive feedback loop. Once this loop is broken, less RNAPII and transcriptional activators are recruited to the promoter and are replaced by transcriptional repressors, which epigenetically silence the promoter. It is expected that the accumulation of certain silencing marks on the HIV genome (eg, histone 3 lysine 9 trimethylation) recruit transcriptional repressors that “read” this mark. This will result in the self-propagating silencing of proviruses, rendering them permanently silenced or at least extremely resistant to reactivation (Figure 2C).
HIV latency is a dynamic and flexible process, and dCA-induced latency is likely far more complex than described above, and more needs to be understood [4]. Although Tat inhibitors can delay and attenuate viral rebound, and prolonged treatment results in a repressive heterochromatin environment, the durability of those epigenetic modifications remains a question. To achieve permanent epigenetic silencing, additional features at the HIV promoter are likely needed, for example, removal of RNAPII at the transcription start site, nucleosome sliding to unfavorable positions, DNA methylation, histone hypoacetylation and hypermethylation, recruitment of repressors such as DNA, and histone methyltransferases. Ultimately, transcription initiation would become very unlikely, leading to further propagation of these marks with the establishment of a silencing feedback loop.
dCA INHIBITS TAT-MEDIATED NEUROINFLAMMATION AND PREVENTS POTENTIATION OF COCAINE REWARD IN TAT TRANSGENIC MICE
In addition to its crucial role in HIV transcription, Tat has also been implicated in HIV-1–associated neurocognitive disorders (HAND) [42]. Excreted Tat from infected cells can be taken in by noninfected cells directly or through membrane receptors, contributing to the release of soluble factors involved in inflammation, oxidative stress, and excitotoxicity, leading to neuronal damage [43]. We investigated the potential for dCA to relieve HIV-related neuropathogenesis [38]. dCA blocked extracellular Tat uptake in microglialike and astrocyte cell line models, as well as Tat-mediated release of inflammatory signaling proteins interleukin 1-β, tumor necrosis factor α, and monocyte chemoattractant protein 1 in an astrocytic cell line [38]. We also observed a reduction in these inflammatory markers in the brains of infected BLT mice treated with dCA. Tat also potentiates cocaine-mediated reward mechanisms by disrupting the dopaminergic system [44], and the neuronal damage observed in HAND is escalated by abuse of psychostimulants. As such, using a GT-tg bigenic mouse model specifically expressing Tat in astrocytes and using conditioned place preference experiments, we demonstrated that dCA reversed Tat potentiation of cocaine-mediated reward [38]. In sum, dCA, in addition to blocking HIV transcription, has the potential to improve Tat-associated outcomes in HAND and substance abuse.
RESISTANCE TO dCA
HIV rapidly escapes selective pressure and has evolved resistance to members of all ARVs classes when used individually, thus the use of combination therapy [45]. We passaged HIV-1 NL4-3 strain in naive HeLa-CD4 cells for 12 months, with increasing doses of dCA to select dCA-resistance virus [46]. Two resistant variants were isolated, MUT1 and MUT2, capable of replicating in 1 μmol/L of dCA (approximately 1000 times the EC50 of dCA), while remaining sensitive to other ARVs. Next-generation sequencing revealed 9 point mutations and a major insertion of 2 NF-kB/1 Sp1 sites in MUT1, and 13 point mutations in MUT2 [46]. These mutations were found in the LTR as well as in Gag, Pol, Vif, Vpr, Tat, Env, and Nef. Mutations in Tat and TAR were not identified, consistent with the high level of conservation of these elements. Through a detailed work of reverse genetics, we identified mutations in HIV-1 LTR, Nef, and Vpr regions as key in the resistance to dCA. The nucleotide changes in the LTR promoted a 10–20-fold increase in basal transcription, while Nef mutations and truncation of Vpr resulted in upregulation of NF-κB activity.
This greater transcriptional fitness results in higher viral production and consequently higher cytopathic effects on the host cell [46]. Furthermore, infection of primary CD4+ T cells with these dCA-resistant virus presented a higher level of capsid expression per cell and showed a trend toward increased susceptibility to CD8+T cell killing [46]. Collectively, we demonstrated that a combination of 13 mutations in the LTRs Nef and Vpr results in superior viral fitness and evasion of dCA, emphasizing the high genetic barrier to dCA resistance. It is uncertain whether these mutant viruses would have been identified in vivo. Their increased transcription fitness, confirmed by higher RNAPII recruitment to the promoter, may ultimately be detrimental. Their inability to control entry into latency may lead to higher cytopathic effects and possibly clearance by the immune system. To our knowledge, the present study describes the first HIV-1 isolates resistant to a Tat inhibitor. Work is ongoing to detail the molecular mechanisms by which the MUT Nef and Vpr regions regulate NF-κB, as well as the landscape of transcription factors associating with mutant LTRs. These results will certainly provide important insight into novel mechanisms that regulate HIV-1 transcription.
dCA INHIBITS SIMIAN IMMUNODEFICIENCY VIRUS REPLICATION AND REACTIVATION
Simian immunodeficiency virus (SIV) infection of rhesus macaques is the best-characterized model to assess viral transmission, latency, and pathogenesis. To characterize the activity of dCA on SIV infections, Hut78 cells were infected with SIVmac251 or SIVmac239 in the presence of dCA [26]. Both biochemical and cellular assays showed that dCA binds directly to SIV Tat’s basic domain, blocking Tat-TAR interaction, neutralizing SIV Tat transactivation and inhibiting acute and chronic SIV infection in the low nanomolar range [26]. dCA excludes SIV Tat from the nucleolus while remaining mostly nuclear, in agreement with results obtained with HIV-1 Tat [23].
In an SIV latency cell model, dCA significantly inhibited SIV replication and viral activation, by reducing RNAPII recruitment to the SIV-LTR [26]. Importantly, adding dCA to ART reduced viral RNA production and viral rebound with LRAs in primary CD4+ T cells from healthy rhesus macaques infected in vitro with SIVmac251 or SIVmac239 [26]. In the more biologically relevant CD4+ T cells explanted from ART-naive SIV-infected rhesus macaques, treatment with dCA blocked viral reactivation with LRAs by 1 log [26]. Thus, the efficacy of dCA on SIV opens the possibility for preclinical evaluation of dCA in the block-and-lock approach in nonhuman primate (NHP) models. This will allow assessment of dCA regimens on infection dynamics, latency, rebound, and reservoir size. Preliminary pharmacokinetics of dCA in NHPs is promising, and efficacy pilot studies are ongoing.
DISCUSSION
Our studies with dCA provided the proof of concept for the use of Tat inhibitors in block-and-lock approaches. Tat inhibitors are unlike any other ARV, in that duration of treatment affects the outcome. The reason being the feedback nature of Tat-TAR activity, where transcriptional silencing allows accumulation of epigenetic marks at the HIV-1 promoter over time [47]. We hypothesized that, ultimately, transcriptional repression could be pushed past a certain threshold where viral reactivation from latency is extremely difficult, permanently locking HIV into sustained latency. The additive activity of dCA to other ARVs also supports adding Tat inhibitors to first-line treatment to accelerate viral suppression, potentially reducing the size of the established reservoir. It is emerging from studies of individuals receiving very early ART that a smaller reservoir size directly translates into better viral control [48].
HIV reservoirs are characterized by high heterogeneity in molecular mechanism, cell type, and tissue distribution [49, 50]. Hence, using combinations of LPAs with different mechanisms of action may be effective in suppressing viral expression. Combinatorial use of LPAs that act synergistically may also allow dose reduction, minimizing long-term toxicity. We suspect that viral resistance to HIV-1 transcriptional inhibitors is less likely to emerge owing to the interplay of both viral and host proteins in HIV-1 transcription. Other potential targets to be explored in the block-and-lock approaches are reviewed elsewhere and shown in Figure 3 [13, 17].
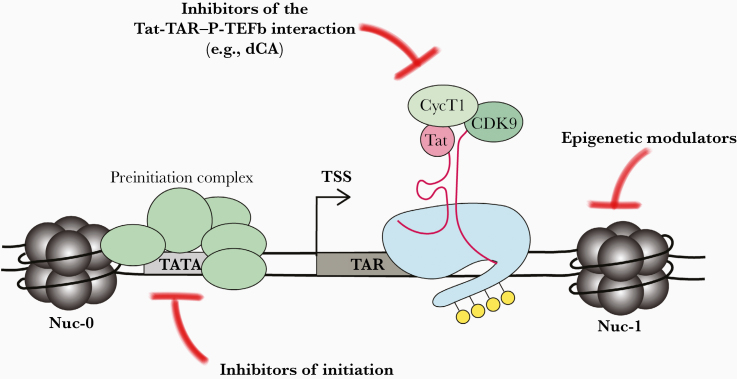
Figure 3.
Potential targets for the block-and-lock approach. Human immunodeficiency virus (HIV) transcription can be blocked at several different steps. Host factors, such as components of the preinitiation complex, may serve as suitable targets to block transcriptional initiation. Alternatively, Tat or other components involved in the Tat–transactivation response element RNA (TAR)–positive elongation factor b (P-TEFb) interaction can be targeted to block transcriptional elongation, eg, didehydro–cortistatin A (dCA). Finally, factors that modulate the chromatin environment of the HIV provirus could be modulated to block transcription. Abbreviation: TSS, transcription start site.
In conclusion, studies using dCA to target Tat opened promise to permanent HIV suppression and ultimately a functional cure. It is now critical to assess the full clinical potential of Tat inhibitors. This will involve identification of additional Tat inhibitors, exploring various LPA combinations, evaluating their in vivo efficacy in NHP models and investigating resistant viruses in vivo. Some important questions that need addressing include (1) the relationship between LPA regimen and residual viremia in tissues and time to rebound after TI; (2) the impact of LPAs as front-line therapy on the size of the viral reservoir; (3) the mechanisms of viral resistance to LPAs in vivo; (4) the impact of Tat inhibitors on immune activation and chronic inflammation associated with HIV infection; and (5) with regard to sustained latency, whether all therapy should be interrupted, or whether an LPA needs to be maintained for virological suppression. Moving an HIV-1 transcriptional inhibitor, such as a Tat inhibitor, into clinical use will help answer these questions, bringing us closer to a functional cure for HIV.
Presented in part: Keystone HIV Persistence, March 20–24, 2016; IAS, Paris, France, July 23–26, 2017; Strategies for an HIV Cure, Washington DC, U.S.A, October 10–12, 2018; Keystone Functional Cures and the Eradication of HIV, Whistler, British Columbia, Canada, March 24–28, 2019; Acute HIV Science Meeting, Bethesda, Maryland, March 28, 2018.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01AI097012, R01AI118432-01A1, R01AI097012-06A1, R21/R33 AI116226-01, and R21AI112462), and the Campbell Foundation.
Supplement sponsorship. This supplement is sponsored by the Harvard University Center for AIDS Research (CFAR), an NIH funded program (P30 AI060354), and the Ragon Institute of MGH, MIT and Harvard. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest.. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 1998; 95:8869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colby DJ, Trautmann L, Pinyakorn S, et al. ; RV411 study group Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med 2018; 24:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rasmussen TA, Lewin SR. Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents? Curr Opin HIV AIDS 2016; 11:394–401. [DOI] [PubMed] [Google Scholar]
- 4. Elsheikh MM, Tang Y, Li D, Jiang G. Deep latency: A new insight into a functional HIV cure. EBioMedicine 2019; 45:624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darcis G, Van Driessche B, Van Lint C. HIV latency: should we shock or lock? Trends Immunol 2017; 38:217–28. [DOI] [PubMed] [Google Scholar]
- 6. Kessing CF, Nixon CC, Li C, et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a “block-and-lock” strategy for HIV-1 treatment. Cell Rep 2017; 21:600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 2003; 33:245–54. [DOI] [PubMed] [Google Scholar]
- 8. Hurst TP, Magiorkinis G. Epigenetic control of human endogenous retrovirus expression: Focus on regulation of long-terminal repeats (LTRs). Viruses 2017; 9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. ; ANRS VISCONTI Study Group Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Namazi G, Fajnzylber JM, Aga E, et al. The control of HIV After antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis 2018; 218:1954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frange P, Faye A, Avettand-Fenoël V, et al. ; ANRS EPF-CO10 Pediatric Cohort and the ANRS EP47 VISCONTI study group HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 3:e49–54. [DOI] [PubMed] [Google Scholar]
- 12. Violari A, Cotton MF, Kuhn L, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun 2019; 10:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mori L, Valente ST. Key players in HIV-1 transcriptional regulation: targets for a functional cure. Viruses 2020; 12:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Razooky BS, Pai A, Aull K, Rouzine IM, Weinberger LS. A hardwired HIV latency program. Cell 2015; 160:990–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khoury G, Darcis G, Lee MY, et al. The molecular biology of HIV latency. Adv Exp Med Biol 2018; 1075:187–212. [DOI] [PubMed] [Google Scholar]
- 16. Hakre S, Chavez L, Shirakawa K, Verdin E. Epigenetic regulation of HIV latency. Curr Opin HIV AIDS 2011; 6:19–24. [DOI] [PubMed] [Google Scholar]
- 17. Vansant G, Bruggemans A, Janssens J, Debyser Z. Block-and-lock strategies to cure HIV infection. Viruses 2020; 12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valente ST, Gilmartin GM, Venkatarama K, Arriagada G, Goff SP. HIV-1 mRNA 3’ end processing is distinctively regulated by eIF3f, CDK11, and splice factor 9G8. Mol Cell 2009; 36:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valente ST, Gilmartin GM, Mott C, Falkard B, Goff SP. Inhibition of HIV-1 replication by eIF3f. Proc Natl Acad Sci U S A 2009; 106:4071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cee VJ, Chen DY, Lee MR, Nicolaou KC. Cortistatin A is a high-affinity ligand of protein kinases ROCK, CDK8, and CDK11. Angew Chem Int Ed Engl 2009; 48:8952–7. [DOI] [PubMed] [Google Scholar]
- 21. Shenvi RA, Guerrero CA, Shi J, Li CC, Baran PS. Synthesis of (+)-cortistatin A. J Am Chem Soc 2008; 130:7241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science 2002; 298:1912–34. [DOI] [PubMed] [Google Scholar]
- 23. Mousseau G, Clementz MA, Bakeman WN, et al. An analog of the natural steroidal alkaloid cortistatin A potently suppresses Tat-dependent HIV transcription. Cell Host Microbe 2012; 12:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mediouni S, Chinthalapudi K, Ekka MK, et al. Didehydro-cortistatin A inhibits HIV-1 by specifically binding to the unstructured basic region of Tat. MBio 2019; 10:e02662-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tahirov TH, Babayeva ND, Varzavand K, Cooper JJ, Sedore SC, Price DH. Crystal structure of HIV-1 Tat complexed with human P-TEFb. Nature 2010; 465:747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mediouni S, Kessing CF, Jablonski JA, et al. The Tat inhibitor didehydro-cortistatin A suppresses SIV replication and reactivation. FASEB J 2019; 33:8280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cary DC, Rheinberger M, Rojc A, Peterlin BM. HIV transcription is independent of mediator kinases. AIDS Res Hum Retroviruses 2019; 35:710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee HM, Nieto-Oberhuber C, Shair MD. Enantioselective synthesis of (+)-cortistatin a, a potent and selective inhibitor of endothelial cell proliferation. J Am Chem Soc 2008; 130:16864–6. [DOI] [PubMed] [Google Scholar]
- 29. Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, Valente ST. The tat inhibitor didehydro-cortistatin a prevents HIV-1 reactivation from latency. MBio 2015; 6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li C, Mousseau G, Valente ST. Tat inhibition by didehydro-cortistatin A promotes heterochromatin formation at the HIV-1 long terminal repeat. Epigenetics Chromatin 2019; 12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takata H, Kessing C, Sy A, et al. Modeling HIV-1 latency using primary CD4+ T cells from HIV-1 infected ART suppressed individuals. J Virol 2019; 93:e02248–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cillo AR, Sobolewski MD, Bosch RJ, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2014; 111:7078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2008; 105:3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 2013; 254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Imamichi H, Dewar RL, Adelsberger JW, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A 2016; 113:8783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pollack RA, Jones RB, Pertea M, et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T Lymphocytes, which shape the proviral landscape. Cell Host Microbe 2017; 21:494–506.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imamichi H, Smith M, Adelsberger JW, et al. Defective HIV-1 proviruses produce viral proteins. Proc Natl Acad Sci U S A 2020; 117:3704–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mediouni S, Jablonski J, Paris JJ, et al. Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res 2015; 13:64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rafati H, Parra M, Hakre S, Moshkin Y, Verdin E, Mahmoudi T. Repressive LTR nucleosome positioning by the BAF complex is required for HIV latency. PLoS Biol 2011; 9:e1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell 2005; 122:169–82. [DOI] [PubMed] [Google Scholar]
- 41. Morton EL, Forst CV, Zheng Y, et al. Transcriptional circuit fragility influences HIV proviral fate. Cell Rep 2019; 27:154–71.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spudich S HIV and neurocognitive dysfunction. Curr HIV/AIDS Rep 2013; 10:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mediouni S, Marcondes MC, Miller C, McLaughlin JP, Valente ST. The cross-talk of HIV-1 Tat and methamphetamine in HIV-associated neurocognitive disorders. Front Microbiol 2015; 6:1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H. HIV, Tat and dopamine transmission. Neurobiol Dis 2017; 105:51–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coffin JM HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 1995; 267:483–9. [DOI] [PubMed] [Google Scholar]
- 46. Mousseau G, Aneja R, Clementz MA, et al. Resistance to the tat inhibitor didehydro-cortistatin a is mediated by heightened basal HIV-1 transcription. MBio 2019; 10:e01750–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karn J The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Curr Opin HIV AIDS 2011; 6:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pardons M, Fromentin R, Pagliuzza A, Routy JP, Chomont N. Latency-reversing agents induce differential responses in distinct memory CD4 T cell subsets in individuals on antiretroviral therapy. Cell Rep 2019; 29:2783–2795.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Darcis G, Kula A, Bouchat S, et al. An in-depth comparison of latency-reversing agent combinations in various in vitro and ex vivo HIV-1 latency models identified bryostatin-1+JQ1 and Ingenol-B+JQ1 to potently reactivate viral gene expression. PLoS Pathog 2015; 11:e1005063. [DOI] [PMC free article] [PubMed] [Google Scholar]